The Symmetry of Viral Sialic Acid Binding Sites–Implications for Antiviral Strategies
Abstract
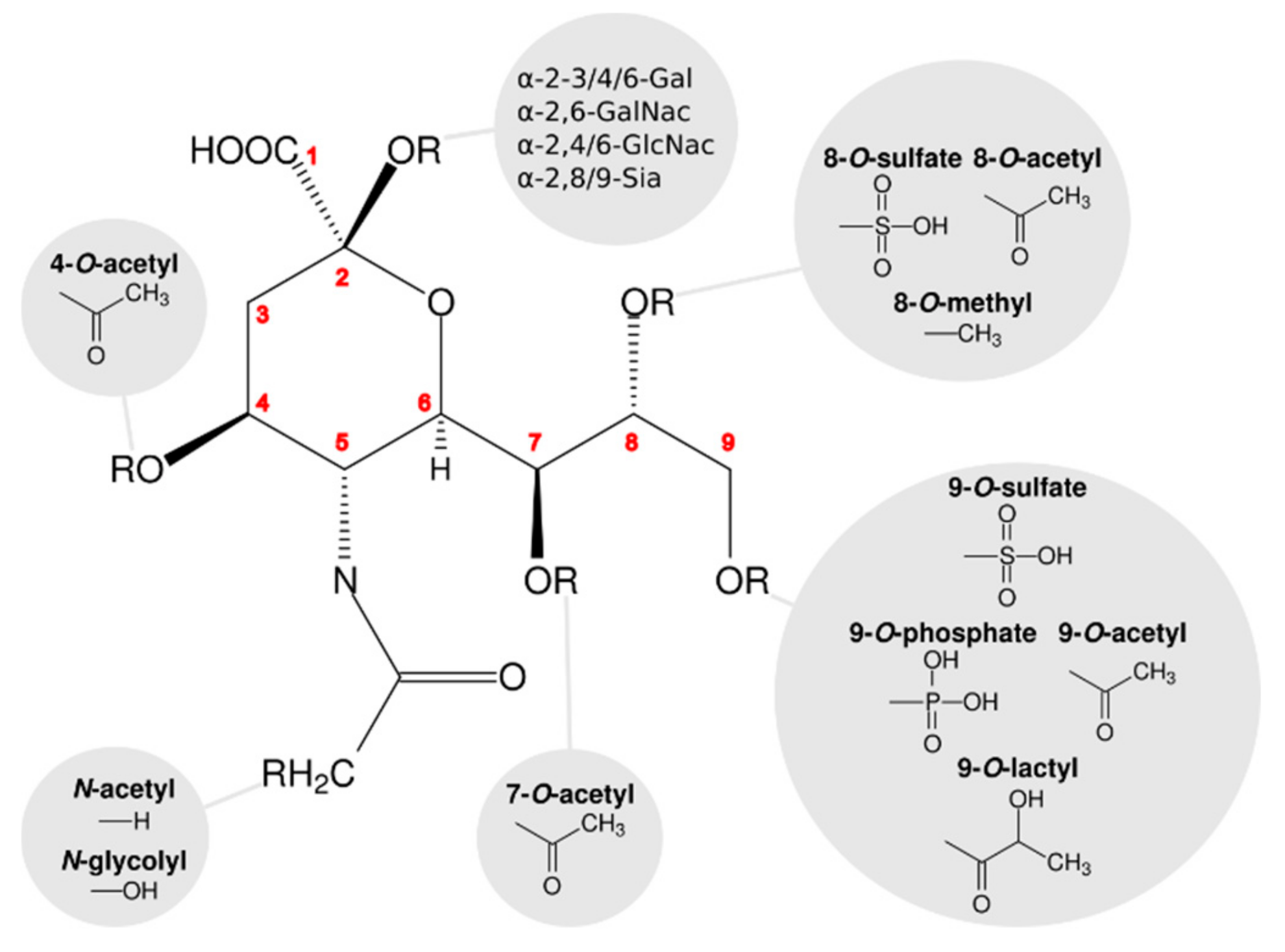
1. Introduction
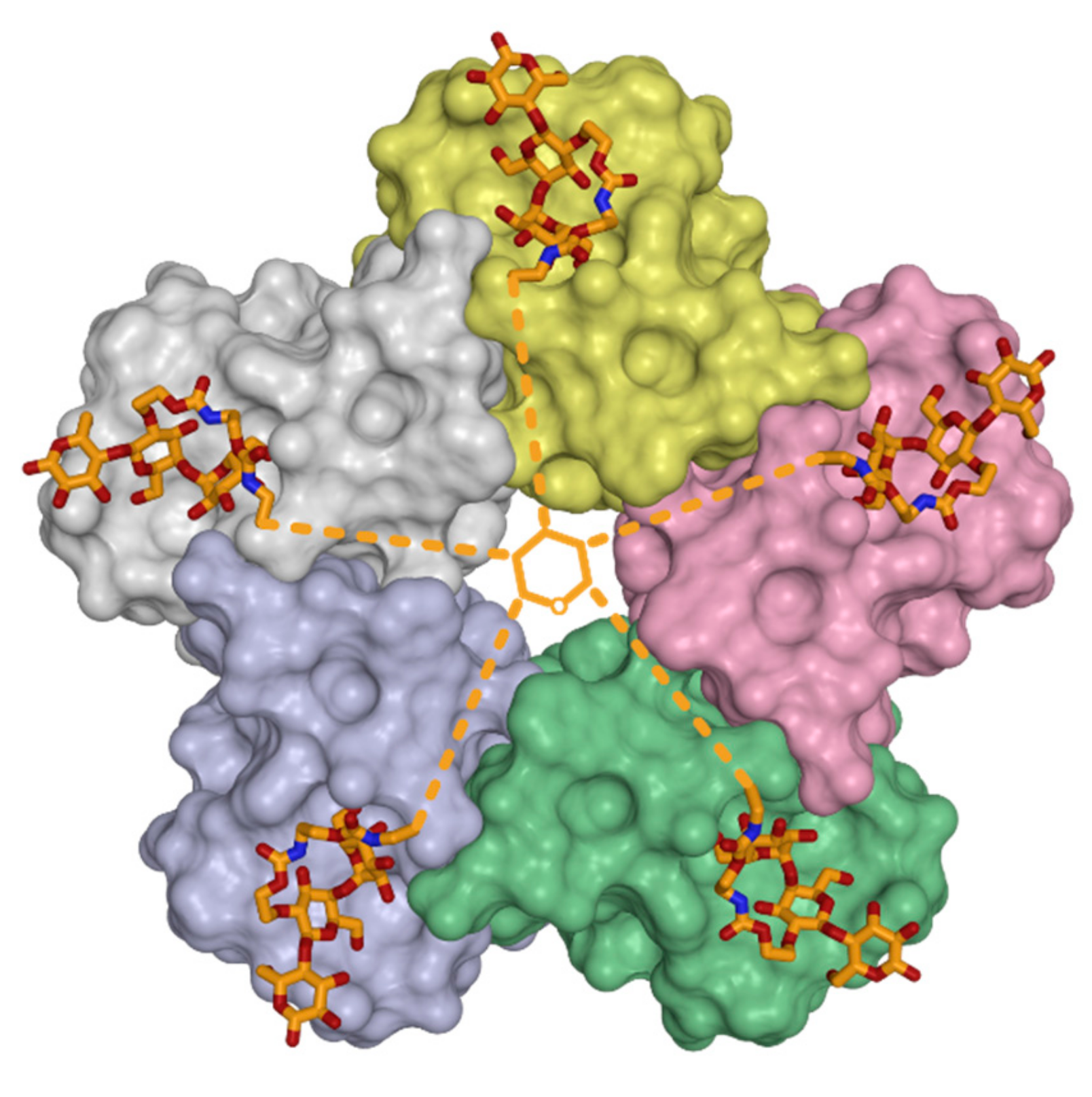
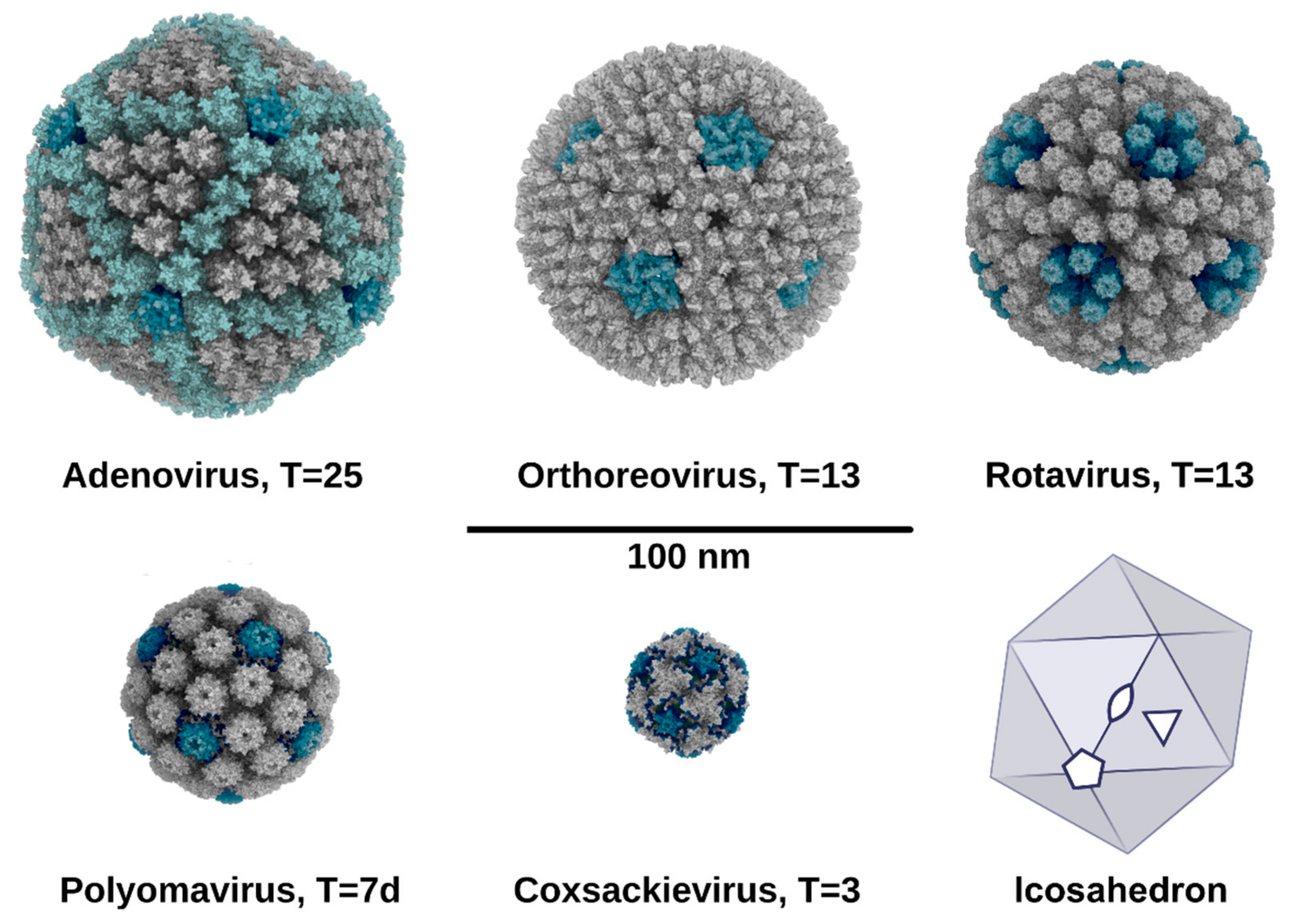
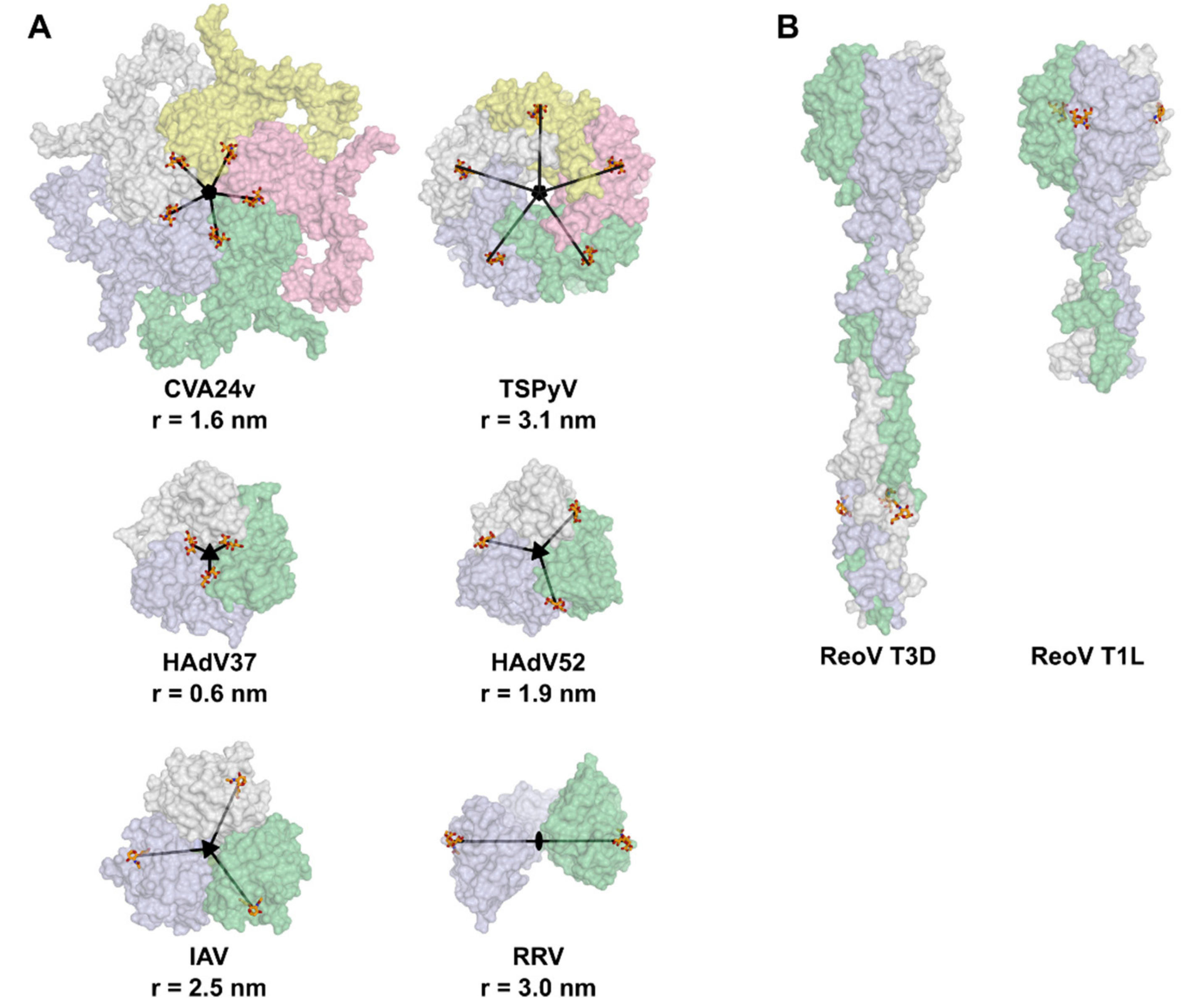
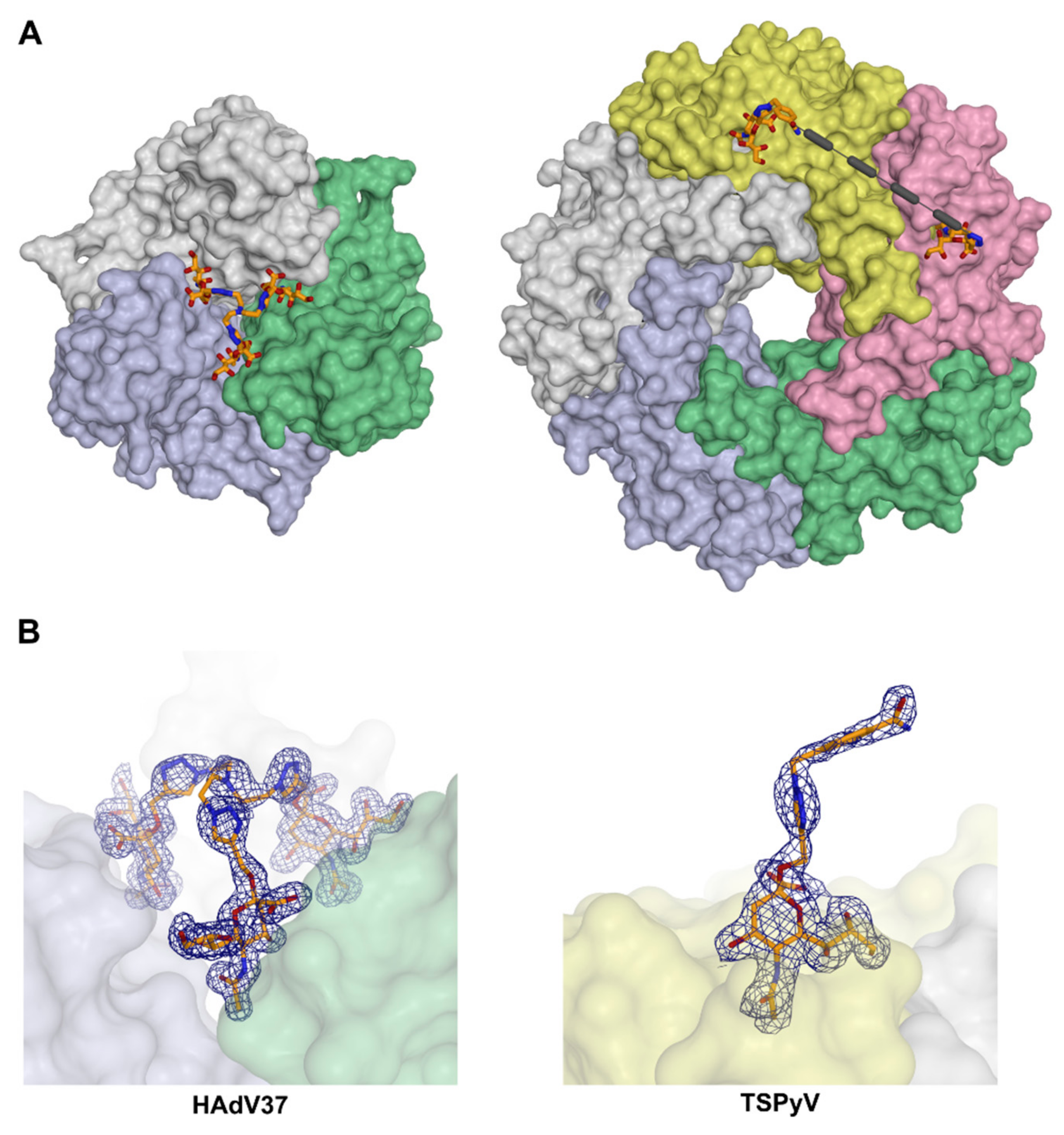
2. Symmetry in Virus Structures and Their Sialic Acid Binding Sites
3. Sialic Acid-Based Design of Anti-Viral Compounds
3.1. Monovalency
3.2. Polyvalency
3.3. Oligovalency
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Varki, A. N-glycolylneuraminic acid deficiency in humans. Biochimie 2001, 83, 615–622. [Google Scholar] [CrossRef]
- Angata, T.; Varki, A. Chemical Diversity in the Sialic Acids and Related α-Keto Acids: An Evolutionary Perspective. Chem. Rev. 2002, 102, 439–470. [Google Scholar] [CrossRef] [PubMed]
- Varki, N.M.; Varki, A. Diversity in cell surface sialic acid presentations: Implications for biology and disease. Lab. Investig. 2007, 87, 851–857. [Google Scholar] [CrossRef] [PubMed]
- Varki, A.; Schauer, R. Sialic Acids. In Essentials of Glycobiology, 2nd ed.; Varki, A., Cummings, R.D., Esko, J.D., Freeze, H.H., Stanley, P., Bertozzi, C.R., Hart, G.W., Etzler, M.E., Eds.; Cold Spring Harbor Laboratory Press: New York, NY, USA, 2009; Volume 1, pp. 199–217. [Google Scholar]
- Kumlin, U.; Olofsson, S.; Dimock, K.; Arnberg, N. Sialic acid tissue distribution and influenza virus tropism. Influ. Other Respir. Viruses 2008, 2, 147–154. [Google Scholar] [CrossRef] [PubMed]
- Shanker, S.; Hu, L.; Ramani, S.; Atmar, R.L.; Estes, M.K.; Prasad, B.V.V. Structural features of glycan recognition among viral pathogens. Curr. Opin. Struct. Biol. 2017, 44, 211–218. [Google Scholar] [CrossRef] [PubMed]
- Ströh, L.J.; Stehle, T. Glycan Engagement by Viruses: Receptor Switches and Specificity. Annu. Rev. Virol. 2014, 1, 285–306. [Google Scholar] [CrossRef]
- Vlasak, R.; Luytjes, W.; Spaan, W.; Palese, P. Human and bovine coronaviruses recognize sialic acid-containing receptors similar to those of influenza C viruses. Proc. Natl. Acad. Sci. USA 1988, 85, 4526–4529. [Google Scholar] [CrossRef]
- Schwegmann-Weßels, C.; Herrler, G. Sialic acids as receptor determinants for coronaviruses. Glycoconj. J. 2006, 23, 51–58. [Google Scholar] [CrossRef]
- Villar, E.; Barroso, I.M. Role of sialic acid-containing molecules in paramyxovirus entry into the host cell: A minireview. Glycoconj. J. 2006, 23, 5–17. [Google Scholar] [CrossRef]
- Weis, W.; Brown, J.H.; Cusack, S.; Paulson, J.C.; Skehel, J.J.; Wiley, D.C. Structure of the influenza virus haemagglutinin complexed with its receptor, sialic acid. Nature 1988, 333, 426–431. [Google Scholar] [CrossRef]
- Suzuki, Y.; Ito, T.; Suzuki, T.; Holland, R.E.; Chambers, T.M.; Kiso, M.; Ishida, H.; Kawaoka, Y. Sialic Acid Species as a Determinant of the Host Range of Influenza A Viruses. J. Virol. 2000, 74, 11825–11831. [Google Scholar] [CrossRef] [PubMed]
