Avian Flavivirus Enters BHK-21 Cells by a Low pH-Dependent Endosomal Pathway
Abstract
1. Introduction
2. Material and Methods
2.1. Cells and Virus
2.2. Cell Infection and Drug Treatments
2.3. RNA Extraction and RT-qPCR
2.4. Cell Viability Assay
2.5. Confocal Microscopy
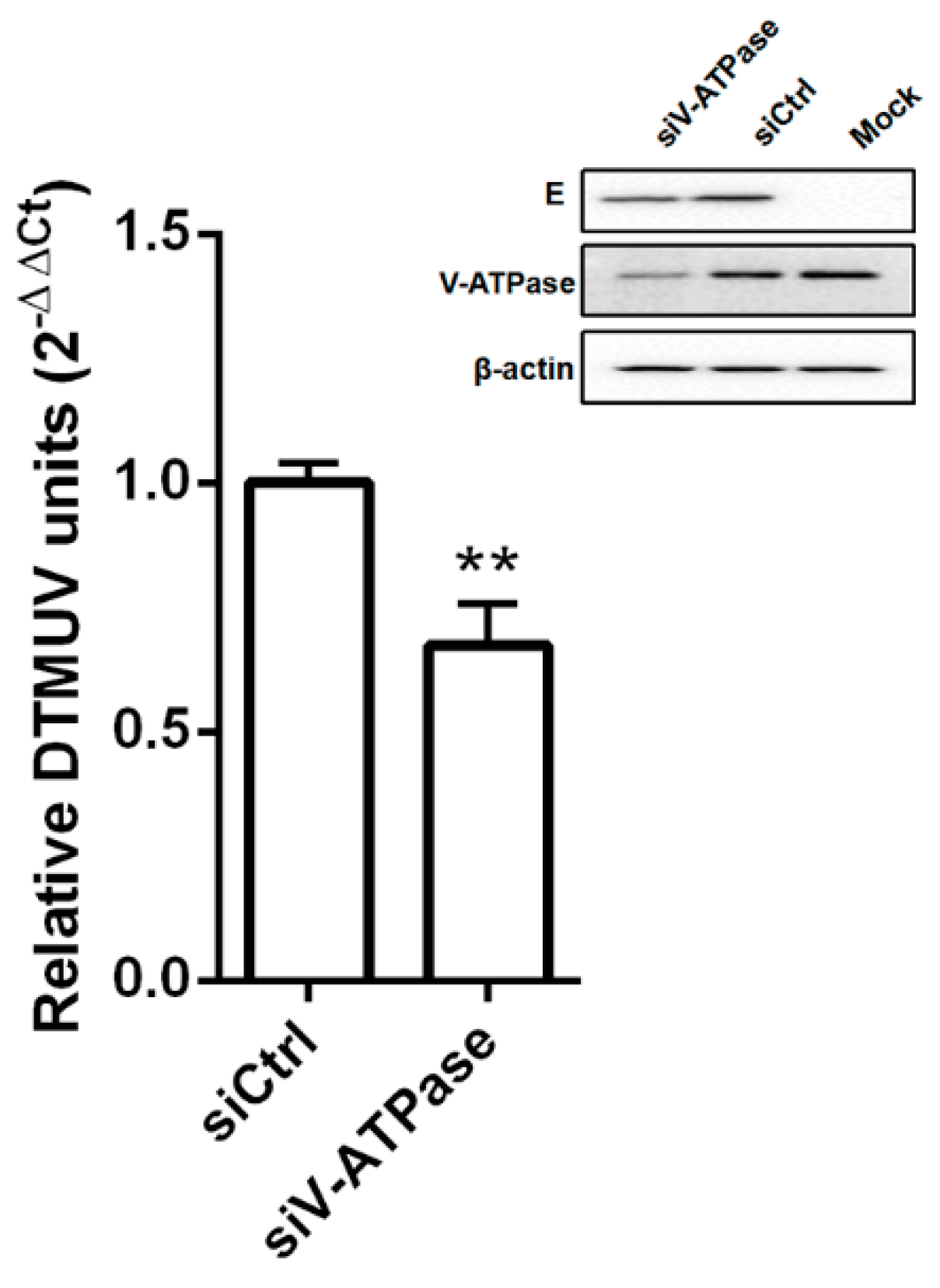
2.6. siRNA Transfections
2.7. Western Blotting
2.8. Statistical Analysis
3. Results
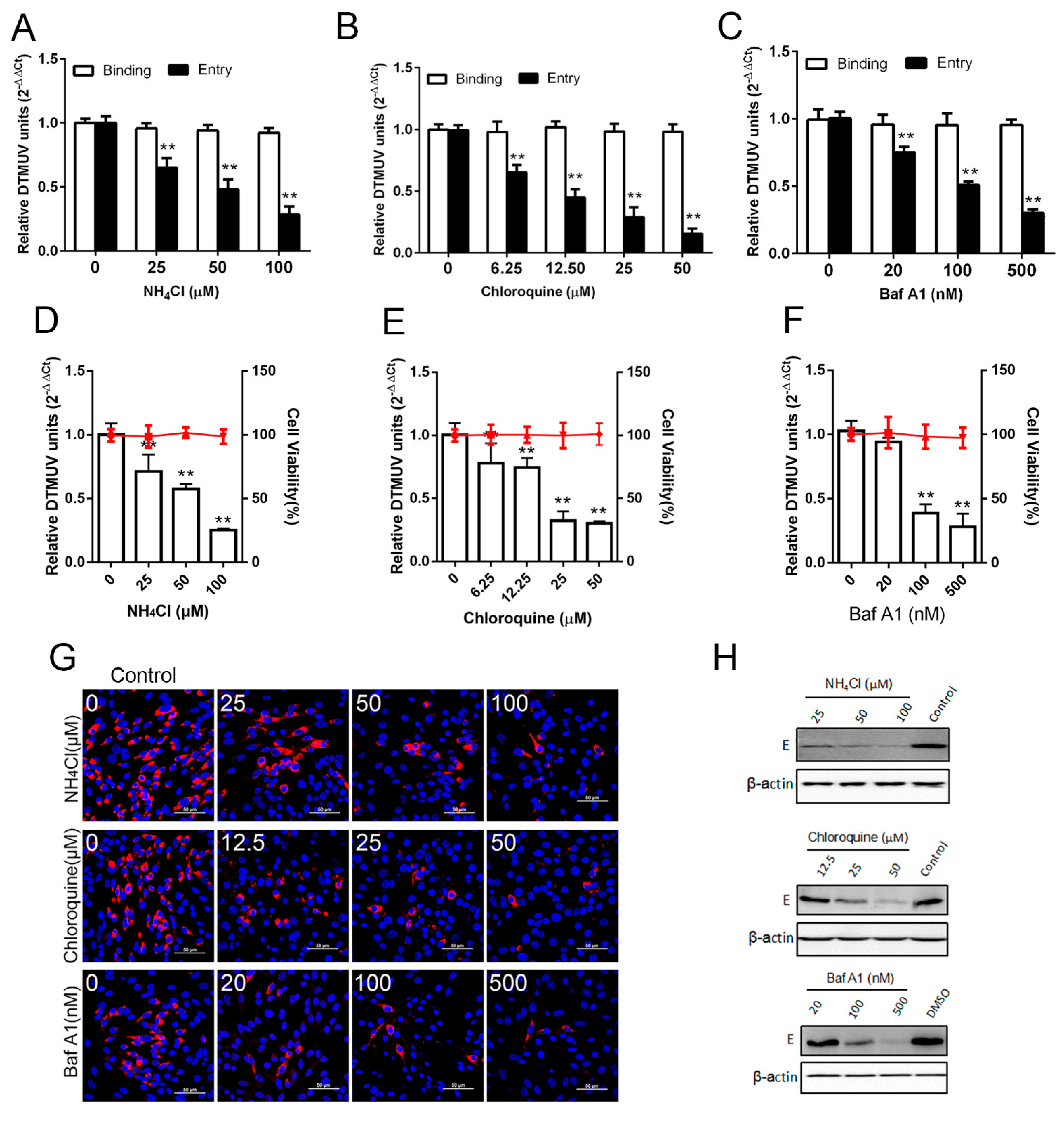
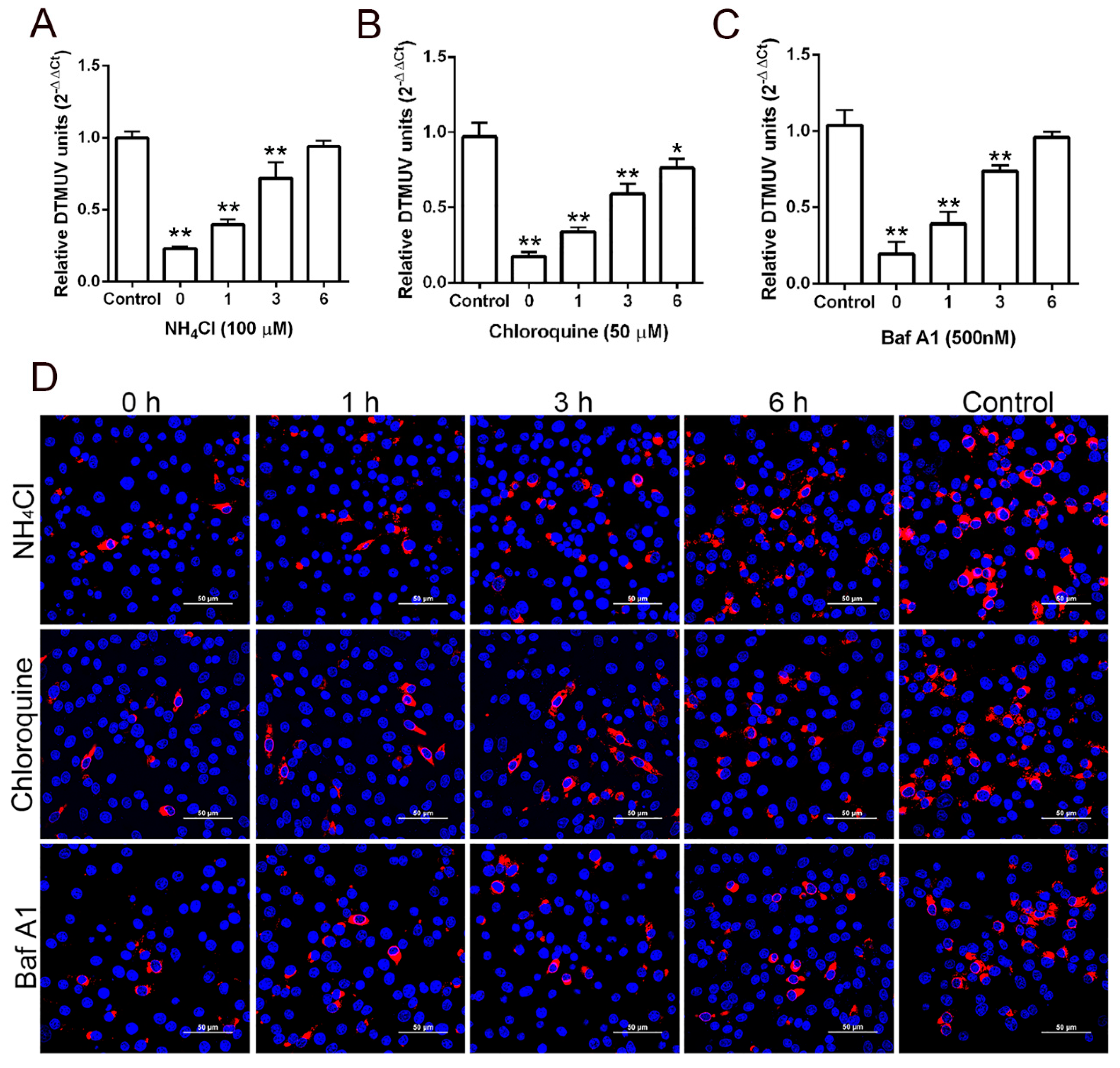
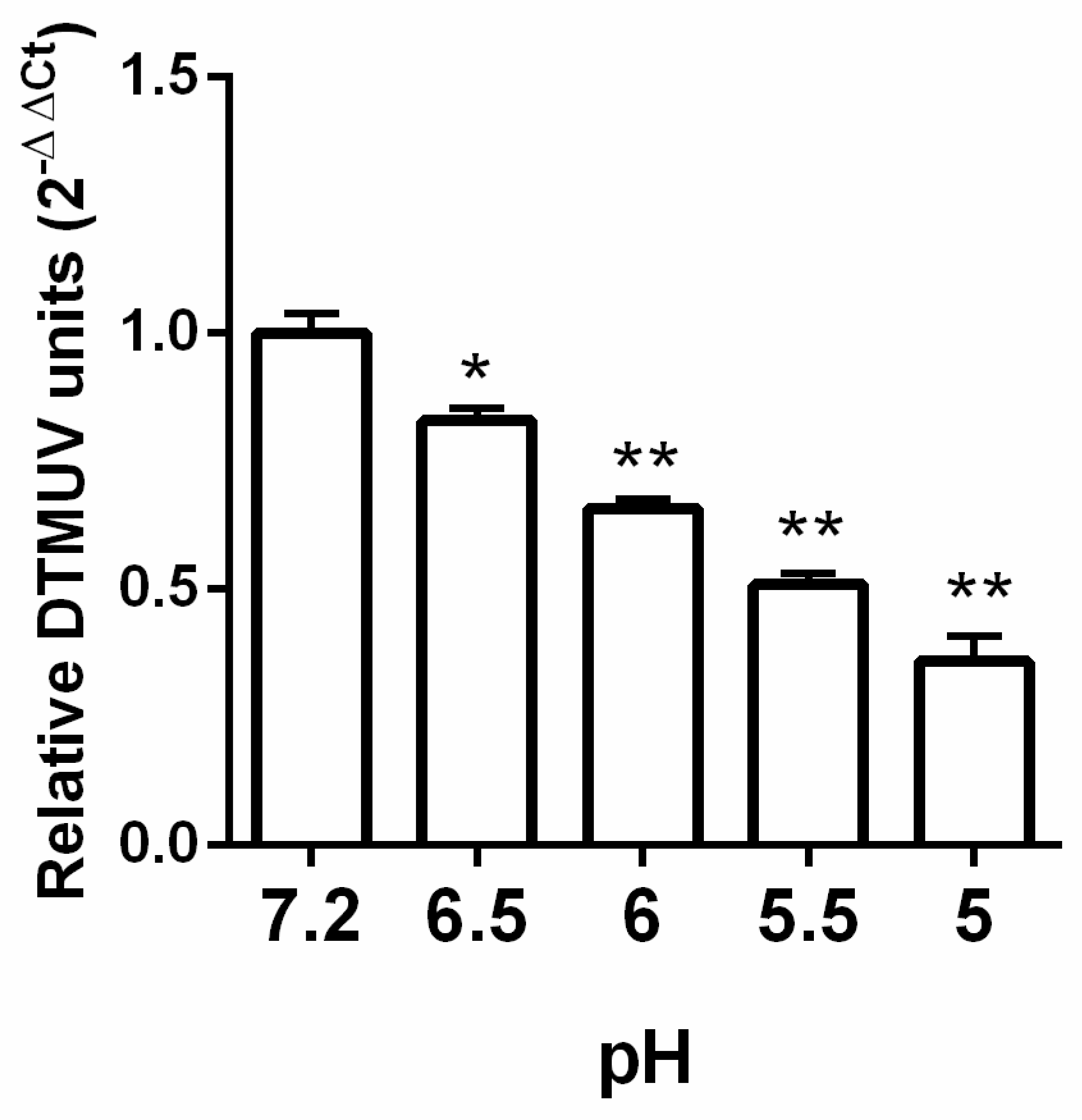
3.1. DTMUV Entry is pH Dependent
3.2. Lysosomotropic Agents Act at an Early Time Point in DTMUV Infection
3.3. Effect of Moderate Acidic pH Pretreatment on DTMUV
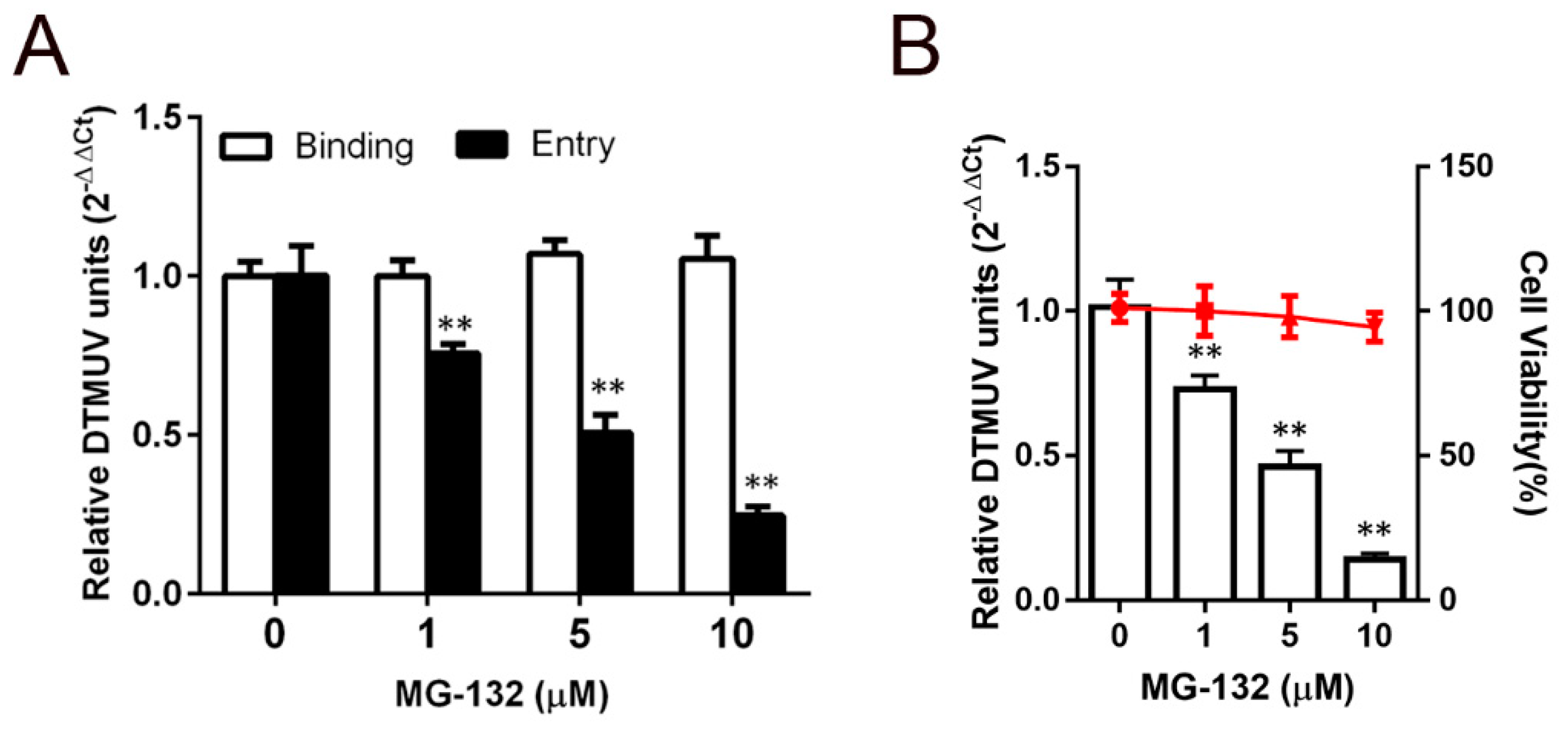
3.4. Effect of the Proteasome on DTMUV Entry
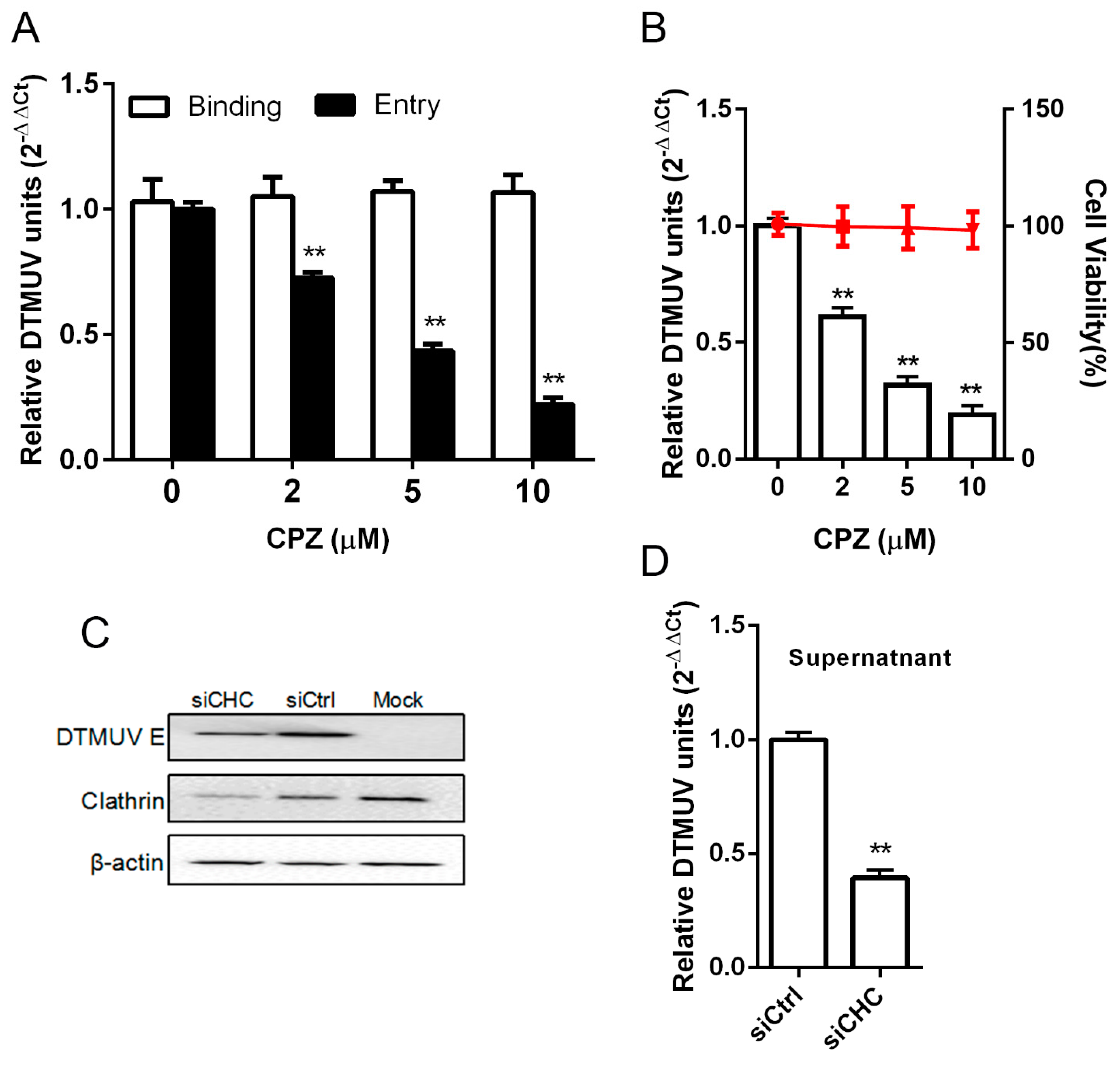
3.5. DTMUV Entry is Clathrin Dependent
4. Discussion
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Liu, M.; Liu, C.; Li, G.; Li, X.; Yin, X.; Chen, Y.; Zhang, Y. Complete Genomic Sequence of Duck Flavivirus from China. J. Virol. 2012, 86, 3398–3399. [Google Scholar] [CrossRef] [PubMed]
- Mukhopadhyay, S.; Kuhn, R.J.; Rossmann, M.G. A structural perspective of the flavivirus life cycle. Nat. Rev. Microbiol. 2005, 3, 13. [Google Scholar] [CrossRef] [PubMed]
