Erythromycin Estolate Inhibits Zika Virus Infection by Blocking Viral Entry as a Viral Inactivator
Abstract
:1. Introduction
2. Materials and Methods
2.1. Cells and Viruses
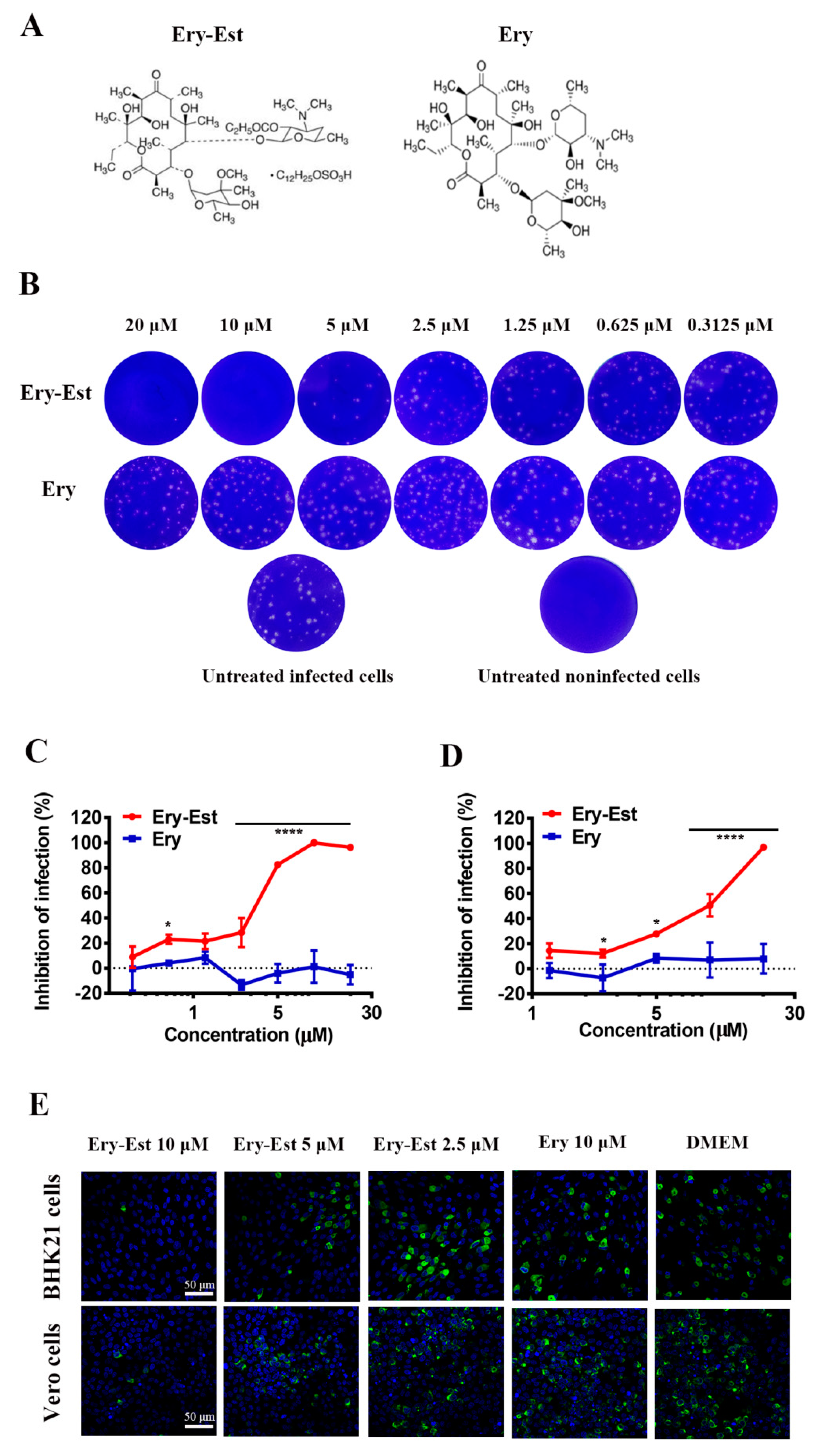
2.2. Compounds
2.3. Plaque Assay
2.4. Drug Cytotoxicity Assay
2.5. Assays for Antiviral Activity
2.6. Immunofluorescence Staining Assay
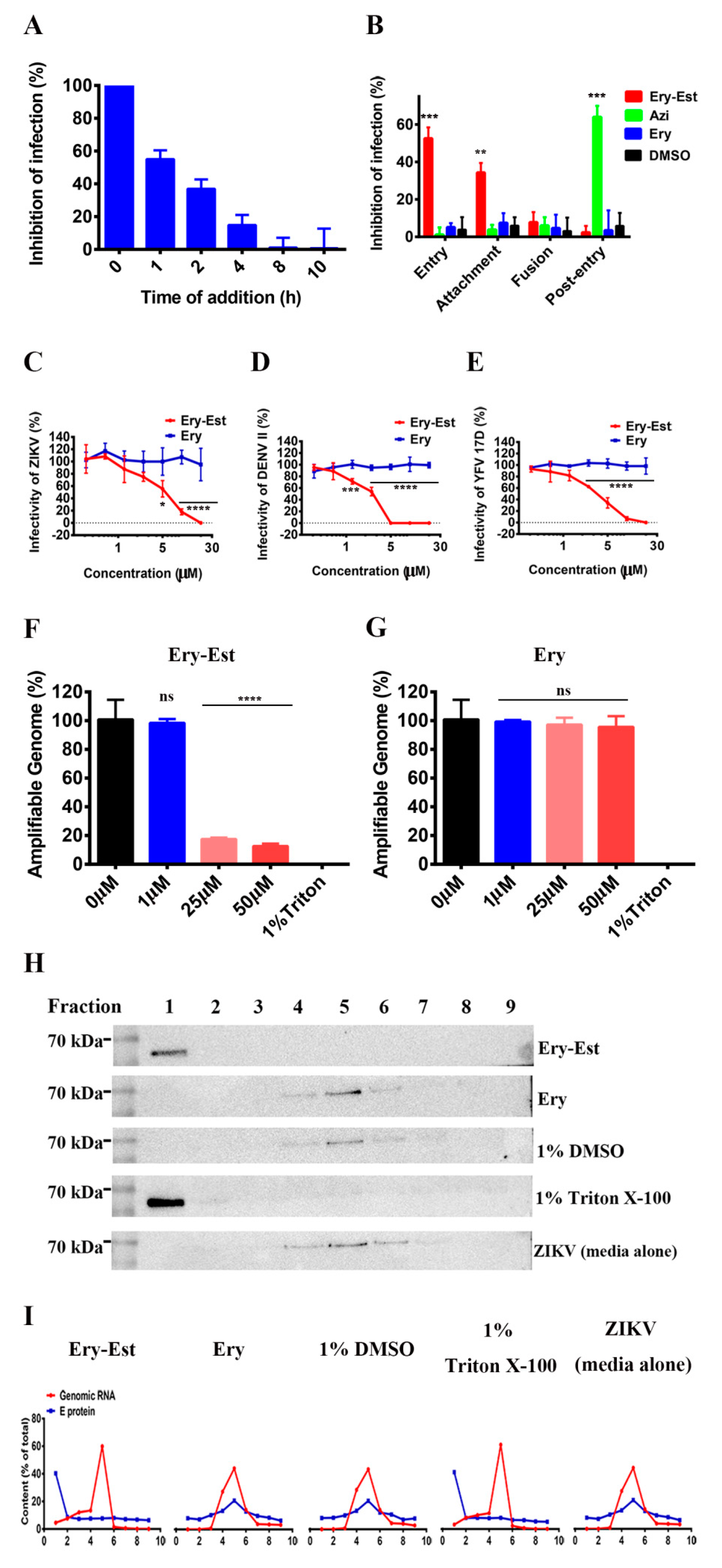
2.7. Time-of-Addition Assay
2.8. Assay to Detect Inactivated Virions
2.9. RNase Digestion Assay and RT-qPCR
2.10. Sucrose Density Gradient Assay
2.11. Ethics Statement
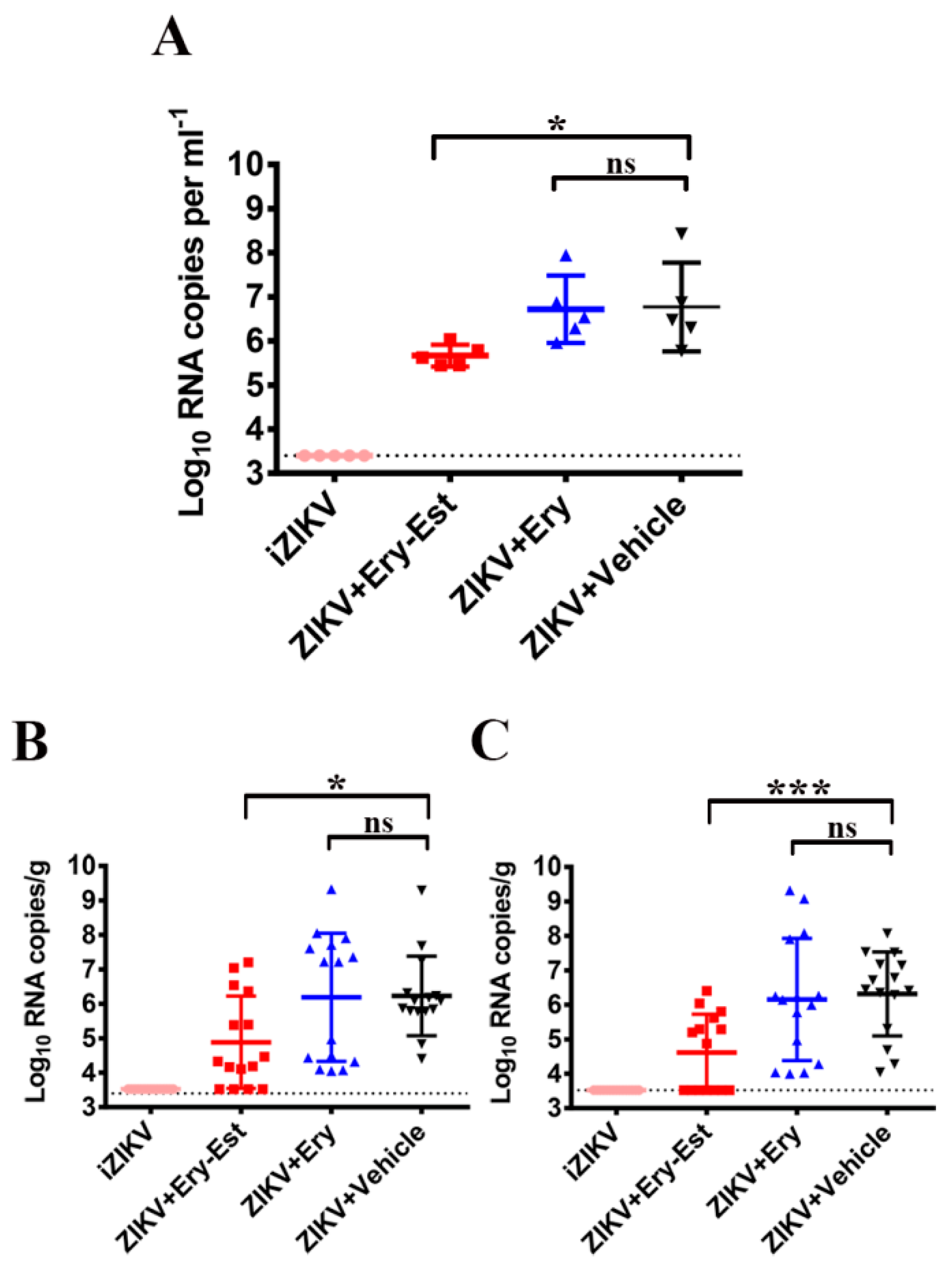
2.12. Antiviral Efficiency of Ery-Est in A129 Mice
2.13. Antiviral Efficiency of Ery-Est in Pregnant C57BL/6 Mice
2.14. Statistical Analysis
3. Results
3.1. Ery-Est Inhibited ZIKV Infection in Different Cell Types
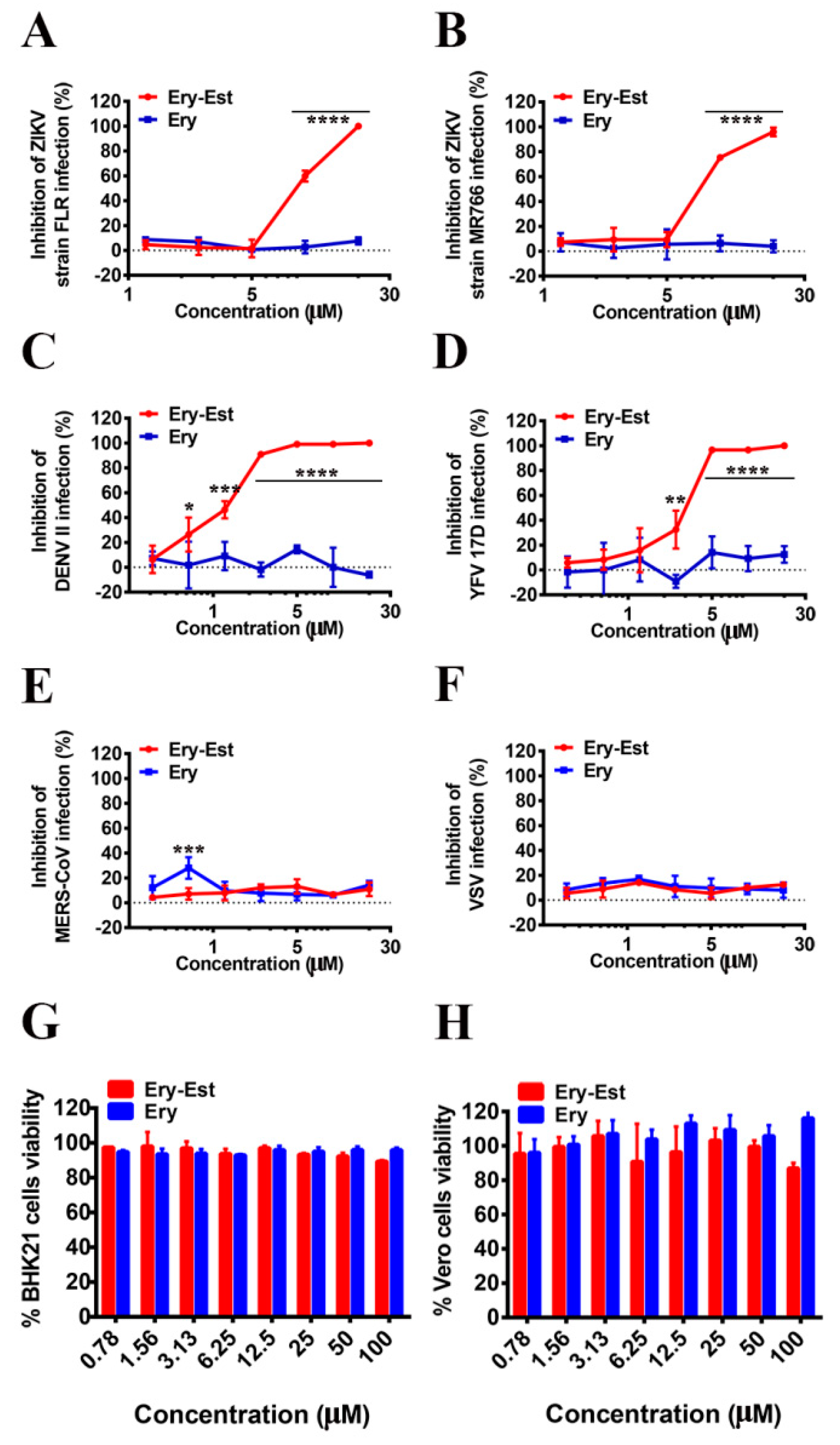
3.2. Ery-Est Inhibited ZIKV Strains FLR and MR766, DENV II, and YFV 17D Infections
3.3. Ery-Est Inhibited ZIKV Infection in the Early Stage
3.4. Ery-Est Inactivated ZIKV Virons
3.5. Ery-Est Protected A129 Mice from Lethal ZIKV Challenge
3.6. Ery-Est Protected Against Vertical Transmission of ZIKV in Pregnant C57BL/6 Mice
4. Discussion
Supplementary Materials
Author Contributions
Acknowledgments
Conflicts of Interest
References
- Dick, G.W.; Kitchen, S.F.; Haddow, A.J. Zika virus. I. Isolations and serological specificity. Trans. R Soc. Trop. Med. Hyg. 1952, 46, 509–520. [Google Scholar] [CrossRef]
