Hepatitis C Direct-Acting Antivirals in the Immunosuppressed Host: Mechanisms, Interactions, and Clinical Outcomes
Abstract
1. Introduction
2. Overview of DAAs
3. Classes of DAAs and Their Mechanisms of Action
3.1. Non-Structural 3/4A (NS3/4A) Protease Inhibitors (PIs)
3.2. NS5A Inhibitors
3.3. NS5B Polymerase Inhibitors
- Nucleotide analog inhibitors such as sofosbuvir are the most widely used and clinically important agents.
- Non-nucleoside inhibitors, such as dasabuvir, are less commonly prescribed.
4. Overview of Immunosuppressive Therapies
5. Common Classes of Immunosuppressive and Biologic Agents and Their Interaction with DAAs
5.1. Calcineurin Inhibitors (CNIs)
5.2. Mammalian Target of Rapamycin (mTOR) Inhibitors
5.3. Antimetabolites
- Azathioprine is a prodrug that is converted to 6-mercaptopurine (6-MP), which blocks purine nucleotide synthesis in rapidly dividing lymphocytes [28].
- MMF is hydrolyzed to mycophenolic acid (MPA), which selectively inhibits inosine monophosphate dehydrogenase (IMPDH), an enzyme essential for de novo purine synthesis in lymphocytes [28].
5.4. Corticosteroids
5.5. Biological Agents
6. Metabolism of DAAs
7. DAA Treatment Outcomes in Immunosuppressed Populations
8. Outcomes of HCV Treatment Using DAAs in Solid Organ Transplant Recipients
9. Outcomes of HCV Treatment Using DAAs in Patients Receiving Biological Agents
10. DDIs Between DAAs and Conventional Immunosuppressants
11. DDIs Between DAAs and Biological Agents
12. Options for DAA Treatment Failure
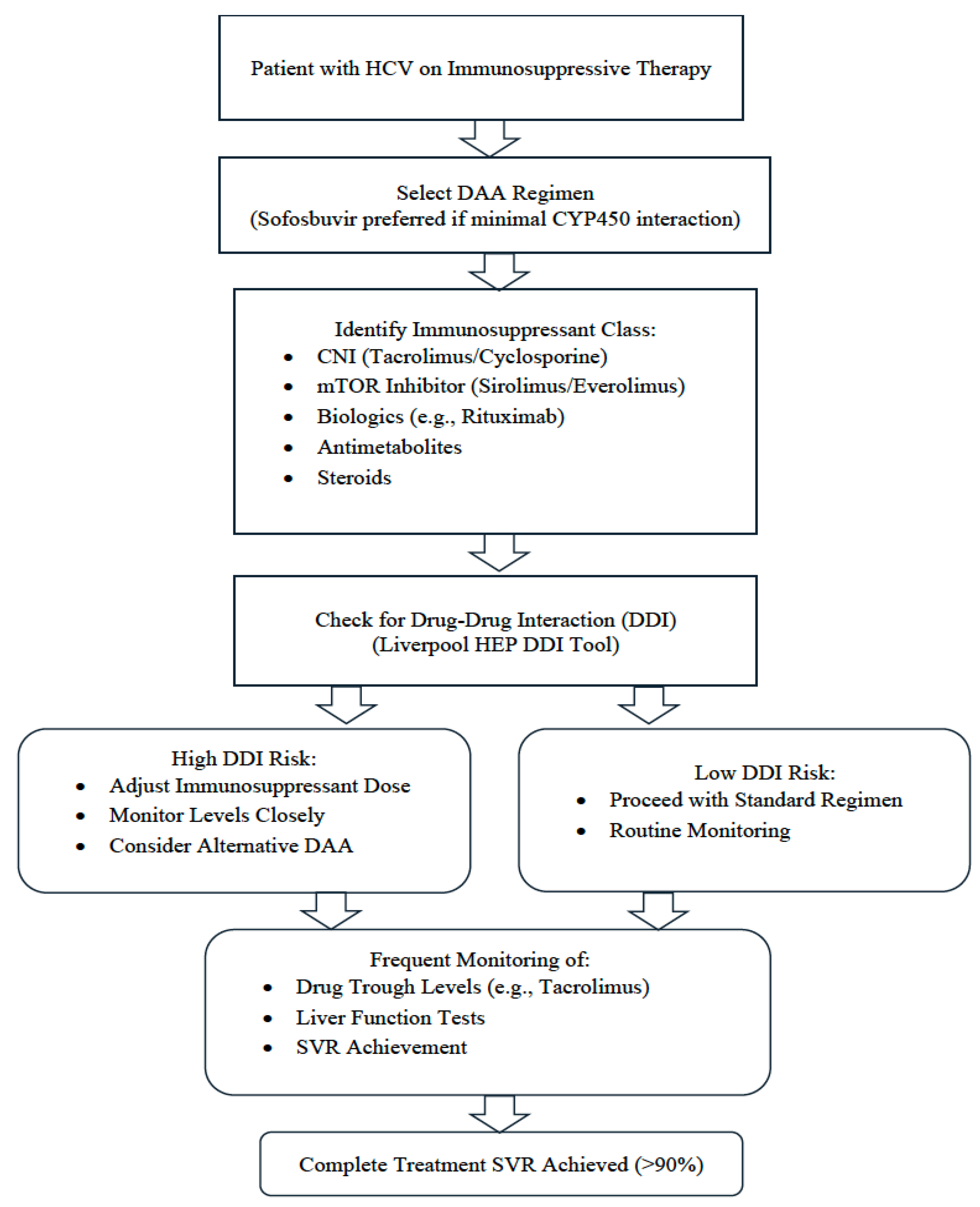
13. Clinical Approach to Managing DAAs in Immunosuppressed Patients
14. Conclusions
Author Contributions
Funding
Conflicts of Interest
Abbreviations
| DAAs | Direct-acting antivirals |
| HCV | Hepatitis C Virus |
| SVR | Sustained virological response |
| mTOR | Mammalian target of rapamycin |
| Peg- IFN | Pegylated interferon |
| NS5A | Non-structural 5A |
| NS5B | Non-structural 5B |
| HIV | Human immunodeficiency virus |
| CYP450 | Cytochrome P450 |
| TNF-α | Tumor necrosis factor-alpha |
| CNIs | Calcineurin inhibitors |
| CYP3A4 | Cytochrome P450 3A4 |
| P-gp | P-glycoprotein |
| HBV | Hepatitis B virus |
| IL-2 | Interleukin-2 |
| mTORC1 | Mammalian target of rapamycin complex 1 |
| MMF | Mycophenolate mofetil |
| 6-MP | 6-mercaptopurine |
| MPA | mycophenolic acid |
| IMPDH | Inosine monophosphate dehydrogenase |
| IBD | Inflammatory bowel disease |
| TPMT | Thiopurine methyltransferase |
| RA | Rheumatoid arthritis |
| BCRP | Breast cancer resistance protein |
| MRP2 | Multidrug resistance-associated protein 2 |
| IFN | Interferon |
| DLQI | Dermatology Quality of Life Index |
