Preparation and Evaluation of Intraperitoneal Long-Acting Oxaliplatin-Loaded Multi-Vesicular Liposomal Depot for Colorectal Cancer Treatment
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials and Animals
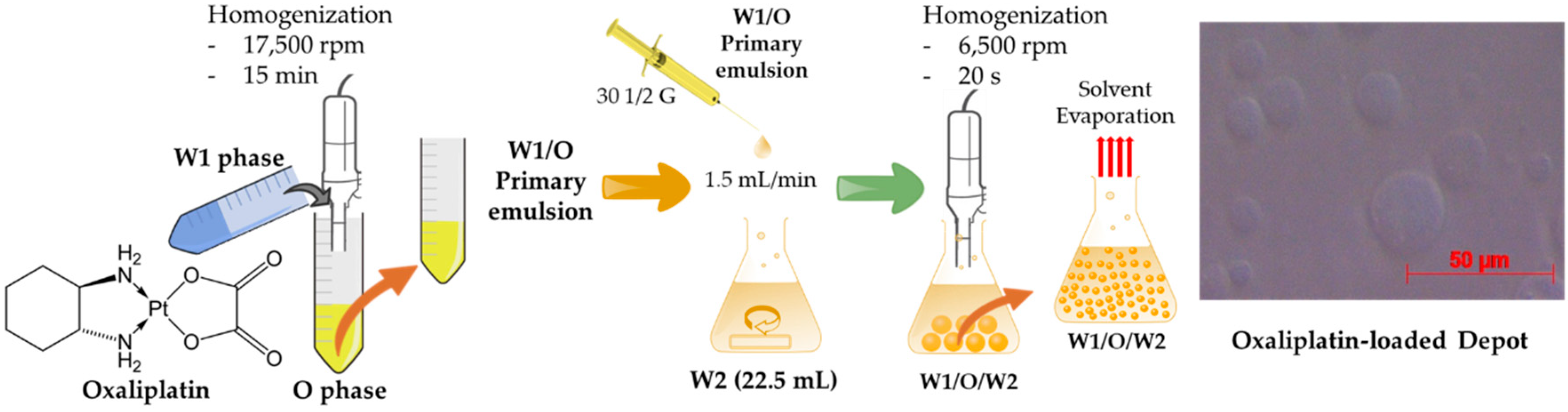
2.2. Preparation of Oxaliplatin-Loaded Liposomal Depot
2.3. Characterization of the Oxaliplatin-Loaded Liposomal Depot
2.3.1. Morphology
2.3.2. Particle Size Analysis
2.3.3. Drug EE
2.4. In Vitro Oxaliplatin Release from the Liposomal Depot
2.5. In Vivo Oxaliplatin Release in the IP Cavity of SD Rats
2.6. HPLC Analysis
2.7. Statistical Analysis
2.8. Stability
3. Results
3.1. Characterization of the Liposomal Depot
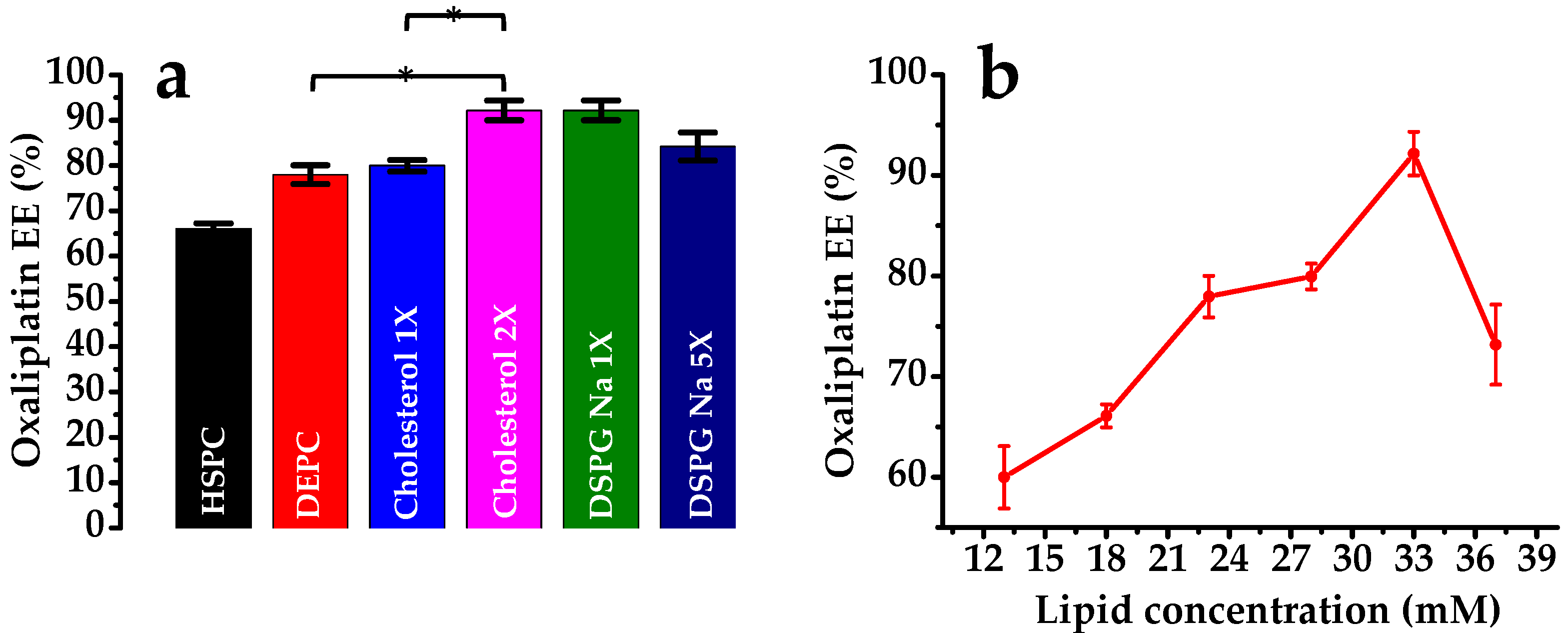
3.1.1. Effect of Lipid Composition
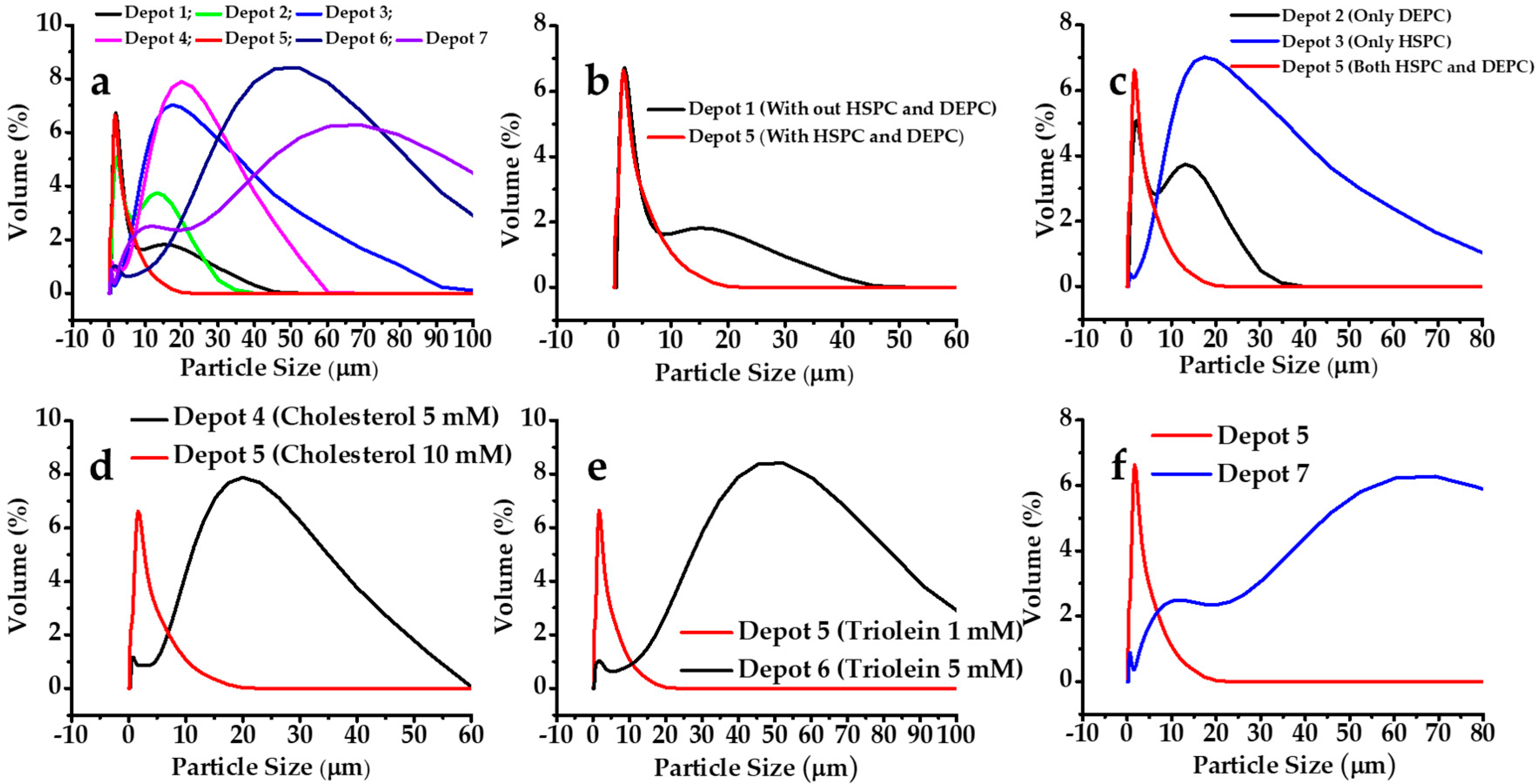
3.1.2. Effect on Particle Size
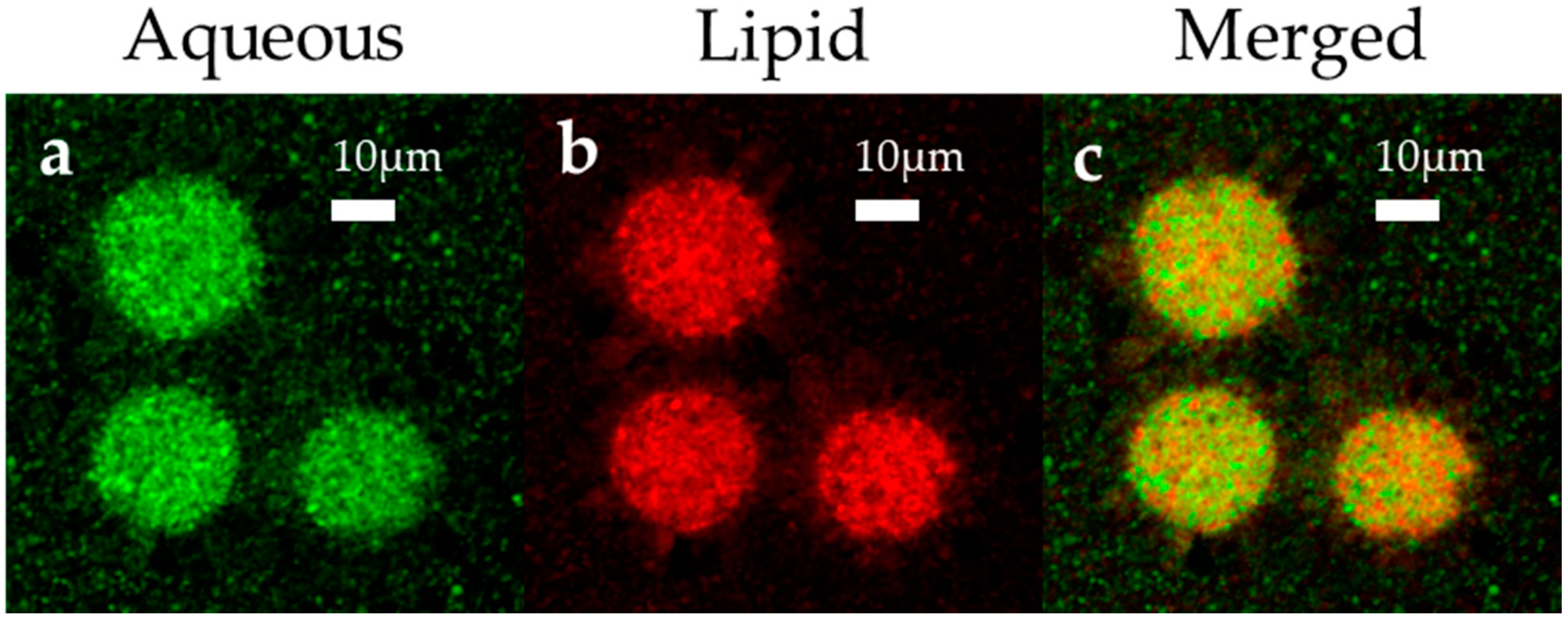
3.1.3. Morphological Analysis
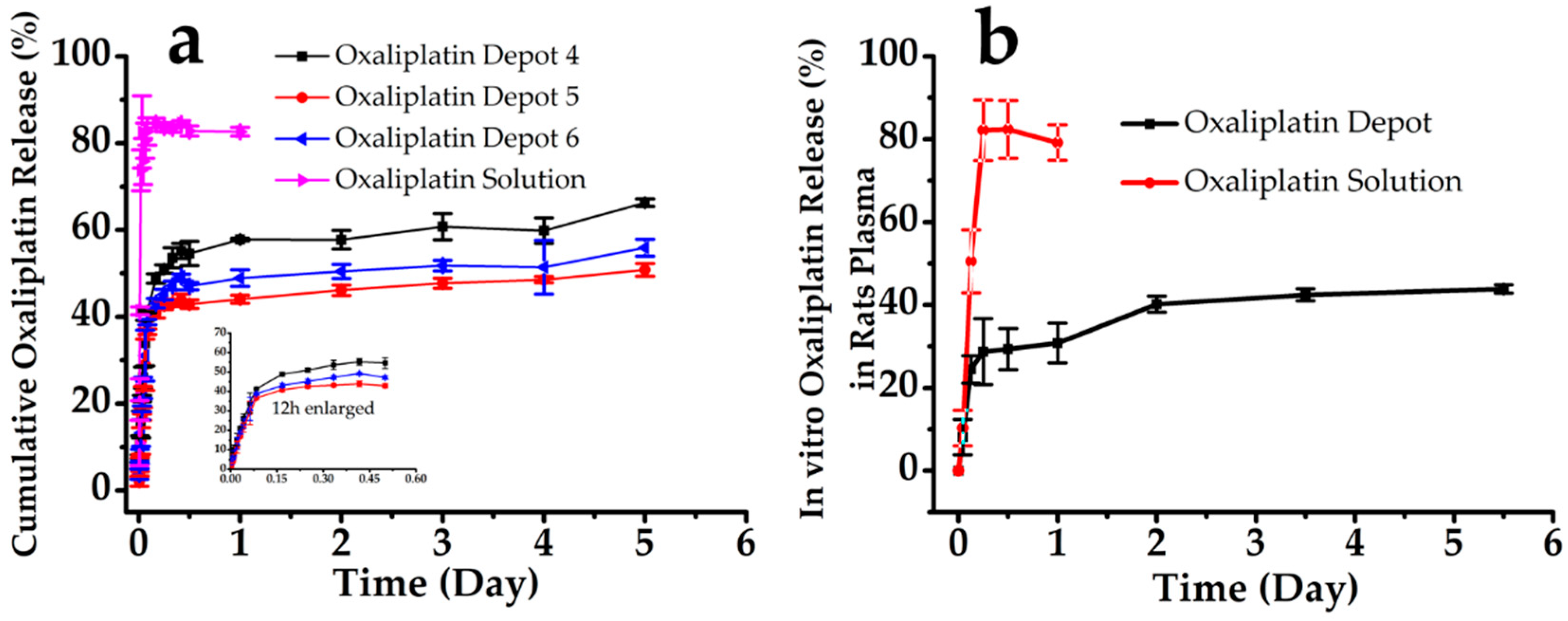
3.2. In Vitro Oxaliplatin Release
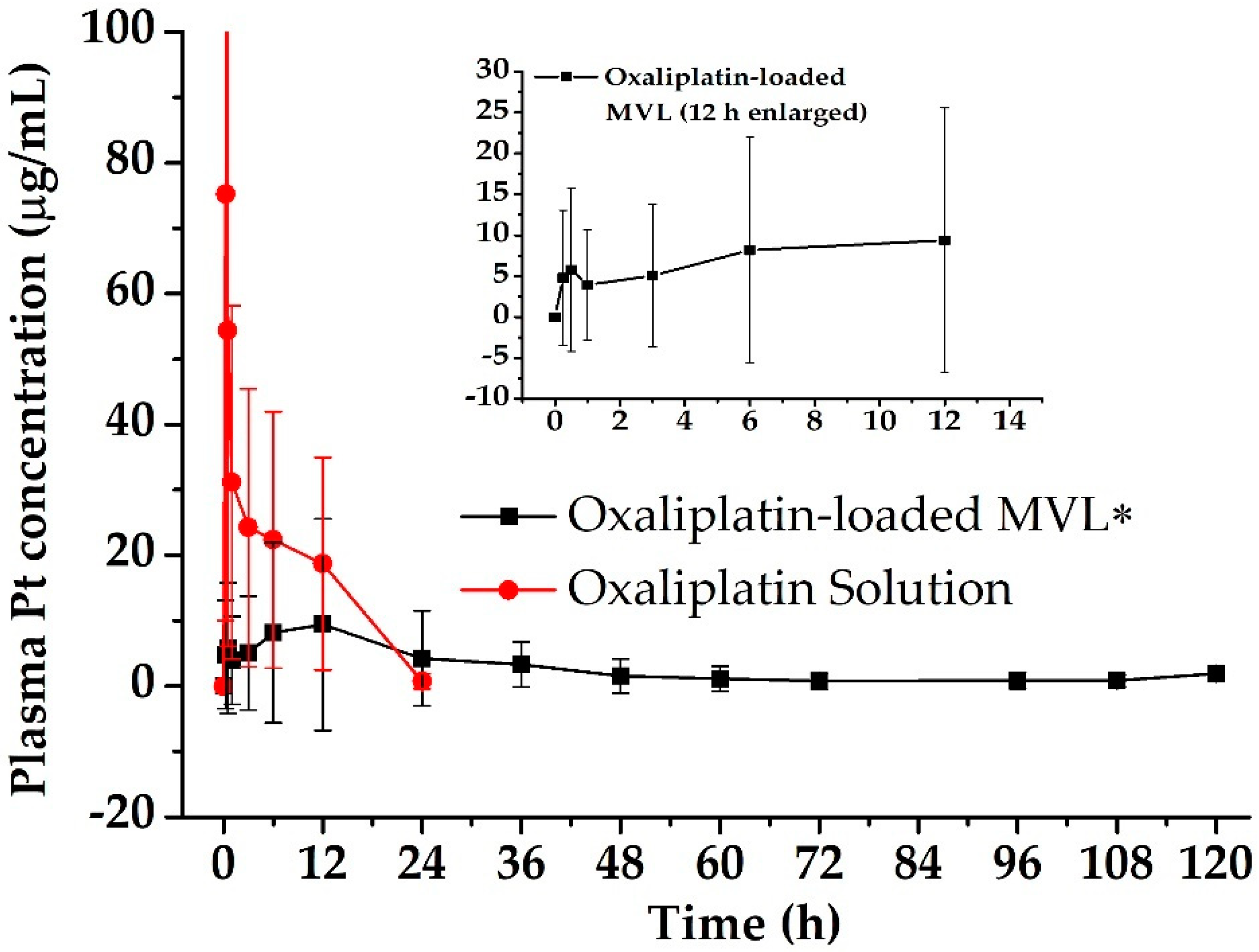
3.3. Pharmacokinetics in Rats
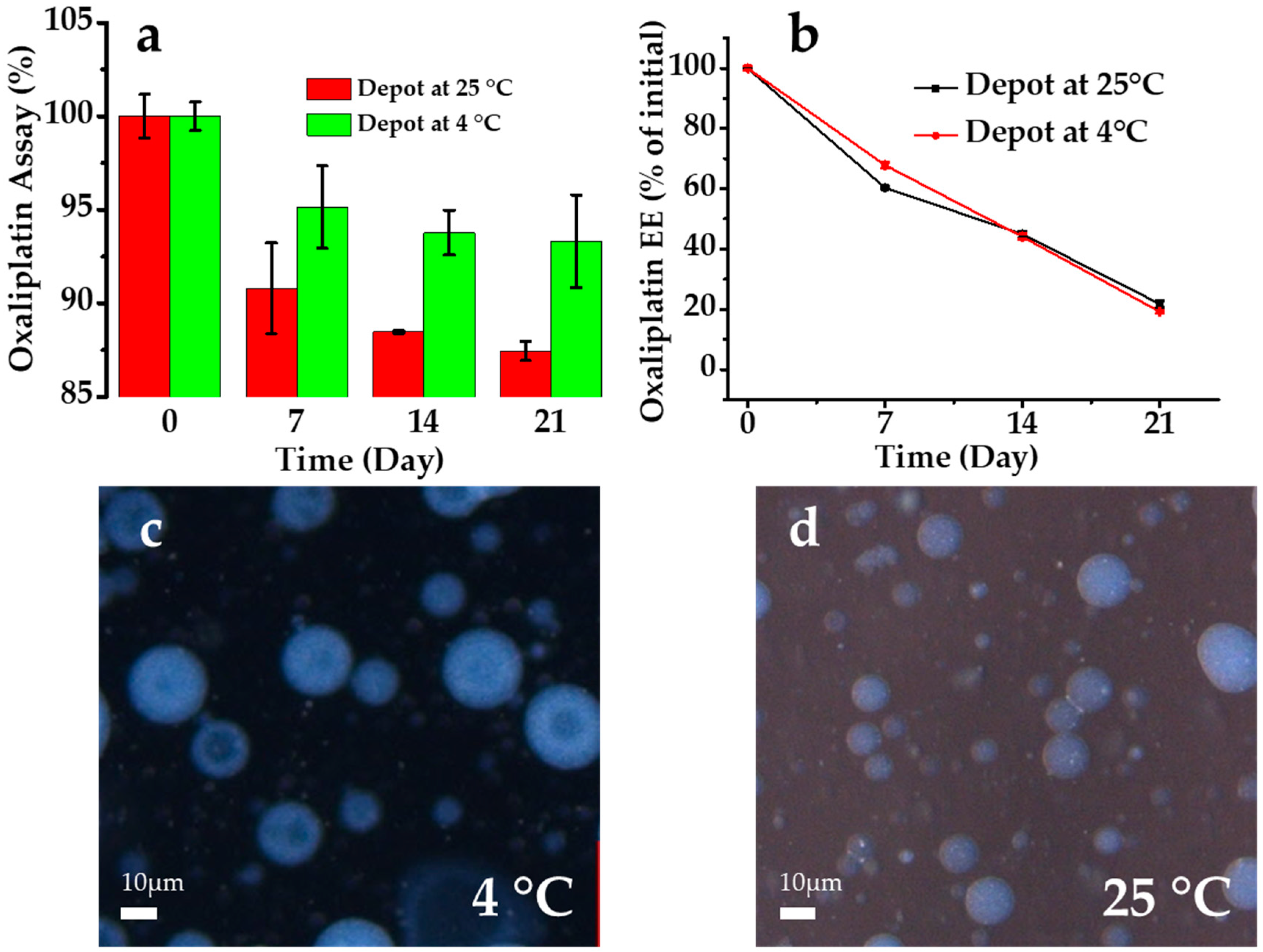
3.4. Stability
4. Discussion
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Zheng, W.; Luo, Z.; Zhang, J.; Min, P.; Li, W.; Xu, D.; Zhang, Z.; Xiong, P.; Liang, H.; Liu, J. Neural precursor cell expressed, developmentally downregulated 8activating enzyme inhibitor MLN4924 sensitizes colorectal cancer cells to oxaliplatin by inducing DNA damage, G2 cell cycle arrest and apoptosis. Mol. Med. Rep. 2017, 15, 2795–2801. [Google Scholar] [CrossRef] [PubMed]
- Yin, W.; Zhong, G.; Fan, H.; Xia, H. The Effect of Compound Sophora on Fluorouracil and Oxaliplatin Resistance in Colorectal Cancer Cells. Evid. Based Complement. Altern. Med. 2019, 2019, 7564232. [Google Scholar] [CrossRef] [PubMed]
- Elias, D.; Raynard, B.; Farkhondeh, F.; Goere, D.; Rouquie, D.; Ciuchendea, R.; Pocard, M.; Ducreux, M. Peritoneal carcinomatosis of colorectal origin—Long-term results of intraperitoneal chemohyperthermia with oxaliplatin following complete cytoreductive surgery. Gastroenterol. Clin. Biol. 2006, 30, 1200–1204. [Google Scholar] [CrossRef]
