Zika Virus in West Africa: A Seroepidemiological Study between 2007 and 2012
Abstract
1. Introduction
2. Materials and Methods
3. Results
4. Discussion
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Musso, D.; Ko, A.I.; Baud, D. Zika Virus Infection—After the Pandemic. N. Engl. J. Med. 2019, 381, 1444–1457. [Google Scholar] [CrossRef]
- Musso, D.; Gubler, D.J. Zika Virus. Clin. Microbiol. Rev. 2016, 29, 487–524. [Google Scholar] [CrossRef] [PubMed]
- Gubler, D.J.; Vasilakis, N.; Musso, D. History and Emergence of Zika Virus. J. Infect. Dis. 2017, 216, S860–S867. [Google Scholar] [CrossRef]
- Duffy, M.R.; Chen, T.H.; Hancock, W.T.; Powers, A.M.; Kool, J.L.; Lanciotti, R.S.; Pretrick, M.; Marfel, M.; Holzbauer, S.; Dubray, C.; et al. Zika virus outbreak on Yap Island, Federated States of Micronesia. N. Engl. J. Med. 2009, 360, 2536–2543. [Google Scholar] [CrossRef] [PubMed]
- Marchi, S.; Trombetta, C.M.; Montomoli, E. Emerging and Re-emerging Arboviral Diseases as a Global Health Problem. In Publich Health. Emerging and Re-Emerging Issues; Majumder, A.A., Kabir, S., Rahman, S., Eds.; IntechOpen: London, UK, 2018. [Google Scholar]
- Gudo, E.S.; Ali, S.; Antonio, V.S.; Chelene, I.R.; Chongo, I.; Demanou, M.; Falk, K.; Guiliche, O.C.; Heinrich, N.; Monteiro, V.; et al. Seroepidemiological Studies of Arboviruses in Africa. Adv. Exp. Med. Biol. 2018, 1062, 361–371. [Google Scholar] [CrossRef] [PubMed]
- Gake, B.; Vernet, M.A.; Leparc-Goffart, I.; Drexler, J.F.; Gould, E.A.; Gallian, P.; de Lamballerie, X. Low seroprevalence of Zika virus in Cameroonian blood donors. Braz. J. Infect. Dis. 2017, 21, 481–483. [Google Scholar] [CrossRef] [PubMed]
- Herrera, B.B.; Chang, C.A.; Hamel, D.J.; Mboup, S.; Ndiaye, D.; Imade, G.; Okpokwu, J.; Agbaji, O.; Bei, A.K.; Kanki, P.J. Continued Transmission of Zika Virus in Humans in West Africa, 1992–2016. J. Infect. Dis. 2017, 215, 1546–1550. [Google Scholar] [CrossRef]
- Mathe, P.; Egah, D.Z.; Muller, J.A.; Shehu, N.Y.; Obishakin, E.T.; Shwe, D.D.; Pam, V.C.; Okolo, M.O.; Yilgwan, C.; Gomerep, S.S.; et al. Low Zika virus seroprevalence among pregnant women in North Central Nigeria, 2016. J. Clin. Virol. 2018, 105, 35–40. [Google Scholar] [CrossRef]
- Grard, G.; Caron, M.; Mombo, I.M.; Nkoghe, D.; Mboui Ondo, S.; Jiolle, D.; Fontenille, D.; Paupy, C.; Leroy, E.M. Zika virus in Gabon (Central Africa)–2007: A new threat from Aedes albopictus? PLoS Negl. Trop. Dis. 2014, 8, e2681. [Google Scholar] [CrossRef]
- World Health Organization. Zika Virus Infection—Cape Verde. Available online: https://www.who.int/csr/don/21-december-2015-zika-cape-verde/en/ (accessed on 15 November 2018).
