Establishment of a Parvovirus B19 NS1-Expressing Recombinant Adenoviral Vector for Killing Megakaryocytic Leukemia Cells
Abstract
1. Introduction
2. Material and Methods
2.1. Cell Lines
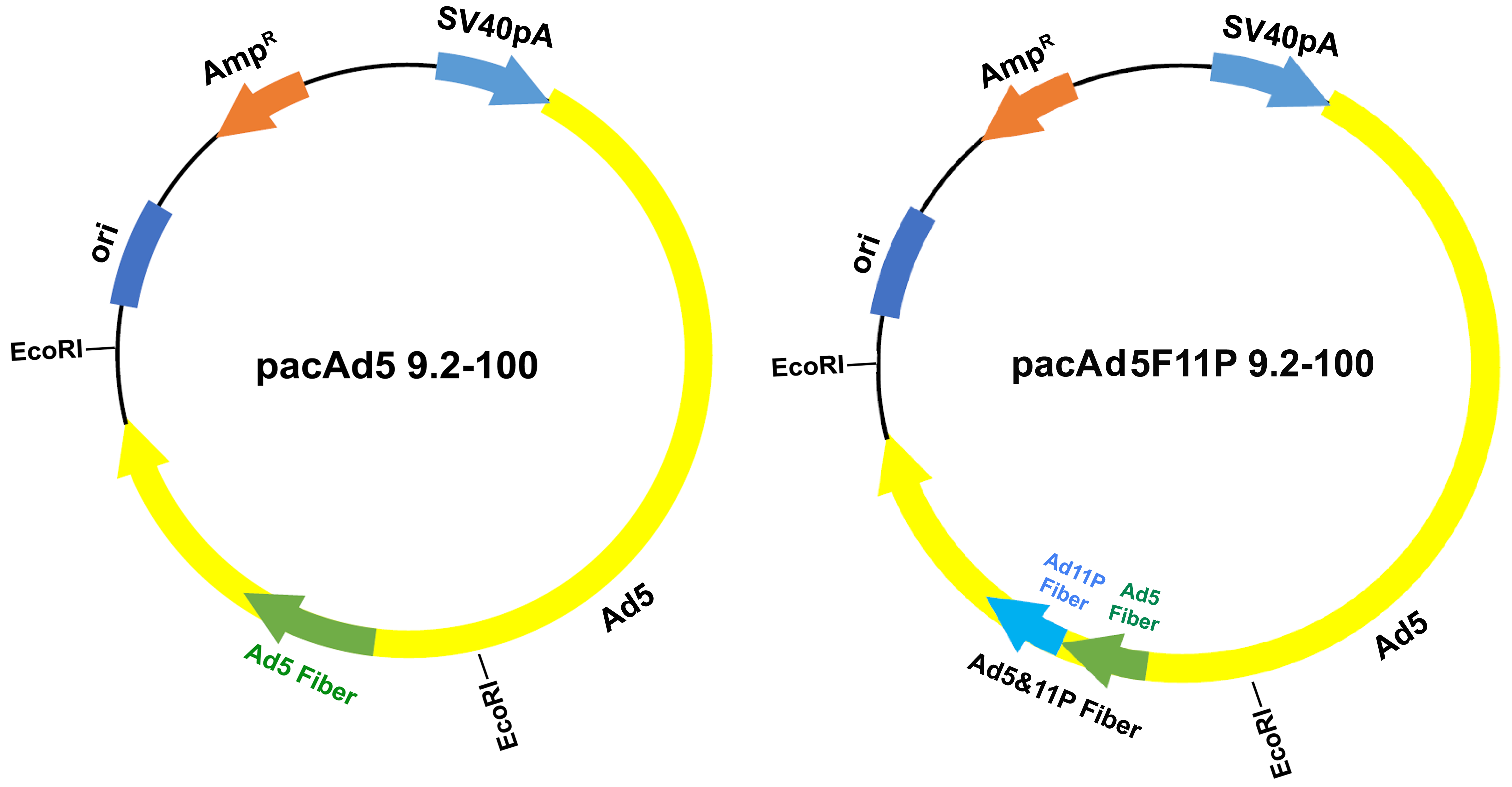
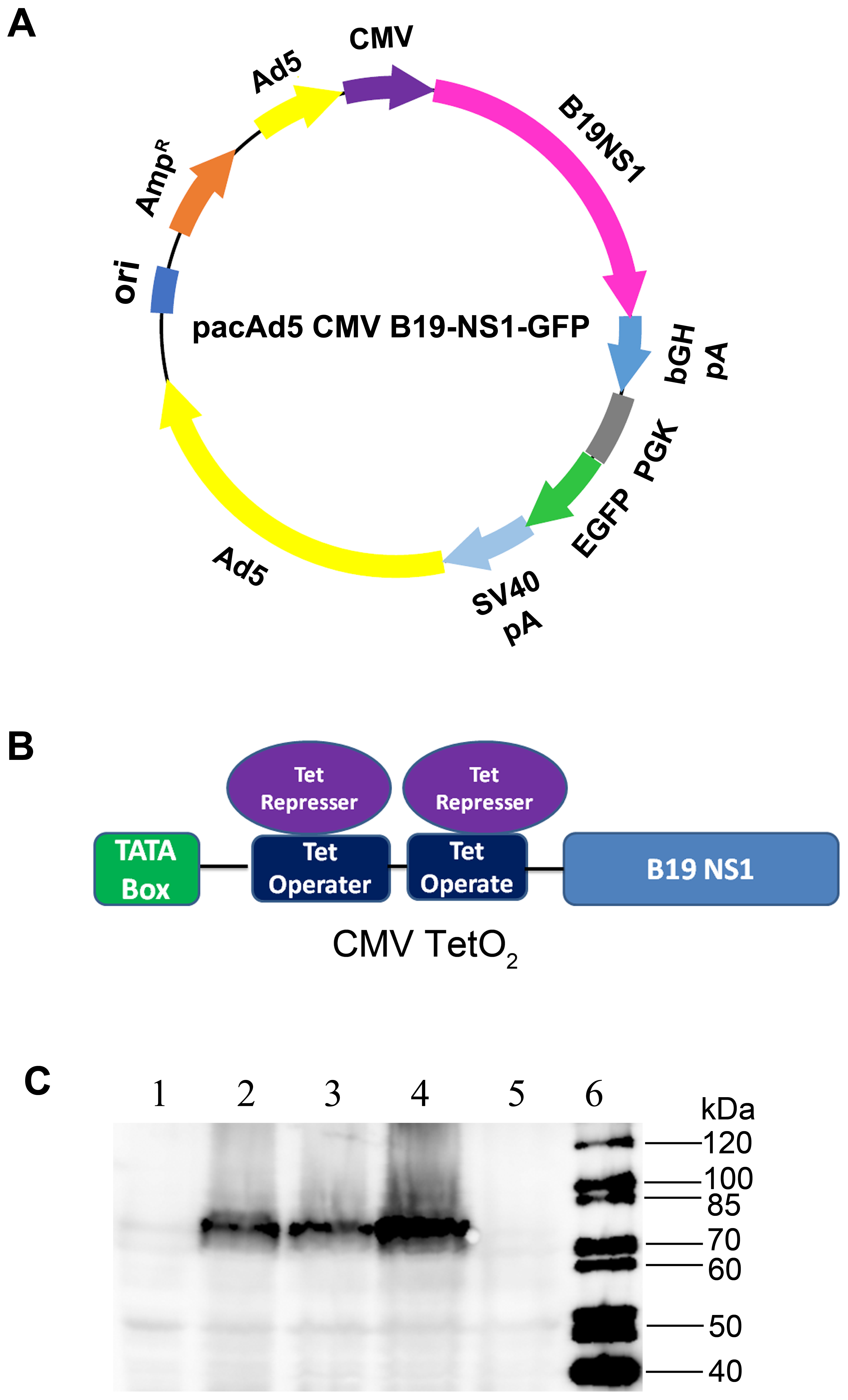
2.2. Plasmids
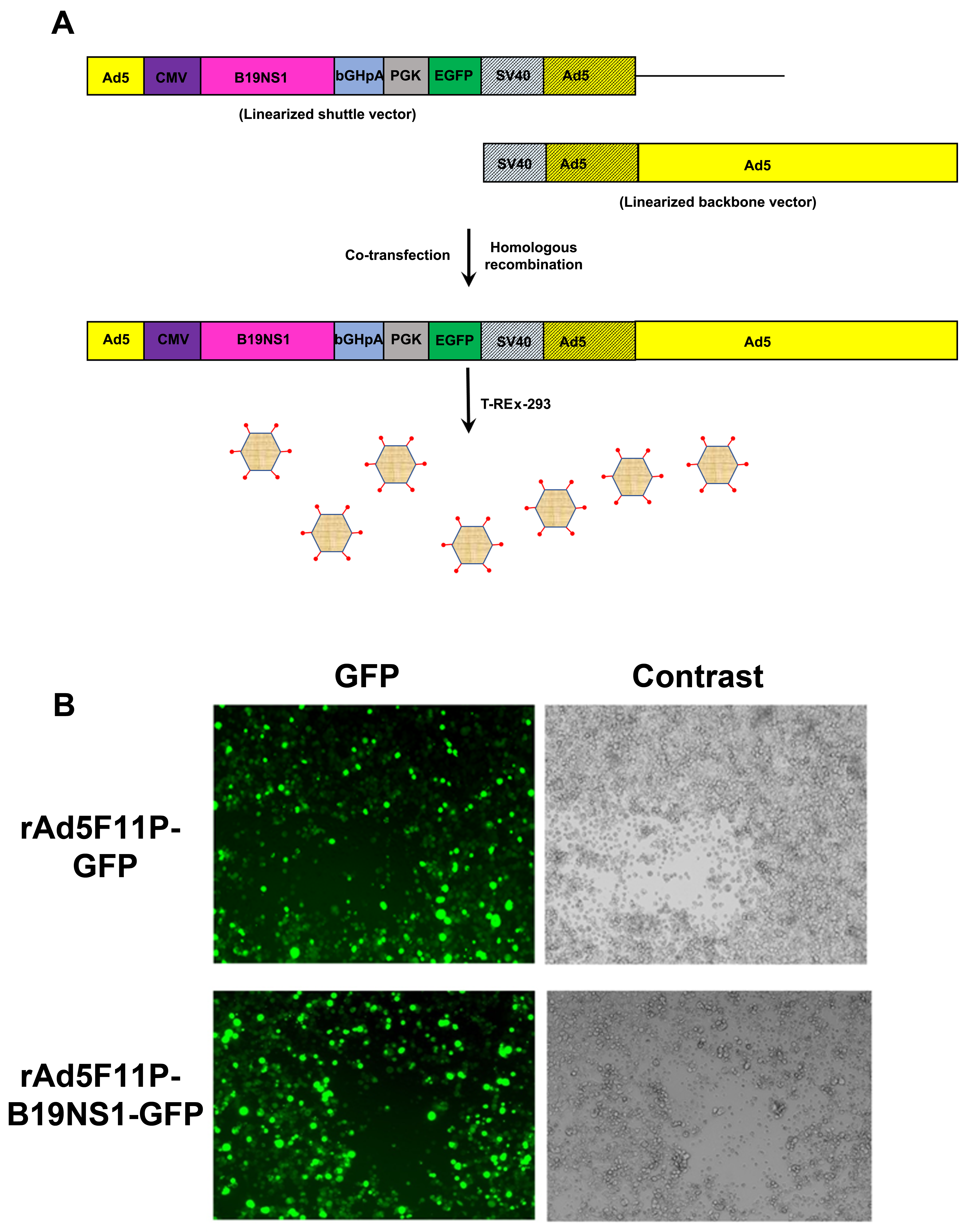