- Franko, J.; Shi, Q.; Meyers, J.P.; Maughan, T.S.; Adams, R.A.; Seymour, M.T.; Saltz, L.; Punt, C.J.A.; Koopman, M.; Tournigand, C.; et al. Prognosis of patients with peritoneal metastatic colorectal cancer given systemic therapy: An analysis of individual patient data from prospective randomised trials from the Analysis and Research in Cancers of the Digestive System (ARCAD) database. Lancet Oncol. 2016, 17, 1709–1719. [Google Scholar] [CrossRef]
- Fedirko, V.; Riboli, E.; Pischon, T.; Tjonneland, A.; Bueno-de-Mesquita, H.B.; Jenab, M.; Grp, E.C.W. Prediagnostic anthropometric measures and survival after diagnosis with colorectal cancer in the European Prospective Investigation into Cancer and Nutrition (EPIC). Cancer Res. 2013, 73. [Google Scholar] [CrossRef]
- Zani, S.; Papalezova, K.; Stinnett, S.; Tyler, D.; Hsu, D.; Blazer Iii, D.G. Modest advances in survival for patients with colorectal-associated peritoneal carcinomatosis in the era of modern chemotherapy. J. Surg. Oncol. 2013, 107, 307–311. [Google Scholar] [CrossRef]
- Goodman, M.D.; McPartland, S.; Detelich, D.; Saif, M.W. Chemotherapy for intraperitoneal use: A review of hyperthermic intraperitoneal chemotherapy and early post-operative intraperitoneal chemotherapy. J. Gastrointest. Oncol. 2016, 7, 45–57. [Google Scholar] [CrossRef]
- Witkamp, A.J.; de Bree, E.; Van Goethem, R.; Zoetmulder, F.A.N. Rationale and techniques of intra-operative hyperthermic intraperitoneal chemotherapy. Cancer Treat. Rev. 2001, 27, 365–374. [Google Scholar] [CrossRef]
- Hildebrandt, B.; Wust, P.; Ahlers, O.; Dieing, A.; Sreenivasa, G.; Kerner, T.; Felix, R.; Riess, H. The cellular and molecular basis of hyperthermia. Crit. Rev. Oncol. Hematol. 2002, 43, 33–56. [Google Scholar] [CrossRef]
- Kemmel, V.; Mercoli, H.A.; Meyer, N.; Brumaru, D.; Romain, B.; Lessinger, J.M.; Brigand, C. Mitomycin C Pharmacokinetics as Predictor of Severe Neutropenia in Hyperthermic Intraperitoneal Therapy. Ann. Surg. Oncol. 2015, 22, S873–S879. [Google Scholar] [CrossRef]
- Tan, G.H.C.; Ong, W.S.; Chia, C.S.; Tham, C.K.; Soo, K.C.; Teo, M.C.C. Does early post-operative intraperitoneal chemotherapy (EPIC) for patients treated with cytoreductive surgery and hyperthermic intraperitoneal chemotherapy (HIPEC) make a difference? Int. J. Hyperth. 2016, 32, 281–288. [Google Scholar] [CrossRef] [PubMed]
