Enhancing Chemical Stability and Bioavailability of Aneratrigine Capsules via Dry Granulation: Addressing Stability Challenges in Sodium Bicarbonate-Containing Formulations for Clinical Development
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Laboratory-Scale Compatibility and Stress Testing
2.3. Preparation of Lab-Scale Dry Granules and Ejection Force Evaluation (Slugging Method)
2.4. Preparation of Production-Scale Dry Granules (Roller Compaction)
2.5. Evaluation of Slugs
2.5.1. Tensile Strength of Slugs
2.5.2. Disintegration Testing of Slugs
2.6. Dissolution Testing of Capsules
2.7. Impurity Analysis
2.8. Statistical Analysis
3. Results
3.1. Root-Cause Analysis of the Wet Granulation Process
3.1.1. Discoloration
3.1.2. Impurities
3.2. Dry Granulation Formulation Study
Screening Experiments
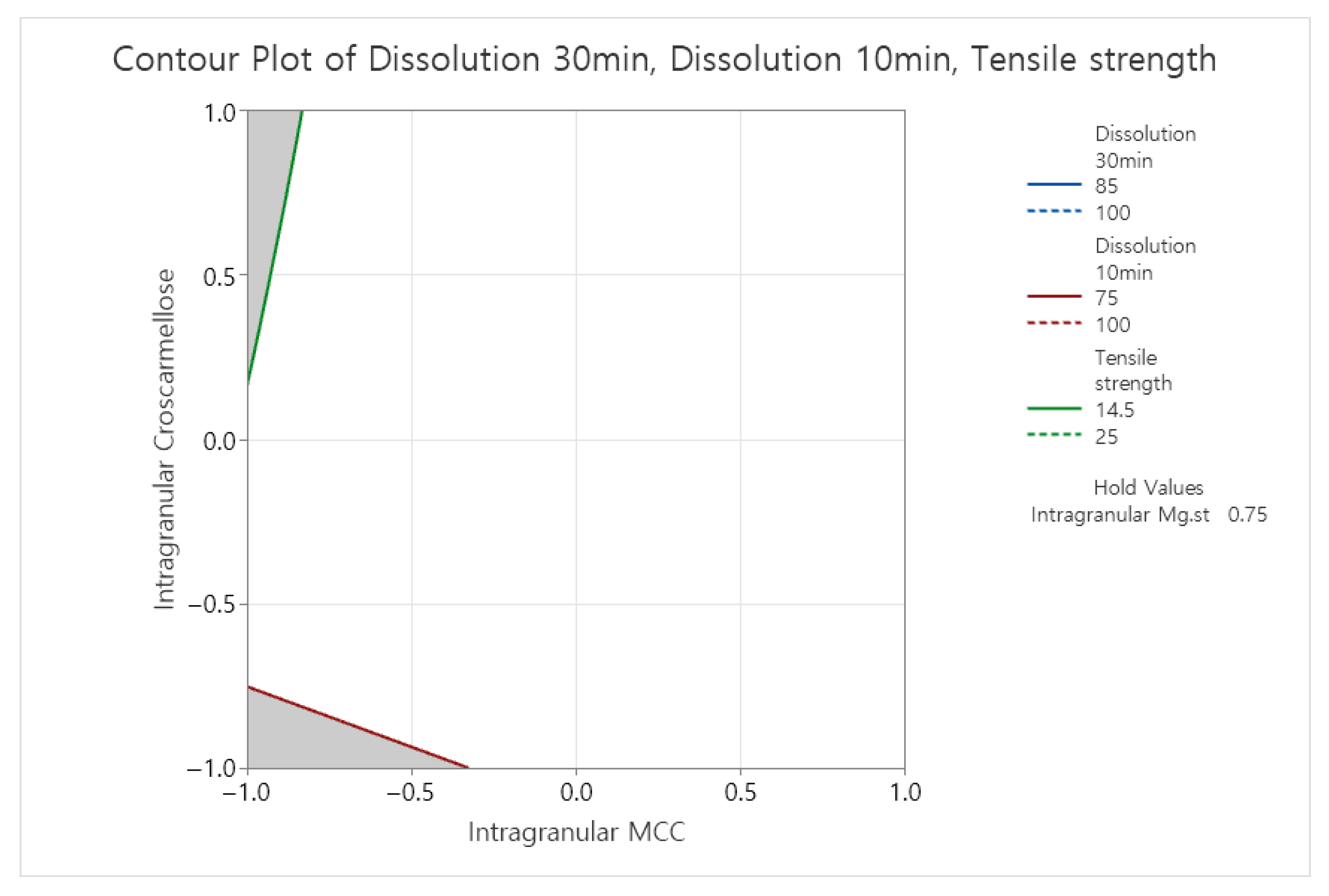
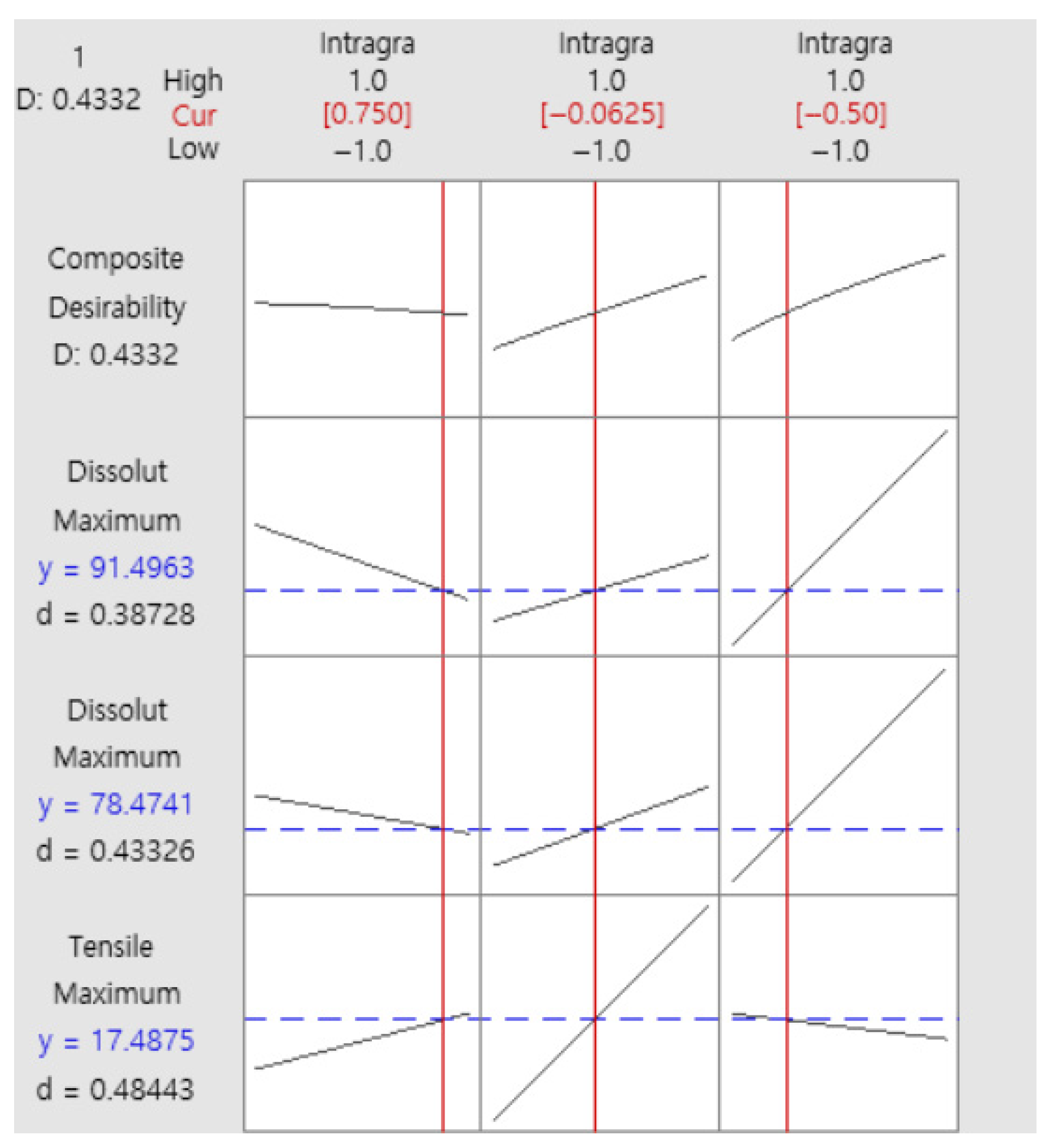
3.3. Formulation Optimization
3.4. Verification of Dry Granulation at Production Scale
3.5. Stability Study Results
3.5.1. Pilot-Scale Stability
3.5.2. Production-Scale Stability
4. Discussion
4.1. Decomposition Mechanism of Sodium Bicarbonate and Its Impact on Formulation Stability
4.2. Scientific Rationale for Process Change and Scalability
4.3. Process-Dependent Functionality of Excipients
4.4. Quality by Design (QbD) Optimization Strategy
4.5. Successful Scale-Up and Technology Transfer
4.6. Stability Profile and Long-Term Quality Assurance
- six-month accelerated/long-term testing [pilot-scale batch (1.5 kg)] (see Section 3.5.1)
- three-month accelerated/long-term testing [Technical (non-GMP) batch (5.4 kg)] (see Section 3.5.2)
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| Nav | Voltage-Gated Sodium Channel |
| DoE | Design of Experiments |
| OECD | Organization for Economic Co-operation and Development |
| U.S | United States |
| FDA | Food and Drug |
| IC50 | Half Maximal Inhibitory Concentration (the concentration of an inhibitor where the response is reduced by half) |
| pKa1,2,3… | Acid Dissociation Constant |
| API | Active pharmaceutical ingredient |
| DW | Distilled water |
| RH | Relative humidity |
| q.s | as much as needed |
| HPLC | High-Performance Liquid Chromatography |
| RC | Regenerated Cellulose |
| MCC | Microcrystalline Cellulose |
| AV | Acceptance value |
| RL | Report Limit |
| CFU | Colony Forming Unit |
| N/D | Not Detected |
| NMT | Not more than |
| N/T | Not Tested |
| TBD | To Be Determined |
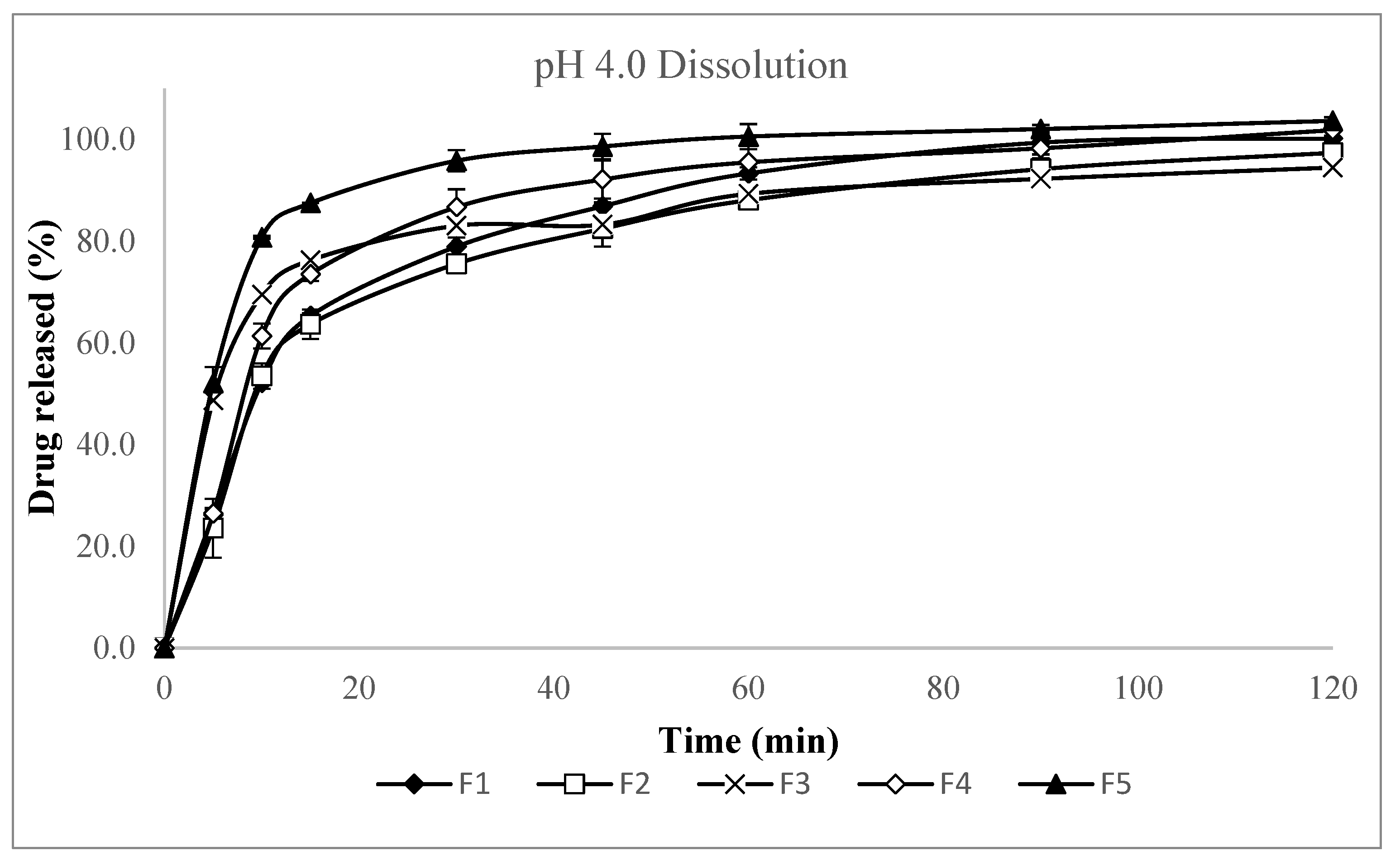
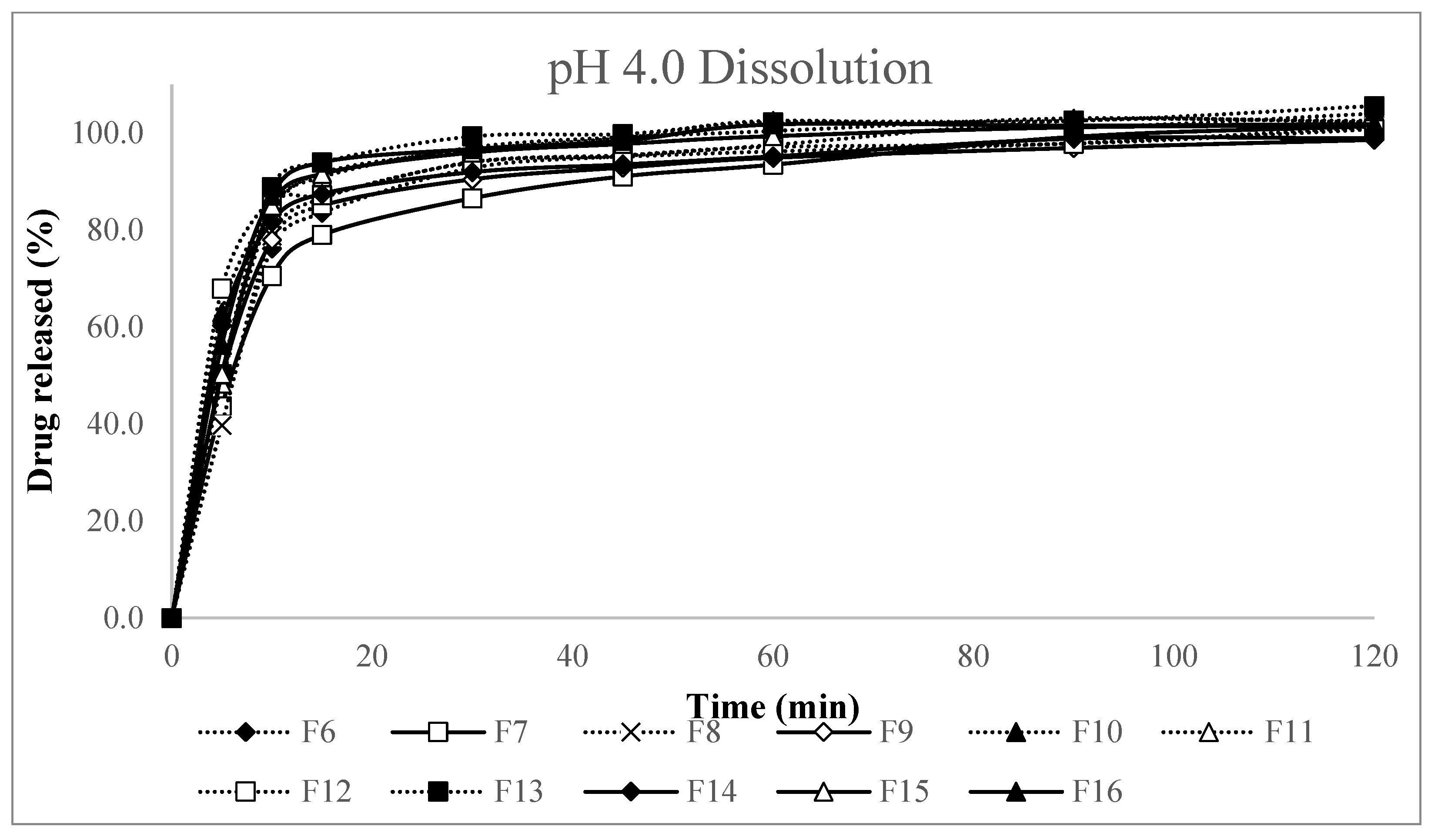
Appendix A. pH 4.0 Dissolution Test Results
Appendix A.1. pH 4.0 Dissolution Test Results: Screening Study (F1–F5), Formulation Study (F6–F16)
| pH 4.0 | Drug Release (%) | |||||||
|---|---|---|---|---|---|---|---|---|
| Formulation No/Time | 5 min | 10 min | 15 min | 30 min | 45 min | 60 min | 90 min | 120 min |
| F1 | 26.2 | 52.2 | 65.3 | 78.9 | 86.9 | 93.3 | 99.4 | 100.2 |
| F2 | 23.6 | 53.5 | 63.6 | 75.5 | 82.5 | 88.1 | 94.2 | 97.4 |
| F3 | 48.7 | 69.5 | 76.2 | 83.0 | 83.3 | 89.3 | 92.3 | 94.5 |
| F4 | 26.4 | 61.3 | 73.5 | 86.7 | 92.1 | 95.5 | 98.2 | 101.8 |
| F5 | 52.1 | 80.8 | 87.5 | 95.8 | 98.6 | 100.6 | 102.0 | 103.7 |
| pH 4.0 | Drug Release (%) | |||||||
|---|---|---|---|---|---|---|---|---|
| Formulation No/Time | 5 min | 10 min | 15 min | 30 min | 45 min | 60 min | 90 min | 120 min |
| F6 | 39.8 | 76.0 | 82.5 | 91.7 | 93.6 | 95.4 | 99.9 | 99.3 |
| F7 | 43.6 | 70.3 | 77.9 | 85.5 | 89.5 | 91.5 | 96.7 | 98.3 |