- Walters, R.W.; Yi, S.M.P.; Keshavjee, S.; Brown, K.E.; Welsh, M.J.; Chiorini, J.A.; Zabner, J. Binding of Adeno-associated Virus Type 5 to 2,3-Linked Sialic Acid Is Required for Gene Transfer. J. Biol. Chem. 2001, 276, 20610–20616. [Google Scholar] [CrossRef] [PubMed]
- Kaludov, N.; Brown, K.E.; Walters, R.W.; Zabner, J.; Chiorini, J.A. Adeno-Associated Virus Serotype 4 (AAV4) and AAV5 Both Require Sialic Acid Binding for Hemagglutination and Efficient Transduction but Differ in Sialic Acid Linkage Specificity. J. Virol. 2001, 75, 6884–6893. [Google Scholar] [CrossRef] [PubMed]
- Alexander, D.A.; Dimock, K. Sialic Acid Functions in Enterovirus 70 Binding and Infection. J. Virol. 2002, 76, 11265–11272. [Google Scholar] [CrossRef] [PubMed]
- Mistry, N.; Inoue, H.; Jamshidi, F.; Storm, R.J.; Oberste, M.S.; Arnberg, N. Coxsackievirus A24 Variant Uses Sialic Acid-Containing O-Linked Glycoconjugates as Cellular Receptors on Human Ocular Cells. J. Virol. 2011, 85, 11283–11290. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Sheng, J.; Baggen, J.; Meng, G.; Xiao, C.; Thibaut, H.J.; Van Kuppeveld, F.J.M.; Rossmann, M.G. Sialic acid-dependent cell entry of human enterovirus D68. Nat. Commun. 2015, 6, 8865. [Google Scholar] [CrossRef]
- Tan, M.; Wei, C.; Huang, P.; Fan, Q.; Quigley, C.; Xia, M.; Fang, H.; Zhang, X.; Zhong, W.; Klassen, J.S.; et al. Tulane virus recognizes sialic acids as cellular receptors. Sci. Rep. 2015, 5, 11784. [Google Scholar] [CrossRef]
- Liu, C.K.; Wei, G.; Atwood, W.J. Infection of Glial Cells by the Human Polyomavirus JC Is Mediated by an N-Linked Glycoprotein Containing Terminal α(2-6)-Linked Sialic Acids. J. Virol. 1998, 72, 4643–4649. [Google Scholar]
- Tsai, B.; Gilbert, J.M.; Stehle, T.; Lencer, W.; Benjamin, T.L.; Rapoport, T.A. Gangliosides are receptors for murine polyoma virus and SV40. EMBO J. 2003, 22, 4346–4355. [Google Scholar] [CrossRef]
- Low, J.A.; Magnuson, B.; Tsai, B.; Imperiale, M.J. Identification of Gangliosides GD1b and GT1b as Receptors for BK Virus. J. Virol. 2006, 80, 1361–1366. [Google Scholar] [CrossRef]
- Erickson, K.D.; Garcea, R.L.; Tsai, B. Ganglioside GT1b Is a Putative Host Cell Receptor for the Merkel Cell Polyomavirus. J. Virol. 2009, 83, 10275–10279. [Google Scholar] [CrossRef] [PubMed]
- Paul, R.W.; Choi, A.H.; Lee, P.W. The α-anomeric form of sialic acid is the minimal receptor determinant recognized by reovirus. Virology 1989, 172, 382–385. [Google Scholar] [CrossRef]
- Ciarlet, M.; Ludert, J.E.; Iturriza-Gómara, M.; Liprandi, F.; Gray, J.J.; Desselberger, U.; Estes, M.K. Initial Interaction of Rotavirus Strains with N-Acetylneuraminic (Sialic) Acid Residues on the Cell Surface Correlates with VP4 Genotype, Not Species of Origin. J. Virol. 2002, 76, 4087–4095. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Arnberg, N.; Edlund, K.; Kidd, A.H.; Wadell, G. Adenovirus Type 37 Uses Sialic Acid as a Cellular Receptor. J. Virol. 2000, 74, 42–48. [Google Scholar] [CrossRef]
