Genomic Diversity of SARS-CoV-2 Omicron Sublineages and Co-Circulation with Respiratory Viruses in Pediatric Patients in Sao Paulo, Brazil
Abstract
1. Introduction
2. Materials and Methods
2.1. Sample Collection
2.2. Virus Detection
2.3. SARS-CoV-2 Whole Genome Sequencing
2.4. Genomic Analysis
2.5. Data Analysis
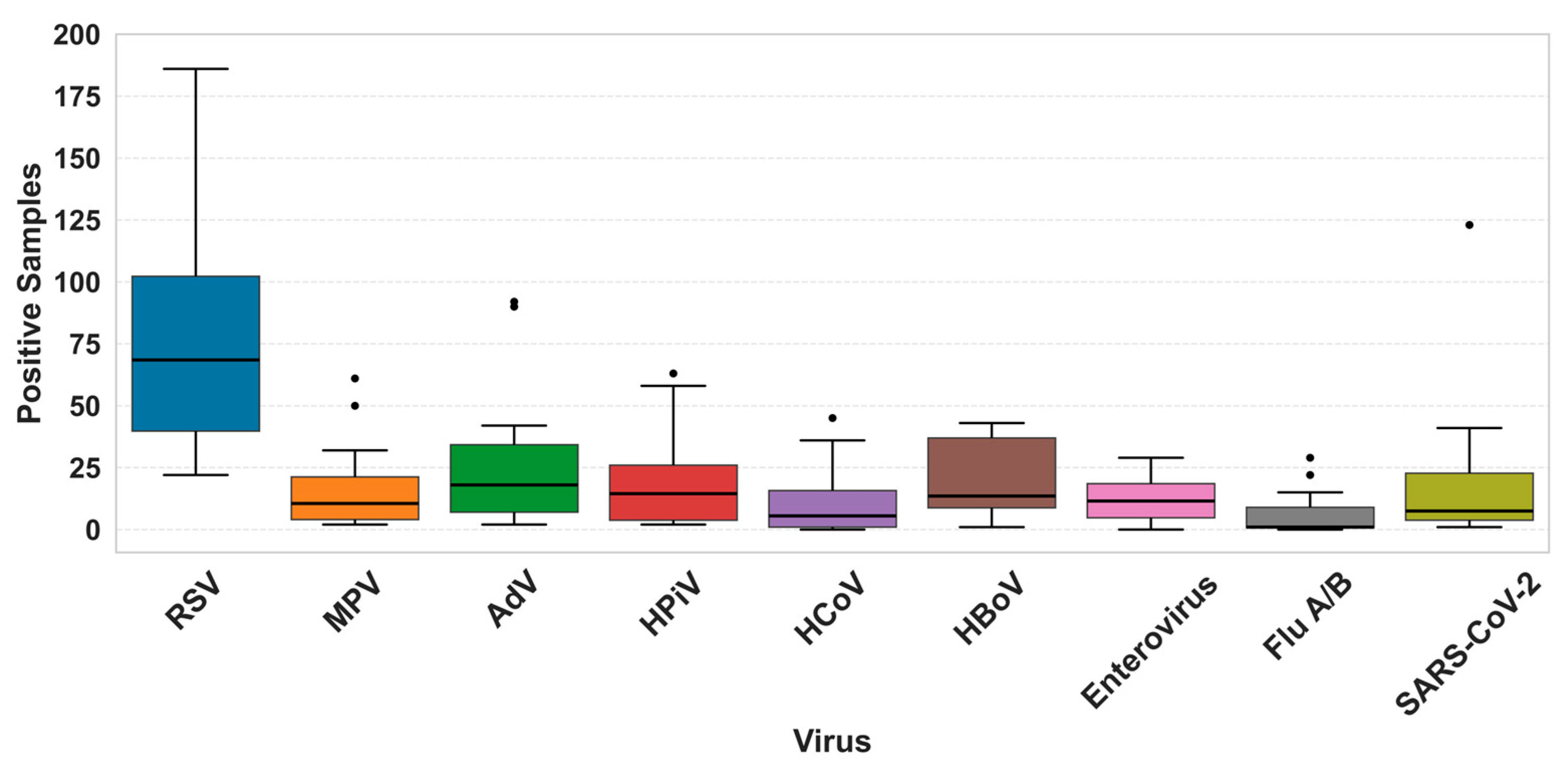
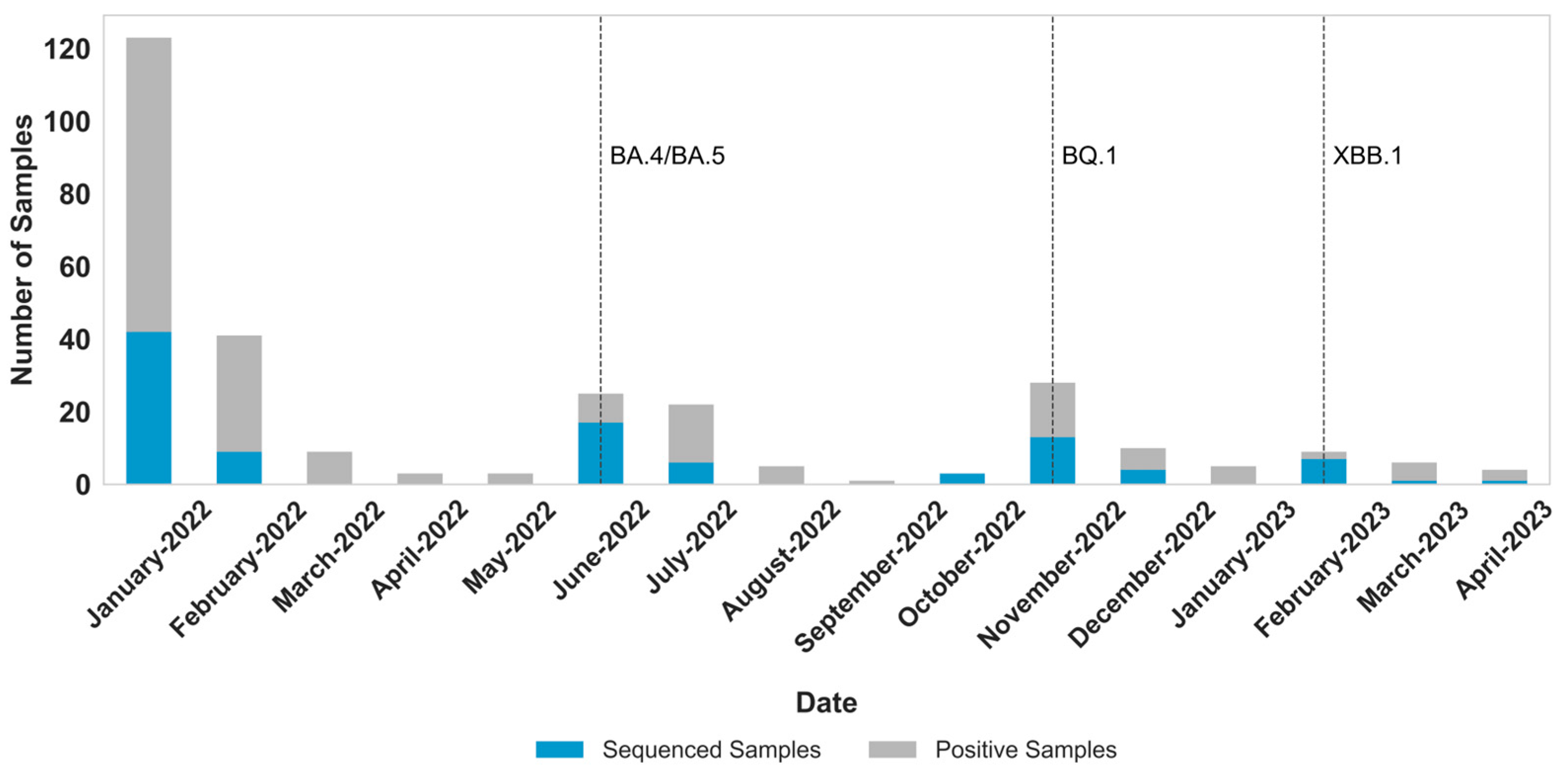
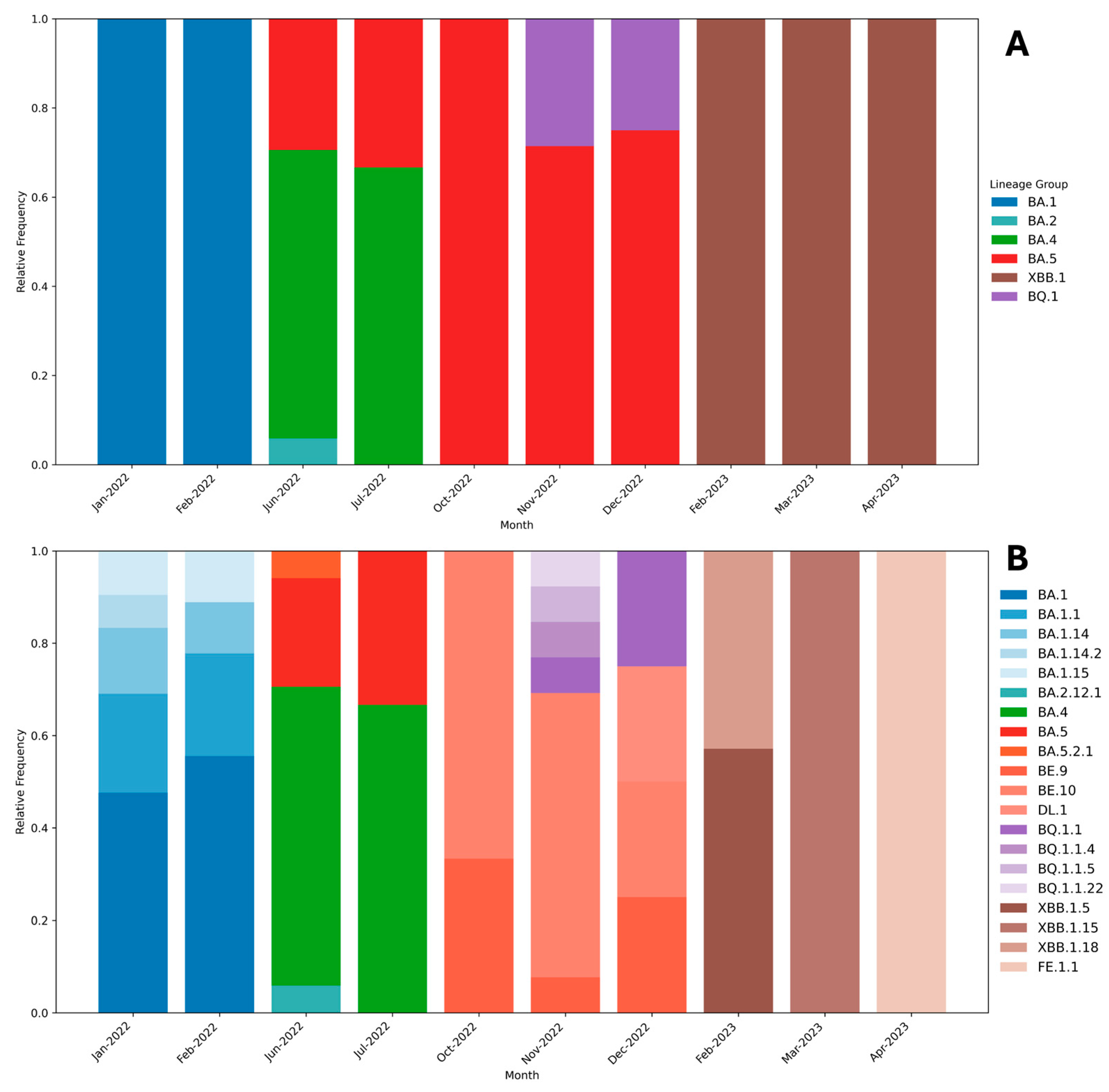
3. Results
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Zhou, P.; Yang, X.L.; Wang, X.G.; Hu, B.; Zhang, L.; Zhang, W.; Si, H.R.; Zhu, Y.; Li, B.; Huang, C.L.; et al. A Pneumonia Outbreak Associated with a New Coronavirus of Probable Bat Origin. Nature 2020, 579, 270–273. [Google Scholar] [CrossRef]
- COVID-19 Cases|WHO COVID-19 Dashboard. Available online: https://data.who.int/dashboards/covid19/cases (accessed on 14 October 2025).
- Coronavírus Brasil. Available online: https://covid.saude.gov.br/ (accessed on 14 October 2025).