| F8 | 39.8 | 78.5 | 85.4 | 92.8 | 93.5 | 94.0 | 95.2 | 98.0 |
| F9 | 49.5 | 77.7 | 83.9 | 89.3 | 91.2 | 92.7 | 94.2 | 95.4 |
| F10 | 63.0 | 83.5 | 90.5 | 95.2 | 97.1 | 100.1 | 98.3 | 100.5 |
| F11 | 48.3 | 83.8 | 89.7 | 95.8 | 97.4 | 98.1 | 100.2 | 98.8 |
| F12 | 68.0 | 85.9 | 85.8 | 92.7 | 93.6 | 94.8 | 94.9 | 97.4 |
| F13 | 50.3 | 88.6 | 92.7 | 98.0 | 98.0 | 99.8 | 99.6 | 102.1 |
| F14 | 60.3 | 81.4 | 86.2 | 90.7 | 91.8 | 92.9 | 96.1 | 95.6 |
| F15 | 50.3 | 84.8 | 90.3 | 94.7 | 96.0 | 97.0 | 98.3 | 98.1 |
| F16 | 56.3 | 86.8 | 92.6 | 95.4 | 96.6 | 99.4 | 98.6 | 98.4 |
Appendix B.2. Statistical Analysis Results (Minitab® 22 Statistic Software): Formulation Study (F6–F16)
Appendix B.2.1. Tensile Strength of Slugs
| Source | DF | Adj SS | Adj MS | F-Value | p-Value |
|---|---|---|---|---|---|
| Model | 5 | 16.6548 | 3.3310 | 5.87 | 0.037 |
| Linear | 3 | 16.2098 | 5.4033 | 9.52 | 0.016 |
| Intragranular Mg.st | 1 | 1.1277 | 1.1277 | 1.99 | 0.218 |
| Intragranular MCC | 1 | 14.8954 | 14.8954 | 26.25 | 0.004 |
| Intragranular Croscarmellose | 1 | 0.1867 | 0.1867 | 0.33 | 0.591 |
| 2-Way Interactions | 2 | 0.4450 | 0.2225 | 0.39 | 0.695 |
| Intragranular Mg.st*Intragranular MCC | 1 | 0.1881 | 0.1881 | 0.33 | 0.590 |
| Intragranular MCC*Intragranular Croscarmellose | 1 | 0.2568 | 0.2568 | 0.45 | 0.531 |
| Error | 5 | 2.8372 | 0.5674 | ||
| Curvature | 1 | 1.3735 | 1.3735 | 3.75 | 0.125 |
| Lack-of-Fit | 2 | 1.4634 | 0.7317 | 4878.83 | 0.000 |
| Pure Error | 2 | 0.0003 | 0.0001 | ||
| Total | 10 | 19.4920 |
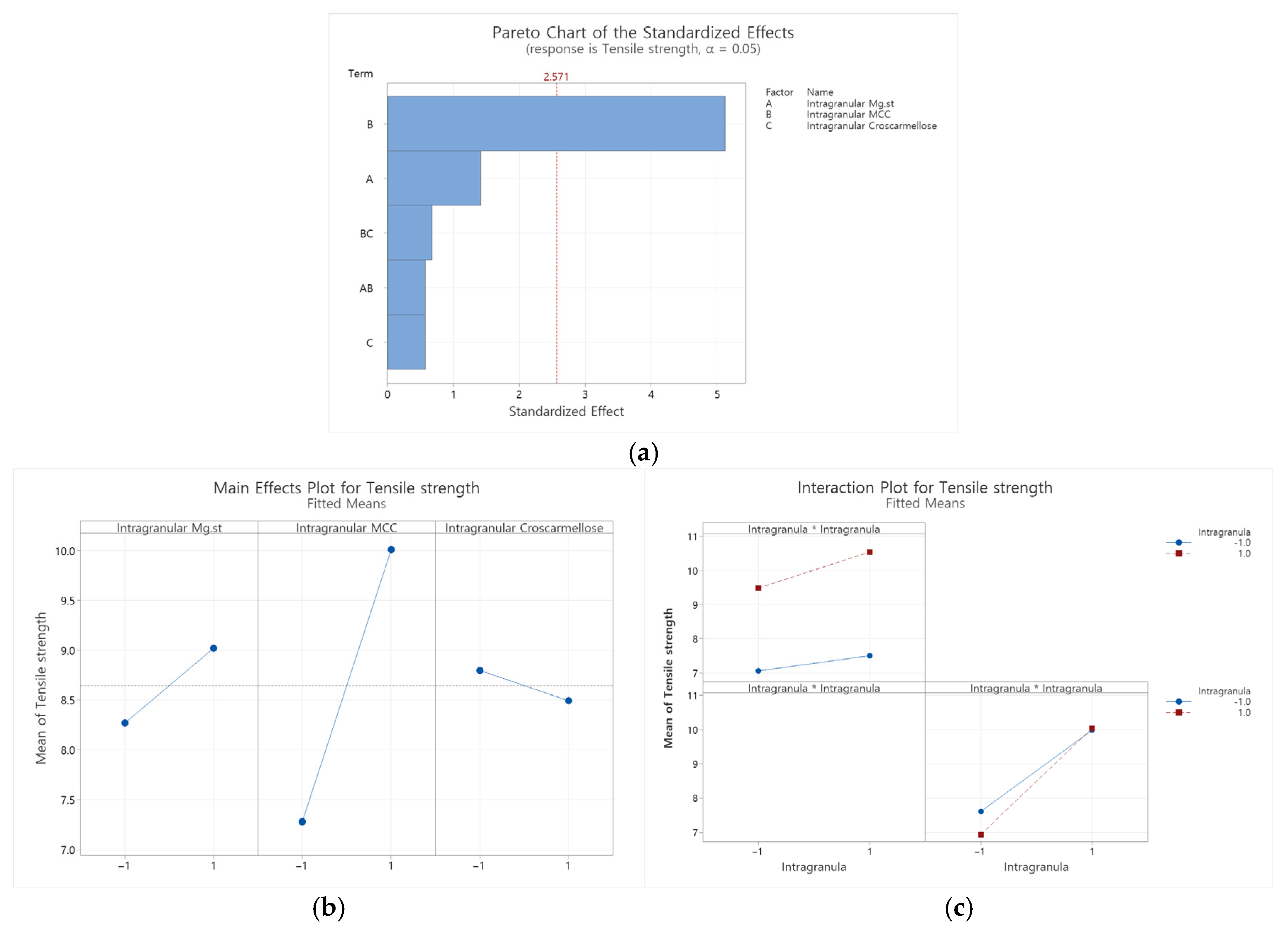
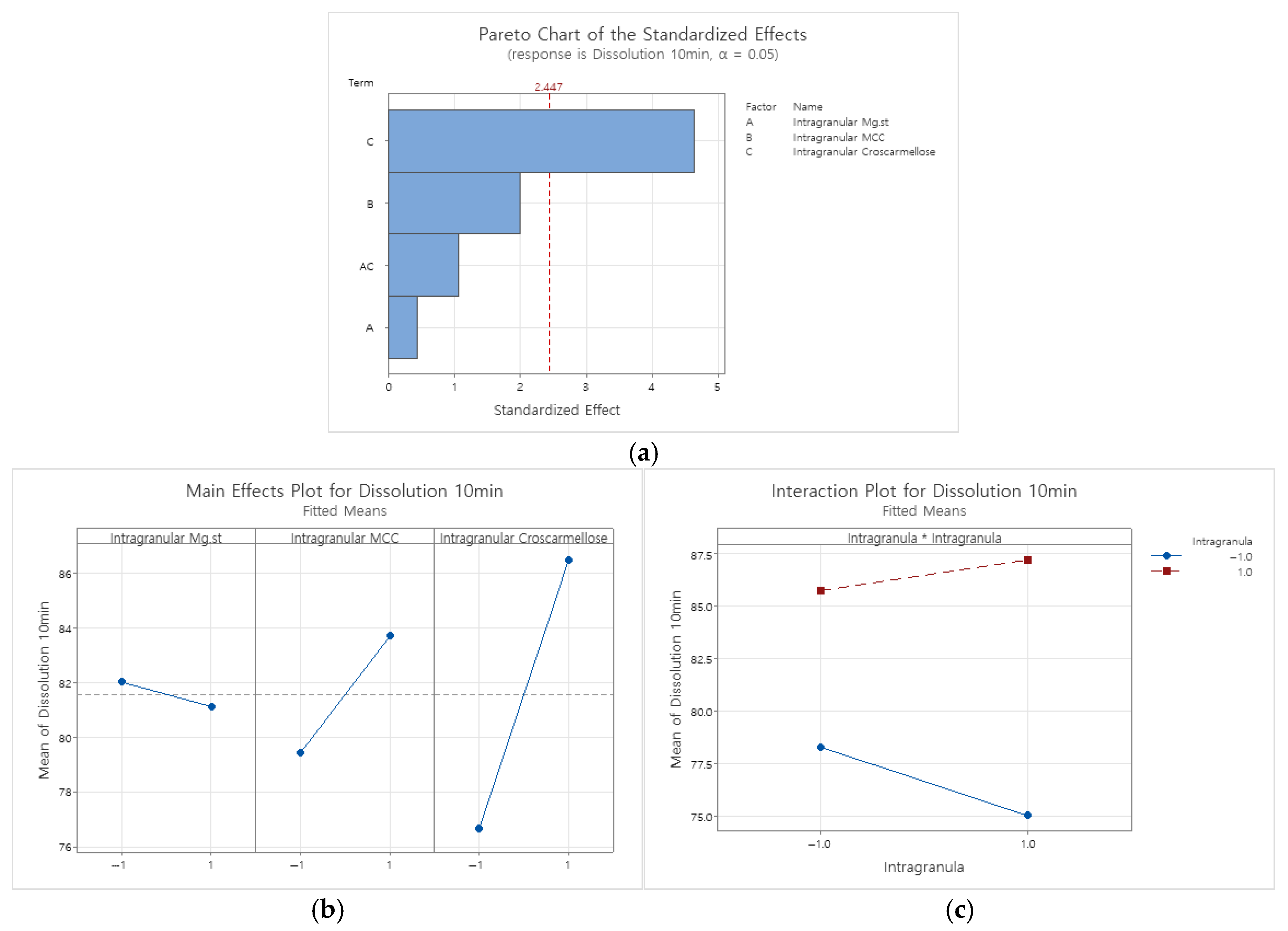
Appendix B.2.2. Dissolution Rate at 10 Min in pH 4.0 Buffer
| Source | DF | Adj SS | Adj MS | F-Value | p-Value |
|---|---|---|---|---|---|
| Model | 4 | 245.020 | 61.255 | 6.72 | 0.021 |
| Linear | 3 | 234.450 | 78.150 | 8.57 | 0.014 |
| Intragranular Mg.st | 1 | 1.711 | 1.711 | 0.19 | 0.680 |
| Intragranular MCC | 1 | 36.683 | 36.683 | 4.02 | 0.092 |
| Intragranular Croscarmellose | 1 | 196.056 | 196.056 | 21.51 | 0.004 |
| 2-Way Interactions | 1 | 10.570 | 10.570 | 1.16 | 0.323 |
| Intragranular Mg.st*Intragranular Croscarmellose | 1 | 10.570 | 10.570 | 1.16 | 0.323 |
| Error | 6 | 54.698 | 9.116 | ||
| Curvature | 1 | 31.633 | 31.633 | 6.86 | 0.047 |
| Lack-of-Fit | 3 | 8.270 | 2.757 | 0.37 | 0.785 |
| Pure Error | 2 | 14.795 | 7.397 | ||
| Total | 10 | 299.718 |
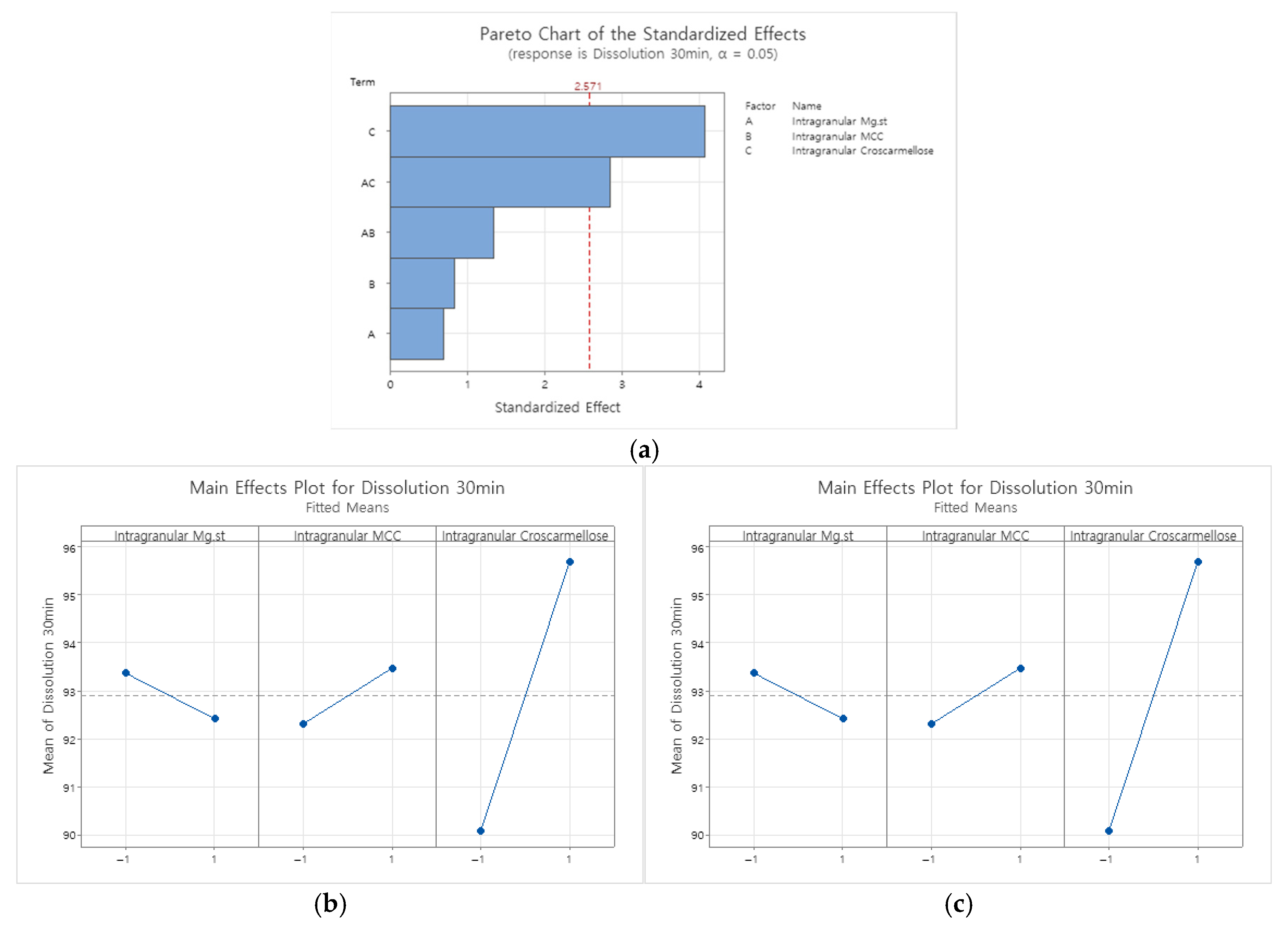
Appendix B.2.3. Dissolution Rate at 30 Min in pH 4.0 Buffer
| Source | DF | Adj SS | Adj MS | F-Value | p-Value |
|---|---|---|---|---|---|
| Model | 5 | 108.992 | 21.798 | 5.60 | 0.041 |
| Linear | 3 | 71.697 | 23.899 | 6.14 | 0.039 |
| Intragranular Mg.st | 1 | 1.944 | 1.944 | 0.50 | 0.511 |
| Intragranular MCC | 1 | 2.938 | 2.938 | 0.75 | 0.425 |
| Intragranular Croscarmellose | 1 | 66.815 | 66.815 | 17.17 | 0.009 |
| 2-Way Interactions | 2 | 37.296 | 18.648 | 4.79 | 0.069 |
| Intragranular Mg.st*Intragranular MCC | 1 | 7.095 | 7.095 | 1.82 | 0.235 |
| Intragranular Mg.st*Intragranular Croscarmellose | 1 | 30.201 | 30.201 | 7.76 | 0.039 |
| Error | 5 | 19.461 | 3.892 | ||
| Curvature | 1 | 2.390 | 2.390 | 0.56 | 0.496 |
| Lack-of-Fit | 2 | 3.890 | 1.945 | 0.30 | 0.772 |
| Pure Error | 2 | 13.181 | 6.590 | ||
| Total | 10 | 128.453 |
References
- Vo, Q.N.; Mahinthichaichan, P.; Shen, J.; Ellis, C.R. How mu-Opioid Receptor Recognizes Fentanyl. Res. Sq 2020. [Google Scholar] [CrossRef]
