Understanding Enterovirus D68-Induced Neurologic Disease: A Basic Science Review
Abstract
1. Introduction
2. Enterovirus D68 (EV-D68): A Re-Emerging Human Pathogen
3. Enterovirus D68 (EV-D68)-Associated Acute Flaccid Myelitis (AFM)
4. Role of Viral Genetics in the Emergence of Enterovirus D68 (EV-D68) Neurovirulence
5. Enterovirus D68 (EV-D68)-Induced Central Nervous System (CNS) Disease in Mouse Models
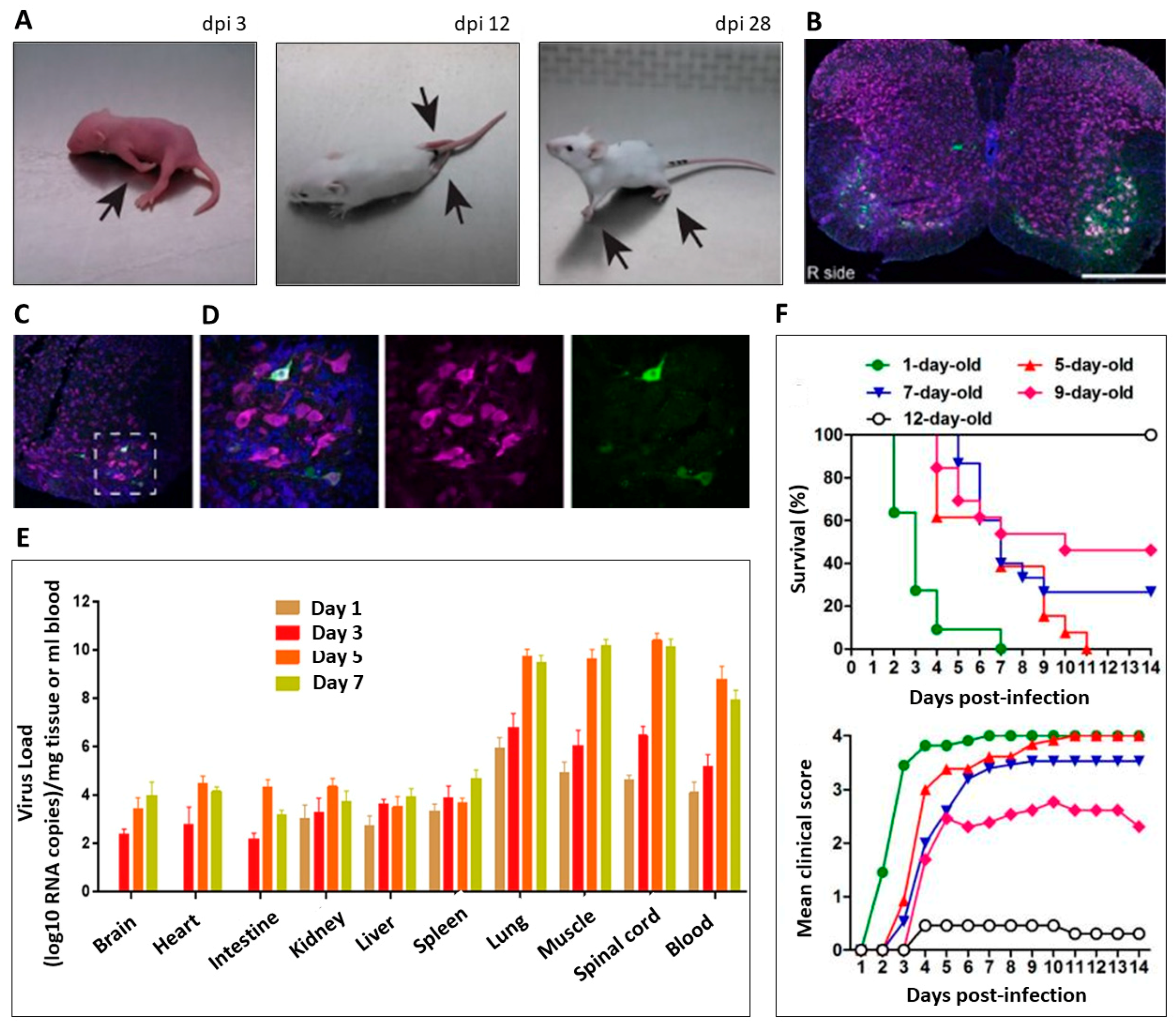
5.1. Strain-Dependent Paralysis
5.2. Enterovirus D68 (EV-D68) Tropism and Spread
5.3. Age-Dependent Paralysis
5.4. Antibody-Mediated Protection
5.5. Anti-Viral Strategies for the Treatment of Enterovirus D68 (EV-D68) Infections
6. Enterovirus D68 (EV-D68) Neurotropism in Neuron Culture Models
6.1. Infection of Neurons
6.2. Receptor Binding
7. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Racaniello, V.R. One hundred years of poliovirus pathogenesis. Virology 2006, 344, 9–16. [Google Scholar] [CrossRef] [PubMed]
- Pons-Salort, M.; Parker, E.P.; Grassly, N.C. The epidemiology of non-polio enteroviruses: Recent advances and outstanding questions. Curr. Opin. Infect. Dis. 2015, 28, 479–487. [Google Scholar] [CrossRef] [PubMed]
- Schieble, H.J.; Fox, V.L.; Lennette, E.H. A probable new human picornavirus associated with respiratory diseases. Am. J. Epidemiol. 1967, 85, 297–310. [Google Scholar] [CrossRef] [PubMed]
- Oberste, M.S.; Maher, K.; Schnurr, D.; Flemister, M.R.; Lovchik, J.C.; Peters, H.; Sessions, W.; Kirk, C.; Chatterjee, N.; Fuller, S.; et al. Enterovirus 68 is associated with respiratory illness and shares biological features with both the enteroviruses and the rhinoviruses. J. Gen. Virol. 2004, 85, 2577–2584. [Google Scholar] [CrossRef] [PubMed]
- Khetsuriani, N.; LaMonte-Fowlkes, A.; Oberst, S.; Pallansch, M.A. Centers for Disease Control and Prevention. Enterovirus surveillance—United States, 1970–2005. MMWR Surveill. Summ 2006, 55, 1–20. [Google Scholar] [PubMed]
- Ishiko, H.; Miura, R.; Shimada, Y.; Hayashi, A.; Nakajima, H.; Yamazaki, S.; Takeda, N. Human rhinovirus 87 identified as human enterovirus 68 by VP4-based molecular diagnosis. Intervirology 2002, 45, 136–141. [Google Scholar] [CrossRef]
