Abstract
Prion diseases are a unique group of rare neurodegenerative disorders characterized by tissue deposition of heterogeneous aggregates of abnormally folded protease-resistant prion protein (PrPSc), a broad spectrum of disease phenotypes and a variable efficiency of disease propagation in vivo. The dominant clinicopathological phenotypes of human prion disease include Creutzfeldt–Jakob disease, fatal insomnia, variably protease-sensitive prionopathy, and Gerstmann–Sträussler–Scheinker disease. Prion disease propagation into susceptible hosts led to the isolation and characterization of prion strains, initially operatively defined as “isolates” causing diseases with distinctive characteristics, such as the incubation period, the pattern of PrPSc distribution, and the regional severity of neuropathological changes after injection into syngeneic hosts. More recently, the structural basis of prion strains has been linked to amyloid polymorphs (i.e., variant amyloid protein conformations) and the concept extended to all protein amyloids showing polymorphic structures and some evidence of in vivo or in vitro propagation by seeding. Despite the significant advances, however, the link between amyloid structure and disease is not understood in many instances. Here we reviewed the most significant contributions of human prion disease studies to current knowledge of the molecular basis of phenotypic variability and the prion strain phenomenon and underlined the unsolved issues from the human disease perspective.
1. Introduction
Prion diseases, also known as transmissible spongiform encephalopathies (TSEs), belong to a group of neurodegenerative disorders of humans and animals, characterized by aggregation and tissue deposition of a misfolded form of the prion protein (PrP). In this pathological process, the physiological cellular PrP (PrPC) converts into abnormal PrP (PrPSc) through post-translational events that lead to an increased β-sheet conformation [1]. Once formed, PrPSc replicates itself by a seeded-conversion mechanism in which PrPSc binds to PrPC and mediates its conversion to PrPSc [2]. Newly converted PrPSc then propagates and accumulates preferentially, but not exclusively, in the central nervous system.
Despite their rarity, prion diseases include a wide range of clinicopathological phenotypes. They also often propagate after inoculation into susceptible hosts, a property that led to the isolation and characterization of different prion strains. These were initially defined as animal or human “isolates” that, after injection into syngeneic hosts, cause diseases with distinctive characteristics, such as the incubation period, the pattern of PrPSc distribution, and the regional severity of neuropathological changes [3]. However, following the demonstration that any given protein in the amyloid state, including those involved in other neurodegenerative diseases, can form ordered aggregates with distinct conformations, the term is frequently applied, with a much broader meaning, to all protein amyloids that show heterogeneous structures and some evidence of in vitro or in vivo propagation by seeding [4].
The characterization of distinct isoforms of PrPSc that strongly correlate with the disease phenotype also led to the concept of molecular strain typing, in which the different PrPSc isoforms or types may serve as molecular markers for prion strains [5].
The clinicopathological spectrum of human prion diseases is traditionally classified in major disease groups, which include Creutzfeldt–Jakob disease (CJD), by far the most common; fatal insomnia (FI); Gerstmann–Sträussler–Scheinker disease (GSS); and variably protease-sensitive prionopathy (VPSPr). However, apart from FI, which truly represents a distinctive disease subtype that is linked to a specific prion strain, all of the others represent heterogeneous groups including multiple distinct disease phenotypes or subtypes.
Human prion diseases also differ in their apparent origin, which is another common criterion used for their classification. Sporadic prion disease (also known as idiopathic) comprises more than 80% of all cases with an overall prevalence of about 2 cases per million and is thought to originate spontaneously from unknown stochastic cellular events leading to PrPC conversion into PrPSc. However, the involvement, at least in some cases, of an exogenous trigger of PrPSc formation has not been completely ruled out. The genetic form (also called inherited or familial prion disease) is linked to autosomal-dominant mutations in the prion protein gene (PRNP) and account for about 10% of cases. Finally, the acquired form may originate from accidental human-to-human transmission as in iatrogenic CJD (iCJD) or ritual cannibalism as in kuru, or from ingestion or inoculation of bovine spongiform encephalopathy (BSE)-derived prions as in variant CJD (vCJD) [6].
Here we have reviewed the most significant contributions of studies on human prion diseases to current knowledge of the molecular basis of phenotypic variability and the prion strain phenomenon and underlined the unsolved issues from the human disease perspective.
2. Historical Background
The origin of the concept of strains in the field of TSEs, a term originally intended to define the variability of an infectious agent due to nucleic acid sequence variations, dates back 60 years. At that time, the successful experimental transmission of ovine scrapie to goats, achieved by Pattison and Millson, led to the description of the “scratchy” and “nervous” (later defined as “drowsy”) phenotypes and the demonstration that they were maintained after serial passages [7]. Follow-up experiments, based on transmissions to rodents, further highlighted the heterogeneity of the scrapie agent and the phenotypic diversity of TSEs [8,9,10].
At variance with scrapie, the study of human prion strains and their characterization started much later. Early studies in non-human primates aimed to prove the transmissibility of human prion disease but did not include detailed comparative studies of the resulting phenotype in the affected animals [11,12]. The difficulties in obtaining efficient propagation in wild-type rodents because of the “species barrier” also significantly contributed to this delay [13,14]. As a consequence, the progression of knowledge concerning the basis of phenotypic heterogeneity in ovine and human prion diseases followed an opposite trajectory. Indeed, in humans, the detailed description of the main phenotypic variants of the disease preceded the characterization of prion strains after experimental transmission [15]. The development of models based on transgenic (Tg) lines allowed researchers to overcome the issue of the species barrier, and it is not surprising that the study of strain-specific properties of human prion isolates has mainly been conducted in Tg mice [16]. As the only exceptions, a few studies analyzed the transmission proprieties of human isolates in various species of non-human primates and in the bank vole (Clethrionomys glareolus), which only recently was proven to be more susceptible than other rodents to human prion strains [17,18,19].
Although knowledge about the phenotypic heterogeneity of human prion diseases dates back several decades, the discovery of the main molecular determinants of this variability was only achieved in the 1990s [20]. At that time, a wealth of evidence was gathered indicating that the clinical and pathological features of prion disease depend on two major molecular determinants: the PRNP genotype, which is specified by mutations and polymorphic sites, and the physicochemical properties (see paragraphs 3 and 4) of the PrPSc aggregates that accumulate in the affected tissues. From a historical perspective, the discovery of GSS-specific PRNP mutations and the demonstration that the genotype at the polymorphic codon 129 in the mutated PRNP allele is responsible for the strikingly different phenotypes between two familial prion diseases linked to the same mutation (i.e., D178N), first demonstrated the role that the PRNP genotype plays in genetic prion disease [21,22]. Similarly, the finding that the codon 129 valine (V) allele specifically correlates with a plaque-like pattern of PrPSc deposition in both sporadic and iatrogenic CJD further highlighted the central role of the polymorphic codon 129 [23,24,25]. Most significantly, the demonstration that, in both CJD and GSS [26,27,28], PrPSc molecules with distinct physicochemical properties correlate with distinct clinicopathological disease variants that are also independent of the PRNP genotype laid the foundation for the first robust classification of the disease and the subsequent characterization of human prion strains by transmission studies.
3. Molecular Basis of Phenotypic Variability and Disease Subtypes
3.1. Creutzfeldt–Jakob Disease (CJD) and Fatal Insomnia (FI)
In CJD and FI, the proteolytic cleavage of PrPSc aggregates by proteinase K (PK) generates two major core fragments, named types 1 and 2 (Figure 1), and a variable amount of less represented, shorter N-terminally truncated fragments.
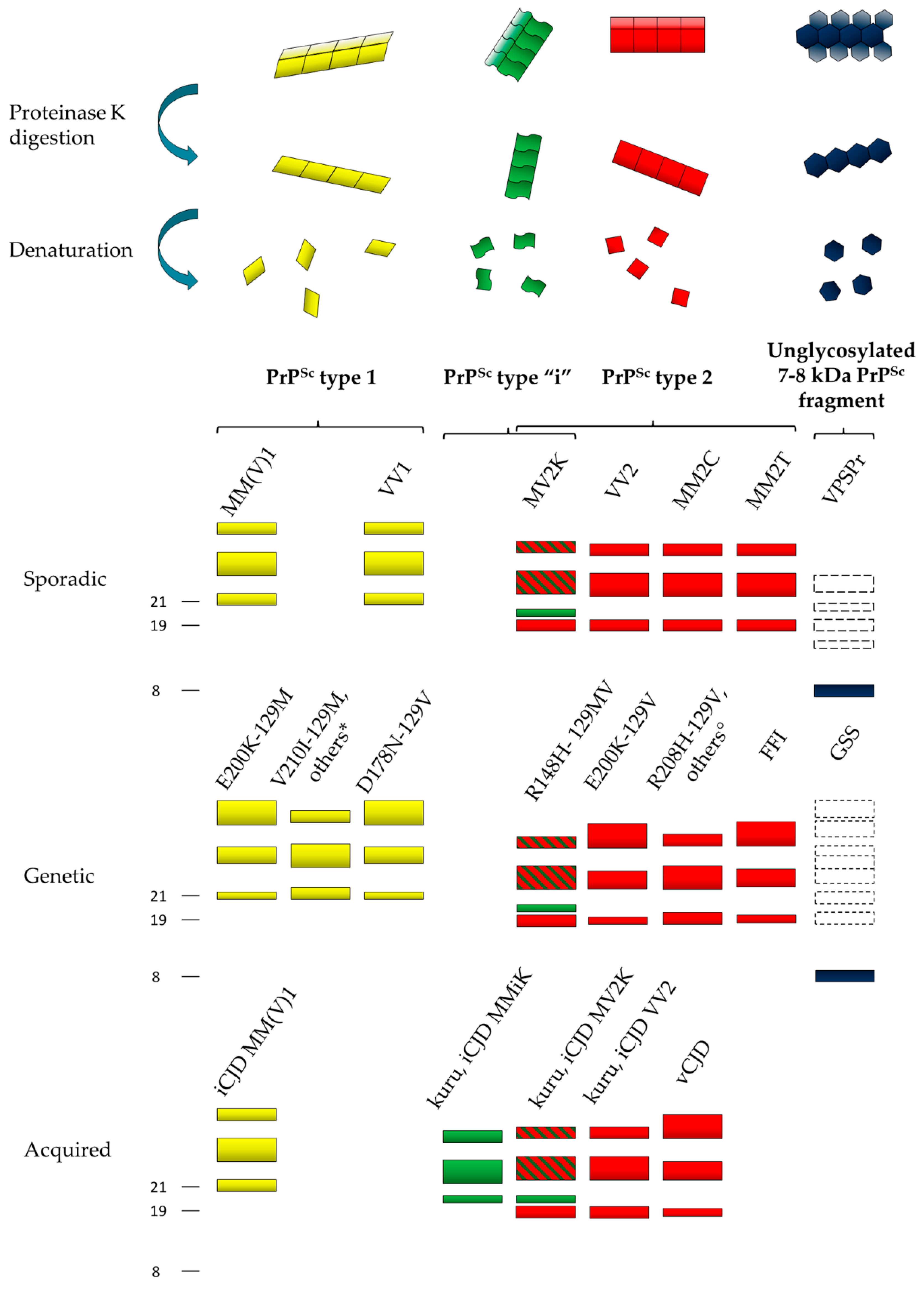
Figure 1.
Schematic representation of the heterogeneity of abnormal prion protein (PrPSc) aggregates and proteinase K (PK)-resistant core fragments in human prion disease. Four dominant human PrPSc fragments can be distinguished by sodium dodecyl sulfate (SDS) electrophoresis and immunoblotting after PK digestion and protein denaturation. They include PrPSc type 1 (21 kDa, in yellow), PrPSc type “i” (20 kDa, in green), PrPSc type 2 (19 kDa, in red) and the 7–8 kDa unglycosylated PrPSc fragments (in dark blue). The bands with dotted lines symbolize PrPSc fragments that are only occasionally seen. PrPSc types 1, 2 and “i” comprise a triad of fragments representing, from top to bottom, the diglycosylated, monoglycosylated, and unglycosylated isoforms of the protein. The predominance of the monoglycosylated or diglycosylated band defines “pattern A” or “pattern B”, respectively. The main disease subtypes associated with the four PrPSc profiles in each etiological disease group (i.e., sporadic, genetic or acquired) are listed. Other mutations include: * PrPSc type 1: G114V-129M, R148H-129M, D178N-129V, T188K-129M, V189I-129M, E196K-129M, E200K-129M, V203I-129M, R208H-129M, V210I-129M, 2-6 OPRIs–129M; ° PrPSc type 2 196K-129V, E200G-129V.
Type 1 PrPSc primarily originates by PK cleavage at residue 82 and has a relative molecular mass of 21 kDa, while type 2 has a molecular mass of 19 kDa and is primarily cleaved at residue 97 [29,30]. Type 1 PrPSc is preferentially associated with methionine (M) at codon 129, and type 2 with V. The six possible combinations between the codon 129 genotype and PrPSc type (i.e., MM1, MV1, VV1, MM2, MV2, and VV2) specify the six sporadic CJD (sCJD) clinicopathological subtypes recognized by current disease classification with only a few exceptions [31,32,33]. These include the MM1 and MV1 cases, which have been merged in a single subtype MM(V)1 because of their similarities, and the MM2 subjects, which, on the contrary, may belong to two subtypes with distinctive histopathological features in the cerebral cortex and the thalamus, designated accordingly as MM2-cortical (MM2C) and MM2-thalamic (MM2T). Furthermore, the MV2 subtype characterized by the amyloid plaques of the kuru type has been designated as “MV2K” to distinguish it from the rare MV2C cases resembling MM2C. PrPSc types 1 and 2 also characterize the genetic and acquired forms of CJD, including vCJD, suggesting a common mechanism of PrPSc formation that is independent of the supposedly distinct etiology of the disease [29,30] (Figure 1).
After PK digestion, the Western blot profile of both types 1 and 2 PrPSc comprises additional heterogeneity, which fits well with the phenotypic subtypes described above. Most significantly, a further PrPSc fragment with an electrophoretic mobility of approximately 20 kDa, intermediate between types 1 and 2, and designated as PrPSc “i”, characterizes the sporadic and iatrogenic CJD cases showing amyloid plaques of the kuru type and carrying at least one M allele at PRNP codon 129 [18,31,32,34,35] (Figure 1). Additional fragments may form in human prion diseases based on the loss of the glycosylphosphatidylinositol (GPI) anchor or the availability of other proteolytic cleavage sites in the C-terminus. They include anchorless fragments of 18.5 or 17 kDa, representing the 21- or 19-kDa PrPSc core fragments without the GPI anchor and the so-called PrP-CTF12/13 that are C-terminal-truncated peptides of 12 and 13 kDa generated by PK cleavage at residues 162/167 and 154/156 [36,37,38,39]. Notably, both the presence and the relative amount of PrP-CTF13 vary significantly among CJD subtypes [39].
The PK-resistant fragments described above refer to the peptidic bone of PrPSc; however, the heterogeneity of PrPSc also extends to the attached glycans. In homology with PrPC, the Western blot profile of PrPSc comprises a triad of fragments, which reflect the non-obligatory addition of one or two sugar chains [40]. It is well established that PrPSc molecules associated with the different subtypes of prion disease manifest distinct ratios of the three differently glycosylated forms (the so-called glycoform ratio). More specifically, while most CJD cases show a predominance of the monoglycosylated band, representing the so-called “pattern A”, vCJD and the familial prion diseases linked to the E200K, P102L, and D178N mutations display a “pattern B” that is characterized by the prevalence of the diglycosylated band [28,29,30,41,42] (Figure 1). However, this is a rather gross classification, given that significant differences in the PrPSc glycoform ratio also feature between subtypes of both groups. For example, within the first group (pattern A), the glycopattern differs significantly between sCJD MM2C and MM2T and between sCJD VV2 and MM(V)1, while it differs between genetic CJD (gCJD) D178N-129M, D178N-129V, and E200K-129M in the second (pattern B) [29,43,44].
