Investigation of Novel Aronia Bioactive Fraction-Alginic Acid Nanocomplex on the Enhanced Modulation of Neuroinflammation and Inhibition of Aβ Aggregation
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Preparation and Characterization of AANCP
2.2.1. Preparation of ABF and AANCP
2.2.2. Physical Characterization of AANCP
2.3. Release Characteristics of AANCP
2.4. Chemical Stability of ABFand AANCP in Plasmas
2.5. Cell Culture and Viability of BV2 Microglial Cells
2.6. In Vitro Analysis of Pro-Inflammatory Cytokine Secretion from Microglia
2.7. In Vitro Analysis via qRT-PCR
2.8. In Vitro Microglial Phagocytosis Assay
2.9. Image Acquisition and Analysis
2.10. In Vitro Thioflavin T Assay for the Aggregation of Aβ42
2.11. In Vivo Animals and Oral Administration in Mice
2.12. Y-Maze Test
2.13. Passive Avoidance Test
2.14. Statistical Analysis
3. Results
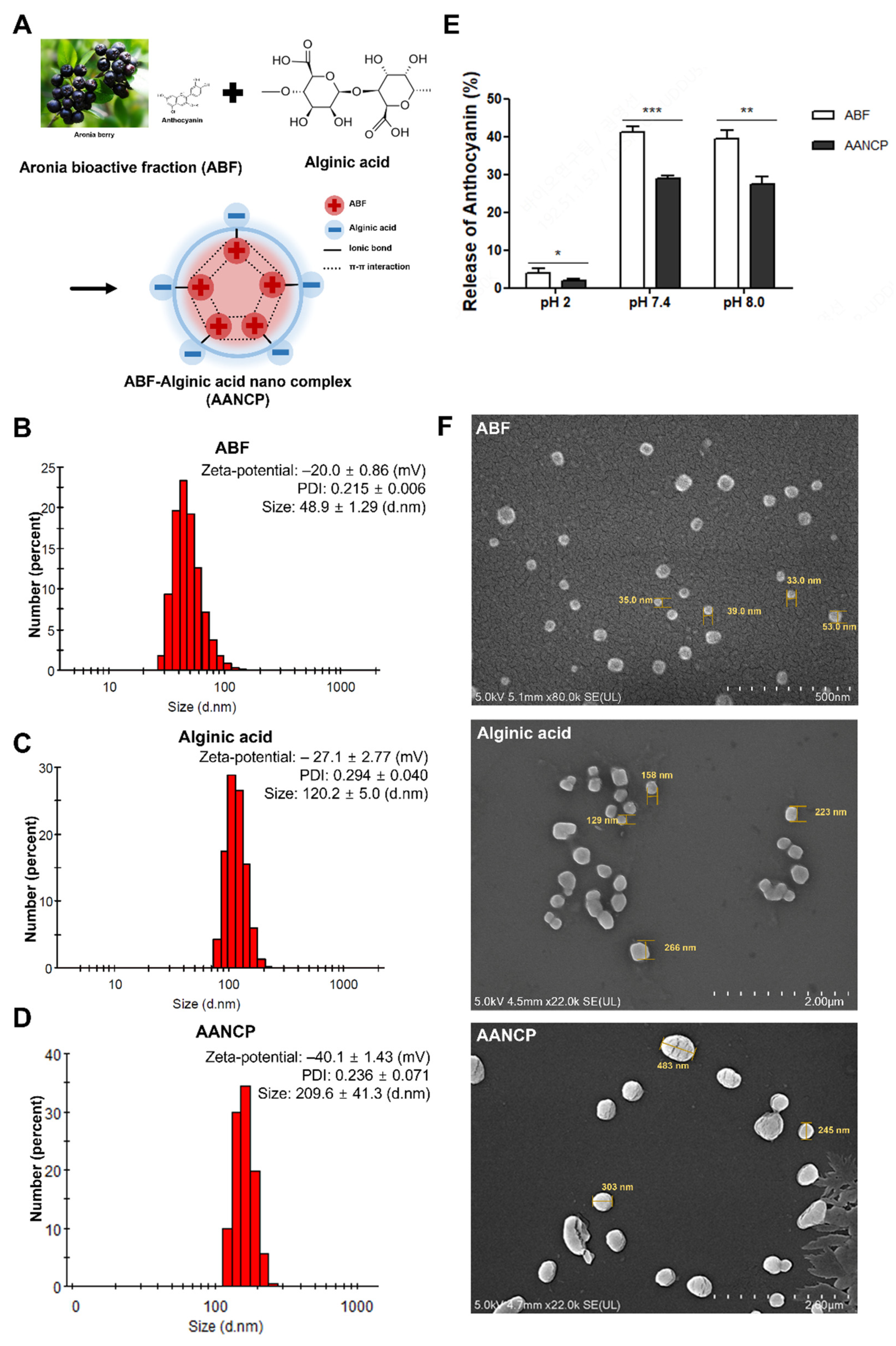
3.1. Physicochemical Characterization of AANCP
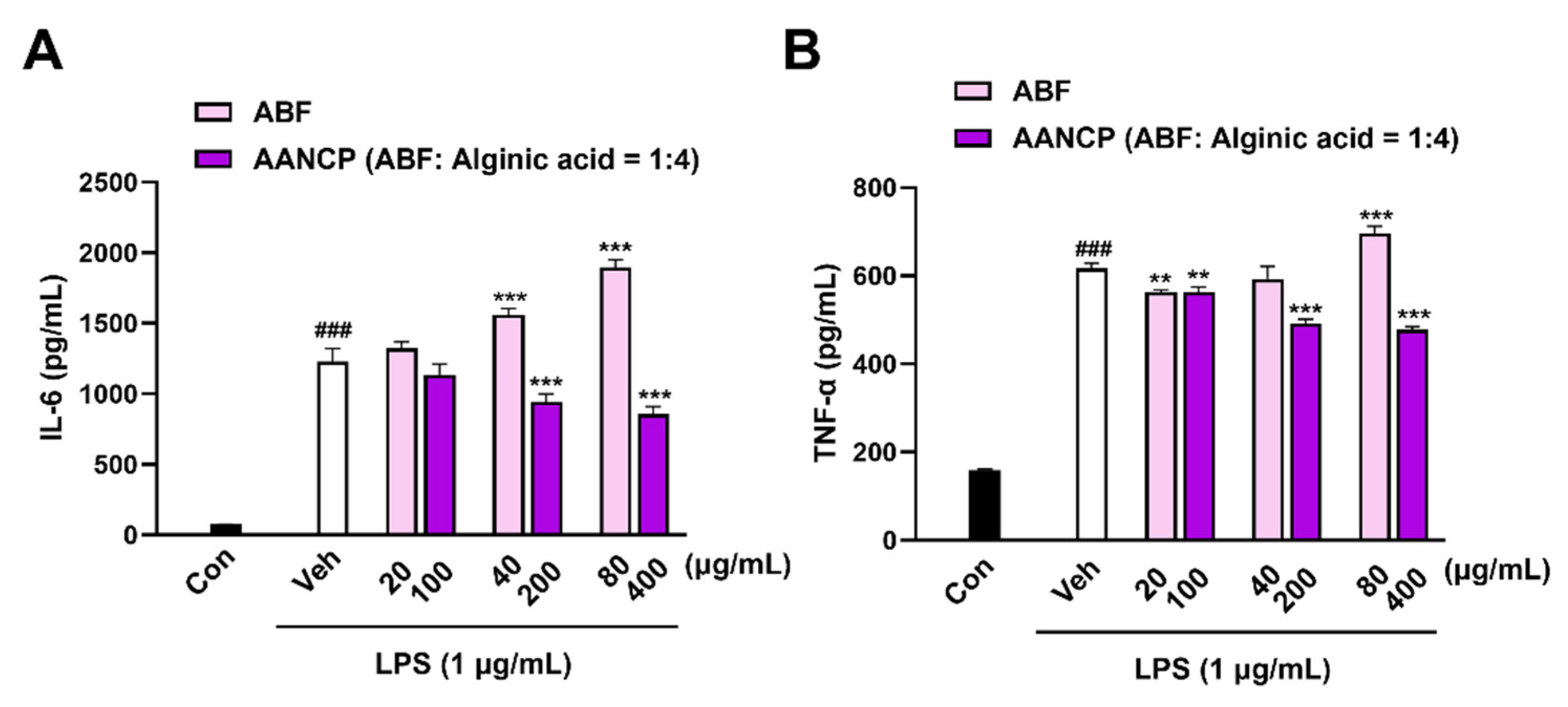
3.2. AANCP Inhibits LPS-Induced Secretion of Pro-Inflammatory Cytokines from Microglia
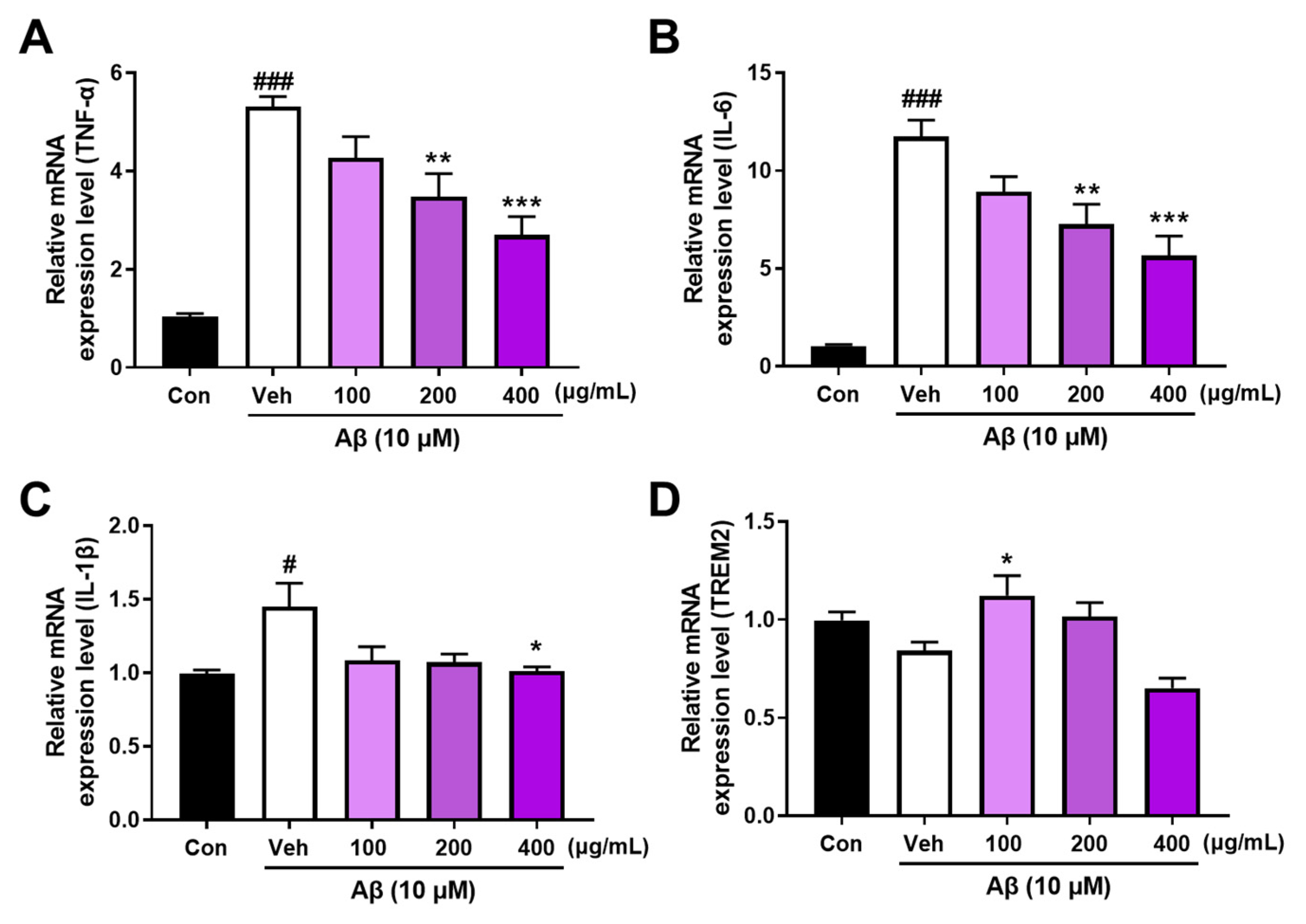
3.3. AANCP Stimulates In Vitro Microglial Polarization from M1 to M2 by Inducing the Expression of TREM2
3.4. AANCP Activates Phagocytosis in BV2 Microglial Cells
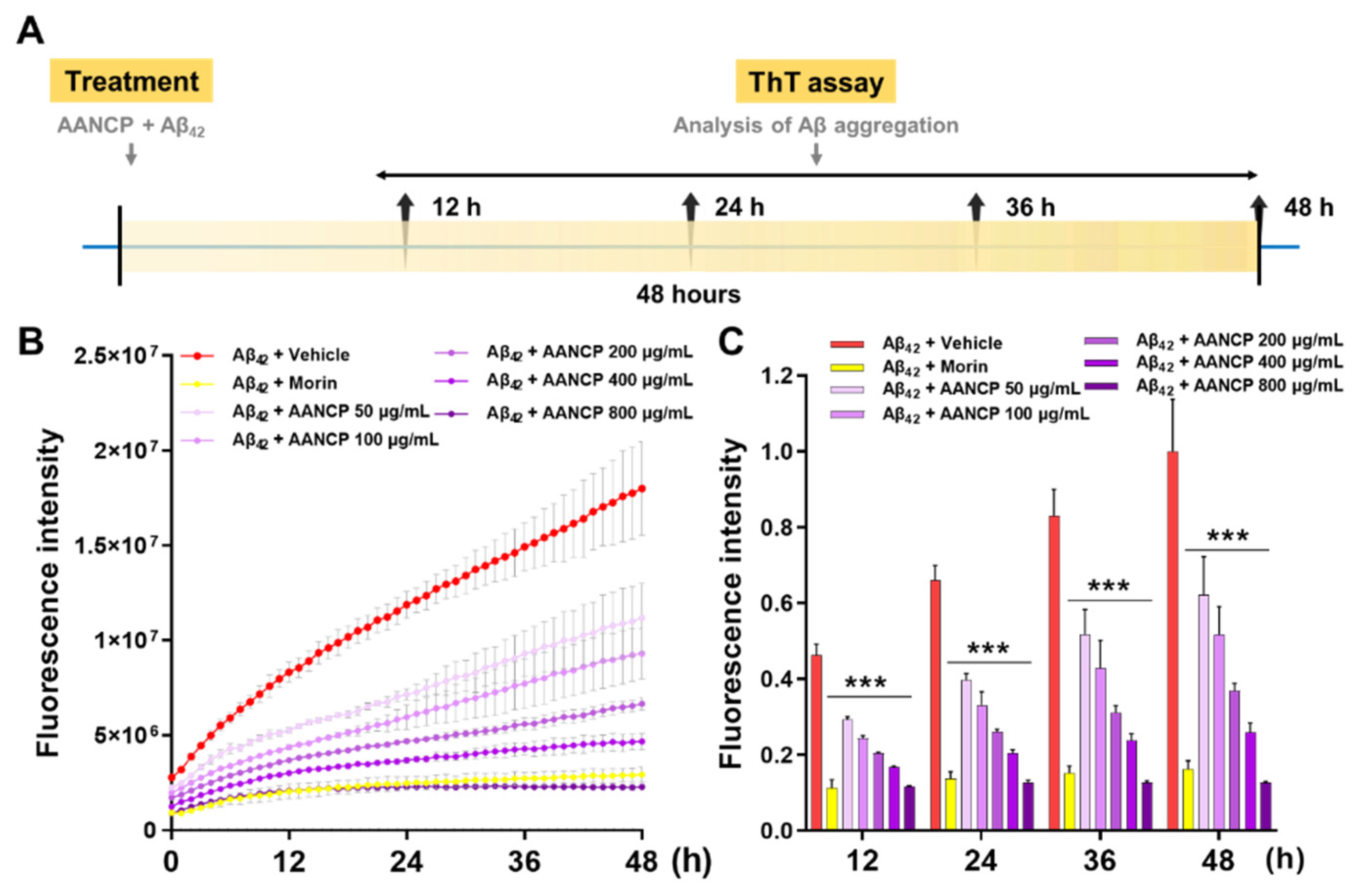
3.5. AANCP Inhibits In Vitro Aggregation of Aβ42
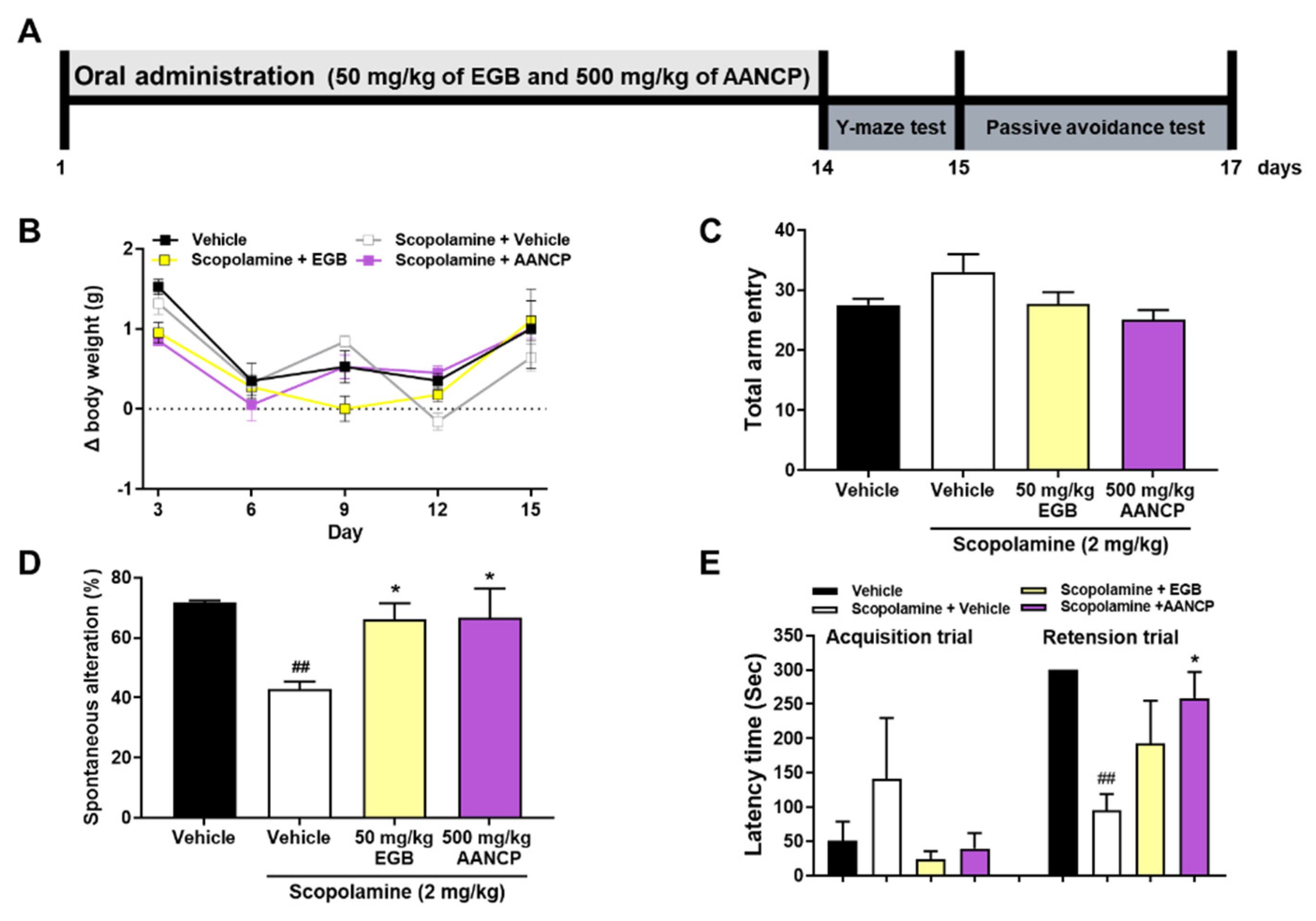
3.6. AANCP Ameliorates Cognitive Impairment in Scopolamine-Induced Mice
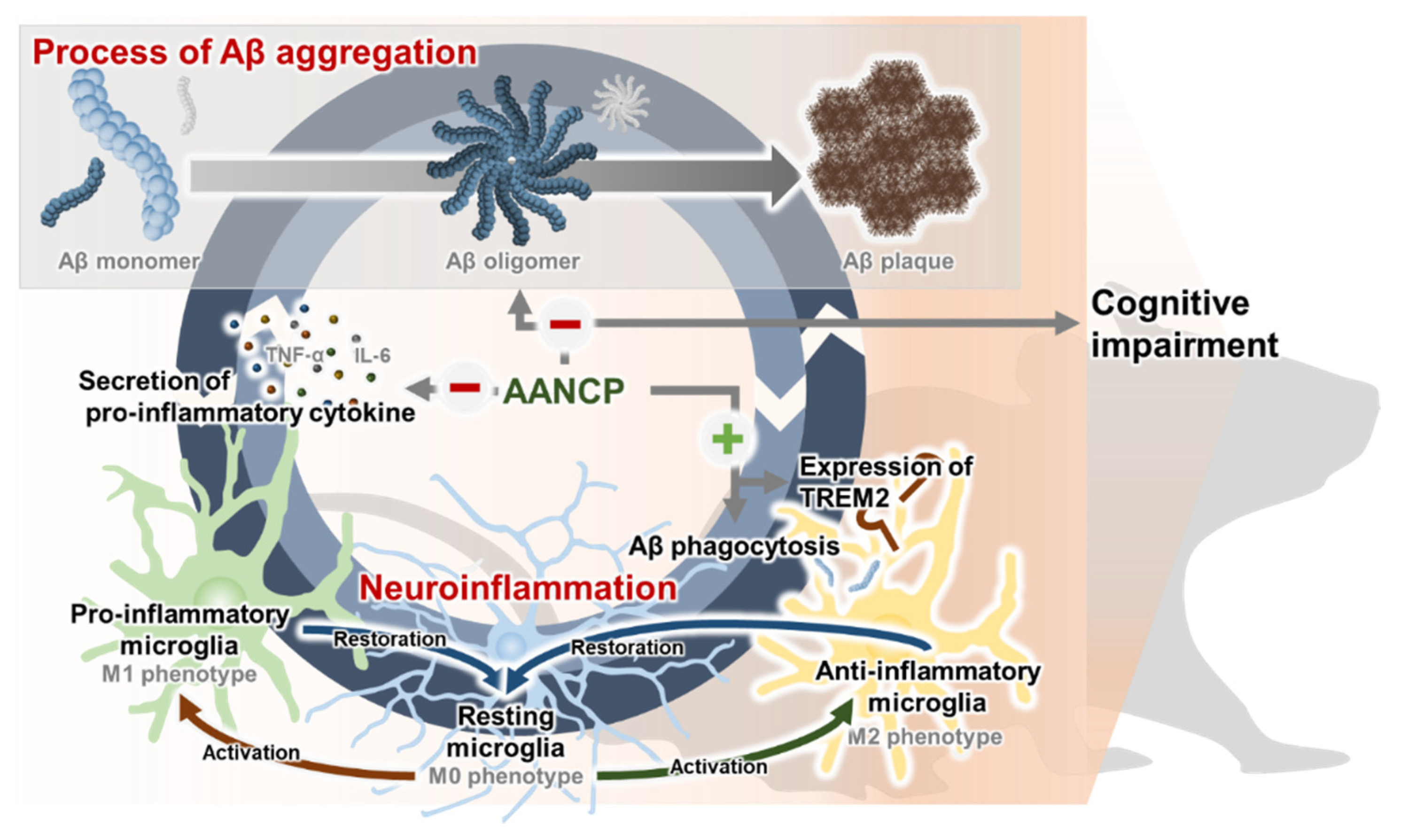
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
References
- Zhao, J.; Zhao, D.; Wang, J.; Luo, X.; Guo, R. Inflammation—Cause or Consequence of Late Onset Alzheimer’s Disease or Both? A Review of the Evidence. Eur. J. Inflamm. 2022, 20, 1721727X221095383. [Google Scholar] [CrossRef]
- Cai, Z.; Hussain, M.D.; Yan, L.-J. Microglia, Neuroinflammation, and Beta-Amyloid Protein in Alzheimer’s Disease. Int. J. Neurosci. 2014, 124, 307–321. [Google Scholar] [CrossRef]
- LaRocca, T.J.; Cavalier, A.N.; Roberts, C.M.; Lemieux, M.R.; Ramesh, P.; Garcia, M.A.; Link, C.D. Amyloid Beta Acts Synergistically as a Pro-Inflammatory Cytokine. Neurobiol. Dis. 2021, 159, 105493. [Google Scholar] [CrossRef]
- Leng, F.; Edison, P. Neuroinflammation and Microglial Activation in Alzheimer Disease: Where Do We Go from Here? Nat. Rev. Neurol. 2021, 17, 157–172. [Google Scholar] [CrossRef] [PubMed]
- Yu, Y.; Ye, R.D. Microglial Aβ Receptors in Alzheimer’s Disease. Cell. Mol. Neurobiol. 2015, 35, 71–83. [Google Scholar] [CrossRef] [PubMed]
- Chen, C.-H.; Zhou, W.; Liu, S.; Deng, Y.; Cai, F.; Tone, M.; Tone, Y.; Tong, Y.; Song, W. Increased NF-κB Signalling up-Regulates BACE1 Expression and Its Therapeutic Potential in Alzheimer’s Disease. Int. J. Neuropsychopharmacol. 2012, 15, 77–90. [Google Scholar] [CrossRef]
- Xie, L.; Lai, Y.; Lei, F.; Liu, S.; Liu, R.; Wang, T. Exploring the Association between Interleukin-1β and Its Interacting Proteins in Alzheimer’s Disease. Mol. Med. Rep. 2015, 11, 3219–3228. [Google Scholar] [CrossRef]
- Cummings, J.L.; Osse, A.M.L.; Kinney, J.W. Alzheimer’s Disease: Novel Targets and Investigational Drugs for Disease Modification. Drugs 2023, 83, 1387–1408. [Google Scholar] [CrossRef] [PubMed]