- Markov, P.V.; Ghafari, M.; Beer, M.; Lythgoe, K.; Simmonds, P.; Stilianakis, N.I.; Katzourakis, A. The Evolution of SARS-CoV-2. Nat. Rev. Microbiol. 2023, 21, 361–379. [Google Scholar] [CrossRef]
- Lamarca, A.P.; Souza, U.J.B.d.; Moreira, F.R.R.; Almeida, L.G.P.d.; de Menezes, M.T.; de Souza, A.B.; de Ferreira, A.C.S.; Gerber, A.L.; de Lima, A.B.; de Guimarães, A.P.C.; et al. The Omicron Lineages BA.1 and BA.2 (Betacoronavirus SARS-CoV-2) Have Repeatedly Entered Brazil through a Single Dispersal Hub. Viruses 2023, 15, 888. [Google Scholar] [CrossRef]
- Viana, R.; Moyo, S.; Amoako, D.G.; Tegally, H.; Scheepers, C.; Althaus, C.L.; Anyaneji, U.J.; Bester, P.A.; Boni, M.F.; Chand, M.; et al. Rapid Epidemic Expansion of the SARS-CoV-2 Omicron Variant in Southern Africa. Nature 2022, 603, 679–686. [Google Scholar] [CrossRef] [PubMed]
- Christensen, P.A.; Olsen, R.J.; Long, S.W.; Snehal, R.; Davis, J.J.; Ojeda Saavedra, M.; Reppond, K.; Shyer, M.N.; Cambric, J.; Gadd, R.; et al. Signals of Significantly Increased Vaccine Breakthrough, Decreased Hospitalization Rates, and Less Severe Disease in Patients with Coronavirus Disease 2019 Caused by the Omicron Variant of Severe Acute Respiratory Syndrome Coronavirus 2 in Houston, Texas. Am. J. Pathol. 2022, 192, 642–652. [Google Scholar] [CrossRef]
- Bálint, G.; Vörös-Horváth, B.; Széchenyi, A. Omicron: Increased Transmissibility and Decreased Pathogenicity. Signal Transduct. Target. Ther. 2022, 7, 151. [Google Scholar] [CrossRef]
- Taylor, L. Covid-19: Omicron Drives Weekly Record High in Global Infections. BMJ 2022, 376, o66. [Google Scholar] [CrossRef]
- Liu, W.; Huang, Z.; Xiao, J.; Wu, Y.; Xia, N.; Yuan, Q. Evolution of the SARS-CoV-2 Omicron Variants: Genetic Impact on Viral Fitness. Viruses 2024, 16, 184. [Google Scholar] [CrossRef]
- Shanmugaraj, B. Emergence of SARS-CoV-2 Variants KP.2 and KP.3 Sparks Concerns: What Should We Do? Health Care Sci. 2024, 3, 211. [Google Scholar] [CrossRef] [PubMed]
- Eldesouki, R.E.; Uhteg, K.; Mostafa, H.H. The Circulation of Non-SARS-CoV-2 Respiratory Viruses and Coinfections with SARS-CoV-2 during the Surge of the Omicron Variant. J. Clin. Virol. 2022, 153, 105215. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Berger, N.; Davis, P.B.; Kaelber, D.C.; Volkow, N.; Xu, R. Time Trend and Seasonality in Medically Attended Respiratory Syncytial Virus (RSV) Infections in US Children Aged 0–5 Years, January 2010–January 2023. Fam. Med. Community Health 2023, 11, 2453. [Google Scholar] [CrossRef]
- Eden, J.S.; Sikazwe, C.; Xie, R.; Deng, Y.M.; Sullivan, S.G.; Michie, A.; Levy, A.; Cutmore, E.; Blyth, C.C.; Britton, P.N.; et al. Off-Season RSV Epidemics in Australia after Easing of COVID-19 Restrictions. Nat. Commun. 2022, 13, 2884. [Google Scholar] [CrossRef]
- Rhoden, J.; Hoffmann, A.T.; Stein, J.F.; da Silva, M.S.; Gularte, J.S.; Filippi, M.; Demoliner, M.; Girardi, V.; Spilki, F.R.; Fleck, J.D.; et al. Diversity of Omicron Sublineages and Clinical Characteristics in Hospitalized Patients in the Southernmost State of Brazil. BMC Infect. Dis. 2024, 24, 193. [Google Scholar] [CrossRef] [PubMed]