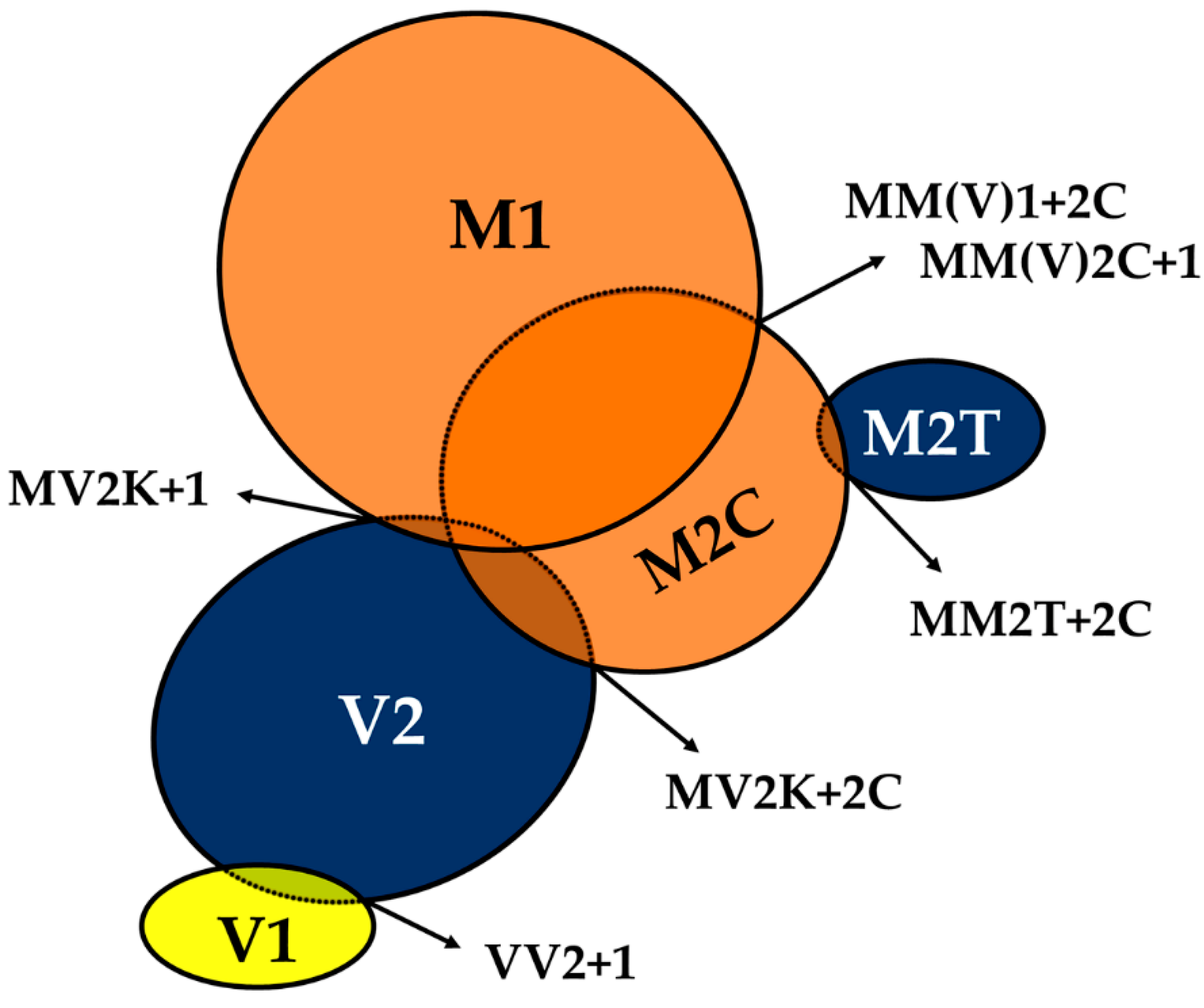
The phenotypic spectrum of CJD includes variants showing the co-occurrence of types 1 and 2 PrPSc or even the coexistence of subtypes linked to the same PrPSc type (e.g., MV2K+2C) (Figure 2).
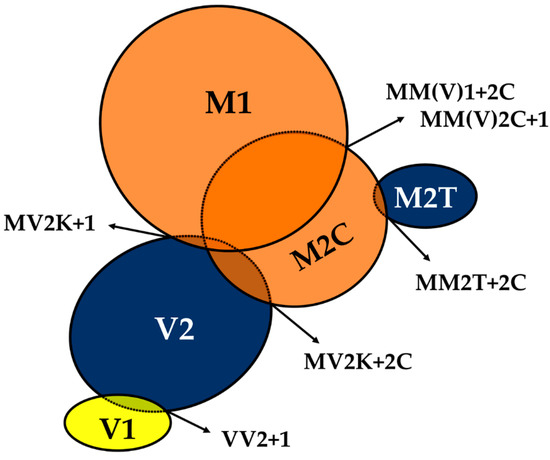
Figure 2.
The spectrum of mixed phenotypes and prion strain co-occurrence in sporadic Creutzfeldt–Jakob disease (CJD). Prion strains are classified as “dominant” or “not-dominant” according to their relative quantitative contribution to the mixed CJD phenotype as assessed by histopathological examination and PrPSc typing. The “dominant-only” strains are highlighted in blue, those either “dominant” or “not-dominant” in orange, and those “not-dominant-only” in yellow. The extent of overlap between circles reflects the relative prevalence of the strain co-occurrence compared to that of the individual “pure” phenotypes. In the M1+M2C strain co-occurrence, M1 dominates over M2C in the majority of cases. For strain nomenclature (e.g. M1, V2) see paragraph 6.
This scenario represents an intriguing and mostly unexplained event, which is likely relevant to the understanding of prion strains. Several studies dealt with it, and the resulting data raised some debate in recent years concerning the extent of the phenomenon. Indeed, the calculated incidence of sCJD cases with the co-occurrence of PrPSc types varied from 12% to 44% among studies that used standard antibodies against PrP C-terminus [45,46,47,48,49,50], but increased to 100% in those that used antibodies, thought to be selective for type 1, that recognize an epitope between residues 82 and 96, which is cleaved off by PK in type 2 PrPSc. These findings led to the proposition that the co-occurrence of PrPSc types 1 and 2 might involve the totality of CJD patients [51,52,53]. Specifically, some researchers argued that the calculated incidence of “mixed” sCJD directly reflects the extent of brain sampling and the sensitivity of detection of a minority PrPSc type in the presence of the larger amounts of the dominant type [51]. However, we and others raised concern about the reliability of “type 1 selective” antibodies, given that, especially in mild digestion conditions, they cannot distinguish the bona fide type 1 and 2 “core” fragments from those generated by an incomplete protease digestion of the N-terminus [54,55]. By applying both an extensive regional brain sampling and an accurate and reliable methodology for the detection of the PrPSc type coexistence, which provided good sensitivity combined with absolute specificity for the bona fide type 1 and 2 “core” fragments, we confirmed an overall prevalence of types 1 and 2 co-existence of 35% in a largely unselected series of 200 cases [32,54]. Although the co-occurrence of types 1 and 2 characterizes all codon 129 genotypes, the two abnormal PrPSc isoforms coexist more frequently in MM (43%) than in MV (23%) or VV (15%) subjects (Figure 2). Moreover, the co-occurrence of subtypes linked to the same PrPSc type only affects subjects carrying MM (MM2T+2C) or MV (MV2K+2C) [32] (Figure 2). Interestingly, the uneven distribution of mixed sCJD cases among subjects carrying distinct codon 129 genotypes appears to be related to the high incidence of the MM(V)2C subtype as a co-occurring subtype (Figure 2).
It is also worth noting that the deposition of either type 1 or 2 PrPSc, when concurrent, as well as the association between CJD subtypes in mixed phenotypes, are not random (e.g., certain subtype combinations have never been observed, and the relative contribution of each strain to the mixed phenotype seems to follow some rules). Furthermore, phenotypic features in “mixed” CJD cases largely reproduce those seen in the “pure” subtypes (i.e., they lack novel, specific traits) with a relative abundance depending on the most prevalent PrPSc type accumulating in the brain. Based on their relative quantitative contribution to the mixed phenotypes, CJD subtypes can be divided into three distinct groups. The first would include the VV2, MV2K, and MM2T subtypes, whose phenotypic traits invariably predominate in the mixed phenotype (“dominant” subtypes), the second the VV1 variant which always co-occurs as “not-dominant” subtype, while the third the MM(V)1 and MM(V)2C subtypes, which manifest either as dominant or “not-dominant” phenotype.
3.2. Gerstmann–Sträussler–Scheinker (GSS) Disease and Variably Protease-Sensitive Prionopathy (VPSPr)
While CJD and FI typically present as rapidly progressive neurological syndromes, GSS and other inherited prion amyloidosis manifest a slower clinical course and show amyloid deposition rather than spongiform change in the affected brain tissue [6]. Interestingly, this significant difference in the distinctive histopathology correlates with the PrPSc properties. Indeed, while in CJD and FI the protease digestion of full-length PrPSc generates N-terminally truncated core fragments mostly retaining the GPI anchor and the attachment to the cell membrane, in GSS PrPSc digestion generates unglycosylated anchorless fragments that form aggregates in the extracellular space [26,27].
Studies showed that the PrPSc obtained by purified amyloid preparations from GSS-affected brains mainly comprise unglycosylated 7–8 kDa PrPSc fragments with ragged N- and C-termini that are primarily composed of mutant PrP [26,27,56,57,58] (Figure 1).
Interestingly, PrPSc in VPSPr also includes a prominent internal “GSS-like” PrPSc fragment migrating at ~8 kDa, besides C-terminally truncated peptides lacking the diglycosylated form of PrPSc (Figure 1). Thus, there are significant similarities in the PrPSc physicochemical properties between VPSPr and GSS, which support the proposition that the former may represent the sporadic variant of the latter [59,60]. Furthermore, GSS brains occasionally show PrPSc fragments of higher molecular weight resembling the primary PrPSc species found in CJD and FI, such as PrPSc types 1 and 2 (Figure 1). Similarly, recent studies documented a regional variability of PrPSc properties also in VPSPr, including the presence of a fully glycosylated (i.e., comprising the diglycosylated form) CJD-like 19-kDa PrPSc fragment in some cases [61,62]. These findings not only corroborate the similarities between GSS and VPSPr, but also indicate that CJD/FI and GSS/VPSPr belong to a disease spectrum rather than being considered as separate disorders with different pathogenesis.
As the most significant example of the coexistence of GSS and CJD features, GSS patients carrying the PRNP P102L-129M haplotype may manifest either a rapidly progressive CJD-like phenotype with both spongiform changes and amyloid plaques or a slowly progressive “pure” GSS phenotype with amyloid plaques, but no spongiform changes. Most significantly, the former phenotype displays the co-occurrence of 21-kDa (type 1-like) and 8-kDa PrPSc fragments in the affected brain, while the latter only shows the 8-kDa PrP fragment [26,27]. It is noteworthy, however, that both vCJD and CJDMV2K show amyloid plaques in the absence of the 7–8 kDa fragments Thus, amyloid plaques in human prion disease are not invariably linked to the 7–8 kDa fragments that are ragged at both termini and lack the N-linked glycosylation, which indicates that other factors must play a significant role in determining the amyloidogenic properties of PrPSc fragments.
4. Characterization of Human Abnormal Prion Protein (PrPSc) by Conformational and Solubility Assays
The described heterogeneity of protease-resistant PrPSc fragments associated with human prion diseases, and the demonstration that they also form in a significant amount in vivo [26,63], raised questions about the pathogenic role of these peptides, including their neurotoxic potential and involvement in the determinism of prion infectivity and strain-specific properties [39]. Concerning the latter issue, most scientists interpreted PrPSc fragment variability as indirect evidence of the structural heterogeneity of PrPSc, in support of the protein conformation hypothesis of prion strains. However, the direct proof of this contention, as well as the demonstration of the level of the protein structure that is primarily involved (i.e., secondary, tertiary, or quaternary conformation), are still lacking [5,30,39,64,65,66].
Pursuing the putative conformational hypothesis, scientists have exploited other biochemical assays that indirectly explore the PrPSc structure to further investigate the basis of the variability among prion disease subtypes and strains.
Original studies on experimentally transmitted scrapie strains demonstrated that PK digestion not only generates protease-resistant core fragments of different lengths but is also variably efficient in digesting the abnormal protein.
We carried out a comprehensive study on the degree of protease resistance of human PrPSc [67]. We performed a PK titration assay on purified PrPSc from the whole spectrum of sporadic prion disease subtypes, and vCJD. Our findings demonstrated that the amount of PK required to digest 50% of PrPSc (ED50) significantly differs among CJD subtypes. Specifically, PrPSc ED50 was lowest for sCJDVV1 and VPSPr and highest for sCJD MV2K/VV2 and vCJD with sCJD MM1, MM2T, and MM2C showing intermediate values. Moreover, the ED50 positively correlated with the relative percentage of diglycosylated PrPSc in both “type 1” (VV1 and MM1) and “type 2” (MM2T, MM2C, MV2K, VV2, and vCJD) CJD groups (Table 1). By contrast, the ED50 inversely correlated with the relative amount of the 13-kDa truncated C-terminal fragment generated by PK digestion [67].

Table 1.
Comparison of clinical, biochemical and transmission data among the sporadic CJD (sCJD) subtypes, variant CJD (vCJD) and variably protease-sensitive prionopathy (VPSPr) (adapted from [68]).
In the same study, we also searched for the so-called PK-sensitive form of PrPSc (sPrPSc), which was previously defined as an isoform of PrPSc characterized by a degree of protease resistance comparable to PrPC. The results showed that, in sCJD, only a minority of the total purified detergent-insoluble PrPSc behaves as sPrPSc. Overall, sPrPSc accounted for less than 10% of the whole PrPSc in all sCJD subtypes, with sCJDMM2C showing the highest and sCJDVV2 the lowest relative amount of sPrPSc [67]. In contrast, Safar and colleagues applied an assay they originally developed capable of discriminating conformational changes (i.e., conformation-dependent immunoassay or CDI) and found surprisingly elevated levels of sPrPSc, reaching up to 80% of total PrPSc in some cases [79,80]. In another CDI study, the results also documented relatively high levels of sPrPSc, although with high variability among the examined cases [81]. Interestingly, despite the different methodologies and the discrepant results, all of these studies uniformly pointed to MM2C being the sCJD subtype showing the highest relative amount of sPrPSc [67,81,82].
The correct understanding of the significant discrepancy in the amount of sPrPSc detected among studies relies on methodological aspects, the operative definition of sPrPSc, and data interpretation.
It is well-established that the quantification of PrPSc, which is, by definition, only partially resistant to protease digestion, is profoundly influenced by the experimental conditions in which the protease digestion is performed. In this regard, the ratio between the PK concentration and the total protein amount in the sample probably differed significantly between studies. Specifically, while Saverioni and co-workers [67] carefully adjusted the PK concentration to the amount of PrPSc, in the other studies an unspecified amount of PrPSc contained in a total brain homogenate was digested with a relatively high PK concentration.
Furthermore, whereas our method separated PrPC based on a physical property (solubility in sarkosyl detergent) [67], Safar and colleagues [80] enriched PrPSc in the sample by sodium phosphotungstic acid precipitation. Similarly, while we obtained the estimate of sPrPSc after the physical separation of a fully PK-sensitive PrPSc fraction forming aggregates of relatively low size, CDI measures sPrPSc as a part of the whole PrPSc without defining the actual structural difference between the two putative PrPSc species. Thus, the issue of whether the sPrPSc signal detected by CDI reflects the properties of a distinct PrPSc molecule explicitly remains unsolved.
Altogether, these considerations underline the limitations of the estimates of sPrPSc, especially in the interlaboratory setting, when based on a single digestion condition and without a structural or physical definition of the putative protein isoform.
Another approach with which to search for indirect evidence of structural PrPSc variants among CJD subtypes exploited the conformational stability of PrPSc aggregates using various methods to measure the degree of conformational loss. CDI, for example, quantifies the affinity of monoclonal antibodies to conformational epitopes of PrPSc. Using this approach, Kim et al. [82] measured the concentration of guanidine hydrochloride (GdnHCl) that is needed to denature 50% of PrPSc ([GdnHCl]1/2) in sCJD MM1, MM2C, and VV2 cases. The results revealed a wide range of values, even within the same sCJD subtype, suggesting that each disease variant is comprised of several distinct PrPSc conformations. They also demonstrated a significant difference in the stability of PrPSc aggregates between sCJD MM1 (the most stable) and MM2C (the least stable). Finally, they showed that PK digestion has a substantial effect on the structural stability of MM2C PrPSc, given that these aggregates more easily solubilize after PK digestion [82]. The extended analysis to sCJD with mixed MM1 + 2C features confirmed that the two PrPSc types have distinct [GdnHCl]1/2 values and PrPSc structural organization at the level of both the polypeptide backbone and the quaternary packing arrangements [83].
Other groups calculated the [GdnHCl]1/2 by measuring the progressive loss of PK-resistance (conformational stability assay or CSA) or the increase in solubility of PrPSc generated by increasing concentrations of GdnHCl (conformational stability and solubility assay, or CSSA). Both methodologies confirmed the higher stability PrPSc aggregates in sCJD MM1 in comparison to MM2C [55,84]. Also, CSSA distinguished between sCJD MM2C and MM2T [85]. Given the discriminatory power of CSSA, Cali and colleagues extended their study to sCJDMM1+2C. Intriguingly, when coexisting in the sample, either naturally or in an artificial mixture, PrPSc types 1 and 2 displayed similar [GdnHCl]1/2 values.
In another study, however, we failed to reveal significant strain-specific differences in the GdnHCl denaturation curve of PrPSc aggregates monitored by CSA, in a comparative analysis of all six sCJD subtypes and vCJD [68].
