Host Immune Response to ZIKV in an Immunocompetent Embryonic Mouse Model of Intravaginal Infection
Abstract
1. Introduction
2. Materials and Methods
2.1. Animals
2.2. Virus
2.3. Intravaginal Infection
2.4. Quantitative PCR (qPCR)
2.5. Statistical Analysis
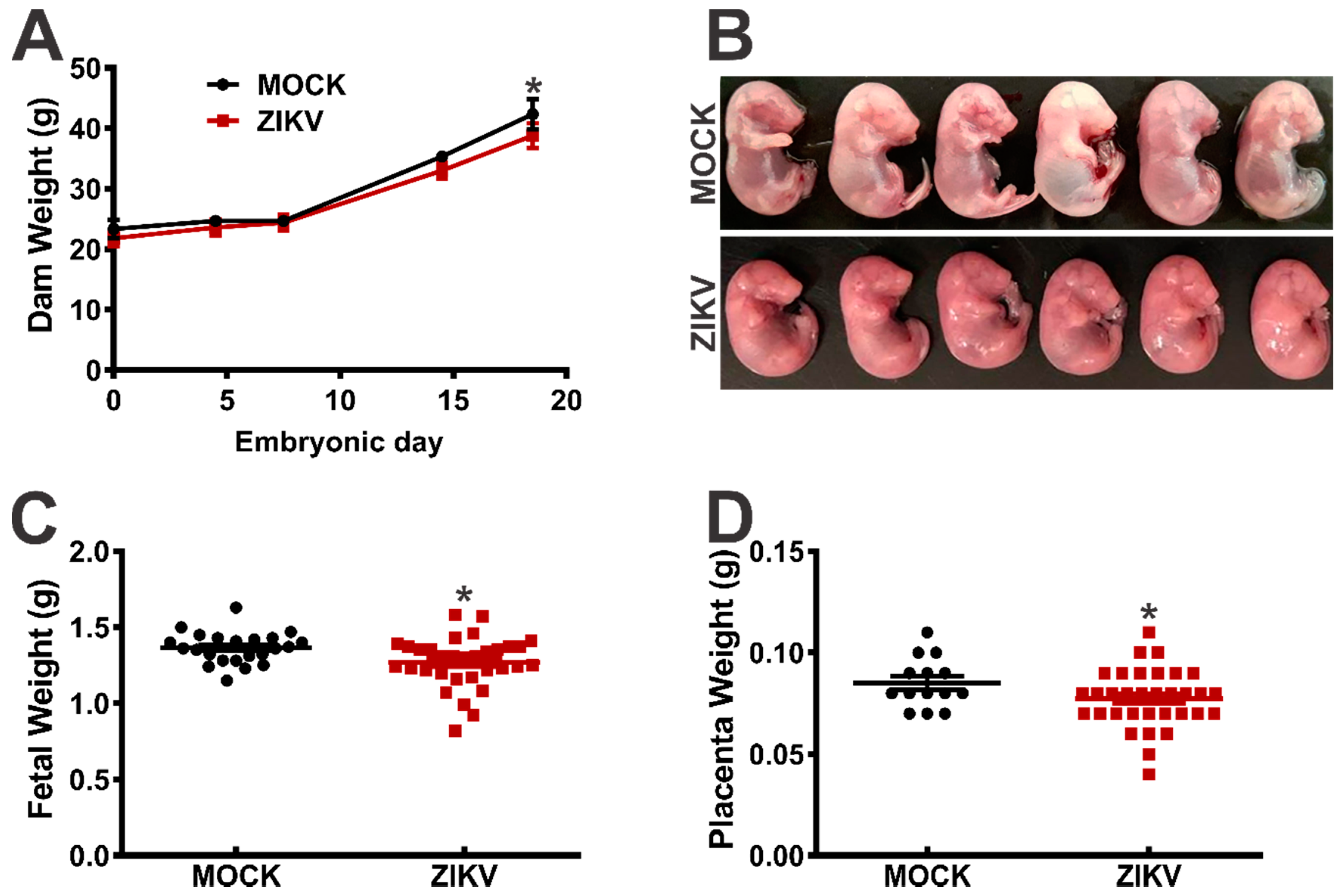
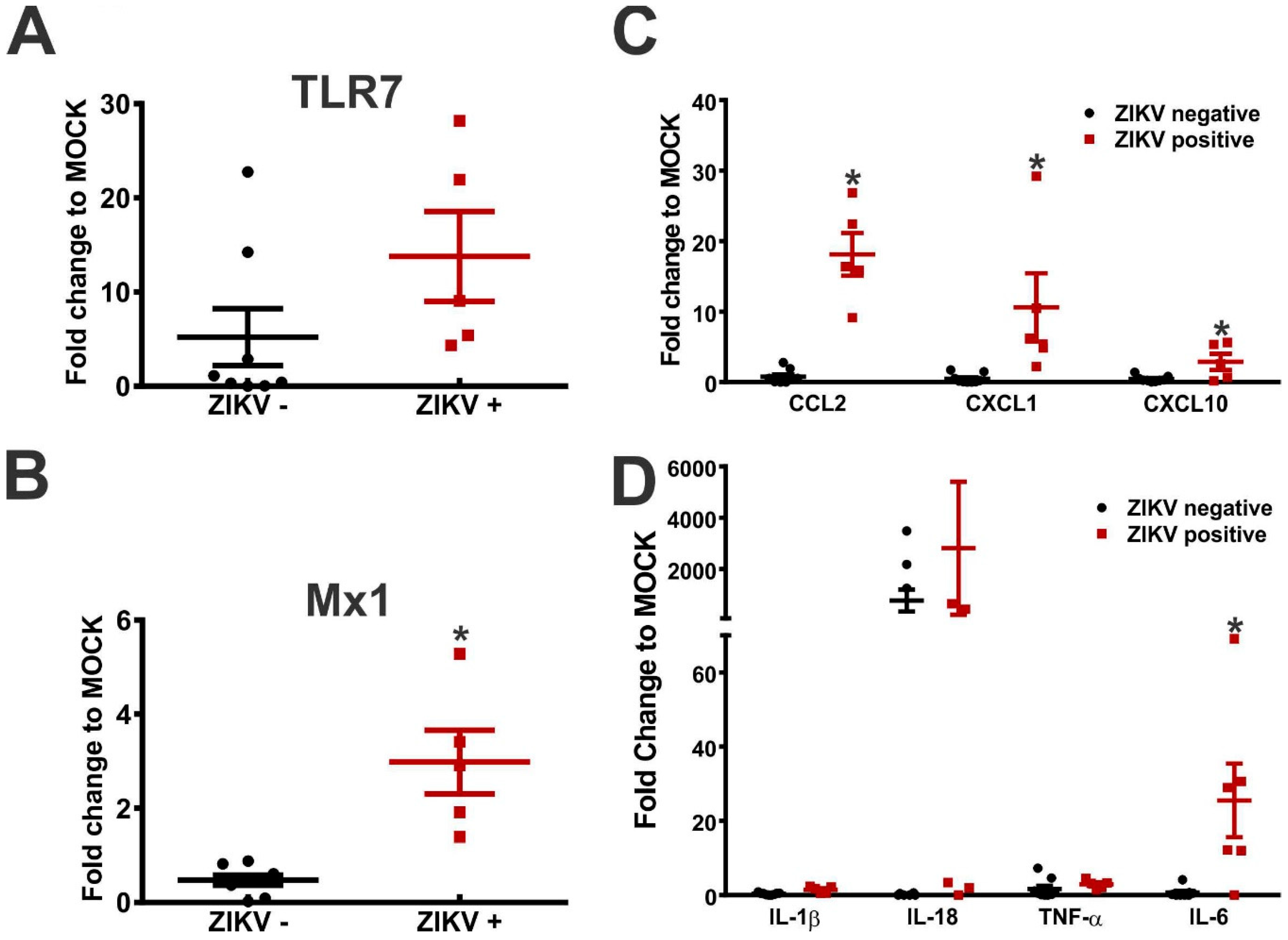
3. Results
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- McCarthy, M. Zika virus was transmitted by sexual contact in Texas, health officials report. BMJ 2016, 352, i720. [Google Scholar] [CrossRef] [PubMed]
- D’Ortenzio, E.; Matheron, S.; Yazdanpanah, Y.; de Lamballerie, X.; Hubert, B.; Piorkowski, G.; Maquart, M.; Descamps, D.; Damond, F.; Leparc-Goffart, I. Evidence of sexual transmission of Zika virus. N. Engl. J. Med. 2016, 374, 2195–2198. [Google Scholar] [CrossRef] [PubMed]
- Hills, S.L.; Russell, K.; Hennessey, M.; Williams, C.; Oster, A.M.; Fischer, M.; Mead, P. Transmission of Zika virus through sexual contact with travelers to areas of ongoing transmission—Continental United States, 2016. Morb. Mortal. Wkly. Rep. 2016, 65, 215–216. [Google Scholar] [CrossRef] [PubMed]
- Venturi, G.; Zammarchi, L.; Fortuna, C.; Remoli, M.E.; Benedetti, E.; Fiorentini, C.; Trotta, M.; Rizzo, C.; Mantella, A.; Rezza, G.; et al. An autochthonous case of Zika due to possible sexual transmission, Florence, Italy, 2014. Eurosurveillance 2016, 21, 30148. [Google Scholar] [CrossRef] [PubMed]
- Foy, B.D.; Kobylinski, K.C.; Chilson Foy, J.L.; Blitvich, B.J.; Travassos da Rosa, A.; Haddow, A.D.; Lanciotti, R.S.; Tesh, R.B. Probable non-vector-borne transmission of Zika virus, Colorado, USA. Emerg. Infect. Dis. 2011, 17, 880–882. [Google Scholar] [CrossRef]
- Davidson, A.; Slavinski, S.; Komoto, K.; Rakeman, J.; Weiss, D. Suspected female-to-male sexual transmission of Zika virus—New York City, 2016. Morb. Mortal. Wkly. Rep. 2016, 65, 716–717. [Google Scholar] [CrossRef]
- Deckard, D.T.; Chung, W.M.; Brooks, J.T.; Smith, J.C.; Woldai, S.; Hennessey, M.; Kwit, N.; Mead, P. Male-to-Male Sexual Transmission of Zika Virus-Texas, January 2016. Mor. Mortal Wkl. Rep. 2016, 65, 372–374. [Google Scholar] [CrossRef]
