Surfactants Significantly Improved the Oral Bioavailability of Curcumin Amorphous Solid Dispersions and Its Underlying Mechanism
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Methods
2.2.1. Preparation of Cur ASDs
- (1)
- Low drug-loading ASDs, including binary surfactant-free Cur/P188 ASD (1:8, w/w; 11 wt.% drug) and ternary surfactant-containing Cur/P188/TW80 and Cur/P188/SLS ASDs (1:8:1, w/w/w; 10 wt.% drug), were designed with higher polymer content to enhance dissolution performance and prolong supersaturation. These formulations were used for in vitro dissolution (Section 2.2.4), cellular uptake (Section 2.2.5), and in vivo pharmacokinetic studies (Section 2.2.6).
- (2)
- High drug-loading ASDs, including Cur/P188 ASD (1:2, w/w; 33 wt.% drug) and Cur/P188/TW80 and Cur/P188/SLS ASDs (1:2:1, w/w/w; 25 wt.% drug), contained less polymer to maintain the amorphous state of the drug while minimizing spectral interference from the polymer. These formulations were specifically used for FT-IR studies (Fourier Transform Infrared Spectroscopy (FT-IR) section).
2.2.2. Particle Characterization Methods
Powder X-Ray Diffraction (PXRD)
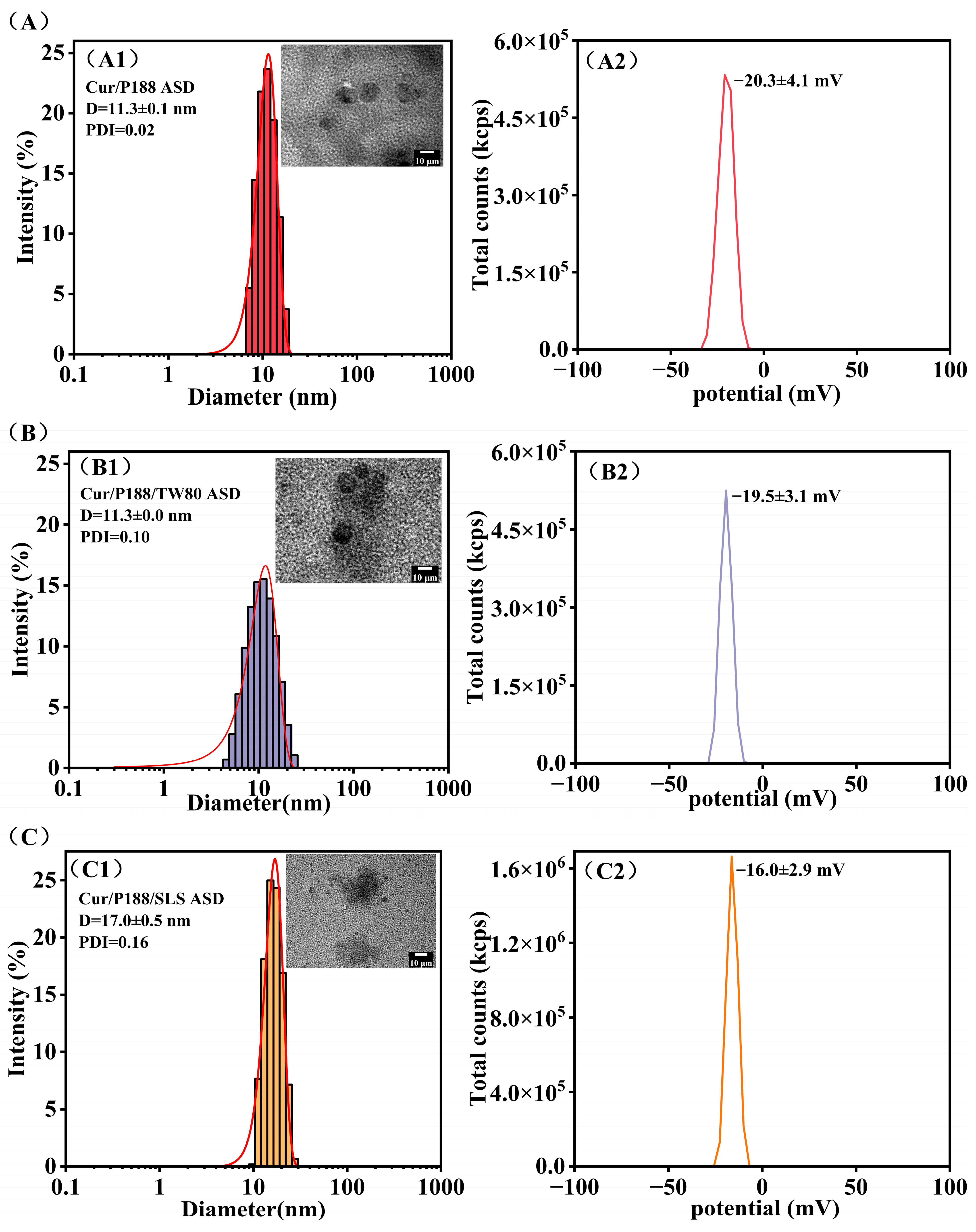
Dynamic Light Scattering (DLS)
Transmission Electron Microscopy (TEM)
2.2.3. Characterization of Intermolecular Interactions
Nuclear Magnetic Resonance (NMR)
Fourier Transform Infrared Spectroscopy (FT-IR)
2.2.4. In Vitro Dissolution Studies
Solubility Measurement
Non-Sink Dissolution
2.2.5. Quantification of Cellular Uptake of Cur ASDs
Cytotoxicity Assay
- As is the absorbance of experimental wells (cells + treatment + CCK-8);
- Ac is the absorbance of control wells (cells + medium + CCK-8, no drug);
- Aᵦ is the absorbance of blank wells (medium + CCK-8, no cells, no drug).
