Ecological Interactions among Thrips, Soybean Plants, and Soybean Vein Necrosis Virus in Pennsylvania, USA
Abstract
1. Introduction
2. Materials and Methods
2.1. Soybean Field Layout
2.1.1. Thrips Abundance
2.1.2. Determination of Virus Presence in Field Plants from 2016–2017
2.1.3. Correlations of Thrips Abundance to Weather Factors during 2016–2017
2.1.4. Assessment of Grain Quality Parameters
2.2. Thrips Species Survey on Soybeans, Field Crops, Weeds, and Ornamentals
2.3. Effect of SVNV on Plant Physiology
2.3.1. Protocol Establishment for SVNV Inoculation of Virus in Soybean
2.3.2. Experimental Set-Up for Physiology Experiment
2.3.3. Plant Physiological Parameters Measurements
2.3.4. Plant Morphological Characters
2.4. Statistical Data Analysis
3. Results
3.1. Population Abundance of Thrips in the Soybean Cultivars during 2016–2017 Field Seasons
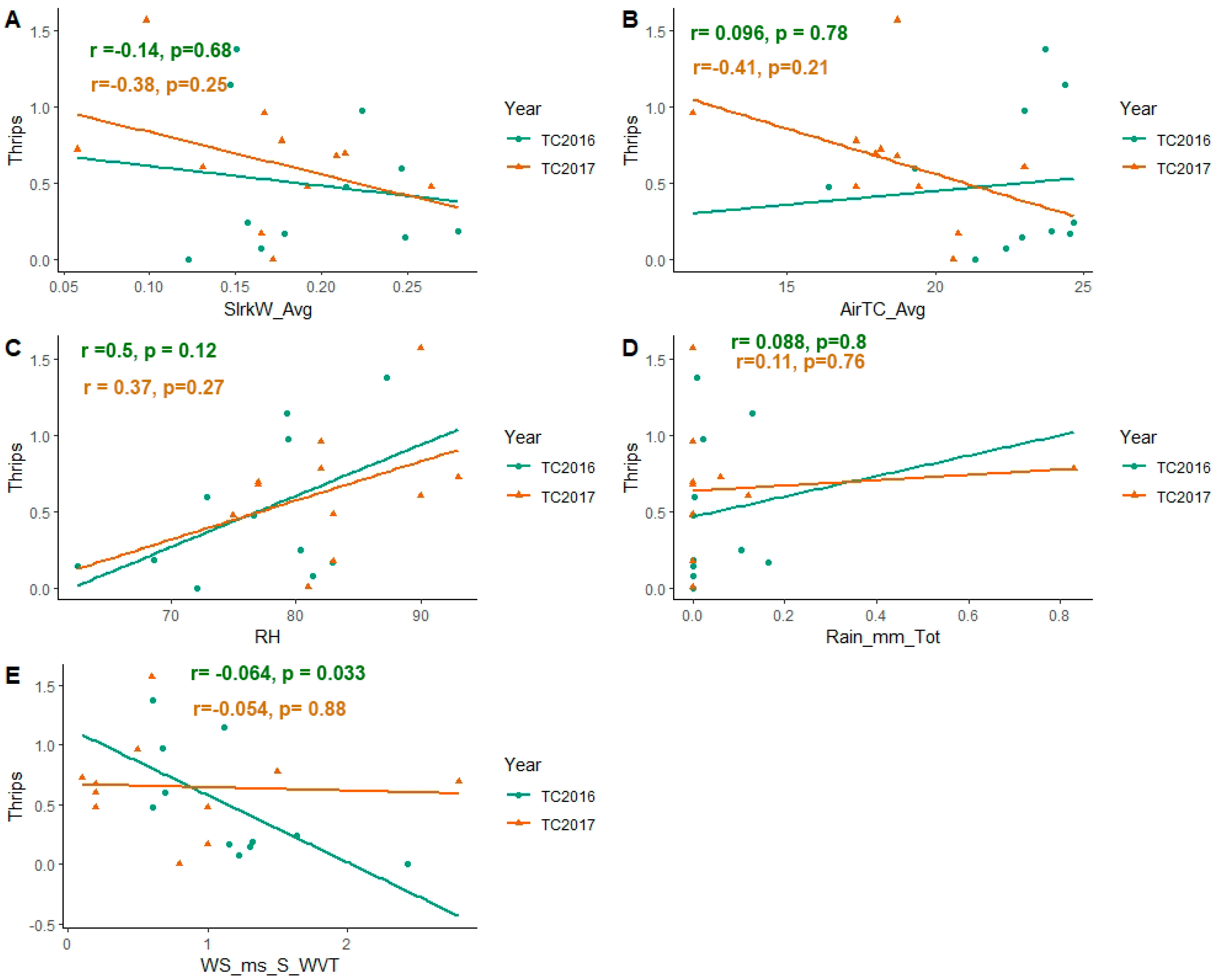
3.2. Effect of Weather Factors on Population Abundance of Soybean Thrips during 2016–2017
3.3. Thrips Species on Soybeans and Nearby Crops in PA in 2018
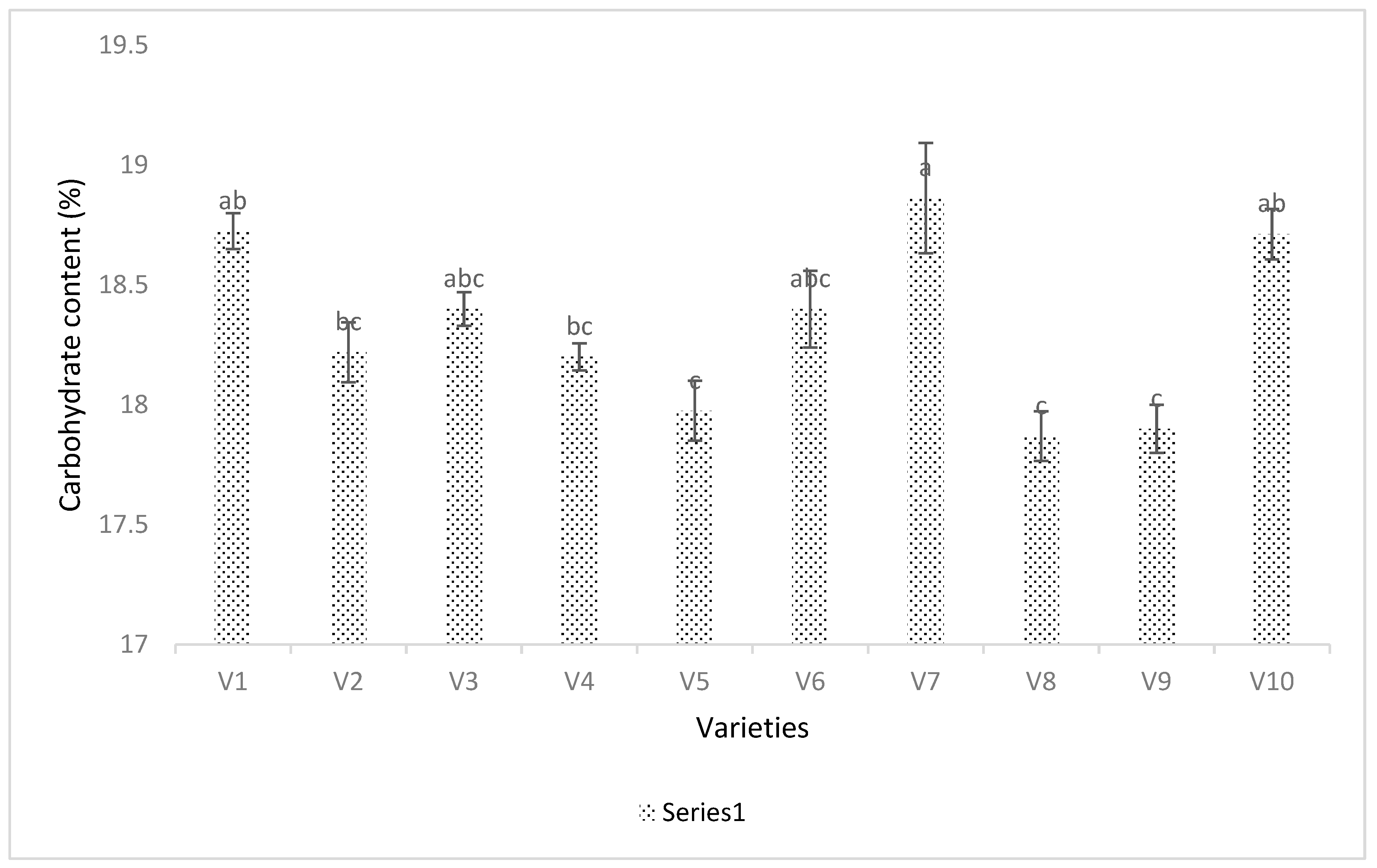
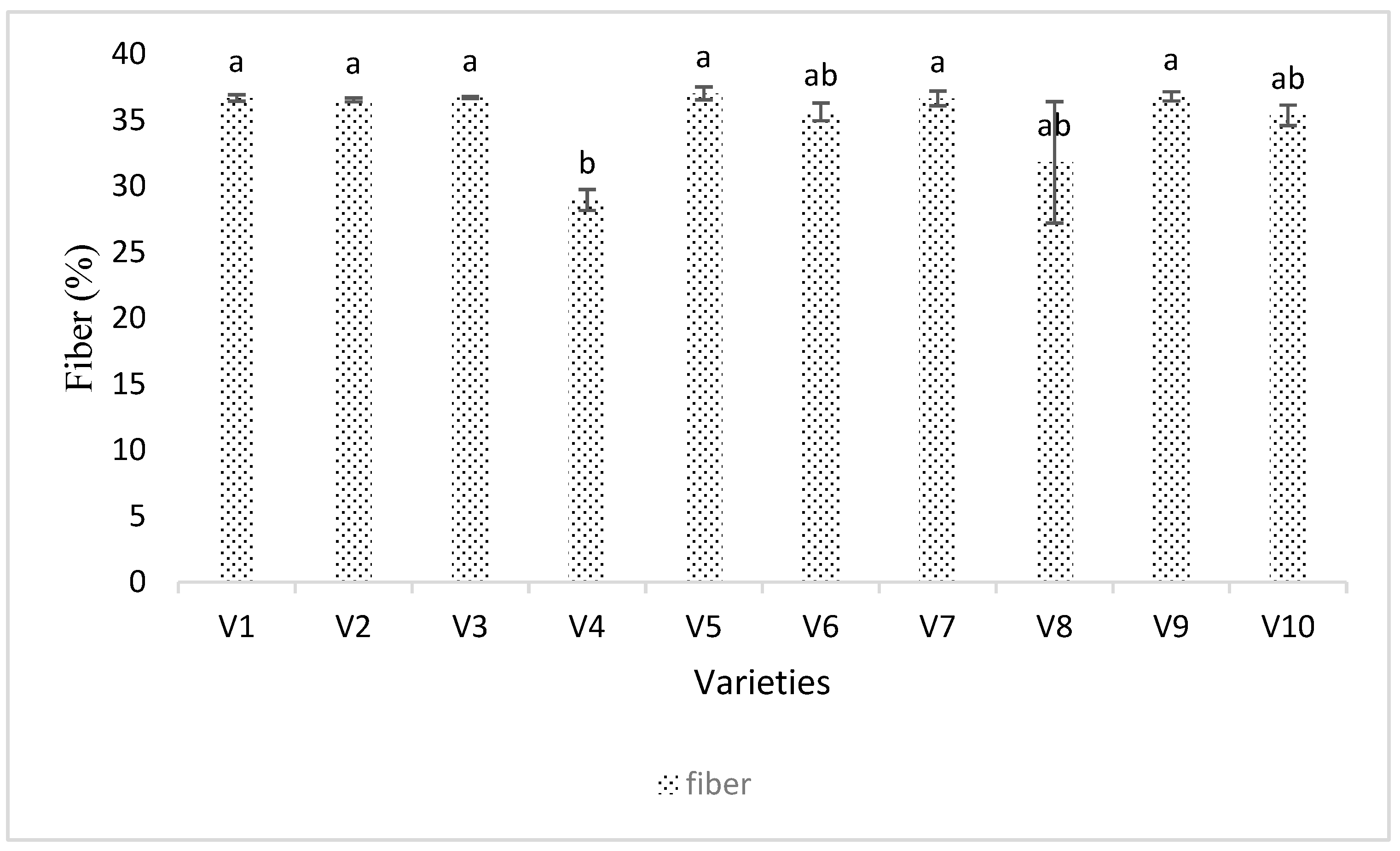
3.4. Seed Analysis Results
3.5. Effect of SVNV on Plant Growth Parameters including Plant Physiological Parameters
3.5.1. Confirmation of Virus Inoculation in the Inoculated and Infected Plants
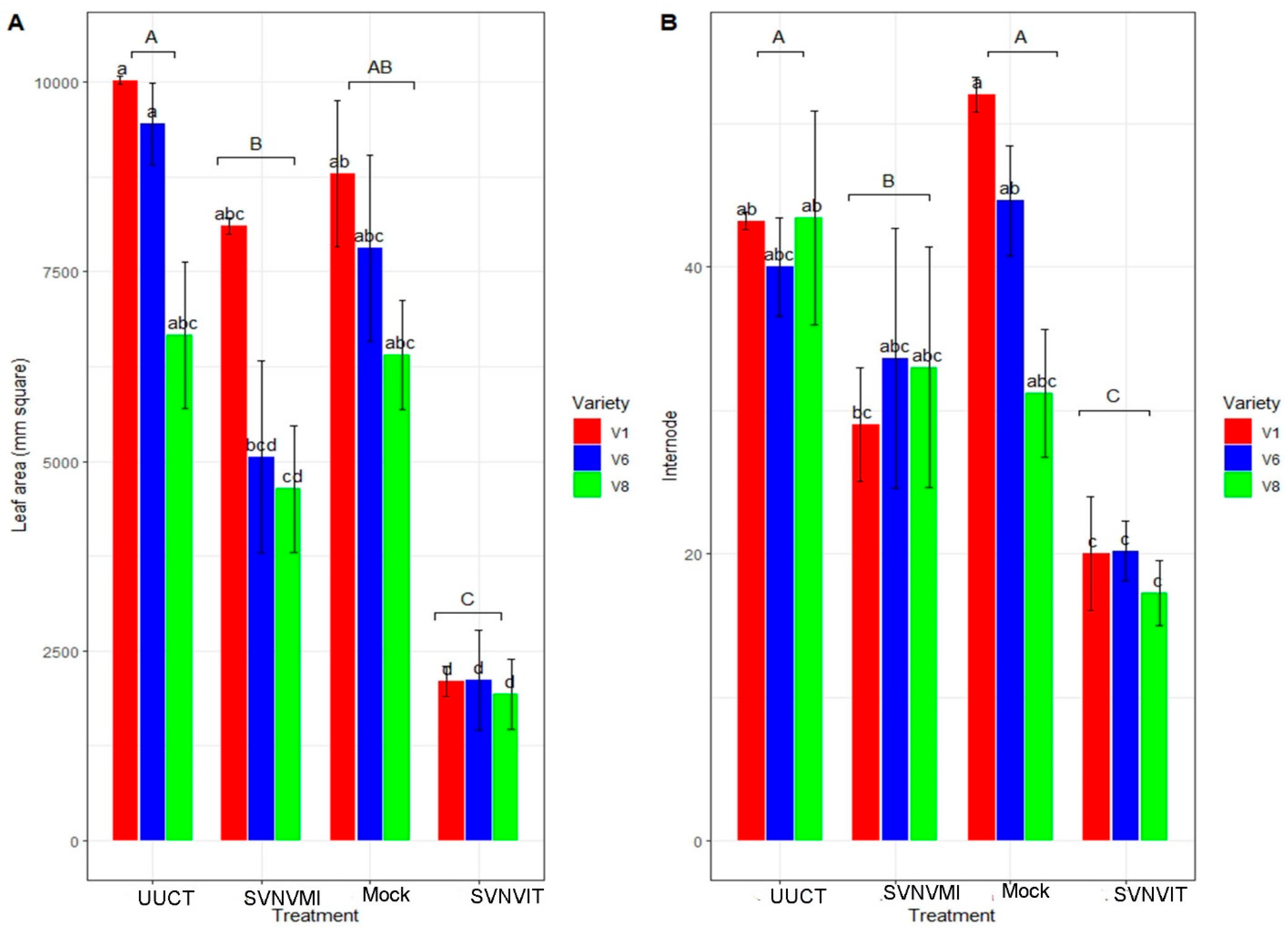
3.5.2. Plant Morphological Characters
3.5.3. Plant Photosynthetic Rate
3.5.4. Stomatal Conductance
3.5.5. Intercellular Carbon Dioxide Content
3.5.6. Transpiration
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Pagano, M.C.; Miransari, M. The importance of soybean production worldwide. In Abiotic and Biotic Stresses in Soybean Production; Elsevier: Amsterdam, The Netherlands, 2016; pp. 1–26. [Google Scholar]
- Hartman, G.L.; West, E.D.; Herman, T.K. Crops that feed the World 2. Soybean—Worldwide production, use, and constraints caused by pathogens and pests. Food Secur. 2011, 3, 5–17. [Google Scholar] [CrossRef]