| PASI | Psoriasis Area Severity Index |
| NHL | Non-Hodgkin lymphoma |
| AUC | Area under the curve |
References
- Yang, J.; Qi, J.L.; Wang, X.X.; Li, H.H.; Jin, R.; Liu, B.Y.; Liu, H.X.; Rao, H.Y. The burden of hepatitis C virus in the world, China, India, and the United States from 1990 to 2019. Front. Public Health 2023, 11, 1041201. [Google Scholar] [CrossRef]
- Néant, N.; Solas, C. Drug-drug interactions potential of direct-acting antivirals for the treatment of chronic hepatitis C virus infection. Int. J. Antimicrob. Agents 2020, 56, 105571. [Google Scholar] [CrossRef]
- Asselah, T.; Marcellin, P.; Schinazi, R.F. Treatment of hepatitis C virus infection with direct-acting antiviral agents: 100% cure? Liver Int. 2018, 38, 7–13. [Google Scholar] [CrossRef]
- Kapadia, S.N.; Johnson, P.; Marks, K.M.; Schackman, B.R.; Bao, Y. Hepatitis C treatment by nonspecialist providers in the direct-acting antiviral era. Med. Care 2021, 59, 795–800. [Google Scholar] [CrossRef]
- Falade-Nwulia, O.; Sulkowski, M.S. Hepatitis C virus treatment: Simplifying the simple and optimizing the difficult. J. Infect. Dis. 2020, 222, S745–S757. [Google Scholar] [CrossRef]
- Syed, T.A.; Bashir, M.H.; Farooqui, S.M.; Chen, A.; Chen, S.; Nusrat, S.; Fazili, J. Treatment outcomes of hepatitis C-infected patients in specialty clinic vs. primary care physician clinic: A comparative analysis. Gastroenterol. Res. Pract. 2019, 2019, 8434602. [Google Scholar] [CrossRef] [PubMed]
- Hong, J.; Wright, R.C.; Partovi, N.; Yoshida, E.M.; Hussaini, T. Review of clinically relevant drug interactions with next generation hepatitis C direct-acting antiviral agents. J. Clin. Transl. Hepatol. 2020, 8, 322. [Google Scholar] [CrossRef] [PubMed]
- Carta, P.; Curci, F.; Caroti, L.; Aida, L.; DiMaria, L.; Cirami, L. Interactions between Immunosuppressive Therapy and Direct-Acting Antivirals in Kidney Transplant Recipient with Hepatitis C Infection. J. Ren. Hepatic Disord. 2020, 4, 31–34. [Google Scholar] [CrossRef]
- Jadoul, M.; Berenguer, M.C.; Doss, W.; Fabrizi, F.; Izopet, J.; Jha, V.; Kamar, N.; Kasiske, B.L.; Lai, C.L.; Morales, J.M.; et al. Executive summary of the 2018 KDIGO Hepatitis C in CKD Guideline: Welcoming advances in evaluation and management. Kidney Int. 2018, 94, 663–673. [Google Scholar] [CrossRef] [PubMed]
- McCauley, J.A.; Rudd, M.T. Hepatitis C virus NS3/4a protease inhibitors. Curr. Opin. Pharmacol. 2016, 30, 84–92. [Google Scholar] [CrossRef]
- Manzano-Robleda, M.D.C.; Ornelas-Arroyo, V.; Barrientos-Gutiérrez, T.; Méndez-Sánchez, N.; Uribe, M.; Chávez-Tapia, N.C. Boceprevir and telaprevir for chronic genotype 1 hepatitis C virus infection. A systematic review and meta-analysis. Ann. Hepatol. 2015, 14, 46–57. [Google Scholar]