- Bai, X.; Lv, R.; Liu, C.; Qiu, N.; He, Y.; Yin, X.; Li, X.; Liu, M.; Zhang, Y. Molecular characterization of a duck Tembusu virus from China. Virus Genes 2013, 47, 478–482. [Google Scholar] [CrossRef] [PubMed]
- Cao, Z.; Zhang, C.; Liu, Y.; Ye, W.; Han, J.; Ma, G.; Zhang, D.; Xu, F.; Gao, X.; Tang, Y. Tembusu virus in ducks, China. Emerg. Infect. Dis. 2011, 17, 1873. [Google Scholar] [CrossRef] [PubMed]
- Liu, P.; Lu, H.; Li, S.; Wu, Y.; Gao, G.F.; Su, J. Duck egg drop syndrome virus: An emerging Tembusu-related flavivirus in China. Sci. China Life Sci. 2013, 56, 701–710. [Google Scholar] [CrossRef] [PubMed]
- Tang, Y.; Gao, X.; Diao, Y.; Feng, Q.; Chen, H.; Liu, X.; Ge, P.; Yu, C. Tembusu virus in human, China. Transbound. Emerg. Dis. 2013, 60, 193–196. [Google Scholar] [CrossRef]
- Chen, S.; Wang, S.; Li, Z.; Lin, F.; Cheng, X.; Zhu, X.; Wang, J.; Chen, S.; Huang, M.; Zheng, M. Isolation and characterization of a Chinese strain of Tembusu virus from Hy-Line Brown layers with acute egg-drop syndrome in Fujian, China. Arch. Virol. 2014, 159, 1099–1107. [Google Scholar] [CrossRef]
- Yu, G.; Lin, Y.; Tang, Y.; Diao, Y. Evolution of Tembusu Virus in Ducks, Chickens, Geese, Sparrows, and Mosquitoes in Northern China. Viruses 2018, 10, 485. [Google Scholar] [CrossRef]