- Savolainen, C.; Blomqvist, S.; Mulders, M.N.; Hovi, T. Genetic clustering of all 102 human rhinovirus prototype strains: Serotype 87 is close to human enterovirus 70. J. Gen. Virol. 2002, 83, 333–340. [Google Scholar] [CrossRef] [PubMed]
- Blomqvist, S.; Savolainen, C.; Råman, L.; Roivainen, M.; Hovi, T. Human rhinovirus 87 and enterovirus 68 represent a unique serotype with rhinovirus and enterovirus features. J. Clin. Microbiol. 2002, 40, 4218–4223. [Google Scholar] [CrossRef]
- Holm-Hansen, C.C.; Midgley, S.E.; Fischer, T.K. Global emergence of enterovirus D68: A systematic review. Lancet Infect. Dis. 2016, 16, e64–e75. [Google Scholar] [CrossRef]
- Messacar, K.; Abzug, M.J.; Dominguez, S.R. The Emergence of Enterovirus-D68. Microbiol. Spectr. 2016, 4, 105–119. [Google Scholar]
- Rahamat-Langendoen, J.; Riezebos-Brilman, A.; Borger, R.; van der Heide, R.; Brandenburg, A.; Schölvinck, E.; Niesters, H.G. Upsurge of human enterovirus 68 infections in patients with severe respiratory tract infections. J. Clin. Virol. 2011, 52, 103–106. [Google Scholar] [CrossRef]
- Tokarz, R.; Firth, C.; Madhi, S.A.; Howie, S.R.; Wu, W.; Sall, A.A.; Haq, S.; Briese, T.; Lipkin, W.I. Worldwide emergence of multiple clades of enterovirus 68. J. Gen. Virol. 2012, 93, 1952–1958. [Google Scholar] [CrossRef] [PubMed]
- Midgley, C.M.; Watson, J.T.; Nix, W.A.; Curns, A.T.; Rogers, S.L.; Brown, B.A.; Conover, C.; Dominguez, S.R.; Feikin, D.R.; Gray, S.; et al. Severe respiratory illness associated with a nationwide outbreak of enterovirus D68 in the USA (2014): A descriptive epidemiological investigation. Lancet Respir. Med. 2015, 3, 879–887. [Google Scholar] [CrossRef]
- Oermann, C.M.; Schuster, J.E.; Conners, G.P.; Newland, J.G.; Selvarangan, R.; Jackson, M.A. Enterovirus d68. A focused review and clinical highlights from the 2014 U.S. Outbreak. Ann. Am. Thorac. Soc. 2015, 12, 775–781. [Google Scholar] [CrossRef] [PubMed]
- Midgley, C.M.; Jackson, M.A.; Selvarangan, R.; Turabelidze, G.; Obringer, E.; Johnson, D.; Giles, B.L.; Patel, A.; Echols, F.; Oberste, M.S.; et al. Severe respiratory illness associated with enterovirus D68—Missouri and Illinois, 2014. MMWR Morb. Mortal. Wkly. Rep. 2014, 63, 798–799. [Google Scholar]
- Centers for Disease Control and Prevention. Enterovirus D68. 19 July 2016. Available online: http://www.cdc.gov/non-polio-enterovirus/about/ev-d68.html (accessed on 17 March 2019).
