Lake Trout (Salvelinus namaycush) Naturally Infected with Salmovirus salmonidallo3 (SalHV-3; Family Alloherpesviridae) Continue to Harbor the Virus for Nearly a Decade
Abstract
1. Introduction
2. Materials and Methods
2.1. Fish and Husbandry
2.2. Fish Handling and Sample Collection
2.2.1. Water Sample Collection to Assess Virus Shedding
2.2.2. Handling and PIT-Tagging
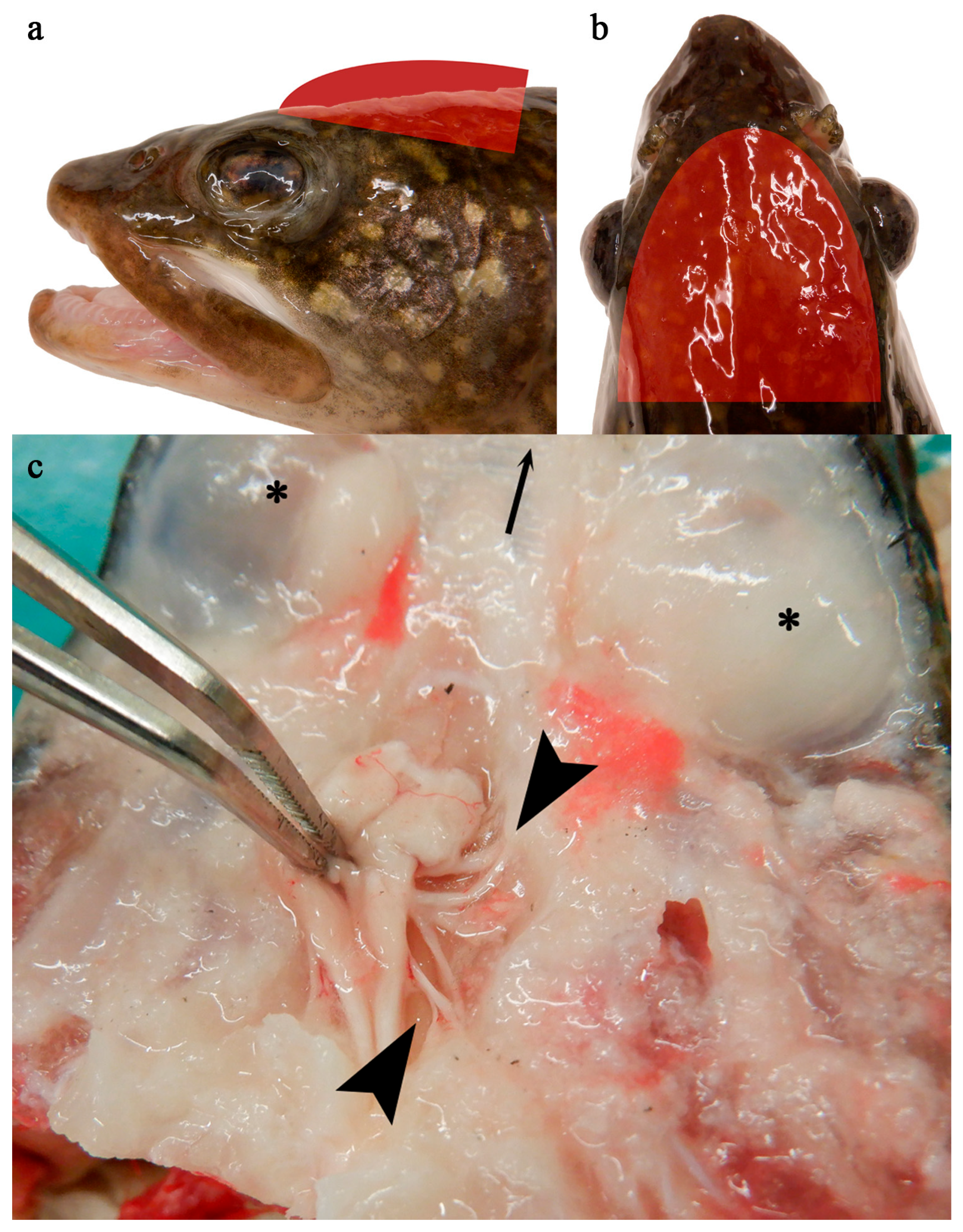
2.2.3. Non-Lethal Tissue Sample Collection
- Mucus—approximately 100 µL of mucus per fish was collected from along the left lateral line and around the base of the left pectoral and pelvic fins, using a 1 mL slip-tip syringe barrel (Becton, Dickinson and Company, Franklin Lakes, NJ, USA).