- Coelho, F.C.; Durovni, B.; Saraceni, V.; Lemos, C.; Codeco, C.T.; Camargo, S.; de Carvalho, L.M.; Bastos, L.; Arduini, D.; Villela, D.A.; et al. Higher incidence of Zika in adult women than adult men in Rio de Janeiro suggests a significant contribution of sexual transmission from men to women. Int. J. Infect. Dis. 2016, 51, 128–132. [Google Scholar] [CrossRef]
- Duffy, M.R.; Chen, T.H.; Hancock, W.T.; Powers, A.M.; Kool, J.L.; Lanciotti, R.S.; Pretrick, M.; Marfel, M.; Holzbauer, S.; Dubray, C.; et al. Zika virus outbreak on Yap Island, Federated States of Micronesia. N. Engl. J. Med. 2009, 360, 2536–2543. [Google Scholar] [CrossRef]
- Atkinson, B.; Hearn, P.; Afrough, B.; Lumley, S.; Carter, D.; Aarons, E.J.; Simpson, A.J.; Brooks, T.J.; Hewson, R. Detection of Zika virus in semen. Emerg. Infect. Dis. 2016, 22, 940. [Google Scholar] [CrossRef]
- Mansuy, J.M.; Dutertre, M.; Mengelle, C.; Fourcade, C.; Marchou, B.; Delobel, P.; Izopet, J.; Martin-Blondel, G. Zika virus: High infectious viral load in semen, a new sexually transmitted pathogen? Lancet Infect. Dis. 2016, 16, 405. [Google Scholar] [CrossRef]
- Mansuy, J.M.; Suberbielle, E.; Chapuy-Regaud, S.; Mengelle, C.; Bujan, L.; Marchou, B.; Delobel, P.; Gonzalez-Dunia, D.; Malnou, C.E.; Izopet, J.; et al. Zika virus in semen and spermatozoa. Lancet Infect. Dis. 2016, 16, 1106–1107. [Google Scholar] [CrossRef]
- Matheron, S.; d’Ortenzio, E.; Leparc-Goffart, I.; Hubert, B.; de Lamballerie, X.; Yazdanpanah, Y. Long-lasting persistence of Zika virus in semen. Clin. Infect. Dis. 2016, 63, 1264. [Google Scholar] [CrossRef] [PubMed]
- Barzon, L.; Pacenti, M.; Franchin, E.; Lavezzo, E.; Trevisan, M.; Sgarabotto, D.; Palu, G. Infection dynamics in a traveller with persistent shedding of Zika virus RNA in semen for six months after returning from Haiti to Italy, January 2016. Eurosurveillance 2016, 21, 30316. [Google Scholar] [CrossRef] [PubMed]