Cellular Uptake Study
2.2.6. In Vivo Pharmacokinetics Study of Cur ASDs
Animals
Pharmacokinetic Study
2.2.7. Quantitative Determination Methods
HPLC/UV-Vis Method
LC-MS/MS Method
2.2.8. Data Analysis
3. Results
3.1. Drug–Polymer–Surfactant Interactions
3.1.1. Monitoring Intermolecular Interactions by NMR Spectroscopy
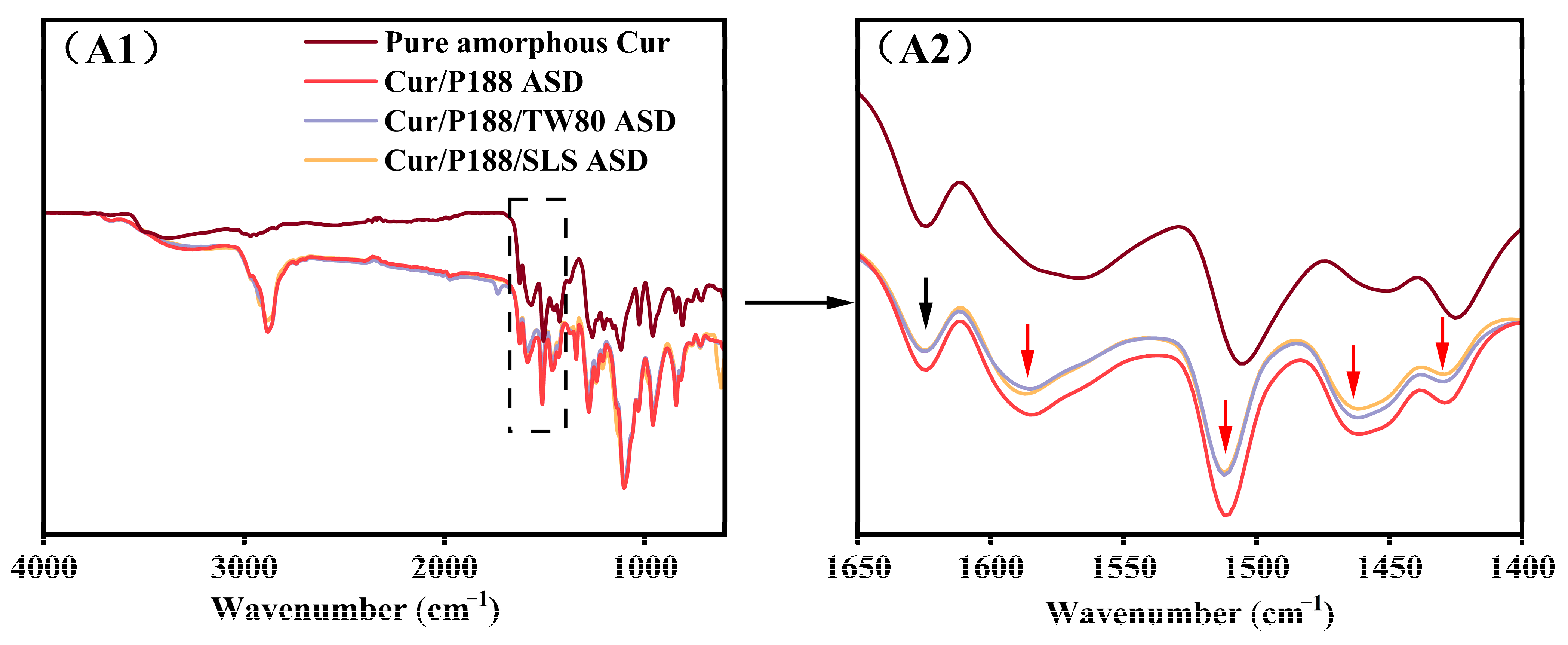
3.1.2. Monitoring Intermolecular Interactions by FT-IR Spectroscopy
3.2. In Vitro Dissolution of Cur Formulations
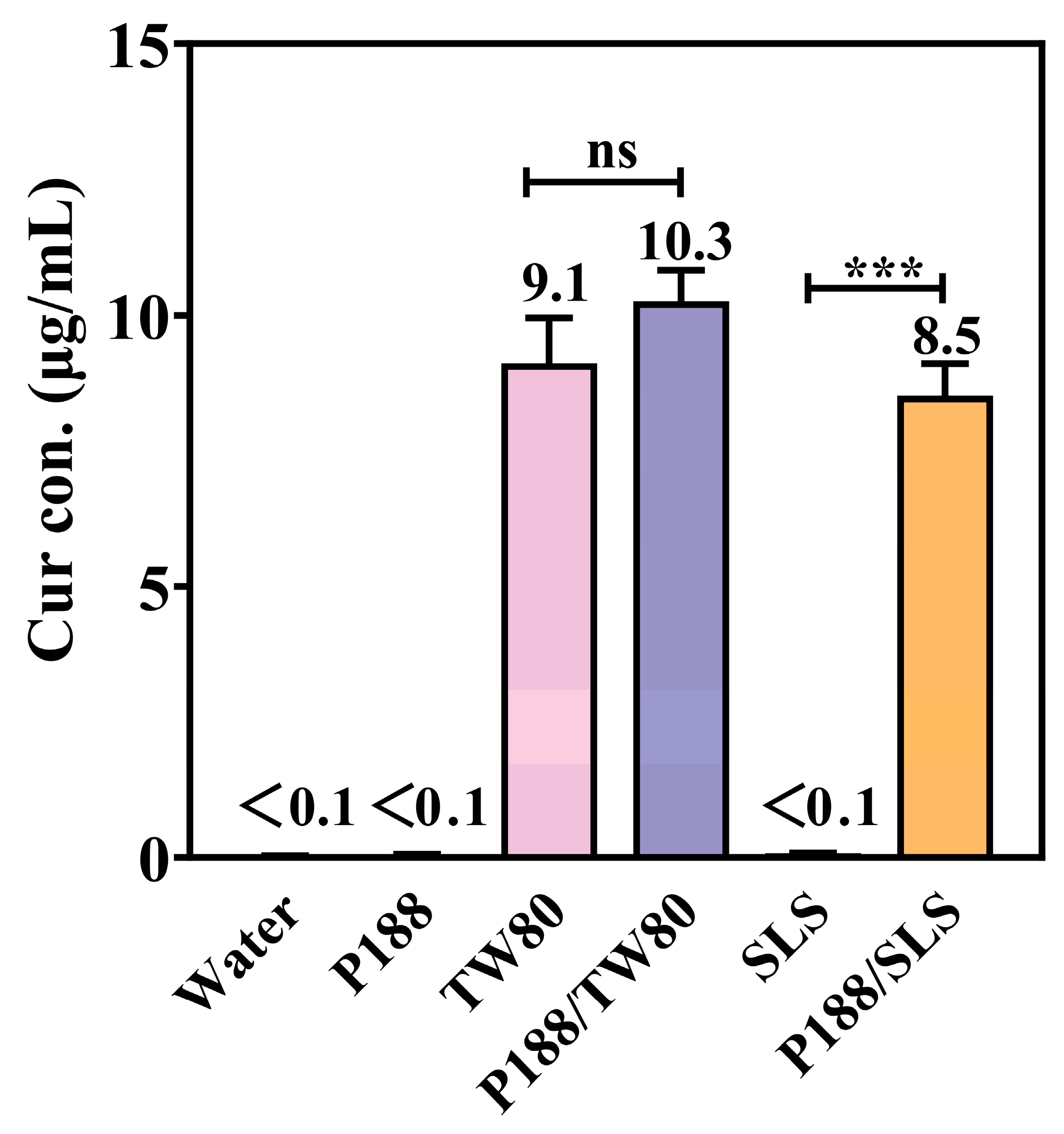
3.2.1. Solubility of Crystalline Cur in Excipient Solutions
3.2.2. Dissolution Performance
3.3. Cellular Uptake of Cur Formulations
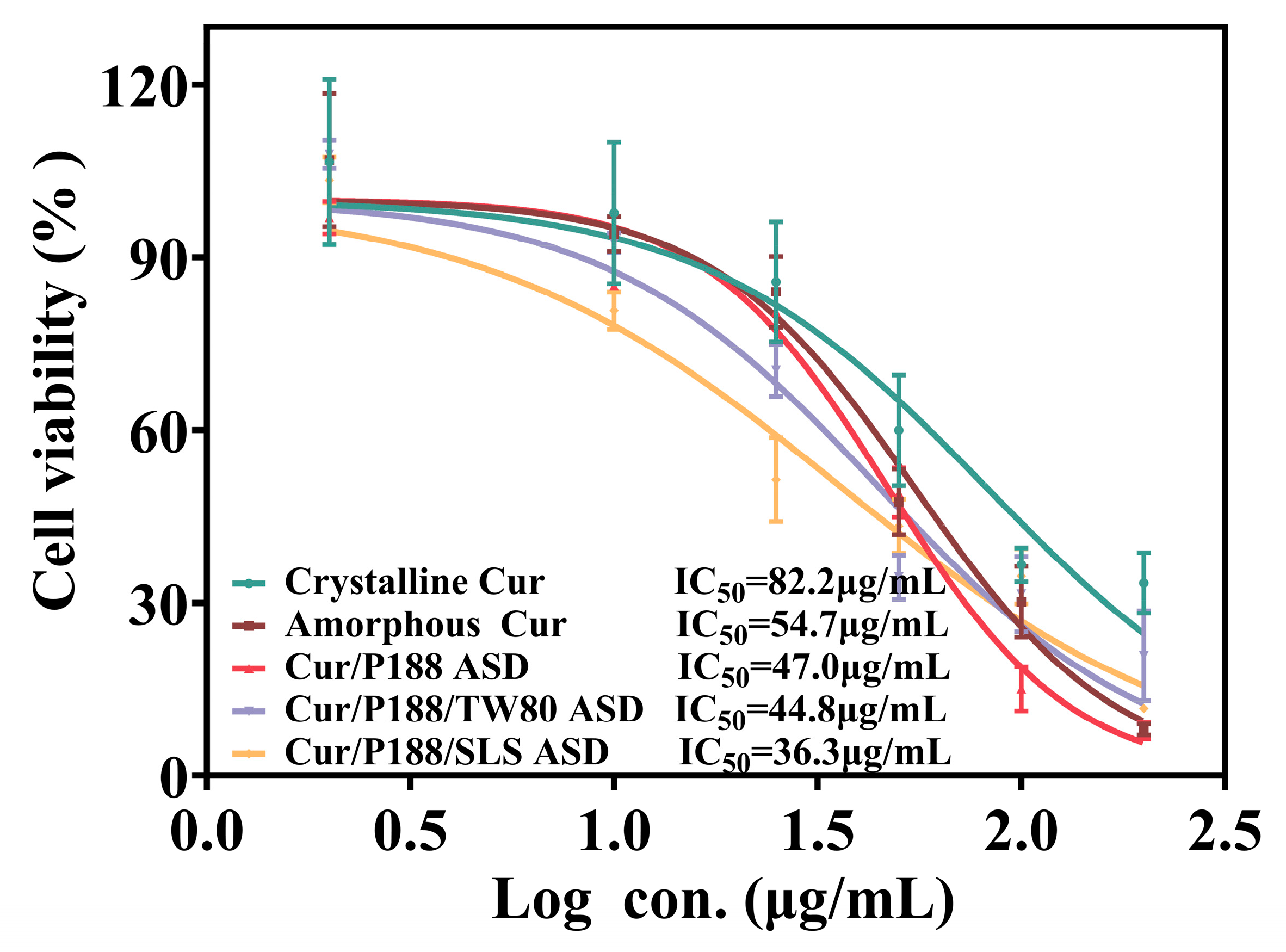
3.3.1. Cytotoxicity in MDCK Cells
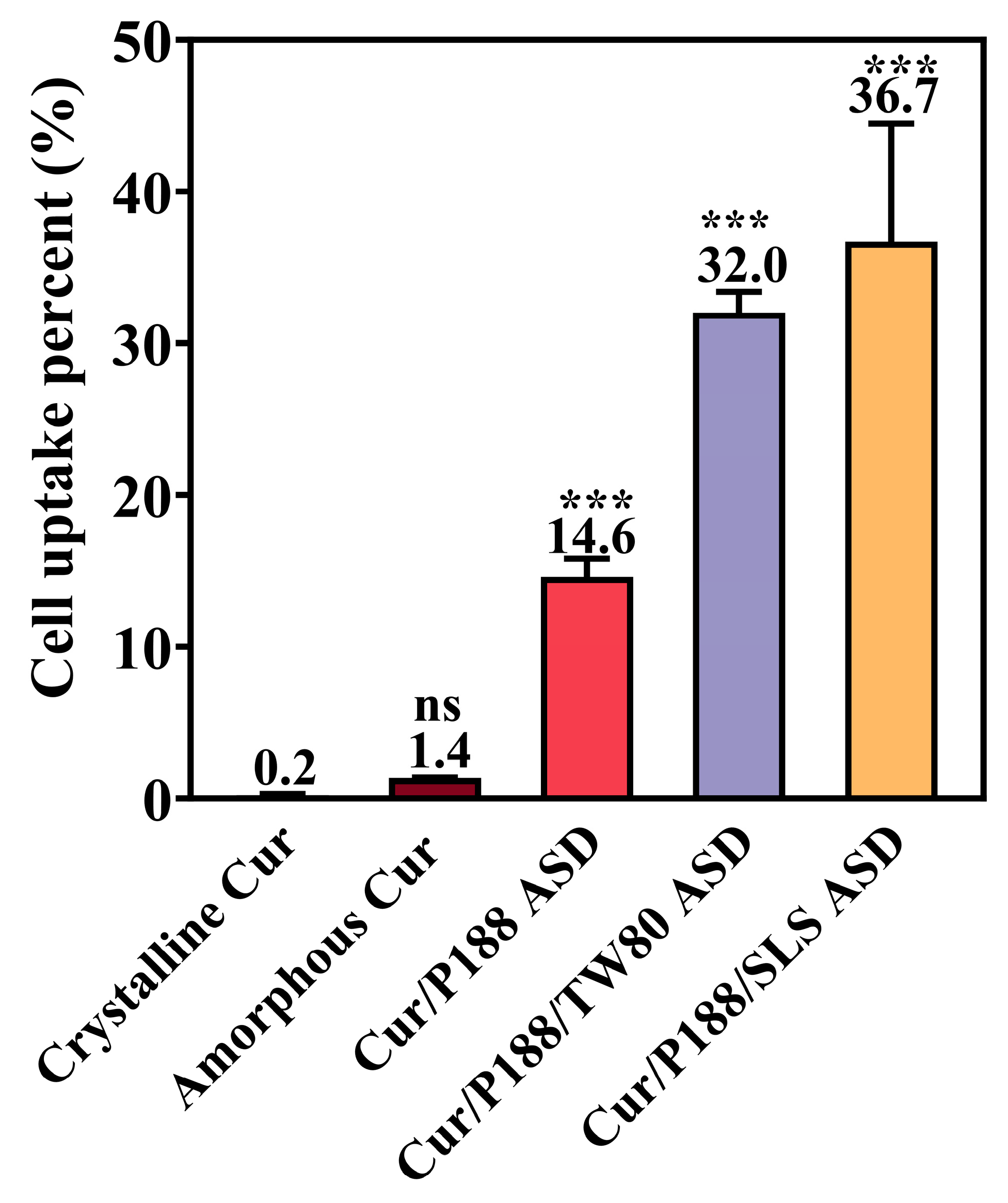
3.3.2. Cellular Uptake of Cur by MDCK Cells
3.4. Pharmacokinetics of Cur Formulations
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Maurya, R.; Vikal, A.; Patel, P.; Narang, R.K.; Kurmi, B.D. Enhancing Oral Drug Absorption: Overcoming Physiological and Pharmaceutical Barriers for Improved Bioavailability. AAPS PharmSciTech 2024, 25, 228. [Google Scholar] [CrossRef]
- Stielow, M.; Witczyńska, A.; Kubryń, N.; Fijałkowski, Ł.; Nowaczyk, J.; Nowaczyk, A. The Bioavailability of Drugs—The Current State of Knowledge. Molecules 2023, 28, 8038. [Google Scholar] [CrossRef]
- Xie, B.; Liu, Y.; Li, X.; Yang, P.; He, W. Solubilization techniques used for poorly water-soluble drugs. Acta Pharm. Sin. B 2024, 14, 4683–4716. [Google Scholar] [CrossRef]
- Schittny, A.; Huwyler, J.; Puchkov, M. Mechanisms of increased bioavailability through amorphous solid dispersions: A review. Drug Deliv. 2020, 27, 110–127. [Google Scholar] [CrossRef]