- Liu, P.; Wang, Y.; Sun, Y.; Peng, G. Neuroinflammation as a Potential Therapeutic Target in Alzheimer’s Disease. Clin. Interv. Aging 2022, 17, 665–674. [Google Scholar] [CrossRef]
- Block, M.L.; Zecca, L.; Hong, J.-S. Microglia-Mediated Neurotoxicity: Uncovering the Molecular Mechanisms. Nat. Rev. Neurosci. 2007, 8, 57–69. [Google Scholar] [CrossRef] [PubMed]
- Chen, Z.; Trapp, B.D. Microglia and Neuroprotection. J. Neurochem. 2016, 136 (Suppl. S1), 10–17. [Google Scholar] [CrossRef] [PubMed]
- Davis, B.M.; Salinas-Navarro, M.; Cordeiro, M.F.; Moons, L.; De Groef, L. Characterizing Microglia Activation: A Spatial Statistics Approach to Maximize Information Extraction. Sci. Rep. 2017, 7, 1576. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.; Chung, H.; Ngoc Mai, H.; Nam, Y.; Shin, S.J.; Park, Y.H.; Chung, M.J.; Lee, J.K.; Rhee, H.Y.; Jahng, G.-H.; et al. Low-Dose Ionizing Radiation Modulates Microglia Phenotypes in the Models of Alzheimer’s Disease. Int. J. Mol. Sci. 2020, 21, 4532. [Google Scholar] [CrossRef]
- Sanjay; Park, M.; Lee, H.-J. Roles of Fatty Acids in Microglial Polarization: Evidence from In Vitro and In Vivo Studies on Neurodegenerative Diseases. Int. J. Mol. Sci. 2022, 23, 7300. [Google Scholar] [CrossRef] [PubMed]
- Lyman, M.; Lloyd, D.G.; Ji, X.; Vizcaychipi, M.P.; Ma, D. Neuroinflammation: The Role and Consequences. Neurosci. Res. 2014, 79, 1–12. [Google Scholar] [CrossRef]
- Mishra, A.; Kim, H.J.; Shin, A.H.; Thayer, S.A. Synapse Loss Induced by Interleukin-1β Requires Pre- and Post-Synaptic Mechanisms. J. Neuroimmune Pharmacol. 2012, 7, 571–578. [Google Scholar] [CrossRef]
- Gervois, P.; Lambrichts, I. The Emerging Role of Triggering Receptor Expressed on Myeloid Cells 2 as a Target for Immunomodulation in Ischemic Stroke. Front. Immunol. 2019, 10, 1668. [Google Scholar] [CrossRef]
- Jiang, T.; Zhang, Y.-D.; Chen, Q.; Gao, Q.; Zhu, X.-C.; Zhou, J.-S.; Shi, J.-Q.; Lu, H.; Tan, L.; Yu, J.-T. TREM2 Modifies Microglial Phenotype and Provides Neuroprotection in P301S Tau Transgenic Mice. Neuropharmacology 2016, 105, 196–206. [Google Scholar] [CrossRef]
- Krasemann, S.; Madore, C.; Cialic, R.; Baufeld, C.; Calcagno, N.; El Fatimy, R.; Beckers, L.; O’Loughlin, E.; Xu, Y.; Fanek, Z.; et al. The TREM2-APOE Pathway Drives the Transcriptional Phenotype of Dysfunctional Microglia in Neurodegenerative Diseases. Immunity 2017, 47, 566–581.e9. [Google Scholar] [CrossRef]
- Zhang, Y.; Feng, S.; Nie, K.; Li, Y.; Gao, Y.; Gan, R.; Wang, L.; Li, B.; Sun, X.; Wang, L.; et al. TREM2 Modulates Microglia Phenotypes in the Neuroinflammation of Parkinson’s Disease. Biochem. Biophys. Res. Commun. 2018, 499, 797–802. [Google Scholar] [CrossRef]
- Carmona, S.; Zahs, K.; Wu, E.; Dakin, K.; Bras, J.; Guerreiro, R. The Role of TREM2 in Alzheimer’s Disease and Other Neurodegenerative Disorders. Lancet Neurol. 2018, 17, 721–730. [Google Scholar] [CrossRef]
- Fan, Y.; Ma, Y.; Huang, W.; Cheng, X.; Gao, N.; Li, G.; Tian, S. Up-Regulation of TREM2 Accelerates the Reduction of Amyloid Deposits and Promotes Neuronal Regeneration in the Hippocampus of Amyloid Beta1-42 Injected Mice. J. Chem. Neuroanat. 2019, 97, 71–79. [Google Scholar] [CrossRef]
- Dhillon, S. Aducanumab: First Approval. Drugs 2021, 81, 1437–1443. [Google Scholar] [CrossRef]
- Reardon, S. FDA Approves Alzheimer’s Drug Lecanemab amid Safety Concerns. Nature 2023, 613, 227–228. [Google Scholar] [CrossRef]