- Brown, B.A.; Nix, W.A.; Sheth, M.; Frace, M.; Oberste, M.S. Seven Strains of Enterovirus D68 Detected in the United States during the 2014 Severe Respiratory Disease Outbreak. Genome Announc. 2014, 2, e01201-14. [Google Scholar] [CrossRef]
- Messacar, K.; Hawkins, S.M.; Baker, J.; Pearce, K.; Tong, S.; Dominguez, S.R.; Parker, S. Resource Burden During the 2014 Enterovirus D68 Respiratory Disease Outbreak at Children’s Hospital Colorado: An Unexpected Strain. JAMA Pediatr. 2016, 170, 294–297. [Google Scholar] [CrossRef][Green Version]
- Cassidy, H.; Poelman, R.; Knoester, M.; Van Leer-Buter, C.C.; Niesters, H.G.M. Enterovirus D68—The New Polio? Front. Microbiol. 2018, 9, 2677. [Google Scholar] [CrossRef]
- Esposito, S.; Bosis, S.; Niesters, H.; Principi, N. Enterovirus D68 Infection. Viruses 2015, 7, 6043–6050. [Google Scholar] [CrossRef]
- Imamura, T.; Oshitani, H. Global reemergence of enterovirus D68 as an important pathogen for acute respiratory infections. Rev. Med. Virol. 2015, 25, 102–114. [Google Scholar] [CrossRef]
- Messacar, K.; Abzug, M.J.; Dominguez, S.R. 2014 outbreak of enterovirus D68 in North America. J. Med. Virol. 2016, 88, 739–745. [Google Scholar] [CrossRef]
- Helfferich, J.; Knoester, M.; Van Leer-Buter, C.C.; Neuteboom, R.F.; Meiners, L.C.; Niesters, H.G.; Brouwer, O.F. Acute flaccid myelitis and enterovirus D68: Lessons from the past and present. Eur. J. Pediatr. 2019, 178, 1305–1315. [Google Scholar] [CrossRef] [PubMed]
- Domingo, F.R.; McMorris, O.; Mersereau, T. Surveillance of the emerging enterovirus D68 in Canada: An evaluation. Can. Commun. Dis. Rep. 2016, 42, 4–8. [Google Scholar] [CrossRef]
- Pariani, E.; Pellegrinelli, L.; Merlone, A.D.; Piralla, A.; Baldanti, F.; Binda, S. Letter to the editor: Need for a European network for enterovirus D68 surveillance after detections of EV-D68 of the new B3 lineage in Sweden and Italy, 2016. Eurosurveillance 2017, 22, 30440. [Google Scholar] [CrossRef]
- Dyda, A.; Stelzer-Braid, S.; Adam, D.; Chughtai, A.A.; MacIntyre, C.R. The association between acute flaccid myelitis (AFM) and Enterovirus D68 (EV-D68)—What is the evidence for causation? Eurosurveillance 2018, 23, 17-00310. [Google Scholar] [CrossRef] [PubMed]
- Messacar, K.; Asturias, E.J.; Hixon, A.M.; Van Leer-Buter, C.; Niesters, H.G.; Tyler, K.L.; Abzug, M.J.; Dominguez, S.R. Enterovirus D68 and acute flaccid myelitis-evaluating the evidence for causality. Lancet Infect. Dis. 2018, 18, e239–e247. [Google Scholar] [CrossRef]
- Kujawski, S.A.; Midgley, C.M.; Rha, B.; Lively, J.Y.; Nix, W.A.; Curns, A.T.; Payne, D.C.; Englund, J.A.; Boom, J.A.; Williams, J.V.; et al. Enterovirus D68-Associated Acute Respiratory Illness—New Vaccine Surveillance Network, United States, July–October, 2017 and 2018. MMWR Morb. Mortal. Wkly. Rep. 2019, 68, 277–280. [Google Scholar] [CrossRef] [PubMed]
- Center for Disease Control and Prevention. New Vaccine Surveillance Network (NVSN). Available online: https://www.cdc.gov/surveillance/nvsn/index.html (accessed on 10 May 2019).
- Center for Disease Control and Prevention. National Enterovirus Surveillance System (NESS). Available online: https://www.cdc.gov/surveillance/ness/index.html (accessed on 10 May 2019).
- Lopez, A. Vital Signs: Surveillance for Acute Flaccid Myelitis—United States, 2018. MMWR Morb. Mortal. Wkly. Rep. 2019, 68, 608–614. [Google Scholar] [CrossRef] [PubMed]
- Messacar, K.; Robinson, C.C.; Pretty, K.; Yuan, J.; Dominguez, S.R. Surveillance for enterovirus D68 in colorado children reveals continued circulation. J. Clin. Virol. 2017, 92, 39–41. [Google Scholar] [CrossRef]
- Srinivasan, M.; Niesen, A.; Storch, G.A. Enterovirus D68 Surveillance, St. Louis, Missouri, USA, 2016. Emerg. Infect. Dis. 2018, 24, 2115–2117. [Google Scholar] [CrossRef]
- Abedi, G.R.; Watson, J.T.; Nix, W.A.; Oberste, M.S.; Gerber, S.I. Enterovirus and Parechovirus Surveillance—United States, 2014–2016. MMWR Morb. Mortal. Wkly. Rep. 2018, 67, 515–518. [Google Scholar] [CrossRef] [PubMed]
- Messacar, K.; Pretty, K.; Reno, S.; Dominguez, S.R. Continued biennial circulation of enterovirus D68 in Colorado. J. Clin. Virol. 2019, 113, 24–26. [Google Scholar] [CrossRef] [PubMed]
- Uprety, P.; Curtis, D.; Elkan, M.; Fink, J.; Rajagopalan, R.; Zhao, C.; Bittinger, K.; Mitchell, S.; Ulloa, E.R.; Hopkins, S.; et al. Association of Enterovirus D68 with Acute Flaccid Myelitis, Philadelphia, Pennsylvania, USA, 2009–2018. Emerg. Infect. Dis. 2019, 25, 1676–1682. [Google Scholar] [CrossRef] [PubMed]