- Wang, J.; Lei, C.-Q.; Ji, Y.; Zhou, H.; Ren, Y.; Peng, Q.; Zeng, Y.; Jia, Y.; Ge, J.; Zhong, B. Duck Tembusu virus nonstructural protein 1 antagonizes IFN-β signaling pathways by targeting VISA. J. Immunol. 2016, 197, 4704–4713. [Google Scholar] [CrossRef]
- Huang, J.; Shen, H.; Jia, R.; Wang, M.; Chen, S.; Zhu, D.; Liu, M.; Zhao, X.; Yang, Q.; Wu, Y. Oral vaccination with a DNA vaccine encoding capsid protein of duck Tembusu virus induces protection immunity. Viruses 2018, 10, 180. [Google Scholar] [CrossRef]
- Kono, Y.; Tsukamoto, K.; Hamid, M.A.; Darus, A.; Lian, T.C.; Sam, L.S.; Yok, C.N.; Di, K.B.; Lim, K.T.; Yamaguchi, S. Encephalitis and retarded growth of chicks caused by Sitiawan virus, a new isolate belonging to the genus Flavivirus. Am. J. Trop. Med. Hyg. 2000, 63, 94–101. [Google Scholar] [CrossRef] [PubMed]
- Yan, D.; Shi, Y.; Wang, H.; Li, G.; Li, X.; Wang, B.; Su, X.; Wang, J.; Teng, Q.; Yang, J. A single mutation at position 156 in the envelope protein of Tembusu virus is responsible for virus tissue tropism and transmissibility in ducks. J. Virol. 2018, 92, e00427-18. [Google Scholar] [CrossRef] [PubMed]
- Alen, M.M.; Schols, D. Dengue virus entry as target for antiviral therapy. J. Trop. Med. 2012, 2012. [Google Scholar] [CrossRef] [PubMed]
