Identification of Novel Yellow Fever Class II Epitopes in YF-17D Vaccinees
Abstract
1. Introduction
2. Materials and Methods
2.1. Human Blood Samples from Vaccinated Individuals
2.2. HLA Typing
2.3. MHC Class II Binding Predictions and Peptide Selection
2.4. In Vitro Expansion of YFV-Specific T Cells
2.5. Ex Vivo IFNγ ELISPOT Assay
2.6. YFV-CD4 Megapool Design and Manufacture
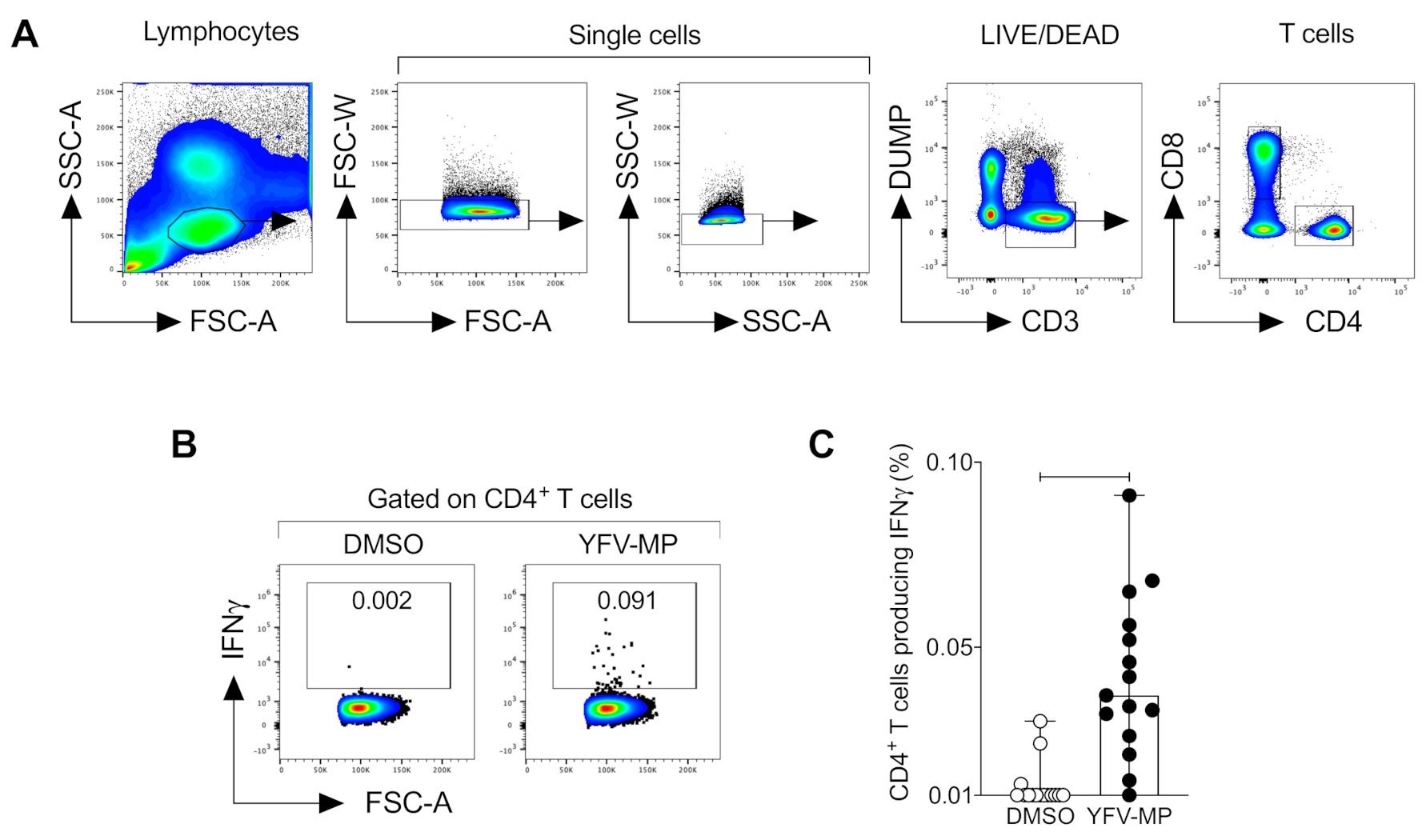
2.7. Flow Cytometry
2.8. Statistical Analysis
3. Results
3.1. Selection of a Panel of DRB1 Alleles that Affords High Population Coverage
3.2. Identification of Yellow Fever Epitopes
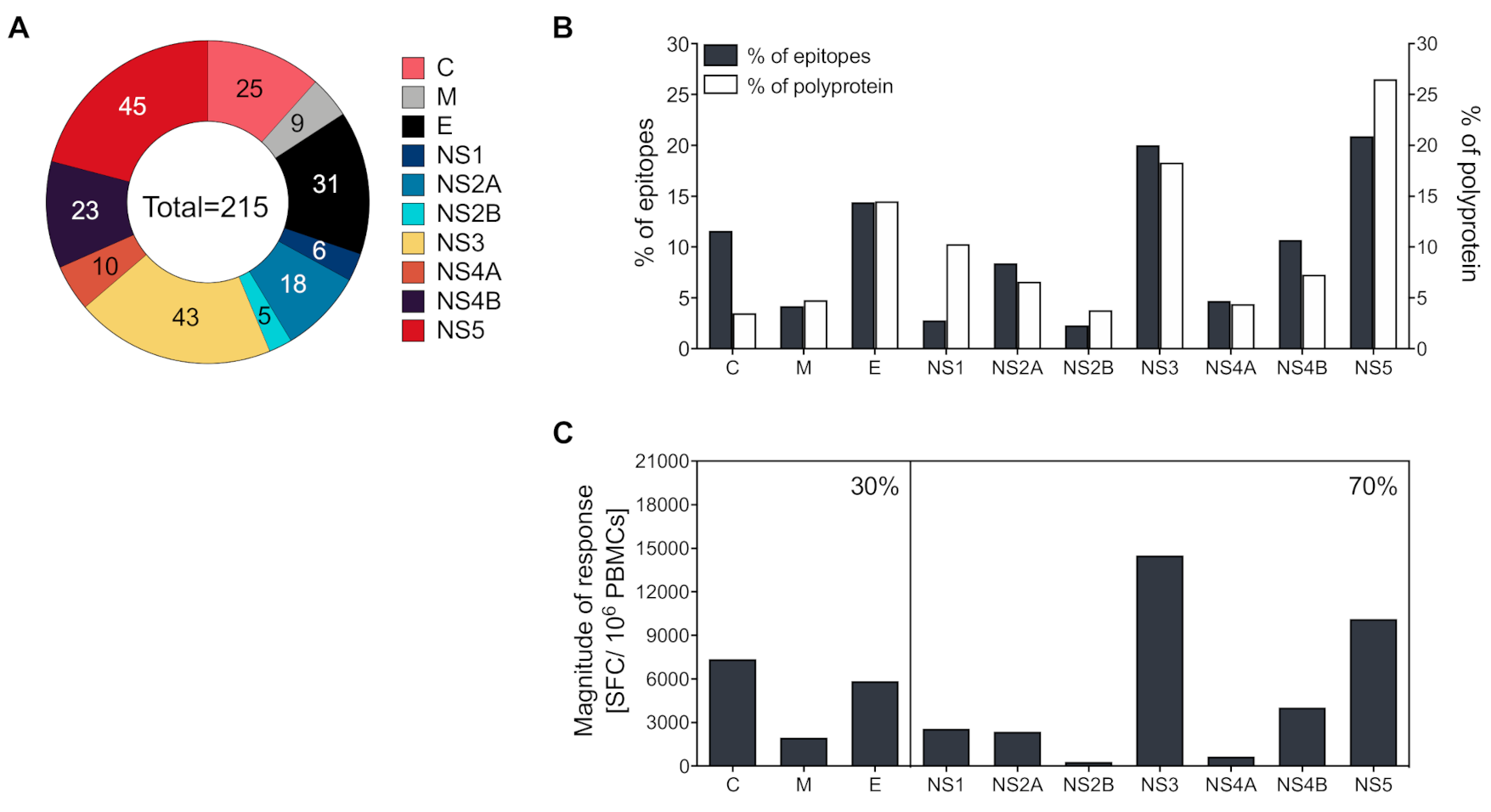
3.3. CD4+ T Cells Recognize both Structural and Non-Structural Proteins
3.4. Magnitude of Responses Varies as a Function of HLA
3.5. Development and Validation of a CD4+ Yellow Fever Epitope Mega Pool
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- WHO. Yellow fever in Africa and the Americas, 2018. Wkly. Epidemiol. Rec. 2019, 33, 365–380. [Google Scholar]
- Hasan, S.S.; Sevvana, M.; Kuhn, R.J.; Rossmann, M.G. Structural biology of Zika virus and other flaviviruses. Nat. Struct. Mol. Biol. 2018, 25, 13–20. [Google Scholar] [CrossRef] [PubMed]
- Douam, F.; Ploss, A. Yellow fever virus: Knowledge gaps impeding the fight against an old foe. Trends Microbiol. 2018, 26, 913–928. [Google Scholar] [CrossRef] [PubMed]
- CDC. Yellow Fever Vaccine. Available online: https://www.cdc.gov/yellowfever/vaccine/index.html (accessed on 1 October 2019).