- Lenman, A.; Liaci, A.M.; Liu, Y.; Årdahl, C.; Rajan, A.; Nilsson, E.; Bradford, W.; Kaeshammer, L.; Jones, M.S.; Frängsmyr, L.; et al. Human Adenovirus 52 Uses Sialic Acid-containing Glycoproteins and the Coxsackie and Adenovirus Receptor for Binding to Target Cells. PLOS Pathog. 2015, 11, e1004657. [Google Scholar] [CrossRef]
- Neu, U.; Woellner, K.; Gauglitz, G.; Stehle, T. Structural basis of GM1 ganglioside recognition by simian virus 40. Proc. Natl. Acad. Sci. USA 2008, 105, 5219–5224. [Google Scholar] [CrossRef]
- Buch, M.H.C.; Liaci, A.M.; O’Hara, S.D.; Garcea, R.L.; Neu, U.; Stehle, T. Structural and Functional Analysis of Murine Polyomavirus Capsid Proteins Establish the Determinants of Ligand Recognition and Pathogenicity. PLOS Pathog. 2015, 11, e1005104. [Google Scholar] [CrossRef]
- Saleeb, M.; Liaci, A.M.; Storm, R.J.; Frängsmyr, L.; Qian, W.; Caraballo, R.; Bauer, J.; Chandra, N.; Stehle, T.; Arnberg, N.; et al. Triazole linker-based trivalent sialic acid inhibitors of adenovirus type 37 infection of human corneal epithelial cells. Org. Biomol. Chem. 2015, 13, 9194–9205. [Google Scholar]
- Sauter, N.K.; Bednarski, M.D.; Wurzburg, B.A.; Hanson, J.E.; Whitesides, G.M.; Skehel, J.J.; Wiley, D.C. Hemagglutinins from two influenza virus variants bind to sialic acid derivatives with millimolar dissociation constants: A 500-MHz proton nuclear magnetic resonance study. Biochemistry 1989, 28, 8388–8396. [Google Scholar] [CrossRef]
- Merritt, E.A.; Hol, W.G. AB5 toxins. Curr. Opin. Struct. Biol. 1995, 5, 165–171. [Google Scholar] [CrossRef]
- Waddell, T.; Head, S.; Petric, M.; Cohen, A.; Lingwood, C. Globotriosyl ceramide is specifically recognized by the escherichia coli verocytotoxin 2. Biochem. Biophys. Res. Commun. 1988, 152, 674–679. [Google Scholar] [CrossRef]
- Ling, H.; Boodhoo, A.; Hazes, B.; Cummings, M.D.; Armstrong, G.D.; Brunton, J.L.; Read, R.J. Structure of the Shiga-like Toxin I B-Pentamer Complexed with an Analogue of Its Receptor Gb3. Biochemistry 1998, 37, 1777–1788. [Google Scholar] [CrossRef] [PubMed]
- Kitov, P.I.; Sadowska, J.M.; Mulvey, G.; Armstrong, G.D.; Ling, H.; Pannu, N.S.; Read, R.J.; Bundle, D.R. Shiga-like toxins are neutralized by tailored multivalent carbohydrate ligands. Nature 2000, 403, 669–672. [Google Scholar] [CrossRef] [PubMed]
- Caspar, D.L.D.; Klug, A. Physical Principles in the Construction of Regular Viruses. Cold Spring Harb. Symp. Quant. Biol. 1962, 27, 1–24. [Google Scholar] [CrossRef]
- Huang, L.-Y.; Patel, A.; Ng, R.; Miller, E.B.; Halder, S.; McKenna, R.; Asokan, A.; Agbandje-McKenna, M. Characterization of the Adeno-Associated Virus 1 and 6 Sialic Acid Binding Site. J. Virol. 2016, 90, 5219–5230. [Google Scholar] [CrossRef]