- Nicastri, E.; Castilletti, C.; Liuzzi, G.; Iannetta, M.; Capobianchi, M.R.; Ippolito, G. Persistent detection of Zika virus RNA in semen for six months after symptom onset in a traveller returning from Haiti to Italy, February 2016. Eurosurveillance 2016, 21, 30314. [Google Scholar] [CrossRef] [PubMed]
- Arsuaga, M.; Bujalance, S.G.; Diaz-Menendez, M.; Vazquez, A.; Arribas, J.R. Probable sexual transmission of Zika virus from a vasectomised man. Lancet Infect. Dis. 2016, 16, 1107. [Google Scholar] [CrossRef]
- Prisant, N.; Bujan, L.; Benichou, H.; Hayot, P.H.; Pavili, L.; Lurel, S.; Herrmann, C.; Janky, E.; Joguet, G. Zika virus in the female genital tract. Lancet Infect. Dis. 2016, 16, 1000–1001. [Google Scholar] [CrossRef]
- Paz-Bailey, G.; Rosenberg, E.S.; Doyle, K.; Munoz-Jordan, J.; Santiago, G.A.; Klein, L.; Perez-Padilla, J.; Medina, F.A.; Waterman, S.H.; Gubern, C.G.; et al. Persistence of Zika virus in body fluids—Preliminary report. N. Engl. J. Med. 2017, 379, 1234–1243. [Google Scholar] [CrossRef] [PubMed]
- Yockey, L.J.; Varela, L.; Rakib, T.; Khoury-Hanold, W.; Fink, S.L.; Stutz, B.; Szigeti-Buck, K.; Van den Pol, A.; Lindenbach, B.D.; Horvath, T.L.; et al. Vaginal exposure to Zika Virus during pregnancy leads to fetal brain infection. Cell 2016, 166, 1247–1256. [Google Scholar] [CrossRef] [PubMed]
- Duggal, N.K.; Ritter, J.M.; Pestorius, S.E.; Zaki, S.R.; Davis, B.S.; Chang, G.J.; Bowen, R.A.; Brault, A.C. Frequent Zika virus sexual transmission and prolonged viral RNA shedding in an immunodeficient mouse model. Cell Rep. 2017, 18, 1751–1760. [Google Scholar] [CrossRef] [PubMed]
- Uraki, R.; Jurado, K.A.; Hwang, J.; Szigeti-Buck, K.; Horvath, T.L.; Iwasaki, A.; Fikrig, E. Fetal growth restriction caused by sexual transmission of Zika virus in mice. J. Infect. Dis. 2017, 215, 1720–1724. [Google Scholar] [CrossRef] [PubMed]
- Winkler, C.W.; Woods, T.A.; Rosenke, R.; Scott, D.P.; Best, S.M.; Peterson, K.E. Sexual and vertical transmission of Zika virus in anti-interferon receptor-treated Rag1-deficient mice. Sci. Rep. 2017, 7, 7176. [Google Scholar] [CrossRef] [PubMed]