In the same study [68], we also compared the thermostability of PrPSc aggregates across the CJD spectrum by applying the so-called thermo-solubilization assays (TSA) [86]. At variance with CSA, the results highlighted significant strain-specific differences allowing a gross classification of CJD subtypes in three groups that were, respectively, resistant (sCJD MM1, VV2, MV2K), sensitive (vCJD, sCJD MM2C and MM2T), and highly sensitive (sCJDVV1) to thermal solubilization (Table 1). Interestingly, the analysis of CJD brains with mixed MM1+2C phenotypes by using an antibody that selectively recognizes the PrPSc types 1 or 2, showed that when coexisting, each subtype maintains the thermal solubility of the corresponding “pure” phenotype, namely sCJD MM1 and MM2C. These findings, at variance with those of a previous study with CSA [55], support the hypothesis that in CJD brains with mixed phenotypes, subtype-specific PrPSc aggregates are spatially segregated and do not influence each other [68].
5. Characterization of Human PrPSc Types Conversion and Seeding Activity by In Vitro Amplification Techniques
Prion amplification techniques, such as the protein misfolding cyclic amplification assay (PMCA) and the real-time quaking-induced conversion (RT-QuIC) method, take advantage of PrPSc ability to induce a conformational change of a PrPC substrate leading to the formation of ordered aggregates, mimicking the process of PrPSc replication that was thought to occur in vivo. The application of these novel techniques led, on the one hand, to the successful detection of PrPSc in tissues and fluids containing a minute amount of the abnormal protein and, on the other, to a better understanding of the molecular mechanisms of prion replication.
PMCA, which was first described in 2001 by Saborio and colleagues [87], fosters PrPSc amplification through a series of alternating sonication and rest cycles that promote the fragmentation of prion aggregates and the emergence of new nucleation sites. Depending on the protocol, the PrPC substrate is provided by normal brain or peripheral tissue homogenates [88,89,90], plasma [91], human platelets [92], cultured cells [93], or recombinant PrP (rPrP) [94].
The significant heterogeneity in the source of PrPC substrates and experimental protocols utilized to date prevents the comparison of current PMCA studies on human prion diseases. Nevertheless, as the most significant finding, most studies revealed a higher seeding activity and amplification efficiency of vCJD and sCJD prions linked to PrPSc type 2 than in those to PrPSc type 1 [88,89,90,91,92,95,96,97,98,99,100,101,102,103]. Accordingly, only a few studies have, to date, accomplished an efficient PMCA-amplification of PrPSc type 1 [88,96,97,101,103].
By exploiting the ability of PMCA to replicate PrPSc type 2 and using specific brain regions as substrates to mimic the regional tropism of prions, Privat and colleagues [102] found that vCJD and sCJDVV2 seeds partially reproduce the specific brain targeting observed in affected brains. Specifically, they found a highly significant relationship between in vivo and in vitro regional brain targeting, suggesting an influence of both PrPC level and locally expressed cofactors in strain tropism [102]. Hence, the study confirmed the utility of PMCA in exploring potential cofactors and general mechanisms of strain replication [104,105,106,107,108]. Haldiman and coworkers also employed the technique for the characterization of sCJDMM1+2C while aiming to investigate the impact of strain coexistence in PrPSc replication and adaptation. Interestingly, by applying consecutive rounds of PMCA, thereby mimicking an evolutionary process, they found that the PrPSc type 1 conformations, presenting lower stability as assessed by CDI, were preferentially amplified in comparison of that of the more stable PrPSc type 2 [96].
While the studies above confirm that PMCA may have a role in the investigation of PrPSc conversion and the molecular events leading to the acquisition of strain-specific features, its application for diagnostic purposes is limited. Indeed, apart from a test for the detection of vCJD prions in human urine and blood [90,98], the implementation of the PMCA technique to date failed to provide a robust and reproducible assay to be applied in sCJD diagnostics.
QuIC is another in vitro conversion assay that exclusively uses recombinant PrP (rPrP) as a substrate and in which sonication is replaced by shaking at a relatively high temperature. RT-QuIC, in particular, is the last adaptation of QuIC in which the seeded conversion of rPrP into aggregates of PrPSc is monitored in real time. The monitoring is permitted by including Thioflavin T (ThT) in the reaction, which binds the aggregated PrPSc causing a change in the ThT emission spectrum. RT-QuIC has ultimately gained more interest than PMCA as a detection technique with diagnostic applicability as it is less affected by the potential drawbacks of PMCA, such as the time taken, the complexity of the substrate, and the reliance on sonication, which is difficult to standardize [109]. Accordingly, the application of RT-QuIC successfully provided a robust and reproducible assay with high sensitivity and specificity for the clinical diagnosis of CJD.
Diagnostic RT-QuIC for CJD uses cerebrospinal fluid (CSF) or olfactory mucosa as seeds [110,111,112] and various sources of rPrP as a substrate, the latter being one of the main factors affecting the diagnostic performance of RT-QuIC. As the most significant example in humans, the use of truncated (90–231) hamster rPrP instead of full-length (23–231) hamster rPrP, in addition to other adjusted parameters (i.e. SDS concentration and temperature), in the so-called second generation RT-QuIC, significantly improved the diagnostic sensitivity of the assay without affecting its specificity [113,114]. The results obtained in several laboratories with the first generation of this assay demonstrated a 79–86% sensitivity and a 99–100% specificity [109,115,116,117], while the diagnostic sensitivity with the RT-QuIC of the second generation increased to 95–97% [114,118,119,120].
Recently, RT-QuIC in humans also detected PrP seeding activity in the skin, peripheral nerve, and eye of CJD patients [121,122,123].
Current results concerning the ability of RT-QuIC to discriminate among prion disease subtypes are limited. The three major sCJD subtypes, namely MM(V)1, VV2, and MV2K, demonstrated overall comparable test sensitivity and kinetics of the reactions (i.e., lag phase and ThT maximum fluorescence) [114,115,116,119,124]. In contrast, CSF seeds from sCJD MM2C, MM2T, and VV1 subtypes, as well as VPSPr, showed a higher percentage of negative responses, a lower fluorescence intensity and, when positive, an extended lag phase in comparison to the most common subtypes [114,115,116,119]. Interestingly, the reduced ability to replicate in vitro parallels the reduced capacity to propagate in vivo by experimental transmission.
Intriguingly, at variance with the amplification efficiency of PMCA, RT-QuIC showed a relatively low seeding activity of vCJD prions [114,116], which can be partially overcome by using bank vole rPrP [122,125].
Finally, several studies suggested that RT-QuIC may also be useful as a diagnostic test for iCJD and at least some gCJD variants [103,114,115,116]. Interestingly, gCJD cases carrying the common and highly penetrant E200K-129M haplotype [126] showed the shortest lag phase among the tested human prion samples [114,115].
In conclusion, the results obtained by these in vitro assays further highlight the complexity of the mechanisms regulating prion conversion and replication, which are thought to transmit structural information by a template model. Indeed, this likely involves PrPC structural features and molecular factors that are unrelated to PrPC, in addition to PrPSc properties.
6. Characterization of Human Prion Strains by Experimental Transmission
Prion strains are operationally defined as infectious isolates that, when transmitted to syngenic hosts, under fixed and controlled conditions, exhibit distinct prion disease phenotypes [127]. The strain-associated phenotypic traits not only include biological and histopathological features (i.e., incubation times, histopathological lesion profiles, and specific neuronal target areas), but also biochemical properties related to the PrPSc structure (i.e., the pattern of electrophoretic mobility, glycosylation, and PK resistance), which, although not invariably, usually persist upon serial transmission [5,128].
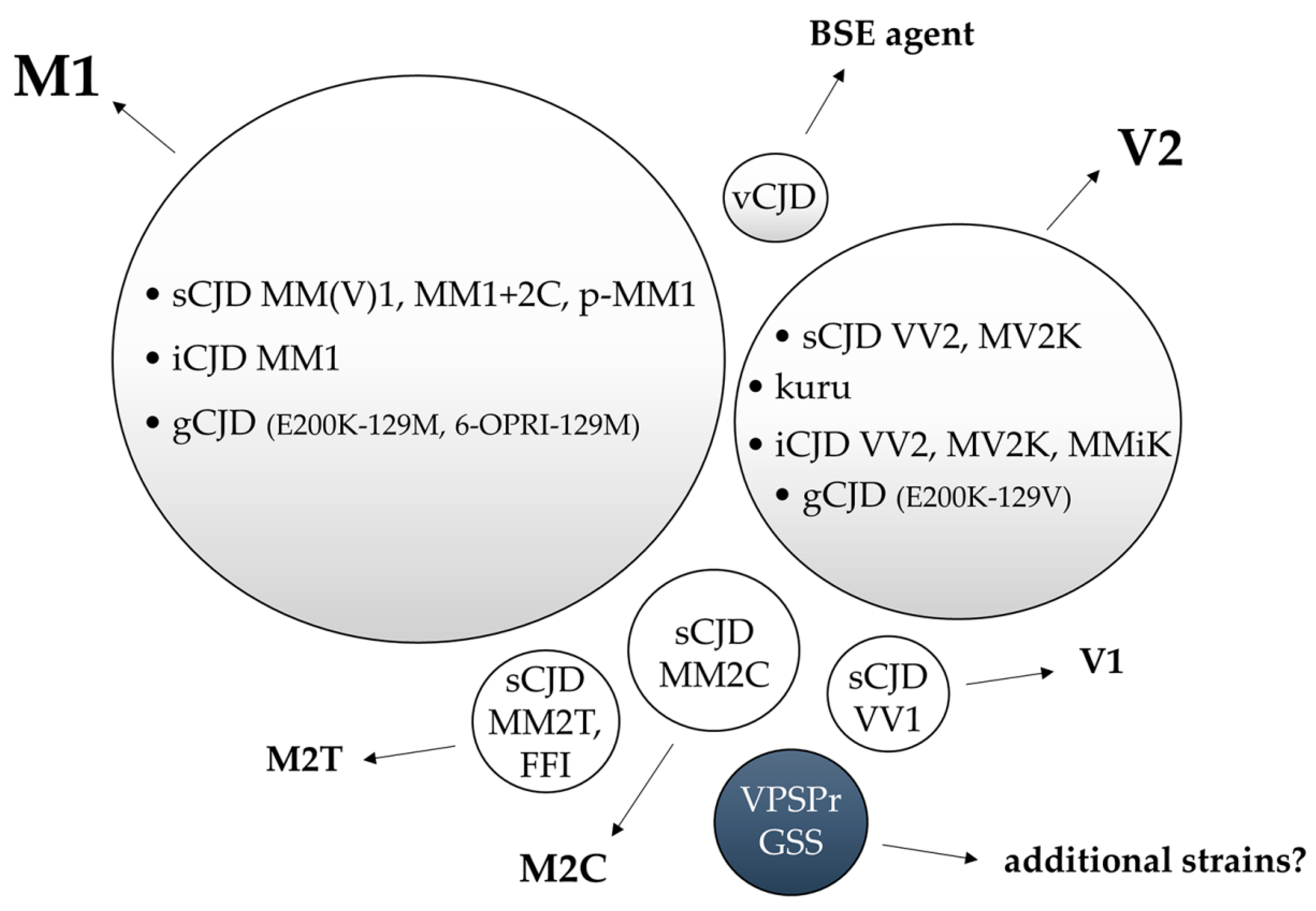
To date, the experimental transmission of human prion disease “isolates” has led to the identification of five different CJD strains named M1, V2, M2C, M2T, and V1 according to the combination of the PRNP codon 129 genotype that is most susceptible to PrPC conversion and the PrPSc type (1 or 2) accumulating in the brain. The same, or highly similar, strains characterize gCJD, kuru, and iCJD. Initial evidence suggests that additional prion strains may originate from GSS and VPSPr transmissions, although further studies are needed to verify this hypothesis (Figure 3).
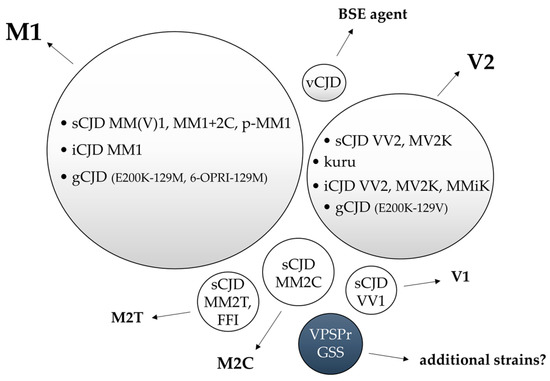
Figure 3.
Representation of the relationship between human prion disease subtypes and the isolated strains by experimental transmission. Bubble sizes reflect the relative prevalence of each disease subtype/strain. In light grey, the phenotypes related to prion strains with documented human-to-human transmission. In white, experimentally transmitted phenotypes lacking documented human-to-human transmission. In blue, the prion disorders that transmitted amyloid-PrP in the absence of clinical disease.
6.1. Strain M1
The MM(V)1 subtype, either pure or mixed with MM(V)2C, often referred to as “typical or classic CJD”, comprises about 65% of all sCJD cases [31,32]. The first successful transmission of sCJDMM(V)1 must be reasonably attributed to Gajdusek’s group because the brain homogenate used to inoculate the first chimpanzee affected by the transmitted disease belonged to a case of sCJD later classified as such [12,18].
Prusiner’s lab first characterized the phenotype of experimentally transmitted sCJDMM(V)1 in Tg mouse lines overexpressing a chimeric mouse-human prion protein (MHu2M). The MM1 inoculum transmitted the disease with a mean incubation time of about 190 days, which decreased by 50% in MHu2M mice with amino acid substitution at residue 165 and 167 (Tg (MHu2M,M165V,E167Q)) [129]. The comparison of histopathological and biochemical PrPSc features in these mice revealed a consistent pattern of PrPSc deposition, distribution of vacuolation, and electrophoretic mobility of a PrPSc unglycosylated fragment (21 kDa, type 1) compatible with a single strain.
In full agreement with the disease epidemiology in humans, the sCJDMM1 inoculum showed a faster transmission in mice expressing human PrP transgene encoding M at codon 129 (Tg(HuPrP,M129)) than in the homologous mouse line with V at the same codon (Tg(HuPrP,V129)) [129], identifying codon 129 MM as the most susceptible genotype to this strain.
Other groups also successfully transmitted sCJDMM(V)1 to transgenic and knock-in mouse lines expressing human PrPC, obtaining results compatible with a single prion strain, designated as M1 [71,72,73,78,130,131] (Figure 3).
As expected, the injection of sCJDMM1(V)1 in lines of Tg mice with the three different genotypic combination at codon 129 (HuMM, HuMV, and HuVV) and expressing normal levels of PrPC within the brain resulted in a more efficient transmission in Tg HuMM [71], confirming the previous results in Tg mice overexpressing PrPC [129].
The review of archival material from the NIH series of transmitted disease, which proved the transmissibility of human prion disease [132,133,134,135], has also allowed the study of the transmission properties of sCJDMM(V)1 in four species of non-human primates, namely squirrel, capuchin, spider, and African green monkeys [18]. The characterization of these primary transmissions revealed strikingly homogenous results regarding the incubation time, type of spongiform changes, lesion profile, and PrPSc properties among more than 30 sCJDMM(V)1 inocula, yielding the most robust support to the contention that sCJDMM(V)1 is linked to a single prion strain.
Studies of the transmission properties of sCJDMM(V)1 in bank voles and transgenic mice expressing bank vole PrP (BvPrP) with either M or isoleucine at the polymorphic codon 109 (BvPrP-109M and BvPrP109I) also showed a highly consistent lesion profile, a pattern of PrPSc deposits and Western blot profile of PK-resistant PrPSc with substantial similarities with those observed in the human phenotype.
A rare atypical sCJD phenotype that is linked to MM at codon 129 and characterized by PrP-amyloid plaques in subcortical white matter, but otherwise very similar to the MM(V)1 subtype [136,137], has also been transmitted to bank voles. The results suggest that the M1 strain is also responsible for this variant, despite the unusual pathological features [137] (Figure 3).
The inoculation of gCJD E200K-129M brain homogenates into Tg(HuPrP,M129) and Tg(MHu2M,M165V,E167Q) showed an incubation time and a size of PrPSc fragments (type 1) comparable to those obtained with sCJDMM1 [129]. Similarly, the results obtained in bank voles inoculated with sCJDMM(V)1 and gCJD linked to E200K-129M and V210I-129M supported the view that the MM(V)1 subtype is related to the M1 strain in all forms of CJD, irrespective of their different etiology (Figure 3). The latter contention is also supported by the finding that squirrel monkeys inoculated with sCJDMM(V)1, gCJD E200K-MM1, and iCJD MM1 showed identical transmission properties [18]. Finally, the inoculation of gCJD linked to a six-octarepeat insertion in cis with M at codon 129 and PrPSc type 1 in 129VV Tg152 mice, a murine model lacking any transmission barrier to sporadic/iatrogenic forms of CJD regardless of the codon 129 genotype of the inoculum [138], also gave results closely resembling that of the sCJDMM(V)1 isolates with a 100% attack rate [139] (Figure 3).
