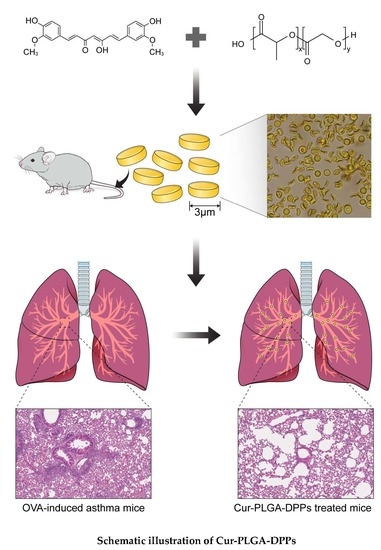
Therapeutic Efficacy of Curcumin Enhanced by Microscale Discoidal Polymeric Particles in a Murine Asthma Model
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Preparation of Curcumin-Loaded PLGA-DPPs
2.3. Characterization of Cur-PLGA-DPPs
2.4. Drug Loading and In Vitro Drug Release Test
2.5. In Vitro Stability
2.6. Murine Asthma Model
2.7. Ex Vivo Imaging
2.8. In Vivo Therapeutic Efficacy
2.9. In Vivo Toxicity
2.10. Bronchoalveolar Lavage Fluid Collection and Cell Counts
2.11. Histopathology
2.12. Statistical Analyses
3. Results and Discussion
3.1. Preparation and Characterization of Cur-PLGA-DPPs
3.1.1. Size and Zeta Potential
3.1.2. Surface Morphology
3.1.3. Loading Capacity and Release Profiles
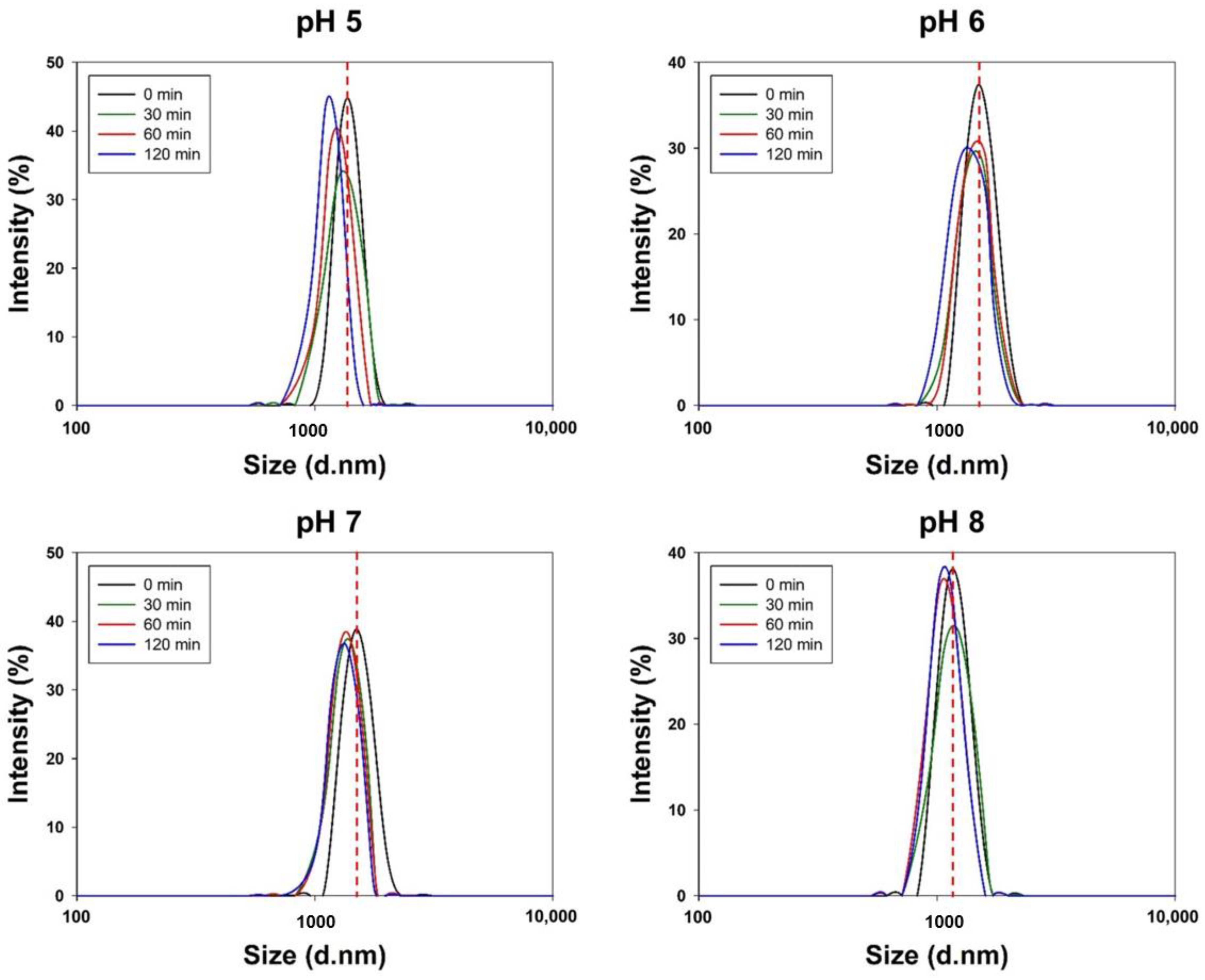
3.2. In Vitro Stability of Cur-PLGA-DPPs
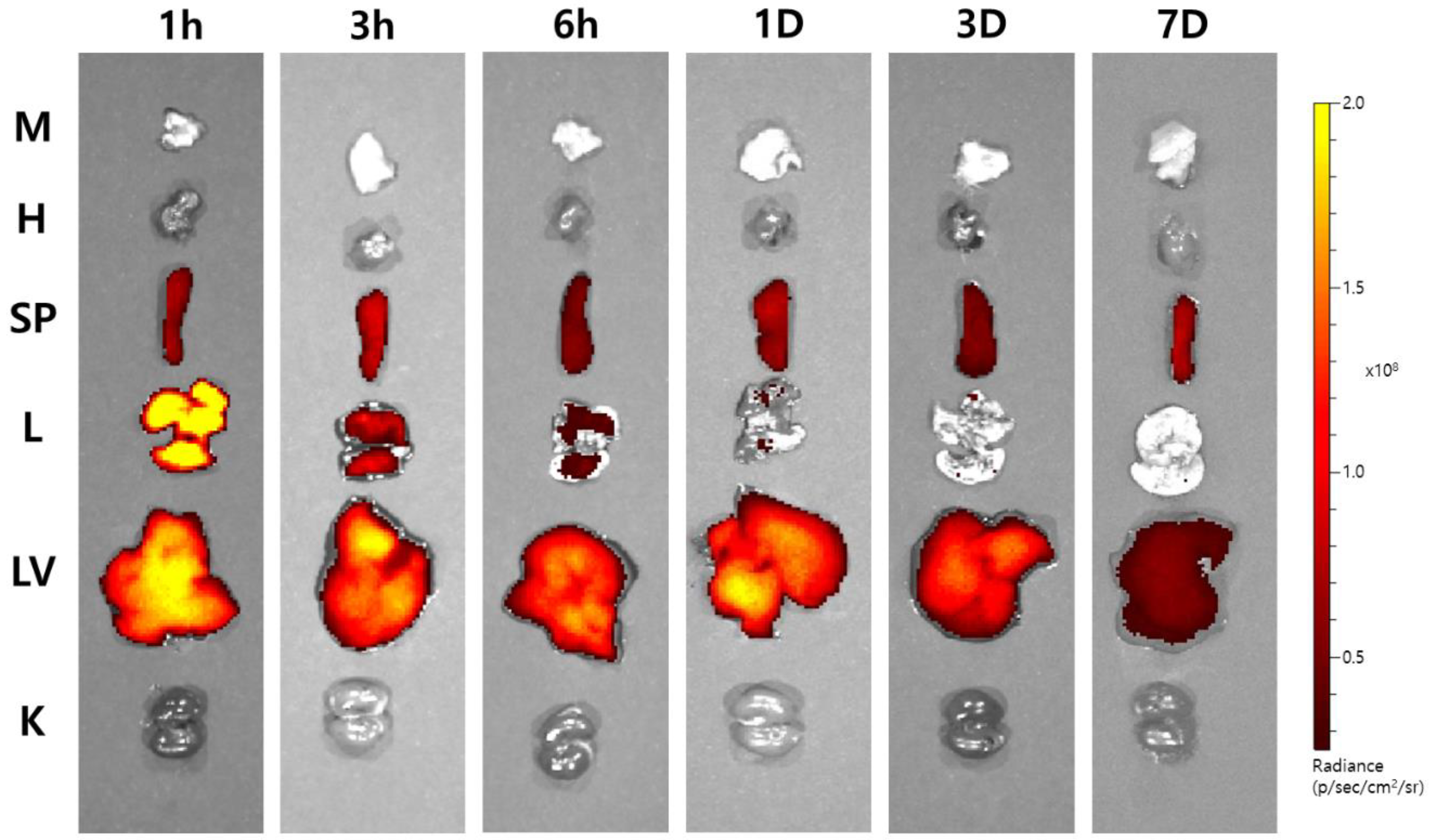
3.3. Ex Vivo Imaging of Cy7-Cur-PLGA-DPPs
3.4. In Vivo Cytotoxicity of Cur-PLGA-DPPs
3.5. In Vivo Therapeutic Efficacy of Cur-PLGA-DPPs
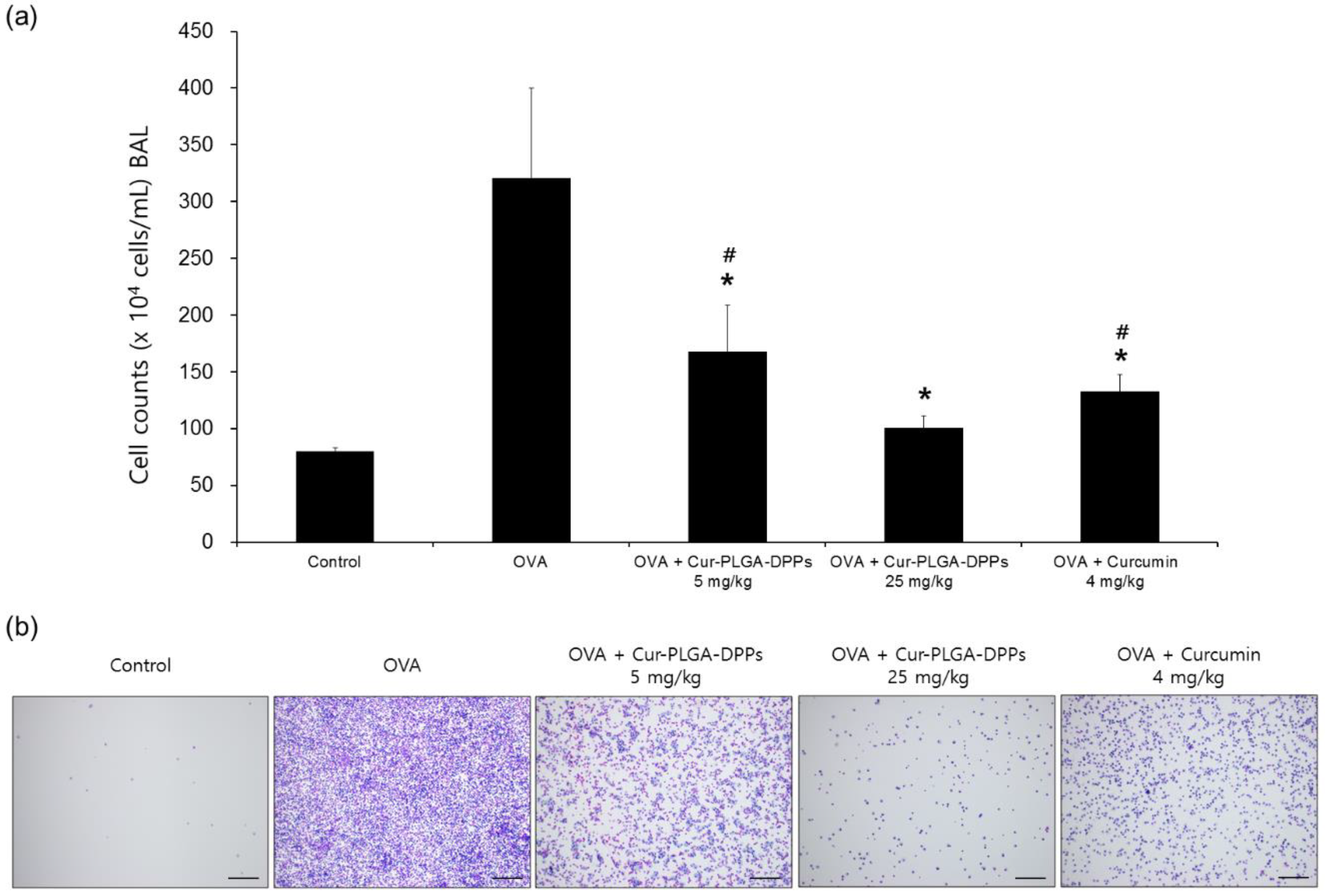
3.5.1. BALF Analysis
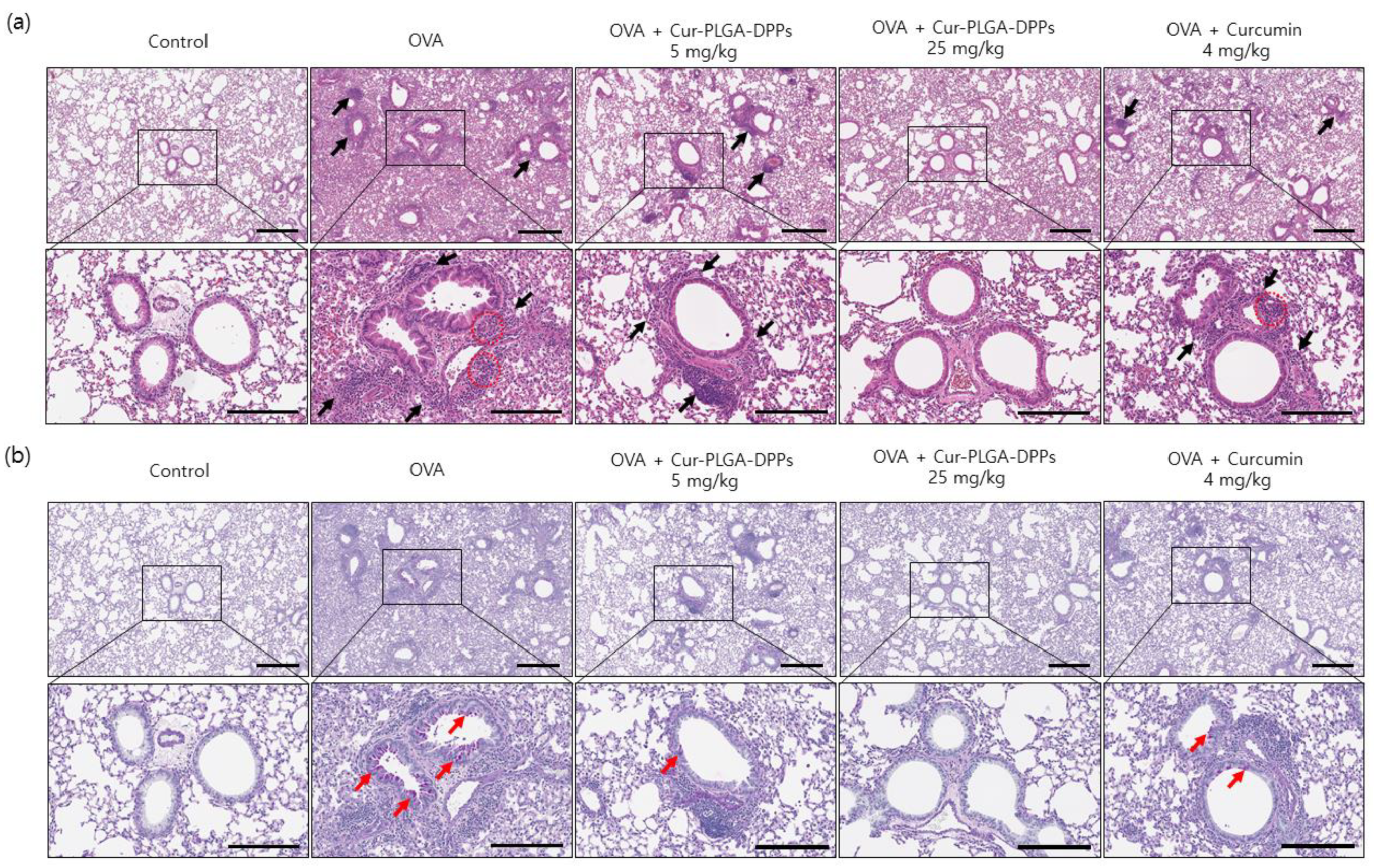
3.5.2. Histological Evaluation
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Holgate, S.T. Innate and adaptive immune responses in asthma. Nat. Med. 2012, 18, 673–683. [Google Scholar] [CrossRef] [PubMed]
- Kudo, M.; Ishigatsubo, Y.; Aoki, I. Pathology of asthma. Front. Microbiol. 2013, 4, 263. [Google Scholar] [CrossRef] [PubMed]