- Souza, U.J.B.d.; Spilki, F.R.; Tanuri, A.; Roehe, P.M.; Campos, F.S. Two Years of SARS-CoV-2 Omicron Genomic Evolution in Brazil (2022–2024): Subvariant Tracking and Assessment of Regional Sequencing Efforts. Viruses 2025, 17, 64. [Google Scholar] [CrossRef]
- Corman, V.M.; Landt, O.; Kaiser, M.; Molenkamp, R.; Meijer, A.; Chu, D.K.W.; Bleicker, T.; Brünink, S.; Schneider, J.; Schmidt, M.L.; et al. Detection of 2019 Novel Coronavirus (2019-NCoV) by Real-Time RT-PCR. Eurosurveillance 2020, 25, 2000045. [Google Scholar] [CrossRef] [PubMed]
- Kodani, M.; Yang, G.; Conklin, L.M.; Travis, T.C.; Whitney, C.G.; Anderson, L.J.; Schrag, S.J.; Taylor, T.H.; Beall, B.W.; Breiman, R.F.; et al. Application of TaqMan Low-Density Arrays for Simultaneous Detection of Multiple Respiratory Pathogens. J Clin Microbiol 2011, 49, 2175–2182. [Google Scholar] [CrossRef]
- Wang, L.; Piedra, P.A.; Avadhanula, V.; Durigon, E.L.; Machablishvili, A.; López, M.R.; Thornburg, N.J.; Peret, T.C.T. Duplex Real-Time RT-PCR Assay for Detection and Subgroup-Specific Identification of Human Respiratory Syncytial Virus. J Virol Methods 2019, 271, 113676. [Google Scholar] [CrossRef] [PubMed]
- Morgan, O.W.; Chittaganpitch, M.; Clague, B.; Chantra, S.; Sanasuttipun, W.; Prapasiri, P.; Naorat, S.; Laosirithavorn, Y.; Peret, T.C.T.; Erdman, D.D.; et al. Hospitalization Due to Human Parainfluenza Virus–Associated Lower Respiratory Tract Illness in Rural Thailand. Influenza Other Respir Viruses 2013, 7, 280–285. [Google Scholar] [CrossRef][Green Version]
- Dare, R.K.; Fry, A.M.; Chittaganpitch, M.; Sawanpanyalert, P.; Olsen, S.J.; Erdman, D.D. Human Coronavirus Infections in Rural Thailand: A Comprehensive Study Using Real-Time Reverse-Transcription Polymerase Chain Reaction Assays. J Infect Dis 2007, 196, 1321–1328. [Google Scholar] [CrossRef]
- Heim, A.; Ebnet, C.; Harste, G.; Pring-Åkerblom, P. Rapid and Quantitative Detection of Human Adenovirus DNA by Real-Time PCR. J Med Virol 2003, 70, 228–239. [Google Scholar] [CrossRef]
- Lu, X.; Holloway, B.; Dare, R.K.; Kuypers, J.; Yagi, S.; Williams, J.V.; Hall, C.B.; Erdman, D.D. Real-Time Reverse Transcription-PCR Assay for Comprehensive Detection of Human Rhinoviruses. J Clin Microbiol 2008, 46, 533–539. [Google Scholar] [CrossRef]
- Lu, X.; Chittaganpitch, M.; Olsen, S.J.; Mackay, I.M.; Sloots, T.P.; Fry, A.M.; Erdman, D.D. Real-Time PCR Assays for Detection of Bocavirus in Human Specimens. J Clin Microbiol 2006, 44, 3231–3235. [Google Scholar] [CrossRef] [PubMed]
- Fan, J.; Cui, D.; Lau, S.; Xie, G.; Guo, X.; Zheng, S.; Huang, X.; Yang, S.; Yang, X.; Huo, Z.; et al. Detection of a Novel Avian Influenza A (H7N9) Virus in Humans by Multiplex One-Step Real-Time RT-PCR Assay. BMC Infect Dis 2014, 14, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Nakauchi, M.; Takayama, I.; Takahashi, H.; Oba, K.; Kubo, H.; Kaida, A.; Tashiro, M.; Kageyama, T. Real-Time RT-PCR Assays for Discriminating Influenza B Virus Yamagata and Victoria Lineages. J Virol Methods 2014, 205, 110–115. [Google Scholar] [CrossRef]