2.3. Recombinant Adenoviral (rAd) Vector Construction and Purification
2.4. Q-PCR
2.5. Fluorescence Images
2.6. Flow Cytometry Analysis
- (i)
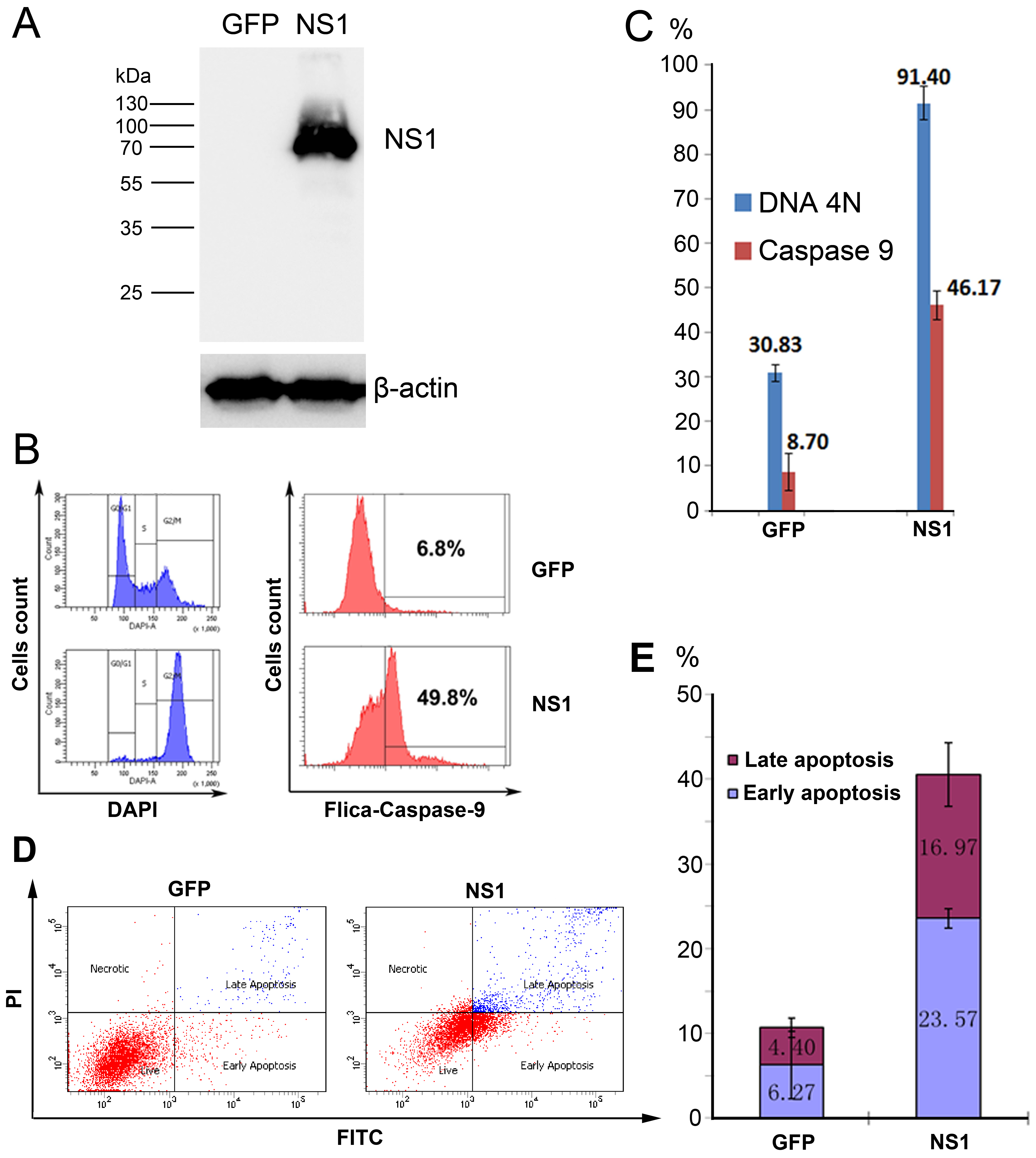
- Cell cycle analysis: Cell cycle analysis was performed using the 4′,6-diamidino-2-phenylindole (DAPI) staining described as before [20]. Briefly, rAd-transduced UT7/Epo-S1 cells were washed with PBS, fixed by 1% Paraformaldehyde (PFA), then permeabilized with 0.4% tween-20 and stained with DAPI at a concentration of 1 μg/mL for 30 min in dark. Samples were analyzed by flow cytometry within 1 h.
- (ii)
- Fluorescent-Labeled Inhibitors of Caspases (FLICA): FLICA Caspase-9 assay kit was purchased from ImmunoChemistry Technologies (Bloomington, MN, USA) and the assay was performed following the manufacturer’s protocol. Briefly, 290 µL of rAd-transduced UT7/Epo-S1 cells was incubated with 10 µL of the working solution of the reagent and then incubated for approximately 1 h, and then analyzed with a flow cytometer.
