Identification of Novel Natural Products as Effective and Broad-Spectrum Anti-Zika Virus Inhibitors
Abstract
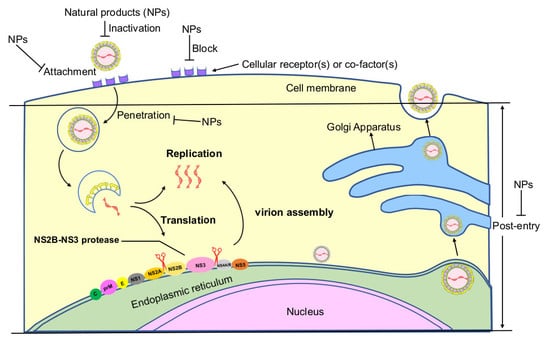
:1. Introduction
2. Materials and Methods
2.1. Cells and Viruses
2.2. Antiviral Activity of Natural Products
2.3. Detection of In Vitro Cytotoxicity
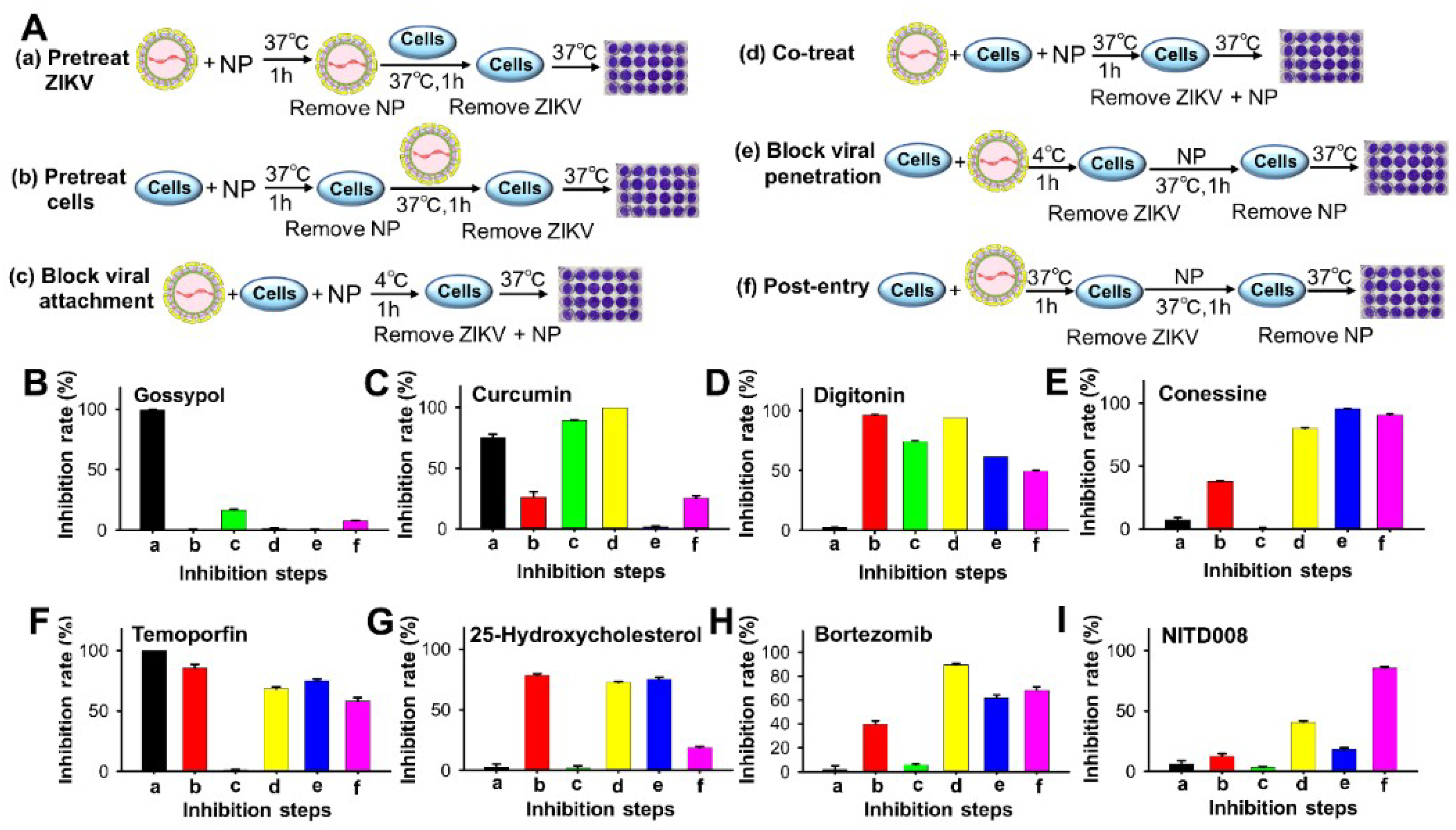
2.4. Time-of-Addition Experiment
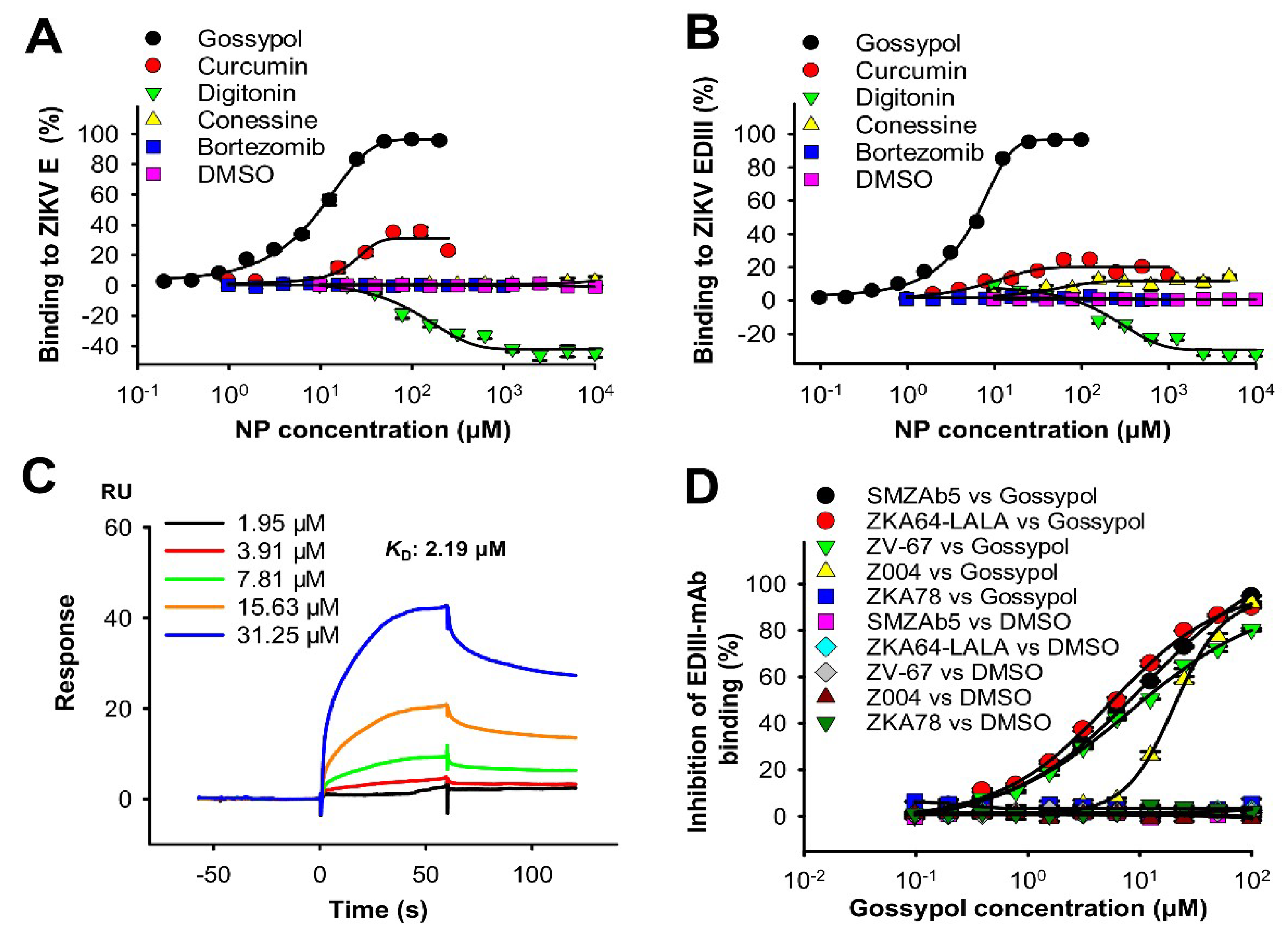
2.5. ELISA
2.6. Surface Plasmon Resonance (SPR)
2.7. Combinatorial Effects of Gossypol with Other Natural Products against ZIKV Infection
3. Results
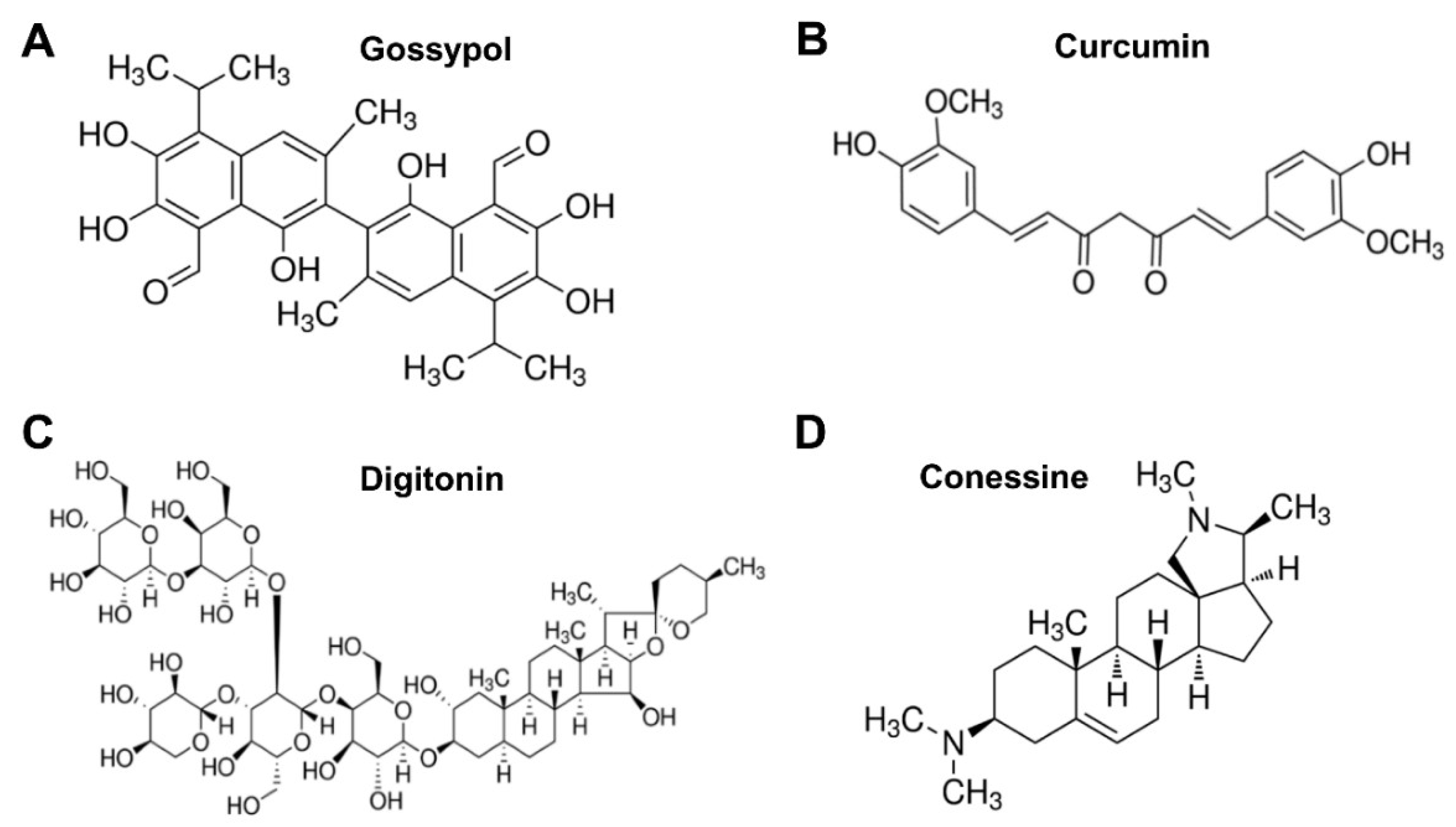
3.1. Identification of Lead Natural Products with Broad-Spectrum Activity against Multiple ZIKV Strains
3.2. Inhibition Mechanisms of Lead Natural Products against ZIKV Infection
3.3. Identification of Potential Binding Regions of Lead Natural Products in ZIKV Proteins
3.4. Combinatorial Effects of the Combinations of Gossypol with Other Natural Products against ZIKV Infection and Their Cytotoxicity to Vero E6 Cells
3.5. Potent Inhibitory Activity of Lead Natural Products, Particularly Gossypol, against Infections of DENV-1–4 Strains
4. Discussion
Supplementary Materials
Author Contributions
Acknowledgments
Conflicts of Interest
References
- Lazear, H.M.; Diamond, M.S. Zika virus: New clinical syndromes and its emergence in the Western Hemisphere. J. Virol. 2016, 90, 4864–4875. [Google Scholar] [CrossRef] [PubMed]
- Dick, G.W.; Kitchen, S.F.; Haddow, A.J. Zika virus (I). Isolations and serological specificity. Trans. R. Soc. Trop. Med. Hyg. 1952, 46, 509–520. [Google Scholar] [CrossRef]
- Zorrilla, C.D.; Garcia Garcia, I.; Garcia Fragoso, L.; De La Vega, A. Zika virus infection in pregnancy: Maternal, fetal, and neonatal considerations. J. Infect. Dis. 2017, 216, S891–S896. [Google Scholar] [CrossRef] [PubMed]
- Castro, M.C.; Han, Q.C.; Carvalho, L.R.; Victora, C.G.; Franca, G.V.A. Implications of Zika virus and congenital Zika syndrome for the number of live births in Brazil. Proc. Natl. Acad. Sci. USA 2018, 115, 6177–6182. [Google Scholar] [CrossRef] [Green Version]
- Lucey, D.; Cummins, H.; Sholts, S. Congenital Zika syndrome in 2017. JAMA 2017, 317, 1368–1369. [Google Scholar] [CrossRef]
- Driggers, R.W.; Ho, C.Y.; Korhonen, E.M.; Kuivanen, S.; Jaaskelainen, A.J.; Smura, T.; Rosenberg, A.; Hill, D.A.; DeBiasi, R.L.; Vezina, G.; et al. Zika virus infection with prolonged maternal viremia and fetal brain abnormalities. N. Engl. J. Med. 2016, 374, 2142–2151. [Google Scholar] [CrossRef]
- Brasil, P.; Pereira, J.P., Jr.; Moreira, M.E.; Ribeiro Nogueira, R.M.; Damasceno, L.; Wakimoto, M.; Rabello, R.S.; Valderramos, S.G.; Halai, U.A.; Salles, T.S.; et al. Zika virus infection in pregnant women in Rio de Janeiro. N. Engl. J. Med. 2016, 375, 2321–2334. [Google Scholar] [CrossRef]