- Costa, V.V.; Del Sarto, J.L.; Rocha, R.F.; Silva, F.R.; Doria, J.G.; Olmo, I.G.; Marques, R.E.; Queiroz-Junior, C.M.; Foureaux, G.; Araujo, J.M.S.; et al. N-methyl-d-aspartate (NMDA) receptor blockade prevents neuronal death induced by Zika virus infection. MBio 2017, 8, e00350-17. [Google Scholar] [CrossRef] [PubMed]
- Dowall, S.D.; Graham, V.A.; Rayner, E.; Atkinson, B.; Hall, G.; Watson, R.J.; Bosworth, A.; Bonney, L.C.; Kitchen, S.; Hewson, R. A susceptible mouse model for Zika virus infection. PLoS Negl. Trop. Dis. 2016, 10, e0004658. [Google Scholar] [CrossRef] [PubMed]
- Cugola, F.R.; Fernandes, I.R.; Russo, F.B.; Freitas, B.C.; Dias, J.L.; Guimaraes, K.P.; Benazzato, C.; Almeida, N.; Pignatari, G.C.; Romero, S.; et al. The Brazilian Zika virus strain causes birth defects in experimental models. Nature 2016, 534, 267–271. [Google Scholar] [CrossRef] [PubMed]
- Xavier-Neto, J.; Carvalho, M.; Pascoalino, B.D.; Cardoso, A.C.; Costa, A.M.; Pereira, A.H.; Santos, L.N.; Saito, A.; Marques, R.E.; Smetana, J.H.; et al. Hydrocephalus and arthrogryposis in an immunocompetent mouse model of Zika teratogeny: A developmental study. PLoS Negl. Trop. Dis. 2017, 11, e0005363. [Google Scholar] [CrossRef] [PubMed]
- Costa, V.V.; Fagundes, C.T.; Valadao, D.F.; Avila, T.V.; Cisalpino, D.; Rocha, R.F.; Ribeiro, L.S.; Ascencao, F.R.; Kangussu, L.M.; Celso, M.Q., Jr.; et al. Subversion of early innate antiviral responses during antibody—Dependent enhancement of Dengue virus infection induces severe disease in immunocompetent mice. Med. Microbiol. Immunol. 2014, 203, 231–250. [Google Scholar] [CrossRef] [PubMed]
- Mesci, P.; Macia, A.; LaRock, C.N.; Tejwani, L.; Fernandes, I.R.; Suarez, N.A.; de, A.Z.P.M.; Beltrao-Braga, P.C.B.; Nizet, V.; Muotri, A.R. Modeling neuro-immune interactions during Zika virus infection. Hum. Mol. Genet. 2018, 27, 41–52. [Google Scholar] [CrossRef] [PubMed]
- Vanwalscappel, B.; Tada, T.; Landau, N.R. Toll-like receptor agonist R848 blocks Zika virus replication by inducing the antiviral protein viperin. Virology 2018, 522, 199–208. [Google Scholar] [CrossRef]
