Harnessing Phytonanotechnology to Tackle Neglected Parasitic Diseases: Focus on Chagas Disease and Malaria
Abstract
1. Introduction
2. Antichagasic PNOs
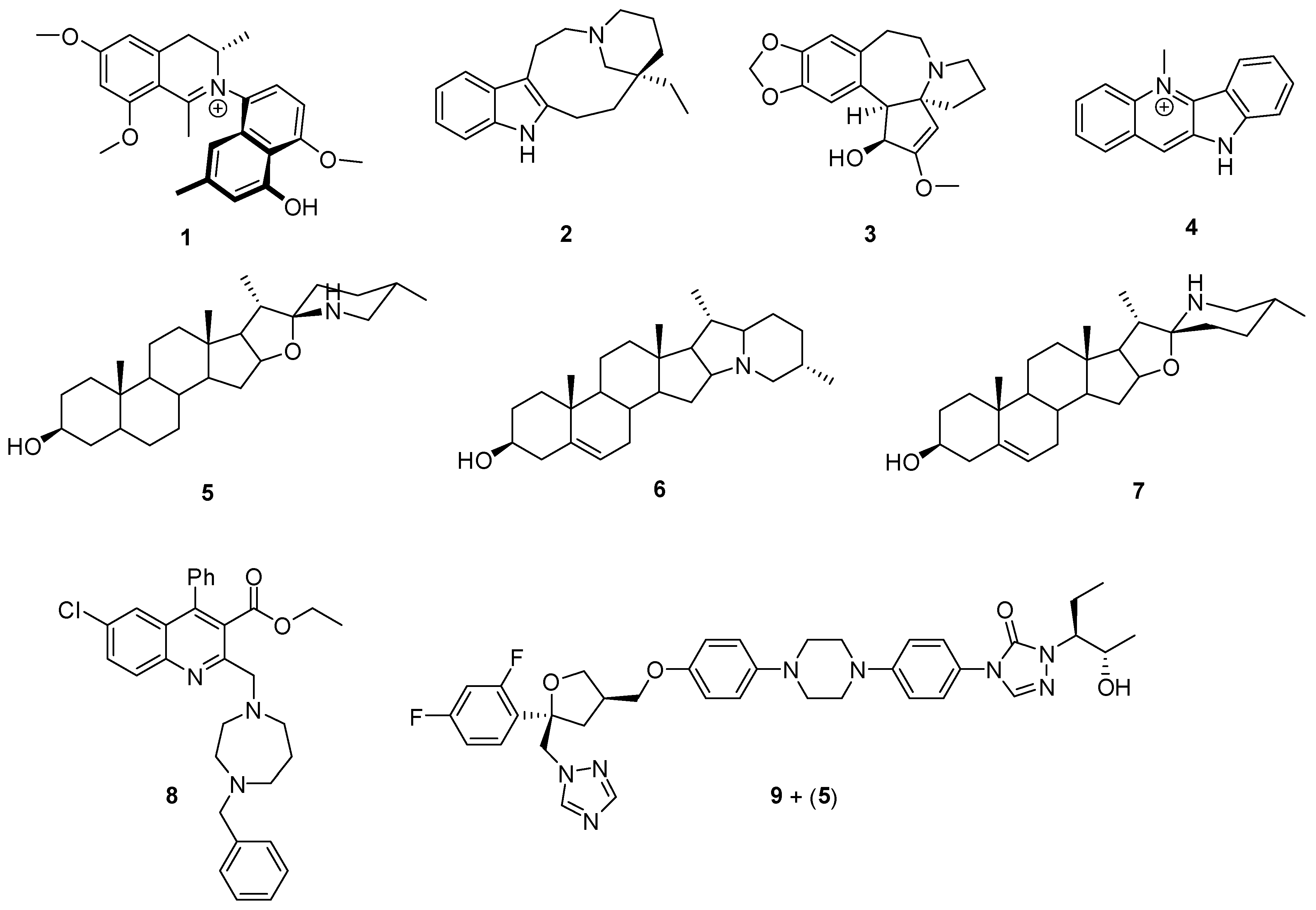
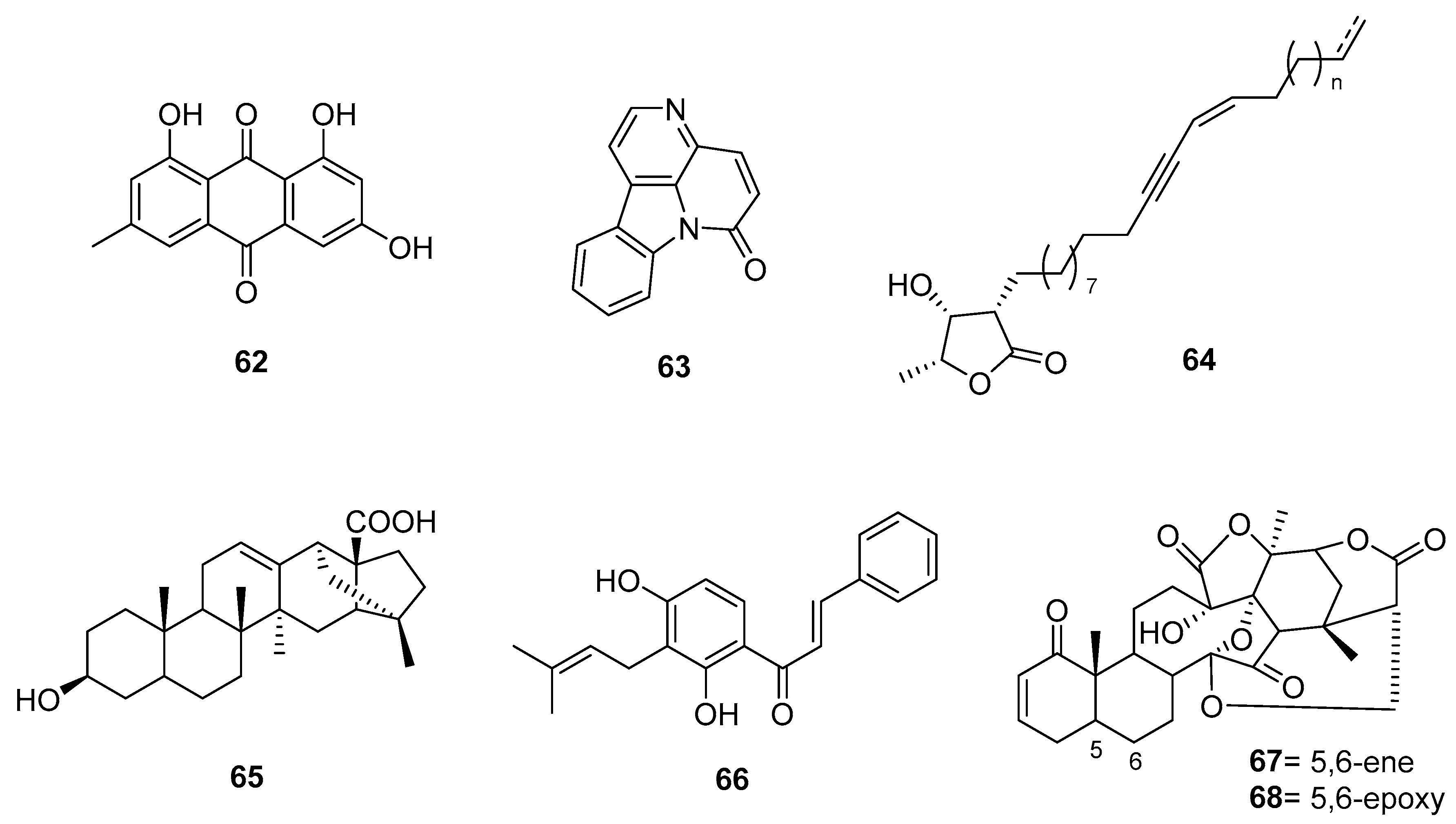
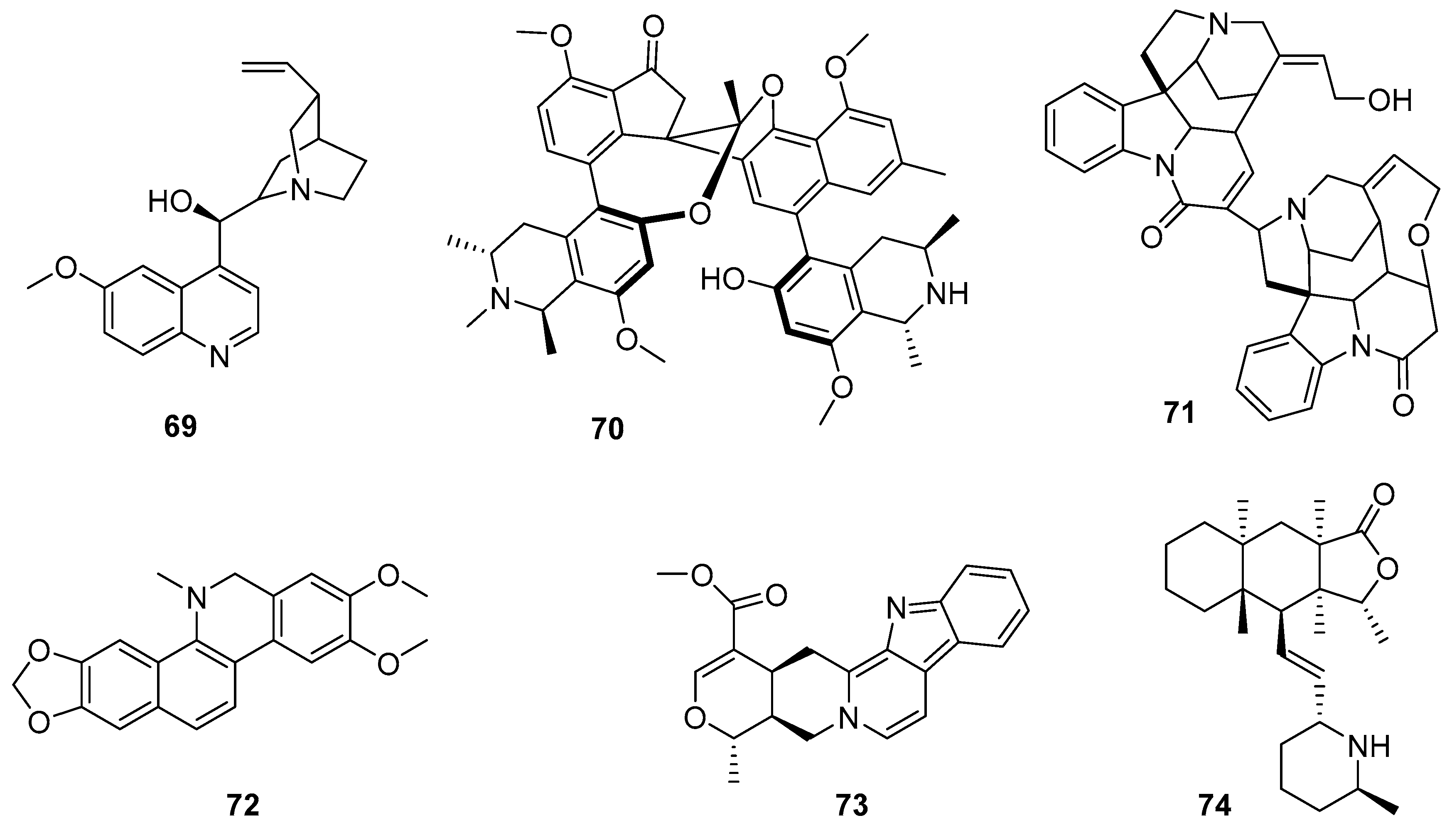
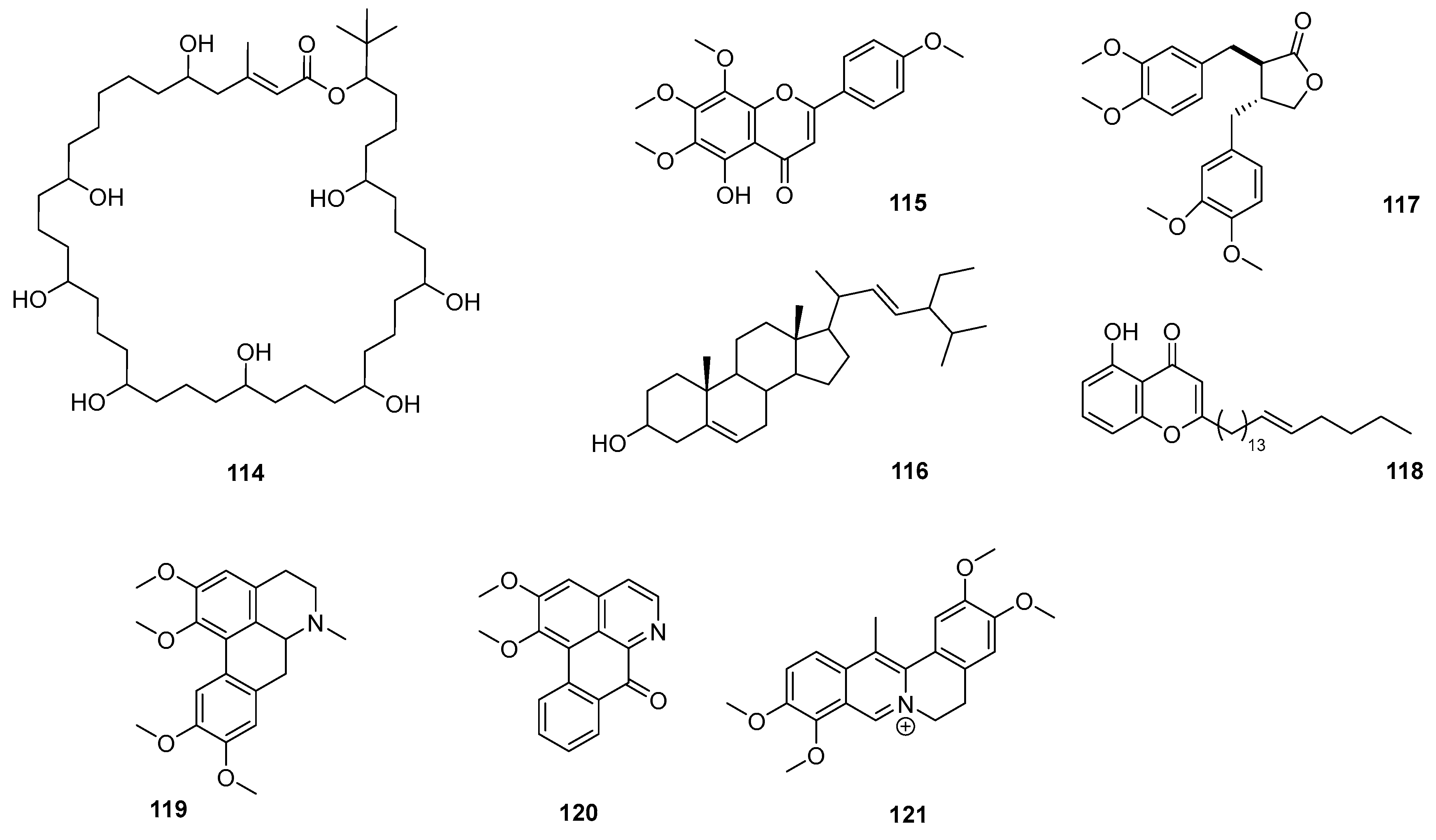
2.1. Alkaloids
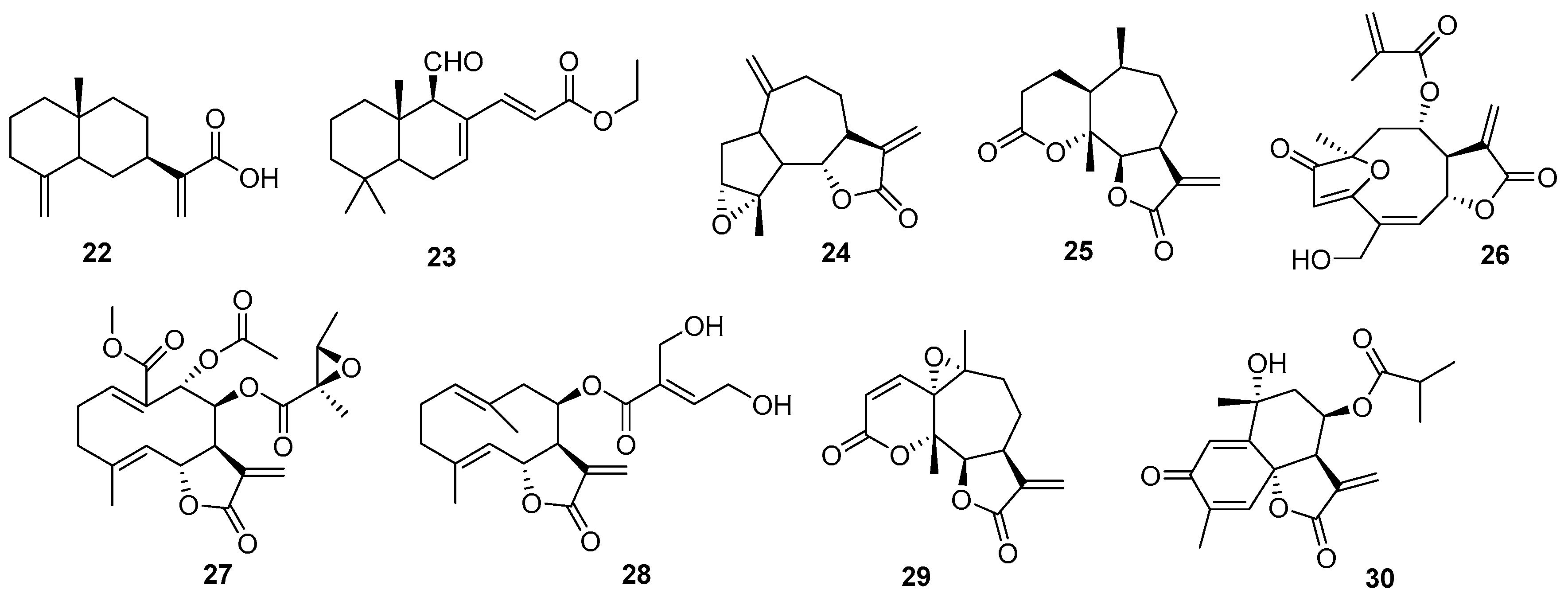
2.2. Terpenes
2.3. Sesquiterpenes
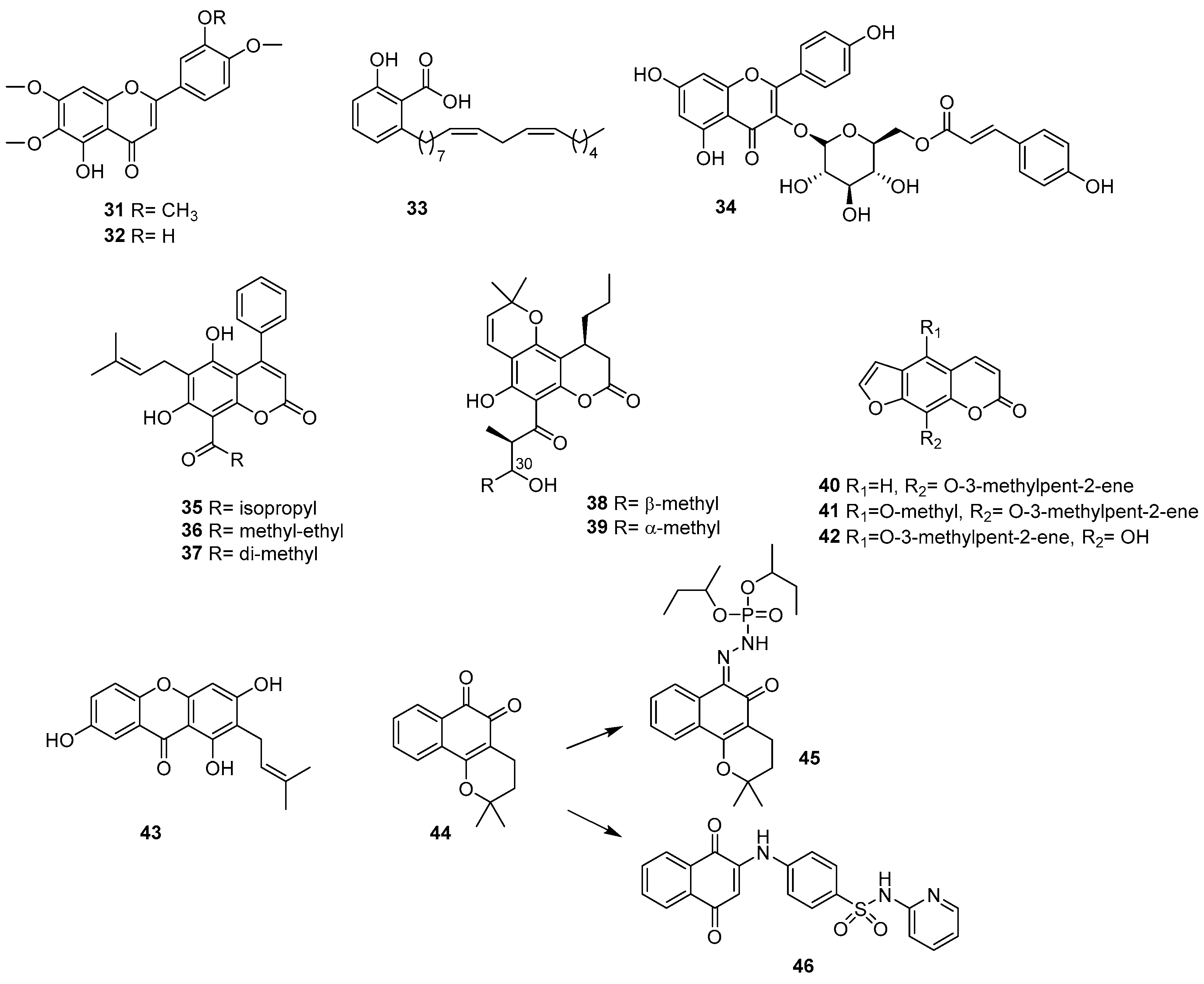
2.4. Phenolic Acids and Related Compounds
2.5. Coumarins
2.6. Quinones
2.7. Lignans
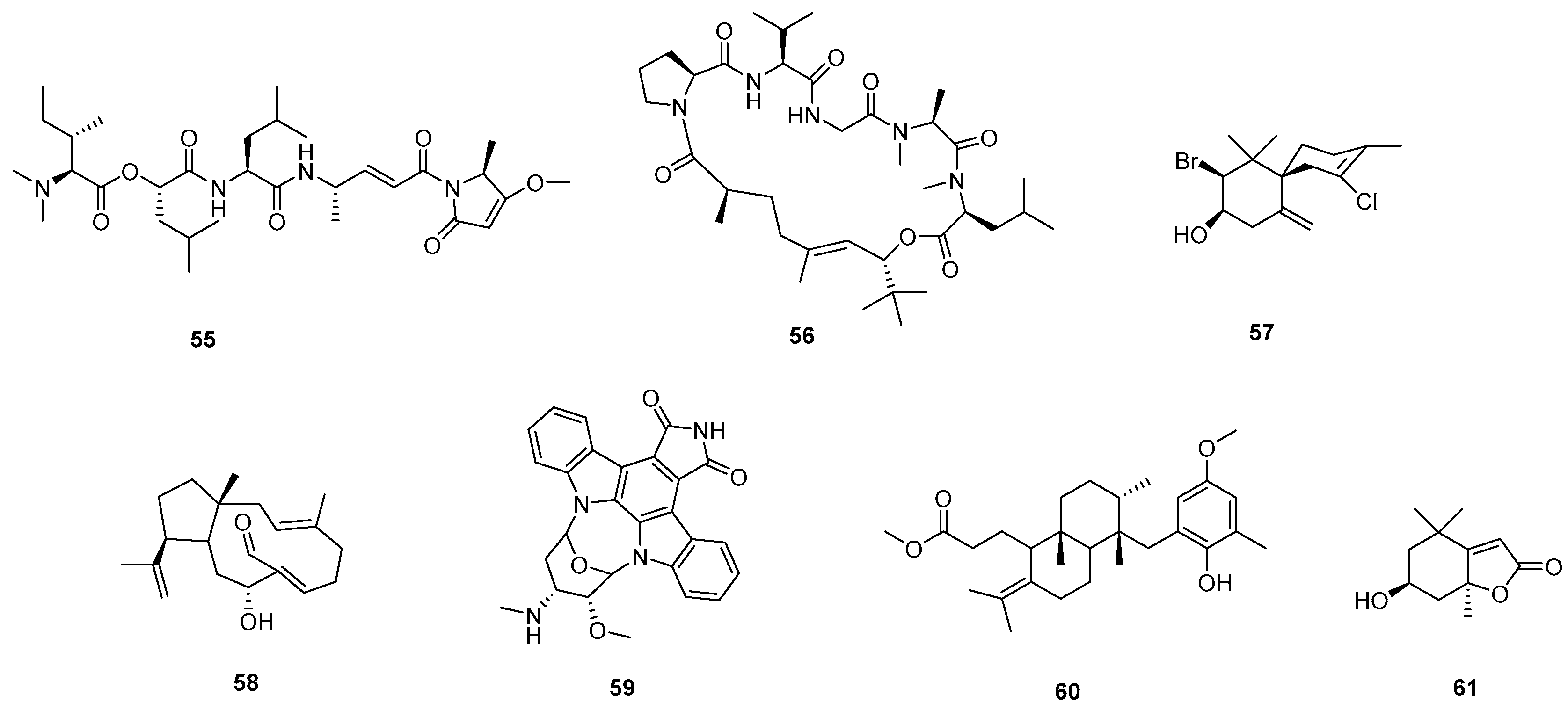
2.8. Marine PNOs
2.9. Extracts and Herbal Drugs
3. Antimalarials PNOs
3.1. Alkaloids
3.2. Terpenoids
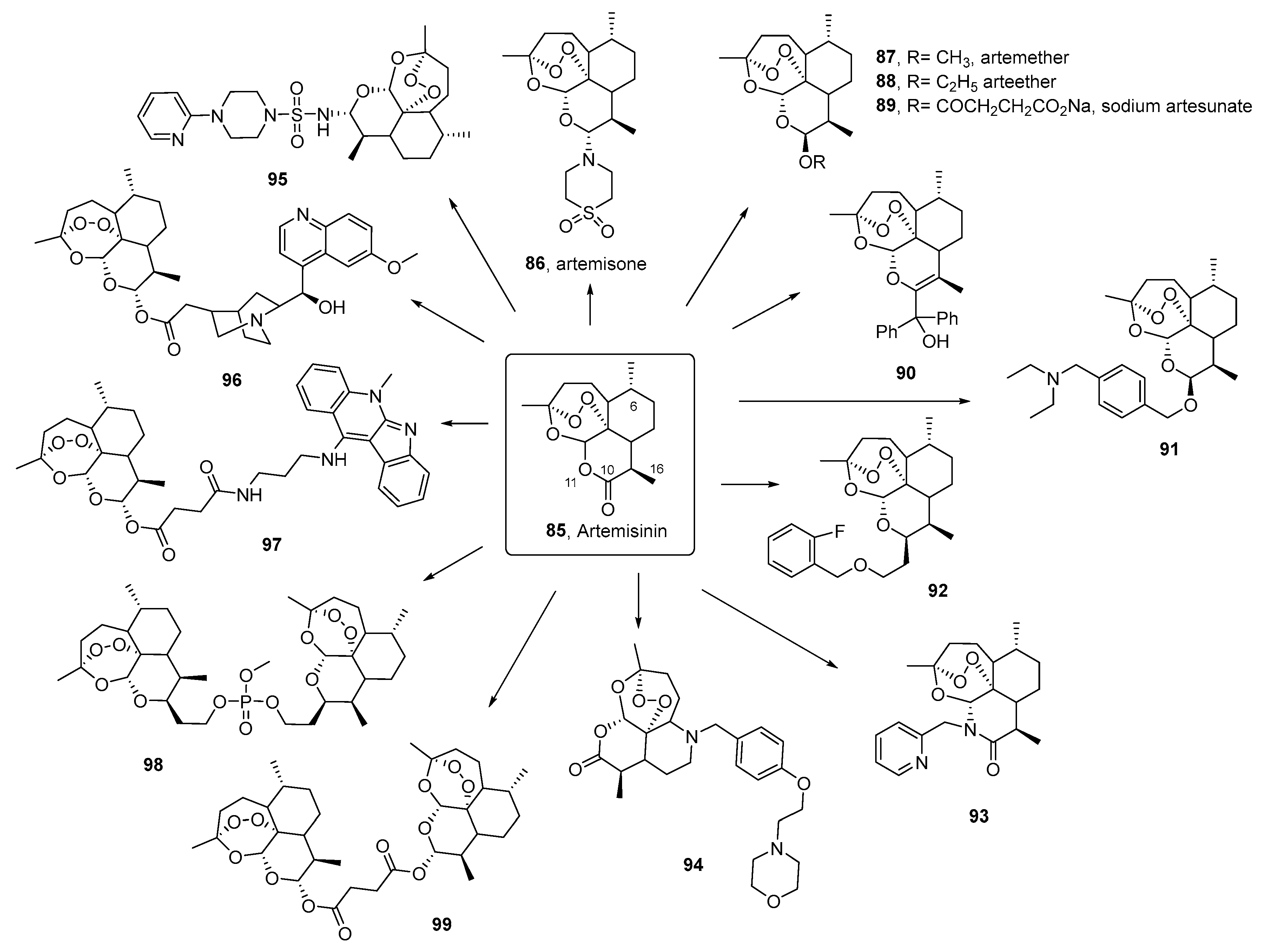
3.3. Artemisinin
3.4. Quinone, Polyphenolic, and Chromane-Related Compounds
3.5. Marine PNO
3.6. Extracts and Herbal Drugs
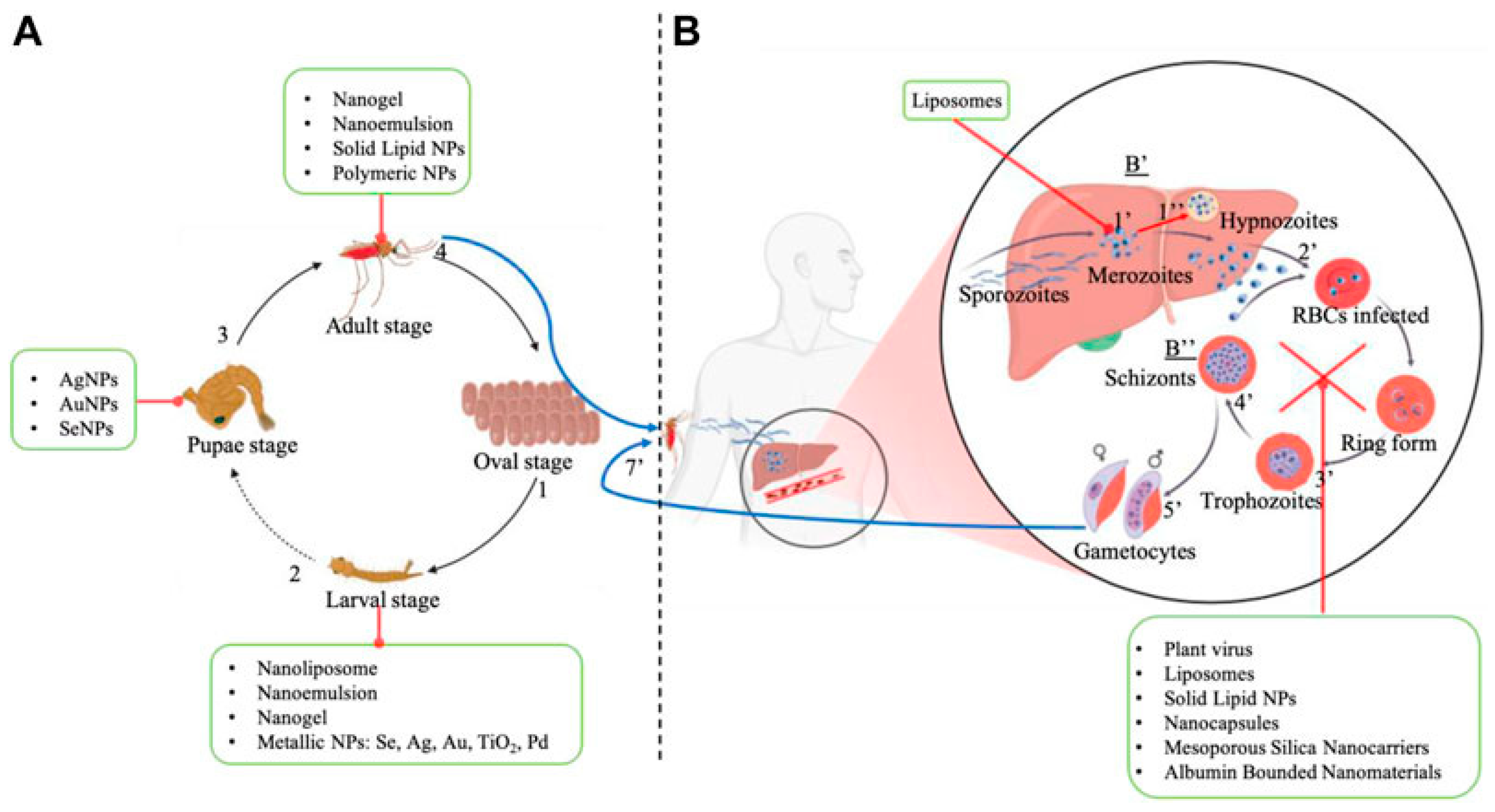
4. Phytonanotechnology to Beat Neglected Parasitosis
4.1. Nanocarriers for Anti-Malarial Phytochemicals
4.1.1. Inorganic Nanocarriers
4.1.2. Organic Nanocarriers
Polymer-Based NPs
Nanoemulsions and Nanogels
Solid Lipid Nanoparticles
Liposomes
Nanostructured Lipid Carriers
Nutriosomes
Self-Microemulsifying Drug Delivery Systems
Micelles and Cyclodextrins
| Nanocarrier | Nanocarrier Composition | Plant (Part/s Used) | Main Chemical Constituents | Size (nm) | Stage of Development | Reference |
|---|---|---|---|---|---|---|
| Inorganic NPs | AuNPs | Cymbopogon citratus | Volatile oils, flavonoids, phenolic acids, phenylpropanoids, alcohols, esters, aldehydes, and alkaloids | 20–50 | In vivo (larvae and pupae of An. stephensi) | [137] |
| AuNPs | Couroupita guianensis (flowers) | Flavonoids, phenolics, alkaloids | 29–44 | In vivo (larvae, pupae, and adults of An. stephensi) | [138] | |
| AgNPs | Annona muricata (leaves) | Acetogenins, flavonoids, alkaloids | 20–53 | In vivo(larvae An. stephensi) | [139] | |
| AgNPs | Azadirachta indica (leaves and bark) | Azadirachtin, nimbin, flavonoids, and terpenoids | <20 | In vitro (3D7 and RKL9 P. falciparum strains) | [146] | |
| AgNPs | Azadirachta indica | 35–60 | In vivo(albino mice) | [143] | ||
| AgNPs | Azadirachta indica and Ocimum sanctum | Phenols, terpenoids | <40 | In vitro (P. falciparum 3D7 parasites) | [145] | |
| AgNPs | Artemisia nilagirica (leaves) | ART, quercetin, apigenin, Β-caryophyllene, luteolin, and simple phenolic acids | <30 | In vivo(larvae I–IV instar, and pupa of An. stephensi) | [140] | |
| AgNPs | Crataegus ambigua (leaves and fruit) | Oligomeric procyanidins, flavonoids, triterpenes, polysaccharides, catecholamines | 30 | In vitro (NF54 strain of P. falciparum parasites) | [141] | |
| CdONPs | Leucaena leucocephala L. (aqueous plant extract) | Tannins, saponins, amino acids, steroids, flavonoids, phenols, carbohydrates, coumarins, cardiac glycoside | 36–57 | In vitro (P. falciparum) | [180] | |
| ZnONPs | Rhazya stricta (leaves extract) | Tannins, saponins, amino acids, gallic acid, and various flavonoids | 19 | In vitro (P. falciparum) | [150] | |
| FeONPs | Nephrolepis exaltata | Saponins, steroids, alkaloids, phenols, and tannins (notably catechin) | 16 | In vitro (P. falciparum) | [151] | |
| SeNPs | Clausena dentata (leaves) | Coumarins, alkaloids, steroids, terpenoids, essential oils, and flavonoids | 46–79 | In vivo(larvae I–IV instar of An. stephensi) | [148] | |
| TiO2NPs | Momordica charantia (leaves) | Alkaloids, saponins, glycosides, phenolic constituents, reducing sugars and free acids | 35–70 | In vivo (larvae I–IV instar, and pupa of An. stephensi) | [181] | |
| PdNPs | Citrus limon (leaves) | Essential oils (limonene), flavonoids, phenolic acid | 2–4.8 | In vivo (3rd instar larvae of An. stephensi) | [182] | |
| Nanoemulsion/nanogels | Tween® 20 and carboxymethylcellulose | Elettaria cardamomum (leaves) | Essential oils (1,8-cineole, terpineol, limonene, terpinyl acetates, linalyl acetate, linalool, sabinene) | 86 | In vivo (larvae An. stephensi) | [161] |
| Tween® 20 and carboxymethylcellulose | Zataria multiflora (leaves) | Essential oils (Thymol and cavacrol) | 8 | In vivo (larvae An. stephensi) | ||
| Tween 80®, Tween 20®, propylene glycol | Eucalyptus globulus and Syzygium aromaticum | Essential oils (1,8-cineole and eugenol) | 11–23 | In vivo (larvae An. stephensi) | [162] | |
| Tween 20® | Anethum graveolens | Essential oils (alpha-Phellandrene, p-Cymene, Carvone) | <20 | In vivo (larvae An. stephensi) | [183] | |
| Tween® 20 | Artemisia dracunculus | Essential oils (estragole, sabinene, methyl eugenol, elemicin) | 152 | In vivo (late-3rd or young-4th instar larvae An. stephensi) | [164] | |
| Tween® 20, carboxymethylcellulose | Artemisia dracunculus | NA | ||||
| SLNs | Stearic acid/Span® 60/Tween® 80 | Zataria multiflora | Essential oils (thymol and cavacrol) | 134 | In vivo (larvae An. stephensi) | [167] |
| Stearic acid/Span® 60/Tween® 80 | Mentha longifolia | Essential oils (pulegone, 1,8-cineole, piperitone, menthone) | 105 | In vivo (larvae An. stephensi) | [166] | |
| Mentha pulegium | Essential oils (pulegone, piperitone, limonene) | 210 | ||||
| Zataria multiflora | Essential oils (thymol and cavacrol) | 137 | ||||
| Nanostructured lipid carriers (NLC) | Coconut oil and cetyl palmitate | Curcuma longa (rhizomes) | Curcumin | 145 | In vivo (mice) | [171] |
| Nanoliposomes | Lecithin, cholesterol, Tween® 20 | Citrus aurantium | Essential oils (limonene) | 42–67 | In vivo (larvae An. stephensi) | [168] |
| Citrus limon | ||||||
| Citrus sinensis | ||||||
| lecithin, cholesterol, Tween® 20 | Artemisia annua | Essential oils (ART, camphor, 1,8-cineole) | 137 | In vivo (larvae An. stephensi) | [169] | |
| Artemisia dracunculus | Essential oils (estragole, cis-ocimene, ɣ-terpinene) | 151 | ||||
| Artemisia sieberi | Essential oils (camphor, α/β-thujone) | 92 | ||||
| Heparin, DOTAP, DOPC, cholesterol | Poupartia borbonica | Poupartone B | 183–256 | In vitro (P. falciparum) | [184] | |
| Lecithin, cholesterol, Tween® 20 | Syzygium aromaticum | Essential oils (eugenol) | 109–158 | In vivo (larvae An. stephensi) | [185] | |
| Cinnamomum zeylanicum | Essential oils (cinnamaldehyde) | 111–195 | ||||
| Polymeric NPs | Chitosan/ Tween® 20 | Elettaria cardamomum and Cinnamomum zeylanicum | Essential oils (1,8-cineole and cinnamaldehyde) | 78–204 | In vivo (larvae An. stephensi) | [157] |
| PLGA | Curcuma longa (rhizomes) | Curcumin | 495 | In vivo (mice) | [155] | |
| PLGA | Curcuma longa (rhizomes) | Curcumin | 291 | In vivo (mice) | [156] | |
| PLGA | Curcuma longa (rhizomes) | Curcumin | 251 | In vivo (mice) | [154] | |
| Zwitterionic self-assembled NPs | PBMA- MESBMA | Curcuma longa (rhizomes) | Curcumin | 20–100 | In vivo (mice) | [186] |
| Nutrisomes | Nutriose FM06®, Eudragit® | Curcuma longa (rhizomes) | Curcumin | 300–337 | In vivo (mice) | [173] |
| Nutriose FM06®, S75 | Curcuma longa (rhizomes) | Curcumin | 108–152 | In vitro (P. falciparum) | [172] | |
| Artemisia annua | ART | 93–95 | ||||
| NA | Quercetin (polyphenol) | 121–125 | ||||
| Artemisia annua and Curcuma longa | ART-curcumin | 111–121 | ||||
| Curcuma longa, NA | ART-quercetin | 119–128 | ||||
| Nanocapsules | PCL, MCT, Span 60®, and (coatings: P80, PEG, Chitosan, or Eudragit RS100®) | Curcuma longa (rhizomes) | Curcumin | 123–250 | In vitro | [187] |
| Micelles | mPEG-PCL | Artemisia annua | ART | 110–143 | In vitro | [178] |
| γ-CD NPs | Sorbitan monooleate, P80, DMPEmPEG2000 | Artemisia annua | ART | 92–188 | In vivo (Wistar rats) | [179] |
4.2. Nanocarriers for Anti-Chagasic Phytochemicals
4.2.1. Inorganic Nanocarriers
4.2.2. Organic Nanocarriers
Polymer-Based NPs
Nanoemulsions
Cyclodextrins
| Nanocarrier | Nanocarrier Composition | Plant Name | Main Chemical Constituent/s | Size (nm) | Stage of Development | Reference |
|---|---|---|---|---|---|---|
| Inorganic NPs | AgNPs | Corn Cobs | Xylan (D-xylose, L-arabinose, 4-O-methyl-D-glucuronic acid) | 40–55.3 | In vitro (epimastigote forms of the Y strain of T. cruzi) | [189] |
| Polymeric nanocapsules | PCL | Lychnophora trichocarpha | LYC | 190.2 | Preclinical (Swiss mice) | [190] |
| 175–245 | Preclinical (Female Swiss albino mice) | [194] | ||||
| PLA-PEG | 106.1 | Preclinical (Swiss mice) | [190] | |||
| 105.1 | Preclinical (C57BL/6 mice) | [192] | ||||
| 105–138 | Preclinical (Female Swiss albino mice) | [194] | ||||
| 107 | Preclinical (Female Swiss albino mice) | [193] | ||||
