Enhancing Patient-Centric Drug Development: Coupling Hot Melt Extrusion with Fused Deposition Modeling and Pressure-Assisted Microsyringe Additive Manufacturing Platforms with Quality by Design
Abstract
1. Introduction
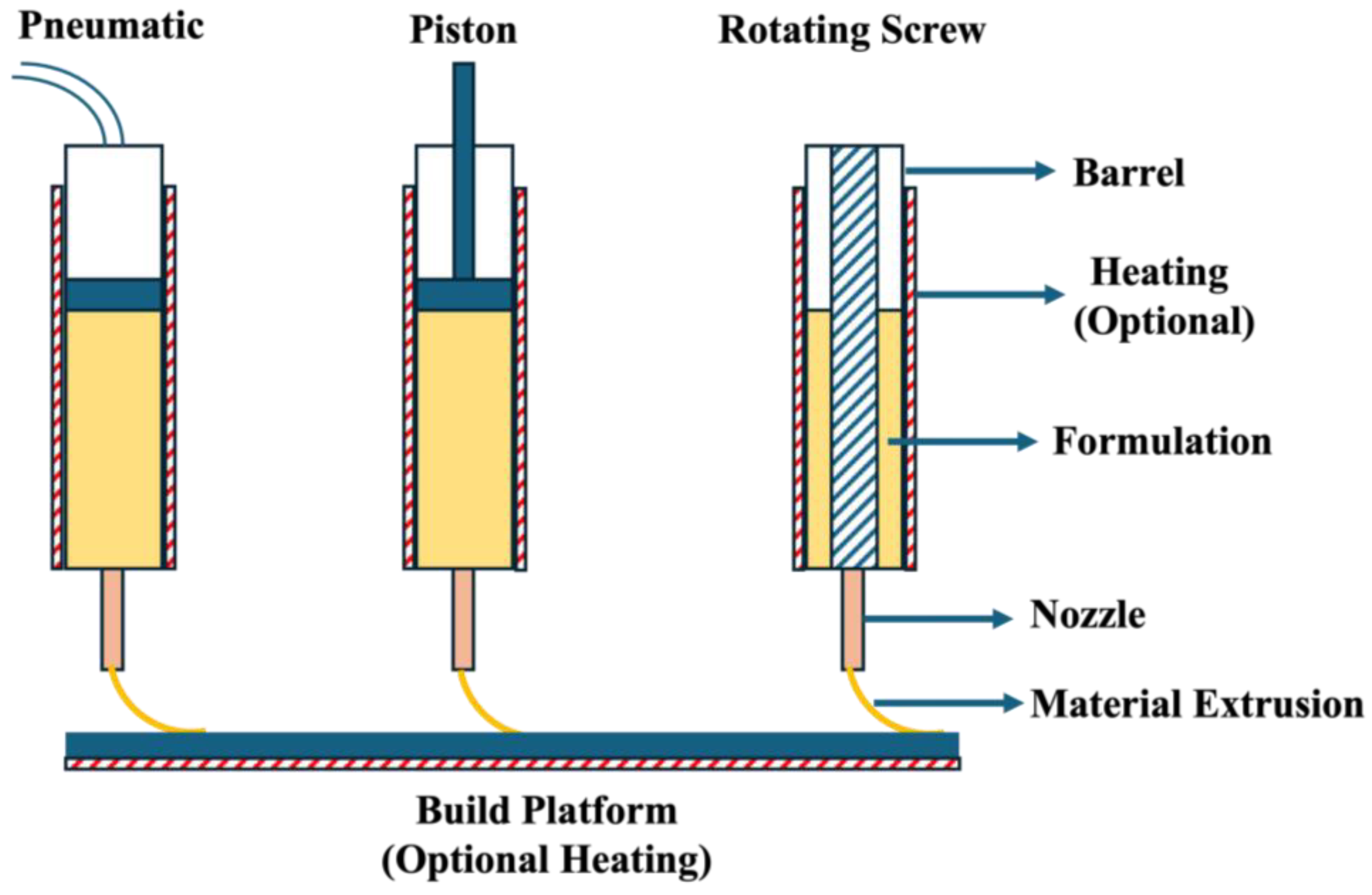
2. Pressure-Assisted Microsyringe (PAM)
2.1. Critical Material Properties
2.1.1. Thermal Properties
2.1.2. Rheological Properties
2.1.3. Miscibility
2.1.4. Density of Material
2.2. Critical Instrument Parameters
2.2.1. Type of Piston
2.2.2. Nozzle Size
2.2.3. Cooling Fan
2.2.4. Build Platform
2.3. Critical Process Parameters
2.3.1. Barrel and Nozzle Temperature
2.3.2. Printing Speed
2.3.3. Piston Pressure
2.3.4. Build Platform Temperature
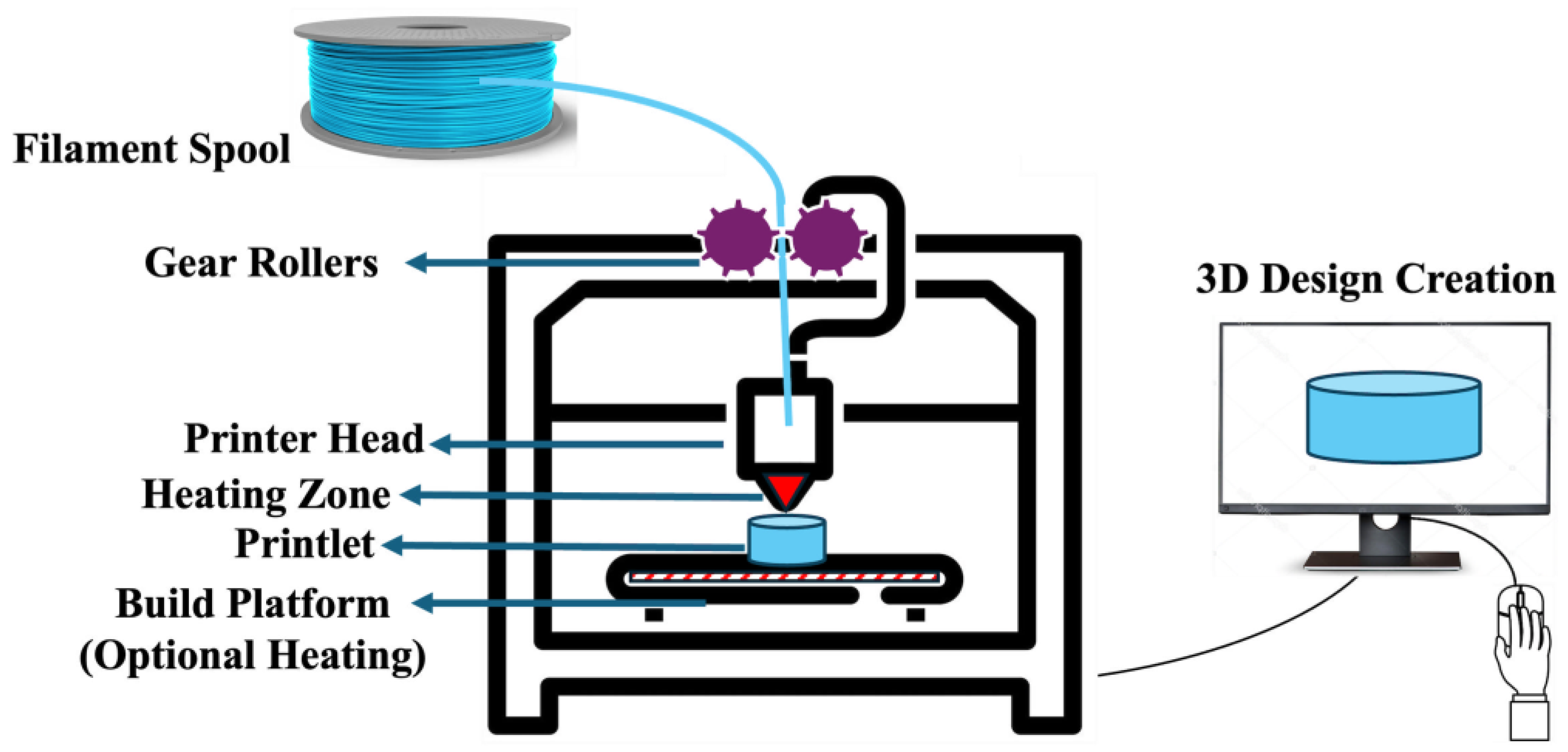
3. FDM 3D Printing
3.1. Critical Material Properties