Taken together, the results from transmission studies indicate that the same M1 prion strain is responsible for all cases of CJD MM(V)1, independent of the supposedly different etiology (i.e., sporadic, genetic, or iatrogenic).
6.2. Strain V2
At variance with all other CJD subtypes, the experimental transmissions of sCJD VV2 and MV2K revealed a convergence to a single propagating phenotype, indicating that a common strain, designated as V2, is linked to both subtypes [18,71] (Figure 3). Consequently, the host codon 129 genotype is the main one responsible for the phenotypic differences between these subtypes in humans. The transmission of the VV2 and MV2K subtypes to Tg mice and non-human primates carrying M at codon 129 showed a longer incubation time and a lower attack rate in comparison to those inoculated with sCJDMM(V)1, indicating a lower virulence [18,129]. However, transmission of sCJD MM(V)1, VV2, and MV2K in Tg(HuPrP,V129) mice revealed the opposite behavior [129]. Likewise, sCJD VV2 and MV2K showed the fastest transmission and the highest attack rate in Tg mice carrying HuVV [71]. Notably, the latter represents, to date, the genotype/agent combination with the shortest incubation time in mice expressing physiological PrP levels, within the spectrum of human prions (Table 1). Interestingly, the demonstration that MV2K transmits more efficiently in Tg HuVV than in Tg HuMV indicates that, regarding prion replication efficiency, the strain tropism for the V allele is more critical than the genotypic homology between the inoculum and the host [71].
Recent observations on iCJD in humans carrying MM, as well as the characterization of the transmission properties of the full spectrum of iCJD cases, have further highlighted the ability of the V2 strain to adapt its properties to host-expressing M at codon 129 [34,140]. At variance with sCJD, iCJD patients carrying MM at codon 129 may display two distinct phenotypes. While the most prevalent “nonplaque type” reproduces the typical sCJDMM(V)1 and is linked to PrPSc type 1, the “plaque type” shows a widespread occurrence of kuru plaques and plaque-like deposits, and is associated with a PrPSc type “i” (hence the name iCJD MMiK). Transmission studies in knock-in mice expressing human PrP with 129 MM and VV showed that nonplaque-type, dura mater graft-associated iCJD transmitted most efficiently in the former, while iCJD MMiK in the latter [141]. Moreover, in iCJD MMiK transmissions, PrPSc type “i” reproduced itself in 129MM mice, whereas it shifted to PrPSc type 2 in the 129VV mice. This observation raised the possibility that the V2 strain causes iCJD MMiK, a hypothesis that was later confirmed through the demonstration that the inoculation of sCJDVV2 in 129MM mice results in an identical phenotype, which is still reversible by reinoculation in 129VV mice [72,141]. This phenomenon, designated as traceback, elegantly demonstrated that the plaque-type iCJD depends on the propagation of V2 prions in 129MM subjects. In line with this observation, the “intermediate” pattern of electrophoretic mobility of PK-resistant PrPSc corresponding to a 20-kDa relative molecular mass of the fragment, also characterized sCJDMV2K, thus represented the hallmark of the conversion of the codon 129M allele by V2 prions.
Notably, kuru transmitted to non-human primates [18] revealed transmission properties equivalent to those of the sCJDVV2 inocula (Figure 3). Moreover, a single case of kuru from a 129MM subject showing the intermediate PrPSc profile revealed PrPSc type 2 and neuropathological features matching those seen after transmission of kuru brains carrying 129VV [142], paralleling the observations in iCJD MMiK. Altogether, these data strongly support the hypothesis that kuru originated from the consumption of brain material of an individual with sCJD VV2 or MV2K and the subsequent serial transmission of V2 prions [18].
Finally, a single gCJD E200K-129V inoculum showing PrPSc type 2 has been transmitted to Tg152, expressing human PrP 129V with a 100% attack rate, short incubation period, and plaque-like focal deposits [143], thus paralleling the results obtained with sCJD VV2 and MV2K inocula. Notably, when inoculated in Tg23 and Tg49 mice expressing human PrP with E200K-129M at 3x and 2x the PrPC levels, the same case showed a marked increase of the incubation time, reduced attack rate, and less abundant PrP plaque during a neuropathological evaluation [143]. Overall, these results demonstrate that, as for the M1 isolate, the V2 strain is responsible for sporadic, genetic, and acquired CJD cases, highlighting, once again, the fact that the strain-specific properties of CJD prions are mainly encoded by PrPSc properties that are independent of the presence of specific PRNP mutations and the disease etiology.
6.3. Other CJD Strains: M2T, M2C and V1
FFI, the genetic form of FI linked to the D178N-129M PRNP haplotype, has a higher incidence than the sporadic form (sFI or MM2T). Accordingly, FFI was successfully transmitted earlier than sFI. FFI induced disease in both wild-type [144] and Tg mice overexpressing human PrP [64,145], whereas sFI into Tg mice expressing a chimeric mouse-human PrP [146]. Neuropathological findings and PrPSc properties in the affected mice showed striking similarities between FFI and MM2T inocula, which is consistent with a common strain (M2T) [146] (Figure 3). Data from subsequent transmissions in murine lines either overexpressing human PrP, such as Tg(MHu2M,M165V,E167Q), Tg(MHu2M), and Tg(HuPrP,M129) [129], or expressing normal PrP levels (HuMM) [74] further corroborated the conclusion.
Among the sCJD subtypes, the MM2C (strain M2C) is the only one that did not successfully transmit to knock-in mice expressing human PrP at physiological levels [71,73,74]. However, the disease propagated successfully in transgenic and knock-in murine lines overexpressing human PrP with 129M by 2 to 6 fold [78,129,130,147], although with a remarkably longer incubation time than M1 prions [17,78,129,130]. The MM2C subtype also transmitted to Tg and knock-in mice carrying the BvPrP-109M transgene [19,148], but not to Tg MHu2M [129]. Like M1 prions, M2C prions transmitted more efficiently in Tg mice expressing 129M than in those carrying 129V [129]. Taken together, these transmission data indicate that the M2C strain has a low transmission efficiency and a reduced infectious potential. In this regard, it is noteworthy that, at variance with an M1 prion, there is no demonstration to date of the human-to-human transmission of M2C prions. Moreover, whether M2C prions are also linked to gCJD, as is the case for M1, V2, and M2T prions, remains to be seen.
No study specifically addressed the transmission proprieties of VV1, the last of the successfully transmitted sCJD subtypes [71]. sCJDVV1 inoculated into Tg HuMM, HuMV, and HuVV mice expressing normal PrPC levels transmitted the disease most efficiently in Tg HuVV than in Tg HuMV, whereas it did not propagate in Tg HuMM [71]. Thus, like V2 prions, V1 prions preferentially transmit to a host expressing at least one 129V allele. However, V1 prions showed significantly longer incubation times and a lower attack rate than V2 prions, which indicate a lower virulence of the former. More recently, VV1 transmitted successfully to Tg mice overexpressing human PrPC levels and carrying either 129V [78] or 129M [130].
6.4. Variant CJD
Shortly after its discovery in 1996, two groups separately transmitted vCJD to wild-type and Tg mice [138,149], leading to the identification of a specific pathological and molecular “signature” [14], and the demonstration that a communal agent strain is responsible for both BSE and vCJD [138,149]. The transmission of vCJD to nonhuman primates [150], as well as the description of the traceback phenomenon after retro-transmission to bovinized transgenic mice (TgBov) [75], further confirmed the origin of human vCJD from BSE prions. Remarkably, while BSE transmitted efficiently to TgBov mice, a substantial species barrier existed between cattle and humans as demonstrated by the resistance of knock-in PRNP-humanized mice expressing normal levels of the PrP to BSE prions [151]. This species barrier only partially disappeared after secondary transmission of vCJD to these mice, indicating that the human-to-human transmission of BSE prions is relatively efficient [151,152]. However, the increased transmission efficiency was lower than expected given that the majority of vCJD infected Tg mice remained asymptomatic, despite prion replication indicated by PrPSc accumulation, a finding which was confirmed in different models [153,154]. Moreover, human vCJD prions maintained efficient transmission to Tg mice expressing ancestral bovine PrPC, without an obvious transmission barrier [155]. This particular behavior of the human-adapted BSE strain has been interpreted as an example of non-adaptive prion amplification [156], in which the causative ancestral BSE prions are not optimally adapted for full pathogenic potential in humans. The incomplete adaptation would possibly occur because dominant PrPSc conformers bypass the need for the selection of optimized prion conformations from larger ensembles, as it would occur for most prion strains during interspecies transmission [157].
To date, all but one vCJD patients carried MM at codon 129 [158,159]. Thus, as for the CJD strains M1, M2C, and M2T, the susceptibility for the vCJD strain is highest in codon MM carriers, while V confers protection to this strain.
vCJD transmission to humanized knock-in mice lines expressing different genotypes at codon 129 (MM, MV, VV) revealed the critical role played by codon 129 in determining the host susceptibility to the disease (i.e., the attack rate and incubation time), being highest in subjects carrying MM and lowest in those carrying VV [75,151]. Nevertheless, the V allele did not confer full protection from the disease, even in the homozygous state. The amount of PrP accumulation and of florid plaques also varied according to codon 129 genotype [75], although the PrPSc Western blot profile did not [75,151].
Transmission experiments with vCJD prions led to other significant findings, including the discovery of substantial infectivity in extraneural tissues, including tonsils, spleen, rectum, and blood components [160,161,162,163,164], and the ability of this prion strain to sustain a chronic asymptomatic infection in lymphoreticular tissue [164].
Given the causal link with the oral exposure to food contaminated with BSE-affected cattle, the experimental transmission of vCJD prions was also carried out through peripheral routes. In non-human primates challenged with brain homogenates from first-passage animals with BSE, the intravenous route showed a transmission efficiency similar to the intracerebral inoculation, but significantly higher than the oral exposure [165]. Moreover, the results documented substantial variability in the incubation periods after oral exposure ranging from 3.7 to 10 years. Intriguingly, the cases with a longer incubation time remained asymptomatic and only accumulated an atypical isoform of PrP in the lumbar spinal cord [166]. At variance with the intracerebral route, the intraperitoneal inoculation of vCJD brain tissue in Tg mice overexpressing PrP (129M) resulted in an asymptomatic infection involving peripheral tissues [164]. Interestingly, the peripheral challenge in these animals led to an early PrPSc accumulation in the spleen, followed by a plateau, while PrPSc became detectable in the brain only late in the course of the disease [164].
An intriguing result of vCJD transmission experiments concerns the claimed emergence of different phenotypes or prion “signatures” in Tg mice, largely overlapping those observed with the sCJD isolates. The phenomenon seems to mainly concern heterotypic transmissions (i.e., discordant PrP sequence between the inoculum and host) to Tg mice expressing 129V [138,153,154,167,168], although this is not the rule [164]. These results should be treated with caution as they might depend on the choice of the construct and genetic backgrounds, as well as on the expression level of the transgene. In support of this view is the finding of a single phenotype and a conserved PrPSc signature after serial transmissions of vCJD prions in wild-type mice [160]. More recently, Comoy et al. reported the emergence of a novel “atypical” phenotype mainly involving the spinal cord in rodents and nonhuman primates challenged with vCJD-infected blood [169]. The authors hypothesized a dissociation between infectivity and toxicity and suggested that the leading cause promoting the phenotypic switch might be related to non-protease-resistant and soluble PrPSc in transfused blood. Evidence of a similar dissociation after primary transmission of BSE to mice (i.e., a 100% attack rate, but PrPSc was not detectable in half of the cases) [150] and the occurrence of an analogous “spinal” phenotype associated with atypical PrP features in few macaques orally exposed to BSE, support the speculation [166].
6.5. Gerstmann–Straüssler–Scheinker (GSS) Disease
At variance with CJD and FI, the early attempts to transmit GSS experimentally had much less success. Indeed, GSS P105L, A117V, and the majority of P102L cases failed to propagate the disease in both rodents and nonhuman primates [134,170,171]. In the late 1990s, the generation of Tg mice with an amino acid alteration (101L) into murine PrP, equivalent to P102L in the human PrP gene, allowed the significant increase of the transmission efficiency of GSS P102L [172]. Nevertheless, GSS P102L cases lacking spongiform change failed to induce clinical disease in these mice, too, although the inoculation elicited PrP deposition in both primary and secondary passages [173]. These observations led to the hypothesis that GSS brains lacking PrPSc 27-30 and spongiform change transmit non-infectious prions, which promote PrP-amyloidogenesis, but no spongiform change [174]. Notably, at the second passage, the 101L-passaged GSS P102L prions transmitted to wild-type mice [172]. The latter finding has questioned results obtained from similar experimental transmissions of GSS P102L prions in humanized Tg overexpressing human PrP with the P102L mutation. Indeed, in these mice, GSS P102L was efficiently transmitted at the first passage (homotypic transmission) [143,175], but the secondary propagation to wild-type mice failed [175]. The results suggest that 101L-passaged GSS P102L prions [172,174] may represent a novel strain generated by the mutant mouse PrP. The finding also highlighted the need for homotypic hosts for genetic prion disorders to obtain accurate and reproducible transmissions and avoid the emergence of novel strains.
Using a similar experimental design, GSS A117V was faithfully transmitted to Tg mice overexpressing human A117V PrP; although after an extremely long incubation period, inducing both clinical disease and spongiform change. Interestingly, immunoblot analysis revealed the coexistence of PrPSc 27–30 kDa and 7-8 kDa fragments [176]. Recently, GSS G131V intracerebrally inoculated into Tg mice expressing human PrP at a level 8–16-fold higher than physiological levels induced PrPSc deposition within the brain detectable at immunohistochemistry; nevertheless, immunoblotting and RT-QuIC failed to reveal abnormal PrP [177].
GSS F198S, A117V, and P102L (the latter including both the phenotype associated with PrPSc type 1-like and that accumulating ~8 kDa fragments) have also successfully transmitted to bank voles expressing isoleucine at codon 109 (Bv109I) [178]. Interestingly, a GSS P102L case with both type 1-like and 8 kDa PrPSc in the frontal cortex transmitted two distinct phenotypes in bank voles: the first characterized by short disease duration and accumulation of 8 kDa PrPSc, and the second by long duration and 21 kDa PrPSc. This result suggested that GSS P102L might transmit different prion strains [178], although this speculation has not been confirmed by serial transmission experiments.
Overall, this evidence raised the question of whether GSS is a genuinely transmissible spongiform encephalopathy and, if this is the case, whether single or multiple prion strains sustain the disease. The available data indicate that various both infectious and non-infectious prion agents with distinctive features may be responsible for the GSS phenotype. The former may induce clinical disease and spongiform change in the host brain and accumulate PrPSc with a molecular mass that is similar to CJD and FI (and, therefore, can be classified as genuine TSE), while the latter does not transmit the clinical disease, but induces PrP-amyloid deposition formed by PrPSc species of lower molecular mass (7–8 kDa).
6.6. Other Inherited Prion Amyloidosis
The Q226X variant is the only PrP-truncating mutation that successfully transmitted the disease in Tg mice overexpressing human PrP. The detection of abnormal PrP in the brain by immunostaining, immunoblot, and PrP amyloid seeding activity assayed by RT-QuIC faithfully demonstrated the disease transmission. Under the same experimental conditions, the Q227X variant failed to propagate the disease [177]. Similar attempts to transmit the Q163X and Y145X stop-codon variants in humanized Tg mice and wild-type rodents failed as well [170,179].
6.7. Variably Protease-Sensitive Prionopathy (VPSPr)
Two recent studies accomplished the transmission of VPSPr in humanized Tg mice expressing either physiological [76] or 2–8-fold PrP levels [78]. Brain inocula representative of all 129 genotypic combinations [78] or from subjects carrying the VV or MV genotype [77] yielded comparable results despite the slightly different study design. While no mice developed the clinical disease, about half of them showed PrPSc accumulation within the brain at immunohistochemistry assessment, and only one third showed detectable abnormal PrP by Western blot analysis [76,78]. Finally, there was no evidence of transmission after a second passage. These results showed striking similarities to those obtained in a subgroup of GSS cases. More recently, VPSPr inocula efficiently transmitted the disease to bank voles (Bv109M, Bv109I) [180]. Despite the low attack rate after the first passage (5% for Bv109M, 35% Bv109I), all inoculated animals developed the disease and showed dramatically reduced survival times (about 50%) after the secondary transmission, suggesting a consistent species barrier between humans and bank voles. Intriguingly, the affected animals showed three histo-molecular phenotypes, apparently unrelated to genotype at codon 129, with transmission characteristics reproducing those of PrPSc type 1 (21 kDa) and 2 (19 kDa) sCJD, and GSS expressing the ~7 kDa PrPSc fragment [180].