- (iii)
- Apoptosis analysis: FITC-conjugated Annexin V and Propidium Iodide (PI) double staining was performed following the manufacturer’s protocol. Briefly, rAd-transduced UT7/Epo-S1 cells were washed twice with cold PBS and then resuspended in 1 × Binding Buffer at a concentration of 1 × 106 cells/mL. A volume of 100 µL of the solution (1 × 105 cells) was transferred to a 1.5 mL tube, with 5 µL of FITC Annexin V and 5 µL of PI. After gentle vertexing, the cells were incubated for 15 min at RT (25 °C) in dark, with 400 µL of 1 × Binding Buffer, and were analyzed by flow cytometry within 1 h.
2.7. Western Blot
3. Results
3.1. Modification of the rAd Vector System
3.2. Production of B19V NS1-Expressing rAd
3.3. Ad5F11p-B19NS1-GFP Is More Effective in the Transduction of Leukemia Cells at a Relatively Low Multiplicity of Infection (MOI)
3.4. rAd5F11p-B19NS1-GFP Induces a Cell Cycle Arrest at G2 Phase and Apoptosis in Transduced Cells
4. Discussion
Author Contributions
Funding
Conflicts of Interest
References
- Russell, W.C. Adenoviruses: Update on structure and function. J. Gen. Virol. 2009, 90, 1–20. [Google Scholar] [CrossRef] [PubMed]
- Ginn, S.L.; Amaya, A.K.; Alexander, I.E.; Edelstein, M.; Abedi, M.R. Gene therapy clinical trials worldwide to 2017: An update. J. Gene Med. 2018, 20, e3015. [Google Scholar] [CrossRef] [PubMed]
- Yamamoto, Y.; Nagasato, M.; Yoshida, T.; Aoki, K. Recent advances in genetic modification of adenovirus vectors for cancer treatment. Cancer Sci. 2017, 108, 831–837. [Google Scholar] [CrossRef] [PubMed]
- Ehrke-Schulz, E.; Zhang, W.; Gao, J.; Ehrhardt, A. Recent Advances in Preclinical Developments Using Adenovirus Hybrid Vectors. Hum. Gene Ther. 2017, 28, 833–841. [Google Scholar] [CrossRef] [PubMed]
- Nilsson, M.; Ljungberg, J.; Richter, J.; Kiefer, T.; Magnusson, M.; Lieber, A.; Widegren, B.; Karlsson, S.; Fan, X. Development of an adenoviral vector system with adenovirus serotype 35 tropism; efficient transient gene transfer into primary malignant hematopoietic cells. J. Gene Med. 2004, 6, 631–641. [Google Scholar] [CrossRef]
- Mei, Y.F.; Segerman, A.; Lindman, K.; Hörnsten, P.; Wahlin, A.; Wadell, G. Human hematopoietic (CD34+) stem cells possess high-affinity receptors for adenovirus type 11p. Virology 2004, 328, 198–207. [Google Scholar] [CrossRef]
- Qiu, J.; Söderlund-Venermo, M.; Young, N.S. Human Parvoviruses. Clin. Microbiol. Rev. 2017, 30, 43–113. [Google Scholar] [CrossRef]
- Chen, A.Y.; Kleiboeker, S.; Qiu, J. Productive Parvovirus B19 Infection of Primary Human Erythroid Progenitor Cells at Hypoxia Is Regulated by STAT5A and MEK Signaling but not HIFa. PLoS Pathog. 2011, 7, e1002088. [Google Scholar] [CrossRef]
- Zhi, N.; Zadori, Z.; Brown, K.E.; Tijssen, P. Construction and sequencing of an infectious clone of the human parvovirus B19. Virology 2004, 318, 142–152. [Google Scholar] [CrossRef]
- Wan, Z.; Zhi, N.; Wong, S.; Keyvanfar, K.; Liu, D.; Raghavachari, N.; Munson, P.J.; Su, S.; Malide, D.; Kajigaya, S.; et al. Human parvovirus B19 causes cell cycle arrest of human erythroid progenitors via deregulation of the E2F family of transcription factors. J. Clin. Investig. 2010, 120, 3530–3544. [Google Scholar] [CrossRef]