- Ferreira, C.C.; Campi-Azevedo, A.C.; Peruhype-Magalhaes, V.; Costa-Pereira, C.; Albuquerque, C.P.; Muniz, L.F.; Yokoy de Souza, T.; Oliveira, A.C.V.; Martins-Filho, O.A.; da Mota, L.M.H. The 17D-204 and 17DD yellow fever vaccines: An overview of major similarities and subtle differences. Expert Rev. Vaccines 2018, 17, 79–90. [Google Scholar] [CrossRef] [PubMed]
- Poland, J.D.; Calisher, C.H.; Monath, T.P.; Downs, W.G.; Murphy, K. Persistence of neutralizing antibody 30-35 years after immunization with 17D yellow fever vaccine. Bull. World Health Organ. 1981, 59, 895–900. [Google Scholar]
- Co, M.D.; Terajima, M.; Cruz, J.; Ennis, F.A.; Rothman, A.L. Human cytotoxic T lymphocyte responses to live attenuated 17D yellow fever vaccine: Identification of HLA-B35-restricted CTL epitopes on nonstructural proteins NS1, NS2b, NS3, and the structural protein E. Virology 2002, 293, 151–163. [Google Scholar] [CrossRef]
- Guy, B.; Nougarede, N.; Begue, S.; Sanchez, V.; Souag, N.; Carre, M.; Chambonneau, L.; Morrisson, D.N.; Shaw, D.; Qiao, M.; et al. Cell-mediated immunity induced by chimeric tetravalent dengue vaccine in naive or flavivirus-primed subjects. Vaccine 2008, 26, 5712–5721. [Google Scholar] [CrossRef]
- de Melo, A.B.; Nascimento, E.J.; Braga-Neto, U.; Dhalia, R.; Silva, A.M.; Oelke, M.; Schneck, J.P.; Sidney, J.; Sette, A.; Montenegro, S.M.; et al. T-cell memory responses elicited by yellow fever vaccine are targeted to overlapping epitopes containing multiple HLA-I and -II binding motifs. PLoS Negl. Trop. Dis. 2013, 7, e1938. [Google Scholar] [CrossRef]
- Blom, K.; Braun, M.; Ivarsson, M.A.; Gonzalez, V.D.; Falconer, K.; Moll, M.; Ljunggren, H.G.; Michaelsson, J.; Sandberg, J.K. Temporal dynamics of the primary human T cell response to yellow fever virus 17D as it matures from an effector- to a memory-type response. J. Immunol. 2013, 190, 2150–2158. [Google Scholar] [CrossRef]
- Wieten, R.W.; Jonker, E.F.; van Leeuwen, E.M.; Remmerswaal, E.B.; Ten Berge, I.J.; de Visser, A.W.; van Genderen, P.J.; Goorhuis, A.; Visser, L.G.; Grobusch, M.P.; et al. A single 17D yellow fever vaccination provides lifelong immunity; characterization of yellow fever-specific neutralizing antibody and T-cell responses after vaccination. PLoS ONE 2016, 11, e0149871. [Google Scholar] [CrossRef]
- Wieten, R.W.; Goorhuis, A.; Jonker, E.F.F.; de Bree, G.J.; de Visser, A.W.; van Genderen, P.J.J.; Remmerswaal, E.B.M.; Ten Berge, I.J.M.; Visser, L.G.; Grobusch, M.P.; et al. 17D yellow fever vaccine elicits comparable long-term immune responses in healthy individuals and immune-compromised patients. J. Infect. 2016, 72, 713–722. [Google Scholar] [CrossRef] [PubMed]
- Lee, E.S.; Thomas, P.G.; Mold, J.E.; Yates, A.J. Identifying T cell receptors from high-throughput sequencing: Dealing with promiscuity in TCRalpha and TCRbeta pairing. PLoS Comput. Biol. 2017, 13, e1005313. [Google Scholar] [CrossRef] [PubMed]
- Pogorelyy, M.V.; Minervina, A.A.; Touzel, M.P.; Sycheva, A.L.; Komech, E.A.; Kovalenko, E.I.; Karganova, G.G.; Egorov, E.S.; Komkov, A.Y.; Chudakov, D.M.; et al. Precise tracking of vaccine-responding T cell clones reveals convergent and personalized response in identical twins. Proc. Natl. Acad. Sci. USA 2018, 115, 12704–12709. [Google Scholar] [CrossRef] [PubMed]