- Vardanyan, R.S.; Hruby, V.J. Fentanyl-related compounds and derivatives: Current status and future prospects for pharmaceutical applications. Future Med. Chem. 2014, 6, 385–412. [Google Scholar] [CrossRef] [PubMed]
- Bird, H.E.; Huhn, A.S.P.; Dunn, K.E.P. Fentanyl Absorption, Distribution, Metabolism, and Excretion: Narrative Review and Clinical Significance Related to Illicitly Manufactured Fentanyl. J. Addict. Med. 2023, 17, 503–508. [Google Scholar] [CrossRef] [PubMed]
- Rauf, U.; Ali, M.; Dehele, I.; Paudyal, V.; Elnaem, M.H.; Cheema, E. Causes, Nature and Toxicology of Fentanyl-Analogues Associated Fatalities: A Systematic Review of Case Reports and Case Series. J. Pain Res. 2021, 14, 2601–2614. [Google Scholar] [CrossRef] [PubMed]
- Sutcliffe, K.J.; Corey, R.A.; Charlton, S.J.; Sessions, R.B.; Henderson, G.; Kelly, E. Fentanyl binds to the μ-opioid receptor via the lipid membrane and transmembrane helices. bioRxiv 2021. [Google Scholar] [CrossRef]
- Williamson, J.; Kermanizadeh, A. A Review of Toxicological Profile of Fentanyl—A 2024 Update. Toxics 2024, 12, 960. [Google Scholar] [CrossRef] [PubMed]
- Castañeda, A.M.; Lee, C.-S.; Kim, Y.-C.; Lee, D.; Moon, J.Y. Addressing Opioid-Related Chemical Coping in Long-Term Opioid Therapy for Chronic Noncancer Pain: A Multicenter, Observational, Cross-Sectional Study. J. Clin. Med. 2018, 7, 354. [Google Scholar] [CrossRef] [PubMed]
- Grand View Research. Non-opioid Pain Treatment Market Size, Share & Trends Analysis Report By Drug Class (NSAIDs, Local Anesthetics), By Pain (Chronic Pain, Post-operative Pain), By Route of Administration, By Distribution Channel, By Region, And Segment Forecasts, 2025–2030. 2023. p. 100. Available online: https://www.grandviewresearch.com/industry-analysis/non-opioid-pain-treatment-market-report# (accessed on 10 June 2025).
- Stewart, R.G.; Osorno, T.; Fujita, A.; Jo, S.; Ferraiuolo, A.; Carlin, K.; Bean, B.P. Modulation of human dorsal root ganglion neuron firing by the Nav1.8 inhibitor suzetrigine. Proc. Natl. Acad. Sci. USA 2025, 122, e2503570122. [Google Scholar] [CrossRef] [PubMed]
- Hameed, S. Na(v)1.7 and Na(v)1.8: Role in the pathophysiology of pain. Mol. Pain 2019, 15, 1744806919858801. [Google Scholar] [CrossRef] [PubMed]
- Le Franc, A.; Da Silva, A.; Lepetre-Mouelhi, S. Nanomedicine and voltage-gated sodium channel blockers in pain management: A game changer or a lost cause? Drug Deliv. Transl. Res. 2024, 14, 2112–2145. [Google Scholar] [CrossRef] [PubMed]
- Middleton, S.J.; Perini, I.; Themistocleous, A.C.; A Weir, G.; McCann, K.; Barry, A.M.; Marshall, A.; Lee, M.; Mayo, L.M.; Bohic, M.; et al. Nav1.7 is required for normal C-low threshold mechanoreceptor function in humans and mice. Brain 2022, 145, 3637–3653. [Google Scholar] [CrossRef] [PubMed]
- Sun, J.; Li, L.; Yang, L.; Duan, G.; Ma, T.; Li, N.; Liu, Y.; Yao, J.; Liu, J.Y.; Zhang, X. Novel SCN9A missense mutations contribute to congenital insensitivity to pain: Unexpected correlation between electrophysiological characterization and clinical phenotype. Mol. Pain 2020, 16, 1744806920923881. [Google Scholar] [CrossRef] [PubMed]
- Adi, T.; Estacion, M.; Schulman, B.R.; Vernino, S.; Dib-Hajj, S.D.; Waxman, S.G. A novel gain-of-function Na(v)1.7 mutation in a carbamazepine-responsive patient with adult-onset painful peripheral neuropathy. Mol. Pain 2018, 14, 1744806918815007. [Google Scholar] [CrossRef] [PubMed]
- Nassar, M.A.; Levato, A.; Stirling, L.C.; Wood, J.N. Neuropathic pain develops normally in mice lacking both Na(v)1.7 and Na(v)1.8. Mol. Pain 2005, 1, 24. [Google Scholar] [CrossRef] [PubMed]