- Hamid, Q.; Tulic, M.K.; Liu, M.C.; Moqbel, R. Inflammatory cells in asthma: Mechanisms and implications for therapy. J. Allergy Clin. Immunol. 2003, 111, S5–S12. [Google Scholar] [CrossRef] [PubMed]
- Chen, L.; Deng, H.; Cui, H.; Fang, J.; Zuo, Z.; Deng, J.; Li, Y.; Wang, X.; Zhao, L. Inflammatory responses and inflammation-associated diseases in organs. Oncotarget 2017, 9, 7204–7218. [Google Scholar] [CrossRef]
- Rowe, B.H.; Edmonds, M.L.; Spooner, C.H.; Diner, B.; Camargo, C.A. Corticosteroid therapy for acute asthma. Respir. Med. 2004, 98, 275–284. [Google Scholar] [CrossRef]
- Marandi, Y.; Farahi, N.; Hashjin, G.S. Asthma: Beyond corticosteroid treatment. Arch. Med. Sci. 2013, 9, 521–526. [Google Scholar] [CrossRef]
- Dahl, R. Systemic side effects of inhaled corticosteroids in patients with asthma. Respir. Med. 2006, 100, 1307–1317. [Google Scholar] [CrossRef]
- Kunnumakkara, A.B.; Anand, P.; Aggarwal, B.B. Curcumin inhibits proliferation, invasion, angiogenesis and metastasis of different cancers through interaction with multiple cell signaling proteins. Cancer Lett. 2008, 269, 199–225. [Google Scholar] [CrossRef] [PubMed]
- Lelli, D.; Sahebkar, A.; Johnston, T.P.; Pedone, C. Curcumin use in pulmonary diseases: State of the art and future perspectives. Pharmacol. Res. 2017, 115, 133–148. [Google Scholar] [CrossRef]