- Pettersson, D.; Pontoppidan, K. Soybean meal and the potential for upgrading its feeding value by enzyme supplementation. In Soybean-bio-Active Compounds; IntechOpen: London, UK, 2013; pp. 288–307. [Google Scholar]
- Gale, F.; Valdes, C.; Ash, M. Interdependence of China, United States, and Brazil in Soybean Trade; USDA: Washington, DC, USA, 2019. [Google Scholar]
- USDA. Crop Production 2019 Summary. National Agricultural Statistics Service; USDA: Washington, DC, USA, 2020. [Google Scholar]
- Zhou, J.; Kantartzi, S.; Wen, R.-H.; Newman, M.; Hajimorad, M.; Rupe, J.; Tzanetakis, I. Molecular characterization of a new tospovirus infecting soybean. Virus Genes 2011, 43, 289. [Google Scholar] [CrossRef] [PubMed]
- Zhou, J.; Tzanetakis, I.E. Epidemiology of Soybean vein necrosis-associated virus. Phytopathology 2013, 103, 966–971. [Google Scholar] [CrossRef] [PubMed]
- Groves, C.; German, T.; Dasgupta, R.; Mueller, D.; Smith, D.L. Seed transmission of Soybean vein necrosis virus: The first Tospovirus implicated in seed transmission. PLoS ONE 2016, 11, e0147342. [Google Scholar] [CrossRef] [PubMed]
- Keough, S.; Han, J.; Shuman, T.; Wise, K.; Nachappa, P. Effects of Soybean vein necrosis virus on life history and host preference of its vector, Neohydatothrips variabilis, and evaluation of vector status of Frankliniella tritici and Frankliniella fusca. J. Econ. Entomol. 2016, 109, 1979–1987. [Google Scholar] [CrossRef] [PubMed]
- Hameed, A. Tripartite Interactions among Soybean Vein Necrosis Orthotospovirus, Vector Thrips and Soybean Plants; Pennsylvania State University: State College, PA, USA, 2021. [Google Scholar]
- Hameed, A.; Rosa, C.; Rajotte, E.G. A Review on Ecology of Interactions in Soybean Vein Necrosis Orthotospovirus (SVNV): Plants, Vectors, Virus Dispersal and Management Perspectives; IntechOpen: London, UK, 2022. [Google Scholar]
- Zhou, J.; Tzanetakis, I.E. Soybean vein necrosis virus: An emerging virus in North America. Virus Genes 2019, 55, 12–21. [Google Scholar] [CrossRef] [PubMed]
- Shazly, E.-W. Identification and characterization of soybean vein necrosis virus (SVNV): A newly Isolated Thrips-Borne Tospovirus in Egypt. J. Virol. Sci. 2017, 1, 76–90. [Google Scholar]
