Antiviral Activity of Essential Oils Against Avian Influenza Virus H7N3 In Vitro and In Ovo Models
Abstract
1. Introduction
2. Materials and Methods
2.1. Essential Oils Formulation and Characterization
2.2. Viruses and Cells
2.3. In Vitro Cytotoxicity Assay of the EOs Formulation Using MDCKs
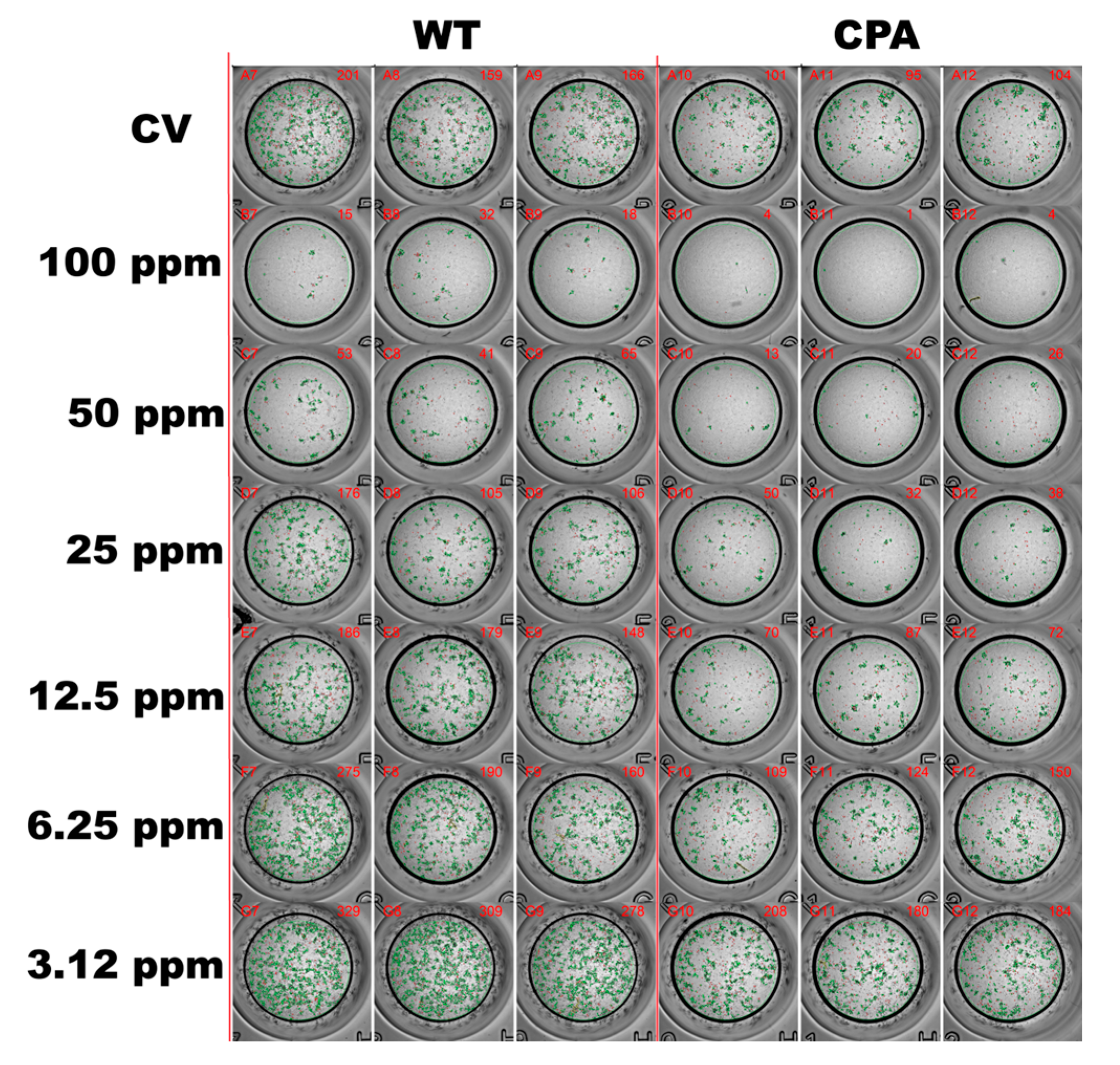
2.4. Antiviral Activity of the EOs Formulation by Focus Reduction Neutralization Test in MDCKs
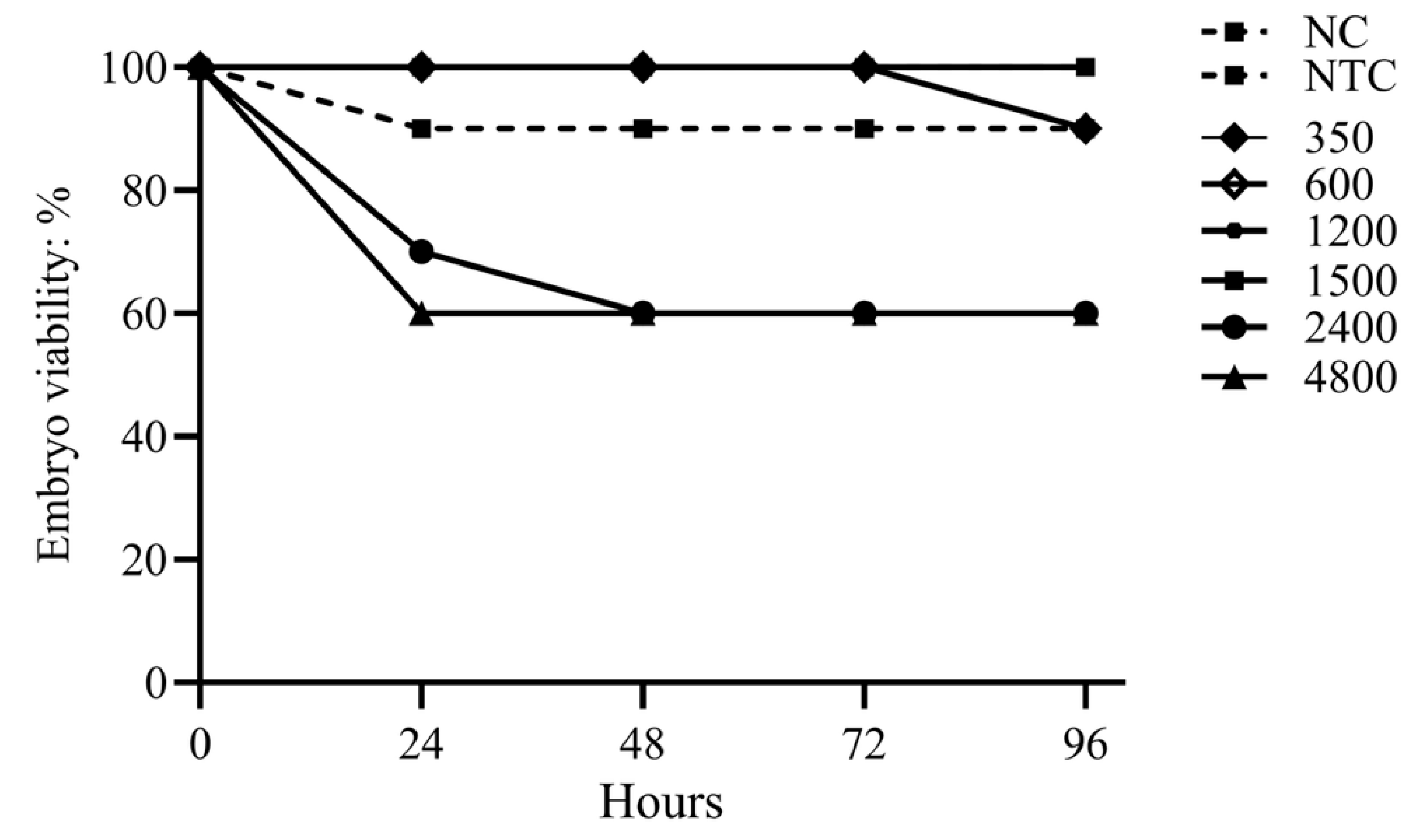
2.5. Assessment of the Toxicity of the EOs Formulation in Chicken Embryos
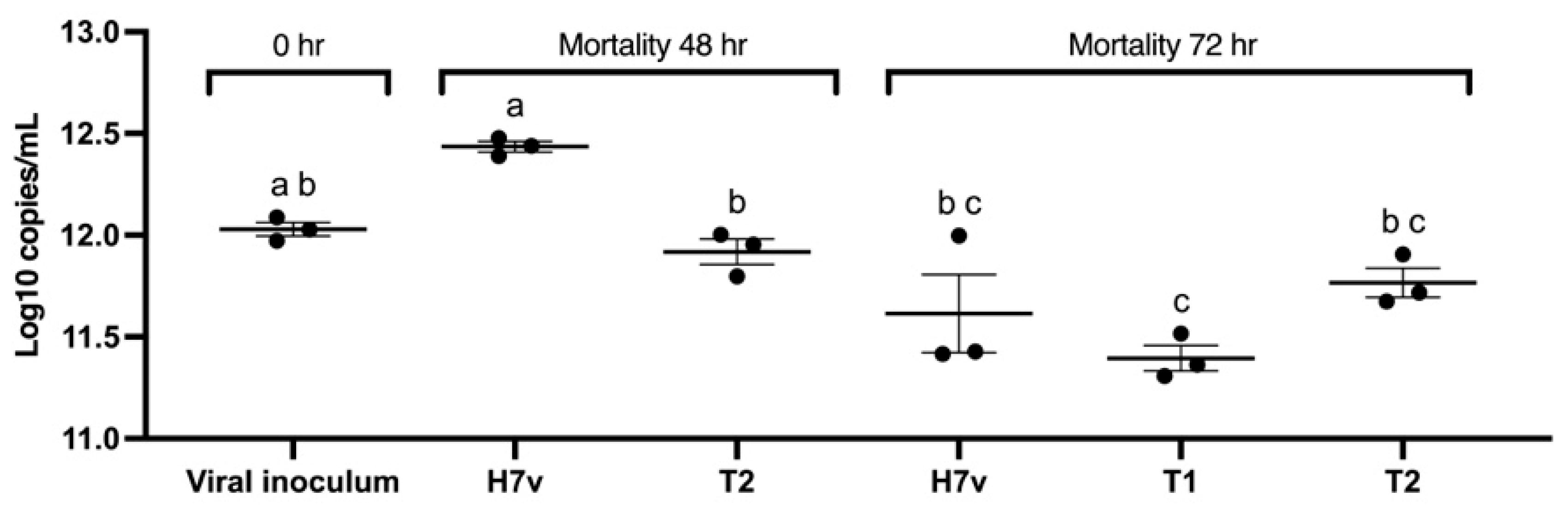
2.6. Viral Inhibition Assay of the EOs Formulation Against AIV H7N3 in Chicken Embryos
2.7. Statistical Analysis
3. Results
3.1. In Vitro Cytotoxicity Assay of the EOs Formulation Using MDCKs
3.2. Assessment of the Toxicity of the EOs Formulation in Chicken Embryos
3.3. Viral Inhibition Assay of the EOs Formulation Against AIV H7N3 in Chicken Embryos

| Compound | RT (min) | Amount (%) |
|---|---|---|
| 1,8 cineole | 20.7 | 7.5 |
| Camphor | 25.5 | 4.1 |
| Geranial | 29.8 | 2.8 |
| β-pinene | 18.2 | 2.2 |
| Thymol | 30.5 | 2.9 |
| Carvacrol | 30.9 | 1.7 |
| α-pinene | 16.3 | 1.9 |
| Caryophyllene oxide | 40.9 | 1.5 |
| p-cimene | 20.3 | 1.8 |
| Treatment | Mean (log10 Copies/mL) ± SEM |
|---|---|
| Viral inoculum (0 h) | 12.03 ± 0.03 a,b |
| H7v (48 h) | 12.43 ± 0.02 a |
| T2 (48 h) | 11.91 ± 0.05 b |
| H7v (72 h) | 11.61 ± 0.16 b,c |
| T1 (72 h) | 11.39 ± 0.054 c |
| T2 (72 h) | 11.77 ± 0.061 b,c |
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| AIV | Avian influenza virus |
| CC50 | Median cytotoxic concentration 50% assay |
| CE | Chicken embryo |
| CPA | Recombinant AIV strain A/chicken/Guanajuato/CPA-06664-18-VS/2018 |
| DMEM | Dulbecco’s modified Eagle media |
| EID50 | Embryo infectious dose 50% |
| ELISA | Enzyme-linked immunosorbent assay |
| EOs | Essential oils |
| FBS | Fetal bovine serum |
| FRNT | Focus reduction neutralization test |