- Zocher, G.; Mistry, N.; Frank, M.; Hähnlein-Schick, I.; Ekström, J.-O.; Arnberg, N.; Stehle, T. A Sialic Acid Binding Site in a Human Picornavirus. PLOS Pathog. 2014, 10, 1004401. [Google Scholar] [CrossRef]
- Modis, Y.; Trus, B.L.; Harrison, S.C. Atomic model of the papillomavirus capsid. EMBO J. 2002, 21, 4754–4762. [Google Scholar] [CrossRef]
- Rayment, I.; Baker, T.S.; Caspar, D.L.D.; Murakami, W.T. Polyoma virus capsid structure at 22.5. A resolution. Nature 1982, 295, 110–115. [Google Scholar] [CrossRef]
- Liddington, R.C.; Yan, Y.; Moulai, J.; Sahli, R.; Benjamin, T.L.; Harrison, S.C. Structure of simian virus 40 at 3.8-Å resolution. Nature 1991, 354, 278–284. [Google Scholar] [CrossRef]
- Chen, X.S.; Garcea, R.L.; Goldberg, I.; Casini, G.; Harrison, S.C. Structure of Small Virus-like Particles Assembled from the L1 Protein of Human Papillomavirus 16. Mol. Cell 2000, 5, 557–567. [Google Scholar] [CrossRef]
- Salunke, D.; Caspar, D.; Garcea, R. Polymorphism in the assembly of polyomavirus capsid protein VP1. Biophys. J. 1989, 56, 887–900. [Google Scholar] [CrossRef]
- Nilsson, J.; Miyazaki, N.; Xing, L.; Wu, B.; Hammar, L.; Li, T.C.; Takeda, N.; Miyamura, T.; Cheng, R.H. Structure and Assembly of a T = 1 Virus-Like Particle in BK Polyomavirus. J. Virol. 2005, 79, 5337–5345. [Google Scholar] [CrossRef] [PubMed]
- Stehle, T.; Harrison, S.C. High-resolution structure of a polyomavirus VP1-oligosaccharide complex: Implications for assembly and receptor binding. EMBO J. 1997, 16, 5139–5148. [Google Scholar] [CrossRef] [PubMed]
- Neu, U.; Maginnis, M.S.; Palma, A.S.; Ströh, L.J.; Nelson, C.D.; Feizi, T.; Atwood, W.J.; Stehle, T. Structure-function analysis of the human JC polyomavirus establishes the LSTc pentasaccharide as a functional receptor motif. Cell Host Microbe 2010, 8, 309–319. [Google Scholar] [CrossRef] [PubMed]
- Neu, U.; Hengel, H.; Blaum, B.S.; Schowalter, R.M.; Macejak, D.; Gilbert, M.; Wakarchuk, W.W.; Imamura, A.; Ando, H.; Kiso, M.; et al. Structures of Merkel Cell Polyomavirus VP1 Complexes Define a Sialic Acid Binding Site Required for Infection. PLOS Pathog. 2012, 8, e1002738. [Google Scholar] [CrossRef] [PubMed]
- Neu, U.; Allen, S.-A.A.; Blaum, B.S.; Liu, Y.; Frank, M.; Palma, A.S.; Ströh, L.J.; Feizi, T.; Peters, T.; Atwood, W.J.; et al. A Structure-Guided Mutation in the Major Capsid Protein Retargets BK Polyomavirus. PLOS Pathog. 2013, 9, e1003688. [Google Scholar] [CrossRef]
- Neu, U.; Khan, Z.M.; Schuch, B.; Palma, A.S.; Liu, Y.; Pawlita, M.; Feizi, T.; Stehle, T. Structures of B-Lymphotropic Polyomavirus VP1 in Complex with Oligosaccharide Ligands. PLOS Pathog. 2013, 9, e1003714. [Google Scholar] [CrossRef]
- Khan, Z.M.; Liu, Y.; Neu, U.; Gilbert, M.; Ehlers, B.; Feizi, T.; Stehle, T. Crystallographic and Glycan Microarray Analysis of Human Polyomavirus 9 VP1 Identifies N-Glycolyl Neuraminic Acid as a Receptor Candidate. J. Virol. 2014, 88, 6100–6111. [Google Scholar] [CrossRef]