- Lourenco, J.; de Lourdes Monteiro, M.; Valdez, T.; Monteiro Rodrigues, J.; Pybus, O.; Rodrigues Faria, N. Epidemiology of the Zika Virus Outbreak in the Cabo Verde Islands, West Africa. PLoS Curr. 2018, 10. [Google Scholar] [CrossRef]
- Sassetti, M.; Ze-Ze, L.; Franco, J.; Cunha, J.D.; Gomes, A.; Tome, A.; Alves, M.J. First case of confirmed congenital Zika syndrome in continental Africa. Trans. R. Soc. Trop. Med. Hyg. 2018, 112, 458–462. [Google Scholar] [CrossRef] [PubMed]
- Kraemer, M.U.G.; Brady, O.J.; Watts, A.; German, M.; Hay, S.I.; Khan, K.; Bogoch, I.I. Zika virus transmission in Angola and the potential for further spread to other African settings. Trans. R. Soc. Trop. Med. Hyg. 2017, 111, 527–529. [Google Scholar] [CrossRef] [PubMed]
- Hamer, D.H.; Wilson, M.E.; Jean, J.; Chen, L.H. Epidemiology, Prevention, and Potential Future Treatments of Sexually Transmitted Zika Virus Infection. Curr. Infect. Dis. Rep. 2017, 19, 16. [Google Scholar] [CrossRef] [PubMed]
- World Health Organization. Regional Office for Africa. Zika Virus Risk Assessment in the WHO African Region: A Technical Report; World Health Organization. Regional Office for Africa: Brazzaville, Republic of Congo, 2016. Available online: https://apps.who.int/iris/handle/10665/204478 (accessed on 3 June 2020).
- Geser, A.; Henderson, B.E.; Christensen, S. A multipurpose serological survey in Kenya. 2. Results of arbovirus serological tests. Bull. World Health Organ. 1970, 43, 539–552. [Google Scholar] [PubMed]
- Shen, S.; Shi, J.; Wang, J.; Tang, S.; Wang, H.; Hu, Z.; Deng, F. Phylogenetic analysis revealed the central roles of two African countries in the evolution and worldwide spread of Zika virus. Virol. Sin. 2016, 31, 118–130. [Google Scholar] [CrossRef] [PubMed]
- LaForce, F.M.; Konde, K.; Viviani, S.; Preziosi, M.P. The Meningitis Vaccine Project. Vaccine 2007, 25 (Suppl. 1), A97–A100. [Google Scholar] [CrossRef]
- Sow, S.O.; Okoko, B.J.; Diallo, A.; Viviani, S.; Borrow, R.; Carlone, G.; Tapia, M.; Akinsola, A.K.; Arduin, P.; Findlow, H.; et al. Immunogenicity and safety of a meningococcal A conjugate vaccine in Africans. N. Engl. J. Med. 2011, 364, 2293–2304. [Google Scholar] [CrossRef]
- Diallo, A.; Sow, S.O.; Idoko, O.T.; Hirve, S.; Findlow, H.; Preziosi, M.P.; Elie, C.; Kulkarni, P.S.; Parulekar, V.; Diarra, B.; et al. Antibody Persistence at 1 and 4 Years Following a Single Dose of MenAfriVac or Quadrivalent Polysaccharide Vaccine in Healthy Subjects Aged 2–29 Years. Clin. Infect. Dis. 2015, 61 (Suppl. 5), S521–S530. [Google Scholar] [CrossRef]
- Nurtop, E.; Villarroel, P.M.S.; Pastorino, B.; Ninove, L.; Drexler, J.F.; Roca, Y.; Gake, B.; Dubot-Peres, A.; Grard, G.; Peyrefitte, C.; et al. Combination of ELISA screening and seroneutralisation tests to expedite Zika virus seroprevalence studies. Virol. J. 2018, 15, 192. [Google Scholar] [CrossRef]
- Saba Villarroel, P.M.; Nurtop, E.; Pastorino, B.; Roca, Y.; Drexler, J.F.; Gallian, P.; Jaenisch, T.; Leparc-Goffart, I.; Priet, S.; Ninove, L.; et al. Zika virus epidemiology in Bolivia: A seroprevalence study in volunteer blood donors. PLoS Negl. Trop. Dis. 2018, 12, e0006239. [Google Scholar] [CrossRef]
- Diallo, A.; Sie, A.; Sirima, S.; Sylla, K.; Ndiaye, M.; Bountogo, M.; Ouedraogo, E.; Tine, R.; Ndiaye, A.; Coulibaly, B.; et al. An epidemiological study to assess Plasmodium falciparum parasite prevalence and malaria control measures in Burkina Faso and Senegal. Malar. J. 2017, 16, 63. [Google Scholar] [CrossRef] [PubMed]
- Sow, S.; (Centre for Vaccine Development, Bamako, Mali). Personal communication, 2020.