- Akondy, R.S.; Johnson, P.L.; Nakaya, H.I.; Edupuganti, S.; Mulligan, M.J.; Lawson, B.; Miller, J.D.; Pulendran, B.; Antia, R.; Ahmed, R. Initial viral load determines the magnitude of the human CD8 T cell response to yellow fever vaccination. Proc. Natl. Acad. Sci. USA 2015, 112, 3050–3055. [Google Scholar] [CrossRef]
- Akondy, R.S.; Monson, N.D.; Miller, J.D.; Edupuganti, S.; Teuwen, D.; Wu, H.; Quyyumi, F.; Garg, S.; Altman, J.D.; Del Rio, C.; et al. The yellow fever virus vaccine induces a broad and polyfunctional human memory CD8+ T cell response. J. Immunol. 2009, 183, 7919–7930. [Google Scholar] [CrossRef]
- Kongsgaard, M.; Bassi, M.R.; Rasmussen, M.; Skjodt, K.; Thybo, S.; Gabriel, M.; Hansen, M.B.; Christensen, J.P.; Thomsen, A.R.; Buus, S.; et al. Adaptive immune responses to booster vaccination against yellow fever virus are much reduced compared to those after primary vaccination. Sci. Rep. 2017, 7, 662. [Google Scholar] [CrossRef]
- Wrammert, J.; Miller, J.; Akondy, R.; Ahmed, R. Human immune memory to yellow fever and smallpox vaccination. J. Clin. Immunol. 2009, 29, 151–157. [Google Scholar] [CrossRef]
- Koblischke, M.; Mackroth, M.S.; Schwaiger, J.; Fae, I.; Fischer, G.; Stiasny, K.; Heinz, F.X.; Aberle, J.H. Protein structure shapes immunodominance in the CD4 T cell response to yellow fever vaccination. Sci. Rep. 2017, 7, 8907. [Google Scholar] [CrossRef]
- da Costa-Rocha, I.A.; Campi-Azevedo, A.C.; Peruhype-Magalhaes, V.; Coelho-Dos-Reis, J.G.; Fradico, J.R.B.; Souza-Lopes, T.; Reis, L.R.; Freire, L.C.; Costa-Pereira, C.; Mambrini, J.V.M.; et al. Duration of humoral and cellular immunity 8 years after administration of reduced doses of the 17DD-yellow fever vaccine. Front. Immunol. 2019, 10, 1211. [Google Scholar] [CrossRef]
- Domingo, C.; Fraissinet, J.; Ansah, P.O.; Kelly, C.; Bhat, N.; Sow, S.O.; Mejia, J.E. Long-term immunity against yellow fever in children vaccinated during infancy: A longitudinal cohort study. Lancet Infect. Dis. 2019. [Google Scholar] [CrossRef]
- Akondy, R.S.; Fitch, M.; Edupuganti, S.; Yang, S.; Kissick, H.T.; Li, K.W.; Youngblood, B.A.; Abdelsamed, H.A.; McGuire, D.J.; Cohen, K.W.; et al. Origin and differentiation of human memory CD8 T cells after vaccination. Nature 2017, 552, 362–367. [Google Scholar] [CrossRef] [PubMed]
- Tian, Y.; Grifoni, A.; Sette, A.; Weiskopf, D. Human T cell response to dengue virus infection. Front. Immunol. 2019, 10, 2125. [Google Scholar] [CrossRef] [PubMed]
- Slon Campos, J.L.; Mongkolsapaya, J.; Screaton, G.R. The immune response against flaviviruses. Nat. Immunol. 2018, 19, 1189–1198. [Google Scholar] [CrossRef]
- Waggoner, J.J.; Rojas, A.; Pinsky, B.A. Yellow fever virus: Diagnostics for a persistent arboviral threat. J. Clin. Microbiol. 2018, 56. [Google Scholar] [CrossRef] [PubMed]
- Ndeffo-Mbah, M.L.; Pandey, A. Global risk and elimination of yellow fever epidemics. J. Infect. Dis. 2019. [Google Scholar] [CrossRef]
- Zhao, S.; Stone, L.; Gao, D.; He, D. Modelling the large-scale yellow fever outbreak in Luanda, Angola, and the impact of vaccination. PLoS Negl. Trop. Dis. 2018, 12, e0006158. [Google Scholar] [CrossRef]