| Polymeric particles | PLGA | Piper cubeba | (−)-Cubebin | 1000 | Preclinical (BALB/c mice) | [140] |
| PCL-Poloxamer 407 | NA | UA | 173.2 | Preclinical (Swiss mice) | [195] | |
| PLGA-PVA | Curcuma longa | Curcumin | 250–300 | Preclinical (C57BL/6 mice) | [197] | |
| Liposomes | DPPC | Cortex Frangula | Hypericum perforatum | NA | Trypomastigote forms of the T. cruzi Y strain | [202] |
| Micelles | Pluronic™ F-127 | |||||
| Pluronic™ F-123 | ||||||
| Nanoemulsion | Capryol® 90, Cremophor® EL/Transcutol® P | NA | UA | 57.3 | In vitro (amastigote forms of the clone CL Brener strain B5 of T. cruzi) | [198] |
| Pluronic F127® | Eugenia caryophyllus and other trypanocidal agent (sulfonamides) | Essential oils (eugenol) | 35–100 | In vitro (Epimastigote forms of the T. cruzi clone Dm28c (lineage TCII)31 and Y (lineage TCI)) | [199] | |
| CDs | 2-hydroxypropyl-β-cyclodextrin | NA | β-lapachone (naphthoquinone) | NA | In vitro trypomastigotes of the Tulahuen strain, intracellular amastigote, epimastigotes, and trypomastigotes (Y strain) | [201] |
| 2-hydroxypropyl-β-cyclodextrin | NA | β-lapachone and nor-β-lapachone | NA | Epimastigote and trypomastigote forms of T. cruzi (strain Y) | [200] |
5. Remarks and Future Perspectives
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviation
References
- Oyeyemi, O.T.; Ogundahunsi, O.; Schunk, M.; Fatem, R.G.; Shollenberger, L.M. Neglected tropical disease (NTD) diagnostics: Current development and operations to advance control. Pathog. Glob. Health 2024, 118, 1–24. [Google Scholar] [CrossRef] [PubMed]
- Patridge, E.; Gareiss, P.; Kinch, M.S.; Hoyer, D. An analysis of FDA-approved drugs: Natural products and their derivatives. Drug Discov. Today 2016, 21, 204–207. [Google Scholar] [CrossRef]
- DeCorte, B.L. Underexplored Opportunities for Natural Products in Drug Discovery. J. Med. Chem. 2016, 59, 9295–9304. [Google Scholar] [CrossRef] [PubMed]
- Newman, D.J.; Cragg, G.M. Natural Products as Sources of New Drugs over the Nearly Four Decades from 01/1981 to 09/2019. J. Nat. Prod. 2020, 83, 770–803. [Google Scholar] [CrossRef] [PubMed]
- Evans, B.E.; Rittle, K.E.; Bock, M.G.; DiPardo, R.M.; Freidinger, R.M.; Whitter, W.L.; Lundell, G.F.; Veber, D.F.; Anderson, P.S.; Chang, R.S.L. Methods for drug discovery: Development of potent, selective, orally effective cholecystokinin antagonists. J. Med. Chem. 1988, 31, 2235–2246. [Google Scholar] [CrossRef]
- Rodrigues, T.; Reker, D.; Schneider, P.; Schneider, G. Counting on natural products for drug design. Nat. Chem. 2016, 8, 531–541. [Google Scholar] [CrossRef]
- Stratton, C.F.; Newman, D.J.; Tan, D.S. Cheminformatic comparison of approved drugs from natural product versus synthetic origins. Bioorganic Med. Chem. Lett. 2015, 25, 4802–4807. [Google Scholar] [CrossRef]
- Welsch, M.E.; Snyder, S.A.; Stockwell, B.R. Privileged scaffolds for library design and drug discovery. Curr. Opin. Chem. Biol. 2010, 14, 347–361. [Google Scholar] [CrossRef]
- Butler, M.S.; Robertson, A.A.; Cooper, M.A. Natural product and natural product derived drugs in clinical trials. Nat. Prod. Rep. 2014, 31, 1612–1661. [Google Scholar] [CrossRef]
- Camp, D.; Garavelas, A.; Campitelli, M. Analysis of Physicochemical Properties for Drugs of Natural Origin. J. Nat. Prod. 2015, 78, 1370–1382. [Google Scholar] [CrossRef]
- Cheuka, P.M.; Mayoka, G.; Mutai, P.; Chibale, K. The Role of Natural Products in Drug Discovery and Development against Neglected Tropical Diseases. Molecules 2016, 22, 58. [Google Scholar] [CrossRef] [PubMed]
- de Oliveira, R.G.; Cruz, L.R.; Mollo, M.C.; Dias, L.C.; Kratz, J.M. Chagas disease drug discovery in Latin America—A mini review of antiparasitic agents explored between 2010 and 2021. Front. Chem. 2021, 9, 771143. [Google Scholar] [CrossRef]
- Ceravolo, I.P.; Aguiar, A.C.; Adebayo, J.O.; Krettli, A.U. Studies on activities and chemical characterization of medicinal plants in search for new Antimalarials: A ten year review on Ethnopharmacology. Front. Pharmacol. 2021, 12, 734263. [Google Scholar] [CrossRef]
- Bilia, A.R.; Bergonzi, M.C.; Guccione, C.; Manconi, M.; Fadda, A.M.; Sinico, C. Vesicles and micelles: Two versatile vectors for the delivery of natural products. J. Drug Deliv. Sci. Technol. 2016, 32, 241–255. [Google Scholar] [CrossRef]
- Chaves, J.B.; de Moraes, B.P.T.; Ferrarini, S.R.; da Fonseca, F.N.; Silva, A.R.; Gonçalves-de-Albuquerque, C.F. Potential of nanoformulations in malaria treatment. Front. Pharmacol. 2022, 13, 999300. [Google Scholar] [CrossRef]
- Kurul, F.; Turkmen, H.; Cetin, A.E.; Topkaya, S.N. Nanomedicine: How nanomaterials are transforming drug delivery, bio-imaging, and diagnosis. Next Nanotechnol. 2025, 7, 100129. [Google Scholar] [CrossRef]
- Lokole, P.B.; Byamungu, G.G.; Mutwale, P.K.; Ngombe, N.K.; Mudogo, C.N.; Krause, R.W.M.; Nkanga, C.I. Plant-based nanoparticles targeting malaria management. Front. Pharmacol. 2024, 15, 1440116. [Google Scholar] [CrossRef]
- Pandian, S.R.K.; Panneerselvam, T.; Pavadai, P.; Govindaraj, S.; Ravishankar, V.; Palanisamy, P.; Sampath, M.; Sankaranarayanan, M.; Kunjiappan, S. Nano based approach for the treatment of neglected tropical diseases. Front. Nanotechnol. 2021, 3, 665274. [Google Scholar] [CrossRef]
- Bringmann, G.; Hertlein-Amslinger, B.; Kajahn, I.; Dreyer, M.; Brun, R.; Moll, H.; Stich, A.; Ioset, K.N.; Schmitz, W.; Ngoc, L.H. Phenolic analogs of the N,C-coupled naphthylisoquinoline alkaloid ancistrocladinium A, from Ancistrocladus cochinchinensis (Ancistrocladaceae), with improved antiprotozoal activities. Phytochemistry 2011, 72, 89–93. [Google Scholar] [CrossRef]
- Argüelles, A.J.; Cordell, G.A.; Maruenda, H. Molecular docking and binding mode analysis of plant alkaloids as in vitro and in silico inhibitors of trypanothione reductase from Trypanosoma cruzi. Nat. Prod. Commun. 2016, 11, 1934578X1601100118. [Google Scholar] [CrossRef]
- Muscia, G.C.; Roldan Pacheco, F.J.; Asis, S.E.; Buldain, G.Y.; Frank, F.M. Hit-to-lead optimization of novel 2-alkylaminomethylquinoline derivatives as anti-chagas agents. Eur. J. Med. Chem. 2020, 186, 111877. [Google Scholar] [CrossRef]
- Rocha-Hasler, M.; de Oliveira, G.M.; da Gama, A.N.; Fiuza, L.F.A.; Fesser, A.F.; Cal, M.; Rocchetti, R.; Peres, R.B.; Guan, X.L.; Kaiser, M.; et al. Combination With Tomatidine Improves the Potency of Posaconazole Against Trypanosoma cruzi. Front. Cell Infect. Microbiol. 2021, 11, 617917. [Google Scholar] [CrossRef]
- Medina, J.M.; Rodrigues, J.C.; Moreira, O.C.; Atella, G.; Souza, W.; Barrabin, H. Mechanisms of growth inhibition of Phytomonas serpens by the alkaloids tomatine and tomatidine. Mem. Inst. Oswaldo Cruz 2015, 110, 48–55. [Google Scholar] [CrossRef] [PubMed]
- Durão, R.; Ramalhete, C.; Madureira, A.M.; Mendes, E.; Duarte, N. Plant terpenoids as hit compounds against trypanosomiasis. Pharmaceuticals 2022, 15, 340. [Google Scholar] [CrossRef]
- Lozano, E.; Barrera, P.; Tonn, C.; Nieto, M.; Sartor, T.; Sosa, M.A. The effect of the diterpene 5-epi-icetexone on the cell cycle of Trypanosoma cruzi. Parasitol. Int. 2012, 61, 275–279. [Google Scholar] [CrossRef] [PubMed]
- Lozano, E.; Strauss, M.; Spina, R.; Cifuente, D.; Tonn, C.; Rivarola, H.W.; Sosa, M.A. The in vivo trypanocidal effect of the diterpene 5-epi-icetexone obtained from Salvia gilliesii. Parasitol. Int. 2016, 65, 23–26. [Google Scholar] [CrossRef]
- Bossolani, G.; Ueda-Nakamura, T.; Silva, S.; Dias Filho, B.; Costa, T.; Quintanilla, R.; Martinez, S.; Veiga-Junior, V.; Pinto, A.; Nakamura, C. Anti-Trypanosoma Activity and Synergistic Effects of Natural and Semi-Synthetic Triterpenes and Predominant Cell Death through Autophagy in Amastigote Forms. J. Braz. Chem. Soc. 2017, 28, 2473–2489. [Google Scholar] [CrossRef]
- Olmo, F.; Guardia, J.J.; Marin, C.; Messouri, I.; Rosales, M.J.; Urbanova, K.; Chayboun, I.; Chahboun, R.; Alvarez-Manzaneda, E.J.; Sanchez-Moreno, M. Prospects of an alternative treatment against Trypanosoma cruzi based on abietic acid derivatives show promising results in Balb/c mouse model. Eur. J. Med. Chem. 2015, 89, 683–690. [Google Scholar] [CrossRef] [PubMed]
- Meira, C.S.; Barbosa-Filho, J.M.; Lanfredi-Rangel, A.; Guimaraes, E.T.; Moreira, D.R.; Soares, M.B. Antiparasitic evaluation of betulinic acid derivatives reveals effective and selective anti-Trypanosoma cruzi inhibitors. Exp. Parasitol. 2016, 166, 108–115. [Google Scholar] [CrossRef]