| GC–MS | Gas chromatography–mass spectrometry |
| HLB | Hydrophilic–lipophilic equilibrium |
| HPAIV | Highly pathogenic avian influenza virus |
| HRP-conjugate | Horseradish peroxidase-conjugated |
| IBDV | Infectious Bursal Disease virus |
| IBV | Infectious bronchitis virus |
| IC50 | Inhibitory concentration 50%. |
| IV | Influenza virus |
| LPAIV | Low-pathogenic avian influenza virus |
| MDCK | Madin-Darby canine kidney |
| MTT | (3-[4,5-dimethylthiazol-2-yl]-2,5-diphenyltetrazolium bromide) |
| NDV | Newcastle disease virus |
| OD | Optical density |
| PBS | Phosphate-buffered saline |
| ppm | Parts per million |
| qPCR | Quantitative real-time PCR |
| SI | Selective index |
| TLR | Toll-like receptor |
| TPCK | L-(tosylamide-2-phenyl) ethyl chloromethyl ketone-treated |
| WT | Recombinant AIV strain A/chicken/Queretaro/01/2018 (H7N3) |
References
- Rafeeq, M.; Bilal, R.M.; Batool, F.; Yameen, K.; Farag, M.R.; Madkour, M.; Elnesr, S.S.; El-Shall, N.A.; Dhama, K.; Alagawany, M. Application of Herbs and Their Derivatives in Broiler Chickens: A Review. Worlds Poult. Sci. J. 2023, 79, 95–117. [Google Scholar] [CrossRef]
- Kumoro, A.C.; Wardhani, D.H.; Retnowati, D.S.; Haryani, K. A Brief Review on the Characteristics, Extraction and Potential Industrial Applications of Citronella Grass (Cymbopogon nardus) and Lemongrass (Cymbopogon citratus) Essential Oils. IOP Conf. Ser. Mater. Sci. Eng. 2021, 1053, 012118. [Google Scholar] [CrossRef]
- Escobedo-Bonilla, C.M. Properties of Mexican Oregano (Lippia Spp.) Essential Oils and Their Use in Aquaculture. In Science of Spices and Culinary Herbs—Latest Laboratory, Pre-Clinical, and Clinical Studies; Bentham Science Publishers: Sharjah, United Arab Emirates, 2021; pp. 93–114. [Google Scholar]
- Krishan, G.; Narang, A. Use of Essential Oils in Poultry Nutrition: A New Approach. Vet. Anim. Res. 2014, 1, 156–162. [Google Scholar] [CrossRef]