- Yamauchi, Y.; Helenius, A. Virus Entry at a Glance; The Company of Biologists Ltd.: Cambridge, UK, 2013. [Google Scholar]
- Burke, D.; Monath, T. Fields Virology; Lippincott Williams & Wilkins: Philadelphia, PA, USA, 2001; pp. 1043–1125. [Google Scholar]
- Smit, J.; Moesker, B.; Rodenhuis-Zybert, I.; Wilschut, J. Flavivirus cell entry and membrane fusion. Viruses 2011, 3, 160–171. [Google Scholar] [CrossRef]
- Brindley, M.A.; Maury, W. Endocytosis and a low-pH step are required for productive entry of equine infectious anemia virus. J. Virol. 2005, 79, 14482–14488. [Google Scholar] [CrossRef]
- Sieczkarski, S.B.; Whittaker, G.R. Dissecting virus entry via endocytosis. J. Gen. Virol. 2002, 83, 1535–1545. [Google Scholar] [CrossRef]
- Nicola, A.V.; Aguilar, H.C.; Mercer, J.; Ryckman, B.; Wiethoff, C.M. Virus entry by endocytosis. Adv. Virol. 2013. [Google Scholar] [CrossRef]
- Livak, K.J.; Schmittgen, T.D. Analysis of relative gene expression data using real-time quantitative PCR and the 2−ΔΔCT method. Methods 2001, 25, 402–408. [Google Scholar] [CrossRef]
- Nicola, A.V.; McEvoy, A.M.; Straus, S.E. Roles for endocytosis and low pH in herpes simplex virus entry into HeLa and Chinese hamster ovary cells. J. Virol. 2003, 77, 5324–5332. [Google Scholar] [CrossRef]