- Kuno, G.; Chang, G.J. Full-length sequencing and genomic characterization of Bagaza, Kedougou, and Zika viruses. Arch. Virol. 2007, 152, 687–696. [Google Scholar] [CrossRef]
- Agrelli, A.; de Moura, R.R.; Crovella, S.; Brandao, L.A.C. ZIKA virus entry mechanisms in human cells. Infect. Genet. Evol. 2019, 69, 22–29. [Google Scholar] [CrossRef]
- Abrams, R.P.M.; Solis, J.; Nath, A. Therapeutic approaches for Zika virus infection of the nervous system. Neurotherapeutics 2017, 14, 1027–1048. [Google Scholar] [CrossRef]
- Lian, W.; Jang, J.; Potisopon, S.; Li, P.C.; Rahmeh, A.; Wang, J.; Kwiatkowski, N.P.; Gray, N.S.; Yang, P.L. Discovery of immunologically inspired small molecules that target the viral envelope protein. ACS Infect. Dis. 2018, 4, 1395–1406. [Google Scholar] [CrossRef] [PubMed]
- De Wispelaere, M.; Lian, W.; Potisopon, S.; Li, P.C.; Jang, J.; Ficarro, S.B.; Clark, M.J.; Zhu, X.; Kaplan, J.B.; Pitts, J.D.; et al. Inhibition of flaviviruses by targeting a conserved pocket on the viral envelope protein. Cell Chem. Biol. 2018, 25, 1006–1016. [Google Scholar] [CrossRef] [PubMed]
- Culshaw, A.; Mongkolsapaya, J.; Screaton, G.R. The immunopathology of dengue and Zika virus infections. Curr. Opin. Immunol. 2017, 48, 1–6. [Google Scholar] [CrossRef] [PubMed]
- Guzman, M.G.; Halstead, S.B.; Artsob, H.; Buchy, P.; Farrar, J.; Gubler, D.J.; Hunsperger, E.; Kroeger, A.; Margolis, H.S.; Martinez, E.; et al. Dengue: A continuing global threat. Nat. Rev. Microbiol. 2010, 8, S7–S16. [Google Scholar] [CrossRef] [PubMed]
- Tai, W.; He, L.; Wang, Y.; Sun, S.; Zhao, G.; Luo, C.; Li, P.; Zhao, H.; Fremont, D.H.; Li, F.; et al. Critical neutralizing fragment of Zika virus EDIII elicits cross-neutralization and protection against divergent Zika viruses. Emerg. Microbes Infect. 2018, 7, 7. [Google Scholar] [CrossRef] [PubMed]
- Barrows, N.J.; Campos, R.K.; Powell, S.T.; Prasanth, K.R.; Schott-Lerner, G.; Soto-Acosta, R.; Galarza-Munoz, G.; McGrath, E.L.; Urrabaz-Garza, R.; Gao, J.; et al. A screen of FDA-approved drugs for inhibitors of Zika virus infection. Cell Host Microbe 2016, 20, 259–270. [Google Scholar] [CrossRef] [PubMed]
- Tai, W.; Chen, J.; Zhao, G.; Geng, Q.; He, L.; Chen, Y.; Zhou, Y.; Li, F.; Du, L. Rational design of Zika virus subunit vaccine with enhanced efficacy. J. Virol. 2019, 93, e02187-18. [Google Scholar] [CrossRef]
- Tai, W.; Voronin, D.; Chen, J.; Bao, W.; Kessler, D.A.; Shaz, B.; Jiang, S.; Yazdanbakhsh, K.; Du, L. Transfusion-transmitted Zika virus infection in pregnant mice leads to broad tissue tropism with severe placental damage and fetal demise. Front. Microbiol. 2019, 10, 29. [Google Scholar] [CrossRef]