- Bautista-Hernández, I.; Aguilar, C.N.; Martínez-Ávila, G.C.G.; Torres-León, C.; Ilina, A.; Flores-Gallegos, A.C.; Kumar Verma, D.; Chávez-González, M.L. Mexican Oregano (Lippia graveolens Kunth) as Source of Bioactive Compounds: A Review. Molecules 2021, 26, 5156. [Google Scholar] [CrossRef]
- Reichling, J. Antiviral and Virucidal Properties of Essential Oils and Isolated Compounds—A Scientific Approach. Planta Med. 2022, 88, 587–603. [Google Scholar] [CrossRef] [PubMed]
- Adil, M.; Tiwari, P.; Chen, J.T. Therapeutic Potential of Essential Oils against SARS-CoV-2 Infection. In Ethnopharmacology and Drug Discovery for COVID-19: Anti-SARS-CoV-2 Agents from Herbal Medicines and Natural Products; Springer: Singapore, 2023; pp. 435–445. [Google Scholar] [CrossRef]
- Schnitzler, P.; Astani, A.; Reichling, J. Screening for Antiviral Activities of Isolated Compounds from Essential Oils. Evid. Based Complement. Altern. Med. 2011, 2011, 253643. [Google Scholar] [CrossRef]
- Cagno, V.; Andreozzi, P.; D’Alicarnasso, M.; Silva, P.J.; Mueller, M.; Galloux, M.; Le Goffic, R.; Jones, S.T.; Vallino, M.; Hodek, J.; et al. Broad-Spectrum Non-Toxic Antiviral Nanoparticles with a Virucidal Inhibition Mechanism. Nat. Mater. 2018, 17, 195–203. [Google Scholar] [CrossRef]
- Aricapa Giraldo, H.J.; Salazar Salazar, D.F.; Uribe Diaz, S.; Angel-Isaza, J. Inhibition of Bird Infectious Bronchitis Virus through the Use of Essential Oils. Rev. Electrónica Vet. 2017, 18, 1–10. [Google Scholar]
- Ma, L.; Yao, L. Antiviral Effects of Plant-Derived Essential Oils and Their Components: An Updated Review. Molecules 2020, 25, 2627. [Google Scholar] [CrossRef]
- Deryabin, P.G.; Garaev, T.M.; Finogenova, M.P.; Botikov, A.G.; Shibnev, V.A. Amino Acid Derivatives of Adamantane Carbocycle Are Capable of Inhibiting Replication of Highly Virulent Avian Influenza A/H5N1 Virus. Bull. Exp. Biol. Med. 2014, 157, 62–65. [Google Scholar] [CrossRef]