- Plitnick, J.; Griesemer, S.; Lasek-Nesselquist, E.; Singh, N.; Lamson, D.M.; George, K.S. Whole-Genome Sequencing of SARS-CoV-2: Assessment of the Ion Torrent AmpliSeq Panel and Comparison with the Illumina MiSeq ARTIC Protocol. J. Clin. Microbiol. 2021, 59, e0064921. [Google Scholar] [CrossRef]
- Sonnleitner, S.T.; Prelog, M.; Sonnleitner, S.; Hinterbichler, E.; Halbfurter, H.; Kopecky, D.B.C.; Almanzar, G.; Koblmüller, S.; Sturmbauer, C.; Feist, L.; et al. Cumulative SARS-CoV-2 Mutations and Corresponding Changes in Immunity in an Immunocompromised Patient Indicate Viral Evolution within the Host. Nat. Commun. 2022, 13, 2560. [Google Scholar] [CrossRef]
- Alessandrini, F.; Caucci, S.; Onofri, V.; Melchionda, F.; Tagliabracci, A.; Bagnarelli, P.; Sante, L.D.; Turchi, C.; Menzo, S. Evaluation of the Ion AmpliSeq SARS-CoV-2 Research Panel by Massive Parallel Sequencing. Genes 2020, 11, 929. [Google Scholar] [CrossRef]
- O’Toole, Á.; Scher, E.; Underwood, A.; Jackson, B.; Hill, V.; McCrone, J.T.; Colquhoun, R.; Ruis, C.; Abu-Dahab, K.; Taylor, B.; et al. Assignment of Epidemiological Lineages in an Emerging Pandemic Using the Pangolin Tool. Virus Evol. 2021, 7, veab064. [Google Scholar] [CrossRef]
- Piedimonte, G.; Perez, M.K. Respiratory Syncytial Virus Infection and Bronchiolitis. Pediatr. Rev. 2014, 35, 519–530. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Reeves, R.M.; Wang, X.; Bassat, Q.; Brooks, W.A.; Cohen, C.; Moore, D.P.; Nunes, M.; Rath, B.; Campbell, H.; et al. Global Patterns in Monthly Activity of Influenza Virus, Respiratory Syncytial Virus, Parainfluenza Virus, and Metapneumovirus: A Systematic Analysis. Lancet Glob. Health 2019, 7, e1031–e1045. [Google Scholar] [CrossRef]
- Boris, J.; Montes, T.; Montes, D.; Miele, A.; Baik-Han, W.; Gulati, G.; Lew, L.Q. The Impact of COVID-19 Pandemic on Respiratory Syncytial Virus Infection in Children. Pulm. Med. 2024, 2024, 2131098. [Google Scholar] [CrossRef]
- Cai, W.; Köndgen, S.; Tolksdorf, K.; Dürrwald, R.; Schuler, E.; Biere, B.; Schweiger, B.; Goerlitz, L.; Haas, W.; Wolff, T.; et al. Atypical Age Distribution and High Disease Severity in Children with RSV Infections during Two Irregular Epidemic Seasons throughout the COVID-19 Pandemic, Germany, 2021 to 2023. Eurosurveillance 2024, 29, 2300465. [Google Scholar] [CrossRef]
- Thindwa, D.; Li, K.; Cooper-Wootton, D.; Zheng, Z.; Pitzer, V.E.; Weinberger, D.M. Global Patterns of Rebound to Normal RSV Dynamics Following COVID-19 Suppression. BMC Infect. Dis. 2024, 24, 635. [Google Scholar] [CrossRef]
- Li, Y.; Wang, X.; Cong, B.; Deng, S.; Feikin, D.R.; Nair, H. Understanding the Potential Drivers for Respiratory Syncytial Virus Rebound During the Coronavirus Disease 2019 Pandemic. J Infect Dis 2022, 225, 957. [Google Scholar] [CrossRef]
- Kivit, C.M.H.J.; Groen, K.; Jongbloed, M.; Linssen, C.F.M.; van Loo, A.; van Gorp, E.C.M.; van Nieuwkoop, S.; den Hoogen, B.v.; Kruif, M.d. An Off-Season Outbreak of Human Metapneumovirus Infections after Ending of a COVID-19 Lockdown. J. Infect. 2022, 84, 722–746. [Google Scholar] [CrossRef]