- Chen, J.; Liang, Y.; Yi, P.; Xu, L.; Hawkins, H.K.; Rossi, S.L.; Soong, L.; Cai, J.; Menon, R.; Sun, J. Outcomes of congenital Zika disease depend on timing of infection and maternal—Fetal interferon action. Cell Rep. 2017, 21, 1588–1599. [Google Scholar] [CrossRef]
- Rossi, S.L.; Tesh, R.B.; Azar, S.R.; Muruato, A.E.; Hanley, K.A.; Auguste, A.J.; Langsjoen, R.M.; Paessler, S.; Vasilakis, N.; Weaver, S.C. Characterization of a Novel Murine model to study Zika virus. Am. J. Trop. Med. Hyg. 2016, 94, 1362–1369. [Google Scholar] [CrossRef] [PubMed]
- Haller, O.; Stertz, S.; Kochs, G. The Mx GTPase family of interferon-induced antiviral proteins. Microbes Infect. 2007, 9, 1636–1643. [Google Scholar] [CrossRef] [PubMed]
- Mogensen, T.H.; Paludan, S.R. Molecular pathways in virus-induced cytokine production. Microbiol. Mol. Biol. Rev. 2001, 65, 131–150. [Google Scholar] [CrossRef] [PubMed]
- Moore, C.A.; Staples, J.E.; Dobyns, W.B.; Pessoa, A.; Ventura, C.V.; Fonseca, E.B.; Ribeiro, E.M.; Ventura, L.O.; Neto, N.N.; Arena, J.F.; et al. Characterizing the pattern of anomalies in congenital Zika syndrome for pediatric clinicians. JAMA Pediatr. 2017, 171, 288–295. [Google Scholar] [CrossRef]
- Brasil, P.; Pereira, J.P., Jr.; Moreira, M.E.; Ribeiro Nogueira, R.M.; Damasceno, L.; Wakimoto, M.; Rabello, R.S.; Valderramos, S.G.; Halai, U.A.; Salles, T.S.; et al. Zika virus infection in pregnant women in Rio de Janeiro. N. Engl. J. Med. 2016, 375, 2321–2334. [Google Scholar] [CrossRef] [PubMed]
- Reynolds, M.R.; Jones, A.M.; Petersen, E.E.; Lee, E.H.; Rice, M.E.; Bingham, A.; Ellington, S.R.; Evert, N.; Reagan-Steiner, S.; Oduyebo, T.; et al. Vital signs: Update on Zika virus-associated birth defects and evaluation of all, U.S. infants with congenital Zika virus exposure—U.S. Zika pregnancy registry, 2016. Morb. Mortal. Wkly. Rep. 2017, 66, 366–373. [Google Scholar] [CrossRef] [PubMed]
- Johansson, M.A.; Mier-y-Teran-Romero, L.; Reefhuis, J.; Gilboa, S.M.; Hills, S.L. Zika and the risk of microcephaly. N. Engl. J. Med. 2016, 375, 1–4. [Google Scholar] [CrossRef]