- Gado, A.R.; Ellakany, H.F.; Elbestawy, A.R.; El-Hack, M.E.A.; Khafaga, A.F.; Taha, A.E.; Arif, M.; Mahgoub, S.A. Herbal Medicine Additives as Powerful Agents to Control and Prevent Avian Influenza Virus in Poultry—A Review. Ann. Anim. Sci. 2019, 19, 905–935. [Google Scholar] [CrossRef]
- Wang, S.; Luo, X.; Yan, R.; Wang, Q.; Qi, Q.; Chi, X.; Zhang, L.; Yu, Z.; Cai, B.; Chen, J.L.; et al. 3-Anhydro-6-Hydroxy-Ophiobolin A Displays High in Vitro and in Vivo Efficacy against Influenza A Virus Infection. Protein Cell 2016, 7, 839–843. [Google Scholar] [CrossRef] [PubMed]
- De Rapper, S.L.; Viljoen, A.; Van Vuuren, S. Optimizing the Antimicrobial Synergism of Melaleuca Alternifolia (Tea Tree) Essential Oil Combinations for Application against Respiratory Related Pathogens. Planta Med. 2023, 89, 454–463. [Google Scholar] [CrossRef]
- Betancur-Galvis, L.; Jimenez-Jarava, O.J.; Rivas, F.; Mendoza-Hernández, W.E.; González-Cardenete, M.A. Synergistic In Vitro Antiviral Effect of Combinations of Ivermectin, Essential Oils, and 18-(Phthalimid-2-Yl)Ferruginol against Arboviruses and Herpesvirus. Pharmaceuticals 2023, 16, 1602. [Google Scholar] [CrossRef] [PubMed]
- Pascual, M.E.; Slowing, K.; Carretero, E.; Sánchez Mata, D.; Villar, A. Lippia: Traditional Uses, Chemistry and Pharmacology: A Review. J. Ethnopharmacol. 2001, 76, 201–214. [Google Scholar] [CrossRef]
- Atkins, S. Verbenaceae. In the Families and Genera of Vascular Plants; Springer: Berlin/Heidelberg, Germany, 2004; pp. 449–468. [Google Scholar] [CrossRef]
- Navrátilová, Z. 1,8-Cineole (Eucalyptol) in a Therapy of Respiratory Diseases. Ceska Slov. Farm. 2024, 73, 181–186. [Google Scholar] [CrossRef]
- Reichling, J.; Stange, R. Antivirale Und Viruzide Eigenschaften von Ätherischen Ölen Und Ihren Isolierten Verbindungen—Stand Der Präklinischen Forschung. Z. Komplementärmedizin 2024, 16, 16–25. [Google Scholar] [CrossRef]
- Shi, J.; Zeng, X.; Cui, P.; Yan, C.; Chen, H. Alarming Situation of Emerging H5 and H7 Avian Influenza and Effective Control Strategies. Emerg. Microbes Infect. 2023, 12, 2155072. [Google Scholar] [CrossRef]