- Thomas, S.J.; Yoon, I.K. A review of Dengvaxia(R): Development to deployment. Hum. Vaccin. Immunother. 2019, 1–20. [Google Scholar] [CrossRef]
- Pham, J.; Oseroff, C.; Hinz, D.; Sidney, J.; Paul, S.; Greenbaum, J.; Vita, R.; Phillips, E.; Mallal, S.; Peters, B.; et al. Sequence conservation predicts T cell reactivity against ragweed allergens. Clin. Exp. Allergy 2016, 46, 1194–1205. [Google Scholar] [CrossRef]
- Vita, R.; Mahajan, S.; Overton, J.A.; Dhanda, S.K.; Martini, S.; Cantrell, J.R.; Wheeler, D.K.; Sette, A.; Peters, B. The Immune Epitope Database (IEDB): 2018 update. Nucleic Acids Res. 2019, 47, D339–D343. [Google Scholar] [CrossRef]
- Kim, Y.; Ponomarenko, J.; Zhu, Z.; Tamang, D.; Wang, P.; Greenbaum, J.; Lundegaard, C.; Sette, A.; Lund, O.; Bourne, P.E.; et al. Immune epitope database analysis resource. Nucleic Acids Res. 2012, 40, W525–W530. [Google Scholar] [CrossRef]
- Wang, P.; Sidney, J.; Dow, C.; Mothe, B.; Sette, A.; Peters, B. A systematic assessment of MHC class II peptide binding predictions and evaluation of a consensus approach. PLoS Comput. Biol. 2008, 4, e1000048. [Google Scholar] [CrossRef] [PubMed]
- Wang, P.; Sidney, J.; Kim, Y.; Sette, A.; Lund, O.; Nielsen, M.; Peters, B. Peptide binding predictions for HLA DR, DP and DQ molecules. BMC Bioinformatics 2010, 11, 568. [Google Scholar] [CrossRef] [PubMed]
- Weiskopf, D.; Angelo, M.A.; Grifoni, A.; O’Rourke, P.H.; Sidney, J.; Paul, S.; De Silva, A.D.; Phillips, E.; Mallal, S.; Premawansa, S.; et al. HLA-DRB1 alleles are associated with different magnitudes of dengue virus-specific CD4+ T-cell responses. J. Infect. Dis. 2016, 214, 1117–1124. [Google Scholar] [CrossRef] [PubMed]
- Grifoni, A.; Angelo, M.A.; Lopez, B.; O’Rourke, P.H.; Sidney, J.; Cerpas, C.; Balmaseda, A.; Silveira, C.G.T.; Maestri, A.; Costa, P.R.; et al. Global assessment of dengue virus-specific CD4(+) T cell responses in dengue-endemic areas. Front. Immunol. 2017, 8, 1309. [Google Scholar] [CrossRef] [PubMed]
- Sidney, J.; Peters, B.; Frahm, N.; Brander, C.; Sette, A. HLA class I supertypes: A revised and updated classification. BMC Immunol. 2008, 9, 1. [Google Scholar] [CrossRef]
- Weiskopf, D.; Yauch, L.E.; Angelo, M.A.; John, D.V.; Greenbaum, J.A.; Sidney, J.; Kolla, R.V.; De Silva, A.D.; de Silva, A.M.; Grey, H.; et al. Insights into HLA-restricted T cell responses in a novel mouse model of dengue virus infection point toward new implications for vaccine design. J. Immunol. 2011, 187, 4268–4279. [Google Scholar] [CrossRef]
- Carrasco Pro, S.; Sidney, J.; Paul, S.; Lindestam Arlehamn, C.; Weiskopf, D.; Peters, B.; Sette, A. Automatic generation of validated specific epitope sets. J. Immunol. Res. 2015, 2015, 763461. [Google Scholar] [CrossRef]
- Bonaldo, M.C.; Gomez, M.M.; Dos Santos, A.A.; Abreu, F.V.S.; Ferreira-de-Brito, A.; Miranda, R.M.; Castro, M.G.; Lourenco-de-Oliveira, R. Genome analysis of yellow fever virus of the ongoing outbreak in Brazil reveals polymorphisms. Mem. Inst. Oswaldo Cruz 2017, 112, 447–451. [Google Scholar] [CrossRef]
- Paul, S.; Lindestam Arlehamn, C.S.; Scriba, T.J.; Dillon, M.B.; Oseroff, C.; Hinz, D.; McKinney, D.M.; Carrasco Pro, S.; Sidney, J.; Peters, B.; et al. Development and validation of a broad scheme for prediction of HLA class II restricted T cell epitopes. J. Immunol. Methods 2015, 422, 28–34. [Google Scholar] [CrossRef]