- Macnamara, F.N. Zika virus: A report on three cases of human infection during an epidemic of jaundice in Nigeria. Trans. R Soc. Trop. Med. Hyg. 1954, 48, 139–145. [Google Scholar] [CrossRef]
- Musso, D.; Nilles, E.J.; Cao-Lormeau, V.M. Rapid spread of emerging Zika virus in the Pacific area. Clin. Microbiol. Infect. 2014, 20, O595–O596. [Google Scholar] [CrossRef]
- Jouannic, J.M.; Friszer, S.; Leparc-Goffart, I.; Garel, C.; Eyrolle-Guignot, D. Zika virus infection in French Polynesia. Lancet 2016, 387, 1051–1052. [Google Scholar] [CrossRef]
- Zika Virus Outbreaks in the Americas. Available online: https://www.ncbi.nlm.nih.gov/pubmed/26552108/ (accessed on 12 November 2019).
- Gulland, A. Zika virus is a global public health emergency, declares WHO. BMJ 2016, 352, i657. [Google Scholar] [CrossRef] [PubMed]
- Tsetsarkin, K.A.; Maximova, O.A.; Liu, G.; Kenney, H.; Teterina, N.; Bloom, M.E.; Grabowski, J.M.; Mlera, L.; Nagata, B.M.; Moore, I.; et al. Routes of Zika virus dissemination in the testis and epididymis of immunodeficient mice. Nat. Commun. 2018, 9, 5350. [Google Scholar] [CrossRef] [PubMed]
- De Moraes, C.G.; Pettito, M.; Yepez, J.B.; Sakuntabhai, A.; Simon-Loriere, E.; Zaidi, M.B.; Prot, M.; Ruffie, C.; Kim, S.S.; Allikmets, R.; et al. Corrigendum: Optic neuropathy and congenital glaucoma associated with probable Zika virus infection in Venezuelan patients. JMM Case Rep. 2018, 5, e005161. [Google Scholar] [CrossRef]