- Kurup, V.P.; Barrios, C.S.; Raju, R.; Johnson, B.D.; Levy, M.B.; Fink, J.N. Immune response modulation by curcumin in a latex allergy model. Clin. Mol. Allergy 2007, 5, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Moon, D.O.; Kim, M.O.; Lee, H.J.; Choi, Y.H.; Park, Y.M.; Heo, M.S.; Kim, G.Y. Curcumin attenuates ovalbumin-induced airway inflammation by regulating nitric oxide. Biochem. Biophys. Res. Commun. 2008, 375, 275–279. [Google Scholar] [CrossRef] [PubMed]
- Oh, S.W.; Cha, J.Y.; Jung, J.E.; Chang, B.C.; Kwon, H.J.; Lee, B.R.; Kim, D.Y. Curcumin attenuates allergic airway inflammation and hyper-responsiveness in mice through NF-kappaB inhibition. J. Ethnopharmacol. 2011, 136, 414–421. [Google Scholar] [CrossRef] [PubMed]
- Gupta, S.C.; Patchva, S.; Aggarwal, B.B. Therapeutic roles of curcumin: Lessons learned from clinical trials. AAPS J. 2013, 15, 195–218. [Google Scholar] [CrossRef] [PubMed]
- Anand, P.; Kunnumakkara, A.B.; Newman, R.A.; Aggarwal, B.B. Bioavailability of curcumin: Problems and promises. Mol. Pharm. 2007, 4, 807–818. [Google Scholar] [CrossRef]
- Bansal, S.S.; Goel, M.; Aqil, F.; Vadhanam, M.V.; Gupta, R.C. Advanced drug delivery systems of curcumin for cancer chemoprevention. Cancer Prev. Res. 2011, 4, 1158–1171. [Google Scholar] [CrossRef]
- Yadav, V.R.; Suresh, S.; Devi, K.; Yadav, S. Effect of cyclodextrin complexation of curcumin on its solubility and antiangiogenic and anti-inflammatory activity in rat colitis model. AAPS Pharmscitech. 2009, 10, 752–762. [Google Scholar] [CrossRef]
