Nifuroxazide and 4-Hydroxybenzhydrazone Derivatives as New Antiparasitic (Trypanosoma cruzi and Leishmania mexicana) and Anti-Mycobacterium tuberculosis Agents
Abstract
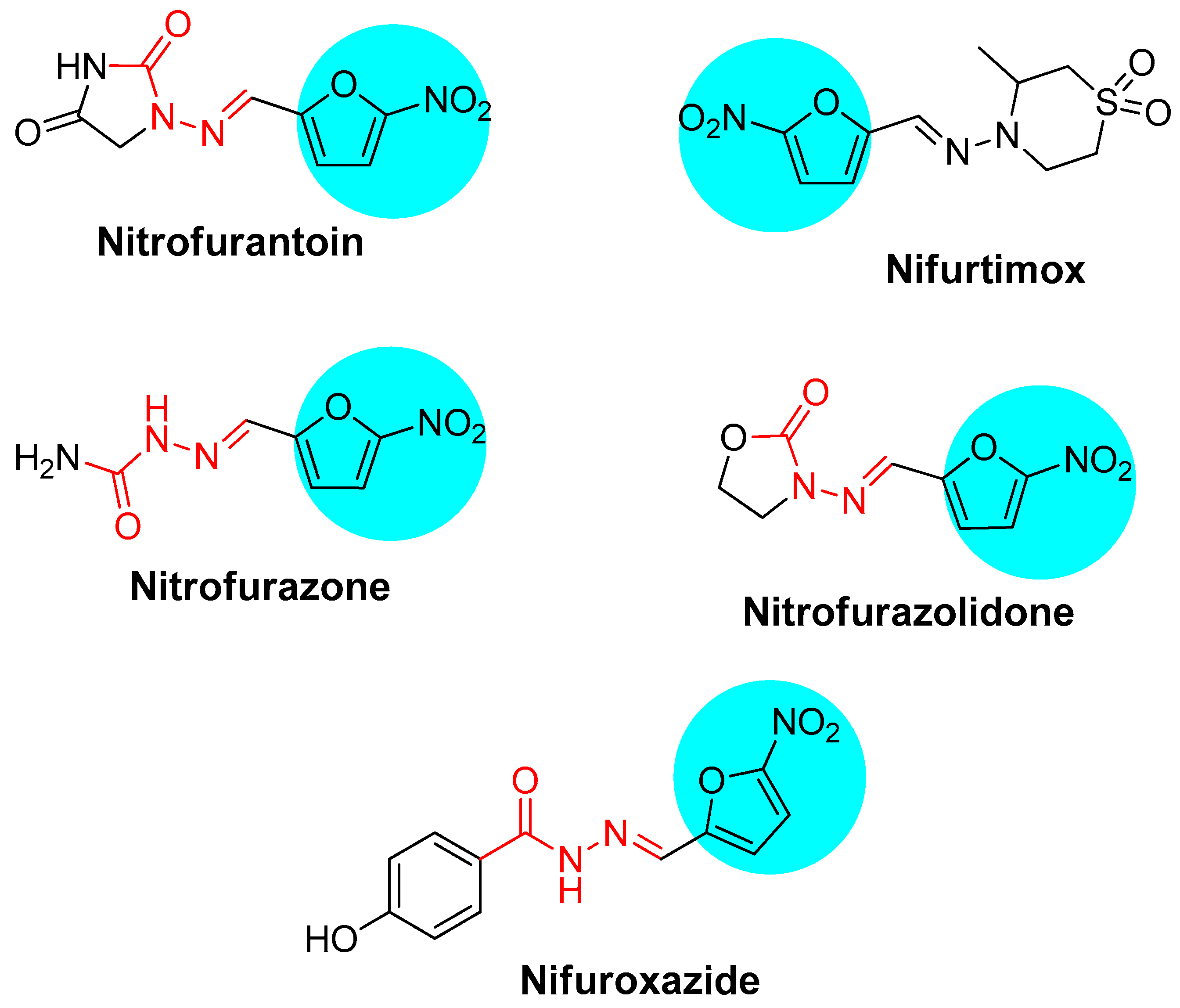
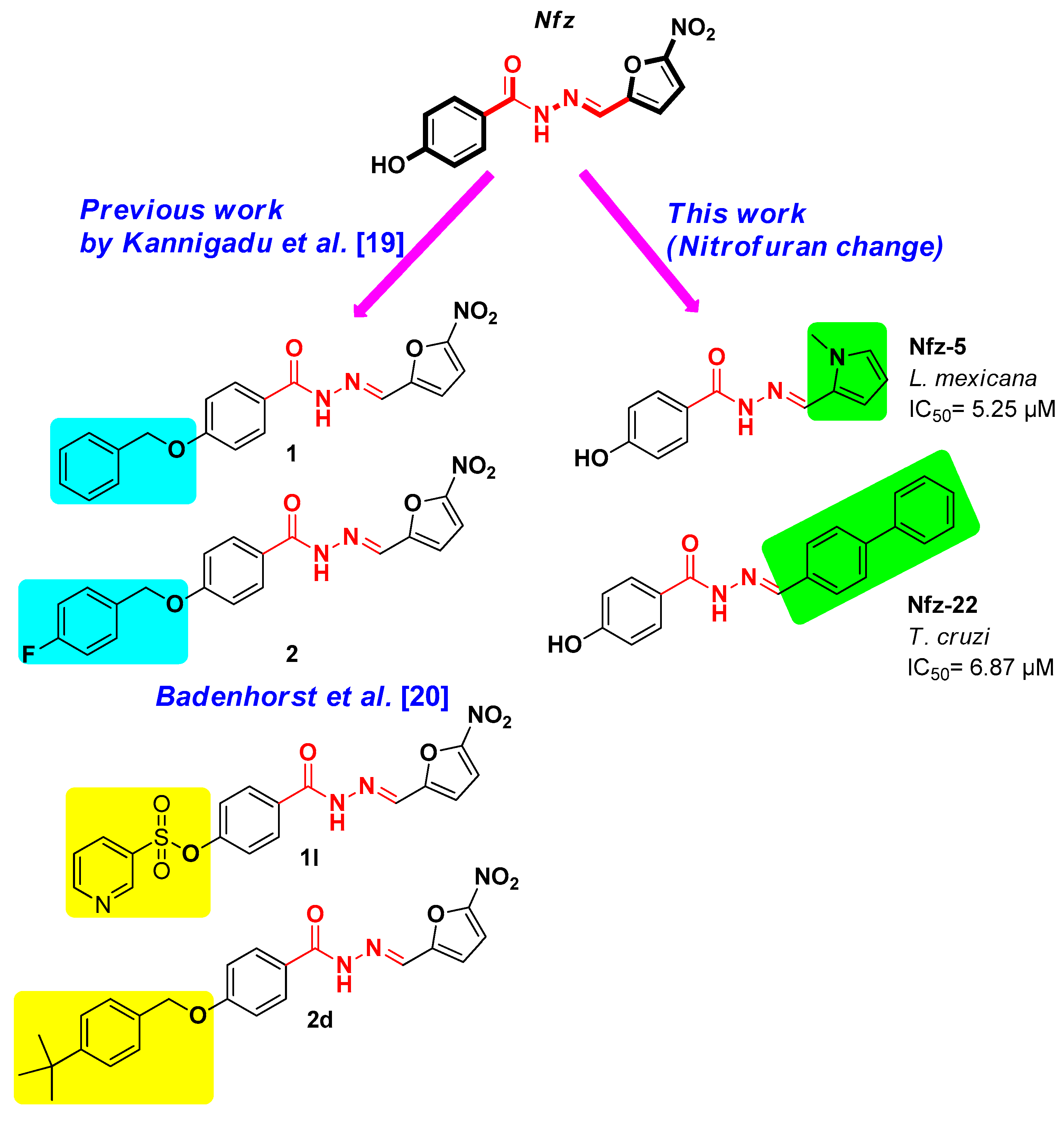
1. Introduction
2. Materials and Methods
2.1. Chemistry
2.2. Biological Evaluations
2.2.1. Trypanocidal Activity
2.2.2. Leishmanicidal Activity
2.2.3. Anti-Mycobacterium Activity
2.2.4. Cytotoxicity
2.2.5. T. cruzi Cysteine Proteases Inhibition Assay
3. Results
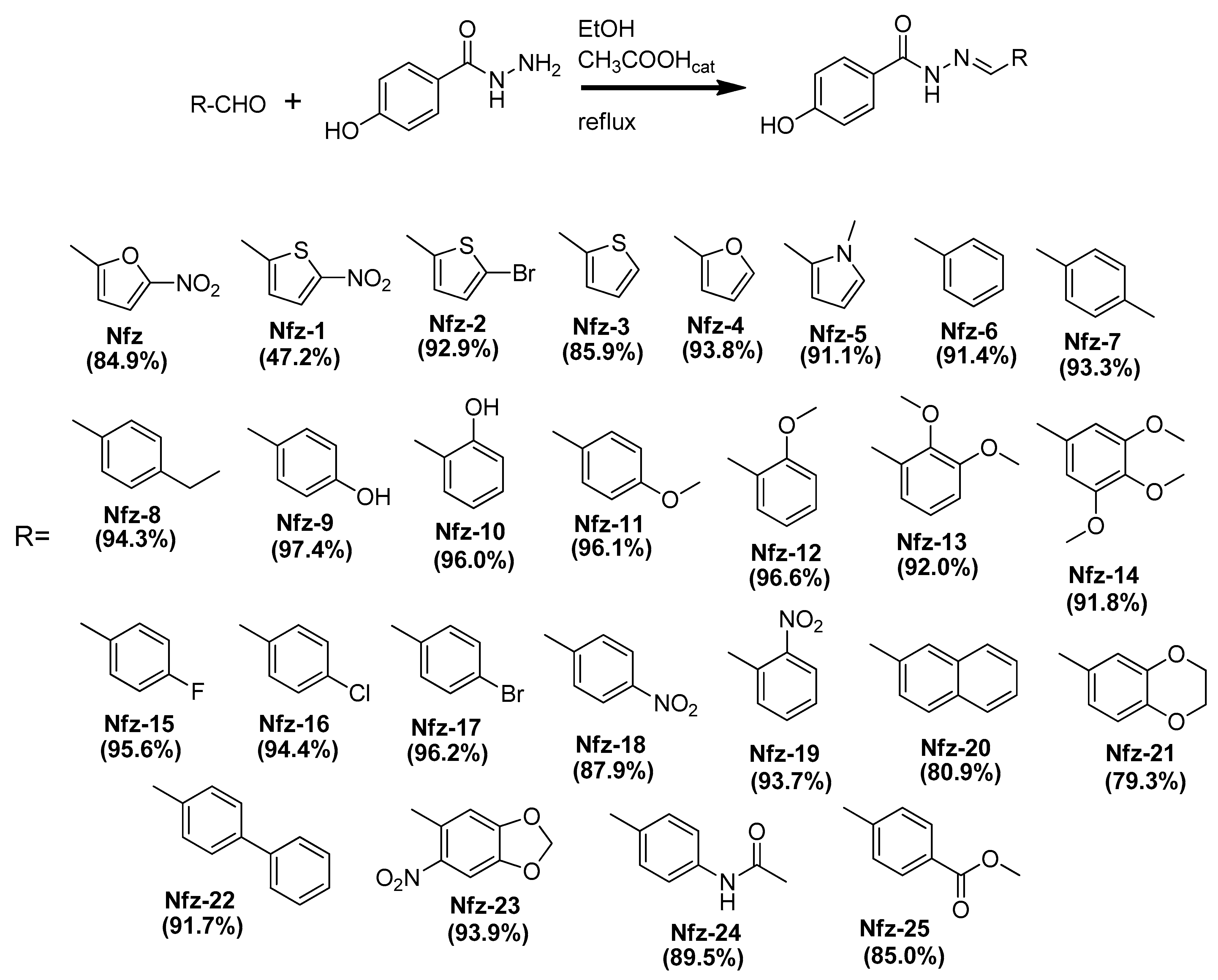
3.1. Synthesis
3.2. Trypanocidal Activity
3.3. Inhibition of T. cruzi Cysteine Protease Activity
3.4. Leishmanicidal Activity
3.5. Cytotoxicity on Macrophages
3.6. Anti-Mycobacterium Activity
4. Discussion
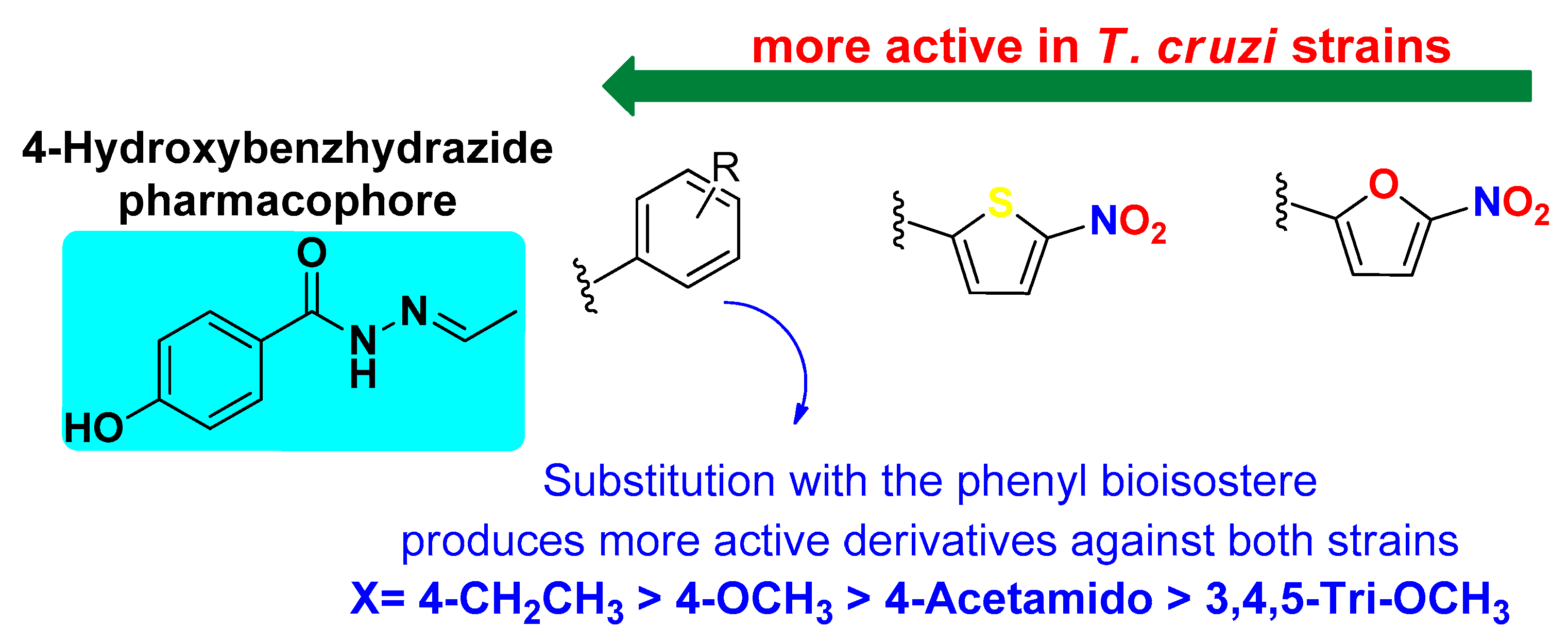
4.1. Structure–Activity Relationship (SAR) Against T. cruzi
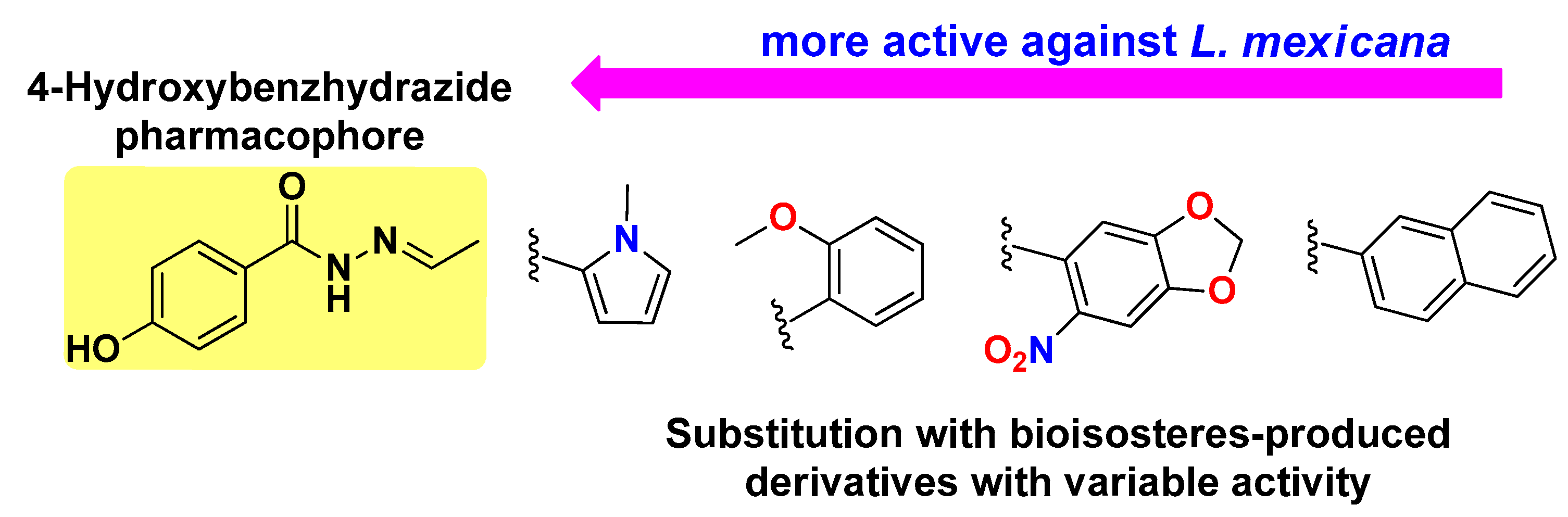
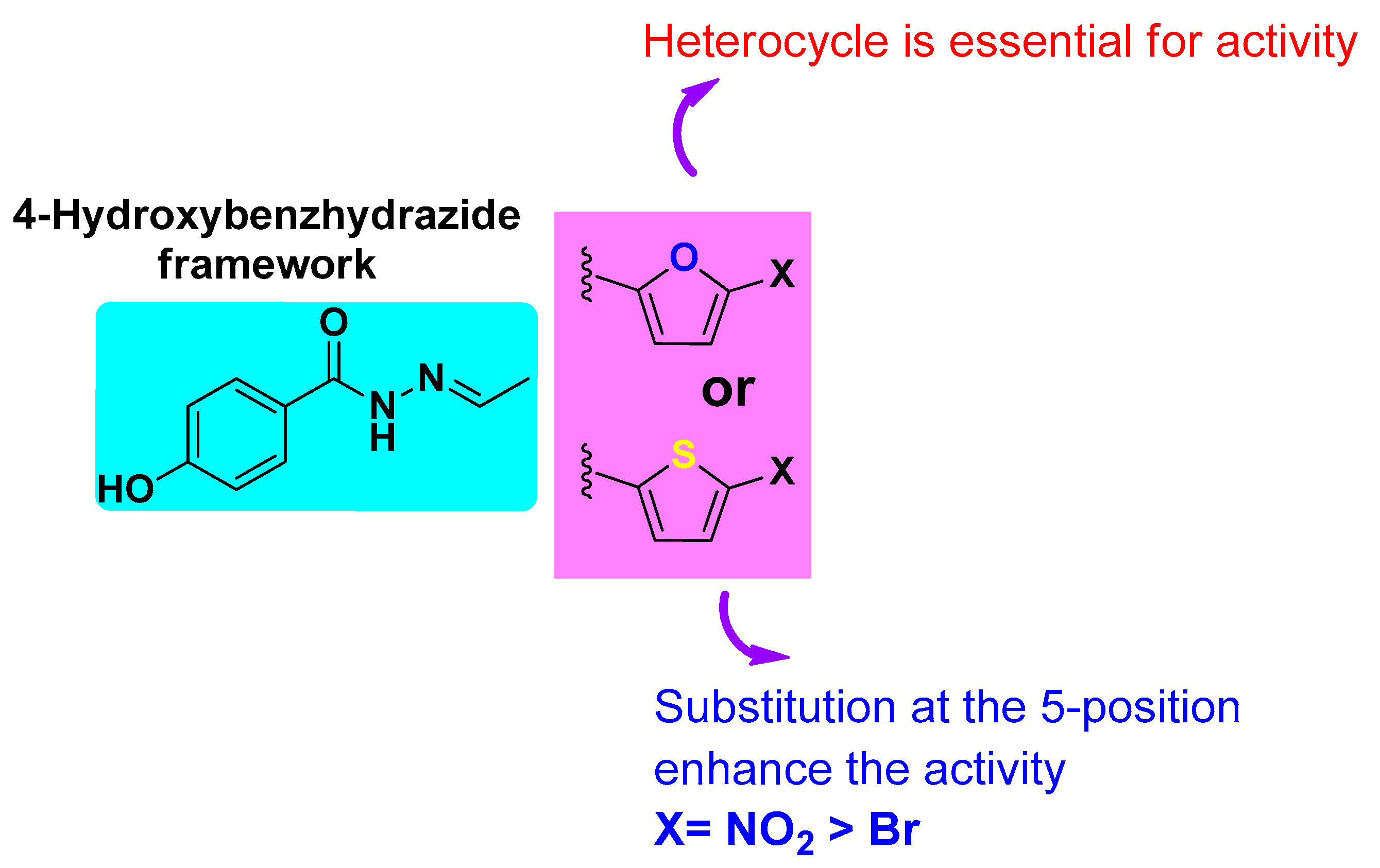
4.2. Structure–Activity Relationship (SAR) Against L. mexicana
4.3. Cytotoxicity and Selectivity Index (SI) on Macrophages
4.4. Inhibition of T. cruzi Cysteine Protease Activity
4.5. Anti-Mycobacterium Activity
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Brune, K. Chemistry and Pharmacology of Nitrofurans. J. Med. Chem. 2022, 65, 530–542. [Google Scholar]
- Gupta, K.; Hooton, T.M.; Naber, K.G. Antimicrobial Use for Urinary Tract Infections: The Role of Nitrofurantoin. Clin. Infect. Dis. 2017, 64, 952–960. [Google Scholar]
- Sok, D.; Hwang, Y.J.; Yoon, J. Safety and Efficacy of Nitrofurantoin in Clinical Practice. Therapeut. Clin. Risk Manag. 2020, 16, 509–517. [Google Scholar]
- Nicolas, M.; McEwan, G.; Brown, J. Advances in Nitrofurans and Their Therapeutic Potential. Expert Rev. Anti-Infect. Ther. 2021, 19, 481–493. [Google Scholar]