- Cabrera-Gaytán, D.A. Avian Influenza: A Latent Challenge. Rev. Med. Inst. Mex. Seguro Soc. 2024, 62, e6383. [Google Scholar] [CrossRef]
- Lopez-Martinez, I.; Balish, A.; Barrera-Badillo, G.; Jones, J.; Nuñez-García, T.E.; Jang, Y.; Aparicio-Antonio, R.; Azziz-Baumgartner, E.; Belser, J.A.; Ramirez-Gonzalez, J.E.; et al. Highly Pathogenic Avian Influenza A(H7N3) Virus in Poultry Workers, Mexico, 2012. Emerg. Infect. Dis. 2013, 19, 1531–1534. [Google Scholar] [CrossRef]
- Swayne, D.E.; Kapczynski, D.R. Vaccines and Vaccination for Avian Influenza in Poultry. In Animal Influenza, 2nd ed.; John Wiley and Sons: Ames, IA, USA, 2016; pp. 378–434. [Google Scholar] [CrossRef]
- Cattoli, G.; Milani, A.; Temperton, N.; Zecchin, B.; Buratin, A.; Molesti, E.; Aly, M.M.; Arafa, A.; Capua, I. Antigenic Drift in H5N1 Avian Influenza Virus in Poultry Is Driven by Mutations in Major Antigenic Sites of the Hemagglutinin Molecule Analogous to Those for Human Influenza Virus. J. Virol. 2011, 85, 8718–8724. [Google Scholar] [CrossRef] [PubMed]
- Stashenko, E.E.; Martínez, J.R.; Cala, M.P.; Durán, D.C.; Caballero, D. Chromatographic and Mass Spectrometric Characterization of Essential Oils and Extracts from Lippia (Verbenaceae) Aromatic Plants. J. Sep. Sci. 2013, 36, 192–202. [Google Scholar] [CrossRef]
- Moradi, S.; Fazlali, A.; Hamedi, H. Microwave-Assisted Hydro-Distillation of Essential Oil from Rosemary: Comparison with Traditional Distillation. Avicenna J. Med. Biotechnol. 2018, 10, 22–28. [Google Scholar]
- Pérez, D.R. (Ed.) Reverse Genetics of RNA Viruses; Methods in Molecular Biology; Springer: New York, NY, USA, 2024; ISBN 978-1-0716-3532-2. [Google Scholar]
- Abdoli, A.; Soleimanjahi, H.; Tavassoti Kheiri, M.; Jamali, A.; Jamaati, A. Determining Influenza Virus Shedding at Different Time Points in Madin-Darby Canine Kidney Cell Line. Cell J. 2013, 15, 130–135. [Google Scholar]