- Sari, T.K.; Pritchard, S.M.; Cunha, C.W.; Wudiri, G.A.; Laws, E.I.; Aguilar, H.C.; Taus, N.S.; Nicola, A.V. Contributions of herpes simplex virus 1 envelope proteins to entry by endocytosis. J. Virol. 2013, 87, 13922–13926. [Google Scholar] [CrossRef]
- De Duve, C.; De Barsy, T.; Poole, B.; Tulkens, P. Lysosomotropic agents. Biochem. Pharmacol. 1974, 23, 2495–2531. [Google Scholar] [CrossRef]
- Lecot, S.; Belouzard, S.; Dubuisson, J.; Rouillé, Y. Bovine viral diarrhea virus entry is dependent on clathrin-mediated endocytosis. J. Virol. 2005, 79, 10826–10829. [Google Scholar] [CrossRef] [PubMed]
- Miller, J.L.; Weed, D.J.; Lee, B.H.; Pritchard, S.M.; Nicola, A.V. Low-pH Endocytic Entry of the Porcine Alphaherpesvirus Pseudorabies Virus. J. Virol. 2019, 93, e01849-18. [Google Scholar] [CrossRef] [PubMed]
- Rosenthal, K.S.; Killius, J.; Hodnichak, C.M.; Venetta, T.M.; Gyurgyik, L.; Janiga, K. Mild acidic pH inhibition of the major pathway of herpes simplex virus entry into HEp-2 cells. J. Gen. Virol. 1989, 70, 857–867. [Google Scholar] [CrossRef] [PubMed]