- Meijer, A.; Benschop, K.S.; Donker, G.A.; van der Avoort, H.G. Continued seasonal circulation of enterovirus D68 in the Netherlands, 2011–2014. Eurosurveillance 2014, 19, 20935. [Google Scholar] [CrossRef] [PubMed]
- Kramer, R.; Sabatier, M.; Wirth, T.; Pichon, M.; Lina, B.; Schuffenecker, I.; Josset, L. Molecular diversity and biennial circulation of enterovirus D68: A systematic screening study in Lyon, France, 2010 to 2016. Eurosurveillance 2018, 23, 1700711. [Google Scholar] [CrossRef]
- Kreuter, J.D.; Barnes, A.; McCarthy, J.E.; Schwartzman, J.D.; Oberste, M.S.; Rhodes, C.H.; Modlin, J.F.; Wright, P.F. A fatal central nervous system enterovirus 68 infection. Arch. Pathol. Lab. Med. 2011, 135, 793–796. [Google Scholar] [PubMed]
- Kirolos, A.; Mark, K.; Shetty, J.; Chinchankar, N.; Mcdougall, C.; Eunson, P.; Stevenson, J.; Templeton, K. NHS Lothian EV-D68 Associated AFM Study Group; Pilley, E.; et al. Outcome of paediatric acute flaccid myelitis associated with enterovirus D68: A case series. Dev. Med. Child. Neurol. 2019, 61, 376–380. [Google Scholar]
- Knoester, M.; Helfferich, J.; Poelman, R.; Van Leer-Buter, C.; Brouwer, O.F.; Niesters, H.G. Twenty-nine Cases of Enterovirus-D68-associated Acute Flaccid Myelitis in Europe 2016: A Case Series and Epidemiologic Overview. Pediatr. Infect. Dis. J. 2019, 38, 16–21. [Google Scholar] [CrossRef]
- Messacar, K.; Schreiner, T.L.; Van Haren, K.; Yang, M.; Glaser, C.A.; Tyler, K.L.; Dominguez, S.R. Acute flaccid myelitis: A clinical review of US cases 2012–2015. Ann. Neurol. 2016, 80, 326–338. [Google Scholar] [CrossRef]
- Maloney, J.A.; Mirsky, D.M.; Messacar, K.; Dominguez, S.R.; Schreiner, T.; Stence, N.V. MRI findings in children with acute flaccid paralysis and cranial nerve dysfunction occurring during the 2014 enterovirus D68 outbreak. AJNR Am. J. Neuroradiol. 2015, 36, 245–250. [Google Scholar] [CrossRef]
- Christy, A.; Messacar, K. Acute Flaccid Myelitis Associated With Enterovirus D68: A Review. J. Child Neurol. 2019, 34, 511–516. [Google Scholar] [CrossRef] [PubMed]
- Hovden, A.I.; Pfeiffer, H.C. Electrodiagnostic findings in acute flaccid myelitis related to enterovirus D68. Muscle Nerve 2015, 52, 909–910. [Google Scholar] [CrossRef] [PubMed]
- Yea, C.; Bitnun, A.; Robinson, J.; Mineyko, A.; Barton, M.; Mah, J.K.; Vajsar, J.; Richardson, S.; Licht, C.; Brophy, J.; et al. Longitudinal Outcomes in the 2014 Acute Flaccid Paralysis Cluster in Canada. J. Child Neurol. 2017, 32, 301–307. [Google Scholar] [CrossRef] [PubMed]
- Martin, J.A.; Messacar, K.; Yang, M.L.; Maloney, J.A.; Lindwall, J.; Carry, T.; Kenyon, P.; Sillau, S.H.; Oleszek, J.; Tyler, K.L.; et al. Outcomes of Colorado children with acute flaccid myelitis at 1 year. Neurology 2017, 89, 129–137. [Google Scholar] [CrossRef] [PubMed]
- Hopkins, S.E. Acute Flaccid Myelitis: Etiologic Challenges, Diagnostic and Management Considerations. Curr. Treat. Options Neurol. 2017, 19, 48. [Google Scholar] [CrossRef] [PubMed]
- Center for Disease Control and Prevention. Acute Flaccid Myelitis - Case Definitions. 2018. Available online: https://www.cdc.gov/acute-flaccid-myelitis/hcp/case-definition.html (accessed on 10 May 2019).
- Messacar, K.; Spence-Davizon, E.; Osborne, C.; Press, C.; Schreiner, T.L.; Martin, J.; Messer, R.; Maloney, J.; Burakoff, A.; Barnes, M.; et al. Clinical Characteristics of Enterovirus A71 Neurologic Disease during an Outbreak in Children in Colorado, 2018. Lancet Infect. Dis. 2019. (under review). [Google Scholar]
- Center for Disease Control and Prevention. Acute Flaccid Myelitis - AFM Confirmed U.S. Cases. 2019. Available online: https://www.cdc.gov/acute-flaccid-myelitis/afm-cases.html (accessed on 10 May 2019).
- Davies, N.W.; Brown, L.J.; Gonde, J.; Irish, D.; Robinson, R.O.; Swan, A.V.; Banatvala, J.; Howard, R.S.; Sharief, M.K.; Muir, P. Factors influencing PCR detection of viruses in cerebrospinal fluid of patients with suspected CNS infections. J. Neurol. Neurosurg. Psychiatry 2005, 76, 82–87. [Google Scholar] [CrossRef]
- Center for Disease Control and Prevention. Acute Flaccid Myelitis. 2019. Available online: https://www.cdc.gov/acute-flaccid-myelitis/index.html (accessed on 10 May 2019).