- Jiang, S.; Lu, H.; Liu, S.; Zhao, Q.; He, Y.; Debnath, A.K. N-substituted pyrrole derivatives as novel human immunodeficiency virus type 1 entry inhibitors that interfere with the gp41 six-helix bundle formation and block virus fusion. Antimicrob. Agents Chemother. 2004, 48, 4349–4359. [Google Scholar] [CrossRef]
- Chou, T.C. Theoretical basis, experimental design, and computerized simulation of synergism and antagonism in drug combination studies. Pharmacol. Rev. 2006, 58, 621–681. [Google Scholar] [CrossRef]
- Si, L.; Meng, K.; Tian, Z.; Sun, J.; Li, H.; Zhang, Z.; Soloveva, V.; Li, H.; Fu, G.; Xia, Q. Triterpenoids manipulate a broad range of virus-host fusion via wrapping the HR2 domain prevalent in viral envelopes. Sci. Adv. 2018, 4, eaau8408. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chen, M.; Aoki-Utsubo, C.; Kameoka, M.; Deng, L.; Terada, Y.; Kamitani, W.; Sato, K.; Koyanagi, Y.; Hijikata, M.; Shindo, K.; et al. Broad-spectrum antiviral agents: Secreted phospholipase A2 targets viral envelope lipid bilayers derived from the endoplasmic reticulum membrane. Sci. Rep. 2017, 7, 15931. [Google Scholar] [CrossRef] [PubMed]
- Basu, A.; Li, B.; Mills, D.M.; Panchal, R.G.; Cardinale, S.C.; Butler, M.M.; Peet, N.P.; Majgier-Baranowska, H.; Williams, J.D.; Patel, I.; et al. Identification of a small-molecule entry inhibitor for filoviruses. J. Virol. 2011, 85, 3106–3119. [Google Scholar] [CrossRef] [PubMed]
- Yu, M.; Si, L.; Wang, Y.; Wu, Y.; Yu, F.; Jiao, P.; Shi, Y.; Wang, H.; Xiao, S.; Fu, G.; et al. Discovery of pentacyclic triterpenoids as potential entry inhibitors of influenza viruses. J. Med. Chem. 2014, 57, 10058–10071. [Google Scholar] [CrossRef] [PubMed]
- Lu, L.; Pan, C.; Li, Y.; Lu, H.; He, W.; Jiang, S. A bivalent recombinant protein inactivates HIV-1 by targeting the gp41 prehairpin fusion intermediate induced by CD4 D1D2 domains. Retrovirology 2012, 9, 104. [Google Scholar] [CrossRef] [PubMed]
- Yu, Y.; Deng, Y.Q.; Zou, P.; Wang, Q.; Dai, Y.; Yu, F.; Du, L.; Zhang, N.N.; Tian, M.; Hao, J.N.; et al. A peptide-based viral inactivator inhibits Zika virus infection in pregnant mice and fetuses. Nat. Commun. 2017, 8, 15672. [Google Scholar] [CrossRef]
- Aoki-Utsubo, C.; Chen, M.; Hotta, H.J.B.-P. Time-of-addition and temperature-shift assays to determine particular step(s) in the viral life cycle that is blocked by antiviral substance(s). Bio-Protocol 2018, 8, e2830. [Google Scholar] [CrossRef]
- Li, Z.; Brecher, M.; Deng, Y.-Q.; Zhang, J.; Sakamuru, S.; Liu, B.; Huang, R.; Koetzner, C.A.; Allen, C.A.; Jones, S.A.; et al. Existing drugs as broad-spectrum and potent inhibitors for Zika virus by targeting NS2B-NS3 interaction. Cell Res. 2017, 27, 1046–1064. [Google Scholar] [CrossRef] [Green Version]