- Teixeira, C.C.; Mendonça, L.M.; Bergamaschi, M.M.; Queiroz, R.H.; Souza, G.E.; Antunes, L.M.; Freitas, L.A. Microparticles containing curcumin solid dispersion: Stability, bioavailability and anti-inflammatory activity. AAPS Pharmscitech. 2016, 17, 252–261. [Google Scholar] [CrossRef]
- Zhang, Q.; Polyakov, N.E.; Chistyachenko, Y.S.; Khvostov, M.V.; Frolova, T.S.; Tolstikova, T.G.; Dushkin, A.V.; Su, W. Preparation of curcumin self-micelle solid dispersion with enhanced bioavailability and cytotoxic activity by mechanochemistry. Drug Deliv. 2018, 25, 198–209. [Google Scholar] [CrossRef]
- Makadia, H.K.; Siegel, S.J. Poly lactic-co-glycolic acid (PLGA) as biodegradable controlled drug delivery carrier. Polymers 2011, 3, 1377–1397. [Google Scholar] [CrossRef]
- Shaikh, J.; Ankola, D.D.; Beniwal, V.; Singh, D.; Kumar, M.N. Nanoparticle encapsulation improves oral bioavailability of curcumin by at least 9-fold when compared to curcumin administered with piperine as absorption enhancer. Eur. J. Pharm. Sci. 2009, 37, 223–230. [Google Scholar] [CrossRef]
- Shahani, K.; Swaminathan, S.K.; Freeman, D.; Blum, A.; Ma, L.; Panyam, J. Injectable sustained release microparticles of curcumin: A new concept for cancer chemoprevention. Cancer Res. 2010, 70, 4443–4452. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Y.; Wang, Y.; Ran, F.; Cui, Y.; Liu, C.; Zhao, Q.; Gao, Y.; Wang, D.; Wang, S. A comparison between sphere and rod nanoparticles regarding their in vivo biological behavior and pharmacokinetics. Sci. Rep. 2017, 7, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Zhu, X.; Vo, C.; Taylor, M.; Smith, B.R. Non-Spherical Micro- and Nanoparticles in Nanomedicine. Mater. Horiz. 2019, 6, 1094–1121. [Google Scholar] [CrossRef]
- Decuzzi, P.; Godin, B.; Tanaka, T.; Lee, S.Y.; Chiappini, C.; Liu, X.; Ferrari, M. Size and shape effects in the biodistribution of intravascularly injected particles. J. Control. Release 2010, 141, 320–327. [Google Scholar] [CrossRef] [PubMed]
- Park, J.Y.; Park, S.; Lee, T.S.; Hwang, Y.H.; Kim, J.Y.; Kang, W.J.; Key, J. Biodegradable micro-sized discoidal polymeric particles for lung-targeted delivery system. Biomaterials 2019, 218, 119331. [Google Scholar] [CrossRef] [PubMed]
- Key, J.; Palange, A.L.; Gentile, F.; Aryal, S.; Stigliano, C.; Di Mascolo, D.; De Rosa, E.; Cho, M.; Lee, Y.; Singh, J.; et al. Soft discoidal polymeric nanoconstructs resist macrophage uptake and enhance vascular targeting in tumors. ACS Nano 2015, 9, 11628–11641. [Google Scholar] [CrossRef]
- Reber, L.L.; Daubeuf, F.; Plantinga, M.; De Cauwer, L.; Gerlo, S.; Waelput, W.; Van Calenbergh, S.; Tavernier, J.; Haegeman, G.; Lambrecht, B.N.; et al. A dissociated glucocorticoid receptor modulator reduces airway hyperresponsiveness and inflammation in a mouse model of asthma. J. Immunol. 2012, 188, 3478–3487. [Google Scholar] [CrossRef]