Further studies should elucidate whether these three phenotypes are related to different VPSPr isolates, which in humans converge in a single phenotype, or, instead, reflect the heterotypic background of the bank vole that sustains limited PrPSc structural variations independent of the predominant conformer of the human inoculum. Whatever the case, and despite the not univocal results, these transmission studies again highlighted the similarities between GSS and VPSPr. Indeed, the two diseases not only show similar PrPSc profiles including internal PrP fragments of low molecular mass and the tendency to form extracellular amyloid aggregates, but they also share the relatively limited transmission efficiency and infectious potential.
7. Summary and Gaps in Our Understanding of Human Prion Strains
The terms “amyloid” and “strain” are increasingly used in the field of neurodegenerative diseases to define shared pathogenic mechanisms between these disorders. Protein scientists now define amyloid as including all protein fibrils that display the cross-β fiber diffraction pattern when examined with X-rays, not only those that bind the dye Congo red and reveal a green birefringence when viewed under polarized light. Most importantly, prion or prion-like strains appear to be a general phenomenon related to amyloid polymorph, namely defined as phenotypic variants encoded by the same protein sequence, which assumes variant protein conformations. Substantial evidence also supports the idea that a prion-like, seeded-polymerization mechanism is responsible for the formation and propagation of protein aggregates in all neurodegenerative disorders [181].
Despite the advances, however, we lack a firm comprehension of the link between amyloid structure and disease in many instances. The full understanding of prion (prion protein) strains, in particular, is challenged by the complexity of human disease, which includes forms with distinct etiology and a broad spectrum of phenotypic variants (including disorders such as GSS that largely overlap with Alzheimer’s disease and other brain amyloidoses). What is the origin of sporadic prion disease? If it is caused by stochastic events related to cell aging, why do some rare CJD subtypes, such as VV1 and MM2T, mostly affect young adults? If the majority of human prion diseases originate spontaneously from random events, why is the anatomical spread of a given disease variant so consistent from case to case? What is the meaning of the ascending course from subcortical areas to the cerebral cortex that is so evident and consistent in some variants (e.g., VV2 and MM2T)? Furthermore, what is the basis of PrPSc types’ co-occurrence in the same brain? Why may the M2C strain coexist with all sCJD variants affecting the M allele and even dominate the M1 in some cases despite being the sCJD subtype that shows the lowest seeding activity? Despite many efforts, uncertainties also remain about the structural properties of amyloid aggregates that correlate with the strain-specific properties (i.e., the incubation time and rate of spread, regional targeting, lesion profile, and distinctive histopathology). What is the structural basis that explains the proteolytic generation of PrPSc type 1, type 2, type “i” and the internal fragments lacking the GPI anchor? What is the role of PrP glycans in determining the seeding and targeting properties of strains? Given the importance of the primary protein sequence in determining the amyloid structure, why is residue 129 so critical, while the majority of mutations linked to genetic CJD do not generate strain-specific properties?
Five decades have passed since Gajdusek’s pioneering studies demonstrated the transmissibility of human spongiform encephalopathies and almost four since Prusiner’s discovery of the prion protein and his formulation of the protein-only hypothesis. We hope that with the powerful tools of structural, molecular, and cellular biology available today, scientists will soon be able to answer many of these and other remaining crucial questions.
Author Contributions
writing—original draft preparation, M.R., S.B. and P.P.; writing—revision and editing, P.P.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Prusiner, S.B. Prions. Proc. Natl. Acad. Sci. USA 1998, 95, 13363–13383. [Google Scholar] [CrossRef]
- Caughey, B.; Baron, G.S.; Chesebro, B.; Jeffrey, M. Getting a grip on prions: oligomers, amyloids, and pathological membrane interactions. Annu. Rev. Biochem. 2009, 78, 177–204. [Google Scholar] [CrossRef]
- Bruce, M.E. TSE strain variation. Br. Med. Bull. 2003, 66, 99–108. [Google Scholar] [CrossRef] [PubMed]
- Eisenberg, D.S.; Sawaya, M.R. Structural Studies of Amyloid Proteins at the Molecular Level. Annu. Rev. Biochem. 2017, 86, 69–95. [Google Scholar] [CrossRef] [PubMed]
- Aguzzi, A.; Heikenwalder, M.; Polymenidou, M. Insights into prion strains and neurotoxicity. Nat. Rev. Mol. Cell Biol. 2007, 8, 552–561. [Google Scholar] [CrossRef]
- Baiardi, S.; Rossi, M.; Capellari, S.; Parchi, P. Recent advances in the histo-molecular pathology of human prion disease. Brain Pathol. 2019, 29, 278–300. [Google Scholar] [CrossRef]
- Pattison, I.H.; Millson, G.C. Scrapie produced experimentally in goats with special reference to the clinical syndrome. J. Comp. Pathol. 1961, 71, 101–109. [Google Scholar] [CrossRef]
- Chandler, R.L.; Fisher, J. Experimental Transmission of Scrapie to Rats. Lancet 1963, 2, 1165. [Google Scholar] [CrossRef]
- Fraser, H.; Dickinson, A.G. Scrapie in mice. Agent-strain differences in the distribution and intensity of grey matter vacuolation. J. Comp. Pathol. 1973, 83, 29–40. [Google Scholar] [CrossRef]
- Bruce, M.E.; Dickinson, A.G. Biological evidence that scrapie agent has an independent genome. J. Gen. Virol. 1987, 68, 79–89. [Google Scholar] [CrossRef]
- Gajdusek, D.C.; Gibbs, C.J.; Alpers, M. Transmission and passage of experimenal “kuru” to chimpanzees. Science 1967, 155, 212–214. [Google Scholar]
- Gibbs, C.J.; Gajdusek, D.C.; Asher, D.M.; Alpers, M.P.; Beck, E.; Daniel, P.M.; Matthews, W.B. Creutzfeldt-Jakob disease (spongiform encephalopathy): transmission to the chimpanzee. Science 1968, 161, 388–389. [Google Scholar] [CrossRef] [PubMed]
- Muramoto, T.; Kitamoto, T.; Hoque, M.Z.; Tateishi, J.; Goto, I. Species barrier prevents an abnormal isoform of prion protein from accumulating in follicular dendritic cells of mice with Creutzfeldt-Jakob disease. J. Virol. 1993, 67, 6808–6810. [Google Scholar] [PubMed]
- Bruce, M.; Chree, A.; McConnell, I.; Foster, J.; Pearson, G.; Fraser, H. Transmission of bovine spongiform encephalopathy and scrapie to mice: strain variation and the species barrier. Philos. Trans. R. Soc. Lond., B, Biol. Sci. 1994, 343, 405–411. [Google Scholar]
- Parchi, P.; Saverioni, D. Molecular pathology, classification, and diagnosis of sporadic human prion disease variants. Folia Neuropathol 2012, 50, 20–45. [Google Scholar] [PubMed]
- Wadsworth, J.D.F.; Asante, E.A.; Collinge, J. Review: contribution of transgenic models to understanding human prion disease. Neuropathol. Appl. Neurobiol. 2010, 36, 576–597. [Google Scholar] [CrossRef]
- Nonno, R.; Di Bari, M.A.; Cardone, F.; Vaccari, G.; Fazzi, P.; Dell’Omo, G.; Cartoni, C.; Ingrosso, L.; Boyle, A.; Galeno, R.; et al. Efficient transmission and characterization of Creutzfeldt-Jakob disease strains in bank voles. PLoS Pathog. 2006, 2, e12. [Google Scholar] [CrossRef] [PubMed]
- Parchi, P.; Cescatti, M.; Notari, S.; Schulz-Schaeffer, W.J.; Capellari, S.; Giese, A.; Zou, W.-Q.; Kretzschmar, H.; Ghetti, B.; Brown, P. Agent strain variation in human prion disease: insights from a molecular and pathological review of the National Institutes of Health series of experimentally transmitted disease. Brain 2010, 133, 3030–3042. [Google Scholar] [CrossRef]
- Watts, J.C.; Giles, K.; Patel, S.; Oehler, A.; DeArmond, S.J.; Prusiner, S.B. Evidence that bank vole PrP is a universal acceptor for prions. PLoS Pathog. 2014, 10, e1003990. [Google Scholar] [CrossRef]
- Parchi, P.; Strammiello, R.; Giese, A.; Kretzschmar, H. Phenotypic variability of sporadic human prion disease and its molecular basis: past, present, and future. Acta Neuropathol. 2011, 121, 91–112. [Google Scholar] [CrossRef]
- Hsiao, K.; Baker, H.F.; Crow, T.J.; Poulter, M.; Owen, F.; Terwilliger, J.D.; Westaway, D.; Ott, J.; Prusiner, S.B. Linkage of a prion protein missense variant to Gerstmann-Sträussler syndrome. Nature 1989, 338, 342–345. [Google Scholar] [CrossRef] [PubMed]
- Goldfarb, L.G.; Petersen, R.B.; Tabaton, M.; Brown, P.; LeBlanc, A.C.; Montagna, P.; Cortelli, P.; Julien, J.; Vital, C.; Pendelbury, W.W. Fatal familial insomnia and familial Creutzfeldt-Jakob disease: disease phenotype determined by a DNA polymorphism. Science 1992, 258, 806–808. [Google Scholar] [CrossRef] [PubMed]
- Deslys, J.P.; Lasmézas, C.; Dormont, D. Selection of specific strains in iatrogenic Creutzfeldt-Jakob disease. Lancet 1994, 343, 848–849. [Google Scholar] [CrossRef]
- De Silva, R.; Ironside, J.W.; McCardle, L.; Esmonde, T.; Bell, J.; Will, R.; Windl, O.; Dempster, M.; Estibeiro, P.; Lathe, R. Neuropathological phenotype and “prion protein” genotype correlation in sporadic Creutzfeldt-Jakob disease. Neurosci. Lett. 1994, 179, 50–52. [Google Scholar] [CrossRef]
- Miyazono, M.; Kitamoto, T.; Doh-ura, K.; Iwaki, T.; Tateishi, J. Creutzfeldt-Jakob disease with codon 129 polymorphism (valine): a comparative study of patients with codon 102 point mutation or without mutations. Acta Neuropathol. 1992, 84, 349–354. [Google Scholar] [CrossRef]
- Parchi, P.; Chen, S.G.; Brown, P.; Zou, W.; Capellari, S.; Budka, H.; Hainfellner, J.; Reyes, P.F.; Golden, G.T.; Hauw, J.J.; et al. Different patterns of truncated prion protein fragments correlate with distinct phenotypes in P102L Gerstmann-Sträussler-Scheinker disease. Proc. Natl. Acad. Sci. USA 1998, 95, 8322–8327. [Google Scholar] [CrossRef]
- Piccardo, P.; Dlouhy, S.R.; Lievens, P.M.; Young, K.; Bird, T.D.; Nochlin, D.; Dickson, D.W.; Vinters, H.V.; Zimmerman, T.R.; Mackenzie, I.R.; et al. Phenotypic variability of Gerstmann-Sträussler-Scheinker disease is associated with prion protein heterogeneity. J. Neuropathol. Exp. Neurol. 1998, 57, 979–988. [Google Scholar] [CrossRef]
- Parchi, P.; Castellani, R.; Capellari, S.; Ghetti, B.; Young, K.; Chen, S.G.; Farlow, M.; Dickson, D.W.; Sima, A.A.; Trojanowski, J.Q.; et al. Molecular basis of phenotypic variability in sporadic Creutzfeldt-Jakob disease. Ann. Neurol. 1996, 39, 767–778. [Google Scholar] [CrossRef] [PubMed]
- Parchi, P.; Capellari, S.; Chen, S.G.; Petersen, R.B.; Gambetti, P.; Kopp, N.; Brown, P.; Kitamoto, T.; Tateishi, J.; Giese, A.; et al. Typing prion isoforms. Nature 1997, 386, 232–234. [Google Scholar] [CrossRef]
- Parchi, P.; Zou, W.; Wang, W.; Brown, P.; Capellari, S.; Ghetti, B.; Kopp, N.; Schulz-Schaeffer, W.J.; Kretzschmar, H.A.; Head, M.W.; et al. Genetic influence on the structural variations of the abnormal prion protein. Proc. Natl. Acad. Sci. USA 2000, 97, 10168–10172. [Google Scholar] [CrossRef]
- Parchi, P.; Giese, A.; Capellari, S.; Brown, P.; Schulz-Schaeffer, W.; Windl, O.; Zerr, I.; Budka, H.; Kopp, N.; Piccardo, P.; et al. Classification of sporadic Creutzfeldt-Jakob disease based on molecular and phenotypic analysis of 300 subjects. Ann. Neurol. 1999, 46, 224–233. [Google Scholar] [CrossRef]
- Parchi, P.; Strammiello, R.; Notari, S.; Giese, A.; Langeveld, J.P.M.; Ladogana, A.; Zerr, I.; Roncaroli, F.; Cras, P.; Ghetti, B.; et al. Incidence and spectrum of sporadic Creutzfeldt-Jakob disease variants with mixed phenotype and co-occurrence of PrPSc types: an updated classification. Acta Neuropathol. 2009, 118, 659–671. [Google Scholar] [CrossRef]
- Parchi, P.; de Boni, L.; Saverioni, D.; Cohen, M.L.; Ferrer, I.; Gambetti, P.; Gelpi, E.; Giaccone, G.; Hauw, J.-J.; Höftberger, R.; et al. Consensus classification of human prion disease histotypes allows reliable identification of molecular subtypes: an inter-rater study among surveillance centres in Europe and USA. Acta Neuropathol. 2012, 124, 517–529. [Google Scholar] [CrossRef]
- Kobayashi, A.; Matsuura, Y.; Mohri, S.; Kitamoto, T. Distinct origins of dura mater graft-associated Creutzfeldt-Jakob disease: past and future problems. Acta Neuropathol. Commun. 2014, 2, 32. [Google Scholar] [CrossRef]
- Notari, S.; Capellari, S.; Giese, A.; Westner, I.; Baruzzi, A.; Ghetti, B.; Gambetti, P.; Kretzschmar, H.A.; Parchi, P. Effects of different experimental conditions on the PrPSc core generated by protease digestion: implications for strain typing and molecular classification of CJD. J. Biol. Chem. 2004, 279, 16797–16804. [Google Scholar] [CrossRef]
- Zou, W.-Q.; Capellari, S.; Parchi, P.; Sy, M.-S.; Gambetti, P.; Chen, S.G. Identification of novel proteinase K-resistant C-terminal fragments of PrP in Creutzfeldt-Jakob disease. J. Biol. Chem. 2003, 278, 40429–40436. [Google Scholar] [CrossRef]
- Satoh, K.; Muramoto, T.; Tanaka, T.; Kitamoto, N.; Ironside, J.W.; Nagashima, K.; Yamada, M.; Sato, T.; Mohri, S.; Kitamoto, T. Association of an 11-12 kDa protease-resistant prion protein fragment with subtypes of dura graft-associated Creutzfeldt-Jakob disease and other prion diseases. J. Gen. Virol. 2003, 84, 2885–2893. [Google Scholar] [CrossRef]