3.1.1. Mechanical Properties
3.1.2. Surface Morphology
3.1.3. Filament Dimension
3.1.4. Rheological and Thermal Properties
3.2. Critical Instrument Parameters
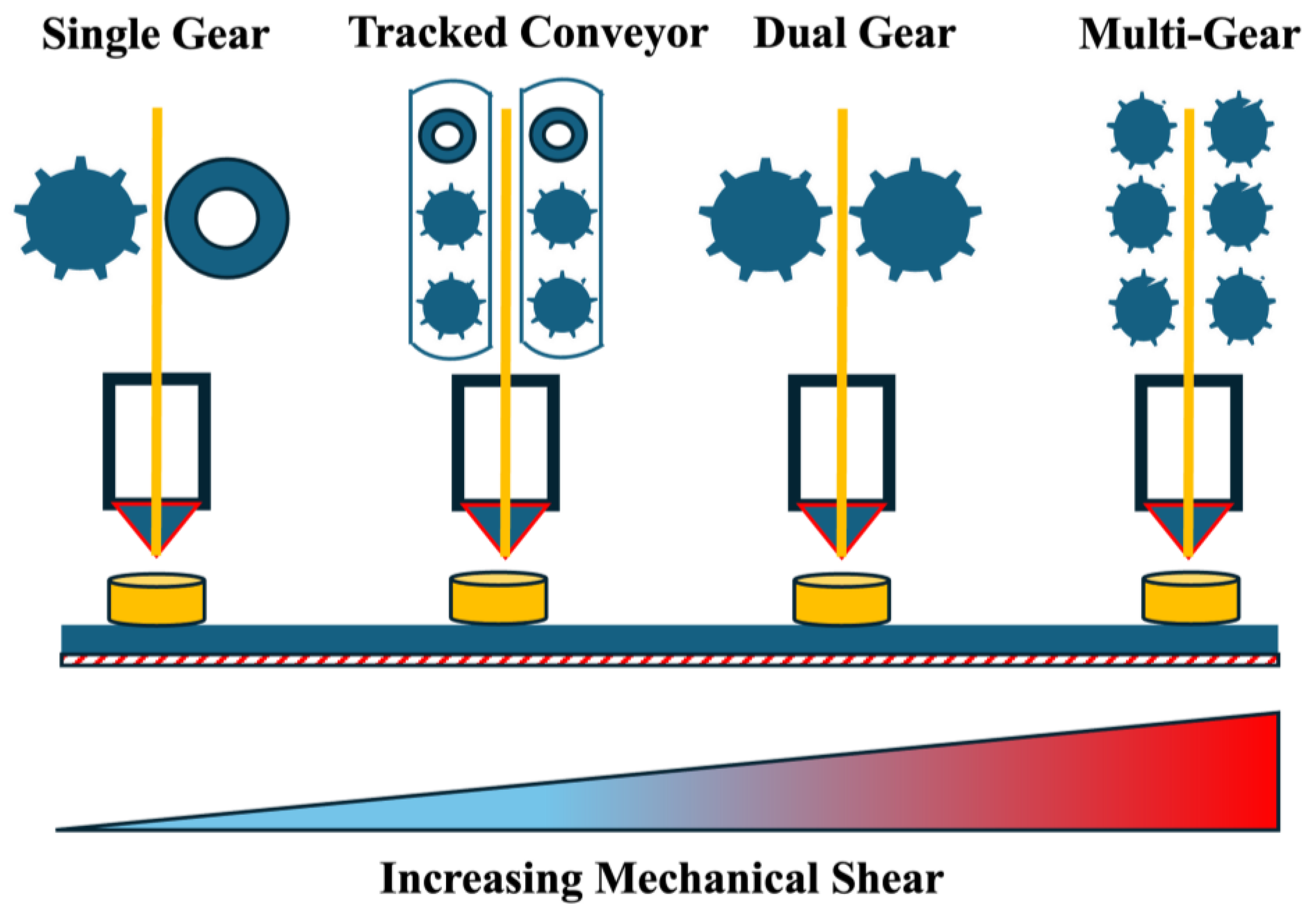
3.2.1. Gear Rollers
3.2.2. Nozzle Size
3.2.3. Cooling Fan and Build Platform
3.3. Critical Process Parameters
3.3.1. Printing Temperature
3.3.2. Printing Speed
3.3.3. Build Platform Temperature
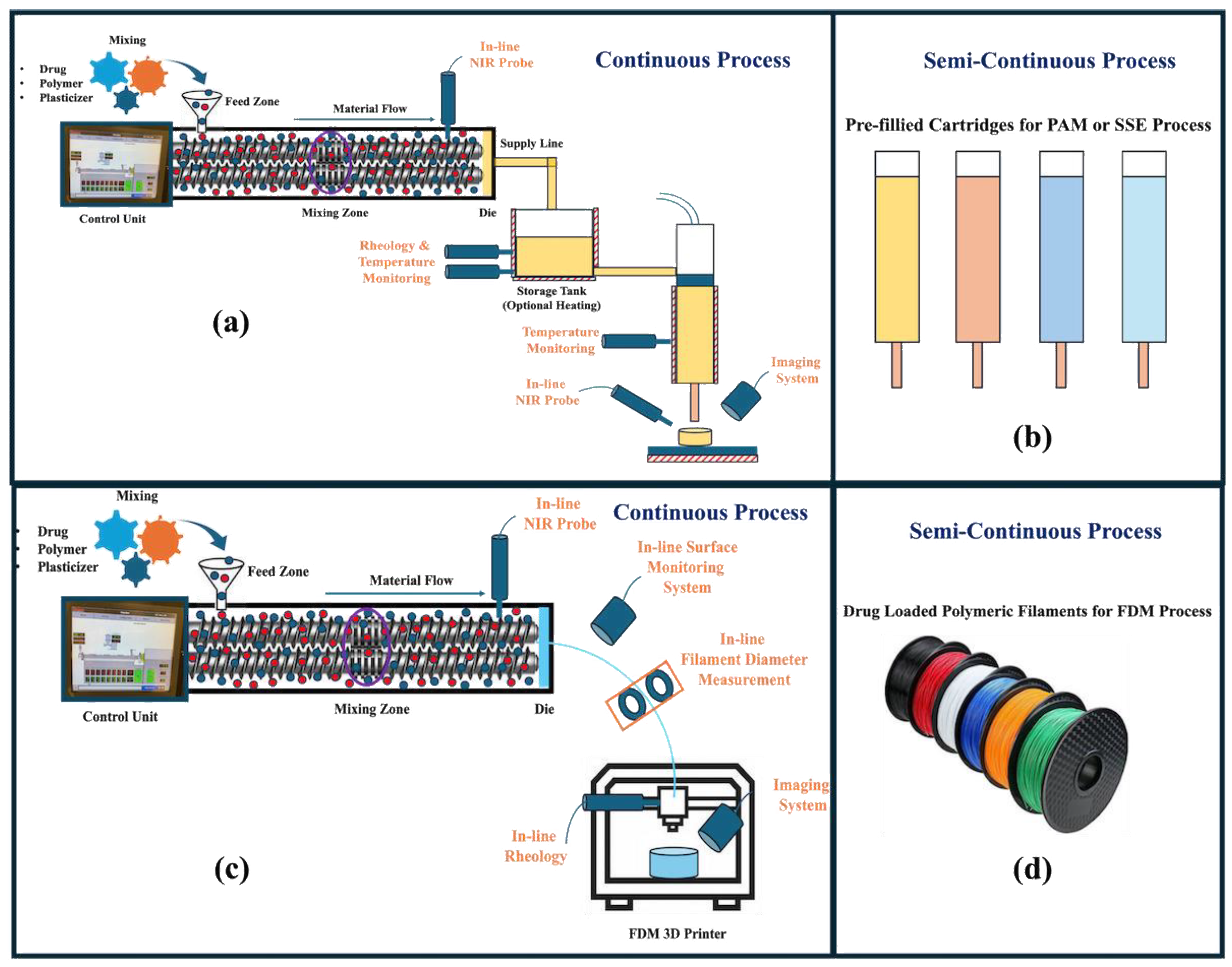
4. Coupling with HME
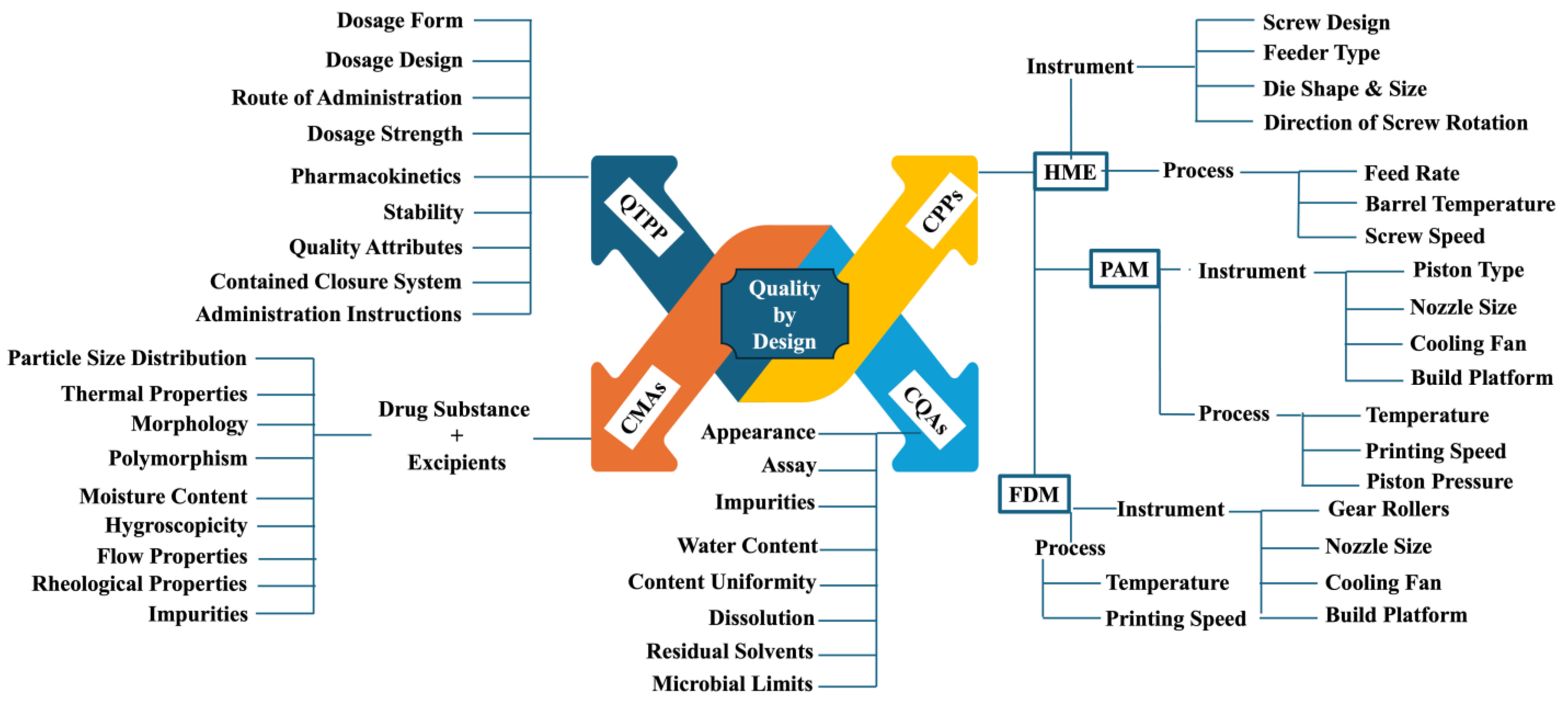
5. Quality by Design (QbD) Elements
5.1. Quality Targeted Product Profile (QTPP)
5.2. CQAs
5.3. CMAs
5.4. CPPs
5.5. Risk Assessment
5.6. Control Strategy and Continuous Improvement
- CQAs: CQAs need to be within the established limits to ensure the quality of the product.