- Keough, S.A. Surveying Indiana Soybean for Soybean Vein Necrosis Virus (SVNV) and Analysis of SVNV on Life History Traits and Host Preference of Thrips Vectors; Purdue University: West Lafayette, IN, USA, 2015. [Google Scholar]
- Han, J.; Nalam, V.J.; Yu, I.; Nachappa, P. Vector Competence of Thrips Species to Transmit Soybean Vein Necrosis Virus. Front. Microbiol. 2019, 10, 431. [Google Scholar] [CrossRef]
- Hameed, A.; Rosa, C.; Rajotte, E.G. The Effect of Species Soybean Vein Necrosis Orthotospovirus (SVNV) on Life Table Parameters of Its Vector, Soybean Thrips (Neohydatothrips variabilis Thysanoptera: Thripidae). Insects 2022, 13, 632. [Google Scholar] [CrossRef]
- Hameed, A.; Ulmer, J.M.; Miko, I.; Rosa, C.; Rajotte, E.G. Morphology of the Female Reproductive System of the Soybean Thrips, Neohydatothrips variabilis (Beach, 1896) (Thysanoptera: Thripidae). Insects 2022, 13, 566. [Google Scholar] [CrossRef]
- Chamberlin, J.; Todd, J.; Beshear, R.; Culbreath, A.; Demski, J. Overwintering hosts and wingform of thrips, Frankliniella spp., in Georgia (Thysanoptera: Thripidae): Implications for management of spotted wilt disease. Environ. Entomol. 1992, 21, 121–128. [Google Scholar] [CrossRef]
- Irizarry, M. Soybean Vein Necrosis Virus: Impacts of Infection on Yield Loss and Seed Quality and Expansion of Plant Host Range; Iowa State University: Ames, IA, USA, 2016. [Google Scholar]
- Irizarry, M.D.; Elmore, M.G.; Batzer, J.C.; Whitham, S.A.; Mueller, D.S. Alternative Hosts for Soybean vein necrosis virus and Feeding Preferences of Its Vector Soybean Thrips. Plant Health Prog. 2018, 19, 176–181. [Google Scholar] [CrossRef]
- Mueller, A.; Higley, L.; Boethel, D. Soybean thrips. In Handbook of Soybean Insect Pests; Entomological Society of America: Annapolis, MD, USA, 1994; pp. 82–83. [Google Scholar]
- Bloomingdale, C.; Irizarry, M.D.; Groves, R.L.; Mueller, D.S.; Smith, D.L. Seasonal population dynamics of Thrips (Thysanoptera) in Wisconsin and Iowa soybean fields. J. Econ. Entomol. 2016, 110, 133–141. [Google Scholar] [CrossRef] [PubMed]