- Iwatsubo, T. Editorial: Clinical Implementation of Lecanemab: Challenges, Questions and Solutions. J. Prev. Alzheimers Dis. 2023, 10, 353–355. [Google Scholar] [CrossRef] [PubMed]
- Alexander, G.C.; Karlawish, J. The Problem of Aducanumab for the Treatment of Alzheimer Disease. Ann. Intern. Med. 2021, 174, 1303–1304. [Google Scholar] [CrossRef]
- Andrade, S.; Ramalho, M.J.; Loureiro, J.A.; Pereira, M.d.C. Natural Compounds for Alzheimer’s Disease Therapy: A Systematic Review of Preclinical and Clinical Studies. Int. J. Mol. Sci. 2019, 20, 2313. [Google Scholar] [CrossRef]
- Olajide, O.A.; Sarker, S.D. Alzheimer’s Disease: Natural Products as Inhibitors of Neuroinflammation. Inflammopharmacology 2020, 28, 1439–1455. [Google Scholar] [CrossRef] [PubMed]
- Pagano, K.; Tomaselli, S.; Molinari, H.; Ragona, L. Natural Compounds as Inhibitors of Aβ Peptide Aggregation: Chemical Requirements and Molecular Mechanisms. Front. Neurosci. 2020, 14, 619667. [Google Scholar] [CrossRef] [PubMed]
- Habtemariam, S. Natural Products in Alzheimer’s Disease Therapy: Would Old Therapeutic Approaches Fix the Broken Promise of Modern Medicines? Molecules 2019, 24, 1519. [Google Scholar] [CrossRef]
- Park, S.-Y. Potential Therapeutic Agents against Alzheimer’s Disease from Natural Sources. Arch. Pharm. Res. 2010, 33, 1589–1609. [Google Scholar] [CrossRef]
- Kulling, S.E.; Rawel, H.M. Chokeberry (Aronia Melanocarpa)—A Review on the Characteristic Components and Potential Health Effects. Planta Med. 2008, 74, 1625–1634. [Google Scholar] [CrossRef]
- Wu, X.; Gu, L.; Prior, R.L.; McKay, S. Characterization of Anthocyanins and Proanthocyanidins in Some Cultivars of Ribes, Aronia, and Sambucus and Their Antioxidant Capacity. J. Agric. Food Chem. 2004, 52, 7846–7856. [Google Scholar] [CrossRef] [PubMed]
- Appel, K.; Meiser, P.; Millán, E.; Collado, J.A.; Rose, T.; Gras, C.C.; Carle, R.; Muñoz, E. Chokeberry (Aronia Melanocarpa (Michx.) Elliot) Concentrate Inhibits NF-κB and Synergizes with Selenium to Inhibit the Release of pro-Inflammatory Mediators in Macrophages. Fitoterapia 2015, 105, 73–82. [Google Scholar] [CrossRef] [PubMed]
- Banach, M.; Wiloch, M.; Zawada, K.; Cyplik, W.; Kujawski, W. Evaluation of Antioxidant and Anti-Inflammatory Activity of Anthocyanin-Rich Water-Soluble Aronia Dry Extracts. Molecules 2020, 25, 4055. [Google Scholar] [CrossRef] [PubMed]
- Iwashima, T.; Kudome, Y.; Kishimoto, Y.; Saita, E.; Tanaka, M.; Taguchi, C.; Hirakawa, S.; Mitani, N.; Kondo, K.; Iida, K. Aronia Berry Extract Inhibits TNF-α-Induced Vascular Endothelial Inflammation through the Regulation of STAT3. Food Nutr. Res. 2019, 63, 3361. [Google Scholar] [CrossRef] [PubMed]
- Zapolska-Downar, D.; Bryk, D.; Małecki, M.; Hajdukiewicz, K.; Sitkiewicz, D. Aronia Melanocarpa Fruit Extract Exhibits Anti-Inflammatory Activity in Human Aortic Endothelial Cells. Eur. J. Nutr. 2012, 51, 563–572. [Google Scholar] [CrossRef]
- Yu, S.-Y.; Kim, M.-B.; Park, Y.-K.; Bae, M.; Kang, H.; Hu, S.; Pham, T.X.; Carpenter, R.; Lee, J.; Lee, O.-H.; et al. Anthocyanin-Rich Aronia Berry Extract Mitigates High-Fat and High-Sucrose Diet-Induced Adipose Tissue Inflammation by Inhibiting Nuclear Factor-κB Activation. J. Med. Food 2021, 24, 586–594. [Google Scholar] [CrossRef] [PubMed]