- CMAs: The drug substance and the excipient should comply with the specification and satisfy the pre-determined requirements to eliminate any associated risk.
- CPPs: The process needs to be operated within the qualified and validated ranges to prevent any unwanted effects on the CQAs of drug products.
- In-process controls: Throughout the manufacturing process, the critical process parameters must be monitored continuously to ensure they stay within the established range and the intermediate product meets the pre-established specification, directly influencing the CQAs of the final product.
- Specifications: An acceptance criterion needs to be established within the specification to ensure product quality.
- PAT: The implementation of PAT tools will help monitor CMAs and CPPs, which are directly associated with the CQAs of drug products.
- Real-time release testing (RTRT): RTRT involves the in-line testing of the intermediate and finished drug product. Implementing RTRT will increase productivity and reduce costs. For example, the uniformity of the drug within the formulation blend can be monitored by mounting a near-infrared (NIR) probe. This process will ensure the development of a quality and safe medication. Any discrepancies can be controlled immediately without being realized towards the end of the manufacturing during end-product testing.
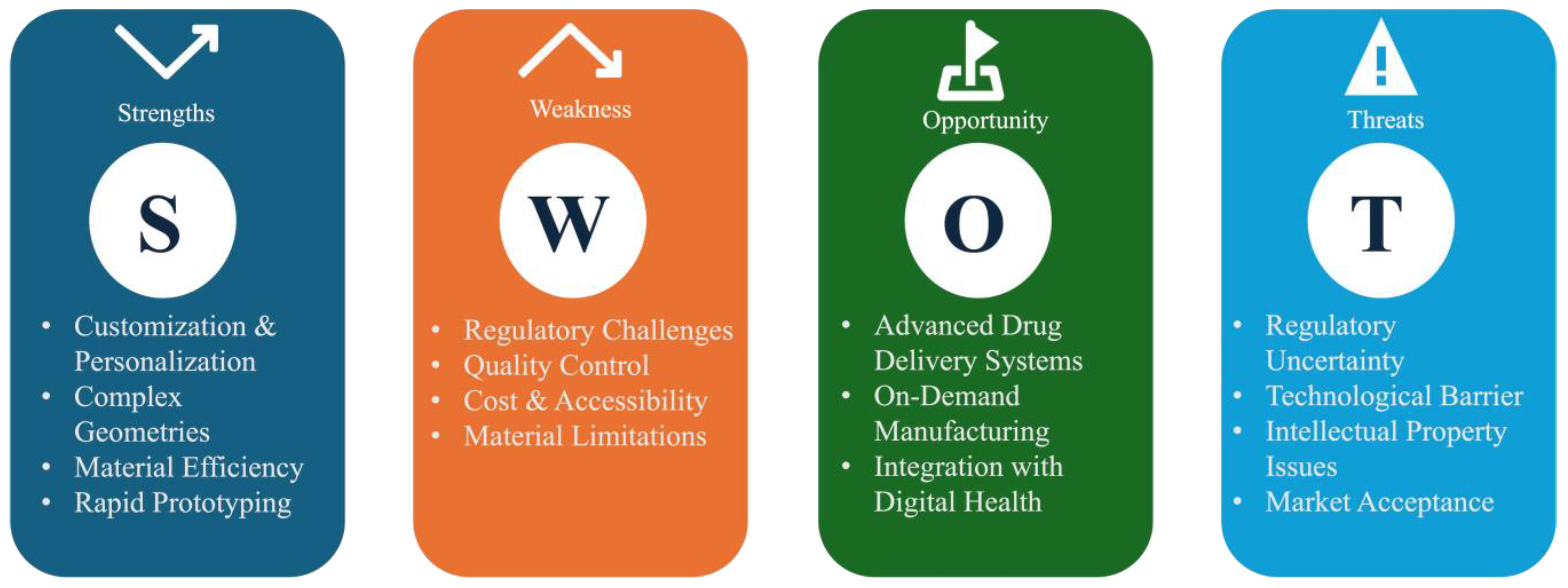
6. Future Perspectives and Expert Opinion
7. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Khan, K.; Minhas, M.; Badshah, S.; Suhail, M.; Sciences, A.A.-L. Overview of Nanoparticulate Strategies for Solubility Enhancement of Poorly Soluble Drugs. Life Sci. 2022, 291, 120301. [Google Scholar] [CrossRef] [PubMed]
- Bhalani, D.V.; Nutan, B.; Kumar, A.; Chandel, A.K.S. Bioavailability Enhancement Techniques for Poorly Aqueous Soluble Drugs and Therapeutics. Biomedicines 2022, 10, 2055. [Google Scholar] [CrossRef] [PubMed]
- da Silva, F.L.O.; Marques, M.B.D.F.; Kato, K.C.; Carneiro, G. Nanonization Techniques to Overcome Poor Water-Solubility with Drugs. Expert Opin. Drug Discov. 2020, 15, 853–864. [Google Scholar] [CrossRef]
- Bazzo, G.C.; Pezzini, B.R.; Stulzer, H.K. Eutectic Mixtures as an Approach to Enhance Solubility, Dissolution Rate and Oral Bioavailability of Poorly Water-Soluble Drugs. Int. J. Pharm. 2020, 588, 119741. [Google Scholar] [CrossRef]
- Das, B.; Baidya, A.T.K.; Mathew, A.T.; Yadav, A.K.; Kumar, R. Structural Modification Aimed for Improving Solubility of Lead Compounds in Early Phase Drug Discovery. Bioorg. Med. Chem. 2022, 56, 116614. [Google Scholar] [CrossRef] [PubMed]
- Tekade, A.R.; Yadav, J.N. A Review on Solid Dispersion and Carriers Used Therein for Solubility Enhancement of Poorly Water Soluble Drugs. Adv. Pharm. Bull. 2020, 10, 359. [Google Scholar] [CrossRef] [PubMed]
- Pires, P.C.; Rodrigues, M.; Alves, G.; Santos, A.O. Strategies to Improve Drug Strength in Nasal Preparations for Brain Delivery of Low Aqueous Solubility Drugs. Pharmaceutics 2022, 14, 588. [Google Scholar] [CrossRef]
- Lee, M.K. Liposomes for Enhanced Bioavailability of Water-Insoluble Drugs: In Vivo Evidence and Recent Approaches. Pharmaceutics 2020, 12, 264. [Google Scholar] [CrossRef] [PubMed]
- Han, J.; Wei, Y.; Lu, Y.; Wang, R.; Zhang, J.; Gao, Y.; Qian, S. Co-Amorphous Systems for the Delivery of Poorly Water-Soluble Drugs: Recent Advances and an Update. Expert Opin. Drug Deliv. 2020, 17, 1411–1435. [Google Scholar] [CrossRef]
- Paredes, A.J.; McKenna, P.E.; Ramöller, I.K.; Naser, Y.A.; Volpe-Zanutto, F.; Li, M.; Abbate, M.T.A.; Zhao, L.; Zhang, C.; Abu-Ershaid, J.M.; et al. Microarray Patches: Poking a Hole in the Challenges Faced When Delivering Poorly Soluble Drugs. Adv. Funct. Mater. 2021, 31, 2005792. [Google Scholar] [CrossRef]
- Rathor, S.; Bhatt, D.C. Formulation, Characterization, and Pharmacokinetic Evaluation of Novel Glipizide-Phospholipid Nano-Complexes with Improved Solubility and Bio-Availability. Pharm. Nanotechnol. 2022, 10, 125–136. [Google Scholar] [CrossRef]
- Barradas, T.N.; Silva, K.G.d.H.e. Nanoemulsions of Essential Oils to Improve Solubility, Stability and Permeability: A Review. Environ. Chem. Lett. 2020, 19, 1153–1171. [Google Scholar] [CrossRef]
- Kim, D.H.; Lee, S.E.; Pyo, Y.C.; Tran, P.; Park, J.S. Solubility Enhancement and Application of Cyclodextrins in Local Drug Delivery. J. Pharm. Investig. 2020, 50, 17–27. [Google Scholar] [CrossRef]
- Ali, M.K.; Moshikur, R.M.; Wakabayashi, R.; Moniruzzaman, M.; Kamiya, N.; Goto, M. Biocompatible Ionic Liquid Surfactant-Based Microemulsion as a Potential Carrier for Sparingly Soluble Drugs. ACS Sustain. Chem. Eng. 2020, 8, 6263–6272. [Google Scholar] [CrossRef]
- Islam, M.R.; Chowdhury, M.R.; Wakabayashi, R.; Kamiya, N.; Moniruzzaman, M.; Goto, M. Ionic Liquid-In-Oil Microemulsions Prepared with Biocompatible Choline Carboxylic Acids for Improving the Transdermal Delivery of a Sparingly Soluble Drug. Pharmaceutics 2020, 12, 392. [Google Scholar] [CrossRef] [PubMed]
- Hwang, D.; Ramsey, J.D.; Kabanov, A.V. Polymeric Micelles for the Delivery of Poorly Soluble Drugs: From Nanoformulation to Clinical Approval. Adv. Drug Deliv. Rev. 2020, 156, 80–118. [Google Scholar] [CrossRef]
- McGuckin, M.B.; Wang, J.; Ghanma, R.; Qin, N.; Palma, S.D.; Donnelly, R.F.; Paredes, A.J. Nanocrystals as a Master Key to Deliver Hydrophobic Drugs via Multiple Administration Routes. J. Control. Release 2022, 345, 334–353. [Google Scholar] [CrossRef] [PubMed]
- Ibrahim, A.H.; Smått, J.H.; Govardhanam, N.P.; Ibrahim, H.M.; Ismael, H.R.; Afouna, M.I.; Samy, A.M.; Rosenholm, J.M. Formulation and Optimization of Drug-Loaded Mesoporous Silica Nanoparticle-Based Tablets to Improve the Dissolution Rate of the Poorly Water-Soluble Drug Silymarin. Eur. J. Pharm. Sci. 2020, 142, 105103. [Google Scholar] [CrossRef]
- Park, H.; Otte, A.; Park, K. Evolution of Drug Delivery Systems: From 1950 to 2020 and Beyond. J. Control. Release 2022, 342, 53–65. [Google Scholar] [CrossRef]