- Gelber, B. The Pennsylvania Weather Book; Rutgers University Press: New Brunswick, NJ, USA, 2002. [Google Scholar]
- Park, Y.; Kim, K.; Kim, Y. Rapid cold hardening of Thrips palmi (Thysanoptera: Thripidae). Environ. Entomol. 2014, 43, 1076–1083. [Google Scholar] [CrossRef] [PubMed]
- Larentzaki, E.; Shelton, A.; Musser, F.; Nault, B.; Plate, J. Overwintering locations and hosts for onion thrips (Thysanoptera: Thripidae) in the onion cropping ecosystem in New York. J. Econ. Entomol. 2007, 100, 1194–1200. [Google Scholar] [CrossRef] [PubMed]
- Sampson, C.; Bennison, J.; Kirk, W.D. Overwintering of the western flower thrips in outdoor strawberry crops. J. Pest Sci. 2019, 94, 143–152. [Google Scholar] [CrossRef]
- Groves, R.L.; Walgenbach, J.F.; Moyer, J.W.; Kennedy, G.G. The role of weed hosts and tobacco thrips, Frankliniella fusca, in the epidemiology of Tomato spotted wilt virus. Plant Dis. 2002, 86, 573–582. [Google Scholar] [CrossRef] [PubMed]
- Duffus, J.E. Role of weeds in the incidence of virus diseases. Annu. Rev. Phytopathol. 1971, 9, 319–340. [Google Scholar] [CrossRef]
- Chitturi, A.; Conner, K.; Sikora, E.J.; Jacobson, A.L. Monitoring seasonal distribution of thrips vectors of soybean vein necrosis virus in Alabama soybeans. J. Econ. Entomol. 2018, 111, 2562–2569. [Google Scholar] [CrossRef]
- Lagos-Kutz, D.M.; Pawlowski, M.L.; Han, J.; Clough, S.J.; Hartman, G.L. Reduction in productivity of soybean plants infested with Neohyadatothrips variabilis (Thysanoptera: Thripidae) with and without soybean vein necrosis virus. Phytoparasitica 2023, 51, 437–445. [Google Scholar] [CrossRef]
- Bos, L. Crop losses caused by viruses. Crop Prot. 1982, 1, 263–282. [Google Scholar] [CrossRef]
- Zhou, J.; Tzanetakis, I.E. Transmission blockage of an orthotospovirus using synthetic peptides. J. Gen. Virol. 2020, 101, 112–121. [Google Scholar] [CrossRef] [PubMed]
- Reynolds, P.E.; Simpson, J.A.; Thevathasan, N.V.; Gordon, A.M. Effects of tree competition on corn and soybean photosynthesis, growth, and yield in a temperate tree-based agroforestry intercropping system in southern Ontario, Canada. Ecol. Eng. 2007, 29, 362–371. [Google Scholar] [CrossRef]