- Malkki, H. CNS infections: Zika virus infection could trigger Guillain-Barre syndrome. Nat. Rev. Neurol. 2016, 12, 187. [Google Scholar] [CrossRef]
- Carteaux, G.; Maquart, M.; Bedet, A.; Contou, D.; Brugieres, P.; Fourati, S.; Cleret de Langavant, L.; de Broucker, T.; Brun-Buisson, C.; Leparc-Goffart, I.; et al. Zika Virus Associated with Meningoencephalitis. N. Engl. J. Med. 2016, 374, 1595–1596. [Google Scholar] [CrossRef]
- Woods, C.G.; Bond, J.; Enard, W. Autosomal recessive primary microcephaly (MCPH): A review of clinical, molecular, and evolutionary findings. Am. J. Hum. Genet. 2005, 76, 717–728. [Google Scholar] [CrossRef]
- McCarthy, M. Zika virus was transmitted by sexual contact in Texas, health officials report. BMJ 2016, 352, i720. [Google Scholar] [CrossRef] [PubMed]
- Deng, Y.Q.; Zhang, N.N.; Li, C.F.; Tian, M.; Hao, J.N.; Xie, X.P.; Shi, P.Y.; Qin, C.F. Adenosine Analog NITD008 Is a Potent Inhibitor of Zika Virus. Open Forum Infect. Dis. 2016, 3, ofw175. [Google Scholar] [CrossRef] [PubMed]
- Delvecchio, R.; Higa, L.M.; Pezzuto, P.; Valadao, A.L.; Garcez, P.P.; Monteiro, F.L.; Loiola, E.C.; Dias, A.A.; Silva, F.J.; Aliota, M.T.; et al. Chloroquine, an Endocytosis Blocking Agent, Inhibits Zika Virus Infection in Different Cell Models. Viruses 2016, 8, 322. [Google Scholar] [CrossRef]
- Li, C.; Deng, Y.Q.; Wang, S.; Ma, F.; Aliyari, R.; Huang, X.Y.; Zhang, N.N.; Watanabe, M.; Dong, H.L.; Liu, P.; et al. 25-Hydroxycholesterol Protects Host against Zika Virus Infection and Its Associated Microcephaly in a Mouse Model. Immunity 2017, 46, 446–456. [Google Scholar] [CrossRef] [PubMed]