- Lin, H.L.; Cheng, W.T.; Chen, L.C.; Ho, H.O.; Lin, S.Y.; Hsieh, C.M. Honokiol/Magnolol-Loaded Self-Assembling Lecithin-Based Mixed Polymeric Micelles (Lb MPMs) for Improving Solubility to Enhance Oral Bioavailability. Int. J. Nanomed. 2021, 16, 651–665. [Google Scholar] [CrossRef] [PubMed]
- Ueda, K.; Moseson, D.E.; Taylor, L.S. Amorphous Solubility Advantage: Theoretical Considerations, Experimental Methods, and Contemporary Relevance. J. Pharm. Sci. 2024, 114, 18–39. [Google Scholar] [CrossRef]
- He, M.; Zheng, W.; Wang, N.; Gao, H.; Ouyang, D.; Huang, Z. Molecular Dynamics Simulation of Drug Solubilization Behavior in Surfactant and Cosolvent Injections. Pharmaceutics 2022, 14, 2366. [Google Scholar] [CrossRef] [PubMed]
- Koch, N.; Jennotte, O.; Gasparrini, Y.; Vandenbroucke, F.; Lechanteur, A.; Evrard, B. Cannabidiol Aqueous Solubility Enhancement: Comparison of Three Amorphous Formulations Strategies Using Different Type of Polymers. Int. J. Pharm. 2020, 589, 119812. [Google Scholar] [CrossRef]
- van der Merwe, J.; Steenekamp, J.; Steyn, D.; Hamman, J. The Role of Functional Excipients in Solid Oral Dosage Forms to Overcome Poor Drug Dissolution and Bioavailability. Pharmaceutics 2020, 12, 393. [Google Scholar] [CrossRef]
- Singh, S.; Kumar, M.; Choudhary, D.; Chopra, S.; Bhatia, A. 3D Printing Technology in Drug Delivery: Polymer Properties and Applications. J. Dispers. Sci. Technol. 2023, 23, 1–35. [Google Scholar] [CrossRef]
- Muehlenfeld, C.; Duffy, P.; Yang, F.; Pérez, D.Z.; El-Saleh, F.; Durig, T. Excipients in Pharmaceutical Additive Manufacturing: A Comprehensive Exploration of Polymeric Material Selection for Enhanced 3D Printing. Pharmaceutics 2024, 16, 317. [Google Scholar] [CrossRef] [PubMed]
- Bishnoi, M.; Ankita; Mody, N.; Jain, A. Additive Manufacturing Strategies for Personalized Drug Delivery Systems and Medical Devices. In Medical Additive Manufacturing: Concepts and Fundamentals; Elsevier: Amsterdam, The Netherlands, 2024; pp. 619–647. ISBN 9780323953832. [Google Scholar]
- Muehlenfeld, C.; Duffy, P.; Yang, F.; Zermeño-Pérez, D.; Durig, T. Polymers for Pharmaceutical 3D Printing: Printability and Critical Insight into Material Properties. In 3D Printing; Springer: Cham, Switzerland, 2024; pp. 97–137. ISBN 978-3-031-46015-9. [Google Scholar]
- Anaya, B.J.; Cerda, J.R.; D’Atri, R.M.; Yuste, I.; Luciano, F.C.; Kara, A.; Ruiz, H.K.; Ballesteros, M.P.; Serrano, D.R. Engineering of 3D Printed Personalized Polypills for the Treatment of the Metabolic Syndrome. Int. J. Pharm. 2023, 642, 123194. [Google Scholar] [CrossRef]
- Ullah, M.; Wahab, A.; Khan, S.U.; Naeem, M.; Rehman, K.U.; Ali, H.; Ullah, A.; Khan, A.; Khan, N.R.; Rizg, W.Y.; et al. 3D Printing Technology: A New Approach for the Fabrication of Personalized and Customized Pharmaceuticals. Eur. Polym. J. 2023, 195, 112240. [Google Scholar] [CrossRef]
- Nizam, M.; Purohit, R.; Taufik, M. 3D Printing in Healthcare: A Review on Drug Printing, Challenges and Future Perspectives. Mater. Today Commun. 2024, 40, 110199. [Google Scholar] [CrossRef]
- Muhindo, D.; Elkanayati, R.; Srinivasan, P.; Repka, M.A.; Ashour, E.A. Recent Advances in the Applications of Additive Manufacturing (3D Printing) in Drug Delivery: A Comprehensive Review. AAPS PharmSciTech 2023, 24, 1–22. [Google Scholar] [CrossRef]
- Mohammed, A.A.; Algahtani, M.S.; Ahmad, M.Z.; Ahmad, J. Optimization of Semisolid Extrusion (Pressure-Assisted Microsyringe)-Based 3D Printing Process for Advanced Drug Delivery Application. Ann. 3D Print. Med. 2021, 2, 100008. [Google Scholar] [CrossRef]
- El Aita, I. Manufacturing of Solid Dosage Forms Using Pressure-Assisted Microsyringe 3D-Printing. Ph.D. Thesis, Heinrich Heine University Düsseldorf, Düsseldorf, Germany, 2021. [Google Scholar]
- Seoane-Viaño, I.; Januskaite, P.; Alvarez-Lorenzo, C.; Basit, A.W.; Goyanes, A. Semi-Solid Extrusion 3D Printing in Drug Delivery and Biomedicine: Personalised Solutions for Healthcare Challenges. J. Control. Release 2021, 332, 367–389. [Google Scholar] [CrossRef] [PubMed]
- El Aita, I.; Rahman, J.; Breitkreutz, J.; Quodbach, J. 3D-Printing with Precise Layer-Wise Dose Adjustments for Paediatric Use via Pressure-Assisted Microsyringe Printing. Eur. J. Pharm. Biopharm. 2020, 157, 59–65. [Google Scholar] [CrossRef]
- Auriemma, G.; Tommasino, C.; Falcone, G.; Esposito, T.; Sardo, C.; Aquino, R.P. Additive Manufacturing Strategies for Personalized Drug Delivery Systems and Medical Devices: Fused Filament Fabrication and Semi Solid Extrusion. Molecules 2022, 27, 2784. [Google Scholar] [CrossRef]
- Gutiérrez, C.L.P.; Cottone, F.; Pagano, C.; di Michele, A.; Puglia, D.; Luzi, F.; Dominici, F.; Sinisi, R.; Ricci, M.; Iborra, C.A.V.; et al. The Optimization of Pressure-Assisted Microsyringe (PAM) 3D Printing Parameters for the Development of Sustainable Starch-Based Patches. Polymers 2023, 15, 3792. [Google Scholar] [CrossRef]
- Panraksa, P.; Zhang, B.; Rachtanapun, P.; Jantanasakulwong, K.; Qi, S.; Jantrawut, P. “Tablet-in-Syringe”: A Novel Dosing Mechanism for Dysphagic Patients Containing Fast-Disintegrating Tablets Fabricated Using Semisolid Extrusion 3D Printing. Pharmaceutics 2022, 14, 443. [Google Scholar] [CrossRef]
- Rahman, J.; Quodbach, J. Versatility on Demand—The Case for Semi-Solid Micro-Extrusion in Pharmaceutics. Adv. Drug Deliv. Rev. 2021, 172, 104–126. [Google Scholar] [CrossRef]
- Kyser, A.J.; Mahmoud, M.Y.; Herold, S.E.; Lewis, W.G.; Lewis, A.L.; Steinbach-Rankins, J.M.; Frieboes, H.B. Formulation and Characterization of Pressure-Assisted Microsyringe 3D-Printed Scaffolds for Controlled Intravaginal Antibiotic Release. Int. J. Pharm. 2023, 641, 123054. [Google Scholar] [CrossRef]
- Ferrero, C.; Urpí, L.; Aguilar-de-Leyva, A.; Mora-Castaño, G.; Linares, V.; Millán-Jiménez, M.; de Ilarduya, A.M.; Caraballo, I. Application of Ultrasound-Assisted Compression and 3D-Printing Semi-Solid Extrusion Techniques to the Development of Sustained-Release Drug Delivery Systems Based on a Novel Biodegradable Aliphatic Copolyester. J. Drug Deliv. Sci. Technol. 2024, 95, 105652. [Google Scholar] [CrossRef]
- Nizam, M.; Purohit, R.; Taufik, M. Extrusion Based Additive Manufacturing of Medicines. AIP Conf. Proc. 2024, 3007, 100046. [Google Scholar]