- Paul, S.; Sidney, J.; Sette, A.; Peters, B. TepiTool: A pipeline for computational prediction of T cell epitope candidates. Curr. Protoc. Immunol. 2016, 114, 18.19.11–18.19.24. [Google Scholar] [CrossRef]
- Dhanda, S.K.; Vaughan, K.; Schulten, V.; Grifoni, A.; Weiskopf, D.; Sidney, J.; Peters, B.; Sette, A. Development of a novel clustering tool for linear peptide sequences. Immunology 2018, 155, 331–345. [Google Scholar] [CrossRef] [PubMed]
- Greenbaum, J.; Sidney, J.; Chung, J.; Brander, C.; Peters, B.; Sette, A. Functional classification of class II human leukocyte antigen (HLA) molecules reveals seven different supertypes and a surprising degree of repertoire sharing across supertypes. Immunogenetics 2011, 63, 325–335. [Google Scholar] [CrossRef] [PubMed]
- Liang, H.; Lee, M.; Jin, X. Guiding dengue vaccine development using knowledge gained from the success of the yellow fever vaccine. Cell. Mol. Immunol. 2016, 13, 36–46. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Monath, T.P. Review of the risks and benefits of yellow fever vaccination including some new analyses. Expert Rev. Vaccines 2012, 11, 427–448. [Google Scholar] [CrossRef]
- Roukens, A.H.E.; van Halem, K.; de Visser, A.W.; Visser, L.G. Long-term protection after fractional-dose yellow fever vaccination: Follow-up study of a randomized, controlled, noninferiority trial. Ann. Intern. Med. 2018, 169, 761–765. [Google Scholar] [CrossRef]
- Grifoni, A.; Moore, E.; Voic, H.; Sidney, J.; Phillips, E.; Jadi, R.; Mallal, S.; De Silva, A.D.; De Silva, A.M.; Peters, B.; et al. Characterization of magnitude and antigen specificity of HLA-DP, DQ, and DRB3/4/5 restricted DENV-specific CD4+ T cell responses. Front. Immunol. 2019, 10, 1568. [Google Scholar] [CrossRef]
- Grifoni, A.; Weiskopf, D.; Lindestam Arlehamn, C.S.; Angelo, M.; Leary, S.; Sidney, J.; Frazier, A.; Phillips, E.; Mallal, S.; Mack, S.J.; et al. Sequence-based HLA-A, B, C, DP, DQ, and DR typing of 714 adults from Colombo, Sri Lanka. Hum. Immunol. 2018, 79, 87–88. [Google Scholar] [CrossRef]
- Kallas, E.G.; Wilder-Smith, A. Managing severe yellow fever in the intensive care: Lessons learnt from Brazil. J. Travel Med. 2019, 26. [Google Scholar] [CrossRef]
- Barrett, A.D.; Teuwen, D.E. Yellow fever vaccine-how does it work and why do rare cases of serious adverse events take place? Curr. Opin. Immunol. 2009, 21, 308–313. [Google Scholar] [CrossRef]
- Grifoni, A.; Pham, J.; Sidney, J.; O’Rourke, P.H.; Paul, S.; Peters, B.; Martini, S.R.; de Silva, A.D.; Ricciardi, M.J.; Magnani, D.M.; et al. Prior dengue virus exposure shapes T cell immunity to Zika virus in humans. J. Virol. 2017, 91. [Google Scholar] [CrossRef]
- Lim, M.Q.; Kumaran, E.A.P.; Tan, H.C.; Lye, D.C.; Leo, Y.S.; Ooi, E.E.; MacAry, P.A.; Bertoletti, A.; Rivino, L. Cross-reactivity and anti-viral function of dengue capsid and NS3-specific memory T cells toward Zika virus. Front. Immunol. 2018, 9, 2225. [Google Scholar] [CrossRef] [PubMed]
- Grifoni, A.; Voic, H.; Dhanda, S.K.; Kidd, C.K.; Brien, J.D.; Buus, S.; Stryhn, A.; Durbin, A.P.; Whitehead, S.; Diehl, S.A.; et al. T cell responses induced by attenuated flavivirus vaccination are specific and show limited cross-reactivity with other Flavivirus species. J. Virol. 2020, 94. [Google Scholar] [CrossRef] [PubMed]