- Li, C.; Deng, Y.Q.; Wang, S.; Ma, F.; Aliyari, R.; Huang, X.Y.; Zhang, N.N.; Watanabe, M.; Dong, H.L.; Liu, P.; et al. 25-Hydroxycholesterol protects host against Zika virus infection and its associated microcephaly in a mouse model. Immunity 2017, 46, 446–456. [Google Scholar] [CrossRef]
- Deng, Y.Q.; Zhang, N.N.; Li, C.F.; Tian, M.; Hao, J.N.; Xie, X.P.; Shi, P.Y.; Qin, C.F. Adenosine analog NITD008 is a potent inhibitor of Zika virus. Open Forum. Infect. Dis. 2016, 3, ofw175. [Google Scholar] [CrossRef]
- Du, L.; Tai, W.; Yang, Y.; Zhao, G.; Zhu, Q.; Sun, S.; Liu, C.; Tao, X.; Tseng, C.K.; Perlman, S.; et al. Introduction of neutralizing immunogenicity index to the rational design of MERS coronavirus subunit vaccines. Nat. Commun. 2016, 7, 13473. [Google Scholar] [CrossRef] [PubMed]
- He, L.; Tai, W.; Li, J.; Chen, Y.; Gao, Y.; Li, J.; Sun, S.; Zhou, Y.; Du, L.; Zhao, G. Enhanced ability of oligomeric nanobodies targeting MERS coronavirus receptor-binding domain. Viruses 2019, 11, 166. [Google Scholar] [CrossRef] [PubMed]
- Stettler, K.; Beltramello, M.; Espinosa, D.A.; Graham, V.; Cassotta, A.; Bianchi, S.; Vanzetta, F.; Minola, A.; Jaconi, S.; Mele, F.; et al. Specificity, cross-reactivity, and function of antibodies elicited by Zika virus infection. Science 2016, 353, 823–826. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhao, G.; He, L.; Sun, S.; Qiu, H.; Tai, W.; Chen, J.; Li, J.; Chen, Y.; Guo, Y.; Wang, Y.; et al. A novel nanobody targeting Middle East respiratory syndrome coronavirus (MERS-CoV) receptor-binding domain has potent cross-neutralizing activity and protective efficacy against MERS-CoV. J. Virol. 2018, 92, e00837-18. [Google Scholar] [CrossRef]
- Qi, Q.; Wang, Q.; Chen, W.; Yu, F.; Du, L.; Dimitrov, D.S.; Lu, L.; Jiang, S. Anti-HIV antibody and drug combinations exhibit synergistic activity against drug-resistant HIV-1 strains. J. Infect. 2017, 75, 68–71. [Google Scholar] [CrossRef]
- Wang, C.; Hua, C.; Xia, S.; Li, W.; Lu, L.; Jiang, S. Combining a fusion inhibitory peptide targeting the MERS-CoV S2 protein HR1 domain and a neutralizing antibody specific for the S1 protein receptor-binding domain (RBD) showed potent synergism against pseudotyped MERS-CoV with or without mutations in RBD. Viruses 2019, 11, 31. [Google Scholar] [CrossRef]
- Mounce, B.C.; Cesaro, T.; Carrau, L.; Vallet, T.; Vignuzzi, M. Curcumin inhibits Zika and chikungunya virus infection by inhibiting cell binding. Antivir. Res. 2017, 142, 148–157. [Google Scholar] [CrossRef]