- Tagami, T.; Okamura, M.; Ogawa, K.; Ozeki, T. Fabrication of Mucoadhesive Films Containing Pharmaceutical Ionic Liquid and Eudragit Polymer Using Pressure-Assisted Microsyringe-Type 3D Printer for Treating Oral Mucositis. Pharmaceutics 2022, 14, 1930. [Google Scholar] [CrossRef]
- Racaniello, G.F.; Silvestri, T.; Pistone, M.; D’Amico, V.; Arduino, I.; Denora, N.; Lopedota, A.A. Innovative Pharmaceutical Techniques for Paediatric Dosage Forms: A Systematic Review on 3D Printing, Prilling/Vibration and Microfluidic Platform. J. Pharm. Sci. 2024, 113, 1726–1748. [Google Scholar] [CrossRef] [PubMed]
- Zhang, B.; Belton, P.; Teoh, X.Y.; Gleadall, A.; Bibb, R.; Qi, S. An Investigation into the Effects of Ink Formulations of Semi-Solid Extrusion 3D Printing on the Performance of Printed Solid Dosage Forms. J. Mater. Chem. B 2023, 12, 131–144. [Google Scholar] [CrossRef]
- Funk, N.L.; Leão, J.; de Oliveira, T.V.; Beck, R.C.R. Semi-Solid Extrusion (SSE) in Pharmaceuticals. In Additive Manufacturing in Pharmaceuticals; Springer: Singapore, Singapore, 2023; pp. 171–200. ISBN 978-981-99-2404-2. [Google Scholar]
- Mathew, E.; Pitzanti, G.; Larrañeta, E.; Lamprou, D.A. 3D Printing of Pharmaceuticals and Drug Delivery Devices. Pharmaceutics 2020, 12, 266. [Google Scholar] [CrossRef]
- Han, X.; Kang, D.; Liu, B.; Zhang, H.; Wang, Z.; Gao, X.; Zheng, A. Feasibility of Developing Hospital Preparation by Semisolid Extrusion 3D Printing: Personalized Amlodipine Besylate Chewable Tablets. Pharm. Dev. Technol. 2022, 27, 164–174. [Google Scholar] [CrossRef]
- Seoane-Viaño, I.; Trenfield, S.J.; Basit, A.W.; Goyanes, A. Translating 3D Printed Pharmaceuticals: From Hype to Real-World Clinical Applications. Adv. Drug Deliv. Rev. 2021, 174, 553–575. [Google Scholar] [CrossRef] [PubMed]
- Dumpa, N.; Butreddy, A.; Wang, H.; Komanduri, N.; Bandari, S.; Repka, M.A. 3D Printing in Personalized Drug Delivery: An Overview of Hot-Melt Extrusion-Based Fused Deposition Modeling. Int. J. Pharm. 2021, 600, 120501. [Google Scholar] [CrossRef] [PubMed]
- Bandari, S.; Nyavanandi, D.; Dumpa, N.; Repka, M.A. Coupling Hot Melt Extrusion and Fused Deposition Modeling: Critical Properties for Successful Performance. Adv. Drug Deliv. Rev. 2021, 172, 52–63. [Google Scholar] [CrossRef] [PubMed]
- Nashed, N.; Lam, M.; Nokhodchi, A. A Comprehensive Overview of Extended Release Oral Dosage Forms Manufactured through Hot Melt Extrusion and Its Combination with 3D Printing. Int. J. Pharm. 2021, 596, 120237. [Google Scholar] [CrossRef]
- Zhang, P.; Xu, P.; Chung, S.; Bandari, S.; Repka, M.A. Fabrication of Bilayer Tablets Using Hot Melt Extrusion-Based Dual-Nozzle Fused Deposition Modeling 3D Printing. Int. J. Pharm. 2022, 624, 121972. [Google Scholar] [CrossRef]
- Viidik, L.; Vesala, J.; Laitinen, R.; Korhonen, O.; Ketolainen, J.; Aruväli, J.; Kirsimäe, K.; Kogermann, K.; Heinämäki, J.; Laidmäe, I.; et al. Preparation and Characterization of Hot-Melt Extruded Polycaprolactone-Based Filaments Intended for 3D-Printing of Tablets. Eur. J. Pharm. Sci. 2021, 158, 105619. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.; Feng, S.; Almotairy, A.; Bandari, S.; Repka, M.A. Development of Multifunctional Drug Delivery System via Hot-Melt Extrusion Paired with Fused Deposition Modeling 3D Printing Techniques. Eur. J. Pharm. Biopharm. 2023, 183, 102–111. [Google Scholar] [CrossRef] [PubMed]
- Omari, S.; Ashour, E.A.; Elkanayati, R.; Alyahya, M.; Almutairi, M.; Repka, M.A. Formulation Development of Loratadine Immediate- Release Tablets Using Hot-Melt Extrusion and 3D Printing Technology. J. Drug Deliv. Sci. Technol. 2022, 74, 103505. [Google Scholar] [CrossRef]
- Nyavanandi, D.; Mandati, P.; Narala, S.; Alzahrani, A.; Kolimi, P.; Pradhan, A.; Bandari, S.; Repka, M.A. Feasibility of High Melting Point Hydrochlorothiazide Processing via Cocrystal Formation by Hot Melt Extrusion Paired Fused Filament Fabrication as a 3D-Printed Cocrystal Tablet. Int. J. Pharm. 2022, 628, 122283. [Google Scholar] [CrossRef] [PubMed]
- Hu, Z.; Xu, P.; Zhang, J.; Bandari, S.; Repka, M.A. Development of Controlled Release Oral Dosages by Density Gradient Modification via Three-Dimensional (3D) Printing and Hot-Melt Extrusion (HME) Technology. J. Drug Deliv. Sci. Technol. 2022, 71, 103355. [Google Scholar] [CrossRef]
- Deshkar, S.; Rathi, M.; Zambad, S.; Gandhi, K. Hot Melt Extrusion and Its Application in 3D Printing of Pharmaceuticals. Curr. Drug Deliv. 2021, 18, 387–407. [Google Scholar] [CrossRef]
- Dumpa, N.R.; Bandari, S.; Repka, M.A. Novel Gastroretentive Floating Pulsatile Drug Delivery System Produced via Hot-Melt Extrusion and Fused Deposition Modeling 3D Printing. Pharmaceutics 2020, 12, 52. [Google Scholar] [CrossRef]
- Pereira, G.G.; Figueiredo, S.; Fernandes, A.I.; Pinto, J.F. Polymer Selection for Hot-Melt Extrusion Coupled to Fused Deposition Modelling in Pharmaceutics. Pharmaceutics 2020, 12, 795. [Google Scholar] [CrossRef]
- Vo, A.Q.; Zhang, J.; Nyavanandi, D.; Bandari, S.; Repka, M.A. Hot Melt Extrusion Paired Fused Deposition Modeling 3D Printing to Develop Hydroxypropyl Cellulose Based Floating Tablets of Cinnarizine. Carbohydr. Polym. 2020, 246, 116519. [Google Scholar] [CrossRef]
- Zhang, P.; Li, J.; Ashour, E.A.; Chung, S.; Wang, H.; Vemula, S.K.; Repka, M.A. Development of Multiple Structured Extended Release Tablets via Hot Melt Extrusion and Dual-Nozzle Fused Deposition Modeling 3D Printing. Int. J. Pharm. 2024, 653, 123905. [Google Scholar] [CrossRef] [PubMed]
- Mandati, P.; Dumpa, N.; Alzahrani, A.; Nyavanandi, D.; Narala, S.; Wang, H.; Bandari, S.; Repka, M.A.; Tiwari, S.; Langley, N. Hot-Melt Extrusion-Based Fused Deposition Modeling 3D Printing of Atorvastatin Calcium Tablets: Impact of Shape and Infill Density on Printability and Performance. AAPS PharmSciTech 2022, 24, 13. [Google Scholar] [CrossRef] [PubMed]
- Ponsar, H.; Wiedey, R.; Quodbach, J. Hot-Melt Extrusion Process Fluctuations and Their Impact on Critical Quality Attributes of Filaments and 3D-Printed Dosage Forms. Pharmaceutics 2020, 12, 511. [Google Scholar] [CrossRef]