- de Almeida, B.C.; Araujo, B.Q.; Carvalho, A.A.; Freitas, S.D.; Maciel, D.D.; Ferreira, A.J.; Tempone, A.G.; Martins, L.F.; Alexandre, T.R.; Chaves, M.H.; et al. Antiprotozoal activity of extracts and isolated triterpenoids of ‘carnauba’ (Copernicia prunifera) wax from Brazil. Pharm. Biol. 2016, 54, 3280–3284. [Google Scholar] [CrossRef]
- Izumi, E.; Ueda-Nakamura, T.; Veiga, V.F., Jr.; Pinto, A.C.; Nakamura, C.V. Terpenes from Copaifera demonstrated in vitro antiparasitic and synergic activity. J. Med. Chem. 2012, 55, 2994–3001. [Google Scholar] [CrossRef] [PubMed]
- Selener, M.G.; Borgo, J.; Sarratea, M.B.; Delfino, M.A.; Laurella, L.C.; Cerny, N.; Gomez, J.; Coll, M.; Malchiodi, E.L.; Bivona, A.E. Trypanocidal and Anti-Inflammatory Effects of Three ent-Kaurane Diterpenoids from Gymnocoronis spilanthoides var. subcordata (Asteraceae). Pharmaceutics 2024, 16, 415. [Google Scholar] [CrossRef] [PubMed]
- Sales Junior, P.A.; Zani, C.L.; de Siqueira, E.P.; Kohlhoff, M.; Marques, F.R.; Caldeira, A.S.P.; Cota, B.B.; Maia, D.N.B.; Tunes, L.G.; Murta, S.M.F.; et al. Trypanocidal Trixikingolides From Trixis Vauthieri. Nat. Prod. Res. 2021, 35, 2691–2699. [Google Scholar] [CrossRef]
- Londero, V.S.; Costa-Silva, T.A.; Tempone, A.G.; Namiyama, G.M.; Thevenard, F.; Antar, G.M.; Baitello, J.B.; Lago, J.H.G. Anti-Trypanosoma cruzi activity of costic acid isolated from Nectandra barbellata (Lauraceae) is associated with alterations in plasma membrane electric and mitochondrial membrane potentials. Bioorganic Chem. 2020, 95, 103510. [Google Scholar] [CrossRef] [PubMed]
- Turner, D.N.; Just, J.; Dasari, R.; Smith, J.A.; Bissember, A.C.; Kornienko, A.; Rogelj, S. Activity of natural and synthetic polygodial derivatives against Trypanosoma cruzi amastigotes, trypomastigotes and epimastigotes. Nat. Prod. Res. 2021, 35, 792–795. [Google Scholar] [CrossRef]
- Sulsen, V.P.; Frank, F.M.; Cazorla, S.I.; Barrera, P.; Freixa, B.; Vila, R.; Sosa, M.A.; Malchiodi, E.L.; Muschietti, L.V.; Martino, V.S. Psilostachyin C: A natural compound with trypanocidal activity. Int. J. Antimicrob. Agents 2011, 37, 536–543. [Google Scholar] [CrossRef]
- Adessi, T.G.; Ana, Y.; Stempin, C.C.; Garcia, M.C.; Bisogno, F.R.; Nicotra, V.E.; Garcia, M.E. Psilostachyins as trypanocidal compounds: Bioguided fractionation of Ambrosia tenuifolia chemically modified extract. Phytochemistry 2022, 194, 113014. [Google Scholar] [CrossRef]
- Sulsen, V.P.; Martino, V.S. Sesquiterpene Lactones: Advances in their Chemistry and Biological Aspects; Springer: Cham, Switzerland, 2018. [Google Scholar]
- Muschietti, L.V.; Ulloa, J.L. Natural sesquiterpene lactones as potential trypanocidal therapeutic agents: A review. Nat. Prod. Commun. 2016, 11, 1934578X1601101036. [Google Scholar] [CrossRef]
- Ulloa, J.L.; Spina, R.; Casasco, A.; Petray, P.B.; Martino, V.; Sosa, M.A.; Frank, F.M.; Muschietti, L.V. Germacranolide-type sesquiterpene lactones from Smallanthus sonchifolius with promising activity against Leishmania mexicana and Trypanosoma cruzi. Parasites Vectors 2017, 10, 567. [Google Scholar] [CrossRef]
- Sulsen, V.P.; Lizarraga, E.F.; Elso, O.G.; Cerny, N.; Sanchez Alberti, A.; Bivona, A.E.; Malchiodi, E.L.; Cazorla, S.I.; Catalan, C.A.N. Activity of Estafietin and Analogues on Trypanosoma cruzi and Leishmania braziliensis. Molecules 2019, 24, 1209. [Google Scholar] [CrossRef]
- Milagre, M.M.; Branquinho, R.T.; Goncalves, M.F.; de Assis, G.; de Oliveira, M.T.; Reis, L.; Saude-Guimaraes, D.A.; de Lana, M. Activity of the sesquiterpene lactone goyazensolide against Trypanosoma cruzi in vitro and in vivo. Parasitology 2020, 147, 108–119. [Google Scholar] [CrossRef]
- Sepulveda-Robles, O.; Espinoza-Gutierrez, B.; Gomez-Verjan, J.C.; Guzman-Gutierrez, S.L.; De Ita, M.; Silva-Miranda, M.; Espitia-Pinzon, C.I.; Fernandez-Ramirez, F.; Herrera-Salazar, A.; Mata-Rocha, M.; et al. Trypanocidal and toxicological assessment in vitro and in silico of three sesquiterpene lactones from Asteraceae plant species. Food Chem. Toxicol. 2019, 125, 55–61. [Google Scholar] [CrossRef]
- Elso, O.G.; Bivona, A.E.; Sanchez Alberti, A.; Cerny, N.; Fabian, L.; Morales, C.; Catalan, C.A.N.; Malchiodi, E.L.; Cazorla, S.I.; Sulsen, V.P. Trypanocidal Activity of Four Sesquiterpene Lactones Isolated from Asteraceae Species. Molecules 2020, 25, 2014. [Google Scholar] [CrossRef] [PubMed]
- Sosa, A.; Salamanca Capusiri, E.; Amaya, S.; Bardón, A.; Giménez-Turba, A.; Vera, N.; Borkosky, S. Trypanocidal activity of South American Vernonieae (Asteraceae) extracts and its sesquiterpene lactones. Nat. Prod. Res. 2021, 35, 5224–5228. [Google Scholar] [CrossRef] [PubMed]
- Goncalves-Santos, E.; Vilas-Boas, D.F.; Diniz, L.F.; Veloso, M.P.; Mazzeti, A.L.; Rodrigues, M.R.; Oliveira, C.M.; Fernandes, V.H.C.; Novaes, R.D.; Chagas-Paula, D.A.; et al. Sesquiterpene lactone potentiates the immunomodulatory, antiparasitic and cardioprotective effects on anti-Trypanosoma cruzi specific chemotherapy. Int. Immunopharmacol. 2019, 77, 105961. [Google Scholar] [CrossRef]
- Schmidt, T.J.; Khalid, S.A.; Romanha, A.J.; Alves, T.M.A.; Biavatti, M.W.; Brun, R.; Da Costa, F.B.; de Castro, S.L.; Ferreira, V.F.; de Lacerda, M.V.G.; et al. The potential of secondary metabolites from plants as drugs or leads against protozoan neglected diseases-Part II. Curr. Med. Chem. 2012, 19, 2176–2228. [Google Scholar] [CrossRef]
- Beer, M.F.; Frank, F.M.; German Elso, O.; Ernesto Bivona, A.; Cerny, N.; Giberti, G.; Luis Malchiodi, E.; Susana Martino, V.; Alonso, M.R.; Patricia Sulsen, V.; et al. Trypanocidal and leishmanicidal activities of flavonoids isolated from Stevia satureiifolia var. satureiifolia. Pharm. Biol. 2016, 54, 2188–2195. [Google Scholar] [CrossRef]
- Umehara, E.; Costa Silva, T.A.; Mendes, V.M.; Guadagnin, R.C.; Sartorelli, P.; Tempone, A.G.; Lago, J.H.G. Differential lethal action of C17:2 and C17:0 anacardic acid derivatives in Trypanosoma cruzi—A mechanistic study. Bioorganic Chem. 2020, 102, 104068. [Google Scholar] [CrossRef] [PubMed]
- Cornelio, V.; Maluf, F.; Fernandes, J.; da Silva, M.F.; Oliva, G.; Guido, R.; Vieira, P. Isolation of Tiliroside from Spiranthera odoratissima as Inhibitor of Trypanosoma cruzi Glyceraldehyde-3-phosphate Dehydrogenase by Using Bioactivity-Guided Fractionation. J. Braz. Chem. Soc. 2017, 28, 512–519. [Google Scholar] [CrossRef]
- Riveiro, M.E.; De Kimpe, N.; Moglioni, A.; Vazquez, R.; Monczor, F.; Shayo, C.; Davio, C. Coumarins: Old compounds with novel promising therapeutic perspectives. Curr. Med. Chem. 2010, 17, 1325–1338. [Google Scholar] [CrossRef]
- Reyes-Chilpa, R.; Estrada-Muñiz, E.; Vega-Avila, E.; Abe, F.; Kinjo, J.; Hernández-Ortega, S. Trypanocidal constituents in plants: 7. Mammea-type coumarins. Mem. Inst. Oswaldo Cruz 2008, 103, 431–436. [Google Scholar] [CrossRef]
- Rodriguez-Hernandez, K.D.; Martinez, I.; Agredano-Moreno, L.T.; Jimenez-Garcia, L.F.; Reyes-Chilpa, R.; Espinoza, B. Coumarins isolated from Calophyllum brasiliense produce ultrastructural alterations and affect in vitro infectivity of Trypanosoma cruzi. Phytomedicine 2019, 61, 152827. [Google Scholar] [CrossRef]
- Silva, L.G.; Gomes, K.S.; Costa-Silva, T.A.; Romanelli, M.M.; Tempone, A.G.; Sartorelli, P.; Lago, J.H.G. Calanolides E1 and E2, two related coumarins from Calophyllum brasiliense Cambess. (Clusiaceae), displayed in vitro activity against amastigote forms of Trypanosoma cruzi and Leishmania infantum. Nat. Prod. Res. 2021, 35, 5373–5377. [Google Scholar] [CrossRef] [PubMed]
- Krishnan, S.R.; Skiba, A.; Luca, S.V.; Marcourt, L.; Wolfender, J.-L.; Skalicka-Woźniak, K.; Gertsch, J. Bioactivity-guided isolation of trypanocidal coumarins and dihydro-pyranochromones from selected Apiaceae plant species. Phytochemistry 2023, 213, 113770. [Google Scholar] [CrossRef] [PubMed]
- Martínez, M.J.A.; Benito, P.B. Biological activity of quinones. Stud. Nat. Prod. Chem. 2005, 30, 303–366. [Google Scholar]
- Caleare Ade, O.; Lazarin-Bidoia, D.; Cortez, D.A.; Ueda-Nakamura, T.; Dias Filho, B.P.; Silva Sde, O.; Nakamura, C.V. Trypanocidal activity of 1,3,7-trihydroxy-2-(3-methylbut-2-enyl)-xanthone isolated from Kielmeyera coriacea. Parasitol. Int. 2013, 62, 405–411. [Google Scholar] [CrossRef] [PubMed]
- Gomes, C.L.; Sales, V.d.A.W.; de Melo, C.G.; da Silva, R.M.F.; Nishimura, R.H.V.; Rolim, L.A.; Neto, P.J.R. Beta-lapachone: Natural occurrence, physicochemical properties, biological activities, toxicity and synthesis. Phytochemistry 2021, 186, 112713. [Google Scholar] [CrossRef]