- Moline, H.; Kalaskar, A.; Pomputius, W.F., III; Lopez, A.; Routh, J.; Kenyon, C.; Griffith, J. Notes from the Field: Six Cases of Acute Flaccid Myelitis in Children—Minnesota, 2018. MMWR Morb. Mortal. Weekly Rep. 2019, 68, 356–358. [Google Scholar] [CrossRef]
- Greninger, A.L.; Naccache, S.N.; Messacar, K.; Clayton, A.; Yu, G.; Somasekar, S.; Federman, S.; Stryke, D.; Anderson, C.; Yagi, S.; et al. A novel outbreak enterovirus D68 strain associated with acute flaccid myelitis cases in the USA (2012–14): A retrospective cohort study. Lancet Infect. Dis. 2015, 15, 671–682. [Google Scholar] [CrossRef]
- Sejvar, J.J.; Lopez, A.S.; Cortese, M.M.; Leshem, E.; Pastula, D.M.; Miller, L.; Glaser, C.; Kambhampati, A.; Shioda, K.; Aliabadi, N.; et al. Acute Flaccid Myelitis in the United States, August-December 2014: Results of Nationwide Surveillance. Clin. Infect. Dis. 2016, 63, 737–745. [Google Scholar] [CrossRef]
- Baltimore, D. Expression of animal virus genomes. Bacteriol. Rev. 1971, 35, 235–241. [Google Scholar]
- Baggen, J.; Thibaut, H.J.; Strating, J.R.; van Kuppeveld, F.J. The life cycle of non-polio enteroviruses and how to target it. Nat. Rev. Microbiol. 2018, 16, 368–381. [Google Scholar] [CrossRef] [PubMed]
- Lulla, V.; Dinan, A.M.; Hosmillo, M.; Chaudhry, Y.; Sherry, L.; Irigoyen, N.; Nayak, K.M.; Stonehouse, N.J.; Zilbauer, M.; Goodfellow, I.; et al. An upstream protein-coding region in enteroviruses modulates virus infection in gut epithelial cells. Nat. Microbiol. 2019, 4, 280–292. [Google Scholar] [CrossRef]
- Imamura, T.; Fuji, N.; Suzuki, A.; Tamaki, R.; Saito, M.; Aniceto, R.; Galang, H.; Sombrero, L.; Lupisan, S.; Oshitani, H. Enterovirus 68 among children with severe acute respiratory infection, the Philippines. Emerg. Infect. Dis. 2011, 17, 1430–1435. [Google Scholar] [CrossRef] [PubMed]
- Kaida, A.; Kubo, H.; Sekiguchi, J.I.; Kohdera, U.; Togawa, M.; Shiomi, M.; Nishigaki, T.; Iritani, N. Enterovirus 68 in children with acute respiratory tract infections, Osaka, Japan. Emerg. Infect. Dis. 2011, 17, 1494–1497. [Google Scholar] [CrossRef] [PubMed]
- Lauinger, I.L.; Bible, J.M.; Halligan, E.P.; Aarons, E.J.; MacMahon, E.; Tong, C.Y. Lineages, sub-lineages and variants of enterovirus 68 in recent outbreaks. PLoS ONE 2012, 7, e36005. [Google Scholar] [CrossRef]
- Linsuwanon, P.; Puenpa, J.; Suwannakarn, K.; Auksornkitti, V.; Vichiwattana, P.; Korkong, S.; Theamboonlers, A.; Poovorawan, Y. Molecular epidemiology and evolution of human enterovirus serotype 68 in Thailand, 2006–2011. PLoS ONE 2012, 7, e35190. [Google Scholar] [CrossRef] [PubMed]
- Ikeda, T.; Mizuta, K.; Abiko, C.; Aoki, Y.; Itagaki, T.; Katsushima, F.; Katsushima, Y.; Matsuzaki, Y.; Fuji, N.; Imamura, T.; et al. Acute respiratory infections due to enterovirus 68 in Yamagata, Japan between 2005 and 2010. Microbiol. Immunol. 2012, 56, 139–143. [Google Scholar] [CrossRef] [PubMed]
- Meijer, A.; van der Sanden, S.; Snijders, B.E.; Jaramillo-Gutierrez, G.; Bont, L.; van der Ent, C.K.; Overduin, P.; Jenny, S.L.; Jusic, E.; van der Avoort, H.G.; et al. Emergence and epidemic occurrence of enterovirus 68 respiratory infections in The Netherlands in 2010. Virology 2012, 423, 49–57. [Google Scholar] [CrossRef]
- Piralla, A.; Girello, A.; Grignani, M.; Gozalo-Margüello, M.; Marchi, A.; Marseglia, G.; Baldanti, F. Phylogenetic characterization of enterovirus 68 strains in patients with respiratory syndromes in Italy. J. Med. Virol. 2014, 86, 1590–1593. [Google Scholar] [CrossRef]
- Renois, F.; Bouin, A.; Andreoletti, L. Enterovirus 68 in pediatric patients hospitalized for acute airway diseases. J. Clin. Microbiol. 2013, 51, 640–643. [Google Scholar] [CrossRef]
- Opanda, S.M.; Wamunyokoli, F.; Khamadi, S.; Coldren, R.; Bulimo, W.D. Genetic diversity of human enterovirus 68 strains isolated in Kenya using the hypervariable 3’-end of VP1 gene. PLoS ONE 2014, 9, e102866. [Google Scholar] [CrossRef] [PubMed]
- Centers for Disease Control Prevention. Clusters of acute respiratory illness associated with human enterovirus 68—Asia, Europe, and United States, 2008–2010. MMWR Morb. Mortal. Wkly. Rep. 2011, 60, 1301–1304. [Google Scholar]
- Gong, Y.N.; Yang, S.L.; Shih, S.R.; Huang, Y.C.; Chang, P.Y.; Huang, C.G.; Kao, K.C.; Hu, H.C.; Liu, Y.C.; Tsao, K.C. Molecular evolution and the global reemergence of enterovirus D68 by genome-wide analysis. Medicine 2016, 95, e4416. [Google Scholar] [CrossRef] [PubMed]
- Tan, Y.; Hassan, F.; Schuster, J.E.; Simenauer, A.; Selvarangan, R.; Halpin, R.A.; Lin, X.; Fedorova, N.; Stockwell, T.B.; Lam, T.T.; et al. Molecular Evolution and Intraclade Recombination of Enterovirus D68 during the 2014 Outbreak in the United States. J. Virol. 2016, 90, 1997–2007. [Google Scholar] [CrossRef] [PubMed]