- Sugarbaker, P.H.; Graves, T.; Debruijn, E.A.; Cunliffe, W.J.; Mullins, R.E.; Hull, W.E.; Oliff, L.; Schlag, P. Early Postoperative Intraperitoneal Chemotherapy as an Adjuvant Therapy to Surgery for Peritoneal Carcinomatosis from Gastrointestinal Cancer—Pharmacological Studies. Cancer Res. 1990, 50, 5790–5794. [Google Scholar] [PubMed]
- Zhao, H.; Su, W.; Kang, Q.; Xing, Z.; Lin, X.; Wu, Z. Natural killer cells inhibit oxaliplatin-resistant colorectal cancer by repressing WBSCR22 via upregulating microRNA-146b-5p. Am. J. Cancer Res. 2018, 8, 824–834. [Google Scholar] [PubMed]
- Zhang, Y.; Huang, L.; Shi, H.; Chen, H.; Tao, J.; Shen, R.; Wang, T. Ursolic acid enhances the therapeutic effects of oxaliplatin in colorectal cancer by inhibition of drug resistance. Cancer Sci. 2018, 109, 94–102. [Google Scholar] [CrossRef]
- Zhang, P.; Chen, Z.; Ning, K.; Jin, J.; Han, X. Inhibition of B7-H3 reverses oxaliplatin resistance in human colorectal cancer cells. Biochem. Biophys. Res. Commun. 2017, 490, 1132–1138. [Google Scholar] [CrossRef]
- Yan, D.; Tu, L.; Yuan, H.; Fang, J.; Cheng, L.; Zheng, X.; Wang, X. WBSCR22 confers oxaliplatin resistance in human colorectal cancer. Sci. Rep. 2017, 7, 15443. [Google Scholar] [CrossRef]
- Song, N.; Pogue-Geile, K.L.; Gavin, P.G.; Yothers, G.; Kim, S.R.; Johnson, N.L.; Lipchik, C.; Allegra, C.J.; Petrelli, N.J.; O’Connell, M.J.; et al. Clinical Outcome From Oxaliplatin Treatment in Stage II/III Colon Cancer According to Intrinsic Subtypes Secondary Analysis of NSABP C-O7/NRG Oncology Randomized Clinical Trial. JAMA Oncol. 2016, 2, 1162–1169. [Google Scholar] [CrossRef]
- Elias, D.; Bonnay, A.; Puizillou, J.M.; Antoun, S.; Demirdjian, S.; El Otmany, A.; Pignon, J.P.; Drouard-Troalen, L.; Ouellet, J.F.; Ducreux, M. Heated intra-operative intraperitoneal oxaliplatin after complete resection of peritoneal carcinomatosis: Pharmacokinetics and tissue distribution. Ann. Oncol. 2002, 13, 267–272. [Google Scholar] [CrossRef]
- Ceelen, W.P.; Hesse, U.; de Hemptinne, B.; Pattyn, P. Hyperthermic intraperitoneal chemoperfusion in the treatment of locally advanced intra-abdominal cancer. Br. J. Surg. 2000, 87, 1006–1015. [Google Scholar] [CrossRef]