| Sequence | Protein | Position | Total SFC (106 PBMCs) | Cumulative % Total | HLA Recognized | HLA Restriction(s) |
|---|---|---|---|---|---|---|
| VRRGVRSLSNKIKQK | C | 16 | 7333 | 5.4 | 4 | DRB1*04:03, DRB1*08:02, DRB1*15:01, DRB1*16:02 |
| LMRRMRRPTGKVTLE | NS5 | 2746 | 6330 | 10.0 | 4 | DRB1*11:01, DRB1*11:04, DRB1*13:01, DRB1*15:02 |
| QTSRLLMRRMRRPTG | NS5 | 2741 | 5313 | 13.8 | 5 | DRB1*08:02, DRB1*11:01, DRB1*11:04, DRB1*13:01, DRB1*15:02 |
| ILAECARRRLRTLVL | NS3 | 1696 | 4983 | 17.5 | 1 | DRB1*16:02 |
| VVVLNRKTFEREYPT | NS3 | 1871 | 4873 | 21.0 | 2 | DRB1*11:04, DRB1*13:01 |
| LRKVKRVVASLMRGL | C | 81 | 4587 | 24.4 | 4 | DRB1*01:01, DRB1*04:03, DRB1*11:01, DRB1*11:04 |
| GEVIGLYGNGILVGD | NS3 | 1631 | 3757 | 27.1 | 2 | DRB1*15:01, DRB1*15:02 |
| RFLPQILAECARRRL | NS3 | 1691 | 3480 | 29.7 | 2 | DRB1*04:07, DRB1*10:01 |
| QGLAVLRKVKRVVAS | C | 76 | 2893 | 31.8 | 4 | DRB1*08:02, DRB1*11:01, DRB1*11:04, DRB1*13:01 |
| DMRLLSLAVSSAVPT | NS5 | 3281 | 2793 | 33.8 | 4 | DRB1*04:07, DRB1*07:01, DRB1*11:01, DRB1*13:01 |
| KAGKSVVVLNRKTFE | NS3 | 1866 | 2773 | 35.8 | 2 | DRB1*11:04, DRB1*13:01 |
| GVTLVRKNRWLLLNV | M | 121 | 2693 | 37.8 | 3 | DRB1*08:02, DRB1*13:01, DRB1*15:01 |
| SLASVAMCRTPFSLA | NS4B | 2436 | 2530 | 39.7 | 4 | DRB1*04:03, DRB1*04:07, DRB1*10:01, DRB1*11:04 |
| NNGGDAMYMALIAAF | NS2A | 1196 | 2523 | 41.5 | 1 | DRB1*13:01 |
| ITAHLKRLWKMLDPR | C | 61 | 2400 | 43.2 | 2 | DRB1*11:01, DRB1*11:04 |
| VRKVCYNAVLTHVKI | E | 341 | 2393 | 45.0 | 3 | DRB1*04:07, DRB1*10:01, DRB1*11:04 |
| GSIVACAKFTCAKSM | E | 396 | 2353 | 46.7 | 2 | DRB1*11:04, DRB1*13:01 |
| NNLYKLHGGHVSCRV | E | 556 | 2287 | 48.4 | 1 | DRB1*01:01 |
| LLIGFGLRTLWSPRE | NS2A | 1216 | 2233 | 50.0 | 2 | DRB1*13:01, DRB1*15:01 |
| GFIFFFLFNILTGKK | C | 46 | 2227 | 51.6 | 5 | DRB1*01:01, DRB1*04:01, DRB1*07:01, DRB1*11:01, DRB1*15:02 |
| VVVQDPKNVYQRGTH | NS1 | 866 | 2123 | 53.2 | 2 | DRB1*03:01, DRB1*13:01 |
| RVVASLMRGLSSRKR | C | 86 | 2087 | 54.7 | 2 | DRB1*04:03, DRB1*13:01 |
| GNTSLLWNGPMAVSM | NS4B | 2466 | 2027 | 56.2 | 1 | DRB1*01:01 |
| KIERWFVRNPFFAVT | M | 241 | 1960 | 57.6 | 2 | DRB1*15:02, DRB1*16:02 |
| EVDISVVVQDPKNVY | NS1 | 861 | 1957 | 59.0 | 1 | DRB1*13:01 |
| FVGVMYNLWKMKTGR | NS4B | 2491 | 1900 | 60.4 | 2 | DRB1*11:01, DRB1*11:04 |
| LIWVGINTRNMTMSM | E | 746 | 1790 | 61.7 | 3 | DRB1*04:01, DRB1*10:01, DRB1*13:01 |
| GTRKIMKVVNRWLFR | NS5 | 2876 | 1720 | 63.0 | 1 | DRB1*15:01 |
| DGDSYYYSEPTSENN | NS3 | 1956 | 1610 | 64.2 | 1 | DRB1*04:01 |
| PKNVYQRGTHPFSRI | NS1 | 871 | 1600 | 65.3 | 1 | DRB1*16:02 |
| FLFNILTGKKITAHL | C | 51 | 1573 | 66.5 | 2 | DRB1*01:01, DRB1*11:01 |
| DEQEILNYMSPHHKK | NS5 | 3056 | 1513 | 67.6 | 3 | DRB1*04:01, DRB1*15:01, DRB1*15:06 |
| LPSIRAANVMAASLR | NS3 | 1851 | 1437 | 68.6 | 5 | DRB1*04:01, DRB1*04:03, DRB1*04:07, DRB1*07:01, DRB1*11:04 |
| GAFLVRNGKKLIPSW | NS3 | 1541 | 1413 | 69.7 | 3 | DRB1*11:01, DRB1*13:01, DRB1*16:02 |
| GVIMMFLSLGVGADQ | E | 766 | 1313 | 70.6 | 2 | DRB1*11:01, DRB1*15:01 |
| QYVIRAQLHVGAKQE | E | 421 | 1270 | 71.5 | 1 | DRB1*16:02 |
| VSMMIAMEVVLRKRQ | NS2A | 1141 | 1243 | 72.5 | 3 | DRB1*04:03, DRB1*13:01, DRB1*16:02 |