- Dollery, S.J.; Wright, C.C.; Johnson, D.C.; Nicola, A.V. Low-pH-dependent changes in the conformation and oligomeric state of the prefusion form of herpes simplex virus glycoprotein B are separable from fusion activity. J. Virol. 2011, 85, 9964–9973. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.; Liu, H.; Zu, X.; Liu, Y.; Chen, L.; Zhu, X.; Zhang, L.; Zhou, Z.; Xiao, G.; Wang, W. The ubiquitin-proteasome system is essential for the productive entry of Japanese encephalitis virus. Virology 2016, 498, 116–127. [Google Scholar] [CrossRef] [PubMed]
- Yang, S.; He, M.; Liu, X.; Li, X.; Fan, B.; Zhao, S. Japanese encephalitis virus infects porcine kidney epithelial PK15 cells via clathrin-and cholesterol-dependent endocytosis. Virol. J. 2013, 10, 258. [Google Scholar] [CrossRef]
- Chuang, C.-K.; Yang, T.-H.; Chen, T.-H.; Yang, C.-F.; Chen, W.-J. Heat shock cognate protein 70 isoform D is required for clathrin-dependent endocytosis of Japanese encephalitis virus in C6/36 cells. J. Gen. Virol. 2015, 96, 793–803. [Google Scholar] [CrossRef]
- Zhang, W.; Chen, S.; Mahalingam, S.; Wang, M.; Cheng, A. An updated review of avian-origin Tembusu virus: A newly emerging avian Flavivirus. J. Gen. Virol. 2017, 98, 2413–2420. [Google Scholar] [CrossRef]
- Wu, L.; Liu, J.; Chen, P.; Jiang, Y.; Ding, L.; Lin, Y.; Li, Q.; He, X.; Chen, Q.; Chen, H. The sequential tissue distribution of duck Tembusu virus in adult ducks. BioMed Res. Int. 2014, 2014. [Google Scholar] [CrossRef]