- Sun, F.; Xiao, G.; Qu, Z. Murine bronchoalveolar lavage. Bio Protoc. 2017, 7, e2287. [Google Scholar] [CrossRef]
- Moussawi, R.N.; Patra, D. Nanoparticle self-assembled grain like curcumin conjugated ZnO: Curcumin conjugation enhances removal of perylene, fluoranthene, and chrysene by ZnO. Sci. Rep. 2016, 6, 1–13. [Google Scholar] [CrossRef]
- Holy, C.E.; Dang, S.M.; Davies, J.E.; Shoichet, M.S. In vitro degradation of a novel poly (lactide-co-glycolide) 75/25 foam. Biomaterials 1999, 20, 1177–1185. [Google Scholar] [CrossRef]
- Zolnik, B.S.; Burgess, D.J. Effect of acidic pH on PLGA microsphere degradation and release. J. Control Release 2007, 122, 338–344. [Google Scholar] [CrossRef] [PubMed]
- Meyer, K.C. Bronchoalveolar lavage as a diagnostic tool. Semin. Respir. Crit. Care. Med. 2007, 28, 546–560. [Google Scholar] [CrossRef]
- Holgate, S.T. Pathogenesis of asthma. Clin. Exp. Allergy 2008, 38, 872–897. [Google Scholar] [CrossRef] [PubMed]
- Lemoine, D.; Francois, C.; Kedzierewicz, F.; Preat, V.; Hoffman, M.; Maincent, P. Stability study of nanoparticles of poly (ɛ-caprolactone), poly (d,l-lactide) and poly (d,l-lactide-co-glycolide). Biomaterials 1996, 17, 2191–2197. [Google Scholar] [CrossRef]