- Dos Anjos, D.O.; Sobral Alves, E.S.; Goncalves, V.T.; Fontes, S.S.; Nogueira, M.L.; Suarez-Fontes, A.M.; Neves da Costa, J.B.; Rios-Santos, F.; Vannier-Santos, M.A. Effects of a novel beta-lapachone derivative on Trypanosoma cruzi: Parasite death involving apoptosis, autophagy and necrosis. Int. J. Parasitol. Drugs Drug Resist. 2016, 6, 207–219. [Google Scholar] [CrossRef]
- Silva, L.R.; Guimaraes, A.S.; do Nascimento, J.; do Santos Nascimento, I.J.; da Silva, E.B.; McKerrow, J.H.; Cardoso, S.H.; da Silva-Junior, E.F. Computer-aided design of 1,4-naphthoquinone-based inhibitors targeting cruzain and rhodesain cysteine proteases. Bioorg Med. Chem. 2021, 41, 116213. [Google Scholar] [CrossRef]
- Suto, Y.; Nakajima-Shimada, J.; Yamagiwa, N.; Onizuka, Y.; Iwasaki, G. Synthesis and biological evaluation of quinones derived from natural product komaroviquinone as anti-Trypanosoma cruzi agents. Bioorganic Med. Chem. Lett. 2015, 25, 2967–2971. [Google Scholar] [CrossRef]
- Uchiyama, N.; Kiuchi, F.; Ito, M.; Honda, G.; Takeda, Y.; Khodzhimatov, O.K.; Ashurmetov, O.A. New icetexane and 20-norabietane diterpenes with trypanocidal activity from Dracocephalum komarovi. J. Nat. Prod. 2003, 66, 128–131. [Google Scholar] [CrossRef]
- Uchiyama, N.; Kabututu, Z.; Kubata, B.K.; Kiuchi, F.; Ito, M.; Nakajima-Shimada, J.; Aoki, T.; Ohkubo, K.; Fukuzumi, S.; Martin, S.K. Antichagasic activity of komaroviquinone is due to generation of reactive oxygen species catalyzed by Trypanosoma cruzi old yellow enzyme. Antimicrob. Agents Chemother. 2005, 49, 5123–5126. [Google Scholar] [CrossRef]
- Costa-Silva, T.A.d.; Grecco, S.S.; De Sousa, F.S.; Lago, J.H.G.; Martins, E.G.A.; Terrazas, C.A.; Varikuti, S.; Owens, K.L.; Beverley, S.M.; Satoskar, A.R. Immunomodulatory and antileishmanial activity of phenylpropanoid dimers isolated from Nectandra leucantha. J. Nat. Prod. 2015, 78, 653–657. [Google Scholar] [CrossRef] [PubMed]
- Grecco, S.S.; Costa-Silva, T.A.; Jerz, G.; de Sousa, F.S.; Alves Conserva, G.A.; Mesquita, J.T.; Galuppo, M.K.; Tempone, A.G.; Neves, B.J.; Andrade, C.H.; et al. Antitrypanosomal activity and evaluation of the mechanism of action of dehydrodieugenol isolated from Nectandra leucantha (Lauraceae) and its methylated derivative against Trypanosoma cruzi. Phytomedicine 2017, 24, 62–67. [Google Scholar] [CrossRef]
- Ferreira, D.D.; Sousa, F.S.; Costa-Silva, T.A.; Reimao, J.Q.; Torrecilhas, A.C.; Johns, D.M.; Sear, C.E.; Honorio, K.M.; Lago, J.H.G.; Anderson, E.A.; et al. Dehydrodieugenol B derivatives as antiparasitic agents: Synthesis and biological activity against Trypanosoma cruzi. Eur. J. Med. Chem. 2019, 176, 162–174. [Google Scholar] [CrossRef]
- Torchelsen, F.; Silva, T.M.; Milagre, M.M.; Silva, R.R.; Reis, L.E.S.; Branquinho, R.T.; Silva, G.N.; de Lana, M. Evaluation of the anti-Trypanosoma cruzi activity in vitro and in vivo of silibinin and silibinin in association to benznidazole. Parasitol. Res. 2021, 120, 1511–1517. [Google Scholar] [CrossRef]
- Nweze, J.A.; Mbaoji, F.N.; Li, Y.M.; Yang, L.Y.; Huang, S.S.; Chigor, V.N.; Eze, E.A.; Pan, L.X.; Zhang, T.; Yang, D.F. Potentials of marine natural products against malaria, leishmaniasis, and trypanosomiasis parasites: A review of recent articles. Infect. Dis. Poverty 2021, 10, 9. [Google Scholar] [CrossRef]
- Tempone, A.G.; Pieper, P.; Borborema, S.E.T.; Thevenard, F.; Lago, J.H.G.; Croft, S.L.; Anderson, E.A. Marine alkaloids as bioactive agents against protozoal neglected tropical diseases and malaria. Nat. Prod. Rep. 2021, 38, 2214–2235. [Google Scholar] [CrossRef] [PubMed]
- Álvarez-Bardón, M.; Pérez-Pertejo, Y.; Ordóñez, C.; Sepúlveda-Crespo, D.; Carballeira, N.M.; Tekwani, B.L.; Murugesan, S.; Martinez-Valladares, M.; García-Estrada, C.; Reguera, R.M. Screening marine natural products for new drug leads against trypanosomatids and malaria. Mar. Drugs 2020, 18, 187. [Google Scholar] [CrossRef] [PubMed]
- Salm, A.; Krishnan, S.R.; Collu, M.; Danton, O.; Hamburger, M.; Leonti, M.; Almanza, G.; Gertsch, J. Phylobioactive hotspots in plant resources used to treat Chagas disease. iScience 2021, 24, 102310. [Google Scholar] [CrossRef]
- Selener, M.G.; Elso, O.; Grosso, C.; Borgo, J.; Clavin, M.; Malchiodi, E.L.; Cazorla, S.I.; Flavia, F.; Sulsen, V.P. Anti-Trypanosoma cruzi Activity of Extracts from Argentinean Asteraceae Species. Iran. J. Pharm. Res. 2019, 18, 1854–1861. [Google Scholar] [CrossRef]
- Ferreira, M.E.; Cebrian-Torrejon, G.; Corrales, A.S.; Vera de Bilbao, N.; Rolon, M.; Gomez, C.V.; Leblanc, K.; Yaluf, G.; Schinini, A.; Torres, S.; et al. Zanthoxylum chiloperone leaves extract: First sustainable Chagas disease treatment. J. Ethnopharmacol. 2011, 133, 986–993. [Google Scholar] [CrossRef]
- de Bilbao, N.V.; Giebelhaus, R.T.; Dias, R.P.; Ferreira, M.E.; Martínez, M.; Velasco-Carneros, L.; Nam, S.L.; de la Mata, A.P.; Maréchal, J.-D.; Adou, A.I. Exploring the Anti-Chagas Activity of Zanthoxylum chiloperone’s Seedlings Through Metabolomics and Protein–Ligand Docking. Plants 2025, 14, 954. [Google Scholar] [CrossRef]
- Charneau, S.; de Mesquita, M.L.; Bastos, I.M.; Santana, J.M.; de Paula, J.E.; Grellier, P.; Espindola, L.S. In vitro investigation of Brazilian Cerrado plant extract activity against Plasmodium falciparum, Trypanosoma cruzi and T. brucei gambiense. Nat. Prod. Res. 2016, 30, 1320–1326. [Google Scholar] [CrossRef]
- Thevenard, F.; Brito, I.A.; Costa-Silva, T.A.; Tempone, A.G.; Lago, J.H.G. Enyne acetogenins from Porcelia macrocarpa displayed anti-Trypanosoma cruzi activity and cause a reduction in the intracellular calcium level. Sci. Rep. 2023, 13, 10254. [Google Scholar] [CrossRef] [PubMed]
- Machado, A.J.T.; Santos, A.T.L.; Martins, G.; Cruz, R.P.; Costa, M.D.S.; Campina, F.F.; Freitas, M.A.; Bezerra, C.F.; Leal, A.; Carneiro, J.N.P.; et al. Antiparasitic effect of the Psidium guajava L. (guava) and Psidium brownianum MART. EX DC. (araca-de-veado) extracts. Food Chem. Toxicol. 2018, 119, 275–280. [Google Scholar] [CrossRef] [PubMed]
- Santos, K.K.; Matias, E.F.; Tintino, S.R.; Souza, C.E.; Braga, M.F.; Guedes, G.M.; Rolon, M.; Vega, C.; de Arias, A.R.; Costa, J.G.; et al. Anti-Trypanosoma cruzi and cytotoxic activities of Eugenia uniflora L. Exp. Parasitol. 2012, 131, 130–132. [Google Scholar] [CrossRef] [PubMed]
- Silva, M.L.; Pereira, A.C.; Ferreira, D.S.; Esperandim, V.R.; Simaro, G.V.; Lima, T.C.; Januario, A.H.; Pauletti, P.M.; Rehder, V.L.; Crevelin, E.J.; et al. In vitro Activities of Pfaffia glomerata Root Extract, Its Hydrolyzed Fractions and Pfaffic Acid Against Trypanosoma cruzi Trypomastigotes. Chem. Biodivers. 2017, 14, e1600175. [Google Scholar] [CrossRef]
- Bortoluzzi, A.A.M.; Staffen, I.V.; Banhuk, F.W.; Griebler, A.; Matos, P.K.; Ayala, T.S.; da Silva, E.A.A.; Sarragiotto, M.H.; Schuquel, I.T.A.; Jorge, T.C.M.; et al. Determination of chemical structure and anti-Trypanosoma cruzi activity of extracts from the roots of Lonchocarpus cultratus (Vell.) A.M.G. Azevedo & H.C. Lima. Saudi J. Biol. Sci. 2021, 28, 99–108. [Google Scholar] [CrossRef]
- Vega Gomez, M.C.; Rolón, M.; Coronel, C.; Pereira Carneiro, J.N.; Lucas dos Santos, A.T.; Almeida-Bezerra, J.W.; Almeida de Menezes, S.; Everson da Silva, L.; Melo Coutinho, H.D.; do Amaral, W.; et al. Antiparasitic effect of essential oils obtained from two species of Piper L. native to the Atlantic forest. Biocatal. Agric. Biotechnol. 2021, 32, 101958. [Google Scholar] [CrossRef]
- Llurba Montesino, N.; Kaiser, M.; Brun, R.; Schmidt, T.J. Search for Antiprotozoal Activity in Herbal Medicinal Preparations; New Natural Leads against Neglected Tropical Diseases. Molecules 2015, 20, 14118–14138. [Google Scholar] [CrossRef]
- Castaneda, J.S.; Suta-Velasquez, M.; Mateus, J.; Pardo-Rodriguez, D.; Puerta, C.J.; Cuellar, A.; Robles, J.; Cuervo, C. Preliminary chemical characterization of ethanolic extracts from Colombian plants with promising anti—Trypanosoma cruzi activity. Exp. Parasitol. 2021, 223, 108079. [Google Scholar] [CrossRef]
- Molina-Garza, Z.J.; Bazaldua-Rodriguez, A.F.; Quintanilla-Licea, R.; Galaviz-Silva, L. Anti-Trypanosoma cruzi activity of 10 medicinal plants used in northeast Mexico. Acta Trop. 2014, 136, 14–18. [Google Scholar] [CrossRef]