- Blood—less than 2 mL of whole blood was collected from each fish (maximum of 0.3% body weight), via caudal vessel puncture using a 3 mL luer lock syringe and 20-gauge needle (Becton, Dickinson and Company, Franklin Lakes, NJ, USA). Whole blood was then centrifuged (5000 rpm, 10 min, 4 °C), after which serum was collected.
- Fin clip—approximately 10 mg tissue was collected using sterile scissors from the left or right pectoral fin.
2.2.4. Lethal Tissue Sample Collection
2.3. Detection and Quantification of SalHV-3
2.3.1. DNA Extraction
2.3.2. Quantitative PCR Analysis
3. Results
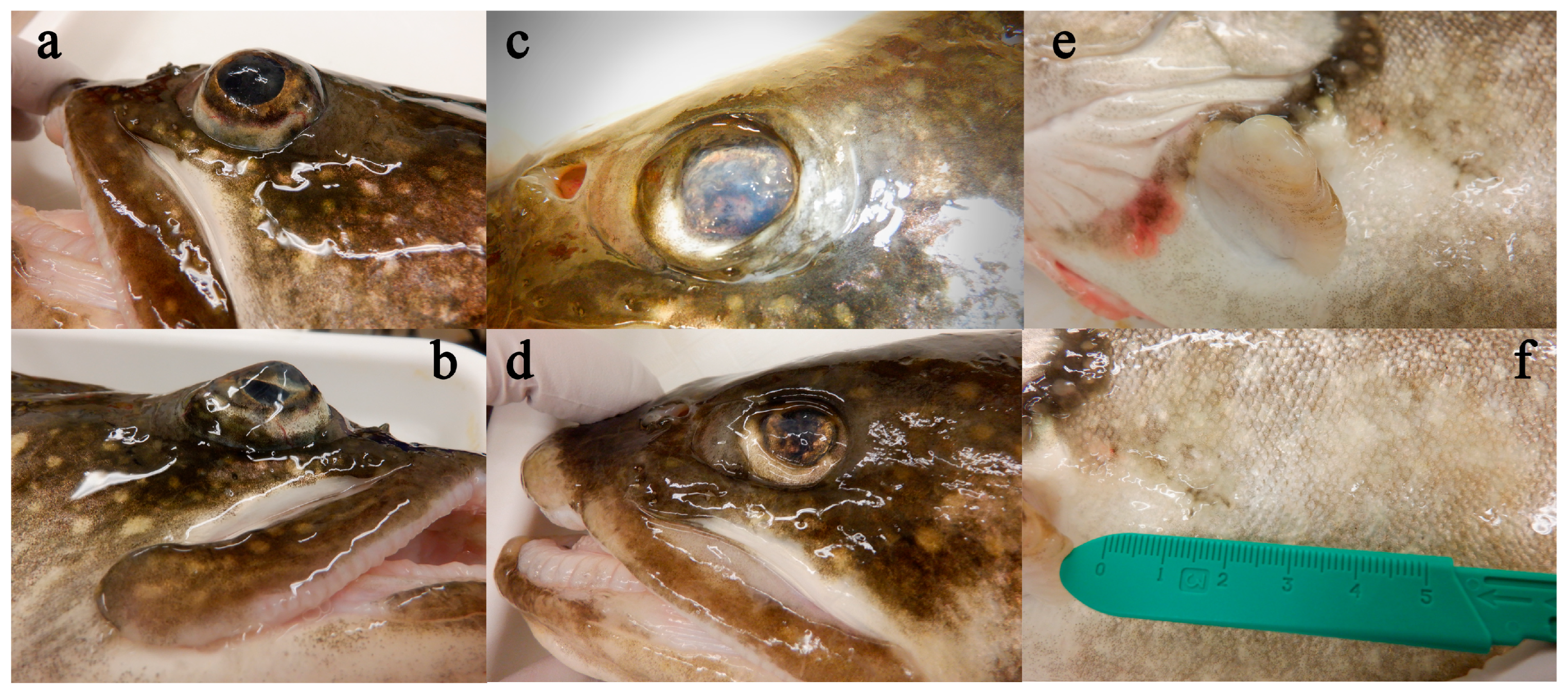
3.1. Clinical Observations
3.2. Non-Lethal Samples—Group 1, Repeated Handling
3.3. Non-Lethal Samples—Group 2, Intermittent Handling
| Fish # | Study Day | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| 0 | 7 | 14 | 21 | 28 | 35 | 42 | 49 | ||
| Group 1—Repeated Handling | 1 | - | 3.88 × 102 | - | - | - | - | - | - |
| 2 | 1.09 × 106 | 1.43 × 106 | 3.99 × 104 | 2.13 × 104 | 1.15 × 105 | 2.33 × 104 | 6.21 × 104 | 3.44 × 105 | |
| 3 | - | - | - | - | - | - | - | - | |
| 4 | - | - | - | 5.87 × 102 | - | - | - | - | |
| 5 | - | - | - | - | - | - | - | - | |
| 6 | - | - | - | - | - | - | - | - | |
| 7 | 3.91 × 106 | 9.41 × 105 | 8.01 × 106 | 2.53 × 106 | - | 5.48 × 105 | 1.00 × 105 | 1.28 × 106 | |
| 8 | - | - | - | - | - | - | - | - | |
| 9 | 5.43 × 102 | - | - | - | - | - | - | - | |
| Group 2—Intermittent Handling | 10 | - | - | ||||||
| 11 | 1.95 × 102 | - | |||||||
| 12 | - | - | |||||||
| 13 | - | - | |||||||
| 14 | - | - | |||||||
| 15 | 5.31 × 105 | - | |||||||
| 16 | 1.22 × 105 | 3.64 × 104 | |||||||
| 17 | - | - | |||||||
| 18 | - | 1.15 × 103 | |||||||
| 19 | - | - | |||||||
| Fish # | Study Day | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| 0 | 7 | 14 | 21 | 28 | 35 | 42 | 49 | ||
| Group 1—Repeated Handling | 1 | - | - | - | - | - | - | - | - |
| 2 | - | - | 6.61 × 103 | 8.90 × 104 | - | 2.89 × 104 | - | - | |
| 3 | - | - | - | - | - | - | - | - | |
| 4 | - | 1.01 × 107 | 6.13 × 106 | 1.07 × 106 | - | 5.40 × 104 | 6.13 × 103 | 9.90 × 106 | |
| 5 | - | - | - | - | - | - | - | - | |
| 6 | - | - | - | - | - | - | - | - | |