- Ströh, L.J.; Gee, G.V.; Blaum, B.S.; Dugan, A.S.; Feltkamp, M.C.W.; Atwood, W.J.; Stehle, T. Trichodysplasia spinulosa-Associated Polyomavirus Uses a Displaced Binding Site on VP1 to Engage Sialylated Glycolipids. PLOS Pathog. 2015, 11, e1005112. [Google Scholar] [CrossRef]
- Joyce, J.G.; Tung, J.-S.; Przysiecki, C.T.; Cook, J.C.; Lehman, E.D.; Sands, J.A.; Jansen, K.U.; Keller, P.M. The L1 Major Capsid Protein of Human Papillomavirus Type 11 Recombinant Virus-like Particles Interacts with Heparin and Cell-surface Glycosaminoglycans on Human Keratinocytes. J. Biol. Chem. 1999, 274, 5810–5822. [Google Scholar] [CrossRef]
- Giroglou, T.; Florin, L.; Schäfer, F.; Streeck, R.E.; Sapp, M. Human Papillomavirus Infection Requires Cell Surface Heparan Sulfate. J. Virol. 2001, 75, 1565–1570. [Google Scholar] [CrossRef] [PubMed]
- Spoden, G.; Kühling, L.; Cordes, N.; Frenzel, B.; Sapp, M.; Boller, K.; Florin, L.; Schelhaas, M. Human Papillomavirus Types 16, 18, and 31 Share Similar Endocytic Requirements for Entry. J. Virol. 2013, 87, 7765–7773. [Google Scholar] [CrossRef] [PubMed]
- Metcalf, P.; Cyrklaff, M.; Adrian, M. The three-dimensional structure of reovirus obtained by cryo-electron microscopy. EMBO J. 1991, 10, 3129–3136. [Google Scholar] [CrossRef] [PubMed]
- Reinisch, K.M.; Nibert, M.L.; Harrison, S.C. Structure of the reovirus core at 3.6? Å resolution. Nature 2000, 404, 960–967. [Google Scholar] [CrossRef] [PubMed]
- McClain, B.; Settembre, E.; Temple, B.R.; Bellamy, A.R.; Harrison, S.C. X-ray crystal structure of the rotavirus inner capsid particle at 3.8 a resolution. J. Mol. Biol. 2010, 397, 587–599. [Google Scholar] [CrossRef]
- Furlong, D.B.; Nibert, M.L.; Fields, B.N. Sigma 1 protein of mammalian reoviruses extends from the surfaces of viral particles. J. Virol. 1988, 62, 246–256. [Google Scholar]
- Reiss, K.; Stencel, J.E.; Liu, Y.; Blaum, B.S.; Reiter, D.M.; Feizi, T.; Dermody, T.S.; Stehle, T. The GM2 Glycan Serves as a Functional Coreceptor for Serotype 1 Reovirus. PLOS Pathog. 2012, 8, 1003078. [Google Scholar] [CrossRef]
- Reiter, D.M.; Frierson, J.M.; Halvorson, E.E.; Kobayashi, T.; Dermody, T.S.; Stehle, T. Crystal structure of reovirus attachment protein sigma1 in complex with sialylated oligosaccharides. PLoS Pathog. 2011, 7, e1002166. [Google Scholar] [CrossRef]
- Shaw, A.; Rothnagel, R.; Chen, D.; Ramig, R.; Chiu, W.; Prasad, B. Three-dimensional visualization of the rotavirus hemagglutinin structure. Cell 1993, 74, 693–701. [Google Scholar] [CrossRef]
- Fukudome, K.; Yoshie, O.; Konno, T. Comparison of human, simian, and bovine rotaviruses for requirement of sialic acid in hemagglutination and cell adsorption. Virology 1989, 172, 196–205. [Google Scholar] [CrossRef]
- Isa, P.; Arias, C.F.; Lopez, S. Role of sialic acids in rotavirus infection. Glycoconj. J. 2006, 23, 27–37. [Google Scholar] [CrossRef] [PubMed]