- Suresh, S.; Begum, R.F.; Singh S, A.; V, C. Anthocyanin as a Therapeutic in Alzheimer’s Disease: A Systematic Review of Preclinical Evidences. Ageing Res. Rev. 2022, 76, 101595. [Google Scholar] [CrossRef] [PubMed]
- Liu, F.; Zhao, F.; Wang, W.; Sang, J.; Jia, L.; Li, L.; Lu, F. Cyanidin-3-O-Glucoside Inhibits Aβ40 Fibrillogenesis, Disintegrates Preformed Fibrils, and Reduces Amyloid Cytotoxicity. Food Funct. 2020, 11, 2573–2587. [Google Scholar] [CrossRef] [PubMed]
- Enaru, B.; Drețcanu, G.; Pop, T.D.; Stǎnilǎ, A.; Diaconeasa, Z. Anthocyanins: Factors Affecting Their Stability and Degradation. Antioxidants 2021, 10, 1967. [Google Scholar] [CrossRef]
- Lee, J.Y.; Jo, Y.-U.; Shin, H.; Lee, J.; Chae, S.U.; Bae, S.K.; Na, K. Anthocyanin-Fucoidan Nanocomplex for Preventing Carcinogen Induced Cancer: Enhanced Absorption and Stability. Int. J. Pharm. 2020, 586, 119597. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.; Nam, Y.; Shin, S.J.; Prajapati, R.; Shin, S.M.; Kim, M.-J.; Soo Kim, H.; Leem, S.H.; Kim, T.-J.; Park, Y.H.; et al. Dual Modulators of Aggregation and Dissociation of Amyloid Beta and Tau: In Vitro, in Vivo, and in Silico Studies of Uncaria Rhynchophylla and Its Bioactive Components. Biomed. Pharmacother. 2022, 156, 113865. [Google Scholar] [CrossRef] [PubMed]
- Lian, H.; Roy, E.; Zheng, H. Microglial Phagocytosis Assay. Bio-Protocol 2016, 6, e1988. [Google Scholar] [CrossRef] [PubMed]
- Beraldo, J.C.; Nogueira, G.F.; Prata, A.S.; Grosso, C.R.F. Effect of molar weight of gelatin in the coating of alginate microparticles. Polímeros 2021, 31, e2021018. [Google Scholar] [CrossRef]
- Cooper-Driver, G.A. Contributions of Jeffrey Harborne and Co-Workers to the Study of Anthocyanins. Phytochemistry 2001, 56, 229–236. [Google Scholar] [CrossRef] [PubMed]
- Bhattacharjee, S. DLS and Zeta Potential—What They Are and What They Are Not? J. Control. Release 2016, 235, 337–351. [Google Scholar] [CrossRef] [PubMed]
- Rigolon, T.C.B.; Silva, R.R.A.; de Oliveira, T.V.; Nascimento, A.L.A.A.; de Barros, F.A.R.; Martins, E.; Campelo, P.H.; Stringheta, P.C. Exploring Anthocyanins-Polysaccharide Synergies in Microcapsule Wall Materials via Spray Drying: Interaction Characterization and Evaluation of Particle Stability. Meas. Food 2024, 13, 100126. [Google Scholar] [CrossRef]
- Olivas-Aguirre, F.J.; Rodrigo-García, J.; Martínez-Ruiz, N.D.R.; Cárdenas-Robles, A.I.; Mendoza-Díaz, S.O.; Álvarez-Parrilla, E.; González-Aguilar, G.A.; de la Rosa, L.A.; Ramos-Jiménez, A.; Wall-Medrano, A. Cyanidin-3-O-Glucoside: Physical-Chemistry, Foodomics and Health Effects. Molecules 2016, 21, 1264. [Google Scholar] [CrossRef] [PubMed]
- Sandoval-Ramírez, B.A.; Catalán, Ú.; Fernández-Castillejo, S.; Pedret, A.; Llauradó, E.; Solà, R. Cyanidin-3-Glucoside as a Possible Biomarker of Anthocyanin-Rich Berry Intake in Body Fluids of Healthy Humans: A Systematic Review of Clinical Trials. Nutr. Rev. 2020, 78, 597–610. [Google Scholar] [CrossRef] [PubMed]
- Biro, A.; Markovich, A.; Homoki, J.R.; Szőllősi, E.; Hegedűs, C.; Tarapcsák, S.; Lukács, J.; Stündl, L.; Remenyik, J. Anthocyanin-Rich Sour Cherry Extract Attenuates the Lipopolysaccharide-Induced Endothelial Inflammatory Response. Molecules 2019, 24, 3427. [Google Scholar] [CrossRef] [PubMed]
- Banji, O.J.F.; Banji, D.; Makeen, H.A.; Alqahtani, S.S.; Alshahrani, S. Neuroinflammation: The Role of Anthocyanins as Neuroprotectants. Curr. Neuropharmacol. 2022, 20, 2156–2174. [Google Scholar] [CrossRef] [PubMed]