- Nain, M.; Abdin, M.Z.; Kalia, M.; Vrati, S. Japanese encephalitis virus invasion of cell: Allies and alleys. Rev. Med. Virol. 2016, 26, 129–141. [Google Scholar] [CrossRef] [PubMed]
- Liu, C.-C.; Zhang, Y.-N.; Li, Z.-Y.; Hou, J.-X.; Zhou, J.; Kan, L.; Zhou, B.; Chen, P.-Y. Rab5 and Rab11 are required for clathrin-dependent endocytosis of Japanese encephalitis virus in BHK-21 cells. J. Virol. 2017, 91, e01113-17. [Google Scholar] [CrossRef] [PubMed]
- Collins, G.A.; Goldberg, A.L. The logic of the 26S proteasome. Cell 2017, 169, 792–806. [Google Scholar] [CrossRef] [PubMed]
- Pastenkos, G.; Lee, B.; Pritchard, S.M.; Nicola, A.V. Bovine herpesvirus 1 entry by a low-pH endosomal pathway. J. Virol. 2018, 92, e00839-18. [Google Scholar] [CrossRef]
- Cheng, S.; Yan, W.; Gu, W.; He, Q. The ubiquitin-proteasome system is required for the early stages of porcine circovirus type 2 replication. Virology 2014, 456, 198–204. [Google Scholar] [CrossRef]
- Wang, H.; Liu, W.; Sun, M.; Chen, D.; Zeng, L.; Lu, L.; Xie, J. Inhibitor analysis revealed that clathrin-mediated endocytosis is involed in cellular entry of type III grass carp reovirus. Virol. J. 2018, 15, 92. [Google Scholar] [CrossRef]