- Ibišević, M.; Pilipović, S.; Husejnović, M.Š.; Dautović, E.; Kozarević, E.C.; Smajlagić, A.; Karić, E.; Horozić, E.; Pehlić, E.; Toromanović, J. Cytotoxicity Evaluation of Origanum Compactum, Melaleuca Alternifolia and Cinnamomum Camphora Essential Oils on Human Carcinoma Cells. Orient. J. Chem. 2025, 41, 518–527. [Google Scholar] [CrossRef]
- Park, Y.; Kim, A.R.; Hwang, Y.H.; Yang, H.; Lee, J.W.; Kim, M.Y.; Kim, H.S.; Chung, G.T.; Yoo, J.S.; Kim, Y.J.; et al. Comparison of Plaque Reduction and Focus Reduction Neutralization Tests for the Measurement of Neutralizing Antibody Titers against Japanese Encephalitis Virus. J. Virol. Methods 2022, 306, 114540. [Google Scholar] [CrossRef]
- Dufour-Zavala, L.; Swayne, D.E.; Glisson, J.R.; Pearson, J.E.; Reed, W.M.; Jackwood, M.W.; Woolcock, P.R. Laboratory Manual for the Isolation and Identificaction of Avian Pathogens, 5th ed.; The American Association of Avian Pathologists: Jacksonville, FL, USA, 2008. [Google Scholar]
- de Aluja, A.S. Animales de Laboratorio y La Norma Oficial Mexicana (NOM-062-ZOO-1999). Gac. Med. Mex. 2002, 138, 295–298. Available online: https://www.anmm.org.mx/bgmm/1864_2007/ (accessed on 25 March 2025).
- Spackman, E.; Senne, D.A.; Myers, T.J.; Bulaga, L.L.; Garber, L.P.; Perdue, M.L.; Lohman, K.; Daum, L.T.; Suarez, D.L. Development of a Real-Time Reverse Transcriptase PCR Assay for Type A Influenza Virus and the Avian H5 and H7 Hemagglutinin Subtypes. J. Clin. Microbiol. 2002, 40, 3256–3260. [Google Scholar] [CrossRef]
- Luczo, J.M.; Spackman, E. Molecular Evolution of the H5 and H7 Highly Pathogenic Avian Influenza Virus Haemagglutinin Cleavage Site Motif. Rev. Med. Virol. 2025, 35, e70012. [Google Scholar] [CrossRef] [PubMed]
- Abaka, A.M.; Hamuel, J.D.; Abdullahi, T.B.; Abubakar, K.B. In-Ovo Antiviral Activity of Hibiscus Sabdariffa against Newcastle Disease Virus. Biol. Med. Nat. Prod. Chem. 2024, 13, 305–310. [Google Scholar] [CrossRef]
- Abou Baker, D.H.; Amarowicz, R.; Kandeil, A.; Ali, M.A.; Ibrahim, E.A. Antiviral Activity of Lavandula angustifolia L. and Salvia officinalis L. Essential Oils against Avian Influenza H5N1 Virus. J. Agric. Food Res. 2021, 4, 100135. [Google Scholar] [CrossRef]