- Faico-Filho, K.S.; Barbosa, G.R.; Bellei, N. Peculiar H3N2 Outbreak in São Paulo during Summer and Emergence of the Omicron Variant. J. Infect. 2022, 85, 90. [Google Scholar] [CrossRef]
- Wong, H.; Sjaarda, C.P.; Rand, B.; Roberts, D.; Tozer, K.; Fattouh, R.; Kozak, R.; Sheth, P.M. The Molecular Epidemiology of Respiratory Syncytial Virus in Ontario, Canada from 2022–2024 Using a Custom Whole Genome Sequencing Assay and Analytics Package. J. Clin. Virol. 2025, 176, 105759. [Google Scholar] [CrossRef]
- Adams, G.; Moreno, G.K.; Petros, B.A.; Uddin, R.; Levine, Z.; Kotzen, B.; Messer, K.S.; Dobbins, S.T.; DeRuff, K.C.; Loreth, C.M.; et al. Viral Lineages in the 2022 RSV Surge in the United States. N. Engl. J. Med. 2023, 388, 1335–1337. [Google Scholar] [CrossRef] [PubMed]
- Wang, W.; Guan, R.; Liu, Z.; Zhang, F.; Sun, R.; Liu, S.; Shi, X.; Su, Z.; Liang, R.; Hao, K.; et al. Epidemiologic and Clinical Characteristics of Human Bocavirus Infection in Children Hospitalized for Acute Respiratory Tract Infection in Qingdao, China. Front. Microbiol. 2022, 13, 935688. [Google Scholar] [CrossRef]
- Krammer, M.; Hoffmann, R.; Ruf, H.G.; Neumann, A.U.; Traidl-Hoffmann, C.; Goekkaya, M.; Gilles, S. Ten-Year Retrospective Data Analysis Reveals Frequent Respiratory Co-Infections in Hospitalized Patients in Augsburg. iScience 2024, 27, 110136. [Google Scholar] [CrossRef] [PubMed]
- Bandeira, T.d.J.P.G.; Oliveira, A.L.S.d.; Martins, L.F.P.; Matos, R.M.; dos Santos, S.R.N.G.; Lopes, M.C.; Sobreira, R.T.P.; Rocha, H.A.L. Molecular Detection of Respiratory Viruses: An Observational Study on Respiratory Co-Infections in Children and Adults. Braz. J. Microbiol. 2025, 56, 537–543. [Google Scholar] [CrossRef]
- Korsun, N.; Trifonova, I.; Madzharova, I.; Alexiev, I.; Uzunova, I.; Ivanov, I.; Velikov, P.; Tcherveniakova, T.; Christova, I. Resurgence of Respiratory Syncytial Virus with Dominance of RSV-B during the 2022–2023 Season. Front. Microbiol. 2024, 15, 1376389. [Google Scholar] [CrossRef] [PubMed]
- Weng, S.; Shang, J.; Cheng, Y.; Zhou, H.; Ji, C.; Yang, R.; Wu, A. Genetic Differentiation and Diversity of SARS-CoV-2 Omicron Variant in Its Early Outbreak. Biosaf. Health 2022, 4, 171–178. [Google Scholar] [CrossRef]
- Cao, Y.; Yisimayi, A.; Jian, F.; Song, W.; Xiao, T.; Wang, L.; Du, S.; Wang, J.; Li, Q.; Chen, X.; et al. BA.2.12.1, BA.4 and BA.5 Escape Antibodies Elicited by Omicron Infection. Nature 2022, 608, 593–602. [Google Scholar] [CrossRef]
- Ao, D.; He, X.; Hong, W.; Wei, X. The Rapid Rise of SARS-CoV-2 Omicron Subvariants with Immune Evasion Properties: XBB.1.5 and BQ.1.1 Subvariants. MedComm 2023, 4, e239. [Google Scholar] [CrossRef]
- Aguilar Ticona, J.P.; Xiao, M.; Li, D.; Nery, N.; Hitchings, M.; Belitardo, E.M.M.A.; Fofana, M.O.; Victoriano, R.; Cruz, J.S.; de Moraes, L.; et al. Extensive Transmission of SARS-CoV-2 BQ.1* Variant in a Population with High Levels of Hybrid Immunity: A Prevalence Survey. Int. J. Infect. Dis. 2024, 139, 159–167. [Google Scholar] [CrossRef]