- Souza, W.V.; Albuquerque, M.; Vazquez, E.; Bezerra, L.C.A.; Mendes, A.; Lyra, T.M.; Araujo, T.V.B.; Oliveira, A.L.S.; Braga, M.C.; Ximenes, R.A.A.; et al. Microcephaly epidemic related to the Zika virus and living conditions in Recife, Northeast Brazil. BMC Public Health 2018, 18, 130. [Google Scholar] [CrossRef]
- Franca, G.V.; Schuler-Faccini, L.; Oliveira, W.K.; Henriques, C.M.; Carmo, E.H.; Pedi, V.D.; Nunes, M.L.; Castro, M.C.; Serruya, S.; Silveira, M.F.; et al. Congenital Zika virus syndrome in Brazil: A case series of the first 1501 livebirths with complete investigation. Lancet 2016, 388, 891–897. [Google Scholar] [CrossRef]
- Duggal, N.K.; McDonald, E.M.; Ritter, J.M.; Brault, A.C. Sexual transmission of Zika virus enhances in utero transmission in a mouse model. Sci. Rep. 2018, 8, 4510. [Google Scholar] [CrossRef]
- Hirsch, A.J.; Roberts, V.H.J.; Grigsby, P.L.; Haese, N.; Schabel, M.C.; Wang, X.; Lo, J.O.; Liu, Z.; Kroenke, C.D.; Smith, J.L.; et al. Zika virus infection in pregnant rhesus macaques causes placental dysfunction and immunopathology. Nat. Commun. 2018, 9, 263. [Google Scholar] [CrossRef] [PubMed]
- Medzhitov, R.; Janeway, C., Jr. Innate immune recognition: Mechanisms and pathways. Immunol. Rev. 2000, 173, 89–97. [Google Scholar] [CrossRef] [PubMed]
- Schneider, W.M.; Chevillotte, M.D.; Rice, C.M. Interferon-stimulated genes: A complex web of host defenses. Annu. Rev. Immunol. 2014, 32, 513–545. [Google Scholar] [CrossRef] [PubMed]
- Cumming, H.E.; Bourke, N.M. Type I IFNs in the female reproductive tract: The first line of defense in an ever-changing battleground. J. Leukoc. Biol. 2019, 105, 353–361. [Google Scholar] [CrossRef] [PubMed]
- McNab, F.; Mayer-Barber, K.; Sher, A.; Wack, A.; O’Garra, A. Type I interferons in infectious disease. Nat. Rev. Immunol. 2015, 15, 87–103. [Google Scholar] [CrossRef] [PubMed]
- Hosking, M.P.; Lane, T.E. The role of chemokines during viral infection of the CNS. PLoS Pathog. 2010, 6, e1000937. [Google Scholar] [CrossRef] [PubMed]
- McGlasson, S.; Jury, A.; Jackson, A.; Hunt, D. Type I interferon dysregulation and neurological disease. Nat. Rev. Neurol. 2015, 11, 515–523. [Google Scholar] [CrossRef] [PubMed]