- Elias, D.; Detroz, B.; Debaene, B.; Damia, E.; Leclercq, B.; Rougier, P.; Lasser, P. Treatment of peritoneal carcinomatosis by intraperitoneal chemo-hyperthermia: Reliable and unreliable concepts. Hepatogastroenterology 1994, 41, 207–213. [Google Scholar]
- Piche, N.; Leblond, F.A.; Sideris, L.; Pichette, V.; Drolet, P.; Fortier, L.P.; Mitchell, A.; Dube, P. Rationale for Heating Oxaliplatin for the Intraperitoneal Treatment of Peritoneal Carcinomatosis A Study of the Effect of Heat on Intraperitoneal Oxaliplatin Using a Murine Model. Ann. Surg. 2011, 254, 138–144. [Google Scholar] [CrossRef] [PubMed]
- Bécouarn, Y.; Ychou, M.; Ducreux, M.; Borel, C.; Bertheault-Cvitkovic, F.; Seitz, J.F.; Nasca, S.; Nguyen, T.D.; Paillot, B.; Raoul, J.L.; et al. Phase II trial of oxaliplatin as first-line chemotherapy in metastatic colorectal cancer patients. Digestive Group of French Federation of Cancer Centers. J. Clin. Oncol. 1998, 16, 2739–2744. [Google Scholar] [CrossRef] [PubMed]
- Ye, Q.; Asherman, J.; Stevenson, M.; Brownson, E.; Katre, N.V. DepoFoam (TM) technology: A vehicle for controlled delivery of protein and peptide drugs. J. Control. Release 2000, 64, 155–166. [Google Scholar] [CrossRef]
- Li, H.; An, J.H.; Park, J.S.; Han, K. Multivesicular liposomes for oral delivery of recombinant human epidermal growth factor. Arch. Pharm. Res. 2005, 28, 988–994. [Google Scholar] [CrossRef] [PubMed]
- Richard, B.M.; Rickert, D.E.; Newton, P.E.; Ott, L.R.; Haan, D.; Brubaker, A.N.; Cole, P.I.; Ross, P.E.; Rebelatto, M.C.; Nelson, K.G. Safety Evaluation of EXPAREL (DepoFoam Bupivacaine) Administered by Repeated Subcutaneous Injection in Rabbits and Dogs: Species Comparison. J. Drug Deliv. 2011, 2011, 467429. [Google Scholar] [CrossRef]
- Xiao, C.J.; Qi, X.R.; Maitani, Y.; Nagai, T. Sustained release of cisplatin from multivesicular liposomes: Potentiation of antitumor efficacy against S180 murine carcinoma. J. Pharm. Sci. 2004, 93, 1718–1724. [Google Scholar] [CrossRef]
- Lu, Z.; Tsai, M.; Lu, D.; Wang, J.; Wientjes, M.G.; Au, J.L. Tumor-penetrating microparticles for intraperitoneal therapy of ovarian cancer. J. Pharmacol. Exp. Ther. 2008, 327, 673–682. [Google Scholar] [CrossRef]