| 7 | - | - | - | 8.41 × 104 | 8.23 × 103 | - | - | - | |
| 8 | - | - | - | - | - | - | - | 1.53 × 105 | |
| 9 | - | - | 3.69 × 104 | - | - | - | - | - | |
| Group 2—Intermittent Handling | 10 | - | 1.05 × 105 | ||||||
| 11 | - | 5.15 × 105 | |||||||
| 12 | - | - | |||||||
| 13 | - | - | |||||||
| 14 | - | - | |||||||
| 15 | - | 4.06 × 105 | |||||||
| 16 | - | 2.04 × 104 | |||||||
| 17 | - | - | |||||||
| 18 | 1.36 ×104 | 4.50 × 106 | |||||||
| 19 | - | - | |||||||
3.4. Lethal Samples—Group 1, Repeated Handling
3.5. SalHV-3 Shedding
| Tissue Type | ||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Non-Lethal | Lethal | |||||||||||||||
| Fish # | Fin | Mucus | Serum | Cerebellum | Cerebrum | Cranial Nerve | Medulla Oblongata | Olfactory | Optic lobe | Optic Nerve | Cornea | Gill | Gonads | |||
| Group 1—Repeated Handling | 1 | 0/8 | 0/8 | 0/8 | 0/1 | 0/1 | 0/1 | 0/1 | 0/1 | 0/1 | 0/1 | 0/1 | 0/1 | 0/1 | ||
| 2 | 8/8 | 3/8 | 0/8 | 0/1 | 0/1 | 1/1 | 0/1 | 0/1 | 1/1 | 1/1 | 1/1 | 0/1 | 0/1 | |||
| 3 | 0/8 | 0/8 | 0/8 | 0/1 | 0/1 | 1/1 | 0/1 | 1/1 | 0/1 | 0/1 | 0/1 | 0/1 | 0/1 | |||
| 4 | 1/8 | 6/8 | 1/8 | 0/1 | 0/1 | 0/1 | 0/1 | - | 0/1 | 0/1 | 0/1 | 0/1 | 0/1 | |||
| 5 | 0/8 | 0/8 | 0/8 | 0/1 | 0/1 | 0/1 | 0/1 | 0/1 | 0/1 | 0/1 | 0/1 | 0/1 | 0/1 | |||
| 6 | 0/8 | 0/8 | 0/8 | 0/1 | 0/1 | 0/1 | 0/1 | 0/1 | 0/1 | 0/1 | 0/1 | 0/1 | 0/1 | |||
| 7 | 7/8 | 2/8 | 0/8 | 0/1 | 0/1 | 1/1 | 0/1 | 0/1 | 0/1 | 0/1 | 1/1 | 0/1 | 0/1 | |||
| 8 | 0/8 | 1/8 | 0/8 | 0/1 | 0/1 | 1/1 | 1/1 | 0/1 | 0/1 | 0/1 | 0/1 | 0/1 | 0/1 | |||
| 9 | 1/8 | 1/8 | 0/8 | 0/1 | 0/1 | 0/1 | 0/1 | 0/1 | 0/1 | 0/1 | 1/1 | 0/1 | 0/1 | |||
| Group 2—Intermittent Handling | 10 | 0/2 | 1/2 | 0/2 | - | - | - | - | - | - | - | - | - | - | ||
| 11 | 1/2 | 1/2 | 0/2 | - | - | - | - | - | - | - | - | - | - | |||
| 12 | 0/2 | 0/2 | 0/2 | - | - | - | - | - | - | - | - | - | - | |||
| 13 | 0/2 | 0/2 | 0/2 | - | - | - | - | - | - | - | - | - | - | |||
| 14 | 0/2 | 0/2 | 0/2 | - | - | - | - | - | - | - | - | - | - | |||
| 15 | 1/2 | 1/2 | 0/2 | - | - | - | - | - | - | - | - | - | - | |||
| 16 | 2/2 | 1/2 | 0/2 | - | - | - | - | - | - | - | - | - | - | |||
| 17 | 0/2 | 0/2 | 0/2 | - | - | - | - | - | - | - | - | - | - | |||