- Pan, T.; Li, R.; Kang, S.-C.; Pastore, M.; Wong, B.-S.; Ironside, J.; Gambetti, P.; Sy, M.-S. Biochemical fingerprints of prion diseases: scrapie prion protein in human prion diseases that share prion genotype and type. J. Neurochem. 2005, 92, 132–142. [Google Scholar] [CrossRef] [PubMed]
- Notari, S.; Strammiello, R.; Capellari, S.; Giese, A.; Cescatti, M.; Grassi, J.; Ghetti, B.; Langeveld, J.P.M.; Zou, W.-Q.; Gambetti, P.; et al. Characterization of truncated forms of abnormal prion protein in Creutzfeldt-Jakob disease. J. Biol. Chem. 2008, 283, 30557–30565. [Google Scholar] [CrossRef]
- Endo, T.; Groth, D.; Prusiner, S.B.; Kobata, A. Diversity of oligosaccharide structures linked to asparagines of the scrapie prion protein. Biochemistry 1989, 28, 8380–8388. [Google Scholar] [CrossRef]
- Monari, L.; Chen, S.G.; Brown, P.; Parchi, P.; Petersen, R.B.; Mikol, J.; Gray, F.; Cortelli, P.; Montagna, P.; Ghetti, B. Fatal familial insomnia and familial Creutzfeldt-Jakob disease: different prion proteins determined by a DNA polymorphism. Proc. Natl. Acad. Sci. USA 1994, 91, 2839–2842. [Google Scholar] [CrossRef] [PubMed]
- Collinge, J.; Sidle, K.C.; Meads, J.; Ironside, J.; Hill, A.F. Molecular analysis of prion strain variation and the aetiology of “new variant” CJD. Nature 1996, 383, 685–690. [Google Scholar] [CrossRef] [PubMed]
- Hill, A.F.; Joiner, S.; Beck, J.A.; Campbell, T.A.; Dickinson, A.; Poulter, M.; Wadsworth, J.D.F.; Collinge, J. Distinct glycoform ratios of protease resistant prion protein associated with PRNP point mutations. Brain 2006, 129, 676–685. [Google Scholar] [CrossRef]
- Pan, T.; Colucci, M.; Wong, B.S.; Li, R.; Liu, T.; Petersen, R.B.; Chen, S.; Gambetti, P.; Sy, M.S. Novel differences between two human prion strains revealed by two-dimensional gel electrophoresis. J. Biol. Chem. 2001, 276, 37284–37288. [Google Scholar] [CrossRef]
- Head, M.W.; Bunn, T.J.R.; Bishop, M.T.; McLoughlin, V.; Lowrie, S.; McKimmie, C.S.; Williams, M.C.; McCardle, L.; MacKenzie, J.; Knight, R.; et al. Prion protein heterogeneity in sporadic but not variant Creutzfeldt-Jakob disease: UK cases 1991-2002. Ann. Neurol. 2004, 55, 851–859. [Google Scholar] [CrossRef] [PubMed]
- Head, M.W.; Ironside, J.W. Sporadic Creutzfeldt-Jakob disease: discrete subtypes or a spectrum of disease? Brain 2009, 132, 2627–2629. [Google Scholar] [CrossRef]
- Lewis, V.; Hill, A.F.; Klug, G.M.; Boyd, A.; Masters, C.L.; Collins, S.J. Australian sporadic CJD analysis supports endogenous determinants of molecular-clinical profiles. Neurology 2005, 65, 113–118. [Google Scholar] [CrossRef]
- Puoti, G.; Giaccone, G.; Rossi, G.; Canciani, B.; Bugiani, O.; Tagliavini, F. Sporadic Creutzfeldt-Jakob disease: co-occurrence of different types of PrP(Sc) in the same brain. Neurology 1999, 53, 2173–2176. [Google Scholar] [CrossRef]
- Schoch, G.; Seeger, H.; Bogousslavsky, J.; Tolnay, M.; Janzer, R.C.; Aguzzi, A.; Glatzel, M. Analysis of prion strains by PrPSc profiling in sporadic Creutzfeldt-Jakob disease. PLoS Med. 2006, 3, e14. [Google Scholar] [CrossRef]
- Uro-Coste, E.; Cassard, H.; Simon, S.; Lugan, S.; Bilheude, J.-M.; Perret-Liaudet, A.; Ironside, J.W.; Haik, S.; Basset-Leobon, C.; Lacroux, C.; et al. Beyond PrP res type 1/type 2 dichotomy in Creutzfeldt-Jakob disease. PLoS Pathog. 2008, 4, e1000029. [Google Scholar] [CrossRef]
- Yull, H.M.; Ritchie, D.L.; Langeveld, J.P.M.; van Zijderveld, F.G.; Bruce, M.E.; Ironside, J.W.; Head, M.W. Detection of type 1 prion protein in variant Creutzfeldt-Jakob disease. Am. J. Pathol. 2006, 168, 151–157. [Google Scholar] [CrossRef]
- Polymenidou, M.; Stoeck, K.; Glatzel, M.; Vey, M.; Bellon, A.; Aguzzi, A. Coexistence of multiple PrPSc types in individuals with Creutzfeldt-Jakob disease. Lancet Neurol. 2005, 4, 805–814. [Google Scholar] [CrossRef]
- Kobayashi, A.; Mizukoshi, K.; Iwasaki, Y.; Miyata, H.; Yoshida, Y.; Kitamoto, T. Co-occurrence of types 1 and 2 PrP(res) in sporadic Creutzfeldt-Jakob disease MM1. Am. J. Pathol. 2011, 178, 1309–1315. [Google Scholar] [CrossRef] [PubMed]
- Notari, S.; Capellari, S.; Langeveld, J.; Giese, A.; Strammiello, R.; Gambetti, P.; Kretzschmar, H.A.; Parchi, P. A refined method for molecular typing reveals that co-occurrence of PrP(Sc) types in Creutzfeldt-Jakob disease is not the rule. Lab. Invest. 2007, 87, 1103–1112. [Google Scholar] [CrossRef] [PubMed]
- Cali, I.; Castellani, R.; Alshekhlee, A.; Cohen, Y.; Blevins, J.; Yuan, J.; Langeveld, J.P.M.; Parchi, P.; Safar, J.G.; Zou, W.-Q.; et al. Co-existence of scrapie prion protein types 1 and 2 in sporadic Creutzfeldt-Jakob disease: its effect on the phenotype and prion-type characteristics. Brain 2009, 132, 2643–2658. [Google Scholar] [CrossRef] [PubMed]
- Piccardo, P.; Liepnieks, J.J.; William, A.; Dlouhy, S.R.; Farlow, M.R.; Young, K.; Nochlin, D.; Bird, T.D.; Nixon, R.R.; Ball, M.J.; et al. Prion proteins with different conformations accumulate in Gerstmann-Sträussler-Scheinker disease caused by A117V and F198S mutations. Am. J. Pathol. 2001, 158, 2201–2207. [Google Scholar] [CrossRef]
- Tagliavini, F.; Prelli, F.; Ghiso, J.; Bugiani, O.; Serban, D.; Prusiner, S.B.; Farlow, M.R.; Ghetti, B.; Frangione, B. Amyloid protein of Gerstmann-Sträussler-Scheinker disease (Indiana kindred) is an 11 kd fragment of prion protein with an N-terminal glycine at codon 58. EMBO J. 1991, 10, 513–519. [Google Scholar] [CrossRef]
- Tagliavini, F.; Lievens, P.M.; Tranchant, C.; Warter, J.M.; Mohr, M.; Giaccone, G.; Perini, F.; Rossi, G.; Salmona, M.; Piccardo, P.; et al. A 7-kDa prion protein (PrP) fragment, an integral component of the PrP region required for infectivity, is the major amyloid protein in Gerstmann-Sträussler-Scheinker disease A117V. J. Biol. Chem. 2001, 276, 6009–6015. [Google Scholar] [CrossRef]
- Pirisinu, L.; Nonno, R.; Esposito, E.; Benestad, S.L.; Gambetti, P.; Agrimi, U.; Zou, W.-Q. Small ruminant nor98 prions share biochemical features with human gerstmann-sträussler-scheinker disease and variably protease-sensitive prionopathy. PLoS ONE 2013, 8, e66405. [Google Scholar] [CrossRef] [PubMed]
- Zou, W.-Q.; Puoti, G.; Xiao, X.; Yuan, J.; Qing, L.; Cali, I.; Shimoji, M.; Langeveld, J.P.M.; Castellani, R.; Notari, S.; et al. Variably protease-sensitive prionopathy: a new sporadic disease of the prion protein. Ann. Neurol. 2010, 68, 162–172. [Google Scholar] [CrossRef]
- Peden, A.H.; Sarode, D.P.; Mulholland, C.R.; Barria, M.A.; Ritchie, D.L.; Ironside, J.W.; Head, M.W. The prion protein protease sensitivity, stability and seeding activity in variably protease sensitive prionopathy brain tissue suggests molecular overlaps with sporadic Creutzfeldt-Jakob disease. Acta Neuropathol. Commun. 2014, 2, 152. [Google Scholar] [CrossRef] [PubMed]
- Vicente-Pascual, M.; Rossi, M.; Gámez, J.; Lladó, A.; Valls, J.; Grau-Rivera, O.; Ávila Polo, R.; Llorens, F.; Zerr, I.; Ferrer, I.; et al. Variably protease-sensitive prionopathy presenting within ALS/FTD spectrum. Ann. Clin. Transl. Neurol. 2018, 5, 1297–1302. [Google Scholar] [CrossRef] [PubMed]
- Chen, S.G.; Teplow, D.B.; Parchi, P.; Teller, J.K.; Gambetti, P.; Autilio-Gambetti, L. Truncated forms of the human prion protein in normal brain and in prion diseases. J. Biol. Chem. 1995, 270, 19173–19180. [Google Scholar] [CrossRef]
- Telling, G.C.; Parchi, P.; DeArmond, S.J.; Cortelli, P.; Montagna, P.; Gabizon, R.; Mastrianni, J.; Lugaresi, E.; Gambetti, P.; Prusiner, S.B. Evidence for the conformation of the pathologic isoform of the prion protein enciphering and propagating prion diversity. Science 1996, 274, 2079–2082. [Google Scholar] [CrossRef]
- Bessen, R.A.; Marsh, R.F. Distinct PrP properties suggest the molecular basis of strain variation in transmissible mink encephalopathy. J. Virol. 1994, 68, 7859–7868. [Google Scholar] [PubMed]
- Wille, H.; Requena, J.R. The Structure of PrPSc Prions. Pathogens 2018, 7. [Google Scholar] [CrossRef] [PubMed]
- Saverioni, D.; Notari, S.; Capellari, S.; Poggiolini, I.; Giese, A.; Kretzschmar, H.A.; Parchi, P. Analyses of protease resistance and aggregation state of abnormal prion protein across the spectrum of human prions. J. Biol. Chem. 2013, 288, 27972–27985. [Google Scholar] [CrossRef]
- Cescatti, M.; Saverioni, D.; Capellari, S.; Tagliavini, F.; Kitamoto, T.; Ironside, J.; Giese, A.; Parchi, P. Analysis of Conformational Stability of Abnormal Prion Protein Aggregates across the Spectrum of Creutzfeldt-Jakob Disease Prions. J. Virol. 2016, 90, 6244–6254. [Google Scholar] [CrossRef]
- Diack, A.B.; Head, M.W.; McCutcheon, S.; Boyle, A.; Knight, R.; Ironside, J.W.; Manson, J.C.; Will, R.G. Variant CJD. 18 years of research and surveillance. Prion 2014, 8, 286–295. [Google Scholar] [CrossRef]
- Abu-Rumeileh, S.; Redaelli, V.; Baiardi, S.; Mackenzie, G.; Windl, O.; Ritchie, D.L.; Didato, G.; Hernandez-Vara, J.; Rossi, M.; Capellari, S.; et al. Sporadic Fatal Insomnia in Europe: Phenotypic Features and Diagnostic Challenges. Ann. Neurol. 2018, 84, 347–360. [Google Scholar] [CrossRef]
- Bishop, M.T.; Will, R.G.; Manson, J.C. Defining sporadic Creutzfeldt-Jakob disease strains and their transmission properties. Proc. Natl. Acad. Sci. USA 2010, 107, 12005–12010. [Google Scholar] [CrossRef] [PubMed]
- Kobayashi, A.; Sakuma, N.; Matsuura, Y.; Mohri, S.; Aguzzi, A.; Kitamoto, T. Experimental verification of a traceback phenomenon in prion infection. J. Virol. 2010, 84, 3230–3238. [Google Scholar] [CrossRef] [PubMed]
- Kobayashi, A.; Iwasaki, Y.; Otsuka, H.; Yamada, M.; Yoshida, M.; Matsuura, Y.; Mohri, S.; Kitamoto, T. Deciphering the pathogenesis of sporadic Creutzfeldt-Jakob disease with codon 129 M/V and type 2 abnormal prion protein. Acta Neuropathol. Commun. 2013, 1, 74. [Google Scholar] [CrossRef] [PubMed]
- Moda, F.; Suardi, S.; Di Fede, G.; Indaco, A.; Limido, L.; Vimercati, C.; Ruggerone, M.; Campagnani, I.; Langeveld, J.; Terruzzi, A.; et al. MM2-thalamic Creutzfeldt-Jakob disease: neuropathological, biochemical and transmission studies identify a distinctive prion strain. Brain Pathol. 2012, 22, 662–669. [Google Scholar] [CrossRef] [PubMed]
- Takeuchi, A.; Kobayashi, A.; Ironside, J.W.; Mohri, S.; Kitamoto, T. Characterization of variant Creutzfeldt-Jakob disease prions in prion protein-humanized mice carrying distinct codon 129 genotypes. J. Biol. Chem. 2013, 288, 21659–21666. [Google Scholar] [CrossRef] [PubMed]
- Diack, A.B.; Ritchie, D.L.; Peden, A.H.; Brown, D.; Boyle, A.; Morabito, L.; Maclennan, D.; Burgoyne, P.; Jansen, C.; Knight, R.S.; et al. Variably protease-sensitive prionopathy, a unique prion variant with inefficient transmission properties. Emerging Infect. Dis. 2014, 20, 1969–1979. [Google Scholar] [CrossRef]
- Kobayashi, A.; Matsuura, Y.; Iwaki, T.; Iwasaki, Y.; Yoshida, M.; Takahashi, H.; Murayama, S.; Takao, M.; Kato, S.; Yamada, M.; et al. Sporadic Creutzfeldt-Jakob Disease MM1+2C and MM1 are Identical in Transmission Properties. Brain Pathol. 2016, 26, 95–101. [Google Scholar] [CrossRef] [PubMed]
- Notari, S.; Xiao, X.; Espinosa, J.C.; Cohen, Y.; Qing, L.; Aguilar-Calvo, P.; Kofskey, D.; Cali, I.; Cracco, L.; Kong, Q.; et al. Transmission characteristics of variably protease-sensitive prionopathy. Emerging Infect. Dis. 2014, 20, 2006–2014. [Google Scholar] [CrossRef] [PubMed]
- Safar, J.; Wille, H.; Itri, V.; Groth, D.; Serban, H.; Torchia, M.; Cohen, F.E.; Prusiner, S.B. Eight prion strains have PrP(Sc) molecules with different conformations. Nat. Med. 1998, 4, 1157–1165. [Google Scholar] [CrossRef] [PubMed]
- Safar, J.G.; Geschwind, M.D.; Deering, C.; Didorenko, S.; Sattavat, M.; Sanchez, H.; Serban, A.; Vey, M.; Baron, H.; Giles, K.; et al. Diagnosis of human prion disease. Proc. Natl. Acad. Sci. USA 2005, 102, 3501–3506. [Google Scholar] [CrossRef]
- Choi, Y.P.; Gröner, A.; Ironside, J.W.; Head, M.W. Comparison of the level, distribution and form of disease-associated prion protein in variant and sporadic Creutzfeldt-Jakob diseased brain using conformation-dependent immunoassay and Western blot. J. Gen. Virol. 2011, 92, 727–732. [Google Scholar] [CrossRef]
- Kim, C.; Haldiman, T.; Cohen, Y.; Chen, W.; Blevins, J.; Sy, M.-S.; Cohen, M.; Safar, J.G. Protease-sensitive conformers in broad spectrum of distinct PrPSc structures in sporadic Creutzfeldt-Jakob disease are indicator of progression rate. PLoS Pathog. 2011, 7, e1002242. [Google Scholar] [CrossRef] [PubMed]
- Safar, J.G.; Xiao, X.; Kabir, M.E.; Chen, S.; Kim, C.; Haldiman, T.; Cohen, Y.; Chen, W.; Cohen, M.L.; Surewicz, W.K. Structural determinants of phenotypic diversity and replication rate of human prions. PLoS Pathog. 2015, 11, e1004832. [Google Scholar] [CrossRef] [PubMed]
- Pirisinu, L.; Di Bari, M.; Marcon, S.; Vaccari, G.; D’Agostino, C.; Fazzi, P.; Esposito, E.; Galeno, R.; Langeveld, J.; Agrimi, U.; et al. A new method for the characterization of strain-specific conformational stability of protease-sensitive and protease-resistant PrPSc. PLoS ONE 2010, 5, e12723. [Google Scholar] [CrossRef] [PubMed]
- Cracco, L.; Notari, S.; Cali, I.; Sy, M.-S.; Chen, S.G.; Cohen, M.L.; Ghetti, B.; Appleby, B.S.; Zou, W.-Q.; Caughey, B.; et al. Novel strain properties distinguishing sporadic prion diseases sharing prion protein genotype and prion type. Sci. Rep. 2017, 7, 38280. [Google Scholar] [CrossRef]