- Yeo, Y.; Ito, T.; Bellas, E.; Highley, C.B.; Marini, R.; Kohane, D.S. In situ cross-linkable hyaluronan hydrogels containing polymeric nanoparticles for preventing postsurgical adhesions. Ann. Surg. 2007, 245, 819–824. [Google Scholar] [CrossRef]
- Abuzar, S.M.; Ahn, J.H.; Park, K.S.; Park, E.J.; Baik, S.H.; Hwang, S.J. Pharmacokinetic Profile and Anti-Adhesive Effect of Oxaliplatin-PLGA Microparticle-Loaded Hydrogels in Rats for Colorectal Cancer Treatment. Pharmaceutics 2019, 11, 392. [Google Scholar] [CrossRef]
- Diamond, M.P.; Freeman, M.L. Clinical implications of postsurgical adhesions. Hum. Reprod Update 2001, 7, 567–576. [Google Scholar] [CrossRef]
- Zeng, C.; Yu, F.; Yang, Y.; Cheng, X.; Liu, Y.; Zhang, H.; Zhao, S.; Yang, Z.; Li, M.; Li, Z.; et al. Preparation and Evaluation of Oxaliplatin Thermosensitive Liposomes with Rapid Release and High Stability. PLoS ONE 2016, 11, e0158517. [Google Scholar] [CrossRef] [PubMed]
- Zhang, B.; Wang, T.; Yang, S.; Xiao, Y.; Song, Y.; Zhang, N.; Garg, S. Development and evaluation of oxaliplatin and irinotecan co-loaded liposomes for enhanced colorectal cancer therapy. J. Control. Release 2016, 238, 10–21. [Google Scholar] [CrossRef] [PubMed]
- Cevenini, A.; Celia, C.; Orru, S.; Sarnataro, D.; Raia, M.; Mollo, V.; Locatelli, M.; Imperlini, E.; Peluso, N.; Peltrini, R.; et al. Liposome-Embedding Silicon Microparticle for Oxaliplatin Delivery in Tumor Chemotherapy. Pharmaceutics 2020, 12, 559. [Google Scholar] [CrossRef] [PubMed]
- Graham, M.A.; Lockwood, G.F.; Greenslade, D.; Brienza, S.; Bayssas, M.; Gamelin, E. Clinical pharmacokinetics of oxaliplatin: A critical review. Clin. Cancer Res. 2000, 6, 1205–1218. [Google Scholar] [PubMed]
- Pestieau, S.R.; Belliveau, J.F.; Griffin, H.; Stuart, O.A.; Sugarbaker, P.H. Pharmacokinetics of intraperitoneal oxaliplatin: Experimental studies. J. Surg. Oncol. 2001, 76, 106–114. [Google Scholar] [CrossRef]
- Ahn, J.H.; Kim, H.D.; Abuzar, S.M.; Lee, J.Y.; Jin, S.E.; Kim, E.K.; Hwang, S.J. Intracorneal melatonin delivery using 2-hydroxypropyl-beta-cyclodextrin ophthalmic solution for granular corneal dystrophy type 2. Int. J. Pharm. 2017, 529, 608–616. [Google Scholar] [CrossRef]
- Mantripragada, S. A lipid based depot (DepoFoam technology) for sustained release drug delivery. Prog. Lipid Res. 2002, 41, 392–406. [Google Scholar] [CrossRef]