- Ganaie, S.S.; Qiu, J. Recent Advances in Replication and Infection of Human Parvovirus B19. Front. Cell. Infect. Microbiol. 2018, 8, 166. [Google Scholar] [CrossRef] [PubMed]
- Poole, B.D.; Zhou, J.; Grote, A.; Schiffenbauer, A.; Naides, S.J. Apoptosis of liver-derived cells induced by parvovirus B19 nonstructural protein. J. Virol. 2006, 80, 4114–4121. [Google Scholar] [CrossRef] [PubMed]
- Thammasri, K.; Rauhamäki, S.; Wang, L.; Filippou, A.; Kivovich, V.; Marjomaki, V.; Naides, S.J.; Gilbert, L. Human parvovirus B19 induced apoptotic bodies contain altered self-antigens that are phagocytosed by antigen presenting cells. PLoS ONE 2013, 8, e67179. [Google Scholar] [CrossRef] [PubMed]
- Xu, P.; Zhou, Z.; Xiong, M.; Zou, W.; Deng, X.; Ganaie, S.S.; Kleiboeker, S.; Peng, J.; Liu, K.; Wang, S.; et al. Parvovirus B19 NS1 protein induces cell cycle arrest at G2 -phase by activating the ATR-CDC25C-CDK1 pathway. PLoS Pathog. 2017, 13, e1006266. [Google Scholar] [CrossRef] [PubMed]
- Morita, E.; Tada, K.; Chisaka, H.; Asao, H.; Sato, H.; Yaegashi, N.; Sugamura, K. Human parvovirus B19 induces cell cycle arrest at G2 phase with accumulation of mitotic cyclins. J. Virol. 2001, 75, 7555–7563. [Google Scholar] [CrossRef] [PubMed]
- Shimomura, S.; Komatsu, N.; Frickhofen, N.; Anderson, S.; Kajigaya, S.; Young, N.S. First continuous propagation of B19 parvovirus in a cell line. Blood 1992, 79, 18–24. [Google Scholar] [PubMed]
- Zhi, N.; Wan, Z.; Liu, X.; Wong, S.; Kim, D.J.; Young, N.S.; Kajigaya, S. Codon optimization of human parvovirus B19 capsid genes greatly increases their expression in nonpermissive cells. J. Virol. 2010, 84, 13059–13062. [Google Scholar] [CrossRef]
- Lu, Z.Z.; Ni, F.; Hu, Z.B.; Wang, L.; Wang, H.; Zhang, Q.W.; Huang, W.R.; Wu, C.T.; Wang, L.S. Efficient gene transfer into hematopoietic cells by a retargeting adenoviral vector system with a chimeric fiber of adenovirus serotype 5 and 11p. Exp. Hematol. 2006, 34, 1171–1182. [Google Scholar] [CrossRef]
- Huang, Q.; Deng, X.; Yan, Z.; Cheng, F.; Luo, Y.; Shen, W.; Lei-Butters, D.C.; Chen, A.Y.; Li, Y.; Tang, L.; et al. Establishment of a reverse genetics system for studying human bocavirus in human airway epithelia. PLoS Pathog. 2012, 8, e1002899. [Google Scholar] [CrossRef]
- Lou, S.; Luo, Y.; Cheng, F.; Huang, Q.; Shen, W.; Kleiboeker, S.; Tisdale, J.F.; Liu, Z.; Qiu, J. Human parvovirus B19 DNA replication induces a DNA damage response that is dispensable for cell cycle arrest at phase G2/M. J. Virol. 2012, 86, 10748–10758. [Google Scholar] [CrossRef]
- Xu, P.; Chen, A.Y.; Ganaie, S.S.; Cheng, F.; Shen, W.; Wang, X.; Kleiboeker, S.; Li, Y.; Qiu, J. The 11-Kilodalton Nonstructural Protein of Human Parvovirus B19 Facilitates Viral DNA Replication by Interacting with Grb2 through Its Proline-Rich Motifs. J. Virol. 2018, 93, e01464-18. [Google Scholar] [CrossRef] [PubMed]