- Kawakami, K.; Ishitsuka, T.; Fukiage, M.; Nishida, Y.; Shirai, T.; Hirai, Y.; Hideshima, T.; Tanabe, F.; Shinoda, K.; Tamate, R.; et al. Long-term physical stability of amorphous solid dispersions: Comparison of detection powers of common evaluation methods for spray-dried and hot-melt extruded formulations. J. Pharm. Sci. 2025, 114, 145–156. [Google Scholar] [CrossRef]
- Li, J.; Wang, Y.; Yu, D. Effects of Additives on the Physical Stability and Dissolution of Polymeric Amorphous Solid Dispersions: A Review. AAPS PharmSciTech 2023, 24, 175. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Zhang, L.; Liu, M.; Zeng, Z. Impact of Surfactants as Formulation Additives and Media Components on the Performance of Amorphous Solid Dispersions. Cryst. Growth Des. 2025, 25, 5561–5583. [Google Scholar] [CrossRef]
- Yang, R.; Zhang, G.G.Z.; Kjoller, K.; Dillon, E.; Purohit, H.S.; Taylor, L.S. Phase separation in surfactant-containing amorphous solid dispersions: Orthogonal analytical methods to probe the effects of surfactants on morphology and phase composition. Int. J. Pharm 2022, 619, 121708. [Google Scholar] [CrossRef] [PubMed]
- Gao, D.; Zhu, D.; Zhou, X.; Dong, S.; Chen, Y. Inhomogeneous Phase Significantly Reduces Oral Bioavailability of Felodipine/PVPVA Amorphous Solid Dispersion. Mol. Pharm. 2023, 20, 409–418. [Google Scholar] [CrossRef]
- Correa-Soto, C.E.; Gao, Y.; Indulkar, A.S.; Zhang, G.G.Z.; Taylor, L.S. Role of surfactants in improving release from higher drug loading amorphous solid dispersions. Int. J. Pharm. 2022, 625, 122120. [Google Scholar] [CrossRef]