- Bett, C.; Joshi-Barr, S.; Lucero, M.; Trejo, M.; Liberski, P.; Kelly, J.W.; Masliah, E.; Sigurdson, C.J. Biochemical properties of highly neuroinvasive prion strains. PLoS Pathog. 2012, 8, e1002522. [Google Scholar] [CrossRef]
- Saborio, G.P.; Permanne, B.; Soto, C. Sensitive detection of pathological prion protein by cyclic amplification of protein misfolding. Nature 2001, 411, 810–813. [Google Scholar] [CrossRef] [PubMed]
- Soto, C.; Anderes, L.; Suardi, S.; Cardone, F.; Castilla, J.; Frossard, M.-J.; Peano, S.; Saa, P.; Limido, L.; Carbonatto, M.; et al. Pre-symptomatic detection of prions by cyclic amplification of protein misfolding. FEBS Lett. 2005, 579, 638–642. [Google Scholar] [CrossRef]
- Rubenstein, R.; Chang, B. Re-assessment of PrP(Sc) distribution in sporadic and variant CJD. PLoS ONE 2013, 8, e66352. [Google Scholar] [CrossRef] [PubMed]
- Concha-Marambio, L.; Pritzkow, S.; Moda, F.; Tagliavini, F.; Ironside, J.W.; Schulz, P.E.; Soto, C. Detection of prions in blood from patients with variant Creutzfeldt-Jakob disease. Sci. Transl. Med. 2016, 8, 370ra183. [Google Scholar] [CrossRef]
- Bougard, D.; Brandel, J.-P.; Bélondrade, M.; Béringue, V.; Segarra, C.; Fleury, H.; Laplanche, J.-L.; Mayran, C.; Nicot, S.; Green, A.; et al. Detection of prions in the plasma of presymptomatic and symptomatic patients with variant Creutzfeldt-Jakob disease. Sci. Transl. Med. 2016, 8, 370ra182. [Google Scholar] [CrossRef]
- Jones, M.; Peden, A.H.; Prowse, C.V.; Gröner, A.; Manson, J.C.; Turner, M.L.; Ironside, J.W.; MacGregor, I.R.; Head, M.W. In vitro amplification and detection of variant Creutzfeldt-Jakob disease PrPSc. J. Pathol. 2007, 213, 21–26. [Google Scholar] [CrossRef]
- Yokoyama, T.; Takeuchi, A.; Yamamoto, M.; Kitamoto, T.; Ironside, J.W.; Morita, M. Heparin enhances the cell-protein misfolding cyclic amplification efficiency of variant Creutzfeldt-Jakob disease. Neurosci. Lett. 2011, 498, 119–123. [Google Scholar] [CrossRef]
- Atarashi, R.; Moore, R.A.; Sim, V.L.; Hughson, A.G.; Dorward, D.W.; Onwubiko, H.A.; Priola, S.A.; Caughey, B. Ultrasensitive detection of scrapie prion protein using seeded conversion of recombinant prion protein. Nat. Methods 2007, 4, 645–650. [Google Scholar] [CrossRef]
- Jones, M.; Peden, A.H.; Yull, H.; Wight, D.; Bishop, M.T.; Prowse, C.V.; Turner, M.L.; Ironside, J.W.; MacGregor, I.R.; Head, M.W. Human platelets as a substrate source for the in vitro amplification of the abnormal prion protein (PrP) associated with variant Creutzfeldt-Jakob disease. Transfusion 2009, 49, 376–384. [Google Scholar] [CrossRef]
- Haldiman, T.; Kim, C.; Cohen, Y.; Chen, W.; Blevins, J.; Qing, L.; Cohen, M.L.; Langeveld, J.; Telling, G.C.; Kong, Q.; et al. Co-existence of distinct prion types enables conformational evolution of human PrPSc by competitive selection. J. Biol. Chem. 2013, 288, 29846–29861. [Google Scholar] [CrossRef]
- Xiao, X.; Yuan, J.; Qing, L.; Cali, I.; Mikol, J.; Delisle, M.-B.; Uro-Coste, E.; Zeng, L.; Abouelsaad, M.; Gazgalis, D.; et al. Comparative Study of Prions in Iatrogenic and Sporadic Creutzfeldt-Jakob Disease. J. Clin. Cell Immunol. 2014, 5, 240. [Google Scholar] [CrossRef]
- Moda, F.; Gambetti, P.; Notari, S.; Concha-Marambio, L.; Catania, M.; Park, K.-W.; Maderna, E.; Suardi, S.; Haïk, S.; Brandel, J.-P.; et al. Prions in the urine of patients with variant Creutzfeldt-Jakob disease. N. Engl. J. Med. 2014, 371, 530–539. [Google Scholar] [CrossRef]
- Takeuchi, A.; Kobayashi, A.; Parchi, P.; Yamada, M.; Morita, M.; Uno, S.; Kitamoto, T. Distinctive properties of plaque-type dura mater graft-associated Creutzfeldt-Jakob disease in cell-protein misfolding cyclic amplification. Lab. Invest. 2016, 96, 581–587. [Google Scholar] [CrossRef]
- Oshita, M.; Yokoyama, T.; Takei, Y.; Takeuchi, A.; Ironside, J.W.; Kitamoto, T.; Morita, M. Efficient propagation of variant Creutzfeldt-Jakob disease prion protein using the cell-protein misfolding cyclic amplification technique with samples containing plasma and heparin. Transfusion 2016, 56, 223–230. [Google Scholar] [CrossRef]
- Redaelli, V.; Bistaffa, E.; Zanusso, G.; Salzano, G.; Sacchetto, L.; Rossi, M.; De Luca, C.M.G.; Di Bari, M.; Portaleone, S.M.; Agrimi, U.; et al. Detection of prion seeding activity in the olfactory mucosa of patients with Fatal Familial Insomnia. Sci. Rep. 2017, 7, 46269. [Google Scholar] [CrossRef]
- Privat, N.; Levavasseur, E.; Yildirim, S.; Hannaoui, S.; Brandel, J.-P.; Laplanche, J.-L.; Béringue, V.; Seilhean, D.; Haïk, S. Region-specific protein misfolding cyclic amplification reproduces brain tropism of prion strains. J. Biol. Chem. 2017, 292, 16688–16696. [Google Scholar] [CrossRef]
- Ritchie, D.L.; Barria, M.A.; Peden, A.H.; Yull, H.M.; Kirkpatrick, J.; Adlard, P.; Ironside, J.W.; Head, M.W. UK Iatrogenic Creutzfeldt-Jakob disease: investigating human prion transmission across genotypic barriers using human tissue-based and molecular approaches. Acta Neuropathol. 2017, 133, 579–595. [Google Scholar] [CrossRef]
- Deleault, N.R.; Lucassen, R.W.; Supattapone, S. RNA molecules stimulate prion protein conversion. Nature 2003, 425, 717–720. [Google Scholar] [CrossRef]
- Deleault, N.R.; Harris, B.T.; Rees, J.R.; Supattapone, S. Formation of native prions from minimal components in vitro. Proc. Natl. Acad. Sci. USA 2007, 104, 9741–9746. [Google Scholar] [CrossRef] [PubMed]
- Deleault, N.R.; Piro, J.R.; Walsh, D.J.; Wang, F.; Ma, J.; Geoghegan, J.C.; Supattapone, S. Isolation of phosphatidylethanolamine as a solitary cofactor for prion formation in the absence of nucleic acids. Proc. Natl. Acad. Sci. USA 2012, 109, 8546–8551. [Google Scholar] [CrossRef] [PubMed]
- Katorcha, E.; Makarava, N.; Savtchenko, R.; Baskakov, I.V. Sialylation of the prion protein glycans controls prion replication rate and glycoform ratio. Sci. Rep. 2015, 5, 16912. [Google Scholar] [CrossRef]
- Imamura, M.; Tabeta, N.; Kato, N.; Matsuura, Y.; Iwamaru, Y.; Yokoyama, T.; Murayama, Y. Heparan Sulfate and Heparin Promote Faithful Prion Replication in Vitro by Binding to Normal and Abnormal Prion Proteins in Protein Misfolding Cyclic Amplification. J. Biol. Chem. 2016, 291, 26478–26486. [Google Scholar] [CrossRef]
- Atarashi, R.; Sano, K.; Satoh, K.; Nishida, N. Real-time quaking-induced conversion: a highly sensitive assay for prion detection. Prion 2011, 5, 150–153. [Google Scholar] [CrossRef]
- Orrú, C.D.; Bongianni, M.; Tonoli, G.; Ferrari, S.; Hughson, A.G.; Groveman, B.R.; Fiorini, M.; Pocchiari, M.; Monaco, S.; Caughey, B.; et al. A test for Creutzfeldt-Jakob disease using nasal brushings. N. Engl. J. Med. 2014, 371, 519–529. [Google Scholar] [CrossRef]
- Cramm, M.; Schmitz, M.; Karch, A.; Mitrova, E.; Kuhn, F.; Schroeder, B.; Raeber, A.; Varges, D.; Kim, Y.-S.; Satoh, K.; et al. Stability and Reproducibility Underscore Utility of RT-QuIC for Diagnosis of Creutzfeldt-Jakob Disease. Mol. Neurobiol. 2016, 53, 1896–1904. [Google Scholar] [CrossRef]
- McGuire, L.I.; Poleggi, A.; Poggiolini, I.; Suardi, S.; Grznarova, K.; Shi, S.; de Vil, B.; Sarros, S.; Satoh, K.; Cheng, K.; et al. Cerebrospinal fluid real-time quaking-induced conversion is a robust and reliable test for sporadic creutzfeldt-jakob disease: An international study. Ann. Neurol. 2016, 80, 160–165. [Google Scholar] [CrossRef] [PubMed]
- Orrú, C.D.; Groveman, B.R.; Hughson, A.G.; Zanusso, G.; Coulthart, M.B.; Caughey, B. Rapid and sensitive RT-QuIC detection of human Creutzfeldt-Jakob disease using cerebrospinal fluid. MBio 2015, 6, e02451-14. [Google Scholar] [CrossRef] [PubMed]
- Franceschini, A.; Baiardi, S.; Hughson, A.G.; McKenzie, N.; Moda, F.; Rossi, M.; Capellari, S.; Green, A.; Giaccone, G.; Caughey, B.; et al. High diagnostic value of second generation CSF RT-QuIC across the wide spectrum of CJD prions. Sci. Rep. 2017, 7, 10655. [Google Scholar] [CrossRef]
- Cramm, M.; Schmitz, M.; Karch, A.; Zafar, S.; Varges, D.; Mitrova, E.; Schroeder, B.; Raeber, A.; Kuhn, F.; Zerr, I. Characteristic CSF prion seeding efficiency in humans with prion diseases. Mol. Neurobiol. 2015, 51, 396–405. [Google Scholar] [CrossRef]
- Lattanzio, F.; Abu-Rumeileh, S.; Franceschini, A.; Kai, H.; Amore, G.; Poggiolini, I.; Rossi, M.; Baiardi, S.; McGuire, L.; Ladogana, A.; et al. Prion-specific and surrogate CSF biomarkers in Creutzfeldt-Jakob disease: diagnostic accuracy in relation to molecular subtypes and analysis of neuropathological correlates of p-tau and Aβ42 levels. Acta Neuropathol. 2017, 133, 559–578. [Google Scholar] [CrossRef] [PubMed]
- McGuire, L.I.; Peden, A.H.; Orrú, C.D.; Wilham, J.M.; Appleford, N.E.; Mallinson, G.; Andrews, M.; Head, M.W.; Caughey, B.; Will, R.G.; et al. Real time quaking-induced conversion analysis of cerebrospinal fluid in sporadic Creutzfeldt-Jakob disease. Ann. Neurol. 2012, 72, 278–285. [Google Scholar] [CrossRef] [PubMed]
- Bongianni, M.; Orrù, C.; Groveman, B.R.; Sacchetto, L.; Fiorini, M.; Tonoli, G.; Triva, G.; Capaldi, S.; Testi, S.; Ferrari, S.; et al. Diagnosis of Human Prion Disease Using Real-Time Quaking-Induced Conversion Testing of Olfactory Mucosa and Cerebrospinal Fluid Samples. JAMA Neurol. 2017, 74, 155–162. [Google Scholar] [CrossRef]
- Foutz, A.; Appleby, B.S.; Hamlin, C.; Liu, X.; Yang, S.; Cohen, Y.; Chen, W.; Blevins, J.; Fausett, C.; Wang, H.; et al. Diagnostic and prognostic value of human prion detection in cerebrospinal fluid. Ann. Neurol. 2017, 81, 79–92. [Google Scholar] [CrossRef]
- Groveman, B.R.; Orrú, C.D.; Hughson, A.G.; Bongianni, M.; Fiorini, M.; Imperiale, D.; Ladogana, A.; Pocchiari, M.; Zanusso, G.; Caughey, B. Extended and direct evaluation of RT-QuIC assays for Creutzfeldt-Jakob disease diagnosis. Ann. Clin. Transl. Neurol. 2017, 4, 139–144. [Google Scholar] [CrossRef]
- Baiardi, S.; Redaelli, V.; Ripellino, P.; Rossi, M.; Franceschini, A.; Moggio, M.; Sola, P.; Ladogana, A.; Fociani, P.; Magherini, A.; et al. Prion-related peripheral neuropathy in sporadic Creutzfeldt-Jakob disease. J. Neurol. Neurosurg. Psychiatry 2018. [Google Scholar] [CrossRef] [PubMed]
- Orrú, C.D.; Yuan, J.; Appleby, B.S.; Li, B.; Li, Y.; Winner, D.; Wang, Z.; Zhan, Y.-A.; Rodgers, M.; Rarick, J.; et al. Prion seeding activity and infectivity in skin samples from patients with sporadic Creutzfeldt-Jakob disease. Sci. Transl. Med. 2017, 9. [Google Scholar] [CrossRef] [PubMed]
- Orrù, C.D.; Soldau, K.; Cordano, C.; Llibre-Guerra, J.; Green, A.J.; Sanchez, H.; Groveman, B.R.; Edland, S.D.; Safar, J.G.; Lin, J.H.; et al. Prion Seeds Distribute throughout the Eyes of Sporadic Creutzfeldt-Jakob Disease Patients. MBio 2018, 9, e02095-18. [Google Scholar] [CrossRef] [PubMed]
- Peden, A.H.; McGuire, L.I.; Appleford, N.E.J.; Mallinson, G.; Wilham, J.M.; Orrú, C.D.; Caughey, B.; Ironside, J.W.; Knight, R.S.; Will, R.G.; et al. Sensitive and specific detection of sporadic Creutzfeldt-Jakob disease brain prion protein using real-time quaking-induced conversion. J. Gen. Virol. 2012, 93, 438–449. [Google Scholar] [CrossRef]
- Orrú, C.D.; Groveman, B.R.; Raymond, L.D.; Hughson, A.G.; Nonno, R.; Zou, W.; Ghetti, B.; Gambetti, P.; Caughey, B. Bank Vole Prion Protein As an Apparently Universal Substrate for RT-QuIC-Based Detection and Discrimination of Prion Strains. PLoS Pathog. 2015, 11, e1004983. [Google Scholar]
- Meiner, Z.; Gabizon, R.; Prusiner, S.B. Familial Creutzfeldt-Jakob disease. Codon 200 prion disease in Libyan Jews. Medicine (Baltimore) 1997, 76, 227–237. [Google Scholar] [CrossRef]
- Bartz, J.C. Prion Strain Diversity. Cold Spring Harb Perspect Med. 2016, 6. [Google Scholar] [CrossRef]
- Morales, R. Prion strains in mammals: Different conformations leading to disease. PLoS Pathog. 2017, 13, e1006323. [Google Scholar] [CrossRef] [PubMed]
- Korth, C.; Kaneko, K.; Groth, D.; Heye, N.; Telling, G.; Mastrianni, J.; Parchi, P.; Gambetti, P.; Will, R.; Ironside, J.; et al. Abbreviated incubation times for human prions in mice expressing a chimeric mouse-human prion protein transgene. Proc. Natl. Acad. Sci. USA 2003, 100, 4784–4789. [Google Scholar] [CrossRef] [PubMed]
- Jaumain, E.; Quadrio, I.; Herzog, L.; Reine, F.; Rezaei, H.; Andréoletti, O.; Laude, H.; Perret-Liaudet, A.; Haïk, S.; Béringue, V. Absence of Evidence for a Causal Link between Bovine Spongiform Encephalopathy Strain Variant L-BSE and Known Forms of Sporadic Creutzfeldt-Jakob Disease in Human PrP Transgenic Mice. J. Virol. 2016, 90, 10867–10874. [Google Scholar] [CrossRef]
- Watts, J.C.; Giles, K.; Serban, A.; Patel, S.; Oehler, A.; Bhardwaj, S.; Guan, S.; Greicius, M.D.; Miller, B.L.; DeArmond, S.J.; et al. Modulation of Creutzfeldt-Jakob disease prion propagation by the A224V mutation. Ann. Neurol. 2015, 78, 540–553. [Google Scholar] [CrossRef]
- Beck, E.; Daniel, P.M.; Asher, D.M.; Gajdusek, D.C.; Gibbs, C.J. Experimental kuru in the chimpanzee. A neuropathological study. Brain 1973, 96, 441–462. [Google Scholar] [CrossRef]
- Brown, P.; Cathala, F.; Castaigne, P.; Gajdusek, D.C. Creutzfeldt-Jakob disease: clinical analysis of a consecutive series of 230 neuropathologically verified cases. Ann. Neurol. 1986, 20, 597–602. [Google Scholar] [CrossRef]