- Moore, T.L.; Rodriguez-Lorenzo, L.; Hirsch, V.; Balog, S.; Urban, D.; Jud, C.; Rothen-Rutishauser, B.; Lattuada, M.; Petri-Fink, A. Nanoparticle colloidal stability in cell culture media and impact on cellular interactions. Chem. Soc. Rev. 2015, 44, 6287–6305. [Google Scholar] [CrossRef] [PubMed]
- Sikora, A.; Bartczak, D.; Geißler, D.; Kestens, V.; Roebben, G.; Ramaye, Y.; Varga, Z.; Palmai, M.; Shard, A.G.; Goenaga-Infante, H.; et al. A systematic comparison of different techniques to determine the zeta potential of silica nanoparticles in biological medium. Anal. Methods 2015, 7, 9835–9843. [Google Scholar] [CrossRef]
- Ali, N.; Teixeira, J.A.; Addali, A. A review on nanofluids: Fabrication, stability, and thermophysical properties. J. Nanomater. 2018, 2018, 33. [Google Scholar] [CrossRef]
- Park, T.G. Degradation of poly (lactic-co-glycolic acid) microspheres: Effect of copolymer composition. Biomaterials 1995, 16, 1123–1130. [Google Scholar] [CrossRef]



| Name | Mean Particle Size (μm) | ζ-Potential (mV) | Loading Content (%) |
|---|---|---|---|
| PLGA-DPPs | 2.5 ± 0.7 | −27.7 ± 4.7 | - |
| Cur-PLGA-DPPs | 2.5 ± 0.4 | −34.6 ± 4.8 | 16.3 ± 0.1 |
| Parameter | Control | OVA | OVA + Cur-PLGA-DPPs 5 mg/kg | OVA + Cur-PLGA-DPPs 25 mg/kg | OVA + Curcumin 4 mg/kg |
|---|---|---|---|---|---|
| ALP (U/L) | 362.0 ± 11.3 | 285.2 ± 36.9 | 296.8 ± 16.6 | 294.0 ± 14.3 | 317.7 ± 26.8 |
| AST (U/L) | 107.5 ± 6.36 | 125.2 ± 53.7 | 116.0 ± 27.4 | 115.3 ± 22.2 | 113.0 ± 9.9 |