- Kawakami, K. Roles of Supersaturation and Liquid-Liquid Phase Separation for Enhanced Oral Absorption of Poorly Soluble Drugs from Amorphous Solid Dispersions. Pharmaceutics 2025, 17, 262. [Google Scholar] [CrossRef]
- Alhalaweh, A.; Sayed, M.E.; Kovac, L.; Bergström, C.A.S. Impact of surfactants on solution behavior and membrane transport of amorphous solid dispersions. J. Pharm. Sci. 2025, 114, 458–467. [Google Scholar] [CrossRef]
- Gumaste, S.G.; Gupta, S.S.; Serajuddin, A.T.M. Investigation of Polymer-Surfactant and Polymer-Drug-Surfactant Miscibility for Solid Dispersion. AAPS J. 2016, 18, 1131–1143. [Google Scholar] [CrossRef] [PubMed]
- Chen, Z.; Liu, Z.; Qian, F. Crystallization of bifonazole and acetaminophen within the matrix of semicrystalline, PEO-PPO-PEO triblock copolymers. Mol. Pharm. 2015, 12, 590–599. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Wang, S.; Wang, S.; Liu, C.; Su, C.; Hageman, M.; Hussain, M.; Haskell, R.; Stefanski, K.; Qian, F. Sodium Lauryl Sulfate Competitively Interacts with HPMC-AS and Consequently Reduces Oral Bioavailability of Posaconazole/HPMC-AS Amorphous Solid Dispersion. Mol. Pharm. 2016, 13, 2787–2795. [Google Scholar] [CrossRef] [PubMed]
- Tung, N.T.; Tran, C.S.; Nguyen, T.L.; Pham, T.M.; Chi, S.C.; Nguyen, H.A.; Bui, Q.D.; Bui, D.N.; Tran, T.Q. Effect of surfactant on the in vitro dissolution and the oral bioavailability of a weakly basic drug from an amorphous solid dispersion. Eur. J. Pharm. Sci. 2021, 162, 105836. [Google Scholar] [CrossRef]
- Kaminaga, Y.; Nagatsu, A.; Akiyama, T.; Sugimoto, N.; Yamazaki, T.; Maitani, T.; Mizukami, H. Production of unnatural glucosides of curcumin with drastically enhanced water solubility by cell suspension cultures of Catharanthus roseus. FEBS Lett. 2003, 11, 311–316. [Google Scholar] [CrossRef]