- Gomes, L.O.; Teixeira, M.R.; Rosa, J.A.D.; Feltrin, A.A.; Rodrigues, J.P.V.; Vecchi, M.D.; Carneiro, J.M.M.; Noblat, L.A.C.B.; Chachá, S.G.F.; Martinelli, A.L.C.; et al. Hepatitis C in Brazil: Lessons learned with boceprevir and telaprevir. Rev. Inst. Med. Trop. São Paulo 2018, 60, e29. [Google Scholar] [CrossRef] [PubMed]
- Matthew, A.N.; Zephyr, J.; Hill, C.J.; Jahangir, M.; Newton, A.; Petropoulos, C.J.; Huang, W.; Kurt-Yilmaz, N.; Schiffer, C.A.; Ali, A.; et al. Hepatitis C virus NS3/4A protease inhibitors incorporating flexible P2 quinoxalines target drug resistant viral variants. J. Med. Chem. 2017, 60, 5699–5716. [Google Scholar] [CrossRef] [PubMed]
- de Leuw, P.; Stephan, C. Protease inhibitors for the treatment of hepatitis C virus infection. GMS Infect. Dis. 2017, 5, Doc08. [Google Scholar]
- Ghany, M.G.; Morgan, T.R.; AASLD-IDSA hepatitis C guidance panel. Hepatitis C guidance 2019 update: American Association for the Study of Liver Diseases–Infectious Diseases Society of America recommendations for testing, managing, and treating hepatitis C virus infection. Hepatology 2020, 71, 686–721. [Google Scholar] [CrossRef] [PubMed]
- Gitto, S.; Gamal, N.; Andreone, P. NS 5A inhibitors for the treatment of hepatitis C infection. J. Viral Hepat. 2017, 24, 180–186. [Google Scholar] [CrossRef]
- Kumar, A.; Narang, R.K.; Bhatia, R. Recent advancements in NS5B inhibitors (2011–2021): Structural insights, SAR studies and clinical status. J. Mol. Struct. 2023, 1293, 136272. [Google Scholar] [CrossRef]
- Kirby, B.J.; Symonds, W.T.; Kearney, B.P.; Mathias, A.A. Pharmacokinetic, pharmacodynamic, and drug-interaction profile of the hepatitis C virus NS5B polymerase inhibitor sofosbuvir. Clin. Pharmacokinet. 2015, 54, 677–690. [Google Scholar] [CrossRef]
- Hussain, Y.; Khan, H. Immunosuppressive drugs. Encycl. Infect. Immun. 2022, 726–740. [Google Scholar]
- Xu, Y.-H.; Zhu, W.-M.; Guo, Z. Current status of novel biologics and small molecule drugs in the individualized treatment of inflammatory bowel disease. World J. Gastroenterol. 2022, 28, 6888. [Google Scholar] [CrossRef]
- Burgess, S.; Partovi, N.; Yoshida, E.M.; Erb, S.R.; Azalgara, V.M.; Hussaini, T. Drug interactions with direct-acting antivirals for hepatitis C: Implications for HIV and transplant patients. Ann. Pharmacother. 2015, 49, 674–687. [Google Scholar] [CrossRef]
- Hernandez, N.; Bessone, F. Hepatotoxicity induced by biological agents: Clinical features and current controversies. J. Clin. Transl. Hepatol. 2022, 10, 486. [Google Scholar] [CrossRef] [PubMed]