- Roquini, V.; Mengarda, A.C.; Cajas, R.A.; Martins-da-Silva, M.F.; Godoy-Silva, J.; Santos, G.A.; Espírito-Santo, M.C.C.; Pavani, T.F.; Melo, V.A.; Salvadori, M.C.; et al. The Existing Drug Nifuroxazide as an Antischistosomal Agent: In Vitro, In Vivo, and In Silico Studies of Macromolecular Targets. Microbiol. Spectrum 2023, 11, e01393-23. [Google Scholar] [CrossRef]
- Toledano-Magaña, Y.; García-Ramos, J.G.; Navarro-Olivarria, M.; Flores-Alamo, M.; Manzanera-Estrada, M.; Ortiz-Frade, L.; Galindo-Murillo, R.; Ruiz-Azuara, L.; Meléndrez-Luevano, R.; Cabrera-Vivas, B.M. Potential Amoebicidal Activity of Hydrazone Derivatives: Synthesis, Characterization, Electrochemical Behavior, Theoretical Study, and Evaluation of the Biological Activity. Molecules 2015, 20, 9929–9948. [Google Scholar] [CrossRef]
- Said, E.; Zaitone, S.A.; Eldosoky, M.; Elsherbiny, N.M. Nifuroxazide, a STAT3 Inhibitor, Mitigates Inflammatory Burden and Protects Against Diabetes-Induced Nephropathy in Rats. Chem.-Biol. Interact. 2018, 281, 111–120. [Google Scholar] [CrossRef]
- Luo, L.; Xu, F.; Peng, H.; Luo, Y.; Tian, X.; Battaglia, G.; Zhang, H.; Gong, Q.; Gu, Z.; Luo, K. Stimuli-Responsive Polymeric Prodrug-Based Nanomedicine Delivering Nifuroxazide and Doxorubicin Against Primary Breast Cancer and Pulmonary Metastasis. J. Control. Release 2020, 318, 124–135. [Google Scholar] [CrossRef]
- Popiołek, Ł. Updated Information on Antimicrobial Activity of Hydrazide–Hydrazones. Int. J. Mol. Sci. 2021, 22, 9389. [Google Scholar] [CrossRef]
- Kannigadu, C.; Aucamp, J.; N’Da, D.D. Exploring Novel Nitrofuranyl Sulfonohydrazides as Anti-Leishmania and Anti-Cancer Agents: Synthesis, In Vitro Efficacy and Hit Identification. Chem. Biol. Drug Des. 2022, 100, 267–279. [Google Scholar] [CrossRef]
- Atta, A.; Fahmy, S.; Rizk, O.; Sriram, D.; Mahran, M.A.; Labouta, I.M. Structure-Based Design of Some Isonicotinic Acid Hydrazide Analogues as Potential Antitubercular Agents. Bioorg. Chem. 2018, 80, 721–732. [Google Scholar] [CrossRef]