- Priyadarsini, K.I. Photophysics, photochemistry and photobiology of curcumin: Studies from organic solutions, bio-mimetics and living cells. J. Photochem. Photobiol. C Photochem. 2009, 10, 81–95. [Google Scholar] [CrossRef]
- Nandiyanto, A.B.D.; Wiryani, A.S.; Rusli, A.; Purnamasari, A.; Abdullah, A.G.; Ana Widiaty, I.; Hurriyati, R. Extraction of Curcumin Pigment from Indonesian Local Turmeric with Its Infrared Spectra and Thermal Decomposition Properties. IOP Conf. Ser. Mater. Sci. Eng. 2017, 180, 012136. [Google Scholar] [CrossRef]
- Gupta, S.C.; Prasad, S.; Kim, J.H.; Patchva, S.; Webb, L.J.; Priyadarsini, I.K.; Aggarwal, B.B. Multitargeting by curcumin as revealed by molecular interaction studies. Nat. Prod. Rep. 2011, 28, 1937–1955. [Google Scholar] [CrossRef]
- Zong, R.; Ruan, H.; Zhu, W.; Zhang, P.; Feng, Z.; Liu, C.; Fan, S.; Liang, H.; Li, J. Curcumin nanocrystals with tunable surface zeta potential: Preparation, characterization and antibacterial study. J. Drug Deliv. Sci. Technol. 2022, 76, 103771. [Google Scholar] [CrossRef]
- Jungblut, S.; Dellago, C. Pathways to self-organization: Crystallization via nucleation and growth. Eur. Phys. J. E 2016, 39, 1–38. [Google Scholar] [CrossRef]
- Indulkar, A.S.; Lou, X.; Zhang, G.G.Z.; Taylor, L.S. Role of Surfactants on Release Performance of Amorphous Solid Dispersions of Ritonavir and Copovidone. Pharm. Res. 2022, 39, 381–397. [Google Scholar] [CrossRef]
- Zhang, Y.; Li, B.; Liu, J.; Han, D.; Rohani, S.; Gao, Z.; Gong, J. Inhibition of crystal nucleation and growth: A review. Cryst. Growth Des. 2024, 24, 2645–2665. [Google Scholar] [CrossRef]