- Kim, S.; Turker, M.S.; Chi, E.Y.; Sela, S.; Martin, G.M. Preparation of multivesicular liposomes. Biochim. Biophys. Acta 1983, 728, 339–348. [Google Scholar] [CrossRef]
- Schneider, M. Process for the Preparation of Liposomes in Aqueous Solution. U.S. Patent Application No. 4,224,179, 23 September 1980. [Google Scholar]
- Manna, S.; Wu, Y.; Wang, Y.; Koo, B.; Chen, L.; Petrochenko, P.; Dong, Y.; Choi, S.; Kozak, D.; Oktem, B.; et al. Probing the mechanism of bupivacaine drug release from multivesicular liposomes. J. Control. Release 2019, 294, 279–287. [Google Scholar] [CrossRef]
- Lim, C.B.; Abuzar, S.M.; Karn, P.R.; Cho, W.; Park, H.J.; Cho, C.W.; Hwang, S.J. Preparation, Characterization, and In Vivo Pharmacokinetic Study of the Supercritical Fluid-Processed Liposomal Amphotericin B. Pharmaceutics 2019, 11, 589. [Google Scholar] [CrossRef]
- Rajvaidya, M.; Gupta, Y.; Jain, A.; Jain, S.K. Development and characterization of multivesicular liposomes bearing serratiopeptidase for sustained delivery. J. Drug Deliv. Sci. Technol. 2007, 17, 315–320. [Google Scholar] [CrossRef]
- Shen, Y.; Ji, Y.; Xu, S.; Chen, D.Q.; Tu, J. Multivesicular liposome formulations for the sustained delivery of ropivacaine hydrochloride: Preparation, characterization, and pharmacokinetics. Drug Deliv. 2011, 18, 361–366. [Google Scholar] [CrossRef] [PubMed]
- Jain, S.K.; Gupta, Y.; Jain, A.; Bhola, M. Multivesicular Liposomes Bearing Celecoxib-β-Cyclodextrin Complex for Transdermal Delivery. Drug Deliv. 2007, 14, 327–335. [Google Scholar] [CrossRef] [PubMed]
- Zuidam, N.J.; Crommelin, D.J.A. Chemical Hydrolysis of Phospholipids. J. Pharm. Sci. 1995, 84, 1113–1119. [Google Scholar] [CrossRef]
- Allen Zhang, J.-A.; Pawelchak, J. Effect of pH, ionic strength and oxygen burden on the chemical stability of EPC/cholesterol liposomes under accelerated conditions: Part 1: Lipid hydrolysis. Eur. J. Pharm. Biopharm. 2000, 50, 357–364. [Google Scholar] [CrossRef]
- Lee, J.E.; Abuzar, S.M.; Seo, Y.; Han, H.; Jeon, Y.; Park, E.J.; Baik, S.H.; Hwang, S.J. Oxaliplatin-loaded chemically cross-linked hydrogels for prevention of postoperative abdominal adhesion and colorectal cancer therapy. Int. J. Pharm. 2019, 565, 50–58. [Google Scholar] [CrossRef]