- Turnes, J.; García-Herola, A.; Morillo-Verdugo, R.; Méndez, M.; Hernández, C.; Sicras-Mainar, A. Impact of potential multiple drug-drug interactions on the adverse event profile of patients with hepatitis C treated with pangenotypic direct-acting antivirals in Spain. Rev. Esp. Sanid. Penit. 2024, 26, 98. [Google Scholar] [CrossRef]
- Lee, H.; Myoung, H.; Kim, S.M. Review of two immunosuppressants: Tacrolimus and cyclosporine. J. Korean Assoc. Oral Maxillofac. Surg. 2023, 49, 311–323. [Google Scholar] [CrossRef]
- Karolin, A.; Genitsch, V.; Sidler, D. Calcineurin inhibitor toxicity in solid organ transplantation. Pharmacology 2021, 106, 347–355. [Google Scholar] [CrossRef]
- Zaza, G.; Granata, S.; Caletti, C.; Signorini, L.; Stallone, G.; Lupo, A. mTOR inhibition role in cellular mechanisms. Transplantation 2018, 102, S3–S16. [Google Scholar] [CrossRef]
- Wagner, M.; Earley, A.K.; Webster, A.C.; Schmid, C.H.; Balk, E.M.; Uhlig, K. Mycophenolic acid versus azathioprine as primary immunosuppression for kidney transplant recipients. Cochrane Database Syst. Rev. 2015, 2015, CD007746. [Google Scholar] [CrossRef] [PubMed]
- Genestier, L.; Paillot, R.; Quemeneur, L.; Izeradjene, K.; Revillard, J.P. Mechanisms of action of methotrexate. Immunopharmacology 2000, 47, 247–257. [Google Scholar] [CrossRef] [PubMed]
- Panackel, C.; Mathew, J.F.; Jacob, M. Immunosuppressive drugs in liver transplant: An insight. J. Clin. Exp. Hepatol. 2022, 12, 1557–1571. [Google Scholar] [CrossRef]
- Parlakpinar, H.; Gunata, M. Transplantation and immunosuppression: A review of novel transplant-related immunosuppressant drugs. Immunopharmacol. Immunotoxicol. 2021, 43, 651–665. [Google Scholar] [CrossRef]
- Dashti-Khavidaki, S.; Saidi, R.; Lu, H. Current status of glucocorticoid usage in solid organ transplantation. World J. Transplant. 2021, 11, 443. [Google Scholar] [CrossRef] [PubMed]
- Ramamoorthy, S.; Cidlowski, J.A. Corticosteroids-mechanisms of action in health and disease. Rheum. Dis. Clin. N. Am. 2016, 42, 15. [Google Scholar] [CrossRef]
- Wood, M.; Whirledge, S. Chapter 9-Mechanism of glucocorticoid action in immunology—Basic concepts. In Reproductive Immunology; Mor, G., Ed.; Academic Press: Cambridge, MA, USA, 2021; pp. 147–170. [Google Scholar]
- de Ruiter, P.E.; Boor, P.P.; de Jonge, J.; Metselaar, H.J.; Tilanus, H.W.; Ijzermans, J.N.; Kwekkeboom, J.; Van der Laan, L.J. Prednisolone does not affect direct-acting antivirals against hepatitis C, but inhibits interferon-alpha production by plasmacytoid dendritic cells. Transpl. Infect. Dis. 2015, 17, 707–715. [Google Scholar] [CrossRef] [PubMed]