- Khaiboullina, S.F.; Uppal, T.; Sarkar, R.; Gorzalski, A.; St Jeor, S.; Verma, S.C. ZIKV infection regulates inflammasomes pathway for replication in monocytes. Sci. Rep. 2017, 7, 16050. [Google Scholar] [CrossRef] [PubMed]
- Foo, S.S.; Chen, W.; Chan, Y.; Lee, W.S.; Lee, S.A.; Cheng, G.; Nielsen-Saines, K.; Brasil, P.; Jung, J.U. Biomarkers and immunoprofiles associated with fetal abnormalities of ZIKV-positive pregnancies. JCI Insight 2018, 3, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Ornelas, A.M.; Pezzuto, P.; Silveira, P.P.; Melo, F.O.; Ferreira, T.A.; Oliveira-Szejnfeld, P.S.; Leal, J.I.; Amorim, M.M.; Hamilton, S.; Rawlinson, W.D.; et al. Immune activation in amniotic fluid from Zika virus-associated microcephaly. Ann. Neurol. 2017, 81, 152–156. [Google Scholar] [CrossRef] [PubMed]
- Naveca, F.G.; Pontes, G.S.; Chang, A.Y.; Silva, G.; Nascimento, V.A.D.; Monteiro, D.; Silva, M.S.D.; Abdalla, L.F.; Santos, J.H.A.; Almeida, T.A.P.; et al. Analysis of the immunological biomarker profile during acute Zika virus infection reveals the overexpression of CXCL10, a chemokine linked to neuronal damage. Mem. Inst. Oswaldo Cruz 2018, 113, e170542. [Google Scholar] [CrossRef] [PubMed]
| Primer Target | Forward | Reverse |
|---|---|---|
| IL-1β | AGCTTCAAATCTCGCAGCAG | TCTCCACAGCCACAATGAGT |
| Il-6 | GACTGATGCTGGTGACAACC | AGACAGGTCTGTTGGGAGTG |
| IL-18 | CTTCTGCAACCTCCAGCATC | GTGAAGTCGGCCAAAGTTGT |
| Ccl2 | AACTGCATCTGCCCTAAGGT | CTGTCACACTGGTCACTCCT |
| Cxcl1 | TGTGGGAGGCTGTGTTTGTA | ACGAGACCAGGAGAAACAGG |
| Cxcl10 | AGCCATGGTCCTGAGACAAA | ACAGAGCTAGGACAGCCATC |
| Mx1 | AGGCAGTGGTATTGTCACCA | AGACTTTGCCTCTCCACTCC |
| TLR7 | ATGTCCTTGGCTCCCTTCTC | ACTGAGCCATGTCTCTTGCT |
| TNFα | CTCATGCACCACCATCAAGG | ACCTGACCACTCTCCCTTTG |
| zika virus 1087-1163 | CCGCTGCCCAACACAAG | CCACTAACGTTCTTTTGCAGACAT |
| Dam | ZIKV | n Embryos | Malformed | Reabsorptions | Total Dead |
|---|---|---|---|---|---|
| Dam 1 | – | 7 | 0 | 0 | 0 |
| Dam 2 | + | 10 | 0 | 1 | 1 |
| Dam 3 | – | 10 | 0 | 0 | 0 |
| Dam 4 | – | 7 | 1 | 0 | 1 |
| Dam 5 | – | 4 | 0 | 0 | 0 |