- Han, C.H.; Khwaounjoo, P.; Hill, A.G.; Miskelly, G.M.; McKeage, M.J. Predicting effects on oxaliplatin clearance: In vitro, kinetic and clinical studies of calcium-and magnesium-mediated oxaliplatin degradation. Sci. Rep. 2017, 7. [Google Scholar] [CrossRef]
- Alberto, M.E.; Lucas, M.F.; Pavelka, M.; Russo, N. The degradation pathways in chloride medium of the third generation anticancer drug oxaliplatin. J. Phys. Chem. B 2008, 112, 10765–10768. [Google Scholar] [CrossRef]
- Luo, Y.; Liu, Z.; Zhang, X.; Huang, J.; Yu, X.; Li, J.; Xiong, D.; Sun, X.; Zhong, Z. Effect of a controlled-release drug delivery system made of oleanolic acid formulated into multivesicular liposomes on hepatocellular carcinoma in vitro and in vivo. Int. J. Nanomed. 2016, 11, 3111–3129. [Google Scholar] [CrossRef]
- Cohen, R.; Steiner, A.; Kanaan, H.; Barenholz, Y. Chemical and physical characterization of remotely loaded bupivacaine liposomes: Comparison between large multivesicular vesicles and small unilamellar vesicles. J. Mater. Chem. B 2013, 1, 4619–4627. [Google Scholar] [CrossRef]
- Kohn, F.R.; Willis, R.C.; Dragani, J.C.; Hartounian, H.; Ricci, J.; Yaksh, T.L. Sustained-release formulations of morphine sulfate (DepoFoam(TM) encapsulated morphine) for epidural administration. Anesthesiology 1996, 85, A756. [Google Scholar]
- Sun, L.; Wang, T.; Gao, L.; Quan, D.; Feng, D. Multivesicular liposomes for sustained release of naltrexone hydrochloride: Design, characterization and in vitro/in vivo evaluation. Pharm. Dev. Technol. 2013, 18, 828–833. [Google Scholar] [CrossRef] [PubMed]
- Katre, N.V.; Asherman, J.; Schaefer, H.; Hora, M. Multivesicular liposome (DepoFoam) technology for the sustained delivery of insulin-like Growth Factor-I (IGF-I). J. Pharm. Sci. 1998, 87, 1341–1346. [Google Scholar] [CrossRef] [PubMed]
| Formulation | Oxaliplatin | β-cyclodextrin | HSPC | DEPC | DSPG-Na | Cholesterol | Triolein | DSPE mPEG-2000 | Lipid Conc. | Span | % EE |
|---|---|---|---|---|---|---|---|---|---|---|---|
| mg | mM | ||||||||||
| Depot-1 | 10 | 3.5 | - | - | 1 | 10 | 1 | 1 | 13 | 5.69 | 60.0 ± 3.10 |
| Depot-2 | 5 | - | 1 | 10 | 1 | 1 | 18 | 4.87 | 66.1 ± 1.15 | ||