- Rausch, K.; Hackett, B.A.; Weinbren, N.L.; Reeder, S.M.; Sadovsky, Y.; Hunter, C.A.; Schultz, D.C.; Coyne, C.B.; Cherry, S. Screening Bioactives Reveals Nanchangmycin as a Broad Spectrum Antiviral Active against Zika Virus. Cell Rep. 2017, 18, 804–815. [Google Scholar] [CrossRef] [PubMed]
- Arabi, Y.M.; Deeb, A.M.; Al-Hameed, F.; Mandourah, Y.; Almekhlafi, G.A.; Sindi, A.A.; Al-Omari, A.; Shalhoub, S.; Mady, A.; Alraddadi, B.; et al. Macrolides in critically ill patients with Middle East Respiratory Syndrome. Int. J. Infect. Dis. 2019, 81, 184–190. [Google Scholar] [CrossRef]
- Yokota, S.; Okabayashi, T.; Hirakawa, S.; Tsutsumi, H.; Himi, T.; Fujii, N. Clarithromycin suppresses human respiratory syncytial virus infection-induced Streptococcus pneumoniae adhesion and cytokine production in a pulmonary epithelial cell line. Mediat. Inflamm. 2012, 2012, 528568. [Google Scholar] [CrossRef]
- Dong, S.; Kang, S. Identification of anti-flaviviral drugs with mosquitocidal and anti-Zika virus activity in Aedes aegypti. PLoS Negl. Trop. Dis. 2019, 13. [Google Scholar] [CrossRef]
- Bosseboeuf, E.; Aubry, M.; Nhan, T.; Pina, J.J.; Rolain, J.M.; Raoult, D.; Musso, D. Azithromycin Inhibits the Replication of Zika Virus. J. Antivir. Antiretrovir. 2018, 10, 6–11. [Google Scholar] [CrossRef]