- Ball, M.C.; Snelling, C.M.; Strachan, A.N.; Strachan, R.M. Thermal decomposition of solid sodium bicarbonate. J. Chem. Soc. Faraday Trans. 1 Phys. Chem. Condens. Phases 1986, 82, 3709–3715. [Google Scholar] [CrossRef]
- Ghaderi, F.; Monajjemzadeh, F. Review of the physicochemical methods applied in the investigation of the maillard reaction in pharmaceutical preparations. J. Drug Deliv. Sci. Technol. 2020, 55, 101362. [Google Scholar] [CrossRef]
- Wirth, D.D.; Baertschi, S.W.; Johnson, R.A.; Maple, S.R.; Miller, M.S.; Hallenbeck, D.K.; Gregg, S.M. Maillard reaction of lactose and fluoxetine hydrochloride, a secondary amine. J. Pharm. Sci. 1998, 87, 31–39. [Google Scholar] [CrossRef] [PubMed]
- Pesonen, T.; Paronen, P.; Ketolainen, J. Disintegrant properties of an agglomerated cellulose powder. Int. J. Pharm. 1989, 57, 139–147. [Google Scholar] [CrossRef]
- Reier, G.E.; Shangraw, R.F. Microcrystalline Cellulose in Tableting. J. Pharm. Sci. 1966, 55, 510–514. [Google Scholar] [CrossRef]
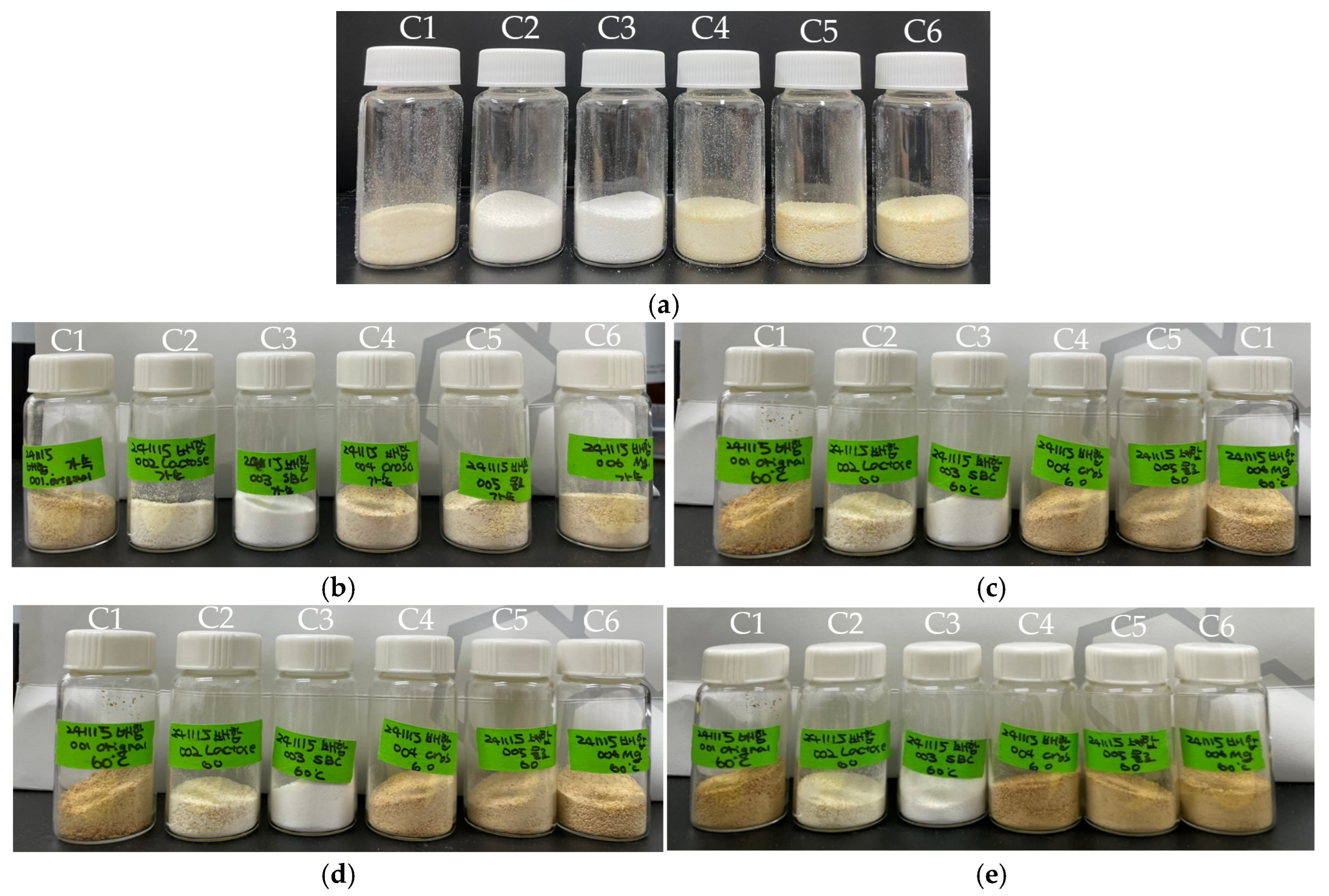
| Component | Total Amount of Batch (g) | ||||||
|---|---|---|---|---|---|---|---|
| C1 | C2 | C3 | C4 | C5 | C6 | ||
| Intragranular | API (g) | 4.79 | 4.79 | 4.79 | 4.79 | 4.79 | 4.79 |
| lactose monohydrate (g) | 1.93 | - | 1.93 | 1.93 | 1.93 | 1.93 | |
| sodium bicarbonate (g) | 0.96 | 0.96 | - | 0.96 | 0.96 | 0.96 | |
| DW (g) | q.s. | ||||||
| Extragranular | sodium bicarbonate (g) | 0.96 | 0.96 | - | 0.96 | 0.96 | 0.96 |
| lactose monohydrate (g) | 0.29 | 0.00 | 0.29 | 0.29 | 0.29 | 0.29 | |
| croscarmellose sodium (g) | 0.48 | 0.48 | 0.48 | - | 0.48 | 0.48 | |
| colloidal silicon dioxide (g) | 0.10 | 0.10 | 0.10 | 0.10 | - | 0.10 | |
| magnesium stearate (g) | 0.10 | 0.10 | 0.10 | 0.10 | 0.10 | - | |
| Total | 9.60 | 7.38 | 7.68 | 9.12 | 9.50 | 9.50 | |
| Storage Condition/Time Point | RRT | Impurities (%) | |||||
|---|---|---|---|---|---|---|---|
| C1 | C2 | C3 | C4 | C5 | C6 | ||
| Initial | 0.49 | 0.29 | 0.05 | - | 0.25 | 0.17 | 0.14 |
| 0.64 | 0.53 | - | - | 0.50 | 0.45 | 0.26 | |
| 1.13 | 0.08 | - | - | 0.06 | - | - | |
| 1.25 | 0.07 | 0.16 | - | 0.28 | 0.08 | ||
| 1.34 | 0.21 | - | - | 0.20 | 0.14 | 0.13 | |
| 2.49 | - | 0.05 | - | - | - | - | |
| 3.03 | 0.41 | 0.17 | - | 0.37 | 0.27 | 0.23 | |
| 3.08 | 0.26 | 0.13 | - | 0.24 | 0.19 | 0.16 | |
| Total impurities | 2.01 | 0.63 | 0.06 | 2.07 | 1.45 | 1.11 | |
| 40 °C/75% RH/ 2 weeks | 0.48 | - | 0.09 | - | - | - | - |
| 0.51 | 0.34 | 0.09 | - | 0.28 | 0.19 | 0.12 | |
| 0.67 | 0.57 | - | - | - | - | - | |
| 0.90 | 0.22 | - | - | 0.15 | 0.13 | 0.09 | |
| 1.22 | 0.11 | - | - | 0.09 | - | - | |
| 1.31 | 0.12 | - | - | - | 0.08 | ||
| 2.24 | 0.06 | 0.06 | - | 0.06 | - | - | |
| 2.25 | - | - | - | 0.11 | - | - | |
| 2.24 | - | - | - | 0.08 | - | - | |
| 2.68 | 0.51 | 0.28 | - | 0.46 | 0.34 | 0.30 | |
| Total impurities | 2.01 | 0.53 | 0.00 | 1.74 | 1.20 | 0.76 | |
| 60 °C/ 2 weeks | 0.51 | 0.38 | 0.14 | - | 0.31 | 0.21 | 0.17 |
| 0.56 | 0.10 | 0.15 | - | - | - | - | |
| 0.60 | 0.30 | - | - | - | 0.06 | - | |
| 0.67 | 0.40 | - | - | 0.41 | 0.38 | 0.21 | |
| 0.90 | 0.28 | - | - | 0.21 | 0.24 | 0.26 | |
| 1.22 | 0.08 | - | - | 0.06 | - | - | |
| 1.32 | - | - | - | 0.11 | - | ||
| 2.17 | - | - | - | 0.06 | - | - | |
| 2.23 | 0.11 | 0.13 | - | 0.08 | - | - | |
| 2.39 | - | - | - | 0.11 | - | - | |
| 2.66 | 0.73 | 0.51 | - | 0.74 | 0.57 | 0.50 | |
| Total impurities | 2.44 | 0.97 | 0.00 | 2.01 | 1.57 | 1.14 | |