- Nyandoro, S.S. In Ovo Antiviral Activity of the Constituents of Artabotrys monteiroae and Artabotrys modestus against Infectious Bursal Disease and Newcastle Disease Viruses. Int. J. Biol. Chem. Sci. 2017, 11, 3075–3085. [Google Scholar] [CrossRef][Green Version]
- Zhang, Y.; Li, X.Y.; Zhang, B.S.; Ren, L.N.; Lu, Y.P.; Tang, J.W.; Lv, D.; Yong, L.; Lin, L.T.; Lin, Z.X.; et al. In Vivo Antiviral Effect of Plant Essential Oils against Avian Infectious Bronchitis Virus. BMC Vet. Res. 2022, 18, 90. [Google Scholar] [CrossRef]
- Vimalanathan, S.; Hudson, J. Anti-Influenza Virus Activity of Essential Oils and Vapors. Am. J. Essent. Oils Nat. Prod. 2014, 2, 47–53. [Google Scholar]
- Dharmayanti, N.L.P.I.; Indriani, R.; Nurjanah, D.; Nuradji, H.; Suyatno, T.; Djufry, F. Antiviral Activity of Eucalyptus Globulus and Eucalyptus Citriodora Essential Oils against H5N1 Avian Influenza Virus and Infectious Bronchitis Virus. Trop. J. Pharm. Res. 2025, 24, 185–193. [Google Scholar] [CrossRef]
- Barjesteh, N.; Brisbin, J.T.; Behboudi, S.; Nagy, É.; Sharif, S. Induction of Antiviral Responses against Avian Influenza Virus in Embryonated Chicken Eggs with Toll-like Receptor Ligands. Viral Immunol. 2015, 28, 192–200. [Google Scholar] [CrossRef] [PubMed]
- Baisalova, G.; Kokorayeva, A.; Klivleyeva, N.; Azhikanova, Z.; Torsykbaeva, B. Anti-Avian Influenza Virus H5N3 Activity of Ethanol Extract of Psoralea drupacea Bge. in Chicken Embryos. Planta Med. 2022, 88, 1567. [Google Scholar] [CrossRef]
- Morris, K.M.; Mishra, A.; Raut, A.A.; Gaunt, E.R.; Borowska, D.; Kuo, R.I.; Wang, B.; Vijayakumar, P.; Chingtham, S.; Dutta, R.; et al. The Molecular Basis of Differential Host Responses to Avian Influenza Viruses in Avian Species with Differing Susceptibility. Front. Cell Infect. Microbiol. 2023, 13, 1067993. [Google Scholar] [CrossRef]