- Khan, M.S.; Ali, T.; Kim, M.W.; Jo, M.H.; Jo, M.G.; Badshah, H.; Kim, M.O. Anthocyanins Protect against LPS-Induced Oxidative Stress-Mediated Neuroinflammation and Neurodegeneration in the Adult Mouse Cortex. Neurochem. Int. 2016, 100, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Zhang, F.; Zhong, R.; Li, S.; Fu, Z.; Cheng, C.; Cai, H.; Le, W. Acute Hypoxia Induced an Imbalanced M1/M2 Activation of Microglia through NF-κB Signaling in Alzheimer’s Disease Mice and Wild-Type Littermates. Front. Aging Neurosci. 2017, 9, 282. [Google Scholar] [CrossRef]
- Kim, S.-M.; Mun, B.-R.; Lee, S.-J.; Joh, Y.; Lee, H.-Y.; Ji, K.-Y.; Choi, H.-R.; Lee, E.-H.; Kim, E.-M.; Jang, J.-H.; et al. TREM2 Promotes Aβ Phagocytosis by Upregulating C/EBPα-Dependent CD36 Expression in Microglia. Sci. Rep. 2017, 7, 11118. [Google Scholar] [CrossRef] [PubMed]
- La Rosa, F.; Agostini, S.; Piancone, F.; Marventano, I.; Hernis, A.; Fenoglio, C.; Galimberti, D.; Scarpini, E.; Saresella, M.; Clerici, M. TREM2 Expression and Amyloid-Beta Phagocytosis in Alzheimer’s Disease. Int. J. Mol. Sci. 2023, 24, 8626. [Google Scholar] [CrossRef] [PubMed]
- Liu, W.; Taso, O.; Wang, R.; Bayram, S.; Graham, A.C.; Garcia-Reitboeck, P.; Mallach, A.; Andrews, W.D.; Piers, T.M.; Botia, J.A.; et al. Trem2 Promotes Anti-Inflammatory Responses in Microglia and Is Suppressed under pro-Inflammatory Conditions. Hum. Mol. Genet. 2020, 29, 3224–3248. [Google Scholar] [CrossRef] [PubMed]
- Sanjay; Shin, J.-H.; Park, M.; Lee, H.-J. Cyanidin-3-O-Glucoside Regulates the M1/M2 Polarization of Microglia via PPARγ and Aβ42 Phagocytosis Through TREM2 in an Alzheimer’s Disease Model. Mol. Neurobiol. 2022, 59, 5135–5148. [Google Scholar] [CrossRef]
- Cásedas, G.; Bennett, A.C.; González-Burgos, E.; Gómez-Serranillos, M.P.; López, V.; Smith, C. Polyphenol-Associated Oxidative Stress and Inflammation in a Model of LPS-Induced Inflammation in Glial Cells: Do We Know Enough for Responsible Compounding? Inflammopharmacology 2019, 27, 189–197. [Google Scholar] [CrossRef]
- Carret-Rebillat, A.-S.; Pace, C.; Gourmaud, S.; Ravasi, L.; Montagne-Stora, S.; Longueville, S.; Tible, M.; Sudol, E.; Chang, R.C.-C.; Paquet, C.; et al. Neuroinflammation and Aβ Accumulation Linked to Systemic Inflammation Are Decreased by Genetic PKR Down-Regulation. Sci. Rep. 2015, 5, 8489. [Google Scholar] [CrossRef]
- Hommet, C.; Mondon, K.; Camus, V.; Ribeiro, M.J.; Beaufils, E.; Arlicot, N.; Corcia, P.; Paccalin, M.; Minier, F.; Gosselin, T.; et al. Neuroinflammation and β Amyloid Deposition in Alzheimer’s Disease: In Vivo Quantification with Molecular Imaging. Dement. Geriatr. Cogn. Disord. 2014, 37, 1–18. [Google Scholar] [CrossRef]
- Minter, M.R.; Taylor, J.M.; Crack, P.J. The Contribution of Neuroinflammation to Amyloid Toxicity in Alzheimer’s Disease. J. Neurochem. 2016, 136, 457–474. [Google Scholar] [CrossRef]
- Zhang, W.; Xiao, D.; Mao, Q.; Xia, H. Role of Neuroinflammation in Neurodegeneration Development. Signal Transduct. Target. Ther. 2023, 8, 267. [Google Scholar] [CrossRef]
- Tarozzi, A.; Morroni, F.; Merlicco, A.; Bolondi, C.; Teti, G.; Falconi, M.; Cantelli-Forti, G.; Hrelia, P. Neuroprotective Effects of Cyanidin 3-O-Glucopyranoside on Amyloid Beta (25-35) Oligomer-Induced Toxicity. Neurosci. Lett. 2010, 473, 72–76. [Google Scholar] [CrossRef]