| 40 °C/75% RH/ 4 weeks | 0.48 | 0.06 | 0.09 | - | - | - | - |
| 0.51 | 0.33 | 0.10 | - | 0.25 | 0.18 | 0.14 | |
| 0.67 | 0.49 | - | - | 0.42 | 0.40 | 0.23 | |
| 0.90 | 0.24 | - | - | - | - | - | |
| 1.21 | 0.16 | - | - | 0.16 | - | 0.12 | |
| 2.23 | - | 0.06 | - | - | - | ||
| 2.40 | 0.08 | 0.08 | - | 0.06 | - | - | |
| 2.67 | 0.50 | 0.36 | - | 0.43 | 0.34 | 0.30 | |
| 2.72 | 0.37 | 0.27 | - | 0.31 | 0.26 | 0.21 | |
| Total impurities | 2.44 | 1.29 | 0.11 | 1.92 | 1.51 | 1.28 | |
| 60 °C_/ 4 weeks | 0.48 | 0.17 | 0.16 | - | 0.12 | 0.08 | 0.09 |
| 0.51 | 0.44 | 0.18 | - | 0.31 | 0.20 | 0.18 | |
| 0.56 | 0.16 | - | - | 0.17 | 0.17 | 0.18 | |
| 0.67 | 0.16 | - | - | 0.23 | 0.18 | 0.11 | |
| 0.90 | 0.14 | - | - | 0.06 | - | 0.07 | |
| 0.96 | - | 0.06 | - | - | - | - | |
| 1.21 | 0.07 | 0.05 | - | 0.06 | 0.05 | 0.05 | |
| 1.45 | 0.09 | - | - | - | - | - | |
| 2.02 | - | 0.07 | - | - | - | - | |
| 2.23 | 0.06 | 0.11 | - | 0.06 | - | - | |
| 2.40 | 0.07 | - | - | 0.05 | - | - | |
| 2.67 | 0.90 | 0.61 | - | 0.77 | 0.59 | 0.54 | |
| 2.72 | 0.65 | 0.45 | - | 0.55 | 0.44 | 0.41 | |
| Total impurities | 3.42 | 2.17 | 0.10 | 2.85 | 2.25 | 2.11 | |
| Component | Composition (mg/cps) | |||||
|---|---|---|---|---|---|---|
| F1 | F2 | F3 | F4 | F5 | ||
| Intragranular (Slug) | API | 239.39 | 239.39 | 239.39 | 239.39 | 239.39 |
| lactose monohydrate (Fast Flo®) | 96.51 | - | 48.51 | 96.51 | 96.51 | |
| lactose monohydrate (Supertab® 30GR) | - | 96.51 | - | - | - | |
| sodium bicarbonate | 48.00 | 48.00 | 48.00 | 96.00 | 48.00 | |
| microcrystalline cellulose | - | - | 48.00 | - | - | |
| croscarmellose sodium | - | - | - | - | 12.00 | |
| magnesium stearate | 4.80 | 4.80 | 4.80 | 4.80 | 4.80 | |
| Extragranular | sodium bicarbonate | 48.00 | 48.00 | 48.00 | - | 48.00 |
| lactose monohydrate (Fast Flo®) | 14.50 | - | 14.50 | 14.50 | 14.50 | |
| lactose monohydrate (Supertab® 30GR) | - | 14.50 | - | - | - | |
| croscarmellose sodium | 24.00 | 24.00 | 24.00 | 24.00 | 12.00 | |
| colloidal silicon dioxide | 4.80 | 4.80 | 4.80 | 4.80 | 4.80 | |
| magnesium stearate | 4.80 | 4.80 | 4.80 | 4.80 | 4.80 | |
| Total | 484.80 | 484.80 | 484.80 | 484.80 | 484.80 | |
| Formulation no. | Slug Weight (mg) | Slug Thickness (mm) | Hardness (kp) | Tensile Strength (MPa) | Slug Disintegration Time | Fines Generation | Ejection Pressure |
|---|---|---|---|---|---|---|---|
| F1 | 824.0 | 3.21 | 20.39 | 12.46 | >30 min (no change) | ++ | − |
| F2 (Supertab 30GR) | 681.6 | 2.72 | 8.26 | 5.96 | >30 min (no change) | +++ | − |
| F3 (Intragranular MCC) | 706.5 | 2.75 | 11.01 | 7.85 | 110 s | − | − |
| F4 (Intragranular Sodium bicarbonate −20%) | 747.0 | 2.79 | 13.66 | 9.60 | >30 min (no change) | + | − |
| F5 (Intragranular croscarmellose sodium) | 663.3 | 2.59 | 13.76 | 10.42 | 30 s | + | +++ |
| Variables | Range | Level | |
|---|---|---|---|
| 1 | intragranular magnesium stearate | 0.25–1.25% (w/w) | −1, 0, +1 |
| 2 | intragranular MCC | 2–18% (w/w) | −1, 0, +1 |
| 3 | intragranular croscarmellose sodium | 1–5% (w/w) | −1, 0, +1 |
| Formulation No. | Standard Order | Run Order | Center Point | Variable 1 | Variable 2 | Variable 3 |
|---|---|---|---|---|---|---|
| F6 | 1 | 7 | 1 | −1 | −1 | −1 |
| F7 | 2 | 3 | 1 | 1 | −1 | −1 |
| F8 | 3 | 1 | 1 | −1 | 1 | −1 |
| F9 | 4 | 6 | 1 | 1 | 1 | −1 |
| F10 | 5 | 4 | 1 | −1 | −1 | 1 |
| F11 | 6 | 11 | 1 | 1 | −1 | 1 |
| F12 | 7 | 8 | 1 | −1 | 1 | 1 |
| F13 | 8 | 5 | 1 | 1 | 1 | 1 |
| F14 | 9 | 2 | 0 | 0 | 0 | 0 |