- Teneva, Y.; Simeonova, R.; Valcheva, V.; Angelova, V.T. Recent Advances in Anti-Tuberculosis Drug Discovery Based on Hydrazide-Hydrazone and Thiadiazole Derivatives Targeting InhA. Pharmaceuticals 2023, 16, 484. [Google Scholar] [CrossRef] [PubMed]
- Sidorov, N.G.; Kravchenko, A.D.; Poddubikov, A.V.; Arzumanian, V.G. Synthesis and Study of the Antimicrobial Activity of Nifuroxazide Derivatives. Microbiol. Indep. Res. J. 2019, 6, 10–17. [Google Scholar] [CrossRef]
- Masunari, A.; Tavares, L.C. A New Class of Nifuroxazide Analogues: Synthesis of 5-Nitrothiophene Derivatives with Antimicrobial Activity Against Multidrug-Resistant Staphylococcus aureus. Bioorg. Med. Chem. 2007, 15, 4229–4236. [Google Scholar] [CrossRef] [PubMed]
- Bailly, C. Toward a Repositioning of the Antibacterial Drug Nifuroxazide for Cancer Treatment. Drug Discov. Today 2019, 24, 1930–1936. [Google Scholar] [CrossRef]
- Gan, C.; Zhang, Q.; Liu, H.; Wang, G.; Wang, L.; Li, Y.; Tan, Z.; Yin, W.; Yao, Y.; Xie, Y.; et al. Nifuroxazide Ameliorates Pulmonary Fibrosis by Blocking Myofibroblast Genesis: A Drug Repurposing Study. Respir. Res. 2022, 23, 32. [Google Scholar] [CrossRef]
- Kaiser, M.; Mäser, P.; Tadoori, L.P.; Ioset, J.R.; Brun, R. Antiprotozoal Activity Profiling of Approved Drugs: A Starting Point Toward Drug Repositioning. PLoS ONE 2015, 10, e0135556. [Google Scholar] [CrossRef]
- Paula, F.R.; Jorge, S.D.; de Almeida, L.V.; Pasqualoto, K.F.; Tavares, L.C. Molecular Modeling Studies and In Vitro Bioactivity Evaluation of a Set of Novel 5-Nitro-Heterocyclic Derivatives as Anti-T. cruzi Agents. Bioorg. Med. Chem. 2009, 17, 2673–2679. [Google Scholar] [CrossRef]
- Kannigadu, C.; Aucamp, J.; N’Da, D.D. Synthesis and In Vitro Antileishmanial Efficacy of Benzyl Analogues of Nifuroxazide. Drug Dev. Res. 2021, 82, 287–295. [Google Scholar] [CrossRef]
- Badenhorst, G.D.; Kannigadu, C.; Aucamp, J.; David, D.D. Probing O-Substituted Nifuroxazide Analogues Against Leishmania: Synthesis, In Vitro Efficacy, and Hit/Lead Identification. Eur. J. Pharm. Sci. 2022, 176, 106242. [Google Scholar] [CrossRef]