- El-Andaloussi, N.; Bonifati, S.; Kaufmann, J.K.; Mailly, L.; Daeffler, L.; Deryckère, F.; Nettelbeck, D.M.; Rommelaere, J.; Marchini, A. Generation of an adenovirus-parvovirus chimera with enhanced oncolytic potential. J. Virol. 2012, 86, 10418–10431. [Google Scholar] [CrossRef] [PubMed]
- Yao, F.; Svensjö, T.; Winkler, T.; Lu, M.; Eriksson, C.; Eriksson, E. Tetracycline repressor, tetR, rather than the tetR-mammalian cell transcription factor fusion derivatives, regulates inducible gene expression in mammalian cells. Hum. Gene Ther. 1998, 9, 1939–1950. [Google Scholar] [CrossRef] [PubMed]
- Li, P.; Zhou, L.; Zhao, T.; Liu, X.; Zhang, P.; Liu, Y.; Zheng, X.; Li, Q. Caspase-9: Structure, mechanisms and clinical application. Oncotarget 2017, 8, 23996–24008. [Google Scholar] [CrossRef] [PubMed]
- Evan, G.I.; Vousden, K.H. Proliferation, cell cycle and apoptosis in cancer. Nature 2001, 411, 342–348. [Google Scholar] [CrossRef] [PubMed]
- Wickham, T.J.; Mathias, P.; Cheresh, D.A.; Nemerow, G.R. Integrins alpha v beta 3 and alpha v beta 5 promote adenovirus internalization but not virus attachment. Cell 1993, 73, 309–319. [Google Scholar] [CrossRef]
- Knaan-Shanzer, S.; Van Der Velde, I.; Havenga, M.J.; Lemckert, A.A.; De Vries, A.A.; Valerio, D. Highly efficient targeted transduction of undifferentiated human hematopoietic cells by adenoviral vectors displaying fiber knobs of subgroup B. Hum. Gene Ther. 2001, 12, 1989–2005. [Google Scholar] [CrossRef]
- Rea, D.; Havenga, M.J.; van Den Assem, M.; Sutmuller, R.P.; Lemckert, A.; Hoeben, R.C.; Bout, A.; Melief, C.J.; Offringa, R. Highly efficient transduction of human monocyte-derived dendritic cells with subgroup B fiber-modified adenovirus vectors enhances transgene-encoded antigen presentation to cytotoxic T cells. J. Immunol. 2001, 166, 5236–5244. [Google Scholar] [CrossRef]
- Sol, N.; Le Junter, J.; Vassias, I.; Freyssinier, J.M.; Thomas, A.; Prigent, A.F.; Rudkin, B.B.; Fichelson, S.; Morinet, F. Possible interactions between the NS-1 protein and tumor necrosis factor alpha pathways in erythroid cell apoptosis induced by human parvovirus B19. J. Virol. 1999, 73, 8762–8770. [Google Scholar]

© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Xu, P.; Wang, X.; Li, Y.; Qiu, J. Establishment of a Parvovirus B19 NS1-Expressing Recombinant Adenoviral Vector for Killing Megakaryocytic Leukemia Cells. Viruses 2019, 11, 820. https://doi.org/10.3390/v11090820
Xu P, Wang X, Li Y, Qiu J. Establishment of a Parvovirus B19 NS1-Expressing Recombinant Adenoviral Vector for Killing Megakaryocytic Leukemia Cells. Viruses. 2019; 11(9):820. https://doi.org/10.3390/v11090820
Chicago/Turabian StyleXu, Peng, Xiaomei Wang, Yi Li, and Jianming Qiu. 2019. "Establishment of a Parvovirus B19 NS1-Expressing Recombinant Adenoviral Vector for Killing Megakaryocytic Leukemia Cells" Viruses 11, no. 9: 820. https://doi.org/10.3390/v11090820
APA StyleXu, P., Wang, X., Li, Y., & Qiu, J. (2019). Establishment of a Parvovirus B19 NS1-Expressing Recombinant Adenoviral Vector for Killing Megakaryocytic Leukemia Cells. Viruses, 11(9), 820. https://doi.org/10.3390/v11090820