| F15 | 10 | 9 | 0 | 0 | 0 | 0 |
| F16 | 11 | 10 | 0 | 0 | 0 | 0 |
| Component | Composition (mg/cps) | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| F6 | F7 | F8 | F9 | F10 | F11 | F12 | F13 | F14 | F15 | F16 | ||
| Intragranular (Slug) | API | 239.39 | 239.39 | 239.39 | 239.39 | 239.39 | 239.39 | 239.39 | 239.39 | 239.39 | 239.39 | 239.39 |
| Lactose Monohydrate, Fast Flo | 86.91 | 86.91 | 10.11 | 10.11 | 86.91 | 86.91 | 10.11 | 10.11 | 48.51 | 48.51 | 48.51 | |
| Sodium Hydrogen Carbonate, Emprove Essential | 48.00 | 48.00 | 48.00 | 48.00 | 48.00 | 48.00 | 48.00 | 48.00 | 48.00 | 48.00 | 48.00 | |
| Microcrystalline cellulose PH102 | 9.60 | 9.60 | 86.40 | 86.40 | 9.60 | 9.60 | 86.40 | 86.40 | 48.00 | 48.00 | 48.00 | |
| Croscarmellose Sodium, Ac-di-sol SD-711 | 4.80 | 4.80 | 4.80 | 4.80 | 24.00 | 24.00 | 24.00 | 24.00 | 14.40 | 14.40 | 14.40 | |
| Magnesium Stearate | 1.20 | 6.00 | 1.20 | 6.00 | 1.20 | 6.00 | 1.20 | 6.00 | 3.60 | 3.60 | 3.60 | |
| Extragranular | Sodium Hydrogen Carbonate, Emprove Essential | 48.00 | 48.00 | 48.00 | 48.00 | 48.00 | 48.00 | 48.00 | 48.00 | 48.00 | 48.00 | 48.00 |
| Lactose Monohydrate, Fast Flo | 14.50 | 14.50 | 14.50 | 14.50 | 14.50 | 14.50 | 14.50 | 14.50 | 14.50 | 14.50 | 14.50 | |
| Croscarmellose Sodium, Ac-di-sol SD-711 | 19.20 | 19.20 | 19.20 | 19.20 | 0.00 | 0.00 | 0.00 | 0.00 | 9.60 | 9.60 | 9.60 | |
| Colloidal Anhydrous Silica, Aerosil 200 Pharma | 4.80 | 4.80 | 4.80 | 4.80 | 4.80 | 4.80 | 4.80 | 4.80 | 4.80 | 4.80 | 4.80 | |
| Magnesium Stearate | 4.80 | 4.80 | 4.80 | 4.80 | 4.80 | 4.80 | 4.80 | 4.80 | 4.80 | 4.80 | 4.80 | |
| Formulation no. | Slug Thickness (mm) | Hardness (kp) | Tensile Strength (MPa) | Disintegration Time (s) | Ejection Pressure |
|---|---|---|---|---|---|
| F6 | 2.75 | 21.6 | 15.4054 | 10 | − |
| F7 | 2.64 | 20.6 | 15.3044 | 2 | − |
| F8 | 2.27 | 20.7 | 17.8854 | 16 | − |
| F9 | 2.50 | 28.2 | 22.1239 | 22 | − |
| F10 | 2.36 | 15.8 | 13.1310 | 70 | ++ |
| F11 | 2.41 | 18.4 | 14.9746 | 65 | ++ |
| F12 | 2.18 | 22.4 | 20.1532 | 55 | ++ |
| F13 | 2.18 | 22.3 | 20.0633 | 55 | ++ |
| F14 | 2.55 | 20.6 | 15.8446 | 25 | + |
| F15 | 2.57 | 20.7 | 15.7976 | 26 | + |
| F16 | 2.54 | 20.5 | 15.8297 | 24 | + |
| Function | Components | Composition (mg/cps) | |
|---|---|---|---|
| Intragranular | API | aneratrigine mesylate | 239.39 mg (equiv. to aneratrigine 200 mg as free base) |
| Diluent | lactose monohydrate | 48.31 mg | |
| Diluent | microcrystalline cellulose | 45.80 mg | |
| Alkalizing agent | sodium bicarbonate | 48.00 mg | |
| Disintegrant | croscarmellose sodium | 12.00 mg | |
| Lubricant | magnesium stearate | 4.80 mg | |
| Extragranular | Diluent | lactose monohydrate | 14.50 mg |
| Alkalizing agent | sodium bicarbonate | 48.00 mg | |
| Disintegrant | croscarmellose sodium | 12.00 mg | |
| Glidant | colloidal silicon dioxide | 4.80 mg | |
| Lubricant | magnesium stearate | 2.40 mg | |
| Total Capsule weight (mg) | 484.80 mg | ||
| Test | Acceptance Criteria | Technical Batch, Non-GMP Batch | GMP Batch, For Clinical Trial |
|---|---|---|---|
| Appearance | White to off-white opaque capsules without deformation containing off-white or pale-yellow powder | Off-white opaque capsules without deformation containing white powder | Off-white opaque capsules without deformation containing white powder |
| Assay | 95.0–105.0% | 99.2% | 97.3% |
| Related Substances | Individual Impurity NMT 0.2% Total Impurities NMT 1.0% | No impurities exceeded RL Total Impurities: <0.05% | No impurities exceeded RLTotal Impurities: <0.05% |