- Nepali, K.; Lee, H.Y.; Liou, J.P. Nitro-Group-Containing Drugs. J. Med. Chem. 2018, 62, 2851–2893. [Google Scholar] [CrossRef] [PubMed]
- Franzblau, S.G.; Witzig, R.S.; McLaughlin, J.C.; Torres, P.; Madico, G.; Hernandez, A.; Degnan, M.T.; Cook, M.B.; Quenzer, V.K.; Ferguson, R.M.; et al. Rapid, Low-Technology MIC Determination with Clinical Mycobacterium tuberculosis Isolates by Using the Microplate Alamar Blue Assay. J. Clin. Microbiol. 1998, 36, 362–366. [Google Scholar] [CrossRef] [PubMed]
- Palos, I.; Luna-Herrera, J.; Lara-Ramírez, E.E.; Loera-Piedra, A.; Fernández-Ramírez, E.; Aguilera-Arreola, M.G.; Paz-González, A.D.; Monge, A.; Wan, B.; Franzblau, S.; et al. Anti-Mycobacterium tuberculosis Activity of Esters of Quinoxaline 1,4-Di-N-Oxide. Molecules 2018, 23, 1453. [Google Scholar] [CrossRef]
- Bradford, M.M. A Rapid and Sensitive Method for the Quantitation of Microgram Quantities of Protein Utilizing the Principle of Protein-Dye Binding. Anal. Biochem. 1976, 72, 248–254. [Google Scholar] [CrossRef]
- Santos, C.C.; Sant’anna, C.; Terres, A.; Cunha-e-Silva, N.L.; Scharfstein, J.; de A. Lima, A.P. Chagasin, the Endogenous Cysteine-Protease Inhibitor of Trypanosoma cruzi, Modulates Parasite Differentiation and Invasion of Mammalian Cells. J. Cell Sci. 2005, 118, 901–915. [Google Scholar] [CrossRef] [PubMed]
- Ritz, C.; Baty, F.; Streibig, J.C.; Gerhard, D. Dose-Response Analysis Using R. PLoS ONE 2016, 10, e0146021. [Google Scholar]
- Rando, D.G.; Avery, M.A.; Tekwani, B.L.; Khan, S.I.; Ferreira, E.I. Antileishmanial Activity Screening of 5-Nitro-2-Heterocyclic Benzylidene Hydrazides. Bioorg. Med. Chem. 2008, 16, 6724–6731. [Google Scholar] [CrossRef]
- Hollósy, F.; Seprödi, J.; Orfi, L.; Erös, D.; Kéri, G.; Idei, M. Evaluation of Lipophilicity and Antitumor Activity of Parallel Carboxamide Libraries. J. Chromatogr. B Analyt. Technol. Biomed. Life Sci. 2002, 780, 355–363. [Google Scholar] [CrossRef]