- Team, R. RStudio: Integrated Development Environment for R; RStudio, Inc.: Boston, MA, USA, 2016. [Google Scholar]
- Wickham, H. ggplot2: Elegant Graphics for Data Analysis; Springer: New York, NY, USA, 2016. [Google Scholar]
- Chellemi, D.; Funderburk, J.; Hall, D. Seasonal abundance of flower-inhabiting Frankliniella species (Thysanoptera: Thripidae) on wild plant species. Environ. Entomol. 1994, 23, 337–342. [Google Scholar] [CrossRef]
- Anderson, N.R.; Irizarry, M.D.; Bloomingdale, C.A.; Smith, D.L.; Bradley, C.A.; Delaney, D.P.; Kleczewski, N.M.; Sikora, E.J.; Mueller, D.S.; Wise, K.A. Effect of soybean vein necrosis on yield and seed quality of soybean. Can. J. Plant Pathol. 2017, 39, 334–341. [Google Scholar] [CrossRef]
- Irwin, M.E.; Yeargan, K.V.; Marston, N.L. Spatial and seasonal patterns of phytophagous thrips in soybean fields with comments on sampling techniques. Environ. Entomol. 1979, 8, 131–140. [Google Scholar] [CrossRef]
- Ananthakrishnan, T. Bionomics of thrips. Annu. Rev. Entomol. 1993, 38, 71–92. [Google Scholar] [CrossRef]
- Nyasani, J.; Meyhöfer, R.; Subramanian, S.; Poehling, H.M. Effect of intercrops on thrips species composition and population abundance on French beans in Kenya. Entomol. Exp. Appl. 2012, 142, 236–246. [Google Scholar] [CrossRef]
- Opiyo, S.O.; Davidson, D.J.; Herman, E.M.; Opiyo, G.A. Importance of Storage Protein in Soybeans A white paper by Stephen O. Opiyo1, Daniel J. Davidson2, Eliot M. Herman3 and Gladys A. Opiyo4; ilsoyadvisor: Bloomington, IL, USA, 2012. [Google Scholar]
- Beckendorf, E.A.; Catangui, M.A.; Riedell, W.E. Soybean aphid feeding injury and soybean yield, yield components, and seed composition. Agron. J. 2008, 100, 237–246. [Google Scholar] [CrossRef]
- Singer, S.D.; Zou, J.; Weselake, R.J. Abiotic factors influence plant storage lipid accumulation and composition. Plant Sci. 2016, 243, 1–9. [Google Scholar] [CrossRef]