- Koshi, E.J.; Young, K.; Mostales, J.C.; Vo, K.B.; Burgess, L.P. Complications of corticosteroid therapy: A comprehensive literature review. J. Pharm. Technol. 2022, 38, 360–367. [Google Scholar]
- Ahmad, M. Clinical pharmacology of biological medicines. Medicine 2023, 52, 51–55. [Google Scholar] [CrossRef]
- Adami, G.; Saag, K.G.; Chapurlat, R.D.; Guañabens, N.; Haugeberg, G.; Lems, W.F.; Matijevic, R.; Peel, N.; Poddubnyy, D.; Geusens, P. Balancing benefits and risks in the era of biologics. Ther. Adv. Musculoskelet. Dis. 2019, 11, 1759720X19883973. [Google Scholar] [CrossRef]
- Geddawy, A.; Ibrahim, Y.F.; Elbahie, N.M.; Ibrahim, M.A. Direct acting anti-hepatitis C virus drugs: Clinical pharmacology and future direction. J. Transl. Intern. Med. 2017, 5, 8–17. [Google Scholar] [CrossRef]
- Salvadori, M.; Tsalouchos, A. Direct-Acting-Antivirals Anti-hepatitis C Virus in Renal Transplant Patients: Relevance of Pharmacologic Interaction. J. Ren. Hepatic Disord. 2020, 4, 29–33. [Google Scholar] [CrossRef]
- Wang, C.S.; Ko, H.H.; Yoshida, E.M.; Marra, C.A.; Richardson, K. Interferon-based combination anti-viral therapy for hepatitis C virus after liver transplantation: A review and quantitative analysis. Am. J. Transplant. 2006, 6, 1586–1599. [Google Scholar] [CrossRef]
- Pascasio, J.M.; Vinaixa, C.; Ferrer, M.T.; Colmenero, J.; Rubin, A.; Castells, L.; Manzano, M.L.; Lorente, S.; Testillano, M.; Xiol, X.; et al. Clinical outcomes of patients undergoing antiviral therapy while awaiting liver transplantation. J. Hepatol. 2017, 67, 1168–1176. [Google Scholar] [CrossRef]
- Belli, L.S.; Duvoux, C.; Berenguer, M.; Berg, T.; Coilly, A.; Colle, I.; Fagiuoli, S.; Khoo, S.; Pageaux, G.P.; Puoti, M.; et al. ELITA consensus statements on the use of DAAs in liver transplant candidates and recipients. J. Hepatol. 2017, 67, 585–602. [Google Scholar] [CrossRef]
- Pacheco, L.S.; Ventura, P.E.; Kist, R.; Garcia, V.D.; Meinerz, G.; Tovo, C.V.; Cantisani, G.P.C.; Zanotelli, M.L.; Mucenic, M.; Keitel, E. Real-world effectiveness and safety of direct-acting antivirals for the treatment of hepatitis C virus in kidney and liver transplant recipients: Experience of a large transplant center in Brazil. Rev. Inst. Med. Trop. São Paulo 2023, 65, e59. [Google Scholar] [CrossRef]
- Sawinski, D.; Wyatt, C.M.; Locke, J.E. Expanding the use of hepatitis C-viremic kidney donors. Kidney Int. 2017, 92, 1031–1033. [Google Scholar] [CrossRef] [PubMed]
- Mansour, M.; Hill, L.; Kerr, J. Safety and effectiveness of direct acting antivirals for treatment of hepatitis C virus in patients with solid organ transplantation. Transpl. Infect. Dis. 2018, 20, e12972. [Google Scholar] [CrossRef] [PubMed]