- Indrayanto, G.; Satrio Putra, G.; Suhud, F. Chapter Six—Validation of In-Vitro Bioassay Methods: Application in Herbal Drug Research. In Profiles of Drug Substances, Excipients and Related Methodology; Al-Majed, A.A., Ed.; Elsevier: Riyadh, Saudi Arabia, 2021; Volume 46, pp. 2–316. [Google Scholar]
- Carvalho, S.A.; Feitosa, L.O.; Soares, M.; Costa, T.E.; Henriques, M.G.; Salomão, K.; de Castro, S.L.; Kaiser, M.; Brun, R.; Wardell, J.L.; et al. Design and Synthesis of New (E)-Cinnamic N-Acylhydrazones as Potent Antitrypanosomal Agents. Eur. J. Med. Chem. 2012, 54, 512–521. [Google Scholar] [CrossRef]
 | |||||
|---|---|---|---|---|---|
| Compound | -R | T. cruzi | L. mexicana | ||
| NINOA (µM) | A1 (µM) | 1 M379 (µM) | 2 FCQEPS (µM) | ||
| Nfz | 5-Nitro-furyl | 36.00 ± 1.2 | 10.41 ± 1.6 | >200 | 51.17 ± 4.2 |
| Nfz-1 | 5-Nitro-thienyl | 11.94 ± 3.2 | 14.00 ± 1.9 | >200 | 32.91 ± 7.0 |
| Nfz-2 | 5-Bromo-thienyl | 4.77 ± 5.6 | >100 | >200 | 98.83 ± 5.2 |
| Nfz-3 | Thienyl | 51.00 ± 2.8 | 19.97 ± 1.9 | >200 | 49.79 ± 5.0 |
| Nfz-4 | Furyl | 18.99 ± 2.1 | 28.89 ± 3.4 | 83.90 ± 6.9 | >200 |
| Nfz-5 | N-Methylpyrrole | >100 | 78.52 ± 4.7 | 5.25 ± 5.8 | >200 |
| Nfz-6 | Phenyl | >100 | 74.39 ± 4.4 | 94.22 ± 0.9 | >200 |
| Nfz-7 | 4-Methylphenyl | 26.02 ± 2.9 | 75.17 ± 8.8 | >200 | 102.77 ± 2.1 |
| Nfz-8 | 4-Ethylphenyl | 14.45 ± 4.4 | 7.28 ± 2.2 | >200 | >200 |
| Nfz-9 | 4-Hidroxyl | 24.88 ± 3.2 | >100 | >200 | 94.28 ± 5.9 |
| Nfz-10 | 2-Hidroxyl | >100 | 8.77 ± 1.6 | 66.71 ± 2.9 | >200 |
| Nfz-11 | 4-Methoxyphenyl | 28.23 ± 1.4 | 18.15 ± 1.4 | >200 | >200 |
| Nfz-12 | 2-Methoxyphenyl | >100 | >100 | 11.56 ± 2.1 | 60.33 ± 4.7 |
| Nfz-13 | 2,3-diMethoxyphenyl | 63.09 ± 1.4 | >100 | 161.69 ± 6.9 | 124.52 ± 1.1 |
| Nfz-14 | 3,4,5-TriMethoxyphenyl | 28.02 ± 1.9 | 29.23 ± 4.4 | >200 | 10.28 ± 0.8 |