- Elena Ferreira, M.; Rojas de Arias, A.; Yaluff, G.; Vera de Bilbao, N.; Nakayama, H.; Torres, S.; Schinini, A.; Torres, S.; Serna, E.; Torrecilhas, A.C.; et al. Helietta apiculata: A tropical weapon against Chagas disease. Nat. Prod. Res. 2019, 33, 3308–3311. [Google Scholar] [CrossRef] [PubMed]
- Dantas Silva, R.P.; Machado, B.A.; Barreto, G.A.; Costa, S.S.; Andrade, L.N.; Amaral, R.G.; Carvalho, A.A.; Padilha, F.F.; Barbosa, J.D.; Umsza-Guez, M.A. Antioxidant, antimicrobial, antiparasitic, and cytotoxic properties of various Brazilian propolis extracts. PLoS ONE 2017, 12, e0172585. [Google Scholar] [CrossRef] [PubMed]
- Ebiloma, G.U.; Ichoron, N.; Siheri, W.; Watson, D.G.; Igoli, J.O.; De Koning, H.P. The Strong Anti-Kinetoplastid Properties of Bee Propolis: Composition and Identification of the Active Agents and Their Biochemical Targets. Molecules 2020, 25, 5155. [Google Scholar] [CrossRef]
- Asfaram, S.; Fakhar, M.; Keighobadi, M.; Akhtari, J. Promising anti-protozoan activities of propolis (bee glue) as natural product: A review. Acta Parasitol. 2021, 66, 1–12. [Google Scholar] [CrossRef]
- Martinez-Peinado, N.; Cortes-Serra, N.; Tallini, L.R.; Pinazo, M.J.; Gascon, J.; Bastida, J.; Alonso-Padilla, J. Amaryllidaceae plants: A potential natural resource for the treatment of Chagas disease. Parasit. Vectors 2021, 14, 337. [Google Scholar] [CrossRef]
- Meira, C.S.; Guimaraes, E.T.; Dos Santos, J.A.; Moreira, D.R.; Nogueira, R.C.; Tomassini, T.C.; Ribeiro, I.M.; de Souza, C.V.; Ribeiro Dos Santos, R.; Soares, M.B. In vitro and in vivo antiparasitic activity of Physalis angulata L. concentrated ethanolic extract against Trypanosoma cruzi. Phytomedicine 2015, 22, 969–974. [Google Scholar] [CrossRef]
- Meira, C.S.; Guimaraes, E.T.; Bastos, T.M.; Moreira, D.R.M.; Tomassini, T.C.B.; Ribeiro, I.M.; Dos Santos, R.R.; Soares, M.B.P. Physalins B and F, seco-steroids isolated from Physalis angulata L., strongly inhibit proliferation, ultrastructure and infectivity of Trypanosoma cruzi. Parasitology 2013, 140, 1811–1821. [Google Scholar] [CrossRef]
- Achan, J.; Talisuna, A.O.; Erhart, A.; Yeka, A.; Tibenderana, J.K.; Baliraine, F.N.; Rosenthal, P.J.; D’Alessandro, U. Quinine, an old anti-malarial drug in a modern world: Role in the treatment of malaria. Malar. J. 2011, 10, 144. [Google Scholar] [CrossRef]
- Pimenta, L.P.; Garcia, G.M.; Goncalves, S.G.; Dionisio, B.L.; Braga, E.M.; Mosqueira, V.C. In vivo antimalarial efficacy of acetogenins, alkaloids and flavonoids enriched fractions from Annona crassiflora Mart. Nat. Prod. Res. 2014, 28, 1254–1259. [Google Scholar] [CrossRef]
- Lombe, B.K.; Feineis, D.; Mudogo, V.; Kaiser, M.; Bringmann, G. Spirombandakamine A3 and Cyclombandakamines A8 and A9, Polycyclic Naphthylisoquinoline Dimers, with Antiprotozoal Activity, from a Congolese Ancistrocladus Plant. J. Nat. Prod. 2021, 84, 1335–1344. [Google Scholar] [CrossRef]
- Beaufay, C.; Ledoux, A.; Jansen, O.; Bordignon, A.; Zhao, S.; Teijaro, C.N.; Andrade, R.B.; Quetin-Leclercq, J.; Frederich, M. In vivo Antimalarial and Antitrypanosomal Activity of Strychnogucine B, a Bisindole Alkaloid from Strychnos icaja. Planta Med. 2018, 84, 881–885. [Google Scholar] [CrossRef]
- Dua, V.K.; Verma, G.; Singh, B.; Rajan, A.; Bagai, U.; Agarwal, D.D.; Gupta, N.C.; Kumar, S.; Rastogi, A. Anti-malarial property of steroidal alkaloid conessine isolated from the bark of Holarrhena antidysenterica. Malar. J. 2013, 12, 194. [Google Scholar] [CrossRef]
- Uche, F.I.; Guo, X.; Okokon, J.; Ullah, I.; Horrocks, P.; Boateng, J.; Huang, C.; Li, W.W. In vivo Efficacy and Metabolism of the Antimalarial Cycleanine and Improved In vitro Antiplasmodial Activity of Semisynthetic Analogues. Antimicrob. Agents Chemother. 2021, 65, 10–1128. [Google Scholar] [CrossRef]
- Goodman, C.D.; Austarheim, I.; Mollard, V.; Mikolo, B.; Malterud, K.E.; McFadden, G.I.; Wangensteen, H. Natural products from Zanthoxylum heitzii with potent activity against the malaria parasite. Malar. J. 2016, 15, 481. [Google Scholar] [CrossRef]
- Arnold, M.S.J.; Macdonald, J.R.; Quinn, R.J.; Skinner-Adams, T.S.; Andrews, K.T.; Fisher, G.M. Antiplasmodial activity of the natural product compounds alstonine and himbeline. Int. J. Parasitol. Drugs Drug Resist. 2021, 16, 17–22. [Google Scholar] [CrossRef]
- Endale, A.; Bisrat, D.; Animut, A.; Bucar, F.; Asres, K. In vivo antimalarial activity of a labdane diterpenoid from the leaves of Otostegia integrifolia Benth. Phytother. Res. 2013, 27, 1805–1809. [Google Scholar] [CrossRef] [PubMed]
- Gbedema, S.Y.; Bayor, M.T.; Annan, K.; Wright, C.W. Clerodane diterpenes from Polyalthia longifolia (Sonn) Thw. var. pendula: Potential antimalarial agents for drug resistant Plasmodium falciparum infection. J. Ethnopharmacol. 2015, 169, 176–182. [Google Scholar] [CrossRef] [PubMed]
- Ramalhete, C.; da Cruz, F.P.; Mulhovo, S.; Sousa, I.J.; Fernandes, M.X.; Prudencio, M.; Ferreira, M.J. Dual-stage triterpenoids from an African medicinal plant targeting the malaria parasite. Bioorganic Med. Chem. 2014, 22, 3887–3890. [Google Scholar] [CrossRef]
- Pereira, T.B.; Rocha e Silva, L.F.; Amorim, R.C.N.; Melo, M.R.S.; Zacardi de Souza, R.C.; Eberlin, M.N.; Lima, E.S.; Vasconcellos, M.C.; Pohlit, A.M. In vitro and in vivo anti-malarial activity of limonoids isolated from the residual seed biomass from Carapa guianensis (andiroba) oil production. Malar. J. 2014, 13, 317. [Google Scholar] [CrossRef]
- Parvatkar, P.T.; Maher, S.P.; Zhao, Y.; Cooper, C.A.; de Castro, S.T.; Péneau, J.; Vantaux, A.; Witkowski, B.; Kyle, D.E.; Manetsch, R. In vitro Antimalarial Activity of Trichothecenes against Liver and Blood Stages of Plasmodium Species. J. Nat. Prod. 2024, 87, 315–321. [Google Scholar] [CrossRef]
- Simelane, M.B.; Shonhai, A.; Shode, F.O.; Smith, P.; Singh, M.; Opoku, A.R. Anti-plasmodial activity of some Zulu medicinal plants and of some triterpenes isolated from them. Molecules 2013, 18, 12313–12323. [Google Scholar] [CrossRef]
- Sol Sol de Medeiros, D.; Tasca Cargnin, S.; Azevedo Dos Santos, A.P.; de Souza Rodrigues, M.; Berton Zanchi, F.; Soares de Maria de Medeiros, P.; de Almeida, E.S.A.; Bioni Garcia Teles, C.; Baggio Gnoatto, S.C. Ursolic and betulinic semisynthetic derivatives show activity against CQ-resistant Plasmodium falciparum isolated from Amazonia. Chem. Biol. Drug Des. 2021, 97, 1038–1047. [Google Scholar] [CrossRef]
- Kong, L.Y.; Tan, R.X. Artemisinin, a miracle of traditional Chinese medicine. Nat. Prod. Rep. 2015, 32, 1617–1621. [Google Scholar] [CrossRef]
- Elfawal, M.A.; Towler, M.J.; Reich, N.G.; Golenbock, D.; Weathers, P.J.; Rich, S.M. Dried whole plant Artemisia annua as an antimalarial therapy. PLoS ONE 2012, 7, e52746. [Google Scholar] [CrossRef] [PubMed]
- Elfawal, M.A.; Towler, M.J.; Reich, N.G.; Weathers, P.J.; Rich, S.M. Dried whole-plant Artemisia annua slows evolution of malaria drug resistance and overcomes resistance to artemisinin. Proc. Natl. Acad. Sci. USA 2015, 112, 821–826. [Google Scholar] [CrossRef] [PubMed]
- Maciuk, A.; Mazier, D.; Duval, R. Future antimalarials from Artemisia? A rationale for natural product mining against drug-refractory Plasmodium stages. Nat. Prod. Rep. 2023, 40, 1130–1144. [Google Scholar] [CrossRef]
- Patel, O.P.; Beteck, R.M.; Legoabe, L.J. Exploration of artemisinin derivatives and synthetic peroxides in antimalarial drug discovery research. Eur. J. Med. Chem. 2021, 213, 113193. [Google Scholar] [CrossRef] [PubMed]
- Patel, O.P.S.; Beteck, R.M.; Legoabe, L.J. Antimalarial application of quinones: A recent update. Eur. J. Med. Chem. 2021, 210, 113084. [Google Scholar] [CrossRef]
- Harel, D.; Schepmann, D.; Prinz, H.; Brun, R.; Schmidt, T.J.; Wunsch, B. Natural product derived antiprotozoal agents: Synthesis, biological evaluation, and structure-activity relationships of novel chromene and chromane derivatives. J. Med. Chem. 2013, 56, 7442–7448. [Google Scholar] [CrossRef]
- Chirawurah, J.D.; Ansah, F.; Blankson, S.; Adikah, B.; Yeboah, S.N.; Amenga-Etego, L.; Awandare, G.A.; Aniweh, Y. Gossypol is a natural product with good antimalarial activity against Plasmodium falciparum clinical isolates. Sci. Rep. 2025, 15, 1469. [Google Scholar] [CrossRef]
- Jun, H.; Han, J.-H.; Hong, M.; Fitriana, F.; Syahada, J.H.; Lee, W.-J.; Mazigo, E.; Louis, J.M.; Nguyen, V.-T.; Cha, S.H. Ellagic Acid from Geranium thunbergii and Antimalarial Activity of Korean Medicinal Plants. Molecules 2025, 30, 359. [Google Scholar] [CrossRef] [PubMed]