| 18 | 1/2 | 2/2 | 0/2 | - | - | - | - | - | - | - | - | - | - | |||
| 19 | 0/2 | 0/2 | 0/2 | - | - | - | - | - | - | - | - | - | - | |||
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| SalHV-3 | Salmovirus salmonidallo3 |
| CyHV-3 | Cyvirus cyprinidallo3 |
| EED | Epizootic Epitheliotropic Disease |
| PIT | Passive Integrated Transponder |
| MS-222 | Tricaine Methanesulfonate |
| qPCR | Quantitative PCR |
References
- Walker, P.J.; Siddell, S.G.; Lefkowitz, E.J.; Mushegian, A.R.; Adriaenssens, E.M.; Alfenas-Zerbini, P.; Dempsey, D.M.; Dutilh, B.E.; García, M.L.; Hendrickson, R.C.; et al. Recent changes to virus taxonomy ratified by the International Committee on Taxonomy of Viruses (2022). Arch. Virol. 2022, 167, 2429–2440. [Google Scholar] [CrossRef] [PubMed]
- Bradley, T.M.; Medina, D.J.; Chang, P.W.; McClain, J. Epizootic epitheliotrophic disease of lake trout (Salvelinus namaycush): History and viral etiology. Dis. Aquat. Org. 1989, 7, 195–201. [Google Scholar] [CrossRef]
- Bradley, T.; Newcomer, C.; Maxwell, K. Epitheliocystis associated with massive mortalities of cultured lake trout Saivelinus namaycush. Dis. Aquat. Org. 1988, 4, 9–17. [Google Scholar] [CrossRef]
- Faisal, M.; Loch, T.P.; Shavalier, M.; VanDeuren, M.G.; Standish, I.; Winters, A.; Glenney, G.; Aho, J.; Wolgamood, M.; VanAmberg, J.; et al. Resurgence of Salmonid Herpesvirus-3 Infection (Epizootic Epitheliotropic Disease) in Hatchery-Propagated Lake Trout in Michigan. J. Aquat. Anim. Health 2019, 31, 31–45. [Google Scholar] [CrossRef]
- Shavalier, M.; Faisal, M.; Loch, T.P.; Fitzgerald, S.D.; Thaiwong, T.; Kiupel, M. Disease Progression in Lake Trout (Salvelinus namaycush) Experimentally Infected With Epizootic Epitheliotropic Disease Virus (Salmonid Herpesvirus-3). Vet. Pathol. 2020, 57, 687–699. [Google Scholar] [CrossRef]
- Purbayu, M.A.; Shavalier, M.A.; Faisal, M.; Loch, T.P. Experimental Evidence of Epizootic Epitheliotropic Disease Virus (Salmoid Herpesvirus-3, Alloherpesviridae) Transmission via Contaminated Fomites and Subsequent Prevention Using a Disinfectant. Pathogens 2021, 10, 724. [Google Scholar] [CrossRef] [PubMed]