| Nfz-15 | 4-Fluorophenyl | >100 | 32.63 ± 2.8 | 35.09 ± 4.7 | 101.72 ± 2.7 |
| Nfz-16 | 4-Chlorophenyl | >100 | 40.22 ± 2.7 | 67.54 ±4.0 | 78.09 ± 3.6 |
| Nfz-17 | 4-Bromophenyl | >100 | 33.59 ± 3.9 | 62.03 ± 2.5 | 52.12 ± 4.8 |
| Nfz-18 | 4-Nitrophenyl | 44.58 ± 6.3 | >100 | >200 | 57.77 ± 2.6 |
| Nfz-19 | 2-Nitrophenyl | 36.45 ± 2.2 | 42.46 ± 6.7 | 146.72 ± 0.7 | >200 |
| Nfz-20 | 2-Naphtyl | >100 | 24.48 ± 2.0 | 28.45 ± 2.8 | >200 |
| Nfz-21 | 2-benzodioxanyl | >100 | 49.04 ± 2.1 | 71.51 ± 4.9 | >200 |
| Nfz-22 | 4-Biphenyl | 6.87 ± 1.2 | >100 | >200 | 133.16 ± 1.4 |
| Nfz-23 | 6-Nitropiperonyl | >100 | 13.52 ± 3.4 | 15.96 ± 2.0 | >200 |
| Nfz-24 | 4-Acetamidophenyl | 14.18 ± 3.5 | 22.19 ± 1.4 | 54.16 ± 4.0 | 55.76 ± 3.5 |
| Nfz-25 | Methyl 4-formylbenzoate | >100 | >100 | 32.53 ± 6.1 | >200 |
| Benznidazole | 30.3 ± 0.3 | 39.08 ± 0.7 | - | - | |
| Nifurtimox | 7.09 ± 0.2 | 19.30 ± 0.8 | |||
| Glucantime | - | - | 133.96 ± 3.1 | 125.23 ± 1.6 | |
| Compound | Structure | IC50 in µM |
|---|---|---|
| Nfz | 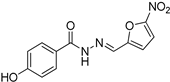 | 89.49 |
| Nfz-1 | 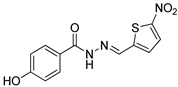 | 17.64 |
| Nfz-4 | 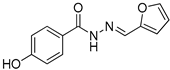 | 69.95 |
| Nfz-8 | 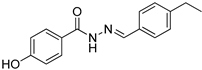 | 4.60 |
| Nfz-11 |  | 3805.1 |
| Compound | −R | Macrophages J774.2 (µM) | T. cruzi SI | L. mexicana SI | ||
|---|---|---|---|---|---|---|
| NINOA | A1 | M379 | FCQEPS | |||
| Nfz | 5-NO2-Furyl | 42.23 ± 2.3 | 1.17 | 4.05 | 0.21 | 0.82 |
| Nfz-1 | 5-NO2-Thienyl | 43.27 ± 2.7 | 3.62 | 3.09 | <0.21 | 1.21 |
| Nfz-2 | 5-Br-Thienyl | 95.48 ± 4.2 | 20.01 | <0.95 | <0.47 | 0.96 |
| Nfz-3 | Thienyl | 213.75 ± 2.9 | 4.19 | 10.70 | <1.06 | 4.29 |
| Nfz-4 | Furyl | >100 | >5.26 | >3.46 | <1.19 | <0.50 |
| Nfz-5 | N-Methylpyrrole | 21.89 ± 4.4 | <0.21 | 0.27 | 4.16 | >0.10 |
| Nfz-6 | C6H5 | 28.77 ± 1.5 | <0.28 | 0.38 | 0.30 | >0.14 |
| Nfz-7 | 4-Methylphenyl | 91.68 ± 5.1 | 3.52 | 1.21 | <0.45 | 0.89 |