- Yamakawa, M.Y.; Uchino, K.; Watanabe, Y.; Adachi, T.; Nakanishi, M.; Ichino, H.; Hongo, K.; Mizobata, T.; Kobayashi, S.; Nakashima, K.; et al. Anthocyanin Suppresses the Toxicity of Aβ Deposits through Diversion of Molecular Forms in in Vitro and in Vivo Models of Alzheimer’s Disease. Nutr. Neurosci. 2016, 19, 32–42. [Google Scholar] [CrossRef] [PubMed]
- Jalbert, J.J.; Daiello, L.A.; Lapane, K.L. Dementia of the Alzheimer Type. Epidemiol. Rev. 2008, 30, 15–34. [Google Scholar] [CrossRef] [PubMed]
- Baek, H.; Sanjay; Park, M.; Lee, H.-J. Cyanidin-3-O-Glucoside Protects the Brain and Improves Cognitive Function in APPswe/PS1ΔE9 Transgenic Mice Model. J. Neuroinflamm. 2023, 20, 268. [Google Scholar] [CrossRef]
- Song, N.; Zhang, L.; Chen, W.; Zhu, H.; Deng, W.; Han, Y.; Guo, J.; Qin, C. Cyanidin 3-O-β-Glucopyranoside Activates Peroxisome Proliferator-Activated Receptor-γ and Alleviates Cognitive Impairment in the APP(Swe)/PS1(ΔE9) Mouse Model. Biochim. Biophys. Acta 2016, 1862, 1786–1800. [Google Scholar] [CrossRef]
- Wen, H.; Cui, H.; Tian, H.; Zhang, X.; Ma, L.; Ramassamy, C.; Li, J. Isolation of Neuroprotective Anthocyanins from Black Chokeberry (Aronia Melanocarpa) against Amyloid-β-Induced Cognitive Impairment. Foods 2020, 10, 63. [Google Scholar] [CrossRef] [PubMed]
- Feng, R.C.; Dong, Y.H.; Hong, X.L.; Su, Y.; Wu, X.V. Effects of Anthocyanin-Rich Supplementation on Cognition of the Cognitively Healthy Middle-Aged and Older Adults: A Systematic Review and Meta-Analysis of Randomized Controlled Trials. Nutr. Rev. 2023, 81, 287–303. [Google Scholar] [CrossRef]
- Kent, K.; Yousefi, M.; do Rosario, V.A.; Fitzgerald, Z.; Broyd, S.; Visentin, D.; Roodenrys, S.; Walton, K.; Charlton, K.E. Anthocyanin Intake Is Associated with Improved Memory in Older Adults with Mild Cognitive Impairment. Nutr. Res. 2022, 104, 36–43. [Google Scholar] [CrossRef]
- Kalt, W.; Blumberg, J.B.; McDonald, J.E.; Vinqvist-Tymchuk, M.R.; Fillmore, S.A.E.; Graf, B.A.; O’Leary, J.M.; Milbury, P.E. Identification of Anthocyanins in the Liver, Eye, and Brain of Blueberry-Fed Pigs. J. Agric. Food Chem. 2008, 56, 705–712. [Google Scholar] [CrossRef]
- Talavéra, S.; Felgines, C.; Texier, O.; Besson, C.; Gil-Izquierdo, A.; Lamaison, J.-L.; Rémésy, C. Anthocyanin Metabolism in Rats and Their Distribution to Digestive Area, Kidney, and Brain. J. Agric. Food Chem. 2005, 53, 3902–3908. [Google Scholar] [CrossRef]
- El Mohsen, M.A.; Marks, J.; Kuhnle, G.; Moore, K.; Debnam, E.; Kaila Srai, S.; Rice-Evans, C.; Spencer, J.P.E. Absorption, Tissue Distribution and Excretion of Pelargonidin and Its Metabolites Following Oral Administration to Rats. Br. J. Nutr. 2006, 95, 51–58. [Google Scholar] [CrossRef] [PubMed]
- Milbury, P.E.; Kalt, W. Xenobiotic Metabolism and Berry Flavonoid Transport across the Blood-Brain Barrier. J. Agric. Food Chem. 2010, 58, 3950–3956. [Google Scholar] [CrossRef] [PubMed]
- Zhong, H.; Xu, J.; Yang, M.; Hussain, M.; Liu, X.; Feng, F.; Guan, R. Protective Effect of Anthocyanins against Neurodegenerative Diseases through the Microbial-Intestinal-Brain Axis: A Critical Review. Nutrients 2023, 15, 496. [Google Scholar] [CrossRef] [PubMed]