- Iannetta, M.; Ippolito, G.; Nicastri, E. Azithromycin Shows Anti-Zika Virus Activity in Human Glial Cells. Antimicrob. Agents Chemother. 2017, 61. [Google Scholar] [CrossRef]
- Retallack, H.; Di Lullo, E.; Arias, C.; Knopp, K.A.; Laurie, M.T.; Sandoval-Espinosa, C.; Mancia Leon, W.R.; Krencik, R.; Ullian, E.M.; Spatazza, J.; et al. Zika virus cell tropism in the developing human brain and inhibition by azithromycin. Am. Proc. Natl. Acad. Sci. USA 2016, 113, 14408–14413. [Google Scholar] [CrossRef] [PubMed]
- Wu, Y.H.; Tseng, C.K.; Lin, C.K.; Wei, C.K.; Lee, J.C.; Young, K.C. ICR suckling mouse model of Zika virus infection for disease modeling and drug validation. PLoS Negl. Trop. Dis. 2018, 12. [Google Scholar] [CrossRef]
- Deng, Y.Q.; Zhao, H.; Li, X.F.; Zhang, N.N.; Liu, Z.Y.; Jiang, T.; Gu, D.Y.; Shi, L.; He, J.A.; Qin, C.F.; et al. Isolation, identification and genomic characterization of the Asian lineage Zika virus imported to China. Sci. China Life Sci. 2016, 59, 428–430. [Google Scholar] [CrossRef]
- Lahon, A.; Arya, R.P.; Kneubehl, A.R.; Vogt, M.B.; Dailey Garnes, N.J.; Rico-Hesse, R. Characterization of a Zika Virus Isolate from Colombia. PLoS Negl. Trop. Dis. 2016, 10, e0005019. [Google Scholar] [CrossRef] [PubMed]
- Deng, Y.Q.; Dai, J.X.; Ji, G.H.; Jiang, T.; Wang, H.J.; Yang, H.O.; Tan, W.L.; Liu, R.; Yu, M.; Qin, C.F.; et al. A broadly flavivirus cross-neutralizing monoclonal antibody that recognizes a novel epitope within the fusion loop of E protein. PLoS ONE 2011, 6, e16059. [Google Scholar] [CrossRef] [PubMed]