- Poorter, H.; Evans, J.R. Photosynthetic nitrogen-use efficiency of species that differ inherently in specific leaf area. Oecologia 1998, 116, 26–37. [Google Scholar] [CrossRef] [PubMed]
- Zamski, E.; Schaffer, A.A. Photoassimilate Distribution in Plants and Crops: Source-Sink Relationships. In Books in Soils; Routledge: Abingdon, UK, 1996. [Google Scholar]
- Nagasuga, K. Soybean Seed Production and Canopy Photosynthesis in Cultivation. In Soybean-Biomass, Yield and Productivity; IntechOpen: London, UK, 2018. [Google Scholar]
- Jin, J.; Liu, X.; Wang, G.; Mi, L.; Shen, Z.; Chen, X.; Herbert, S.J. Agronomic and physiological contributions to the yield improvement of soybean cultivars released from 1950 to 2006 in Northeast China. Field Crops Res. 2010, 115, 116–123. [Google Scholar] [CrossRef]
- Board, J. Reduced lodging for soybean in low plant population is related to light quality. Crop Sci. 2001, 41, 379–384. [Google Scholar] [CrossRef]
- Nielsen, D. Scheduling irrigations for soybeans with the crop water stress index (CWSI). Field Crops Res. 1990, 23, 103–116. [Google Scholar] [CrossRef]
- Berkowitz, G. Water and salt stress. In Photosynthesis: A Comprehensive Treatise; Raghav, A.S., Ed.; Cambridge University Press: Cambridge, UK, 1998; pp. 226–237. [Google Scholar]
- Egli, D. Comparison of corn and soybean yields in the United States: Historical trends and future prospects. Agron. J. 2008, 100, S-79–S-88. [Google Scholar] [CrossRef]
- Egli, D.B.; Crafts-Brandner, S.J. Soybean. In Photoassimilate Distribution Plants and Crops Source-Sink Relationships; Routledge: Abingdon, UK, 2017; pp. 595–624. [Google Scholar]
- Clover, G.; Azam-Ali, S.; Jaggard, K.; Smith, H. The effects of beet yellows virus on the growth and physiology of sugar beet (Beta vulgaris). Plant Pathol. 1999, 48, 129–138. [Google Scholar] [CrossRef]
- Bertamini, M.; Muthuchelian, K.; Nedunchezhian, N. Effect of grapevine leafroll on the photosynthesis of field grown grapevine plants (Vitis vinifera L. cv. Lagrein). J. Phytopathol. 2004, 152, 145–152. [Google Scholar] [CrossRef]