- Weetman, D.; Kamgang, B.; Badolo, A.; Moyes, C.L.; Shearer, F.M.; Coulibaly, M.; Pinto, J.; Lambrechts, L.; McCall, P.J. Aedes Mosquitoes and Aedes-Borne Arboviruses in Africa: Current and Future Threats. Int. J. Environ. Res. Public Health 2018, 15, 220. [Google Scholar] [CrossRef] [PubMed]
- Henderson, A.D.; Aubry, M.; Kama, M.; Vanhomwegen, J.; Teissier, A.; Mariteragi-Helle, T.; Paoaafaite, T.; Teissier, Y.; Manuguerra, J.C.; Edmunds, J.; et al. Zika seroprevalence declines and neutralizing antibodies wane in adults following outbreaks in French Polynesia and Fiji. Elife 2020, 9. [Google Scholar] [CrossRef] [PubMed]
- Pomar, L.; Vouga, M.; Lambert, V.; Pomar, C.; Hcini, N.; Jolivet, A.; Benoist, G.; Rousset, D.; Matheus, S.; Malinger, G.; et al. Maternal-fetal transmission and adverse perinatal outcomes in pregnant women infected with Zika virus: Prospective cohort study in French Guiana. BMJ 2018, 363, k4431. [Google Scholar] [CrossRef]
- Posen, H.J.; Keystone, J.S.; Gubbay, J.B.; Morris, S.K. Epidemiology of Zika virus, 1947–2007. BMJ Glob. Health 2016, 1, e000087. [Google Scholar] [CrossRef]
- World Health Organization. Regional Office for Africa, Health Emergencies Programme. Weekly Bulletin on Outbreaks and other Emergencies: Week 48: 25 November–01 December 2017. In Weekly Bulletin on Outbreaks and Other Emergencies: 1-23; World Health Organization. Regional Office for Africa: Brazzaville, Republic of Congo, 2017. Available online: https://apps.who.int/iris/handle/10665/259557 (accessed on 3 June 2020).
- Wetsman, N. The missing pieces: Lack of Zika data from Africa complicates search for answers. Nat. Med. 2017, 23, 904–906. [Google Scholar] [CrossRef]
- Musso, D.; Nilles, E.J.; Cao-Lormeau, V.M. Rapid spread of emerging Zika virus in the Pacific area. Clin. Microbiol. Infect. 2014, 20, O595–O596. [Google Scholar] [CrossRef]
- Cao-Lormeau, V.M.; Roche, C.; Teissier, A.; Robin, E.; Berry, A.L.; Mallet, H.P.; Sall, A.A.; Musso, D. Zika virus, French polynesia, South pacific, 2013. Emerg. Infect. Dis. 2014, 20, 1085–1086. [Google Scholar] [CrossRef]
- Haddow, A.D.; Schuh, A.J.; Yasuda, C.Y.; Kasper, M.R.; Heang, V.; Huy, R.; Guzman, H.; Tesh, R.B.; Weaver, S.C. Genetic characterization of Zika virus strains: Geographic expansion of the Asian lineage. PLoS Negl. Trop. Dis. 2012, 6, e1477. [Google Scholar] [CrossRef]