- Yu, Y.; Deng, Y.Q.; Zou, P.; Wang, Q.; Dai, Y.; Yu, F.; Du, L.; Qin, C.F.; Jiang, S.; Lu, L.; et al. A peptide-based viral inactivator inhibits Zika virus infection in pregnant mice and fetuses. Nat. Commun. 2017, 8, 15672. [Google Scholar] [CrossRef] [PubMed]
- Xie, J.; Liu, J.H.; Liu, H.; Liao, X.Z.; Chen, Y.; Lin, M.G.; Gu, Y.Y.; Liu, T.L.; Wang, D.M.; Ge, H.; et al. Tanshinone IIA combined with adriamycin inhibited malignant biological behaviors of NSCLC A549 cell line in a synergistic way. BMC Cancer 2016, 16, 899. [Google Scholar] [CrossRef]
- Espano, E.; Nam, J.H.; Song, E.J. Lipophilic statins inhibit Zika virus production in Vero cells. Sci. Rep. 2019, 9, 11461. [Google Scholar] [CrossRef]
- Mounce, B.C.; Cesaro, T.; Carrau, L.; Vallet, T.; Vignuzzi, M. Curcumin inhibits Zika and chikungunya virus infection by inhibiting cell binding. Antivir. Res. 2017, 142, 148–157. [Google Scholar] [CrossRef]
- Lu, L.; Liu, Q.; Zhu, Y.; Chan, K.H.; Qin, L.; Li, Y.; Wang, Q.; Chan, J.F.; Jiang, S.; Yu, F.; et al. Structure-based discovery of Middle East respiratory syndrome coronavirus fusion inhibitor. Nat. Commun. 2014, 5, 3067. [Google Scholar] [CrossRef]
- Zhao, G.; Du, L.; Ma, C.; Li, Y.; Li, L.; Poon, V.K.; Wang, L.; Zheng, B.J.; Jiang, S.; Zhou, Y.; et al. A safe and convenient pseudovirus-based inhibition assay to detect neutralizing antibodies and screen for viral entry inhibitors against the novel human coronavirus MERS-CoV. Virol. J. 2013, 10, 266. [Google Scholar] [CrossRef] [Green Version]