- Prashar, C.; Thakur, N.; Chakraborti, S.; Areeb Hussain, S.S.; Vashisht, K.; Pandey, K.C. The landscape of nature-derived antimalarials-potential of marine natural products in countering the evolving Plasmodium. Front. Drug Discov. 2022, 2, 1065231. [Google Scholar] [CrossRef]
- Conroy, T.; Guo, J.T.; Linington, R.G.; Hunt, N.H.; Payne, R.J. Total synthesis, stereochemical assignment, and antimalarial activity of gallinamide A. Chemistry 2011, 17, 13544–13552. [Google Scholar] [CrossRef] [PubMed]
- Stolze, S.C.; Deu, E.; Kaschani, F.; Li, N.; Florea, B.I.; Richau, K.H.; Colby, T.; van der Hoorn, R.A.; Overkleeft, H.S.; Bogyo, M.; et al. The antimalarial natural product symplostatin 4 is a nanomolar inhibitor of the food vacuole falcipains. Chem. Biol. 2012, 19, 1546–1555. [Google Scholar] [CrossRef]
- Keller, L.; Siqueira-Neto, J.L.; Souza, J.M.; Eribez, K.; LaMonte, G.M.; Smith, J.E.; Gerwick, W.H. Palstimolide A: A Complex Polyhydroxy Macrolide with Antiparasitic Activity. Molecules 2020, 25, 1604. [Google Scholar] [CrossRef]
- Rossella Gagliano, C.; Rosselli, S.; Bruno, M.; Fontana, G. A review of the phytochemistry, traditional uses and biological activities of the essential oils of genus Teucrium. Planta Medica 2020, 87, 432–479. [Google Scholar] [CrossRef]
- Clement, O.A.; Anthony, A.E.; Félicien, M.K.; Mercy, G.T.; Hedmon, O.; Anke, W.; Casim, U.T.; Patrick, E.O. A review for selecting medicinal plants commonly used for malaria in Uganda. Afr. J. Pharm. Pharmacol. 2020, 14, 347–361. [Google Scholar] [CrossRef]
- Mohammadi, S.; Jafari, B.; Asgharian, P.; Martorell, M.; Sharifi-Rad, J. Medicinal plants used in the treatment of Malaria: A key emphasis to Artemisia, Cinchona, Cryptolepis, and Tabebuia genera. Phytother. Res. 2020, 34, 1556–1569. [Google Scholar] [CrossRef] [PubMed]
- Noronha, M.; Pawar, V.; Prajapati, A.; Subramanian, R.B. A literature review on traditional herbal medicines for malaria. S. Afr. J. Bot. 2020, 128, 292–303. [Google Scholar] [CrossRef]
- Ribeiro, G.d.J.G.; Rei Yan, S.L.; Palmisano, G.; Wrenger, C. Plant extracts as a source of natural products with potential antimalarial effects: An update from 2018 to 2022. Pharmaceutics 2023, 15, 1638. [Google Scholar] [CrossRef] [PubMed]
- Muganga, R.; Angenot, L.; Tits, M.; Frederich, M. Antiplasmodial and cytotoxic activities of Rwandan medicinal plants used in the treatment of malaria. J. Ethnopharmacol. 2010, 128, 52–57. [Google Scholar] [CrossRef] [PubMed]
- Gogoi, N.; Gogoi, B.; Chetia, D. In vitro antimalarial activity evaluation of two ethnomedicinal plants against chloroquine sensitive and resistant strains of Plasmodium falciparum. Clin. Phytoscience 2021, 7, 42. [Google Scholar] [CrossRef]
- Ngemenya, M.; Metuge, H.; Mbah, J.; Zofou, D.; Babiaka, S.; Titanji, V. Isolation of Natural Product Hits from Peperomia species with Synergistic Activity against Resistant Plasmodium falciparum Strains. Eur. J. Med. Plants 2015, 5, 77–87. [Google Scholar] [CrossRef]
- Alaribe, S.C.; Oladipupo, A.R.; Uche, G.C.; Onumba, M.U.; Ota, D.; Awodele, O.; Oyibo, W.A. Suppressive, curative, and prophylactic potentials of an antimalarial polyherbal mixture and its individual components in Plasmodium berghei-Infected mice. J. Ethnopharmacol. 2021, 277, 114105. [Google Scholar] [CrossRef]
- Rasoanaivo, P.; Wright, C.W.; Willcox, M.L.; Gilbert, B. Whole plant extracts versus single compounds for the treatment of malaria: Synergy and positive interactions. Malar. J. 2011, 10, S4. [Google Scholar] [CrossRef]
- Obidike, I.C.; Amodu, B.; Emeje, M.O. Antimalarial properties of SAABMAL®: An ethnomedicinal polyherbal formulation for the treatment of uncomplicated malaria infection in the tropics. Indian J. Med. Res. 2015, 141, 221. [Google Scholar]
- Gontijo, D.C.; Leite, J.P.V.; Nascimento, M.; Brandao, G.C.; Oliveira, A.B. Bioprospection for antiplasmodial activity, and identification of bioactive metabolites of native plants species from the Mata Atlantica biome, Brazil. Nat. Prod. Res. 2021, 35, 1732–1737. [Google Scholar] [CrossRef]
- Kumatia, E.K.; Ayertey, F.; Appiah-Opong, R.; Bagyour, G.K.; Asare, K.O.; Mbatcho, V.C.; Dabo, J. Intervention of standardized ethanol leaf extract of Annickia polycarpa, (DC.) Setten and Maas ex I.M. Turner. (Annonaceae), in Plasmodium berghei infested mice produced anti-malaria action and normalized gross hematological indices. J. Ethnopharmacol. 2021, 267, 113449. [Google Scholar] [CrossRef] [PubMed]
- Newman, D.J.; Cragg, G.M. Natural products as sources of new drugs over the last 25 years. J. Nat. Prod. 2007, 70, 461–477. [Google Scholar] [CrossRef]
- Dos Santos, D.B.; Lemos, J.A.; Miranda, S.E.M.; Di Filippo, L.D.; Duarte, J.L.; Ferreira, L.A.M.; Barros, A.L.B.; Oliveira, A.E. Current applications of plant-based drug delivery nano systems for Leishmaniasis treatment. Pharmaceutics 2022, 14, 2339. [Google Scholar] [CrossRef]
- Sato, S. Plasmodium—A brief introduction to the parasites causing human malaria and their basic biology. J. Physiol. Anthropol. 2021, 40, 1. [Google Scholar] [CrossRef]
- Kekani, L.N.; Witika, B.A. Current advances in nanodrug delivery systems for malaria prevention and treatment. Discov. Nano 2023, 18, 66. [Google Scholar] [CrossRef]
- Murugan, K.; Benelli, G.; Panneerselvam, C.; Subramaniam, J.; Jeyalalitha, T.; Dinesh, D.; Nicoletti, M.; Hwang, J.-S.; Suresh, U.; Madhiyazhagan, P. Cymbopogon citratus-synthesized gold nanoparticles boost the predation efficiency of copepod Mesocyclops aspericornis against malaria and dengue mosquitoes. Exp. Parasitol. 2015, 153, 129–138. [Google Scholar] [CrossRef]
- Subramaniam, J.; Murugan, K.; Panneerselvam, C.; Kovendan, K.; Madhiyazhagan, P.; Dinesh, D.; Kumar, P.M.; Chandramohan, B.; Suresh, U.; Rajaganesh, R. Multipurpose effectiveness of Couroupita guianensis-synthesized gold nanoparticles: High antiplasmodial potential, field efficacy against malaria vectors and synergy with Aplocheilus lineatus predators. Environ. Sci. Pollut. Res. 2016, 23, 7543–7558. [Google Scholar] [CrossRef]
- Santhosh, S.B.; Yuvarajan, R.; Natarajan, D. Annona muricata leaf extract-mediated silver nanoparticles synthesis and its larvicidal potential against dengue, malaria and filariasis vector. Parasitol. Res. 2015, 114, 3087–3096. [Google Scholar] [CrossRef] [PubMed]
- Nalini, M.; Lena, M.; Sumathi, P.; Sundaravadivelan, C. Effect of phyto-synthesized silver nanoparticles on developmental stages of malaria vector, Anopheles stephensi and dengue vector, Aedes aegypti. Egypt. J. Basic Appl. Sci. 2017, 4, 212–218. [Google Scholar] [CrossRef]
- Ojemaye, M.O.; Okoh, S.O.; Okoh, A.I. Silver nanoparticles (AgNPs) facilitated by plant parts of Crataegus ambigua Becker AK extracts and their antibacterial, antioxidant and antimalarial activities. Green Chem. Lett. Rev. 2021, 14, 51–61. [Google Scholar] [CrossRef]
- Babatimehin, A.M.; Ajayi, G.O.; Ogunbamowo, O.E.; El-Rayyes, A.; Albedair, L.A.; Alsuhaibani, A.M.; Ofudje, E.A. Synthesis of silver nanoparticles using Azadirachta indica leaf extracts for heavy metal sensing. BioResources 2025, 20, 334. [Google Scholar] [CrossRef]
- Murugan, K.; Panneerselvam, C.; Samidoss, C.M.; Madhiyazhagan, P.; Suresh, U.; Roni, M.; Chandramohan, B.; Subramaniam, J.; Dinesh, D.; Rajaganesh, R. In vivo and in vitro effectiveness of Azadirachta indica-synthesized silver nanocrystals against Plasmodium berghei and Plasmodium falciparum, and their potential against malaria mosquitoes. Res. Vet. Sci. 2016, 106, 14–22. [Google Scholar] [CrossRef] [PubMed]
- Kaur, R.; Kaur, H. Plant derived antimalarial agents. J. Med. Plants Stud. 2017, 5, 346–363. [Google Scholar]
- Sardana, M.; Agarwal, V.; Pant, A.; Kapoor, V.; Pandey, K.C.; Kumar, S. Antiplasmodial activity of silver nanoparticles: A novel green synthesis approach. Asian Pac. J. Trop. Biomed. 2018, 8, 268–272. [Google Scholar] [CrossRef]
- Hawadak, J.; Kojom Foko, L.P.; Pande, V.; Singh, V. In vitro antiplasmodial activity, hemocompatibility and temporal stability of Azadirachta indica silver nanoparticles. Artif. Cells Nanomed. Biotechnol. 2022, 50, 286–300. [Google Scholar] [CrossRef] [PubMed]
- Chen, L.Q.; Fang, L.; Ling, J.; Ding, C.Z.; Kang, B.; Huang, C.Z. Nanotoxicity of silver nanoparticles to red blood cells: Size dependent adsorption, uptake, and hemolytic activity. Chem. Res. Toxicol. 2015, 28, 501–509. [Google Scholar] [CrossRef] [PubMed]