- Haselhorst, T.; Fleming, F.E.; Dyason, J.C.; Hartnell, R.D.; Yu, X.; Holloway, G.; Santegoets, K.; Kiefel, M.J.; Blanchard, H.; Coulson, B.S.; et al. Sialic acid dependence in rotavirus host cell invasion. Nat. Chem. Biol. 2009, 5, 91–93. [Google Scholar] [CrossRef] [PubMed]
- Dormitzer, P.R.; Sun, Z.J.; Wagner, G.; Harrison, S.C. The rhesus rotavirus VP4 sialic acid binding domain has a galectin fold with a novel carbohydrate binding site. EMBO J. 2002, 21, 885–897. [Google Scholar] [CrossRef] [PubMed]
- Settembre, E.C.; Chen, J.Z.; Dormitzer, P.R.; Grigorieff, N.; Harrison, S.C. Atomic model of an infectious rotavirus particle. EMBO J. 2011, 30, 408–416. [Google Scholar] [CrossRef] [PubMed]
- Burnett, R.M.; Grütter, M.G.; White, J.L. The structure of the adenovirus capsid. J. Mol. Biol. 1985, 185, 105–123. [Google Scholar] [CrossRef]
- Stewart, P.L.; Burnett, R.M.; Cyrklaff, M.; Fuller, S.D. Image reconstruction reveals the complex molecular organization of adenovirus. Cell 1991, 67, 145–154. [Google Scholar] [CrossRef]
- Burmeister, W.P.; Guilligay, D.; Cusack, S.; Wadell, G.; Arnberg, N. Crystal Structure of Species D Adenovirus Fiber Knobs and Their Sialic Acid Binding Sites. J. Virol. 2004, 78, 7727–7736. [Google Scholar] [CrossRef]
- Xu, R.; Ekiert, D.C.; Krause, J.C.; Hai, R.; Crowe, J.E.; Wilson, I.A. Structural basis of preexisting immunity to the 2009 H1N1 pandemic influenza virus. Science 2010, 328, 357–360. [Google Scholar] [CrossRef]
- Seto, J.; Rott, R. Functional significance of sialidase during influenza virus multiplication. Virology 1966, 30, 731–737. [Google Scholar] [CrossRef]
- Seto, J.T.; Chang, F.S. Functional Significance of Sialidase during Influenza Virus Multiplication: An Electron Microscope Study. J. Virol. 1969, 4, 58–66. [Google Scholar]
- Meindl, P.; Bodo, G.; Palese, P.; Schulman, J.; Tuppy, H. Inhibition of neuraminidase activity by derivatives of 2-deoxy-2,3-dehydro-N-acetylneuraminic acid. Virology 1974, 58, 457–463. [Google Scholar] [CrossRef]
- Palese, P.; Schulman, J.; Bodo, G.; Meindl, P. Inhibition of influenza and parainfluenza virus replication in tissue culture by 2-deoxy-2,3-dehydro-N-trifluoroacetylneuraminic acid (FANA). Virology 1974, 59, 490–498. [Google Scholar] [CrossRef]
- Von Itzstein, M.; Wu, W.Y.; Kok, G.B.; Pegg, M.S.; Dyason, J.C.; Jin, B.; Van Phan, T.; Smythe, M.L.; White, H.F.; Oliver, S.W.; et al. Rational design of potent sialidase-based inhibitors of influenza virus replication. Nature 1993, 363, 418–423. [Google Scholar] [CrossRef] [PubMed]
- Woods, J.M.; Bethell, R.C.; A Coates, J.; Healy, N.; A Hiscox, S.; A Pearson, B.; Ryan, D.M.; Ticehurst, J.; Tilling, J.; Walcott, S.M. 4-Guanidino-2,4-dideoxy-2,3-dehydro-N-acetylneuraminic acid is a highly effective inhibitor both of the sialidase (neuraminidase) and of growth of a wide range of influenza A and B viruses in vitro. Antimicrob. Agents Chemother. 1993, 37, 1473–1479. [Google Scholar] [CrossRef]