- Faisal, M.; Purbayu, M.; Shavalier, M.A.; Marsh, T.L.; Loch, T.P. Shedding of the Salmonid Herpesvirus-3 by Infected Lake Trout (Salvelinus namaycush). Viruses 2019, 11, 580. [Google Scholar] [CrossRef]
- Glenney, G.W.; Barbash, P.A.; Coll, J.A. A Quantitative Polymerase Chain Reaction Assay for the Detection and Quantification of Epizootic Epitheliotropic Disease Virus (EEDV; Salmonid Herpesvirus 3). J. Aquat. Anim. Health 2016, 28, 56–67. [Google Scholar] [CrossRef]
- Wei, C.; Iida, H.; Chuah, Q.; Tanaka, M.; Kato, G.; Sano, M. Persistence of cyprinid herpesvirus 2 in asymptomatic goldfish Carassius auratus (L.) that survived an experimental infection. J. Fish Dis. 2019, 42, 913–921. [Google Scholar] [CrossRef] [PubMed]
- Lepa, A.; Siwicki, A.K. Fish herpesvirus diseases: A short review of current knowledge. Acta Vet. Brno 2013, 81, 383–389. [Google Scholar] [CrossRef]
- Baumer, A.; Fabian, M.; Wilkens, M.R.; Steinhagen, D.; Runge, M. Epidemiology of cyprinid herpesvirus-3 infection in latently infected carp from aquaculture. Dis. Aquat. Org. 2013, 105, 101–108. [Google Scholar] [CrossRef]
- Davison, A.J.; Eberle, R.; Ehlers, B.; Hayward, G.S.; McGeoch, D.J.; Minson, A.C.; Pellett, P.E.; Roizman, B.; Studdert, M.J.; Thiry, E. The order Herpesvirales. Arch. Virol. 2009, 154, 171–177. [Google Scholar] [CrossRef]
- Quijano Cardé, E.M.; Soto, E. A review of latency in the Alloherpesviridae family. J. Fish Dis. 2024, 47, e14016. [Google Scholar] [CrossRef]
- Eide, K.E.; Miller-Morgan, T.; Heidel, J.R.; Kent, M.L.; Bildfell, R.J.; LaPatra, S.; Watson, G.; Jin, L. Investigation of koi herpesvirus latency in koi. J. Virol. 2011, 85, 4954–4962. [Google Scholar] [CrossRef]
- Wei, C.; Xu, C.; Sun, Y.; Li, J.; Sano, M.; Li, Q. Investigation of the latency of Cyprinid herpesvirus 2 in apparently healthy farmed gibel carp, Carassius auratus gibelio. Aquaculture 2023, 562, 738854. [Google Scholar] [CrossRef]
- Hanson, L.; Dishon, A.; Kotler, M. Herpesviruses that infect fish. Viruses 2011, 3, 2160–2191. [Google Scholar] [CrossRef]
- Cano, I.; Mulhearn, B.; Akter, S.; Paley, R. Seroconversion and Skin Mucosal Parameters during Koi Herpesvirus Shedding in Common carp, Cyprinus carpio. Int. J. Mol. Sci. 2020, 21, 8482. [Google Scholar] [CrossRef] [PubMed]