| Dissolution | Meets the requirement in USP <711>: NLT 80% (Q = 75) in 30 min | Minimum: 92% Maximum 97% Average: 94% | Minimum: 96% Maximum 98% Average: 97% |
| Uniformity of dosage units by mass variation | Meets the requirement in USP <905>: Report AV Value. | AV = 5.0 | AV = 3.0 |
| Microbial Limit | Total Aerobic Microbial Count (TAMC) ≤ 103 CFU/g | <100 CFU/g | <100 CFU/g |
| Total Yeast and Mold Count (TYMC) ≤ 102 CFU/g | <100 CFU/g | <100 CFU/g | |
| Escherichia coli Absent/1g | Absent | Absent |
| Test/Attribute | Acceptance Criteria | T = 0 | T = 2W | T = 1M | T = 3M | T = 6M | T = 9M | T = 12M |
|---|---|---|---|---|---|---|---|---|
| Appearance | White to off-white opaque capsules | Off white opaque capsules | Off white opaque capsules | Off white opaque capsules | Off white opaque capsules | Off white opaque capsules | TBD | TBD |
| Assay | 95.0–105.0% | 102.8% | 100.2% | 99.3% | 99.7% | 100.3% | TBD | TBD |
| Impurities | Individual Impurity NMT 0.2% | N/D | N/D | N/D | N/D | N/D | TBD | TBD |
| Total Impurities NMT 1.0% | N/D | N/D | N/D | 0.14% | N/D | TBD | TBD | |
| Dissolution | Meets the requirement in USP <711>: NLT 80% (Q = 75) in 30 min | Minimum: 90% Maximum: 99% Average: 93% | Minimum: 93% Maximum: 100% Average: 96% | Minimum: 90% Maximum: 95% Average: 92% | Minimum: 98% Maximum: 106% Average: 102% | Minimum: 87% Maximum: 98% Average: 93% | TBD | TBD |
| Uniformity of dosage units by mass variation | Meets the Requirement in USP <905>: Report AV Value | 14.2 (L1) | N/T | N/T | N/T | N/T | N/T | N/T |
| Test/Attribute | Acceptance Criteria | T = 0 | T = 2W | T = 1M | T = 3M | T = 6M |
|---|---|---|---|---|---|---|
| Appearance | White to off-white opaque capsules | Off white opaque capsules | Off white opaque capsules | Off white opaque capsules | Off white opaque capsules | Off white opaque capsules |
| Assay | 95.0–105.0% | 102.8% | 101.3% | 98.0% | 99.8% | 97.7% |
| Impurities | Individual Impurity NMT 0.2% | N/D | N/D | N/D | N/D | N/D |
| Total Impurities NMT 1.0% | N/D | N/D | N/D | 0.17% | N/D | |
| Dissolution | Meets the requirement in USP <711>: NLT 80% (Q = 75) in 30 min | Minimum: 90% Maximum: 99% Average: 93% | Minimum: 86% Maximum: 93% Average: 90% | Minimum: 91% Maximum: 95% Average: 92% | Minimum: 91% Maximum: 102% Average: 95% | Minimum: 90% Maximum: 100% Average: 94% |
| Uniformity of dosage units by mass variation | Meets the Requirement in USP <905>: Report AV Value | 14.2 (L1) | N/T | N.T | N.T | N.T |
| Test/Attribute | Acceptance Criteria | T = 0 | T = 1M | T = 2M | T = 3M | T = 5M | T = 6M | T = 9M | T = 12M |
|---|---|---|---|---|---|---|---|---|---|
| Appearance | White to off-white opaque capsules containing off-white or pale-yellow powder | Off-white opaque capsules without deformation containing white powder | Off-white opaque capsules without deformation containing white powder | Off-white opaque capsules without deformation containing white powder | Off-white opaque capsules without deformation containing white powder | TBD | TBD | TBD | TBD |
| Assay | 95.0–105.0% | 99.2% | 95.7% | 96.4% | 98.0% | TBD | TBD | TBD | TBD |
| Impurities | Individual Impurity NMT 0.2% | N/D | N/D | N/D | N/D | TBD | TBD | TBD | TBD |
| Total Impurities NMT 1.0% | N/D | N/D | N/D | N/D | TBD | TBD | TBD | TBD | |