- Sowndarya, P.; Ramkumar, G.; Shivakumar, M.S. Green synthesis of selenium nanoparticles conjugated Clausena dentata plant leaf extract and their insecticidal potential against mosquito vectors. Artif. Cells Nanomed. Biotechnol. 2017, 45, 1490–1495. [Google Scholar] [CrossRef]
- Nassar, A.-R.A.; Eid, A.M.; Atta, H.M.; El Naghy, W.S.; Fouda, A. Exploring the antimicrobial, antioxidant, anticancer, biocompatibility, and larvicidal activities of selenium nanoparticles fabricated by endophytic fungal strain Penicillium verhagenii. Sci. Rep. 2023, 13, 9054. [Google Scholar] [CrossRef]
- Najoom, S.; Fozia, F.; Ahmad, I.; Wahab, A.; Ahmad, N.; Ullah, R.; Gul, A.; Bari, A.; Khan, M.Y.; Khan, A.A. Effective antiplasmodial and cytotoxic activities of synthesized zinc oxide nanoparticles using Rhazya stricta leaf extract. Evid.-Based Complement. Altern. Med. 2021, 2021, 5586740. [Google Scholar] [CrossRef]
- Nadeem, F.; Fozia, F.; Aslam, M.; Ahmad, I.; Ahmad, S.; Ullah, R.; Almutairi, M.H.; Aleya, L.; Abdel-Daim, M.M. Characterization, antiplasmodial and cytotoxic activities of green synthesized iron oxide nanoparticles using Nephrolepis exaltata aqueous extract. Molecules 2022, 27, 4931. [Google Scholar] [CrossRef]
- Xiao, L.; Mertens, M.; Wortmann, L.; Kremer, S.; Valldor, M.; Lammers, T.; Kiessling, F.; Mathur, S. Enhanced in vitro and in vivo cellular imaging with green tea coated water-soluble iron oxide nanocrystals. ACS Appl. Mater. Interfaces 2015, 7, 6530–6540. [Google Scholar] [CrossRef] [PubMed]
- Mhlwatika, Z.; Aderibigbe, B.A. Polymeric nanocarriers for the delivery of antimalarials. Molecules 2018, 23, 2527. [Google Scholar] [CrossRef] [PubMed]
- Oyeyemi, O.; Morenkeji, O.; Afolayan, F.; Dauda, K.; Busari, Z.; Meena, J.; Panda, A. Curcumin-artesunate based polymeric nanoparticle; antiplasmodial and toxicological evaluation in murine model. Front. Pharmacol. 2018, 9, 562. [Google Scholar] [CrossRef]
- Dende, C.; Meena, J.; Nagarajan, P.; Nagaraj, V.A.; Panda, A.K.; Padmanaban, G. Nanocurcumin is superior to native curcumin in preventing degenerative changes in Experimental Cerebral Malaria. Sci. Rep. 2017, 7, 10062. [Google Scholar] [CrossRef] [PubMed]
- Busari, Z.A.; Dauda, K.A.; Morenikeji, O.A.; Afolayan, F.; Oyeyemi, O.T.; Meena, J.; Sahu, D.; Panda, A.K. Antiplasmodial activity and toxicological assessment of curcumin PLGA-encapsulated nanoparticles. Front. Pharmacol. 2017, 8, 622. [Google Scholar] [CrossRef]
- Sanei-Dehkordi, A.; Moemenbellah-Fard, M.D.; Sereshti, H.; Shahriari-Namadi, M.; Zarenezhad, E.; Osanloo, M. Chitosan nanoparticles containing Elettaria cardamomum and Cinnamomum zeylanicum essential oils; repellent and larvicidal effects against a malaria mosquito vector, and cytotoxic effects on a human skin normal cell line. Chem. Pap. 2021, 75, 6545–6556. [Google Scholar] [CrossRef]
- Solans, C.; Izquierdo, P.; Nolla, J.; Azemar, N.; Garcia-Celma, M.J. Nano-emulsions. Curr. Opin. Colloid Interface Sci. 2005, 10, 102–110. [Google Scholar] [CrossRef]
- Gupta, J.; Sharma, G. Nanogel: A versatile drug delivery system for the treatment of various diseases and their future perspective. Drug Deliv. Transl. Res. 2025, 15, 455–482. [Google Scholar] [CrossRef]
- Esmaili, F.; Sanei-Dehkordi, A.; Amoozegar, F.; Osanloo, M. A review on the use of essential oil-based nanoformulations in control of mosquitoes. Biointerface Res. Appl. Chem. 2021, 11, 12516–12529. [Google Scholar] [CrossRef]
- Moemenbellah-Fard, M.D.; Firoozian, S.; Shahriari-Namadi, M.; Zarenezhad, E.; Roozitalab, G.; Osanloo, M. A natural nanogel with higher efficacy than a standard repellent against the primary malaria mosquito vector, Anopheles stephensi Liston. Chem. Pap. 2022, 76, 1767–1776. [Google Scholar] [CrossRef]
- Sheikh, Z.; Amani, A.; Basseri, H.R.; MoosaKazemi, S.H.; Sedaghat, M.M.; Azam, K.; Yousefpoor, Y.; Amirmohammadi, F.; Azizi, M. Development of mosquito protective textiles using nanoemulsion of Eucalyptus globulus and Syzygium aromaticum essential oils against malaria vector, Anopheles stephensi (liston). Res. Sq. 2021. [Google Scholar] [CrossRef]
- Echeverría, J.; Duarte Galhardo de Albuquerque, R.D. Nanoemulsions of essential oils: New tool for control of vector-borne diseases and in vitro effects on some parasitic agents. Medicines 2019, 6, 42. [Google Scholar] [CrossRef] [PubMed]
- Osanloo, M.; Firooziyan, S.; Abdollahi, A.; Hatami, S.; Nematollahi, A.; Elahi, N.; Zarenezhad, E. Nanoemulsion and nanogel containing Artemisia dracunculus essential oil; larvicidal effect and antibacterial activity. BMC Res. Notes 2022, 15, 276. [Google Scholar] [CrossRef] [PubMed]
- Mehta, M.; Bui, T.A.; Yang, X.; Aksoy, Y.; Goldys, E.M.; Deng, W. Lipid-based nanoparticles for drug/gene delivery: An overview of the production techniques and difficulties encountered in their industrial development. ACS Mater. Au 2023, 3, 600–619. [Google Scholar] [CrossRef] [PubMed]
- Sanei-Dehkordi, A.; Agholi, M.; Shafiei, M.; Osanloo, M. Promising larvicidal efficacy of solid lipid nanoparticles containing mentha longifolia L., mentha pulegium L., and Zataria multiflora boiss. Essential oils against the main malaria vector, Anopheles stephensi liston. Acta Parasitol. 2022, 67, 1265–1272. [Google Scholar] [CrossRef]
- Kelidari, H.R.; Moemenbellah-Fard, M.D.; Morteza-Semnani, K.; Amoozegar, F.; Shahriari-Namadi, M.; Saeedi, M.; Osanloo, M. Solid-lipid nanoparticles (SLN) s containing Zataria multiflora essential oil with no-cytotoxicity and potent repellent activity against Anopheles stephensi. J. Parasit. Dis. 2021, 45, 101–108. [Google Scholar] [CrossRef]
- Sanei-Dehkordi, A.; Moemenbellah-Fard, M.D.; Saffari, M.; Zarenezhad, E.; Osanloo, M. Nanoliposomes containing limonene and limonene-rich essential oils as novel larvicides against malaria and filariasis mosquito vectors. BMC Complement. Med. Ther. 2022, 22, 140. [Google Scholar] [CrossRef]
- Sanei-Dehkordi, A.; Ghasemian, A.; Zarenezhad, E.; Qasemi, H.; Nasiri, M.; Osanloo, M. Nanoliposomes containing three essential oils from the Artemisia genus as effective larvicides against Aedes aegypti and Anopheles stephensi. Sci. Rep. 2023, 13, 11002. [Google Scholar] [CrossRef]
- Doktorovova, S.; Kovačević, A.B.; Garcia, M.L.; Souto, E.B. Preclinical safety of solid lipid nanoparticles and nanostructured lipid carriers: Current evidence from in vitro and in vivo evaluation. Eur. J. Pharm. Biopharm. 2016, 108, 235–252. [Google Scholar] [CrossRef]
- Rashidzadeh, H.; Salimi, M.; Sadighian, S.; Rostamizadeh, K.; Ramazani, A. In vivo antiplasmodial activity of curcumin-loaded nanostructured lipid carriers. Curr. Drug Deliv. 2019, 16, 923–930. [Google Scholar] [CrossRef]
- Fulgheri, F.; Aroffu, M.; Ramírez, M.; Román-Álamo, L.; Peris, J.E.; Usach, I.; Nacher, A.; Manconi, M.; Fernàndez-Busquets, X.; Manca, M.L. Curcumin or quercetin loaded nutriosomes as oral adjuvants for malaria infections. Int. J. Pharm. 2023, 643, 123195. [Google Scholar] [CrossRef]
- Manconi, M.; Manca, M.L.; Escribano-Ferrer, E.; Coma-Cros, E.M.; Biosca, A.; Lantero, E.; Fernàndez-Busquets, X.; Fadda, A.M.; Caddeo, C. Nanoformulation of curcumin-loaded eudragit-nutriosomes to counteract malaria infection by a dual strategy: Improving antioxidant intestinal activity and systemic efficacy. Int. J. Pharm. 2019, 556, 82–88. [Google Scholar] [CrossRef]
- Akula, S.; Gurram, A.K.; Devireddy, S.R. Self-Microemulsifying Drug Delivery Systems: An Attractive Strategy for Enhanced Therapeutic Profile. Int. Sch. Res. Not. 2014, 2014, 964051. [Google Scholar] [CrossRef]
- Shah, A.; Thakkar, V.; Gohel, M.; Baldaniya, L.; Gandhi, T. Optimization of self micro emulsifying drug delivery system containing curcumin and artemisinin using D-optimal mixture design. Saudi J. Med. Pharm. Sci. 2017, 3, 388–398. [Google Scholar]
- Ghezzi, M.; Pescina, S.; Padula, C.; Santi, P.; Del Favero, E.; Cantù, L.; Nicoli, S. Polymeric micelles in drug delivery: An insight of the techniques for their characterization and assessment in biorelevant conditions. J. Control. Release 2021, 332, 312–336. [Google Scholar] [CrossRef]
- Kali, G.; Haddadzadegan, S.; Bernkop-Schnürch, A. Cyclodextrins and derivatives in drug delivery: New developments, relevant clinical trials, and advanced products. Carbohydr. Polym. 2024, 324, 121500. [Google Scholar] [CrossRef]