- Si, L.; Meng, K.; Tian, Z.; Sun, J.; Li, H.; Zhang, Z.; Soloveva, V.; Xiao, S.; Zhang, L.; Zhou, D.; et al. Triterpenoids manipulate a broad range of virus-host fusion via wrapping the HR2 domain prevalent in viral envelopes. Sci. Adv. 2018, 4, eaau8408. [Google Scholar] [CrossRef] [Green Version]
- Lu, L.; Pan, C.; Li, Y.; Lu, H.; He, W.; Jiang, S. A bivalent recombinant protein inactivates HIV-1 by targeting the gp41 prehairpin fusion intermediate induced by CD4 D1D2 domains. Retrovirology 2012, 9, 104. [Google Scholar] [CrossRef] [Green Version]
- Lok, S.M.; Costin, J.M.; Hrobowski, Y.M.; Hoffmann, A.R.; Rowe, D.K.; Kukkaro, P.; Wimley, W.C.; Isern, S.; Rossmann, M.G.; Michael, S.F.; et al. Release of dengue virus genome induced by a peptide inhibitor. PLoS ONE 2012, 7, e50995. [Google Scholar] [CrossRef]
- Aliota, M.T.; Caine, E.A.; Walker, E.C.; Larkin, K.E.; Camacho, E.; Osorio, J.E. Characterization of Lethal Zika Virus Infection in AG129 Mice. PLoS Negl. Trop. Dis. 2016, 10, e0004682. [Google Scholar] [CrossRef] [Green Version]
- Li, S.; Armstrong, N.; Zhao, H.; Hou, W.; Liu, J.; Chen, C.; Zhong, C.; Liu, C.; Zhu, H.; Xia, N.; et al. Zika Virus Fatally Infects Wild Type Neonatal Mice and Replicates in Central Nervous System. Viruses 2018, 10, 49. [Google Scholar] [CrossRef] [Green Version]