| Dissolution | Meets the requirement in USP <711>: NLT 80% (Q = 75) in 30 min | Minimum: 92% Maximum: 97% Average: 94% | Minimum: 91% Maximum: 96% Average: 93% | Minimum: 89% Maximum: 100% Average: 97% | Minimum: 94% Maximum: 97% Average: 96% | TBD | TBD | TBD | TBD |
| Uniformity of dosage units by mass variation | Meets the Requirement in USP <905>: Report AV Value | AV= 5.0 | N/T | N/T | N/T | N/T | N/T | N/T | N/T |
| Microbial Limit | Total Aerobic Microbial Count: NMT 103 CFU/g | <100 CFU/g | N/T | N/T | N/T | N/T | TBD | N/T | TBD |
| Total Yeast and Mold Count: NMT 102 CFU/g | <100 CFU/g | N/T | N/T | N/T | N/T | TBD | N/T | TBD | |
| Escherichia coli: Absent | Complies | N/T | N/T | N/T | N/T | TBD | N/T | TBD |
| Test/Attribute | Acceptance Criteria | T = 0 | T = 1M | T = 2M | T = 3M | T = 5M | T = 6M |
|---|---|---|---|---|---|---|---|
| Appearance | White to off-white opaque capsules containing off-white or pale-yellow powder | Off-white opaque capsules without deformation containing white powder | Off-white opaque capsules without deformation containing white powder | Off-white opaque capsules without deformation containing white powder | Off-white opaque capsules without deformation containing white powder | TBD | TBD |
| Assay | 95.0–105.0% | 99.2% | 95.5% | 95.4% | 97.2% | TBD | TBD |
| Impurities | Individual Impurity NMT 0.2% | N/D | N/D | N/D | N/D | TBD | TBD |
| Total Impurities NMT 1.0% | N/D | N/D | N/D | N/D | TBD | TBD | |
| Dissolution | Meets the requirement in USP <711>: NLT 80% (Q = 75) in 30 min | Minimum: 92% Maximum: 97% Average: 94% | Minimum: 87% Maximum: 99% Average: 95% | Minimum: 82% Maximum: 100% Average: 96% | Minimum: 93% Maximum: 98% Average: 96% | TBD | TBD |
| Uniformity of dosage units by mass variation | Meets the Requirement in USP <905>: Report AV Value | AV = 5.0 | N/T | N/T | N/T | N/T | N/T |
| Microbial Limit | Total Aerobic Microbial Count: NMT 103 CFU/g | <100 CFU/g | N/T | N/T | N/T | N/T | TBD |
| Total Yeast and Mold Count: NMT 102 CFU/g | <100 CFU/g | N/T | N/T | N/T | N/T | TBD | |
| Escherichia coli: Absent | Complies | N/T | N/T | N/T | N/T | TBD |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cha, K.-I.; Kim, G.-E.; Seol, J.-H.; Kim, D.-W.; Lee, S. Enhancing Chemical Stability and Bioavailability of Aneratrigine Capsules via Dry Granulation: Addressing Stability Challenges in Sodium Bicarbonate-Containing Formulations for Clinical Development. Pharmaceutics 2025, 17, 1047. https://doi.org/10.3390/pharmaceutics17081047
Cha K-I, Kim G-E, Seol J-H, Kim D-W, Lee S. Enhancing Chemical Stability and Bioavailability of Aneratrigine Capsules via Dry Granulation: Addressing Stability Challenges in Sodium Bicarbonate-Containing Formulations for Clinical Development. Pharmaceutics. 2025; 17(8):1047. https://doi.org/10.3390/pharmaceutics17081047
Chicago/Turabian StyleCha, Kwan-Ik, Ga-Eon Kim, Ji-Hyung Seol, Dong-Woo Kim, and Seungbeom Lee. 2025. "Enhancing Chemical Stability and Bioavailability of Aneratrigine Capsules via Dry Granulation: Addressing Stability Challenges in Sodium Bicarbonate-Containing Formulations for Clinical Development" Pharmaceutics 17, no. 8: 1047. https://doi.org/10.3390/pharmaceutics17081047
APA StyleCha, K.-I., Kim, G.-E., Seol, J.-H., Kim, D.-W., & Lee, S. (2025). Enhancing Chemical Stability and Bioavailability of Aneratrigine Capsules via Dry Granulation: Addressing Stability Challenges in Sodium Bicarbonate-Containing Formulations for Clinical Development. Pharmaceutics, 17(8), 1047. https://doi.org/10.3390/pharmaceutics17081047