- Lee, J.Y.; Lee, S.; Choi, J.H.; Na, K. ι-Carrageenan nanocomposites for enhanced stability and oral bioavailability of curcumin. Biomater. Res. 2021, 25, 32. [Google Scholar] [CrossRef] [PubMed]
- Peng, Y.; Li, X.; Gu, P.; Cheng, W.; Zhang, R.; Hu, K. Curcumin-loaded zein/pectin nanoparticles: Caco-2 cellular uptake and the effects on cell cycle arrest and apoptosis of human hepatoma cells (HepG2). J. Drug Deliv. Sci. Technol. 2022, 74, 103497. [Google Scholar] [CrossRef]
- Wang, H.; Zheng, C.; Tian, F.; Xiao, Z.; Sun, Z.; Lu, L.; Dai, W.; Zhang, Q.; Mei, X. Improving the Dissolution Rate and Bioavailability of Curcumin via Co-Crystallization. Pharmaceuticals 2024, 17, 489. [Google Scholar] [CrossRef]
- Ishtiaq, M.; Asghar, S.; Khan, I.U.; Iqbal, M.S.; Khalid, S.H. Development of the amorphous solid dispersion of curcumin: A rational selection of polymers for enhanced solubility and dissolution. Crystals 2022, 12, 1606. [Google Scholar] [CrossRef]
- Wang, S.; Xie, Y.; Su, H.; Luo, Y.; Wang, M.; Li, T.; Fu, Y. Delivery of curcumin in a carboxymethyl cellulose and hydroxypropyl methyl cellulose carrier: Physicochemical properties and biological activity. Int. J. Biol. Macromol. 2023, 239, 124203. [Google Scholar] [CrossRef]
- Paul, S.K.; Kumari, D.; Destino, J.; Chauhan, H. Design, Development, and Characterization of High Drug-Loaded Drug-Drug-Polymer Ternary Amorphous Solid Dispersions. AAPS PharmSciTech 2025, 26, 125. [Google Scholar] [CrossRef]
- Wdowiak, K.; Miklaszewski, A.; Cielecka-Piontek, J. Amorphous Polymer-Phospholipid Solid Dispersions for the Co-Delivery of Curcumin and Piperine Prepared via Hot-Melt Extrusion. Pharmaceutics 2024, 16, 999. [Google Scholar] [CrossRef]