- Brown, P.; Gibbs, C.J.; Rodgers-Johnson, P.; Asher, D.M.; Sulima, M.P.; Bacote, A.; Goldfarb, L.G.; Gajdusek, D.C. Human spongiform encephalopathy: The National Institutes of Health series of 300 cases of experimentally transmitted disease. Ann. Neurol. 1994, 35, 513–529. [Google Scholar] [CrossRef]
- Cervenáková, L.; Goldfarb, L.G.; Garruto, R.; Lee, H.S.; Gajdusek, D.C.; Brown, P. Phenotype-genotype studies in kuru: implications for new variant Creutzfeldt-Jakob disease. Proc. Natl. Acad. Sci. USA 1998, 95, 13239–13241. [Google Scholar] [CrossRef]
- Kobayashi, A.; Arima, K.; Ogawa, M.; Murata, M.; Fukuda, T.; Kitamoto, T. Plaque-type deposition of prion protein in the damaged white matter of sporadic Creutzfeldt-Jakob disease MM1 patients. Acta Neuropathol. 2008, 116, 561–566. [Google Scholar] [CrossRef]
- Rossi, M.; Saverioni, D.; Di Bari, M.; Baiardi, S.; Lemstra, A.W.; Pirisinu, L.; Capellari, S.; Rozemuller, A.; Nonno, R.; Parchi, P. Atypical Creutzfeldt-Jakob disease with PrP-amyloid plaques in white matter: molecular characterization and transmission to bank voles show the M1 strain signature. Acta Neuropathol. Commun. 2017, 5, 87. [Google Scholar] [CrossRef]
- Hill, A.F.; Desbruslais, M.; Joiner, S.; Sidle, K.C.; Gowland, I.; Collinge, J.; Doey, L.J.; Lantos, P. The same prion strain causes vCJD and BSE. Nature 1997, 389, 448–450, 526. [Google Scholar] [CrossRef]
- Mead, S.; Poulter, M.; Beck, J.; Webb, T.E.F.; Campbell, T.A.; Linehan, J.M.; Desbruslais, M.; Joiner, S.; Wadsworth, J.D.F.; King, A.; et al. Inherited prion disease with six octapeptide repeat insertional mutation--molecular analysis of phenotypic heterogeneity. Brain 2006, 129, 2297–2317. [Google Scholar] [CrossRef]
- Kobayashi, A.; Asano, M.; Mohri, S.; Kitamoto, T. A traceback phenomenon can reveal the origin of prion infection. Neuropathology 2009, 29, 619–624. [Google Scholar] [CrossRef]
- Kobayashi, A.; Asano, M.; Mohri, S.; Kitamoto, T. Cross-sequence transmission of sporadic Creutzfeldt-Jakob disease creates a new prion strain. J. Biol. Chem. 2007, 282, 30022–30028. [Google Scholar] [CrossRef]
- Wadsworth, J.D.F.; Joiner, S.; Linehan, J.M.; Desbruslais, M.; Fox, K.; Cooper, S.; Cronier, S.; Asante, E.A.; Mead, S.; Brandner, S.; et al. Kuru prions and sporadic Creutzfeldt-Jakob disease prions have equivalent transmission properties in transgenic and wild-type mice. Proc. Natl. Acad. Sci. USA 2008, 105, 3885–3890. [Google Scholar] [CrossRef]
- Asante, E.A.; Gowland, I.; Grimshaw, A.; Linehan, J.M.; Smidak, M.; Houghton, R.; Osiguwa, O.; Tomlinson, A.; Joiner, S.; Brandner, S.; et al. Absence of spontaneous disease and comparative prion susceptibility of transgenic mice expressing mutant human prion proteins. J. Gen. Virol. 2009, 90, 546–558. [Google Scholar] [CrossRef] [PubMed]
- Tateishi, J.; Brown, P.; Kitamoto, T.; Hoque, Z.M.; Roos, R.; Wollman, R.; Cervenáková, L.; Gajdusek, D.C. First experimental transmission of fatal familial insomnia. Nature 1995, 376, 434–435. [Google Scholar] [CrossRef]
- Collinge, J.; Palmer, M.S.; Sidle, K.C.; Gowland, I.; Medori, R.; Ironside, J.; Lantos, P. Transmission of fatal familial insomnia to laboratory animals. Lancet 1995, 346, 569–570. [Google Scholar] [CrossRef]
- Mastrianni, J.A.; Nixon, R.; Layzer, R.; Telling, G.C.; Han, D.; DeArmond, S.J.; Prusiner, S.B. Prion protein conformation in a patient with sporadic fatal insomnia. N. Engl. J. Med. 1999, 340, 1630–1638. [Google Scholar] [CrossRef]
- Chapuis, J.; Moudjou, M.; Reine, F.; Herzog, L.; Jaumain, E.; Chapuis, C.; Quadrio, I.; Boulliat, J.; Perret-Liaudet, A.; Dron, M.; et al. Emergence of two prion subtypes in ovine PrP transgenic mice infected with human MM2-cortical Creutzfeldt-Jakob disease prions. Acta Neuropathol. Commun. 2016, 4, 10. [Google Scholar] [CrossRef]
- Kobayashi, A.; Matsuura, Y.; Takeuchi, A.; Yamada, M.; Miyoshi, I.; Mohri, S.; Kitamoto, T. A domain responsible for spontaneous conversion of bank vole prion protein. Brain Pathol. 2019, 29, 155–163. [Google Scholar] [CrossRef]
- Bruce, M.E.; Will, R.G.; Ironside, J.W.; McConnell, I.; Drummond, D.; Suttie, A.; McCardle, L.; Chree, A.; Hope, J.; Birkett, C.; et al. Transmissions to mice indicate that “new variant” CJD is caused by the BSE agent. Nature 1997, 389, 498–501. [Google Scholar] [CrossRef]
- Lasmézas, C.I.; Deslys, J.P.; Robain, O.; Jaegly, A.; Beringue, V.; Peyrin, J.M.; Fournier, J.G.; Hauw, J.J.; Rossier, J.; Dormont, D. Transmission of the BSE agent to mice in the absence of detectable abnormal prion protein. Science 1997, 275, 402–405. [Google Scholar] [CrossRef]
- Bishop, M.T.; Hart, P.; Aitchison, L.; Baybutt, H.N.; Plinston, C.; Thomson, V.; Tuzi, N.L.; Head, M.W.; Ironside, J.W.; Will, R.G.; et al. Predicting susceptibility and incubation time of human-to-human transmission of vCJD. Lancet Neurol. 2006, 5, 393–398. [Google Scholar] [CrossRef]
- Asano, M.; Mohri, S.; Ironside, J.W.; Ito, M.; Tamaoki, N.; Kitamoto, T. vCJD prion acquires altered virulence through trans-species infection. Biochem. Biophys. Res. Commun. 2006, 342, 293–299. [Google Scholar] [CrossRef]
- Wadsworth, J.D.F.; Asante, E.A.; Desbruslais, M.; Linehan, J.M.; Joiner, S.; Gowland, I.; Welch, J.; Stone, L.; Lloyd, S.E.; Hill, A.F.; et al. Human prion protein with valine 129 prevents expression of variant CJD phenotype. Science 2004, 306, 1793–1796. [Google Scholar] [CrossRef]
- Asante, E.A.; Linehan, J.M.; Desbruslais, M.; Joiner, S.; Gowland, I.; Wood, A.L.; Welch, J.; Hill, A.F.; Lloyd, S.E.; Wadsworth, J.D.F.; et al. BSE prions propagate as either variant CJD-like or sporadic CJD-like prion strains in transgenic mice expressing human prion protein. EMBO J. 2002, 21, 6358–6366. [Google Scholar] [CrossRef]
- Scott, M.R.; Will, R.; Ironside, J.; Nguyen, H.O.; Tremblay, P.; DeArmond, S.J.; Prusiner, S.B. Compelling transgenetic evidence for transmission of bovine spongiform encephalopathy prions to humans. Proc. Natl. Acad. Sci. USA 1999, 96, 15137–15142. [Google Scholar] [CrossRef]
- Bian, J.; Khaychuk, V.; Angers, R.C.; Fernández-Borges, N.; Vidal, E.; Meyerett-Reid, C.; Kim, S.; Calvi, C.L.; Bartz, J.C.; Hoover, E.A.; et al. Prion replication without host adaptation during interspecies transmissions. Proc. Natl. Acad. Sci. USA 2017, 114, 1141–1146. [Google Scholar] [CrossRef]
- Collinge, J.; Clarke, A.R. A general model of prion strains and their pathogenicity. Science 2007, 318, 930–936. [Google Scholar] [CrossRef]
- Mok, T.; Jaunmuktane, Z.; Joiner, S.; Campbell, T.; Morgan, C.; Wakerley, B.; Golestani, F.; Rudge, P.; Mead, S.; Jäger, H.R.; et al. Variant Creutzfeldt-Jakob Disease in a Patient with Heterozygosity at PRNP Codon 129. N. Engl. J. Med. 2017, 376, 292–294. [Google Scholar] [CrossRef]
- Cooper, J.D.; Bird, S.M. Predicting incidence of variant Creutzfeldt-Jakob disease from UK dietary exposure to bovine spongiform encephalopathy for the 1940 to 1969 and post-1969 birth cohorts. Int. J. Epidemiol. 2003, 32, 784–791. [Google Scholar] [CrossRef]
- Ritchie, D.L.; Boyle, A.; McConnell, I.; Head, M.W.; Ironside, J.W.; Bruce, M.E. Transmissions of variant Creutzfeldt-Jakob disease from brain and lymphoreticular tissue show uniform and conserved bovine spongiform encephalopathy-related phenotypic properties on primary and secondary passage in wild-type mice. J. Gen. Virol. 2009, 90, 3075–3082. [Google Scholar] [CrossRef]
- Bruce, M.E.; McConnell, I.; Will, R.G.; Ironside, J.W. Detection of variant Creutzfeldt-Jakob disease infectivity in extraneural tissues. Lancet 2001, 358, 208–209. [Google Scholar] [CrossRef]
- Cervenakova, L.; Yakovleva, O.; McKenzie, C.; Kolchinsky, S.; McShane, L.; Drohan, W.N.; Brown, P. Similar levels of infectivity in the blood of mice infected with human-derived vCJD and GSS strains of transmissible spongiform encephalopathy. Transfusion 2003, 43, 1687–1694. [Google Scholar] [CrossRef]
- Wadsworth, J.D.F.; Joiner, S.; Fox, K.; Linehan, J.M.; Desbruslais, M.; Brandner, S.; Asante, E.A.; Collinge, J. Prion infectivity in variant Creutzfeldt-Jakob disease rectum. Gut 2007, 56, 90–94. [Google Scholar] [CrossRef]
- Béringue, V.; Le Dur, A.; Tixador, P.; Reine, F.; Lepourry, L.; Perret-Liaudet, A.; Haïk, S.; Vilotte, J.-L.; Fontés, M.; Laude, H. Prominent and persistent extraneural infection in human PrP transgenic mice infected with variant CJD. PLoS ONE 2008, 3, e1419. [Google Scholar] [CrossRef]
- Herzog, C.; Salès, N.; Etchegaray, N.; Charbonnier, A.; Freire, S.; Dormont, D.; Deslys, J.-P.; Lasmézas, C.I. Tissue distribution of bovine spongiform encephalopathy agent in primates after intravenous or oral infection. Lancet 2004, 363, 422–428. [Google Scholar] [CrossRef]
- Holznagel, E.; Yutzy, B.; Schulz-Schaeffer, W.; Kruip, C.; Hahmann, U.; Bierke, P.; Torres, J.-M.; Kim, Y.-S.; Thomzig, A.; Beekes, M.; et al. Foodborne transmission of bovine spongiform encephalopathy to nonhuman primates. Emerging Infect. Dis. 2013, 19, 712–720. [Google Scholar] [CrossRef]
- Asante, E.A.; Linehan, J.M.; Gowland, I.; Joiner, S.; Fox, K.; Cooper, S.; Osiguwa, O.; Gorry, M.; Welch, J.; Houghton, R.; et al. Dissociation of pathological and molecular phenotype of variant Creutzfeldt-Jakob disease in transgenic human prion protein 129 heterozygous mice. Proc. Natl. Acad. Sci. USA 2006, 103, 10759–10764. [Google Scholar] [CrossRef]
- Fernández-Borges, N.; Espinosa, J.C.; Marín-Moreno, A.; Aguilar-Calvo, P.; Asante, E.A.; Kitamoto, T.; Mohri, S.; Andréoletti, O.; Torres, J.M. Protective Effect of Val129-PrP against Bovine Spongiform Encephalopathy but not Variant Creutzfeldt-Jakob Disease. Emerging Infect. Dis. 2017, 23, 1522–1530. [Google Scholar] [CrossRef]
- Comoy, E.E.; Mikol, J.; Jaffré, N.; Lebon, V.; Levavasseur, E.; Streichenberger, N.; Sumian, C.; Perret-Liaudet, A.; Eloit, M.; Andreoletti, O.; et al. Experimental transfusion of variant CJD-infected blood reveals previously uncharacterised prion disorder in mice and macaque. Nat. Commun. 2017, 8, 1268. [Google Scholar] [CrossRef]
- Tateishi, J.; Kitamoto, T. Inherited prion diseases and transmission to rodents. Brain Pathol. 1995, 5, 53–59. [Google Scholar] [CrossRef]
- Masters, C.L.; Gajdusek, D.C.; Gibbs, C.J. Creutzfeldt-Jakob disease virus isolations from the Gerstmann-Sträussler syndrome with an analysis of the various forms of amyloid plaque deposition in the virus-induced spongiform encephalopathies. Brain 1981, 104, 559–588. [Google Scholar] [CrossRef]
- Manson, J.C.; Jamieson, E.; Baybutt, H.; Tuzi, N.L.; Barron, R.; McConnell, I.; Somerville, R.; Ironside, J.; Will, R.; Sy, M.S.; et al. A single amino acid alteration (101L) introduced into murine PrP dramatically alters incubation time of transmissible spongiform encephalopathy. EMBO J. 1999, 18, 6855–6864. [Google Scholar] [CrossRef]
- Piccardo, P.; Manson, J.C.; King, D.; Ghetti, B.; Barron, R.M. Accumulation of prion protein in the brain that is not associated with transmissible disease. Proc. Natl. Acad. Sci. USA 2007, 104, 4712–4717. [Google Scholar] [CrossRef]
- Piccardo, P.; King, D.; Telling, G.; Manson, J.C.; Barron, R.M. Dissociation of prion protein amyloid seeding from transmission of a spongiform encephalopathy. J. Virol. 2013, 87, 12349–12356. [Google Scholar] [CrossRef]
- Asante, E.A.; Grimshaw, A.; Smidak, M.; Jakubcova, T.; Tomlinson, A.; Jeelani, A.; Hamdan, S.; Powell, C.; Joiner, S.; Linehan, J.M.; et al. Transmission Properties of Human PrP 102L Prions Challenge the Relevance of Mouse Models of GSS. PLoS Pathog. 2015, 11, e1004953. [Google Scholar] [CrossRef]
- Asante, E.A.; Linehan, J.M.; Smidak, M.; Tomlinson, A.; Grimshaw, A.; Jeelani, A.; Jakubcova, T.; Hamdan, S.; Powell, C.; Brandner, S.; et al. Inherited prion disease A117V is not simply a proteinopathy but produces prions transmissible to transgenic mice expressing homologous prion protein. PLoS Pathog. 2013, 9, e1003643. [Google Scholar] [CrossRef]
- Race, B.; Williams, K.; Hughson, A.G.; Jansen, C.; Parchi, P.; Rozemuller, A.J.M.; Chesebro, B. Familial human prion diseases associated with prion protein mutations Y226X and G131V are transmissible to transgenic mice expressing human prion protein. Acta Neuropathol. Commun. 2018, 6, 13. [Google Scholar] [CrossRef]
- Pirisinu, L.; Di Bari, M.A.; D’Agostino, C.; Marcon, S.; Riccardi, G.; Poleggi, A.; Cohen, M.L.; Appleby, B.S.; Gambetti, P.; Ghetti, B.; et al. Gerstmann-Sträussler-Scheinker disease subtypes efficiently transmit in bank voles as genuine prion diseases. Sci. Rep. 2016, 6, 20443. [Google Scholar] [CrossRef]
- Mead, S.; Gandhi, S.; Beck, J.; Caine, D.; Gallujipali, D.; Carswell, C.; Hyare, H.; Joiner, S.; Ayling, H.; Lashley, T.; et al. A novel prion disease associated with diarrhea and autonomic neuropathy. N. Engl. J. Med. 2013, 369, 1904–1914. [Google Scholar] [CrossRef]
- Nonno, R.; Notari, S.; Di Bari, M.A.; Cali, I.; Pirisinu, L.; d’Agostino, C.; Cracco, L.; Kofskey, D.; Vanni, I.; Lavrich, J.; et al. Variable Protease-Sensitive Prionopathy Transmission to Bank Voles. Emerging Infect. Dis. 2019, 25, 73–81. [Google Scholar] [CrossRef]
- Scheckel, C.; Aguzzi, A. Prions, prionoids and protein misfolding disorders. Nat. Rev. Genet. 2018, 19, 405–418. [Google Scholar] [CrossRef]
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).