- Yuasa, K.; Ito, T.; Sano, M. Effect of water temperature on mortality and virus shedding in carp experimentally infected with koi herpesvirus. Fish Pathol. 2008, 43, 83–85. [Google Scholar] [CrossRef]
- Kancharla, S.R.; Hanson, L.A. Production and shedding of channel catfish virus (CCV) and thymidine kinase negative CCV in immersion exposed channel catfish fingerlings. Dis. Aquat. Org. 1996, 27, 25–34. [Google Scholar] [CrossRef]
- Hubbard, L.E.; Stelzer, E.A.; Poulson, R.L.; Kolpin, D.W.; Szablewski, C.M.; Givens, C.E. Development of a Large-Volume Concentration Method to Recover Infectious Avian Influenza Virus from the Aquatic Environment. Viruses 2024, 16, 1898. [Google Scholar] [CrossRef] [PubMed]
- Ip, Y.C.A.; Chen, J.; Tan, L.Y.; Lau, C.; Chan, Y.H.; Balasubramaniam, R.S.; Wong, W.Y.J.; Ng, K.; Tan, Z.Y.B.; Fernandez, C.J.; et al. Establishing environmental DNA and RNA protocols for the simultaneous detection of fish viruses from seawater. Environ. DNA 2024, 6, e418. [Google Scholar] [CrossRef]
- Wolf, K.; Darlington, R.; Taylor, W.; Quimby, M.; Nagabayashi, T. Herpesvirus salmonis: Characterization of a new pathogen of rainbow trout. J. Virol. 1978, 27, 659–666. [Google Scholar] [CrossRef]
- Hanson, L.A.; Doszpoly, A.; van Beurden, S.; de Oliveira Viadanna, P.H.; Waltzek, T. Alloherpesviruses of fish. In Aquaculture Virology; Elsevier: Amsterdam, The Netherlands, 2024; pp. 165–189. [Google Scholar]
- Kurobe, T.; Marcquenski, S.; Hedrick, R. PCR assay for improved diagnostics of epitheliotropic disease virus (EEDV) in lake trout Salvelinus namaycush. Dis. Aquat. Org. 2009, 84, 17–24. [Google Scholar] [CrossRef]
- Grinde, B. Herpesviruses: Latency and reactivation–viral strategies and host response. J. Oral Microbiol. 2013, 5, 22766. [Google Scholar] [CrossRef]
- Bergmann, S.; Kempter, J. Detection of koi herpesvirus (KHV) after re-activation in persistently infected common carp (Cyprinus carpio L.) using non-lethal sampling methods. Bull. Eur. Assoc. Fish Pathol. 2011, 31, 92–100. [Google Scholar]
- Bordeleau, X.; Hatcher, B.G.; Denny, S.; Fast, M.D.; Whoriskey, F.G.; Patterson, D.A.; Crossin, G.T. Consequences of captive breeding: Fitness implications for wild-origin, hatchery-spawned Atlantic salmon kelts upon their return to the wild. Biol. Conserv. 2018, 225, 144–153. [Google Scholar] [CrossRef]