| Nfz-8 | 4-Ethylphenyl | >100 | >6.92 | >13.73 | <0.50 | <0.50 |
| Nfz-9 | 4-Hidroxyl | 82.71 ± 4.4 | 3.32 | <0.82 | <0.41 | 0.80 |
| Nfz-10 | 2-Hidroxyl | 137.17 ± 2.6 | <1.37 | 15.64 | 2.05 | >0.68 |
| Nfz-11 | 4-Methoxyphenyl | 46.59 ± 4.9 | 1.65 | 2.56 | <0.23 | <0.23 |
| Nfz-12 | 2-Methoxyphenyl | 76.45 ± 3.6 | >0.76 | >0.76 | 6.61 | 1.26 |
| Nfz-13 | 2,3-diMethoxyphenyl | 120.29 ± 0.2 | 1.91 | <1.20 | 0.74 | 0.96 |
| Nfz-14 | 3,4,5-TriMethoxyphenyl | 126.75 ± 6.5 | 4.52 | 4.33 | <0.63 | 12.32 |
| Nfz-15 | 4-Fluorine | 74.00 ± 3.4 | <0.74 | 2.26 | 2.10 | 0.72 |
| Nfz-16 | 4-Chlorine | 83.24 ± 3.2 | <0.83 | 2.06 | 1.23 | 1.06 |
| Nfz-17 | 4-Bromine | >100 | 1.00 | >2.97 | <1.61 | <1.91 |
| Nfz-18 | 4-Nitro | 2.45 ± 2.9 | 0.05 | <0.02 | <0.01 | 0.04 |
| Nfz-19 | 2-Nitro | >100 | >2.74 | >2.35 | <0.68 | <0.50 |
| Nfz-20 | 2-Naphtyl | 88.30 ± 3.9 | <0.88 | 3.60 | 3.10 | >0.44 |
| Nfz-21 | 2-benzodioxanyl | >100 | 1.00 | >2.03 | <1.39 | <0.50 |
| Nfz-22 | 4-Biphenyl | 43.49 ± 2.5 | 6.33 | <0.43 | <0.21 | 0.32 |
| Nfz-23 | 6-Nitropiperonyl | 68.87 ± 6.6 | <0.68 | 5.09 | 4.31 | >0.34 |
| Nfz-24 | 4-Acetamidophenyl | 173.37 ± 5.2 | 12.22 | 7.81 | 3.20 | 3.10 |
| Nfz-25 | Methyl 4-formylbenzoate | >100 | 1.00 | 1.00 | <3.07 | <0.50 |
| Benznidazole | 133.90 ± 0.2 | 4.41 | 3.42 | - | - | |
| Glucantime | >200 | - | - | >2.03 | >2.18 | |
| Compound | MABA MIC (µg/mL) |
|---|---|
| Nfz | 12.3 |
| Nfz-1 | 5.1 |
| Nfz-2 | 18.8 |
| Nfz-3 | >100 |
| Nfz-4 | >100 |
| Nfz-5 | >100 |
| Nfz-6 | >100 |
| Nfz-7 | 98.3 |
| Nfz-8 | 79.4 |
| Nfz-9 | 94.1 |
| Nfz-10 | 47.6 |
| Nfz-11 | >100 |
| Nfz-12 | >100 |
| Nfz-13 | 157.8 |
| Nfz-14 | >200 |
| Nfz-15 | >100 |
| Nfz-16 | 46.4 |
| Nfz-17 | 43.6 |
| Nfz-18 | 186.4 |
| Nfz-19 | >100 |
| Nfz-20 | >100 |
| Nfz-21 | 68.7 |
| Nfz-22 | >100 |
| Nfz-23 | >100 |
| Nfz-24 | >100 |
| Nfz-25 | 45.9 |
| Isoniazid | 0.039 |
| Rifampin | 0.074 |
| Moxifloxacin | 0.184 |