- Manjili, H.R.K.; Malvandi, H.; Mosavi, M.-S.; Danafar, H. Preparation and physicochemical characterization of biodegradable mPEG-PCL core-shell micelles for delivery of artemisinin. Pharm. Sci. 2016, 22, 234–243. [Google Scholar] [CrossRef]
- Yaméogo, J.B.G.; Mazet, R.; Wouessidjewe, D.; Choisnard, L.; Godin-Ribuot, D.; Putaux, J.-L.; Semdé, R.; Gèze, A. Pharmacokinetic study of intravenously administered artemisinin-loaded surface-decorated amphiphilic γ-cyclodextrin nanoparticles. Mater. Sci. Eng. C 2020, 106, 110281. [Google Scholar] [CrossRef] [PubMed]
- Savale, A.; Ghotekar, S.; Pansambal, S.; Pardeshi, O. Green synthesis of fluorescent CdO nanoparticles using Leucaena leucocephala L. extract and their biological activities. J. Bacteriol. Mycol. Open Access 2017, 5, 00148. [Google Scholar] [CrossRef]
- Gandhi, P.R.; Jayaseelan, C.; Kamaraj, C.; Rajasree, S.R.R.; Mary, R.R. In vitro antimalarial activity of synthesized TiO2 nanoparticles using Momordica charantia leaf extract against Plasmodium falciparum. J. Appl. Biomed. 2018, 16, 378–386. [Google Scholar] [CrossRef]
- Minal, S.P.; Prakash, S. Characterization and nano-efficacy study of palladium nanoparticles against Larvae of Anopheles stephensi (Liston). Int. J. Adv. Eng. Nanotechnol. 2018, 3, 1–5. [Google Scholar]
- Osanloo, M.; Sereshti, H.; Sedaghat, M.M.; Amani, A. Nanoemulsion of Dill essential oil as a green and potent larvicide against Anopheles stephensi. Environ. Sci. Pollut. Res. 2018, 25, 6466–6473. [Google Scholar] [CrossRef]
- Ledoux, A.; Mamede, L.; Palazzo, C.; Furst, T.; Jansen, O.; De Tullio, P.; Kagisha, V.; Pendeville, H.; Fillet, M.; Piel, G. Heparin-coated liposomes improve antiplasmodial activity and reduce the toxicity of poupartone B. Planta Medica Int. Open 2020, 7, e73–e80. [Google Scholar] [CrossRef]
- Sanei-Dehkordi, A.; Heiran, R.; Roozitalab, G.; Elahi, N.; Osanloo, M. Larvicidal effects of nanoliposomes containing clove and cinnamon essential oils, eugenol, and cinnamaldehyde against the main malaria vector, Anopheles stephensi Liston. Psyche: A J. Entomol. 2022, 2022, 9991238. [Google Scholar] [CrossRef]
- Biosca, A.; Cabanach, P.; Abdulkarim, M.; Gumbleton, M.; Gómez-Canela, C.; Ramírez, M.; Bouzón-Arnáiz, I.; Avalos-Padilla, Y.; Borros, S.; Fernàndez-Busquets, X. Zwitterionic self-assembled nanoparticles as carriers for Plasmodium targeting in malaria oral treatment. J. Control. Release 2021, 331, 364–375. [Google Scholar] [CrossRef]
- Dos Santos, R.B.; Nakama, K.A.; Pacheco, C.O.; de Gomes, M.G.; de Souza, J.F.; de Souza Pinto, A.C.; de Oliveira, F.A.; da Fonseca, A.L.; Varotti, F.; Fajardo, A.R. Curcumin-loaded nanocapsules: Influence of surface characteristics on technological parameters and potential antimalarial activity. Mater. Sci. Eng. C 2021, 118, 111356. [Google Scholar] [CrossRef] [PubMed]
- Morilla, M.J.; Ghosal, K.; Romero, E.L. Nanomedicines against Chagas disease: A critical review. Beilstein J. Nanotechnol. 2024, 15, 333–349. [Google Scholar] [CrossRef] [PubMed]
- Brito, T.K.; Silva Viana, R.L.; Gonçalves Moreno, C.J.; da Silva Barbosa, J.; Lopes de Sousa Júnior, F.; Campos de Medeiros, M.J.; Melo-Silveira, R.F.; Almeida-Lima, J.; de Lima Pontes, D.; Sousa Silva, M. Synthesis of silver nanoparticle employing corn cob xylan as a reducing agent with anti-Trypanosoma cruzi activity. Int. J. Nanomed. 2020, 15, 965–979. [Google Scholar] [CrossRef]
- de Mello, C.G.C.; Branquinho, R.T.; Oliveira, M.T.; Milagre, M.M.; Saúde-Guimarães, D.A.; Mosqueira, V.C.F.; Lana, M.d. Efficacy of lychnopholide polymeric nanocapsules after oral and intravenous administration in murine experimental Chagas disease. Antimicrob. Agents Chemother. 2016, 60, 5215–5222. [Google Scholar] [CrossRef]
- Gomes, D.C.; Medeiros, T.S.; Alves Pereira, E.L.; da Silva, J.F.O.; de Freitas Oliveira, J.W.; Fernandes-Pedrosa, M.d.F.; de Sousa da Silva, M.; da Silva-Júnior, A.A. From Benznidazole to new drugs: Nanotechnology contribution in Chagas disease. Int. J. Mol. Sci. 2023, 24, 13778. [Google Scholar] [CrossRef] [PubMed]
- Branquinho, R.T.; Roy, J.; Farah, C.; Garcia, G.M.; Aimond, F.; Le Guennec, J.-Y.; Saude-Guimarães, D.A.; Grabe-Guimaraes, A.; Mosqueira, V.C.F.; de Lana, M. Biodegradable polymeric nanocapsules prevent cardiotoxicity of anti-trypanosomal lychnopholide. Sci. Rep. 2017, 7, 44998. [Google Scholar] [CrossRef] [PubMed]
- Branquinho, R.T.; de Mello, C.G.C.; Oliveira, M.T.; Reis, L.E.S.; Vieira, P.M.d.A.; Saúde-Guimarães, D.A.; Mosqueira, V.C.F.; de Lana, M. Lychnopholide in poly (D, L-lactide)-block-polyethylene glycol nanocapsules cures infection with a drug-resistant Trypanosoma cruzi strain at acute and chronic phases. Antimicrob. Agents Chemother. 2020, 64, 10–1128. [Google Scholar] [CrossRef]
- Branquinho, R.T.; Pound-Lana, G.; Marques Milagre, M.; Saúde-Guimarães, D.A.; Vilela, J.M.C.; Spangler Andrade, M.; de Lana, M.; Mosqueira, V.C.F. Increased body exposure to new anti-trypanosomal through nanoencapsulation. Sci. Rep. 2017, 7, 8429. [Google Scholar] [CrossRef]
- Neres, N.B.R.; Montagnini, D.; Ferreira, D.S.; Parreira, R.L.T.; Orenha, R.P.; Lima, T.C.; Molina, E.F.; Cunha, W.R.; Silva, M.L.A.; Esperandim, V.R. In vivo and in Silico Trypanocidal Activity Evaluation of (−)-Cubebin Encapsulated in PLGA Microspheres as Potential Treatment in Acute Phase. Chem. Biodivers. 2021, 18, e2100052. [Google Scholar] [CrossRef]
- Abriata, J.P.; Eloy, J.O.; Riul, T.B.; Campos, P.M.; Baruffi, M.D.; Marchetti, J.M. Poly-epsilon-caprolactone nanoparticles enhance ursolic acid in vivo efficacy against Trypanosoma cruzi infection. Mater. Sci. Eng. C 2017, 77, 1196–1203. [Google Scholar] [CrossRef]
- Hernández, M.; Wicz, S.; Caballero, E.P.; Santamaría, M.H.; Corral, R.S. Dual chemotherapy with benznidazole at suboptimal dose plus curcumin nanoparticles mitigates Trypanosoma cruzi-elicited chronic cardiomyopathy. Parasitol. Int. 2021, 81, 102248. [Google Scholar] [CrossRef]
- De Oliveira, E.C.V.; Carneiro, Z.A.; de Albuquerque, S.; Marchetti, J.M. Development and evaluation of a nanoemulsion containing ursolic acid: A promising trypanocidal agent. AAPS PharmSciTech 2017, 18, 2551–2560. [Google Scholar] [CrossRef]
- Vermelho, A.B.; da Silva Cardoso, V.; Ricci Junior, E.; Dos Santos, E.P.; Supuran, C.T. Nanoemulsions of sulfonamide carbonic anhydrase inhibitors strongly inhibit the growth of Trypanosoma cruzi. J. Enzym. Inhib. Med. Chem. 2018, 33, 139–146. [Google Scholar] [CrossRef]
- Nicoletti, C.D.; Faria, A.F.M.; de Sá Haddad Queiroz, M.; dos Santos Galvão, R.M.; Souza, A.L.A.; Futuro, D.O.; Faria, R.X.; Ferreira, V.F. Synthesis and biological evaluation of β-lapachone and nor-β-lapachone complexes with 2-hydroxypropyl-β-cyclodextrin as trypanocidal agents. J. Bioenerg. Biomembr. 2020, 52, 185–197. [Google Scholar] [CrossRef] [PubMed]
- Barbosa, J.M.C.; Nicoletti, C.D.; da Silva, P.B.; Melo, T.G.; Futuro, D.O.; Ferreira, V.F.; Salomão, K. Characterization and trypanocidal activity of a β-lapachone-containing drug carrier. PLoS ONE 2021, 16, e0246811. [Google Scholar] [CrossRef] [PubMed]
- de Morais, F.A.P.; Enumo, A.; Gonçalves, R.S.; Cesar, G.B.; Miranda, N.; Vilsinski, B.H.; da Silva Junior, R.C.; Nakamura, C.V.; Hioka, N.; Caetano, W. Hypericin photodynamic activity. Part III: In vitro evaluation in different nanocarriers against trypomastigotes of Trypanosoma cruzi. Photochem. Photobiol. Sci. 2019, 18, 487–494. [Google Scholar] [CrossRef] [PubMed]






Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
García, M.; Magi, M.S.; García, M.C. Harnessing Phytonanotechnology to Tackle Neglected Parasitic Diseases: Focus on Chagas Disease and Malaria. Pharmaceutics 2025, 17, 1043. https://doi.org/10.3390/pharmaceutics17081043
García M, Magi MS, García MC. Harnessing Phytonanotechnology to Tackle Neglected Parasitic Diseases: Focus on Chagas Disease and Malaria. Pharmaceutics. 2025; 17(8):1043. https://doi.org/10.3390/pharmaceutics17081043
Chicago/Turabian StyleGarcía, Manuela, María S. Magi, and Mónica C. García. 2025. "Harnessing Phytonanotechnology to Tackle Neglected Parasitic Diseases: Focus on Chagas Disease and Malaria" Pharmaceutics 17, no. 8: 1043. https://doi.org/10.3390/pharmaceutics17081043
APA StyleGarcía, M., Magi, M. S., & García, M. C. (2025). Harnessing Phytonanotechnology to Tackle Neglected Parasitic Diseases: Focus on Chagas Disease and Malaria. Pharmaceutics, 17(8), 1043. https://doi.org/10.3390/pharmaceutics17081043