| HATLTYRMLEPTRVV | NS3 | 1751 | 1213 | 73.3 | 2 | DRB1*01:01, DRB1*10:01 |
| RWLFRHLAREKNPRL | NS5 | 2886 | 1140 | 74.2 | 1 | DRB1*13:01 |
| FIAKVRSHAAIGAYL | NS5 | 2906 | 1127 | 75.0 | 3 | DRB1*01:01, DRB1*04:03, DRB1*04:07 |
| HLA DRB1 Allele | No. 1 | Total Response Magnitude/Donor | Average of Epitopes/Donor | C | M | E | NS1 | NS2A | NS2B | NS3 | NS4A | NS4B | NS5 |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 01:01 | 3 | 4644 | 8.3 | 820 | 140 | 876 | 0 | 300 | 171 | 831 | 0 | 988 | 519 |
| 03:01 | 2 | 850 | 4.5 | 0 | 0 | 93 | 200 | 0 | 0 | 413 | 0 | 47 | 97 |
| 04:01 | 5 | 2280 | 4.6 | 179 | 0 | 297 | 0 | 0 | 64 | 1211 | 116 | 213 | 199 |
| 04:03 | 3 | 2093 | 3.3 | 1307 | 0 | 38 | 0 | 160 | 0 | 98 | 0 | 322 | 169 |
| 04:05 | 1 | 0 | 0.0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| 04:07 | 3 | 2577 | 4.3 | 0 | 0 | 647 | 0 | 51 | 0 | 1080 | 164 | 351 | 283 |
| 07:01 | 3 | 984 | 1.7 | 29 | 0 | 76 | 0 | 0 | 0 | 180 | 0 | 0 | 700 |
| 08:02 | 1 | 2993 | 6.0 | 1320 | 87 | 0 | 0 | 0 | 0 | 320 | 0 | 0 | 1267 |
| 10:01 | 1 | 4560 | 13.0 | 0 | 0 | 833 | 0 | 300 | 0 | 2253 | 0 | 793 | 380 |
| 11:01 | 4 | 1642 | 4.8 | 180 | 0 | 313 | 0 | 0 | 58 | 125 | 38 | 73 | 854 |
| 11:04 | 3 | 5185 | 5.8 | 1625 | 0 | 747 | 0 | 0 | 0 | 493 | 0 | 672 | 1648 |
| 12:01 | 2 | 577 | 1.5 | 0 | 0 | 70 | 0 | 0 | 0 | 80 | 0 | 0 | 427 |
| 13:01 | 5 | 5746 | 5.6 | 889 | 453 | 314 | 784 | 1023 | 0 | 1604 | 0 | 0 | 678 |
| 15:01 | 8 | 1767 | 2.1 | 391 | 72 | 93 | 0 | 240 | 0 | 556 | 108 | 16 | 292 |
| 15:02 | 2 | 3105 | 6.5 | 57 | 893 | 0 | 0 | 0 | 0 | 182 | 237 | 182 | 1555 |
| 15:06 | 1 | 820 | 2.0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 250 | 570 |
| 16:02 | 1 | 9950 | 10.0 | 573 | 320 | 1450 | 1600 | 290 | 0 | 5097 | 0 | 140 | 480 |
| TOTAL | 48 | 49,773 | - | 7369 | 1965 | 5846 | 2584 | 2365 | 293 | 14,523 | 662 | 4047 | 10,117 |
| % | - | 100 | - | 14.8 | 3.9 | 11.7 | 5.2 | 4.8 | 0.6 | 29.2 | 1.3 | 8.1 | 20.3 |
| Mean | - | 2928 | 5 | 433 | 116 | 344 | 152 | 139 | 17 | 854 | 39 | 238 | 595 |
| SD | - | 2485.9 | 3.3 | 553.9 | 238.6 | 421.6 | 420.0 | 258.4 | 44.5 | 1261.6 | 72.3 | 305.0 | 486.7 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mateus, J.; Grifoni, A.; Voic, H.; Angelo, M.A.; Phillips, E.; Mallal, S.; Sidney, J.; Sette, A.; Weiskopf, D. Identification of Novel Yellow Fever Class II Epitopes in YF-17D Vaccinees. Viruses 2020, 12, 1300. https://doi.org/10.3390/v12111300
Mateus J, Grifoni A, Voic H, Angelo MA, Phillips E, Mallal S, Sidney J, Sette A, Weiskopf D. Identification of Novel Yellow Fever Class II Epitopes in YF-17D Vaccinees. Viruses. 2020; 12(11):1300. https://doi.org/10.3390/v12111300
Chicago/Turabian StyleMateus, Jose, Alba Grifoni, Hannah Voic, Michael A. Angelo, Elizabeth Phillips, Simon Mallal, John Sidney, Alessandro Sette, and Daniela Weiskopf. 2020. "Identification of Novel Yellow Fever Class II Epitopes in YF-17D Vaccinees" Viruses 12, no. 11: 1300. https://doi.org/10.3390/v12111300
APA StyleMateus, J., Grifoni, A., Voic, H., Angelo, M. A., Phillips, E., Mallal, S., Sidney, J., Sette, A., & Weiskopf, D. (2020). Identification of Novel Yellow Fever Class II Epitopes in YF-17D Vaccinees. Viruses, 12(11), 1300. https://doi.org/10.3390/v12111300