- Padilla, S.L.; Rodriguez, A.; Gonzales, M.M.; Gallego, G.J.; Castano, O.J. Inhibitory effects of curcumin on dengue virus type 2-infected cells in vitro. Arch. Virol. 2014, 159, 573–579. [Google Scholar] [CrossRef]
- Ravichandran, S.; Hahn, M.; Belaunzarán-Zamudio, P.F.; Ramos-Castañeda, J.; Nájera-Cancino, G.; Caballero-Sosa, S.; Navarro-Fuentes, K.R.; Ruiz-Palacios, G.; Golding, H.; Beigel, J.H. Differential human antibody repertoires following Zika infection and the implications for serodiagnostics and disease outcome. Nat. Commun. 2019, 10, 1943. [Google Scholar] [CrossRef]
- Zhao, H.; Fernandez, E.; Dowd, K.A.; Speer, S.D.; Platt, D.J.; Gorman, M.J.; Govero, J.; Nelson, C.A.; Pierson, T.C.; Diamond, M.S. Structural basis of Zika virus-specific antibody protection. Cell 2016, 166, 1016–1027. [Google Scholar] [CrossRef]
- Robbiani, D.F.; Bozzacco, L.; Keeffe, J.R.; Khouri, R.; Olsen, P.C.; Gazumyan, A.; Schaefer-Babajew, D.; Avila-Rios, S.; Nogueira, L.; Patel, R. Recurrent potent human neutralizing antibodies to Zika virus in Brazil and Mexico. Cell 2017, 169, 597–609. [Google Scholar] [CrossRef] [PubMed]
- Cauchemez, S.; Besnard, M.; Bompard, P.; Dub, T.; Guillemette-Artur, P.; Eyrolle-Guignot, D.; Salje, H.; Van Kerkhove, M.D.; Abadie, V.; Garel, C.J.T.L. Association between Zika virus and microcephaly in French Polynesia, 2013–2015: A retrospective study. Lancet 2016, 387, 2125–2132. [Google Scholar] [CrossRef]
- De Oliveira, W.K.; de Franca, G.V.A.; Carmo, E.H.; Duncan, B.B.; de Souza Kuchenbecker, R.; Schmidt, M.I. Infection-related microcephaly after the 2015 and 2016 Zika virus outbreaks in Brazil: A surveillance-based analysis. Lancet 2017, 390, 861–870. [Google Scholar] [CrossRef]
- Krauer, F.; Riesen, M.; Reveiz, L.; Oladapo, O.T.; Martinez-Vega, R.; Porgo, T.V.; Haefliger, A.; Broutet, N.J.; Low, N.; WHO Zika Causality Working Group. Zika virus infection as a cause of congenital brain abnormalities and Guillain–Barré syndrome: Systematic review. PLoS Med. 2017, 14, e1002203. [Google Scholar] [CrossRef] [PubMed]
- Salinas, J.L.; Walteros, D.M.; Styczynski, A.; Garzón, F.; Quijada, H.; Bravo, E.; Chaparro, P.; Madero, J.; Acosta-Reyes, J.; Ledermann, J.; et al. Zika virus disease-associated Guillain-Barré syndrome—Barranquilla, Colombia 2015–2016. J. Neurol. Sci. 2017, 381, 272–277. [Google Scholar] [CrossRef] [PubMed]
- Dorsett, P.H.; Kerstine, E.E.; Powers, L.J. Letter: Antiviral activity of gossypol and apogossypol. J. Pharm. Sci. 1975, 64, 1073–1075. [Google Scholar] [CrossRef]
- Royer, R.E.; Deck, L.M.; Vander Jagt, T.J.; Martinez, F.J.; Mills, R.G.; Young, S.A.; Vander Jagt, D.L. Synthesis and anti-HIV activity of 1,1′-dideoxygossypol and related compounds. J. Med. Chem. 1995, 38, 2427–2432. [Google Scholar] [CrossRef]