- Feng, S.; Bandari, S.; Repka, M.A. Investigation of Poly(2-Ethyl-2-Oxazoline) as a Novel Extended Release Polymer for Hot-Melt Extrusion Paired with Fused Deposition Modeling 3D Printing. J. Drug Deliv. Sci. Technol. 2022, 74, 103558. [Google Scholar] [CrossRef]
- Nyavanandi, D.; Narala, S.; Repka, M.A. Hot-Melt Extrusion Paired Fused Deposition Modeling 3D Printing: Development of Pharmaceutical Medications. In 3D Printing; Springer: Cham, Switzerland, 2024; pp. 169–194. ISBN 978-3-031-46015-9. [Google Scholar]
- Thanawuth, K.; Sutthapitaksakul, L.; Konthong, S.; Suttiruengwong, S.; Huanbutta, K.; Dass, C.R.; Sriamornsak, P. Impact of Drug Loading Method on Drug Release from 3D-Printed Tablets Made from Filaments Fabricated by Hot-Melt Extrusion and Impregnation Processes. Pharmaceutics 2021, 13, 1607. [Google Scholar] [CrossRef] [PubMed]
- Digkas, T.; Porfire, A.; Van Renterghem, J.; Samaro, A.; Borodi, G.; Vervaet, C.; Crișan, A.G.; Iurian, S.; De Beer, T.; Tomuta, I. Development of Diclofenac Sodium 3D Printed Cylindrical and Tubular-Shaped Tablets through Hot Melt Extrusion and Fused Deposition Modelling Techniques. Pharmaceuticals 2023, 16, 1062. [Google Scholar] [CrossRef]
- Zhang, J.; Lu, A.; Thakkar, R.; Zhang, Y.; Maniruzzaman, M. Development and Evaluation of Amorphous Oral Thin Films Using Solvent-Free Processes: Comparison between 3D Printing and Hot-Melt Extrusion Technologies. Pharmaceutics 2021, 13, 1613. [Google Scholar] [CrossRef]
- Zavřel, F.; Novák, M.; Kroupová, J.; Beveridge, C.; Štěpánek, F.; Ruphuy, G. Development of Hot-Melt Extrusion Method to Produce Hydroxyapatite/Polycaprolactone Composite Filaments. Adv. Eng. Mater. 2021, 24, 2100820. [Google Scholar] [CrossRef]
- Almotairy, A.; Alyahya, M.; Althobaiti, A.; Almutairi, M.; Bandari, S.; Ashour, E.A.; Repka, M.A. Disulfiram 3D Printed Film Produced via Hot-Melt Extrusion Techniques as a Potential Anticervical Cancer Candidate. Int. J. Pharm. 2023, 635, 122709. [Google Scholar] [CrossRef]
- Chung, S.; Srinivasan, P.; Zhang, P.; Bandari, S.; Repka, M.A. Development of Ibuprofen Tablet with Polyethylene Oxide Using Fused Deposition Modeling 3D-Printing Coupled with Hot-Melt Extrusion. J. Drug Deliv. Sci. Technol. 2022, 76, 103716. [Google Scholar] [CrossRef]
- Huang, L.; Yang, W.; Bu, Y.; Yu, M.; Xu, M.; Guo, J.; Ni, W.; Jia, Y.; Zhang, J. Patient-Focused Programable Release Indomethacin Tablets Prepared via Conjugation of Hot Melt Extrusion (HME) and Fused Depositional Modeling (FDM)—3D Printing Technologies. J. Drug Deliv. Sci. Technol. 2024, 97, 105797. [Google Scholar] [CrossRef]
- Nashed, N.; Chan, S.; Lam, M.; Ghafourian, T.; Nokhodchi, A. Effect of PH, Ionic Strength and Agitation Rate on Dissolution Behaviour of 3D-Printed Tablets, Tablets Prepared from Ground Hot-Melt Extruded Filaments and Physical Mixtures. Biomedicines 2023, 11, 375. [Google Scholar] [CrossRef]
- Tabriz, A.G.; Scoutaris, N.; Gong, Y.; Hui, H.W.; Kumar, S.; Douroumis, D. Investigation on Hot Melt Extrusion and Prediction on 3D Printability of Pharmaceutical Grade Polymers. Int. J. Pharm. 2021, 604, 120755. [Google Scholar] [CrossRef] [PubMed]
- dos Santos, J.; da Silva, G.S.; Velho, M.C.; Beck, R.C.R. Eudragit®: A Versatile Family of Polymers for Hot Melt Extrusion and 3D Printing Processes in Pharmaceutics. Pharmaceutics 2021, 13, 1424. [Google Scholar] [CrossRef]
- Giri, B.R.; Song, E.S.; Kwon, J.; Lee, J.H.; Park, J.B.; Kim, D.W. Fabrication of Intragastric Floating, Controlled Release 3D Printed Theophylline Tablets Using Hot-Melt Extrusion and Fused Deposition Modeling. Pharmaceutics 2020, 12, 77. [Google Scholar] [CrossRef] [PubMed]
- Zhang, P.; Wang, H.; Chung, S.; Li, J.; Vemula, S.K.; Repka, M.A. Fabrication of Suppository Shells via Hot-Melt Extrusion Paired with Fused Deposition Modeling 3D Printing Techniques. J. Drug Deliv. Sci. Technol. 2024, 94, 105491. [Google Scholar] [CrossRef]
- Bian, Y.H.; Yu, G.; Zhao, X.; Li, S.X.; He, X.L.; Tian, C.X.; Li, Z.Y. Exit Morphology and Mechanical Property of FDM Printed PLA: Influence of Hot Melt Extrusion Process. Adv. Manuf. 2023, 11, 56–74. [Google Scholar] [CrossRef]
- Mamidi, H.K.; Palekar, S.; Nukala, P.K.; Mishra, S.M.; Patki, M.; Fu, Y.; Supner, P.; Chauhan, G.; Patel, K. Process Optimization of Twin-Screw Melt Granulation of Fenofibrate Using Design of Experiment (DoE). Int. J. Pharm. 2021, 593, 120101. [Google Scholar] [CrossRef]
- Mamidi, H.K.; Rohera, B.D. Application of Thermodynamic Phase Diagrams and Gibbs Free Energy of Mixing for Screening of Polymers for Their Use in Amorphous Solid Dispersion Formulation of a Non-Glass-Forming Drug. J. Pharm. Sci. 2021, 110, 2703–2717. [Google Scholar] [CrossRef]
- Arden, N.S.; Fisher, A.C.; Tyner, K.; Yu, L.X.; Lee, S.L.; Kopcha, M. Industry 4.0 for Pharmaceutical Manufacturing: Preparing for the Smart Factories of the Future. Int. J. Pharm. 2021, 602, 120554. [Google Scholar] [CrossRef] [PubMed]
- Kumar, R.; Kumar, M.; Chohan, J.S. The Role of Additive Manufacturing for Biomedical Applications: A Critical Review. J. Manuf. Process. 2021, 64, 828–850. [Google Scholar] [CrossRef]
- Beg, S.; Almalki, W.H.; Malik, A.; Farhan, M.; Aatif, M.; Rahman, Z.; Alruwaili, N.K.; Alrobaian, M.; Tarique, M.; Rahman, M. 3D Printing for Drug Delivery and Biomedical Applications. Drug Discov. Today 2020, 25, 1668–1681. [Google Scholar] [CrossRef]
- Parupathi, P.; Dhoppalapudi, S. Downstream Processing of Amorphous Solid Dispersions into Tablets. GSC Biol. Pharm. Sci. 2023, 22, 170–177. [Google Scholar] [CrossRef]
- Dhoppalapudi, S.; Parupathi, P. Hot Melt Extrusion: A Single-Step Continuous Manufacturing Process for Developing Amorphous Solid Dispersions of Poorly Soluble Drug Substances. GSC Adv. Res. Rev. 2022, 13, 126–135. [Google Scholar] [CrossRef]
- Parupathi, P.; Dhoppalapudi, S. The Role of Surfactants in Preserving the Stability of Amorphous Solid Dispersions: A Review. GSC Biol. Pharm. Sci. 2022, 21, 039–047. [Google Scholar] [CrossRef]
- Operti, M.C.; Bernhardt, A.; Grimm, S.; Engel, A.; Figdor, C.G.; Tagit, O. PLGA-Based Nanomedicines Manufacturing: Technologies Overview and Challenges in Industrial Scale-Up. Int. J. Pharm. 2021, 605, 120807. [Google Scholar] [CrossRef]