- Gaur, N.; Malhotra, V.; Agrawal, D.; Singh, S.K.; Beniwal, P.; Sharma, S.; Jhorawat, R.; Rathore, V.; Joshi, H. Sofosbuvir–velpatasvir fixed drug combination for the treatment of chronic hepatitis C infection in patients with end-stage renal disease and kidney transplantation. J. Clin. Exp. Hepatol. 2020, 10, 189–193. [Google Scholar] [CrossRef] [PubMed]
- Akin, M.; Akin, M.; Buldukoglu, O.C.; Adanir, H.; Suleymanlar, I.; Dincer, D.; Yildirim, B. Effectiveness and safety of sofosbuvir/ledipasvir±ribavirin treatment in liver and/or renal transplant patients with chronic hepatitis C: A single-center experience. SAGE Open Med. 2018, 6, 2050312118781416. [Google Scholar] [CrossRef]
- Bixby, A.L.; Fitzgerald, L.; Leek, R.; Mellinger, J.; Sharma, P.; Tischer, S. Impact of direct-acting antivirals for hepatitis C virus therapy on tacrolimus dosing in liver transplant recipients. Transpl. Infect. Dis. 2019, 21, e13078. [Google Scholar] [CrossRef]
- Raschzok, N.; Schott, E.; Reutzel-Selke, A.; Damrah, I.; Gül-Klein, S.; Strücker, B.; Sauer, I.M.; Pratschke, J.; Eurich, D.; Stockmann, M. The impact of directly acting antivirals on the enzymatic liver function of liver transplant recipients with recurrent hepatitis C. Transpl. Infect. Dis. 2016, 18, 896–903. [Google Scholar] [CrossRef]
- Roccatello, D.; Fenoglio, R.; Sciascia, S. The dilemma of treating hepatitis C virus-associated cryoglobulinemia. Curr. Opin. Rheumatol. 2019, 31, 499–504. [Google Scholar] [CrossRef]
- Liao, T.L.; Chen, I.C.; Chen, H.W.; Tang, K.T.; Huang, W.N.; Chen, Y.H.; Chen, Y.M. Exosomal microRNAs as biomarkers for viral replication in tofacitinib-treated rheumatoid arthritis patients with hepatitis C. Sci. Rep. 2024, 14, 937. [Google Scholar] [CrossRef]
- Zhou, X.; Lisenko, K.; Lehners, N.; Egerer, G.; Ho, A.D.; Witzens-Harig, M. The influence of rituximab-containing chemotherapy on HCV load in patients with HCV-associated non-Hodgkin’s lymphomas. Ann. Hematol. 2017, 96, 1501–1507. [Google Scholar] [CrossRef] [PubMed]
- Zhang, M.; Gao, F.; Peng, L.; Shen, L.; Zhao, P.; Ni, B.; Hou, J.; Huang, H. Distinct clinical features and prognostic factors of hepatitis C virus-associated non-Hodgkin’s lymphoma: A systematic review and meta-analysis. Cancer Cell Int. 2021, 21, 524. [Google Scholar] [CrossRef] [PubMed]
- Peveling-Oberhag, J.; Arcaini, L.; Bankov, K.; Zeuzem, S.; Herrmann, E. The anti-lymphoma activity of antiviral therapy in HCV-associated B-cell non-Hodgkin lymphomas: A meta-analysis. J. Viral Hepat. 2016, 23, 536–544. [Google Scholar] [CrossRef]