- Goriunova, L.V.; Vichkanova, S.A. Study of antiviral effect of gossypol on chick embryo model. Farmakol. Toksikol. 1969, 32, 615–617. [Google Scholar]
- Lin, T.S.; Schinazi, R.; Griffith, B.; August, E.; Eriksson, B.; Zheng, D.; Huang, L.; Prusoff, W. Selective inhibition of human immunodeficiency virus type 1 replication by the (-) but not the (+) enantiomer of gossypol. Antimicrob. Agents Chemother. 1989, 33, 2149–2151. [Google Scholar] [CrossRef] [Green Version]
- Dodou, K.; Anderson, R.J.; Small, D.A.; Groundwater, P.W. Investigations on gossypol: Past and present developments. Expert Opin. Investig. Drugs 2005, 14, 1419–1434. [Google Scholar] [CrossRef]
- Kovacic, P. Mechanism of drug and toxic actions of gossypol: Focus on reactive oxygen species and electron transfer. Curr. Med. Chem. 2003, 10, 2711–2718. [Google Scholar] [CrossRef] [PubMed]
- Zbidah, M.; Lupescu, A.; Shaik, N.; Lang, F. Gossypol-induced suicidal erythrocyte death. Toxicology 2012, 302, 101–105. [Google Scholar] [CrossRef] [PubMed]
- Esparis-Ogando, A.; Zurzolo, C.; Rodriguez-Boulan, E. Permeabilization of MDCK cells with cholesterol binding agents: Dependence on substratum and confluency. Am. J. Physiol. 1994, 267, C166–C176. [Google Scholar] [CrossRef] [PubMed]
- Keukens, E.A.; de Vrije, T.; Jansen, L.A.; de Boer, H.; Janssen, M.; de Kroon, A.I.; Jongen, W.M.; de Kruijff, B. Glycoalkaloids selectively permeabilize cholesterol containing biomembranes. Biochim. Biophys. Acta 1996, 1279, 243–250. [Google Scholar] [CrossRef] [Green Version]
- Xie, M.; Low, M.G. Streptolysin-O induces release of glycosylphosphatidylinositol-anchored alkaline phosphatase from ROS cells by vesiculation independently of phospholipase action. Biochem. J. 1995, 305, 529–537. [Google Scholar] [CrossRef]
- Banerjee, P.; Joo, J.B.; Buse, J.T.; Dawson, G. Differential solubilization of lipids along with membrane proteins by different classes of detergents. Chem. Phys. Lipids 1995, 77, 65–78. [Google Scholar] [CrossRef]
- Harder, T.; Kellner, R.; Parton, R.G.; Gruenberg, J. Specific release of membrane-bound annexin II and cortical cytoskeletal elements by sequestration of membrane cholesterol. Mol. Biol. Cell 1997, 8, 533–545. [Google Scholar] [CrossRef]
- Dua, V.K.; Verma, G.; Singh, B.; Rajan, A.; Bagai, U.; Agarwal, D.D.; Gupta, N.C.; Kumar, S.; Rastogi, A. Anti-malarial property of steroidal alkaloid conessine isolated from the bark of Holarrhena antidysenterica. Malar. J. 2013, 12, 194. [Google Scholar] [CrossRef]
- Merin, N.; Kelly, K. Clinical use of proteasome inhibitors in the treatment of multiple myeloma. Pharmaceuticals 2015, 8, 1–20. [Google Scholar] [CrossRef]

| Parameters | No. (%) |
|---|---|