| Dam 6 | + | 10 | 0 | 0 | 0 |
| Dam 7 | + | 6 | 0 | 1 | 1 |
| ZIKV+ (n = 6) | ZIKV- (n = 8) | Total (n = 14) | |
|---|---|---|---|
| TLR7 | 6/6 | 4/8 | 10/14 |
| Mx1 | 5/6 | 1/8 | 6/14 |
| CCL2 | 6/6 | 2/8 | 8/14 |
| CXCL1 | 6/6 | 0/8 | 6/14 |
| CXCL10 | 4/6 | 0/8 | 4/14 |
| IL-1β | 3/6 | 0/8 | 3/14 |
| IL-18 | 5/6 | 3/8 | 8/14 |
| TNF-α | 5/6 | 2/8 | 7/14 |
| IL-6 | 5/6 | 1/8 | 6/14 |
| Dam | ZIKV+ (n = 13) | ZIKV- (n = 1) | Total (n = 14) |
|---|---|---|---|
| TLR7 | 10/13 | 1/1 | 11/14 |
| Mx1 | 5/13 | 0/1 | 5/14 |
| CCL2 | 7/13 | 0/1 | 7/14 |
| CXCL1 | 7/13 | 0/1 | 7/14 |
| CXCL10 | 3/13 | 0/1 | 3/14 |
| IL-1β | 2/13 | 0/1 | 2/14 |
| IL-18 | 9/13 | 1/1 | 10/14 |
| TNF-α | 6/13 | 0/1 | 6/14 |
| IL-6 | 8/13 | 0/1 | 8/14 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Khaiboullina, S.F.; Lopes, P.; de Carvalho, T.G.; Real, A.L.C.V.; Souza, D.G.; Costa, V.V.; Teixeira, M.M.; Bloise, E.; Verma, S.C.; Ribeiro, F.M. Host Immune Response to ZIKV in an Immunocompetent Embryonic Mouse Model of Intravaginal Infection. Viruses 2019, 11, 558. https://doi.org/10.3390/v11060558
Khaiboullina SF, Lopes P, de Carvalho TG, Real ALCV, Souza DG, Costa VV, Teixeira MM, Bloise E, Verma SC, Ribeiro FM. Host Immune Response to ZIKV in an Immunocompetent Embryonic Mouse Model of Intravaginal Infection. Viruses. 2019; 11(6):558. https://doi.org/10.3390/v11060558
Chicago/Turabian StyleKhaiboullina, Svetlana F., Priscila Lopes, Toniana G. de Carvalho, Ana Luiza C. V. Real, Danielle G. Souza, Vivian V. Costa, Mauro M. Teixeira, Enrrico Bloise, Subhash C. Verma, and Fabiola M. Ribeiro. 2019. "Host Immune Response to ZIKV in an Immunocompetent Embryonic Mouse Model of Intravaginal Infection" Viruses 11, no. 6: 558. https://doi.org/10.3390/v11060558
APA StyleKhaiboullina, S. F., Lopes, P., de Carvalho, T. G., Real, A. L. C. V., Souza, D. G., Costa, V. V., Teixeira, M. M., Bloise, E., Verma, S. C., & Ribeiro, F. M. (2019). Host Immune Response to ZIKV in an Immunocompetent Embryonic Mouse Model of Intravaginal Infection. Viruses, 11(6), 558. https://doi.org/10.3390/v11060558