- Lanciotti, R.S.; Kosoy, O.L.; Laven, J.J.; Velez, J.O.; Lambert, A.J.; Johnson, A.J.; Stanfield, S.M.; Duffy, M.R. Genetic and serologic properties of Zika virus associated with an epidemic, Yap State, Micronesia, 2007. Emerg. Infect. Dis. 2008, 14, 1232–1239. [Google Scholar] [CrossRef]
- Esposito, D.; Ferreira, M.; Moraes, F.; Ribeiro, B.; Persona, M.; Fonseca, B. Differences in “in vitro” infectivity of Zika virus lineages reveal cellular preference that could implicate in outbreak intensity. Virology 2018, 15, 27–39. [Google Scholar]
- Rayner, J.O.; Kalkeri, R.; Goebel, S.; Cai, Z.; Green, B.; Lin, S.; Snyder, B.; Hagelin, K.; Walters, K.B.; Koide, F. Comparative Pathogenesis of Asian and African-Lineage Zika Virus in Indian Rhesus Macaque’s and Development of a Non-Human Primate Model Suitable for the Evaluation of New Drugs and Vaccines. Viruses 2018, 10, 229. [Google Scholar] [CrossRef] [PubMed]
- Simonin, Y.; van Riel, D.; Van de Perre, P.; Rockx, B.; Salinas, S. Differential virulence between Asian and African lineages of Zika virus. PLoS Negl. Trop. Dis. 2017, 11, e0005821. [Google Scholar] [CrossRef] [PubMed]
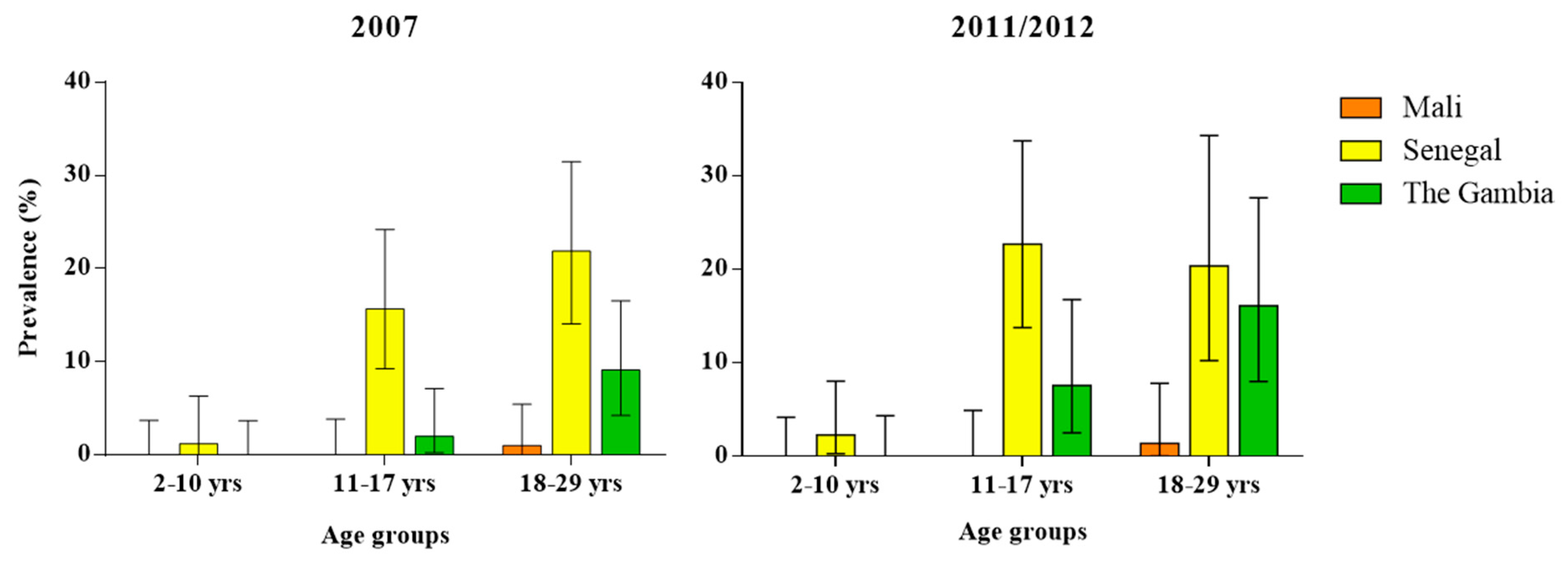
| Study Site | Bamako, Mali | Niakhar, Senegal | Basse Santa Su, The Gambia | ||||
|---|---|---|---|---|---|---|---|
| Year(s) of Collection | 2007 | 2011/2012 | 2007 | 2011/2012 | 2007 | 2011/2012 | |
| Age | 2–10 y | 97 (33.3) | 86 (37.7) | 86 (30.3) | 87 (41.2) | 98 (33.1) | 83 (39.3) |
| 11–17 y | 94 (32.3) | 73 (32.0) | 102 (35.9) | 75 (35.5) | 99 (33.4) | 66 (31.3) | |
| 18–29 y | 100 (34.4) | 69 (30.3) | 96 (33.8) | 49 (23.2) | 99 (33.4) | 62 (29.4) | |
| Sex | M | 171 (58.8) | 128 (56.1) | 158 (55.6) | 111 (52.6) | 179 (60.5) | 128 (60.7) |
| F | 120 (41.2) | 100 (43.9) | 126 (44.4) | 100 (47.4) | 117 (39.5) | 83 (39.3) | |
| Total | 291 | 228 | 284 | 211 | 296 | 211 | |
| Study Site | Bamako, Mali | Niakhar, Senegal | Basse Santa Su, The Gambia | ||||
|---|---|---|---|---|---|---|---|
| Year of Collection | 2007 %(95%CI) | 2011/2012 % (95%CI) | 2007 % (95%CI) | 2011/2012 % (95%CI) | 2007 % (95%CI) | 2011/2012 % (95%CI) | |
| Age | 2–10 y | 0.0 (0.0–3.73) | 0.0 (0.0–4.2) | 1.2 (0.03–6.31) | 2.3 (0.28–8.06) | 0.0 (0.0–3.69) | 0.0 (0.0–4.35) |
| 11–17 y | 0.0 (0.0–3.85) | 0.0 (0.0–4.93) | 15.7 (9.24–24.22) | 22.7 (13.79–33.79) | 2.0 (0.25–7.11) | 7.6 (2.51–16.8) | |
| 18–29 y | 1.0 (0.03–5.45) | 1.4 (0.04–7.81) | 21.9 (14.08–31.47) | 20.4 (10.24–34.34) | 9.1 (4.24–16.56) | 16.1 (8.02–27.67) | |