- Martin-Cardona, A.; Horta, D.; Florez-Diez, P.; Vela, M.; Mesonero, F.; Ramos Belinchón, C.; García, M.J.; Masnou, H.; de la Peña-Negro, L.; Suarez Ferrer, C.; et al. Safety and effectiveness of direct-acting antiviral drugs in the treatment of hepatitis C in patients with inflammatory bowel disease. Dig. Liver Dis. 2024, 56, 468–476. [Google Scholar] [CrossRef]
- Kosloski, M.P.; Zhao, W.; Li, H.; Pugatch, D.; Asatryan, A.; Kort, J.; Mensa, F.J.; Liu, W. Drug-drug interactions of tacrolimus or cyclosporine with glecaprevir and pibrentasvir in healthy subjects. Clin. Pharmacol. Drug Dev. 2019, 8, 779–789. [Google Scholar] [CrossRef]
- Frey, A.; Piras-Straub, K.; Walker, A.; Timm, J.; Gerken, G.; Herzer, K. The influence of immunosuppressants on direct-acting antiviral therapy is dependent on the hepatitis C virus genotype. Transpl. Infect. Dis. 2018, 20, e12803. [Google Scholar] [CrossRef]
- Moretti, M.; Ferro, F.; Baldini, C.; Mosca, M.; Talarico, R. Cryoglobulinemic vasculitis: A 2023 update. Curr. Opin. Rheumatol. 2024, 36, 27–34. [Google Scholar] [CrossRef] [PubMed]
- Sulejmani, N.; Jafri, S.-M. Grazoprevir/elbasvir for the treatment of adults with chronic hepatitis C: A short review on the clinical evidence and place in therapy. Hepatic Med. Evid. Res. 2018, 10, 33–42. [Google Scholar] [CrossRef]
- Pawlotsky, J.-M.; Negro, F.; Aghemo, A.; Berenguer, M.; Dalgard, O.; Dusheiko, G.; Marra, F.; Puoti, M.; Wedemeyer, H. EASL recommendations on treatment of hepatitis C: Final update of the series. J. Hepatol. 2020, 73, 1170–1218. [Google Scholar] [CrossRef]
- Wyles, D.L.; Luetkemeyer, A.F. Understanding Hepatitis C Virus Drug Resistance: Clinical Implications for Current and Future Regimens. Top. Antivir. Med. 2017, 25, 103–109. [Google Scholar]
- Li, H.C.; Yang, C.H.; Lo, S.Y. Hepatitis C Viral Replication Complex. Viruses 2021, 13, 520. [Google Scholar] [CrossRef] [PubMed]
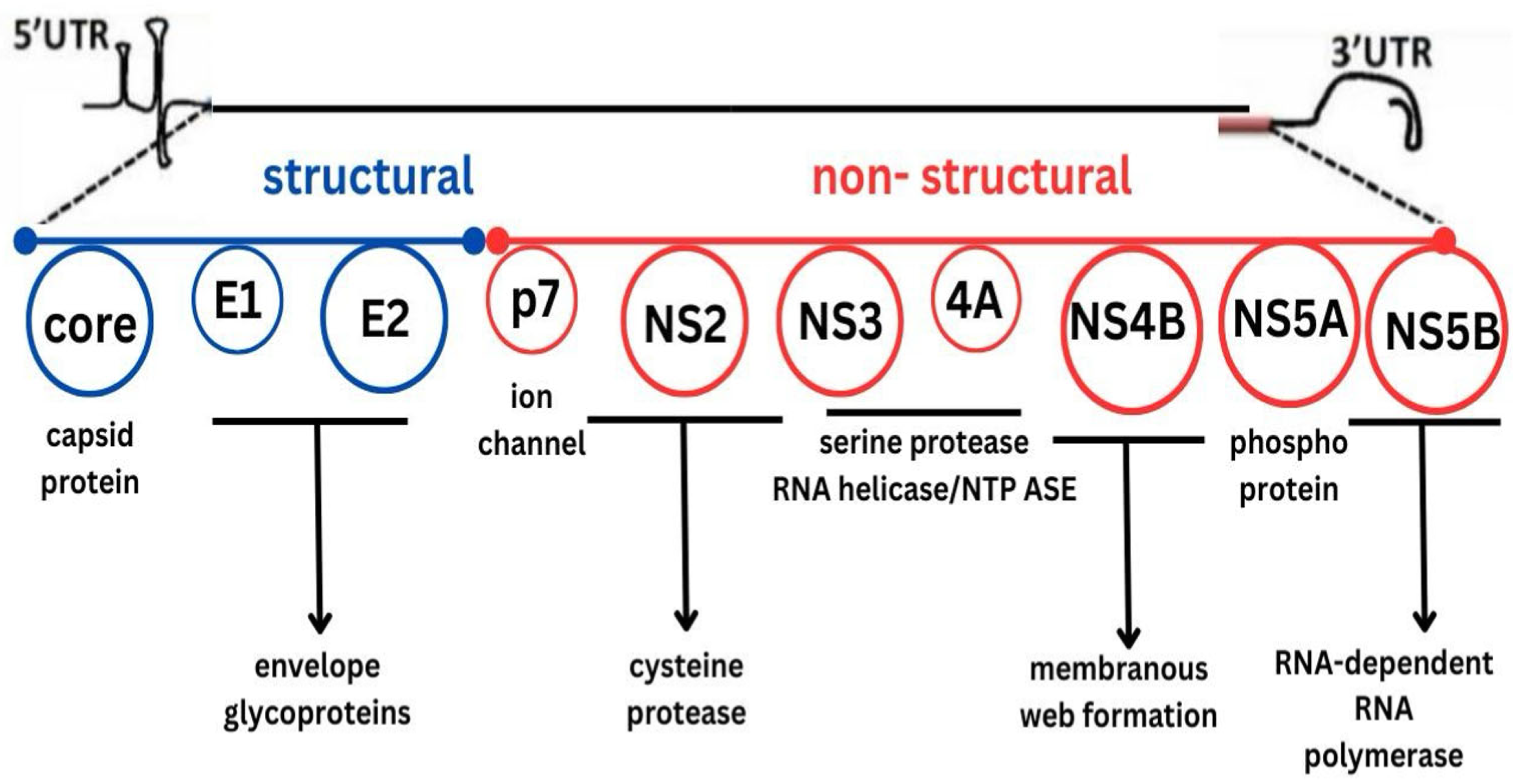
| Class | Target | Example Drugs | Main Clinical Use |
|---|---|---|---|
| NS3/4A Protease Inhibitors (PI) | NS3/4A serine protease | First generation:
| First generation limited by side effects and genotype specificity. Second generation effective even in difficult patients (cirrhosis, renal impairment, transplant, prior failure). |
| NS5A Inhibitors | NS5A protein (phosphoprotein involved in replication and assembly) | Daclatasvir, Ledipasvir, Elbasvir, Velpatasvir, Pibrentasvir | Effective in advanced fibrosis, renal disease, and prior treatment failure. Part of most modern HCV regimens. |
| NS5B Polymerase Inhibitors | NS5B RNA-dependent RNA polymerase | Sofosbuvir (nucleotide analog), Dasabuvir (non-nucleotide) | Used widely in combination with NS5A inhibitors or PIs. Effective in patients with decompensated cirrhosis, HIV coinfection, and transplant. |