- Tort, L.; Balasch, J.C.; Mackenzie, S. Fish immune system. A crossroads between innate and adaptive responses. Inmunología 2003, 22, 277–286. [Google Scholar]
- Cohen, J.I. Herpesvirus latency. J. Clin. Investig. 2020, 130, 3361–3369. [Google Scholar] [CrossRef] [PubMed]
- Sano, N.; Moriwake, M.; Hondo, R.; Sano, T. Herpesvirus cyprini: A search for viral genome in infected fish by infected fish by in situ hybridization. J. Fish Dis. 1993, 16, 495–499. [Google Scholar] [CrossRef]
- Chai, W.; Qi, L.; Zhang, Y.; Hong, M.; Jin, L.; Li, L.; Yuan, J. Evaluation of Cyprinid herpesvirus 2 latency and reactivation in Carassius gibel. Microorganisms 2020, 8, 445. [Google Scholar] [CrossRef]
- Tolo, I.E.; Bajer, P.G.; Mor, S.K.; Phelps, N.B. Disease ecology and host range of Cyprinid herpesvirus 3 (CyHV-3) in CyHV-3 endemic lakes of North America. J. Fish Dis. 2023, 46, 679–696. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Shavalier, M.A.; Faisal, M.; Loch, T.P. Lake Trout (Salvelinus namaycush) Naturally Infected with Salmovirus salmonidallo3 (SalHV-3; Family Alloherpesviridae) Continue to Harbor the Virus for Nearly a Decade. Viruses 2025, 17, 1466. https://doi.org/10.3390/v17111466
Shavalier MA, Faisal M, Loch TP. Lake Trout (Salvelinus namaycush) Naturally Infected with Salmovirus salmonidallo3 (SalHV-3; Family Alloherpesviridae) Continue to Harbor the Virus for Nearly a Decade. Viruses. 2025; 17(11):1466. https://doi.org/10.3390/v17111466
Chicago/Turabian StyleShavalier, Megan A., Mohamed Faisal, and Thomas P. Loch. 2025. "Lake Trout (Salvelinus namaycush) Naturally Infected with Salmovirus salmonidallo3 (SalHV-3; Family Alloherpesviridae) Continue to Harbor the Virus for Nearly a Decade" Viruses 17, no. 11: 1466. https://doi.org/10.3390/v17111466
APA StyleShavalier, M. A., Faisal, M., & Loch, T. P. (2025). Lake Trout (Salvelinus namaycush) Naturally Infected with Salmovirus salmonidallo3 (SalHV-3; Family Alloherpesviridae) Continue to Harbor the Virus for Nearly a Decade. Viruses, 17(11), 1466. https://doi.org/10.3390/v17111466