- Schweiger, J.; Edelhoff, D.; Güth, J.F. 3D Printing in Digital Prosthetic Dentistry: An Overview of Recent Developments in Additive Manufacturing. J. Clin. Med. 2021, 10, 2010. [Google Scholar] [CrossRef]
- Bolla, G.; Sarma, B.; Nangia, A.K. Crystal Engineering of Pharmaceutical Cocrystals in the Discovery and Development of Improved Drugs. Chem. Rev. 2022, 122, 11514–11603. [Google Scholar] [CrossRef] [PubMed]
- Alghamdi, S.S.; John, S.; Choudhury, N.R.; Dutta, N.K. Additive Manufacturing of Polymer Materials: Progress, Promise and Challenges. Polymers 2021, 13, 753. [Google Scholar] [CrossRef] [PubMed]
- Salmi, M. Additive Manufacturing Processes in Medical Applications. Materials 2021, 14, 191. [Google Scholar] [CrossRef]
- Xu, X.; Awad, A.; Robles-Martinez, P.; Gaisford, S.; Goyanes, A.; Basit, A.W. Vat Photopolymerization 3D Printing for Advanced Drug Delivery and Medical Device Applications. J. Control. Release 2021, 329, 743–757. [Google Scholar] [CrossRef] [PubMed]
- Ashima, R.; Haleem, A.; Bahl, S.; Javaid, M.; Mahla, S.K.; Singh, S. Automation and Manufacturing of Smart Materials in Additive Manufacturing Technologies Using Internet of Things towards the Adoption of Industry 4.0. Mater. Today Proc. 2021, 45, 5081–5088. [Google Scholar] [CrossRef]
- Mamidi, H.K.; Mishra, S.M.; Rohera, B.D. Determination of Maximum Flowable Liquid-Loading Potential of Neusilin® US2 and Investigation of Compressibility and Compactibility of Its Liquisolid Blends with PEG (400). J. Drug Deliv. Sci. Technol. 2019, 54, 101285. [Google Scholar] [CrossRef]
- Nazir, A.; Gokcekaya, O.; Billah, K.M.M.; Ertugrul, O.; Jiang, J.; Sun, J.; Hussain, S. Multi-Material Additive Manufacturing: A Systematic Review of Design, Properties, Applications, Challenges, and 3D Printing of Materials and Cellular Metamaterials. Mater. Des. 2023, 226, 111661. [Google Scholar] [CrossRef]
- Zhang, F.; Zhu, L.; Li, Z.; Wang, S.; Shi, J.; Tang, W.; Li, N.; Yang, J. The Recent Development of Vat Photopolymerization: A Review. Addit. Manuf. 2021, 48, 102423. [Google Scholar] [CrossRef]
- Javaid, M.; Haleem, A.; Singh, R.P.; Suman, R.; Rab, S. Role of Additive Manufacturing Applications towards Environmental Sustainability. Adv. Ind. Eng. Polym. Res. 2021, 4, 312–322. [Google Scholar] [CrossRef]
- Mamidi, H.K.; Rohera, B.D. Material-Sparing Approach Using Differential Scanning Calorimeter and Response Surface Methodology for Process Optimization of Hot-Melt Extrusion. J. Pharm. Sci. 2021, 110, 3838–3850. [Google Scholar] [CrossRef]
- Tambe, S.; Jain, D.; Meruva, S.K.; Rongala, G.; Juluri, A.; Nihalani, G.; Mamidi, H.K.; Nukala, P.K.; Bolla, P.K. Recent Advances in Amorphous Solid Dispersions: Preformulation, Formulation Strategies, Technological Advancements and Characterization. Pharmaceutics 2022, 14, 2203. [Google Scholar] [CrossRef] [PubMed]
- Ilyas, R.A.; Sapuan, S.M.; Harussani, M.M.; Hakimi, M.Y.A.Y.; Haziq, M.Z.M.; Atikah, M.S.N.; Asyraf, M.R.M.; Ishak, M.R.; Razman, M.R.; Nurazzi, N.M.; et al. Polylactic Acid (PLA) Biocomposite: Processing, Additive Manufacturing and Advanced Applications. Polymers 2021, 13, 1326. [Google Scholar] [CrossRef]
- Bhujbal, S.V.; Mitra, B.; Jain, U.; Gong, Y.; Agrawal, A.; Karki, S.; Taylor, L.S.; Kumar, S.; Zhou, Q. Pharmaceutical Amorphous Solid Dispersion: A Review of Manufacturing Strategies. Acta Pharm. Sin. B 2021, 11, 2505–2536. [Google Scholar] [CrossRef] [PubMed]
- Ryan, D.; Harris, E.; O’Connor, G.M. Explainable Machine Learning for the Regulatory Environment: A Case Study in Micro-Droplet Printing. Addit. Manuf. 2024, 88, 104237. [Google Scholar] [CrossRef]
- Zhu, J.; Zhou, H.; Wang, C.; Zhou, L.; Yuan, S.; Zhang, W. A Review of Topology Optimization for Additive Manufacturing: Status and Challenges. Chin. J. Aeronaut. 2021, 34, 91–110. [Google Scholar] [CrossRef]
- Treffer, D.; Wahl, P.; Markl, D.; Koscher, G.; Roblegg, E.; Khinast, J.G. Hot Melt Extrusion as a Continuous Pharmaceutical Manufacturing Process. In Melt Extrusion; Springer: New York, NY, USA, 2013; Volume 1, pp. 363–396. ISBN 978-1-4614-8432-5. [Google Scholar]
- Spoerk, M.; Koutsamanis, I.; Kottlan, A.; Makert, C.; Piller, M.; Rajkovaca, M.; Paudel, A.; Khinast, J. Continuous Processing of Micropellets via Hot-Melt Extrusion. AAPS PharmSciTech 2022, 23, 264. [Google Scholar] [CrossRef]
- Chivate, A.; Garkal, A.; Dhas, N.; Mehta, T. Three Dimensional Printing by Hot-Melt Extrusion; New Era for Development of Personalized Medicines and Continuous Manufacturing of Pharmaceuticals. Int. J. Pharm. Investig. 2020, 10, 233. [Google Scholar] [CrossRef]
- Maniruzzaman, M. Pharmaceutical Applications of Hot-Melt Extrusion: Continuous Manufacturing, Twin-Screw Granulations, and 3D Printing. Pharmaceutics 2019, 11, 218. [Google Scholar] [CrossRef] [PubMed]
- Genina, N.; Hadi, B.; Löbmann, K. Hot Melt Extrusion as Solvent-Free Technique for a Continuous Manufacturing of Drug-Loaded Mesoporous Silica. J. Pharm. Sci. 2018, 107, 149–155. [Google Scholar] [CrossRef]
- Thiry, J.; Krier, F.; Ratwatte, S.; Thomassin, J.M.; Jerome, C.; Evrard, B. Hot-Melt Extrusion as a Continuous Manufacturing Process to Form Ternary Cyclodextrin Inclusion Complexes. Eur. J. Pharm. Sci. 2017, 96, 590–597. [Google Scholar] [CrossRef]
- Patil, H.; Kulkarni, V.; Majumdar, S.; Repka, M.A. Continuous Manufacturing of Solid Lipid Nanoparticles by Hot Melt Extrusion. Int. J. Pharm. 2014, 471, 153–156. [Google Scholar] [CrossRef] [PubMed]
- Melocchi, A.; Loreti, G.; del Curto, M.D.; Maroni, A.; Gazzaniga, A.; Zema, L. Evaluation of Hot-Melt Extrusion and Injection Molding for Continuous Manufacturing of Immediate-Release Tablets. J. Pharm. Sci. 2015, 104, 1971–1980. [Google Scholar] [CrossRef] [PubMed]
- Maniruzzaman, M.; Nokhodchi, A. Continuous Manufacturing via Hot-Melt Extrusion and Scale up: Regulatory Matters. Drug Discov. Today 2017, 22, 340–351. [Google Scholar] [CrossRef] [PubMed]