| Linezolid | 0.330 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Delgado-Maldonado, T.; Navarrete-Carriola, D.V.; Vázquez-Jiménez, L.K.; Paz-González, A.D.; Wan, B.; Franzblau, S.; Mohammed, O.M.; Rodríguez-Páez, L.; Aguirre-Alvarado, C.; Alcántara-Farfán, V.; et al. Nifuroxazide and 4-Hydroxybenzhydrazone Derivatives as New Antiparasitic (Trypanosoma cruzi and Leishmania mexicana) and Anti-Mycobacterium tuberculosis Agents. Pharmaceutics 2025, 17, 621. https://doi.org/10.3390/pharmaceutics17050621
Delgado-Maldonado T, Navarrete-Carriola DV, Vázquez-Jiménez LK, Paz-González AD, Wan B, Franzblau S, Mohammed OM, Rodríguez-Páez L, Aguirre-Alvarado C, Alcántara-Farfán V, et al. Nifuroxazide and 4-Hydroxybenzhydrazone Derivatives as New Antiparasitic (Trypanosoma cruzi and Leishmania mexicana) and Anti-Mycobacterium tuberculosis Agents. Pharmaceutics. 2025; 17(5):621. https://doi.org/10.3390/pharmaceutics17050621
Chicago/Turabian StyleDelgado-Maldonado, Timoteo, Diana V. Navarrete-Carriola, Lenci K. Vázquez-Jiménez, Alma D. Paz-González, Baojie Wan, Scott Franzblau, Othman Mueen Mohammed, Lorena Rodríguez-Páez, Charmina Aguirre-Alvarado, Verónica Alcántara-Farfán, and et al. 2025. "Nifuroxazide and 4-Hydroxybenzhydrazone Derivatives as New Antiparasitic (Trypanosoma cruzi and Leishmania mexicana) and Anti-Mycobacterium tuberculosis Agents" Pharmaceutics 17, no. 5: 621. https://doi.org/10.3390/pharmaceutics17050621
APA StyleDelgado-Maldonado, T., Navarrete-Carriola, D. V., Vázquez-Jiménez, L. K., Paz-González, A. D., Wan, B., Franzblau, S., Mohammed, O. M., Rodríguez-Páez, L., Aguirre-Alvarado, C., Alcántara-Farfán, V., Cordero-Martínez, J., Bandyopadhyay, D., Moreno-Rodríguez, A., & Rivera, G. (2025). Nifuroxazide and 4-Hydroxybenzhydrazone Derivatives as New Antiparasitic (Trypanosoma cruzi and Leishmania mexicana) and Anti-Mycobacterium tuberculosis Agents. Pharmaceutics, 17(5), 621. https://doi.org/10.3390/pharmaceutics17050621