- Murray, R.R.; Emblow, M.S.; Hetherington, A.M.; Foster, G.D. Plant virus infections control stomatal development. Sci. Rep. 2016, 6, 34507. [Google Scholar] [CrossRef]
- Balachandran, S.; Hurry, V.; Kelley, S.; Osmond, C.; Robinson, S.; Rohozinski, J.; Seaton, G.; Sims, D. Concepts of plant biotic stress. Some insights into the stress physiology of virus-infected plants, from the perspective of photosynthesis. Physiol. Plant. 1997, 100, 203–213. [Google Scholar] [CrossRef]
- Rahoutei, J.; García-Luque, I.; Barón, M. Inhibition of photosynthesis by viral infection: Effect on PSII structure and function. Physiol. Plant. 2000, 110, 286–292. [Google Scholar] [CrossRef]
- Kangasjärvi, S.; Tikkanen, M.; Durian, G.; Aro, E.-M. Photosynthetic light reactions–An adjustable hub in basic production and plant immunity signaling. Plant Physiol. Biochem. 2014, 81, 128–134. [Google Scholar] [CrossRef]
- Lehrer, A.T.; Moore, P.H.; Komor, E. Impact of sugarcane yellow leaf virus (ScYLV) on the carbohydrate status of sugarcane: Comparison of virus-free plants with symptomatic and asymptomatic virus-infected plants. Physiol. Mol. Plant Pathol. 2007, 70, 180–188. [Google Scholar] [CrossRef]
- Olesinski, A.A.; Almon, E.; Navot, N.; Perl, A.; Galun, E.; Lucas, W.J.; Wolf, S. Tissue-specific expression of the tobacco mosaic virus movement protein in transgenic potato plants alters plasmodesmal function and carbohydrate partitioning. Plant Physiol. 1996, 111, 541–550. [Google Scholar] [CrossRef][Green Version]
- Israel, H.; Ross, A. The fine structure of local lesions induced by tobacco mosaic virus in tobacco. Virology 1967, 33, 272–286. [Google Scholar] [CrossRef]
- Dhindsa, R.S.; Plumb-Dhindsa, P.; Thorpe, T.A. Leaf Senescence: Correlated with Increased Levels of Membrane Permeability and Lipid Peroxidation, and Decreased Levels of Superoxide Dismutase and Catalase. J. Exp. Bot. 1981, 32, 93–101. [Google Scholar] [CrossRef]
- Sutherland, M.W. The generation of oxygen radicals during host plant responses to infection. Physiol. Mol. Plant Pathol. 1991, 39, 79–93. [Google Scholar] [CrossRef]









| Host Plant | Species |