| Immunosuppressant Class | Metabolism Pathway | Class of DAA | Interaction Risk | Potential Clinical Impact |
|---|---|---|---|---|
| CNI | CYP3A4, P-gp | NS3/4A Protease Inhibitors | High | High CNI levels associated with nephrotoxicity, and neurotoxicity |
| mTOR | CYP3A4, P-gp | NS3/4A Protease Inhibitors | High | High mTORi levels associated with impaired wound healing, and toxicity |
| Biologics (Rituximab, TNF-α Inhibitors) | Minimal CYP involvement | Minimal interaction | Low | Generally safe; monitor as needed |
| Class | Target Molecules | Example Drugs | Main Clinical Use |
|---|---|---|---|
| IL-1 inhibitors | IL-1 | Anakinra | RA, Systemic juvenile idiopathic arthritis |
| IL-6 inhibitors | IL-6 | Tocilizumab, Sarilumab | RA, Giant cell arteritis |
| IL-17/IL-23 inhibitors | IL-17/IL-23 | Secukinumab (IL-17), Ustekinumab (IL-12/23) | Psoriasis, Psoriatic arthritis, Ankylosing spondylitis |
| TNF-α inhibitors | TNF-α | Infliximab, Adalimumab, Etanercept | RA, IBD, Psoriasis, Ankylosing spondylitis |
| B-cell depleting agents | CD20 | Rituximab | B-cell lymphomas, RA, ANCA-associated vasculitis |
| T cell co-stimulation modulators | CD80/CD86 | Abatacept | RA, Juvenile idiopathic arthritis |
| JAK inhibitors (small molecules) | Janus kinase pathway | Tofacitinib, Baricitinib | RA, Psoriatic arthritis, Ulcerative colitis |
| Integrin inhibitors | α4β7 integrin (gut-specific) | Vedolizumab | IBD |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
AlKaabi, H.; AlSinani, S.; El-Kassas, M.; Alswat, K.A.; AlNaamani, K.M. Hepatitis C Direct-Acting Antivirals in the Immunosuppressed Host: Mechanisms, Interactions, and Clinical Outcomes. Viruses 2025, 17, 1422. https://doi.org/10.3390/v17111422
AlKaabi H, AlSinani S, El-Kassas M, Alswat KA, AlNaamani KM. Hepatitis C Direct-Acting Antivirals in the Immunosuppressed Host: Mechanisms, Interactions, and Clinical Outcomes. Viruses. 2025; 17(11):1422. https://doi.org/10.3390/v17111422
Chicago/Turabian StyleAlKaabi, Hoor, Siham AlSinani, Mohamed El-Kassas, Khalid A. Alswat, and Khalid M. AlNaamani. 2025. "Hepatitis C Direct-Acting Antivirals in the Immunosuppressed Host: Mechanisms, Interactions, and Clinical Outcomes" Viruses 17, no. 11: 1422. https://doi.org/10.3390/v17111422
APA StyleAlKaabi, H., AlSinani, S., El-Kassas, M., Alswat, K. A., & AlNaamani, K. M. (2025). Hepatitis C Direct-Acting Antivirals in the Immunosuppressed Host: Mechanisms, Interactions, and Clinical Outcomes. Viruses, 17(11), 1422. https://doi.org/10.3390/v17111422