- Guo, S.; Wang, H.; Yin, Y. Microglia Polarization From M1 to M2 in Neurodegenerative Diseases. Front. Aging Neurosci. 2022, 14, 815347. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.; Zong, S.; Cui, X.; Wang, X.; Wu, S.; Wang, L.; Liu, Y.; Lu, Z. The Effects of Microglia-Associated Neuroinflammation on Alzheimer’s Disease. Front. Immunol. 2023, 14, 1117172. [Google Scholar] [CrossRef]
- Gao, H.-M.; Jiang, J.; Wilson, B.; Zhang, W.; Hong, J.-S.; Liu, B. Microglial Activation-Mediated Delayed and Progressive Degeneration of Rat Nigral Dopaminergic Neurons: Relevance to Parkinson’s Disease. J. Neurochem. 2002, 81, 1285–1297. [Google Scholar] [CrossRef]
- Jimenez, S.; Baglietto-Vargas, D.; Caballero, C.; Moreno-Gonzalez, I.; Torres, M.; Sanchez-Varo, R.; Ruano, D.; Vizuete, M.; Gutierrez, A.; Vitorica, J. Inflammatory Response in the Hippocampus of PS1M146L/APP751SL Mouse Model of Alzheimer’s Disease: Age-Dependent Switch in the Microglial Phenotype from Alternative to Classic. J. Neurosci. 2008, 28, 11650–11661. [Google Scholar] [CrossRef] [PubMed]
- Shen, Z.; Li, X.; Bao, X.; Wang, R. Microglia-Targeted Stem Cell Therapies for Alzheimer Disease: A Preclinical Data Review. J. Neurosci. Res. 2017, 95, 2420–2429. [Google Scholar] [CrossRef] [PubMed]
- Porro, C.; Cianciulli, A.; Panaro, M.A. The Regulatory Role of IL-10 in Neurodegenerative Diseases. Biomolecules 2020, 10, 1017. [Google Scholar] [CrossRef] [PubMed]
- Rostam, H.M.; Reynolds, P.M.; Alexander, M.R.; Gadegaard, N.; Ghaemmaghami, A.M. Image Based Machine Learning for Identification of Macrophage Subsets. Sci. Rep. 2017, 7, 3521. [Google Scholar] [CrossRef] [PubMed]
- Gray, S.C.; Kinghorn, K.J.; Woodling, N.S. Shifting Equilibriums in Alzheimer’s Disease: The Complex Roles of Microglia in Neuroinflammation, Neuronal Survival and Neurogenesis. Neural Regen. Res. 2020, 15, 1208–1219. [Google Scholar] [CrossRef]
- Long, H.-Z.; Zhou, Z.-W.; Cheng, Y.; Luo, H.-Y.; Li, F.-J.; Xu, S.-G.; Gao, L.-C. The Role of Microglia in Alzheimer’s Disease From the Perspective of Immune Inflammation and Iron Metabolism. Front. Aging Neurosci. 2022, 14, 888989. [Google Scholar] [CrossRef]
- Ismail, R.; Parbo, P.; Madsen, L.S.; Hansen, A.K.; Hansen, K.V.; Schaldemose, J.L.; Kjeldsen, P.L.; Stokholm, M.G.; Gottrup, H.; Eskildsen, S.F.; et al. The Relationships between Neuroinflammation, Beta-Amyloid and Tau Deposition in Alzheimer’s Disease: A Longitudinal PET Study. J. Neuroinflamm. 2020, 17, 151. [Google Scholar] [CrossRef] [PubMed]
- Montoliu-Gaya, L.; Mulder, S.D.; Herrebout, M.A.C.; Baayen, J.C.; Villegas, S.; Veerhuis, R. Aβ-Oligomer Uptake and the Resulting Inflammatory Response in Adult Human Astrocytes Are Precluded by an Anti-Aβ Single Chain Variable Fragment in Combination with an apoE Mimetic Peptide. Mol. Cell. Neurosci. 2018, 89, 49–59. [Google Scholar] [CrossRef]
- McDonald, C.L.; Hennessy, E.; Rubio-Araiz, A.; Keogh, B.; McCormack, W.; McGuirk, P.; Reilly, M.; Lynch, M.A. Inhibiting TLR2 Activation Attenuates Amyloid Accumulation and Glial Activation in a Mouse Model of Alzheimer’s Disease. Brain Behav. Immun. 2016, 58, 191–200. [Google Scholar] [CrossRef]
- Zhou, C.; Sun, X.; Hu, Y.; Song, J.; Dong, S.; Kong, D.; Wang, Y.; Hua, X.; Han, J.; Zhou, Y.; et al. Genomic Deletion of TLR2 Induces Aggravated White Matter Damage and Deteriorated Neurobehavioral Functions in Mouse Models of Alzheimer’s Disease. Aging 2019, 11, 7257–7273. [Google Scholar] [CrossRef] [PubMed]