| Natural products from MicroSource Discovery Systems for screening | 720 |
| Primary hits (with inhibition against ZIKV strain PAN2016) a | 61 (8.5) |
| Primary hits (with high cytotoxicity in Vero E6 cells) b | 38 (5.3) |
| Specific hits (primary hits with low cytotoxicity) | 23 (3.2) |
| Specific hits (available to purchase) | 16 (2.2) |
| Specific hits (ordered from Sigma and retested to confirm anti-ZIKV activity) | 6 (0.8) |
| Specific hits displaying IC50 < CC50 | 4 (0.6) |
| Natural | IC50 (μM) | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Products | CC50 (μM) | PAN2016 | R116265 | PAN2015 | FLR | R103451 | PRVABC59 | PLCal_ZV | IbH 30656 | MEX 2–81 | MR 766 |
| Gossypol | 14.17 ± 0.74 | 3.48 ± 0.03 | 4.20 ± 0.08 | 3.95 ± 0.05 | 0.21 ± 0.01 | 2.28 ± 0.10 | 4.31 ± 0.02 | 1.98 ± 0.07 | 3.31 ± 0.11 | 2.79 ± 0.01 | 3.75 ± 0.01 |
| Curcumin | 52.86 ± 0.52 | 13.67 ± 0.72 | 14.04 ± 0.15 | 13.71 ± 0.37 | 16.57 ± 0.34 | 11.22 ± 0.37 | 12.85 ± 0.35 | 10.84 ± 0.73 | 13.63 ± 0.31 | 5.62 ± 0.52 | 11.42 ± 0.29 |
| Digitonin | 56.29 ± 1.20 | 4.31 ± 0.23 | 6.52 ± 0.59 | 5.00 ± 0.01 | 3.34 ± 0.22 | 4.30 ± 0.43 | 3.76 ± 1.12 | 3.19 ± 0.25 | 5.5.30 ± 0.13 | 3.84 ± 0.12 | 3.77 ± 0.31 |
| Conessine | 323.71 ± 0.25 | 9.75 ± 0.26 | 7.18 ± 0.13 | 7.98 ± 0.29 | 9.65 ± 0.58 | 11.60 ± 0.33 | 9.08 ± 0.33 | 8.11 ± 0.37 | 10.25 ± 0.41 | 10.94 ± 0.06 | 7.44 ± 0.11 |
| Bortezomib | 16.96 ± 0.20 | 9.75 ± 0.03 | 8.94 ± 0.10 | 9.88 ± 0.12 | 9.62 ± 0.59 | 14.14 ± 0.85 | 11.72 ± 0.82 | 31.04 ± 0.71 | 9.35 ± 0.23 | 7.67 ± 0.31 | 9.51 ± 0.26 |
| Natural Product | IC50 (μM) | Fold of Enhancement | Natural Products | IC50 (μM) | Fold of Enhancement | CI | ||
|---|---|---|---|---|---|---|---|---|
| Alone | In Mixture | Alone | In Mixture | |||||
| Gossypol | 3.79 ± 0.01 | 0.93 ± 0.04 | 3.08 | Curcumin | 13.20 ± 0.81 | 3.67 ± 0.18 | 2.60 | 0.52 |
| 3.79 ± 0.01 | 1.08 ± 0.19 | 2.51 | Digitonin | 4.85 ± 0.24 | 1.51 ± 0.27 | 2.21 | 0.60 | |
| 3.79 ± 0.01 | 0.81 ± 0.11 | 3.68 | Conessine | 10.04 ± 0.25 | 2.26 ± 0.30 | 3.44 | 0.44 | |
| 3.79 ± 0.01 | 1.00 ± 0.02 | 2.79 | Bortezomib | 10.65 ± 0.01 | 2.79 ± 0.06 | 2.82 | 0.53 | |
| Natural Product | IC50 (μM) | Fold of Enhancement | Natural Products | IC50 (μM) | Fold of Enhancement | CI | ||
|---|---|---|---|---|---|---|---|---|
| Alone | In Mixture | Alone | In Mixture | |||||
| Gossypol | 0.26 ± 0.01 | 0.06 ± 0.01 | 3.33 | Curcumin | 17.05 ± 0.08 | 4.44 ± 0.74 | 2.84 | 0.49 |
| 0.26 ± 0.01 | 0.12 ± 0.01 | 1.17 | Digitonin | 3.86 ± 0.02 | 1.89 ± 0.13 | 12.04 | 0.95 | |
| 0.26 ± 0.01 | 0.10 ± 0.01 | 2.60 | Conessine | 10.07 ± 0.45 | 4.73 ± 0.08 | 1.13 | 0.85 | |
| 0.26 ± 0.01 | 0.05 ± 0.01 | 4.20 | Bortezomib | 9.70 ± 0.76 | 2.40 ± 0.28 | 3.04 | 0.44 | |