- Du, J.; Zheng, B.; Zheng, W.; Li, P.; Kang, J.; Hou, J.; Markham, R.; Zhao, K.; Yu, X.F. Analysis of Enterovirus 68 Strains from the 2014 North American Outbreak Reveals a New Clade, Indicating Viral Evolution. PLoS ONE 2015, 10, e0144208. [Google Scholar] [CrossRef] [PubMed]
- Pellegrinelli, L.; Giardina, F.; Lunghi, G.; Renteria, S.C.; Greco, L.; Fratini, A.; Galli, C.; Piralla, A.; Binda, S.; Pariani, E.; et al. Emergence of divergent enterovirus (EV) D68 sub-clade D1 strains, northern Italy, September to October 2018. Eurosurveillance 2019, 24, 1900090. [Google Scholar] [CrossRef] [PubMed]
- Yip, C.C.Y.; Lo, J.; Sridhar, S.; Lung, D.; Luk, S.; Chan, K.H.; Chan, J.; Cheng, V.; Woo, P.; Yuen, K.Y.; et al. First Report of a Fatal Case Associated with EV-D68 Infection in Hong Kong and Emergence of an Interclade Recombinant in China Revealed by Genome Analysis. Int. J. Mol. Sci. 2017, 18, 1065. [Google Scholar] [CrossRef] [PubMed]
- Rohll, J.B.; Percy, N.; Ley, R.; Evans, D.J.; Almond, J.W.; Barclay, W.S. The 5’-untranslated regions of picornavirus RNAs contain independent functional domains essential for RNA replication and translation. J. Virol. 1994, 68, 4384–4391. [Google Scholar] [PubMed]
- Yeh, M.T.; Wang, S.W.; Yu, C.K.; Lin, K.H.; Lei, H.Y.; Su, I.J.; Wang, J.R. A single nucleotide in stem loop II of 5’-untranslated region contributes to virulence of enterovirus 71 in mice. PLoS ONE 2011, 6, e27082. [Google Scholar] [CrossRef]
- De Jesus, N.; Franco, D.; Paul, A.; Wimmer, E.; Cello, J. Mutation of a single conserved nucleotide between the cloverleaf and internal ribosome entry site attenuates poliovirus neurovirulence. J. Virol. 2005, 79, 14235–14243. [Google Scholar] [CrossRef]
- Yuan, L.; Huang, X.Y.; Liu, Z.Y.; Zhang, F.; Zhu, X.L.; Yu, J.Y.; Ji, X.; Xu, Y.P.; Li, G.; Li, C.; et al. A single mutation in the prM protein of Zika virus contributes to fetal microcephaly. Science 2017, 358, 933–936. [Google Scholar] [CrossRef]
- Zhang, Y.; Cao, J.; Zhang, S.; Lee, A.J.; Sun, G.; Larsen, C.N.; Zhao, H.; Gu, Z.; He, S.; Klem, E.B. Genetic changes found in a distinct clade of Enterovirus D68 associated with paralysis during the 2014 outbreak. Virus Evol. 2016, 2, vew015. [Google Scholar] [CrossRef] [PubMed]
- Dyrdak, R.; Grabbe, M.; Hammas, B.; Ekwall, J.; Hansson, K.E.; Luthander, J.; Naucler, P.; Reinius, H.; Rotzén-Östlund, M.; Albert, J. Outbreak of enterovirus D68 of the new B3 lineage in Stockholm, Sweden, August to September 2016. Eurosurveillance 2016, 21, 30403. [Google Scholar] [CrossRef] [PubMed]
- Chen, I.J.; Hu, S.C.; Hung, K.L.; Lo, C.W. Acute flaccid myelitis associated with enterovirus D68 infection: A case report. Medicine 2018, 97, e11831. [Google Scholar] [CrossRef] [PubMed]
- Piralla, A.; Principi, N.; Ruggiero, L.; Girello, A.; Giardina, F.; De Sando, E.; Caimmi, S.; Bianchini, S.; Marseglia, G.L.; Lunghi, G.; et al. Enterovirus-D68 (EV-D68) in pediatric patients with respiratory infection: The circulation of a new B3 clade in Italy. J. Clin. Virol. 2018, 99–100, 91–96. [Google Scholar] [CrossRef]
- Wang, G.; Zhuge, J.; Huang, W.; Nolan, S.M.; Gilrane, V.L.; Yin, C.; Dimitrova, N.; Fallon, J.T. Enterovirus D68 Subclade B3 Strain Circulating and Causing an Outbreak in the United States in 2016. Sci. Rep. 2017, 7, 1242. [Google Scholar] [CrossRef]
- Esposito, S.; Chidini, G.; Cinnante, C.; Napolitano, L.; Giannini, A.; Terranova, L.; Niesters, H.; Principi, N.; Calderini, E. Acute flaccid myelitis associated with enterovirus-D68 infection in an otherwise healthy child. Virol. J. 2017, 14, 4. [Google Scholar] [CrossRef]
- Knoester, M.; Schölvinck, E.H.; Poelman, R.; Smit, S.; Vermont, C.L.; Niesters, H.G.; Van Leer-Buter, C.C. Upsurge of Enterovirus D68, the Netherlands, 2016. Emerg. Infect. Dis. 2017, 23, 140–143. [Google Scholar] [CrossRef]
- Carballo, C.M.; Erro, M.G.; Sordelli, N.; Vazquez, G.; Mistchenko, A.S.; Cejas, C.; Rodriguez, M.; Cisterna, D.M.; Freire, M.C.; Contrini, M.M.; et al. Acute Flaccid Myelitis Associated with Enterovirus D68 in Children, Argentina, 2016. Emerg. Infect. Dis. 2019, 25, 573–576. [Google Scholar] [CrossRef]
- Bal, A.; Sabatier, M.; Wirth, T.; Coste-Burel, M.; Lazrek, M.; Stefic, K.; Brengel-Pesce, K.; Morfin, F.; Lina, B.; Schuffenecker, I.; et al. Emergence of enterovirus D68 clade D1, France, August to November 2018. Eurosurveillance 2019, 24, 1800699. [Google Scholar] [CrossRef] [PubMed]
- Center for Disease Control and Prevention. Specimen Collection, Storage, & Shipment. Available online: https://www.cdc.gov/non-polio-enterovirus/lab-testing/specimen-collection.html (accessed on 28 July 2019).