|---|---|
| Soybean | Frankliniella fusca |
| Soybean | Frankliniella sp. |
| Soybean | Neohydatothrips variabilis (Beach) |
| Soybean | Frankliniella schultzei |
| Soybean | Frankliniella tritici |
| Soybean | Frankliniella occidentalis |
| Soybean | Thrips tabaci |
| Weeds in soybean | Frankliniella tritici |
| Weeds in soybean | Anaphothrips obscurus |
| Weeds in soybean | Frankliniella schultzei |
| Weeds in soybean | Frankliniella sp. |
| Weeds in soybean | Neohydatothrips variabilis (Beach) |
| Squash | Frankliniella sp. |
| Squash | Thrips tabaci |
| Squash | Frankliniella tritici |
| Melon | Frankliniella tritici |
| Melon | Frankliniella sp. |
| Melon | Neohydatothrips variabilis |
| Petunia | Frankliniella sp. |
| Petunia | Frankliniella tritici |
| Onions | Frankliniella sp. |
| Onions | Thrips tabaci |
| Peony | Haplothrips gowdeyi |
| Peony | Frankliniella tritici |
| Red clover | Frankliniella tritici |
| Nasturtium | Frankliniella tritici |
| Nasturtium | Frankliniella fusca |
| White aster daisy | Frankliniella tritici |
| Viburnum | Frankliniella tritici |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hameed, A.; Rosa, C.; O’Donnell, C.A.; Rajotte, E.G. Ecological Interactions among Thrips, Soybean Plants, and Soybean Vein Necrosis Virus in Pennsylvania, USA. Viruses 2023, 15, 1766. https://doi.org/10.3390/v15081766
Hameed A, Rosa C, O’Donnell CA, Rajotte EG. Ecological Interactions among Thrips, Soybean Plants, and Soybean Vein Necrosis Virus in Pennsylvania, USA. Viruses. 2023; 15(8):1766. https://doi.org/10.3390/v15081766
Chicago/Turabian StyleHameed, Asifa, Cristina Rosa, Cheryle A. O’Donnell, and Edwin G. Rajotte. 2023. "Ecological Interactions among Thrips, Soybean Plants, and Soybean Vein Necrosis Virus in Pennsylvania, USA" Viruses 15, no. 8: 1766. https://doi.org/10.3390/v15081766
APA StyleHameed, A., Rosa, C., O’Donnell, C. A., & Rajotte, E. G. (2023). Ecological Interactions among Thrips, Soybean Plants, and Soybean Vein Necrosis Virus in Pennsylvania, USA. Viruses, 15(8), 1766. https://doi.org/10.3390/v15081766