- Rantanen, J. Quality by Design (QbD) Approach for Individualized Products Based on Additive Manufacturing. In 3D & 4D Printing Methods for Pharmaceutical Manufacturing and Personalised Drug Delivery; Springer: Cham, Switzerland, 2023; pp. 113–129. ISBN 978-3-031-34119-9. [Google Scholar]
- Ansari, M.T.; Alahmed, T.A.A.; Sami, F. Quality by Design (QbD) Concept for Formulation of Oral Formulations for Tablets. In Introduction to Quality by Design (QbD); Springer: Singapore, 2024; pp. 161–184. ISBN 978-981-99-8034-5. [Google Scholar]
- Pesode, P.; Barve, S. Additive Manufacturing of Magnesium Alloys and Its Biocompatibility. Bioprinting 2023, 36, e00318. [Google Scholar] [CrossRef]
- Dedeloudi, A.; Weaver, E.; Lamprou, D.A. Machine Learning in Additive Manufacturing & Microfluidics for Smarter and Safer Drug Delivery Systems. Int. J. Pharm. 2023, 636, 122818. [Google Scholar] [CrossRef] [PubMed]
- Ghante, M.; Dargude, S.; Zambre, V.; Sawant, S. Six-Sigma Model in Pharma Industry: Part—II. In Modern Aspects of Pharmaceutical Quality Assurance; Springer Nature: Singapore, 2024; pp. 21–50. [Google Scholar]
- AL-Jawhari, I.F.H. Medical Additive Manufacturing in Pharmacy. In Medical Additive Manufacturing: Concepts and Fundamentals; Elsevier: Amsterdam, The Netherlands, 2024; pp. 525–536. ISBN 9780323953832. [Google Scholar]
- Jain, N.; Triveni; Kaith, A.; Sinha, A.; Mathur, T.; Kaul, S.; Pandey, M.; Nagaich, U. QbD and Artificial Intelligence in Nanoparticulate Drug Delivery Systems: Recent Advances. In Computational Drug Delivery: Molecular Simulation for Pharmaceutical Formulation; De Gruyter: Berlin, Germany, 2024; Volume 2, pp. 163–182. ISBN 9783111208671. [Google Scholar]
- Prasad, E.; Robertson, J.; Halbert, G.W. An Additive Manufacturing MicroFactory: Overcoming Brittle Material Failure and Improving Product Performance through Tablet Micro-Structure Control for an Immediate Release Dose Form. Polymers 2024, 16, 2566. [Google Scholar] [CrossRef]
- Yu, Q.; Zhang, M.; Bhandari, B.; Li, J. Future Perspective of Additive Manufacturing of Food for Children. Trends Food Sci. Technol. 2023, 136, 120–134. [Google Scholar] [CrossRef]
- Ghidini, T.; Grasso, M.; Gumpinger, J.; Makaya, A.; Colosimo, B.M. Additive Manufacturing in the New Space Economy: Current Achievements and Future Perspectives. Prog. Aerosp. Sci. 2023, 142, 100959. [Google Scholar] [CrossRef]
- Velu, R.; Tulasi, R.; Ramachandran, M.K. Environmental Impact, Challenges for Industrial Applications and Future Perspectives of Additive Manufacturing. In Nanotechnology-Based Additive Manufacturing: Product Design, Properties, and Applications; John Wiley & Sons, Ltd.: Hoboken, NJ, USA, 2022; Volume 2, pp. 691–709. ISBN 9783527835478. [Google Scholar]
- Zhou, Q.; Su, X.; Wu, J.; Zhang, X.; Su, R.; Ma, L.; Sun, Q.; He, R. Additive Manufacturing of Bioceramic Implants for Restoration Bone Engineering: Technologies, Advances, and Future Perspectives. ACS Biomater. Sci. Eng. 2023, 9, 1164–1189. [Google Scholar] [CrossRef] [PubMed]
- Aliyu, A.A.A.; Panwisawas, C.; Shinjo, J.; Puncreobutr, C.; Reed, R.C.; Poungsiri, K.; Lohwongwatana, B. Laser-Based Additive Manufacturing of Bulk Metallic Glasses: Recent Advances and Future Perspectives for Biomedical Applications. J. Mater. Res. Technol. 2023, 23, 2956–2990. [Google Scholar] [CrossRef]
- Peng, B.; Xu, H.; Song, F.; Wen, P.; Tian, Y.; Zheng, Y. Additive Manufacturing of Porous Magnesium Alloys for Biodegradable Orthopedic Implants: Process, Design, and Modification. J. Mater. Sci. Technol. 2024, 182, 79–110. [Google Scholar] [CrossRef]
- Jiang, J. A Survey of Machine Learning in Additive Manufacturing Technologies. Int. J. Comput. Integr. Manuf. 2023, 36, 1258–1280. [Google Scholar] [CrossRef]
- Kouhi, M.; Araújo, I.J.d.S.; Asa’ad, F.; Zeenat, L.; Bojedla, S.S.R.; Pati, F.; Zolfagharian, A.; Watts, D.C.; Bottino, M.C.; Bodaghi, M. Recent Advances in Additive Manufacturing of Patient-Specific Devices for Dental and Maxillofacial Rehabilitation. Dent. Mater. 2024, 40, 700–715. [Google Scholar] [CrossRef]
- Rahman, M.A.; Saleh, T.; Jahan, M.P.; McGarry, C.; Chaudhari, A.; Huang, R.; Tauhiduzzaman, M.; Ahmed, A.; Mahmud, A.A.; Bhuiyan, M.S.; et al. Review of Intelligence for Additive and Subtractive Manufacturing: Current Status and Future Prospects. Micromachines 2023, 14, 508. [Google Scholar] [CrossRef]
- Karyappa, R.; Zhang, D.; Zhu, Q.; Ji, R.; Suwardi, A.; Liu, H. Newtonian Liquid-Assisted Material Extrusion 3D Printing: Progress, Challenges and Future Perspectives. Addit. Manuf. 2024, 79, 103903. [Google Scholar] [CrossRef]
| Process | Pros | Cons |
|---|---|---|
| HME |
|
|
| FDM |
|
|
| PAM |
|
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nyavanandi, D.; Mandati, P.; Vidiyala, N.; Parupathi, P.; Kolimi, P.; Mamidi, H.K. Enhancing Patient-Centric Drug Development: Coupling Hot Melt Extrusion with Fused Deposition Modeling and Pressure-Assisted Microsyringe Additive Manufacturing Platforms with Quality by Design. Pharmaceutics 2025, 17, 14. https://doi.org/10.3390/pharmaceutics17010014
Nyavanandi D, Mandati P, Vidiyala N, Parupathi P, Kolimi P, Mamidi HK. Enhancing Patient-Centric Drug Development: Coupling Hot Melt Extrusion with Fused Deposition Modeling and Pressure-Assisted Microsyringe Additive Manufacturing Platforms with Quality by Design. Pharmaceutics. 2025; 17(1):14. https://doi.org/10.3390/pharmaceutics17010014
Chicago/Turabian StyleNyavanandi, Dinesh, Preethi Mandati, Nithin Vidiyala, Prashanth Parupathi, Praveen Kolimi, and Hemanth Kumar Mamidi. 2025. "Enhancing Patient-Centric Drug Development: Coupling Hot Melt Extrusion with Fused Deposition Modeling and Pressure-Assisted Microsyringe Additive Manufacturing Platforms with Quality by Design" Pharmaceutics 17, no. 1: 14. https://doi.org/10.3390/pharmaceutics17010014
APA StyleNyavanandi, D., Mandati, P., Vidiyala, N., Parupathi, P., Kolimi, P., & Mamidi, H. K. (2025). Enhancing Patient-Centric Drug Development: Coupling Hot Melt Extrusion with Fused Deposition Modeling and Pressure-Assisted Microsyringe Additive Manufacturing Platforms with Quality by Design. Pharmaceutics, 17(1), 14. https://doi.org/10.3390/pharmaceutics17010014