- Singh, S.; Jedrzejas, M.J.; Air, G.M.; Luo, M.; Laver, W.G.; Brouillette, W.J. Structure-Based Inhibitors of Influenza Virus Sialidase. A Benzoic Acid Lead with Novel Interaction. J. Med. Chem. 1995, 38, 3217–3225. [Google Scholar] [CrossRef]
- Williams, M.; Bischofberger, N.; Swaminathan, S.; Kim, C.U. Synthesis and influenza neuraminidase inhibitory activity of aromatic analogs of sialic-acid. Bioorganic Med. Chem. Lett. 1995, 5, 2251–2254. [Google Scholar] [CrossRef]
- Kim, C.U.; Lew, W.; Williams, M.A.; Liu, H.; Zhang, L.; Swaminathan, S.; Bischofberger, N.; Chen, M.S.; Mendel, D.B.; Tai, C.Y.; et al. Influenza Neuraminidase Inhibitors Possessing a Novel Hydrophobic Interaction in the Enzyme Active Site: Design, Synthesis, and Structural Analysis of Carbocyclic Sialic Acid Analogues with Potent Anti-Influenza Activity. J. Am. Chem. Soc. 1997, 119, 681–690. [Google Scholar] [CrossRef]
- Bianco, A.; Brufani, M.; Manna, F.; Melchioni, C. Synthesis of a carbocyclic sialic acid analogue for the inhibition of influenza virus neuraminidase. Carbohydr. Res. 2001, 332, 23–31. [Google Scholar] [CrossRef]
- Chong, A.K.J.; Pegg, M.S.; Taylor, N.R.; Itzstein, M. Evidence for a sialosyl cation transition-state complex in the reaction of sialidase from influenza virus. JBIC J. Biol. Inorg. Chem. 1992, 207, 335–343. [Google Scholar] [CrossRef]
- Von Itzstein, M.; Dyason, J.C.; Oliver, S.W.; White, H.F.; Wu, W.Y.; Kok, G.B.; Pegg, M.S. A study of the active site of influenza virus sialidase: An approach to the rational design of novel anti-influenza drugs. J. Med. Chem. 1996, 39, 388–391. [Google Scholar] [CrossRef]
- Spaltenstein, A.; Whitesides, G.M. Polyacrylamides bearing pendant α-sialoside groups strongly inhibit agglutination of erythrocytes by influenza-virus. J. Am. Chem. Soc. 1991, 113, 686–687. [Google Scholar] [CrossRef]
- Mammen, M.; Dahmann, G.; Whitesides, G.M. Effective inhibitors of hemagglutination by influenza virus synthesized from polymers having active ester groups. Insight into mechanism of inhibition. J. Med. Chem. 1995, 38, 4179–4190. [Google Scholar] [CrossRef] [PubMed]
- Choi, S.K.; Mammen, M.; Whitesides, G.M. Generation and in situ evaluation of libraries of poly(acrylic acid) presenting sialosides as side chains as polyvalent inhibitors of influenza-mediated hemagglutination. J. Am. Chem. Soc. 1997, 119, 4103–4111. [Google Scholar] [CrossRef]
- Reuter, J.D.; Myc, A.; Hayes, M.M.; Gan, Z.; Roy, R.; Qin, D.; Yin, R.; Piehler, L.T.; Esfand, R.; Tomalia, D.A.; et al. Inhibition of Viral Adhesion and Infection by Sialic-Acid-Conjugated Dendritic Polymers. Bioconjugate Chem. 1999, 10, 271–278. [Google Scholar] [CrossRef] [PubMed]
- Gambaryan, A.S.; Tuzikov, A.B.; A Chinarev, A.; Juneja, L.R.; Bovin, N.V.; Matrosovich, M.N. Polymeric inhibitor of influenza virus attachment protects mice from experimental influenza infection. Antivir. Res. 2002, 55, 201–205. [Google Scholar] [CrossRef]