- Harvala, H.; Broberg, E.; Benschop, K.; Berginc, N.; Ladhani, S.; Susi, P.; Christiansen, C.; McKenna, J.; Allen, D.; Makiello, P.; et al. Recommendations for enterovirus diagnostics and characterisation within and beyond Europe. J. Clin. Virol. 2018, 101, 11–17. [Google Scholar] [CrossRef] [PubMed]
- Hixon, A.M.; Yu, G.; Leser, J.S.; Yagi, S.; Clarke, P.; Chiu, C.Y.; Tyler, K.L. A mouse model of paralytic myelitis caused by enterovirus D68. PLoS Pathog. 2017, 13, e1006199. [Google Scholar] [CrossRef] [PubMed]
- Morrey, J.D.; Wang, H.; Hurst, B.; Zukor, K.; Siddharthan, V.; Van Wettere, A.; Sinex, D.; Tarbet, E. Causation of Acute Flaccid Paralysis by Myelitis and Myositis in Enterovirus-D68 Infected Mice Deficient in Interferon alphabeta/gamma Receptor Deficient Mice. Viruses 2018, 10, 33. [Google Scholar] [CrossRef] [PubMed]
- Brown, D.M.; Hixon, A.M.; Oldfield, L.M.; Zhang, Y.; Novotny, M.; Wang, W.; Das, S.R.; Shabman, R.S.; Tyler, K.L.; Scheuermann, R.H. Contemporary Circulating Enterovirus D68 Strains Have Acquired the Capacity for Viral Entry and Replication in Human Neuronal Cells. MBio 2018, 9, e01954-18. [Google Scholar] [CrossRef] [PubMed]
- Sun, S.; Bian, L.; Gao, F.; Du, R.; Hu, Y.; Fu, Y.; Su, Y.; Wu, X.; Mao, Q.; Liang, Z. A neonatal mouse model of Enterovirus D68 infection induces both interstitial pneumonia and acute flaccid myelitis. Antivir. Res. 2019, 161, 108–115. [Google Scholar] [CrossRef]
- Zhang, C.; Zhang, X.; Dai, W.; Liu, Q.; Xiong, P.; Wang, S.; Geng, L.; Gong, S.; Huang, Z. A Mouse Model of Enterovirus D68 Infection for Assessment of the Efficacy of Inactivated Vaccine. Viruses 2018, 10, 58. [Google Scholar] [CrossRef]
- Hixon, A.M.; Clarke, P.; Tyler, K.L. Evaluating Treatment Efficacy in a Mouse Model of Enterovirus D68-Associated Paralytic Myelitis. J. Infect. Dis. 2017, 216, 1245–1253. [Google Scholar] [CrossRef]
- Tyler, K.L.; McPhee, D.A.; Fields, B.N. Distinct pathways of viral spread in the host determined by reovirus S1 gene segment. Science 1986, 233, 770–774. [Google Scholar] [CrossRef]
- Sun, J.; Hu, X.Y.; Yu, X.F. Current Understanding of Human Enterovirus D68. Viruses 2019, 11, 490. [Google Scholar] [CrossRef]
- Dai, W.; Zhang, C.; Zhang, X.; Xiong, P.; Liu, Q.; Gong, S.; Geng, L.; Zhou, D.; Huang, Z. A virus-like particle vaccine confers protection against enterovirus D68 lethal challenge in mice. Vaccine 2018, 36, 653–659. [Google Scholar] [CrossRef] [PubMed]
- Harrison, C.J.; Weldon, W.C.; Pahud, B.A.; Jackson, M.A.; Oberste, M.S.; Selvarangan, R. Neutralizing Antibody against Enterovirus D68 in Children and Adults before 2014 Outbreak, Kansas City, Missouri, USA(1). Emerg. Infect. Dis. 2019, 25, 585–588. [Google Scholar] [CrossRef]
- Zhang, Y.; Moore, D.D.; Nix, W.A.; Oberste, M.S.; Weldon, W.C. Neutralization of Enterovirus D68 isolated from the 2014 US outbreak by commercial intravenous immune globulin products. J. Clin. Virol. 2015, 69, 172–175. [Google Scholar] [CrossRef] [PubMed]
- Royston, L.; Essaidi-Laziosi, M.; Pérez-Rodríguez, F.J.; Piuz, I.; Geiser, J.; Krause, K.H.; Huang, S.; Constant, S.; Kaiser, L.; Garcin, D.; et al. Viral chimeras decrypt the role of enterovirus capsid proteins in viral tropism, acid sensitivity and optimal growth temperature. PLoS Pathog. 2018, 14, e1006962. [Google Scholar] [CrossRef] [PubMed]
- Hixon, A.M.; Clarke, P.; Tyler, K.L. Contemporary circulating enterovirus D68 strains infect and undergo retrograde axonal transport in spinal motor neurons independent of sialic acid. J. Virol. 2019. [Google Scholar] [CrossRef]
- Walther, T.; Karamanska, R.; Chan, R.W.; Chan, M.C.; Jia, N.; Air, G.; Hopton, C.; Wong, M.P.; Dell, A.; Peiris, J.M.; et al. Glycomic analysis of human respiratory tract tissues and correlation with influenza virus infection. PLoS Pathog. 2013, 9, e1003223. [Google Scholar] [CrossRef] [PubMed]