- Anjudikkal, J.; Shukla, A.; Pulikkal, A.K. Effects of micellar media on the bioavailability, solubility, and stability of curcumin. Next Nanotechnol. 2025, 7, 100124. [Google Scholar] [CrossRef]
- Pan-On, S.; Tiyaboonchai, W. Development, characterization and Caco-2 cells absorption of curcumin solid dispersion for oral administration. J. Drug Deliv. Sci. Technol. 2023, 86, 104574. [Google Scholar] [CrossRef]
- Xi, Z.; Fei, Y.; Wang, Y.; Lin, Q.; Ke, Q.; Feng, G.; Xu, L. Solubility improvement of curcumin by crystallization inhibition from polymeric surfactants in amorphous solid dispersions. J. Drug Deliv. Sci. Technol. 2023, 83, 104351. [Google Scholar] [CrossRef]
- Ramachandran, G.; Chacko, I.A.; Mishara, M.G.; Khopade, A.J.; Sabitha, M.; Sudheesh, M.S. A review on design rules for formulating amorphous solid dispersions based on drug-polymer interactions in aqueous environment. Int. J. Pharm. 2025, 675, 125541. [Google Scholar] [CrossRef]
- Pokhrel, D.R.; Sah, M.K.; Gautam, B.; Basak, H.K.; Bhattarai, A.; Chatterjee, A. A recent overview of surfactant-drug interactions and their importance. RSC Adv. 2023, 13, 17685–17704. [Google Scholar] [CrossRef] [PubMed]
- Friesen, D.T.; Shanker, R.; Crew, M.; Smithey, D.T.; Curatolo, W.J.; Nightingale, J.A. Hydroxypropyl methylcellulose acetate succinate-based spray-dried dispersions: An overview. Mol. Pharm. 2008, 5, 1003–1019. [Google Scholar] [CrossRef]
- Scott, D.O.; Ghosh, A.; Di, L.; Maurer, T.S. Passive drug permeation through membranes and cellular distribution. Pharmacol. Res. 2017, 117, 94–102. [Google Scholar] [CrossRef]




| Parameters | Solution Cur (i.v.) | Crystalline Cur (o.p.) | Cur/P188 ASD (o.p.) | Cur/P188/TW80 ASD (o.p.) | Cur/P188/SLS ASD (o.p.) |