| Natural Product | IC50 (μM) | Fold of Enhancement | Natural Products | IC50 (μM) | Fold of Enhancement | CI | ||
|---|---|---|---|---|---|---|---|---|
| Alone | In Mixture | Alone | In Mixture | |||||
| Gossypol | 4.38 ± 0.08 | 0.65 ± 0.06 | 5.74 | Curcumin | 12.46 ± 0.05 | 1.93 ± 0.16 | 5.46 | 0.30 |
| 4.38 ± 0.08 | 0.63 ± 0.10 | 5.95 | Digitonin | 3.84 ± 0.81 | 0.55 ± 0.09 | 5.98 | 0.29 | |
| 4.38 ± 0.08 | 0.62 ± 0.01 | 6.06 | Conessine | 9.40 ± 0.21 | 1.29 ± 0.01 | 6.29 | 0.28 | |
| 4.38 ± 0.08 | 0.45 ± 0.02 | 8.73 | Bortezomib | 12.17 ± 0.07 | 1.07 ± 0.04 | 10.37 | 0.19 | |
| Natural Product | CC50 (μM) | Fold of Enhancement | Natural Products | CC50 (μM) | Fold of Enhancement | CI | ||
|---|---|---|---|---|---|---|---|---|
| Alone | In Mixture | Alone | In Mixture | |||||
| Gossypol | 14.84 ± 0.42 | 13.70 ± 0.05 | 0.08 | Curcumin | 53.12 ± 1.83 | 41.36± 1.93 | 0.28 | 1.70 |
| 14.84 ± 0.42 | 16.01 ± 0.40 | ‒0.07 | Digitonin | 50.63 ± 0.22 | 14.05 ± 0.35 | 2.60 | 1.36 | |
| 14.84 ± 0.42 | 15.16 ± 0.80 | ‒0.02 | Conessine | 314.57 ± 2.32 | 46.39 ± 2.94 | 5.78 | 1.17 | |
| 14.84 ± 0.42 | 6.31 ± 0.62 | 1.35 | Bortezomib | 17.77 ± 0.17 | 17.53 ± 1.73 | 0.01 | 1.41 | |
| Natural Products | IC50 (μM) | ||||
|---|---|---|---|---|---|
| CC50 (μM) | DENV-1-V1792 | DENV-2-V594 | DENV-3-V1043 | DENV-4-PR 06-65-740 | |
| Gossypol | 14.54 ± 0.59 | 1.87 ± 0.01 | 1.89 ± 0.21 | 3.70 ± 0.59 | 2.60 ± 0.12 |
| Curcumin | 59.42 ± 1.18 | 9.37 ± 0.47 | 3.07 ± 0.07 | 2.09 ± 0.12 | 4.83 ± 0.24 |
| Digitonin | 59.02 ± 0.33 | 5.21 ± 0.35 | 6.56 ± 0.21 | 4.07 ± 0.83 | 6.44 ± 0.34 |
| Conessine | 302.69 ± 13.40 | 7.09 ± 0.08 | 6.61 ± 0.60 | 7.41 ± 0.04 | 7.27 ± 0.31 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gao, Y.; Tai, W.; Wang, N.; Li, X.; Jiang, S.; Debnath, A.K.; Du, L.; Chen, S. Identification of Novel Natural Products as Effective and Broad-Spectrum Anti-Zika Virus Inhibitors. Viruses 2019, 11, 1019. https://doi.org/10.3390/v11111019
Gao Y, Tai W, Wang N, Li X, Jiang S, Debnath AK, Du L, Chen S. Identification of Novel Natural Products as Effective and Broad-Spectrum Anti-Zika Virus Inhibitors. Viruses. 2019; 11(11):1019. https://doi.org/10.3390/v11111019
Chicago/Turabian StyleGao, Yaning, Wanbo Tai, Ning Wang, Xiang Li, Shibo Jiang, Asim K. Debnath, Lanying Du, and Shizhong Chen. 2019. "Identification of Novel Natural Products as Effective and Broad-Spectrum Anti-Zika Virus Inhibitors" Viruses 11, no. 11: 1019. https://doi.org/10.3390/v11111019
APA StyleGao, Y., Tai, W., Wang, N., Li, X., Jiang, S., Debnath, A. K., Du, L., & Chen, S. (2019). Identification of Novel Natural Products as Effective and Broad-Spectrum Anti-Zika Virus Inhibitors. Viruses, 11(11), 1019. https://doi.org/10.3390/v11111019