- Zhang, Y.-N.; Liu, Y.-Y.; Xiao, F.-C.; Liu, C.-C.; Liang, X.-D.; Chen, J.; Zhou, J.; Baloch, A.S.; Kan, L.; Zhou, B.; et al. Rab5, Rab7, and Rab11 Are Required for Caveola-Dependent Endocytosis of Classical Swine Fever Virus in Porcine Alveolar Macrophages. J. Virol. 2018, 92, e00797-18. [Google Scholar] [CrossRef]
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Baloch, A.S.; Liu, C.; Liang, X.; Liu, Y.; Chen, J.; Cao, R.; Zhou, B. Avian Flavivirus Enters BHK-21 Cells by a Low pH-Dependent Endosomal Pathway. Viruses 2019, 11, 1112. https://doi.org/10.3390/v11121112
Baloch AS, Liu C, Liang X, Liu Y, Chen J, Cao R, Zhou B. Avian Flavivirus Enters BHK-21 Cells by a Low pH-Dependent Endosomal Pathway. Viruses. 2019; 11(12):1112. https://doi.org/10.3390/v11121112
Chicago/Turabian StyleBaloch, Abdul Sattar, Chunchun Liu, Xiaodong Liang, Yayun Liu, Jing Chen, Ruibing Cao, and Bin Zhou. 2019. "Avian Flavivirus Enters BHK-21 Cells by a Low pH-Dependent Endosomal Pathway" Viruses 11, no. 12: 1112. https://doi.org/10.3390/v11121112
APA StyleBaloch, A. S., Liu, C., Liang, X., Liu, Y., Chen, J., Cao, R., & Zhou, B. (2019). Avian Flavivirus Enters BHK-21 Cells by a Low pH-Dependent Endosomal Pathway. Viruses, 11(12), 1112. https://doi.org/10.3390/v11121112