- Heneka, M.T.; Kummer, M.P.; Stutz, A.; Delekate, A.; Schwartz, S.; Vieira-Saecker, A.; Griep, A.; Axt, D.; Remus, A.; Tzeng, T.-C.; et al. NLRP3 Is Activated in Alzheimer’s Disease and Contributes to Pathology in APP/PS1 Mice. Nature 2013, 493, 674–678. [Google Scholar] [CrossRef]
- Novoa, C.; Salazar, P.; Cisternas, P.; Gherardelli, C.; Vera-Salazar, R.; Zolezzi, J.M.; Inestrosa, N.C. Inflammation Context in Alzheimer’s Disease, a Relationship Intricate to Define. Biol. Res. 2022, 55, 39. [Google Scholar] [CrossRef] [PubMed]
- Cummings, J.; Zhou, Y.; Lee, G.; Zhong, K.; Fonseca, J.; Cheng, F. Alzheimer’s Disease Drug Development Pipeline: 2023. Alzheimers Dement. 2023, 9, e12385. [Google Scholar] [CrossRef] [PubMed]
- MacPherson, K.P.; Sompol, P.; Kannarkat, G.T.; Chang, J.; Sniffen, L.; Wildner, M.E.; Norris, C.M.; Tansey, M.G. Peripheral Administration of the Soluble TNF Inhibitor XPro1595 Modifies Brain Immune Cell Profiles, Decreases Beta-Amyloid Plaque Load, and Rescues Impaired Long-Term Potentiation in 5xFAD Mice. Neurobiol. Dis. 2017, 102, 81–95. [Google Scholar] [CrossRef]
- Price, B.R.; Sudduth, T.L.; Weekman, E.M.; Johnson, S.; Hawthorne, D.; Woolums, A.; Wilcock, D.M. Therapeutic Trem2 Activation Ameliorates Amyloid-Beta Deposition and Improves Cognition in the 5XFAD Model of Amyloid Deposition. J. Neuroinflamm. 2020, 17, 238. [Google Scholar] [CrossRef]
- Wang, S.; Mustafa, M.; Yuede, C.M.; Salazar, S.V.; Kong, P.; Long, H.; Ward, M.; Siddiqui, O.; Paul, R.; Gilfillan, S.; et al. Anti-Human TREM2 Induces Microglia Proliferation and Reduces Pathology in an Alzheimer’s Disease Model. J. Exp. Med. 2020, 217, e20200785. [Google Scholar] [CrossRef] [PubMed]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jang, B.-K.; Shin, S.J.; Park, H.H.; Kumar, V.; Park, Y.H.; Kim, J.-Y.; Kang, H.-Y.; Park, S.; Kwon, Y.; Shin, S.-E.; et al. Investigation of Novel Aronia Bioactive Fraction-Alginic Acid Nanocomplex on the Enhanced Modulation of Neuroinflammation and Inhibition of Aβ Aggregation. Pharmaceutics 2025, 17, 13. https://doi.org/10.3390/pharmaceutics17010013
Jang B-K, Shin SJ, Park HH, Kumar V, Park YH, Kim J-Y, Kang H-Y, Park S, Kwon Y, Shin S-E, et al. Investigation of Novel Aronia Bioactive Fraction-Alginic Acid Nanocomplex on the Enhanced Modulation of Neuroinflammation and Inhibition of Aβ Aggregation. Pharmaceutics. 2025; 17(1):13. https://doi.org/10.3390/pharmaceutics17010013
Chicago/Turabian StyleJang, Bong-Keun, Soo Jung Shin, Hyun Ha Park, Vijay Kumar, Yong Ho Park, Jeom-Yong Kim, Hye-Yeon Kang, Sunyoung Park, Youngsun Kwon, Sang-Eun Shin, and et al. 2025. "Investigation of Novel Aronia Bioactive Fraction-Alginic Acid Nanocomplex on the Enhanced Modulation of Neuroinflammation and Inhibition of Aβ Aggregation" Pharmaceutics 17, no. 1: 13. https://doi.org/10.3390/pharmaceutics17010013
APA StyleJang, B.-K., Shin, S. J., Park, H. H., Kumar, V., Park, Y. H., Kim, J.-Y., Kang, H.-Y., Park, S., Kwon, Y., Shin, S.-E., Moon, M., & Lee, B.-J. (2025). Investigation of Novel Aronia Bioactive Fraction-Alginic Acid Nanocomplex on the Enhanced Modulation of Neuroinflammation and Inhibition of Aβ Aggregation. Pharmaceutics, 17(1), 13. https://doi.org/10.3390/pharmaceutics17010013