- Bhatia, S.; Lauster, D.; Bardua, M.; Ludwig, K.; Angioletti-Uberti, S.; Popp, N.; Hoffmann, U.; Paulus, F.; Budt, M.; Stadtmüller, M.; et al. Linear polysialoside outperforms dendritic analogs for inhibition of influenza virus infection in vitro and in vivo. Biomaterials 2017, 138, 22–34. [Google Scholar] [CrossRef]
- Bhatia, S.; Camacho, L.C.; Haag, R. Pathogen Inhibition by Multivalent Ligand Architectures. J. Am. Chem. Soc. 2016, 138, 8654–8666. [Google Scholar] [CrossRef]
- Lu, W.; Pieters, R.J. Carbohydrate–protein interactions and multivalency: Implications for the inhibition of influenza A virus infections. Expert Opin. Drug Discov. 2019, 14, 387–395. [Google Scholar] [CrossRef]
- Haraldsson, B.; Nystrom, J.; Deen, W.M. Properties of the Glomerular Barrier and Mechanisms of Proteinuria. Physiol. Rev. 2008, 88, 451–487. [Google Scholar] [CrossRef]
- Lu, W.; Du, W.; Somovilla, V.J.; Yu, G.; Haksar, D.; De Vries, E.; Boons, G.-J.; De Vries, R.P.; De Haan, C.A.M.; Pieters, R.J. Enhanced Inhibition of Influenza A Virus Adhesion by Di- and Trivalent Hemagglutinin Inhibitors. J. Med. Chem. 2019, 62, 6398–6404. [Google Scholar] [CrossRef]
- Nilsson, E.C.; Storm, R.J.; Bauer, J.; Johansson, S.M.; Lookene, A.; Ångström, J.; Hedenström, M.; Eriksson, T.L.; Frängsmyr, L.; Rinaldi, S.; et al. The GD1a glycan is a cellular receptor for adenoviruses causing epidemic keratoconjunctivitis. Nat. Med. 2011, 17, 105–109. [Google Scholar] [CrossRef] [PubMed]
- Spjut, S.; Qian, W.; Bauer, J.; Storm, R.; Frängsmyr, L.; Stehle, T.; Arnberg, N.; Elofsson, M. A Potent Trivalent Sialic Acid Inhibitor of Adenovirus Type 37 Infection of Human Corneal Cells. Angew. Chem. Int. Ed. 2011, 50, 6519–6521. [Google Scholar] [CrossRef] [PubMed]
- Baier, M.; Rustmeier, N.H.; Harr, J.; Cyrus, N.; Reiss, G.J.; Grafmüller, A.; Blaum, B.S.; Stehle, T.; Hartmann, L. Divalent Sialylated Precision Glycooligomers Binding to Polyomaviruses and the Effect of Different Linkers. Macromol. Biosci. 2019, 19, e1800426. [Google Scholar] [CrossRef] [PubMed]
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rustmeier, N.H.; Strebl, M.; Stehle, T. The Symmetry of Viral Sialic Acid Binding Sites–Implications for Antiviral Strategies. Viruses 2019, 11, 947. https://doi.org/10.3390/v11100947
Rustmeier NH, Strebl M, Stehle T. The Symmetry of Viral Sialic Acid Binding Sites–Implications for Antiviral Strategies. Viruses. 2019; 11(10):947. https://doi.org/10.3390/v11100947
Chicago/Turabian StyleRustmeier, Nils H., Michael Strebl, and Thilo Stehle. 2019. "The Symmetry of Viral Sialic Acid Binding Sites–Implications for Antiviral Strategies" Viruses 11, no. 10: 947. https://doi.org/10.3390/v11100947
APA StyleRustmeier, N. H., Strebl, M., & Stehle, T. (2019). The Symmetry of Viral Sialic Acid Binding Sites–Implications for Antiviral Strategies. Viruses, 11(10), 947. https://doi.org/10.3390/v11100947