|---|---|---|---|---|---|
| AUC(0–24)/h | 697.8 ± 78.4 | 6.8 ± 1.9 | 109.3 ± 15.7 ### | 129.8 ± 13.1 ###, ns | 392.3 ± 56.2 ###, *** |
| Cmax/(ng/mL) | 334.5 ± 9.6 | 2.4 ± 0.4 | 90.1 ± 10.8 ### | 108.2 ± 6.7 ###, ns | 289.0 ± 29.8 ###, *** |
| MRT(0–24)/h | 5.2 ± 0.2 | 6.3 ± 2.0 | 3.6 ± 1.1 | 5.6 ± 0.7 | 4.6 ± 1.4 |
| Tmax/h | 0.1 ± 0.0 | 0.2 ± 0.0 | 0.2 ± 0.0 | 0.2 ± 0.0 | 0.2 ± 0.0 |
| T1/2/h | 1.8 ± 0.2 | 1.8 ± 0.4 | 1.9 ± 0.9 | 1.2 ± 0.2 | 1.4 ± 0.4 |
| F (%) | 0.97 | 15.66 | 18.60 | 56.22 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yuan, J.; Mao, S.; Ma, X.; Liu, X.; Chen, Y. Surfactants Significantly Improved the Oral Bioavailability of Curcumin Amorphous Solid Dispersions and Its Underlying Mechanism. Pharmaceutics 2025, 17, 1541. https://doi.org/10.3390/pharmaceutics17121541
Yuan J, Mao S, Ma X, Liu X, Chen Y. Surfactants Significantly Improved the Oral Bioavailability of Curcumin Amorphous Solid Dispersions and Its Underlying Mechanism. Pharmaceutics. 2025; 17(12):1541. https://doi.org/10.3390/pharmaceutics17121541
Chicago/Turabian StyleYuan, Jinhua, Siyi Mao, Xiuzhen Ma, Xiaoling Liu, and Yuejie Chen. 2025. "Surfactants Significantly Improved the Oral Bioavailability of Curcumin Amorphous Solid Dispersions and Its Underlying Mechanism" Pharmaceutics 17, no. 12: 1541. https://doi.org/10.3390/pharmaceutics17121541
APA StyleYuan, J., Mao, S., Ma, X., Liu, X., & Chen, Y. (2025). Surfactants Significantly Improved the Oral Bioavailability of Curcumin Amorphous Solid Dispersions and Its Underlying Mechanism. Pharmaceutics, 17(12), 1541. https://doi.org/10.3390/pharmaceutics17121541