| ALT (U/L) | 72.0 ± 32.5 | 57.2 ± 32.9 | 65.5 ± 34.8 | 55.8 ± 7.4 | 48.0 ± 28.3 |
| CRE (mg/dL) | 0.2 ± 0.0 | 0.3 ± 0.1 | 0.2 ± 0.1 | 0.2 ± 0.1 | 0.2 ± 0.1 |
| BUN (mg/dL) | 27.4 ± 0.1 | 26.4 ± 3.5 | 31.2 ± 3.3 | 20.3 ± 3.4 | 23.7 ± 2.8 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Park, J.Y.; Chu, G.E.; Park, S.; Park, C.; Aryal, S.; Kang, W.J.; Cho, W.G.; Key, J. Therapeutic Efficacy of Curcumin Enhanced by Microscale Discoidal Polymeric Particles in a Murine Asthma Model. Pharmaceutics 2020, 12, 739. https://doi.org/10.3390/pharmaceutics12080739
Park JY, Chu GE, Park S, Park C, Aryal S, Kang WJ, Cho WG, Key J. Therapeutic Efficacy of Curcumin Enhanced by Microscale Discoidal Polymeric Particles in a Murine Asthma Model. Pharmaceutics. 2020; 12(8):739. https://doi.org/10.3390/pharmaceutics12080739
Chicago/Turabian StylePark, Jun Young, Ga Eul Chu, Sanghyo Park, Chaewon Park, Susmita Aryal, Won Jun Kang, Won Gil Cho, and Jaehong Key. 2020. "Therapeutic Efficacy of Curcumin Enhanced by Microscale Discoidal Polymeric Particles in a Murine Asthma Model" Pharmaceutics 12, no. 8: 739. https://doi.org/10.3390/pharmaceutics12080739
APA StylePark, J. Y., Chu, G. E., Park, S., Park, C., Aryal, S., Kang, W. J., Cho, W. G., & Key, J. (2020). Therapeutic Efficacy of Curcumin Enhanced by Microscale Discoidal Polymeric Particles in a Murine Asthma Model. Pharmaceutics, 12(8), 739. https://doi.org/10.3390/pharmaceutics12080739