- Machado, R.R.G.; Candido, É.D.; Aguiar, A.S.; Chalup, V.N.; Sanches, P.R.; Dorlass, E.G.; Amgarten, D.E.; Pinho, J.R.R.; Durigon, E.L.; Oliveira, D.B.L. Immune Evasion of SARS-CoV-2 Omicron Subvariants XBB.1.5, XBB.1.16 and EG.5.1 in a Cohort of Older Adults after ChAdOx1-S Vaccination and BA.4/5 Bivalent Booster. Vaccines 2024, 12, 144. [Google Scholar] [CrossRef] [PubMed]
- Bankers, L.; O’Brien, S.C.; Tapay, D.M.; Ho, E.; Armistead, I.; Burakoff, A.; Dominguez, S.R.; Matzinger, S.R. SARS-CoV-2 Disease Severity and Cycle Threshold Values in Children Infected during Pre-Delta, Delta, and Omicron Periods, Colorado, USA, 2021–2022. Emerg. Infect. Dis. 2024, 30, 1182–1192. [Google Scholar] [CrossRef] [PubMed]
- Ng, D.C.E.; Liew, C.H.; Tan, K.K.; Chin, L.; Awang, E.H.B.; Chandirasekharan, D.A.; Surdi Roslan, M.J.B.; Jamil, M.B.; Zainol Abidin, N.Z.; Cheah, Y.K.; et al. Clinical Severity of Omicron and Delta SARS-CoV-2 Infections in Children. Pediatr. Int. 2024, 66, e15777. [Google Scholar] [CrossRef]
- Li, Y.; Liang, H.Y.; Ding, X.; Cao, Y.; Yang, D.; Duan, Y. Effectiveness of COVID-19 Vaccine in Children and Adolescents with the Omicron Variant: A Systematic Review and Meta-Analysis. J. Infect. 2023, 86, e64–e66. [Google Scholar] [CrossRef]
- Lu, W.; Zeng, S.; Yao, Y.; Luo, Y.; Ruan, T. The Effect of COVID-19 Vaccine to the Omicron Variant in Children and Adolescents: A Systematic Review and Meta-Analysis. Front. Public Health 2024, 12, 1338208. [Google Scholar] [CrossRef] [PubMed]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dorlass, E.G.; Scagion, G.P.; Oliveira, F.B.L.d.; Telezynski, B.L.; Eisen, A.K.A.; Caleiro, G.S.; Assis, I.B.d.; Valério, C.A.; Chalup, V.N.; Oliveira, C.M.d.; et al. Genomic Diversity of SARS-CoV-2 Omicron Sublineages and Co-Circulation with Respiratory Viruses in Pediatric Patients in Sao Paulo, Brazil. Viruses 2025, 17, 1421. https://doi.org/10.3390/v17111421
Dorlass EG, Scagion GP, Oliveira FBLd, Telezynski BL, Eisen AKA, Caleiro GS, Assis IBd, Valério CA, Chalup VN, Oliveira CMd, et al. Genomic Diversity of SARS-CoV-2 Omicron Sublineages and Co-Circulation with Respiratory Viruses in Pediatric Patients in Sao Paulo, Brazil. Viruses. 2025; 17(11):1421. https://doi.org/10.3390/v17111421
Chicago/Turabian StyleDorlass, Erick Gustavo, Guilherme Pereira Scagion, Fabyano Bruno Leal de Oliveira, Bruna Larotonda Telezynski, Ana Karolina Antunes Eisen, Giovana Santos Caleiro, Isabela Barbosa de Assis, Camila Araújo Valério, Vanessa Nascimento Chalup, Cairo Monteiro de Oliveira, and et al. 2025. "Genomic Diversity of SARS-CoV-2 Omicron Sublineages and Co-Circulation with Respiratory Viruses in Pediatric Patients in Sao Paulo, Brazil" Viruses 17, no. 11: 1421. https://doi.org/10.3390/v17111421
APA StyleDorlass, E. G., Scagion, G. P., Oliveira, F. B. L. d., Telezynski, B. L., Eisen, A. K. A., Caleiro, G. S., Assis, I. B. d., Valério, C. A., Chalup, V. N., Oliveira, C. M. d., Morais, C. O. d., Otsuka, M., Bain, V., Soledade, M. P., Thomazelli, L. M., Sucupira, C., Mau, L. B., Aguiar, A. S., Almeida, F. J., ... Durigon, E. L. (2025). Genomic Diversity of SARS-CoV-2 Omicron Sublineages and Co-Circulation with Respiratory Viruses in Pediatric Patients in Sao Paulo, Brazil. Viruses, 17(11), 1421. https://doi.org/10.3390/v17111421