- Mieres-Castro, D.; Ahmar, S.; Shabbir, R.; Mora-Poblete, F. Antiviral Activities of Eucalyptus Essential Oils: Their Effectiveness as Therapeutic Targets against Human Viruses. Pharmaceuticals 2021, 14, 1210. [Google Scholar] [CrossRef]
- Arooj, B.; Asghar, S.; Saleem, M.; Khalid, S.H.; Asif, M.; Chohan, T.; Khan, I.U.; Zubair, H.M.; Yaseen, H.S. Anti-Inflammatory Mechanisms of Eucalyptol Rich Eucalyptus Globulus Essential Oil Alone and in Combination with Flurbiprofen. Inflammopharmacology 2023, 31, 1849–1862. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Castellanos-Huerta, I.; Ángel-Isaza, J.A.; Bacab-Cab, L.; Yam-Trujillo, K.; Aranda, A.; Velandia-Cruz, S.A.; Mendez, L.; Petrone-Garcia, V.M.; Ayora-Talavera, G.; Uribe, Á.J. Antiviral Activity of Essential Oils Against Avian Influenza Virus H7N3 In Vitro and In Ovo Models. Viruses 2025, 17, 1464. https://doi.org/10.3390/v17111464
Castellanos-Huerta I, Ángel-Isaza JA, Bacab-Cab L, Yam-Trujillo K, Aranda A, Velandia-Cruz SA, Mendez L, Petrone-Garcia VM, Ayora-Talavera G, Uribe ÁJ. Antiviral Activity of Essential Oils Against Avian Influenza Virus H7N3 In Vitro and In Ovo Models. Viruses. 2025; 17(11):1464. https://doi.org/10.3390/v17111464
Chicago/Turabian StyleCastellanos-Huerta, Inkar, Jaime A. Ángel-Isaza, Lucio Bacab-Cab, Kevin Yam-Trujillo, Alejandro Aranda, Sindi Alejandra Velandia-Cruz, Loufrantz Mendez, Victor M. Petrone-Garcia, Guadalupe Ayora-Talavera, and Álvaro José Uribe. 2025. "Antiviral Activity of Essential Oils Against Avian Influenza Virus H7N3 In Vitro and In Ovo Models" Viruses 17, no. 11: 1464. https://doi.org/10.3390/v17111464
APA StyleCastellanos-Huerta, I., Ángel-Isaza, J. A., Bacab-Cab, L., Yam-Trujillo, K., Aranda, A., Velandia-Cruz, S. A., Mendez, L., Petrone-Garcia, V. M., Ayora-Talavera, G., & Uribe, Á. J. (2025). Antiviral Activity of Essential Oils Against Avian Influenza Virus H7N3 In Vitro and In Ovo Models. Viruses, 17(11), 1464. https://doi.org/10.3390/v17111464