| Depot-3 | - | 10 | 1 | 10 | 1 | 1 | 23 | 2.27 | 77.96 ± 2.05 | ||
| Depot-4 | 10 | 10 | 1 | 5 | 1 | 1 | 28 | 2.08 | 79.95 ± 1.27 | ||
| Depot-5 | 10 | 10 | 1 | 10 | 1 | 1 | 33 | 1.99 | 92.16 ± 2.17 | ||
| Depot-6 | 10 | 10 | 1 | 10 | 5 | 1 | 37 | 2.66 | 73.17 ± 3.97 | ||
| Depot-7 | 10 | 10 | 5 | 10 | 1 | 1 | 37 | 3.05 | 84.19 ± 3.09 | ||
| Higuchi | Weibull | Hixcon-Crowell | |
|---|---|---|---|
| Oxaliplatin Depot | Q = 16.530t1/2 + 12.134 r = 0.876 | LnLn(1 /(1 − Q) = 2.065Ln(t) − 7.430 r = 0.928 | (1 − Q)1/3 = 0.138t + 3.954 r = 0.943 |
| Oxaliplatin Solution | Q = 91.827t1/2 + 6.443 r = 0.847 | LnLn(1/(1 − Q) = 1.196Ln(t) − 5.175 r = 0.903 | (1 − Q)1/3 = 2.284t + 2.701 r = 0.897 |
| Group | AUC 1 (µg·h/mL) | AUMC 2 (µg·h2/mL) | Cmax 3 (µg/mL) | Tmax 4 (h) | MRT 5 (h) |
|---|---|---|---|---|---|
| Oxaliplatin-Solution | 412.00 ± 58.54 | 2963.25 ± 315.79 | 75.21 ± 8.78 | 0.25 | 7.19 |
| Oxaliplatin-loaded MVL | 316.59 ± 25.29 | 10,690.22 ± 930.67 | 9.38 ± 16.19 | 12 | 29.20 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Abuzar, S.M.; Park, E.J.; Seo, Y.; Lee, J.; Baik, S.H.; Hwang, S.-J. Preparation and Evaluation of Intraperitoneal Long-Acting Oxaliplatin-Loaded Multi-Vesicular Liposomal Depot for Colorectal Cancer Treatment. Pharmaceutics 2020, 12, 736. https://doi.org/10.3390/pharmaceutics12080736
Abuzar SM, Park EJ, Seo Y, Lee J, Baik SH, Hwang S-J. Preparation and Evaluation of Intraperitoneal Long-Acting Oxaliplatin-Loaded Multi-Vesicular Liposomal Depot for Colorectal Cancer Treatment. Pharmaceutics. 2020; 12(8):736. https://doi.org/10.3390/pharmaceutics12080736
Chicago/Turabian StyleAbuzar, Sharif Md, Eun Jung Park, Yeji Seo, Juseung Lee, Seung Hyuk Baik, and Sung-Joo Hwang. 2020. "Preparation and Evaluation of Intraperitoneal Long-Acting Oxaliplatin-Loaded Multi-Vesicular Liposomal Depot for Colorectal Cancer Treatment" Pharmaceutics 12, no. 8: 736. https://doi.org/10.3390/pharmaceutics12080736
APA StyleAbuzar, S. M., Park, E. J., Seo, Y., Lee, J., Baik, S. H., & Hwang, S.-J. (2020). Preparation and Evaluation of Intraperitoneal Long-Acting Oxaliplatin-Loaded Multi-Vesicular Liposomal Depot for Colorectal Cancer Treatment. Pharmaceutics, 12(8), 736. https://doi.org/10.3390/pharmaceutics12080736