- Baggen, J.; Thibaut, H.J.; Staring, J.; Jae, L.T.; Liu, Y.; Guo, H.; Slager, J.J.; de Bruin, J.W.; van Vliet, A.L.; Blomen, V.A.; et al. Enterovirus D68 receptor requirements unveiled by haploid genetics. Proc. Natl. Acad. Sci. USA 2016, 113, 1399–1404. [Google Scholar] [CrossRef]
- Liu, Y.; Sheng, J.; Baggen, J.; Meng, G.; Xiao, C.; Thibaut, H.J.; Van Kuppeveld, F.J.; Rossmann, M.G. Sialic acid-dependent cell entry of human enterovirus D68. Nat. Commun. 2015, 6, 8865. [Google Scholar] [CrossRef] [PubMed]
- Wei, W.; Guo, H.; Chang, J.; Yu, Y.; Liu, G.; Zhang, N.; Willard, S.H.; Zheng, S.; Yu, X.F. ICAM-5/Telencephalin Is a Functional Entry Receptor for Enterovirus D68. Cell Host Microbe 2016, 20, 631–641. [Google Scholar] [CrossRef]
- Kolatkar, P.R.; Bella, J.; Olson, N.H.; Bator, C.M.; Baker, T.S.; Rossmann, M.G. Structural studies of two rhinovirus serotypes complexed with fragments of their cellular receptor. EMBO J. 1999, 18, 6249–6259. [Google Scholar] [CrossRef]
- Xiao, C.; Bator, C.M.; Bowman, V.D.; Rieder, E.; He, Y.; Hébert, B.; Bella, J.; Baker, T.S.; Wimmer, E.; Kuhn, R.J.; et al. Interaction of coxsackievirus A21 with its cellular receptor, ICAM-1. J. Virol. 2001, 75, 2444–2451. [Google Scholar] [CrossRef] [PubMed]
| Strain | Clade | Route of Admin | Accession # | Source | Reference | |
|---|---|---|---|---|---|---|
| Paralytic | MO/14-18947 | B1 | IC, IM, IN, IP | KM851225 | 2014, USA, Respiratory | Hixon et al. 2017 Zhang et al. 2018 |
| MO/14-18949 | B1 | IP | MH708882 | 2014, USA, Respiratory | Morrey et al. 2018 | |
| CA/14-4232 | B1 | IC | KU844180 | 2014, USA, Respiratory | Hixon et al. 2017 | |
| IL/14-18952 | B2 | IC, IM | KM851230 | 2014, USA, Respiratory | Hixon et al. 2017 PLoS Hixon et al. 2017 JID | |
| Beijing-R0132 | B2 | IP | KP240936 | 2014, China, Respiratory | Sun et al. 2019 | |
| KY/14-18953 | D1 | IC, IP | KM851231 | 2014, USA, Respiratory | Hixon et al. 2017 | |
| Non-paralytic | CA/14-4231 | B2 | IC | KU844181 | 2014, USA, Respiratory | Hixon et al. 2017 |
| USA/N0051U5/2012 | A1 | IM | KT347280 | 2012, USA, Respiratory | Brown et al. 2019 | |
| VR1197 * | Proto type | IM | KT725431 | Respiratory | Brown et al. 2019 | |
| Fermon | Proto type | IC, IM | AY426531 | 1962, USA, Respiratory | Scheible et al. 1967 Hixon et al. 2017 Zhang et al. 2018 | |
| Rhyne | Proto type | IC, IM | KU844178 | 1962, USA, Respiratory | Scheible et al. 1967 Hixon et al. 2017 | |
| Franklin | Proto type | IC | 1962, USA, Respiratory | Scheible et al. 1967 | ||
| Robinson | Proto type | IC | 1962, USA, Respiratory | Scheible et al. 1967 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hixon, A.M.; Frost, J.; Rudy, M.J.; Messacar, K.; Clarke, P.; Tyler, K.L. Understanding Enterovirus D68-Induced Neurologic Disease: A Basic Science Review. Viruses 2019, 11, 821. https://doi.org/10.3390/v11090821
Hixon AM, Frost J, Rudy MJ, Messacar K, Clarke P, Tyler KL. Understanding Enterovirus D68-Induced Neurologic Disease: A Basic Science Review. Viruses. 2019; 11(9):821. https://doi.org/10.3390/v11090821
Chicago/Turabian StyleHixon, Alison M., Joshua Frost, Michael J. Rudy, Kevin Messacar, Penny Clarke, and Kenneth L. Tyler. 2019. "Understanding Enterovirus D68-Induced Neurologic Disease: A Basic Science Review" Viruses 11, no. 9: 821. https://doi.org/10.3390/v11090821
APA StyleHixon, A. M., Frost, J., Rudy, M. J., Messacar, K., Clarke, P., & Tyler, K. L. (2019). Understanding Enterovirus D68-Induced Neurologic Disease: A Basic Science Review. Viruses, 11(9), 821. https://doi.org/10.3390/v11090821