| Sex | M | 0.6 (0.01–3.22) | 0.8 (0.02–4.28) | 12.0 (7.4–18.14) | 11.7 (6.39–19.19) | 3.4 (1.24–7.15) | 7.8 (3.81–13.9) |
| F | 0.0 (0.0–3.03) | 0.0 (0.0–3.62) | 15.1 (9.33–22.54) | 16.0 (9.43–24.68) | 4.3 (1.4–9.69) | 6.0 (1.98–13.5) | |
| Total | 0.3 (0.01–1.90) | 0.4 (0.01–2.42) | 13.4 (9.65–17.9) | 13.7 (9.4–19.14) | 3.7 (1.87–6.55) | 7.1 (4.03–11.45) | |
| Study Site | Bamako, Mali n (%, 95% CI) | Niakhar, Senegal n (%, 95% CI) | Basse Santa Su, The Gambia n (%, 95% CI) | |
|---|---|---|---|---|
| Age | 2–10 y | 0 (0.0, 0.0–4.35) | 1 (1.4, 0.03–7.30) | 0 (0.0, 0.0–4.45) |
| 11–17 y | 0 (0.0, 0.0–5.21) | 6 (8.0, 2.99–16.60) | 3 (4.5, 0.95–12.71) | |
| 18–29 y | 1 (1.4, 0.04–7.81) | 1 (2.0, 0.05–10.85) | 3 (4.9, 1.03–13.71) | |
| Sex | M | 1 (0.8, 0.02–4.48) | 5 (4.8, 1.58–10.86) | 5 (4.0, 1.31–9.09) |
| F | 0 (0.0, 0.0–3.66) | 3 (3.2, 0.66–9.04) | 1 (1.2, 0.03–6.53) | |
| Total | 1 (0.5, 0.01–2.50) | 8 (4.0, 1.76–7.81) | 6 (2.9, 1.07–6.17) | |
| Study Site | Niakhar, Senegal n | Basse Santa Su, The Gambia n | Total n (%, 95% CI) |
|---|---|---|---|
| Same MNT titer | 15 | 4 | 19 (52.8, 37.0–68.02) |
| Increase | 4 | 3 | 7 (19.4, 9.45–35.33) |
| Decrease | 8 | 2 | 10 * (27.8, 15.7–44.14) |
| Total | 27 | 9 | 36 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Marchi, S.; Viviani, S.; Montomoli, E.; Tang, Y.; Boccuto, A.; Vicenti, I.; Zazzi, M.; Sow, S.; Diallo, A.; Idoko, O.T.; et al. Zika Virus in West Africa: A Seroepidemiological Study between 2007 and 2012. Viruses 2020, 12, 641. https://doi.org/10.3390/v12060641
Marchi S, Viviani S, Montomoli E, Tang Y, Boccuto A, Vicenti I, Zazzi M, Sow S, Diallo A, Idoko OT, et al. Zika Virus in West Africa: A Seroepidemiological Study between 2007 and 2012. Viruses. 2020; 12(6):641. https://doi.org/10.3390/v12060641
Chicago/Turabian StyleMarchi, Serena, Simonetta Viviani, Emanuele Montomoli, Yuxiao Tang, Adele Boccuto, Ilaria Vicenti, Maurizio Zazzi, Samba Sow, Aldiouma Diallo, Olubukola T. Idoko, and et al. 2020. "Zika Virus in West Africa: A Seroepidemiological Study between 2007 and 2012" Viruses 12, no. 6: 641. https://doi.org/10.3390/v12060641
APA StyleMarchi, S., Viviani, S., Montomoli, E., Tang, Y., Boccuto, A., Vicenti, I., Zazzi, M., Sow, S., Diallo, A., Idoko, O. T., Bhat, N., & Trombetta, C. M. (2020). Zika Virus in West Africa: A Seroepidemiological Study between 2007 and 2012. Viruses, 12(6), 641. https://doi.org/10.3390/v12060641