- Chan, J.F.; Yip, C.C.; Tsang, J.O.; Tee, K.M.; Cai, J.P.; Chik, K.K.; Zhu, Z.; Jin, D.Y.; Chan, K.H.; Yuen, K.Y.; et al. Differential cell line susceptibility to the emerging Zika virus: Implications for disease pathogenesis, non-vector-borne human transmission and animal reservoirs. Emerg. Microbes Infect. 2016, 5, e93. [Google Scholar] [CrossRef] [Green Version]
- Conzelmann, C.; Zou, M.; Gross, R.; Harms, M.; Rocker, A.; Riedel, C.U.; Munch, J.; Muller, J.A. Storage-Dependent Generation of Potent Anti-ZIKV Activity in Human Breast Milk. Viruses 2019, 11, 591. [Google Scholar] [CrossRef] [Green Version]
- Dai, L.; Song, J.; Lu, X.; Deng, Y.Q.; Musyoki, A.M.; Cheng, H.; Shi, Y.; Qin, C.F.; Qi, J.; Gao, G.F.; et al. Structures of the Zika Virus Envelope Protein and Its Complex with a Flavivirus Broadly Protective Antibody. Cell Host Microbe 2016, 19, 696–704. [Google Scholar] [CrossRef]
- Park, J.G.; Avila-Perez, G.; Madere, F.; Hilimire, T.A.; Nogales, A.; Almazan, F.; Martinez-Sobrido, L. Potent Inhibition of Zika Virus Replication by Aurintricarboxylic Acid. Front. Microbiol. 2019, 10, 718. [Google Scholar] [CrossRef]
- Raza, S.; Abbas, G.; Azam, S.S. Screening pipeline for Flavivirus based Inhibitors for Zika Virus NS1. IEEE/ACM Trans. Comput. Biol. Bioinform. 2019. [Google Scholar] [CrossRef]
- Gorshkov, K.; Shiryaev, S.A.; Fertel, S.; Lin, Y.W.; Huang, C.T.; Pinto, A.; Farhy, C.; Strongin, A.Y.; Zheng, W.; Terskikh, A.V. Zika Virus: Origins, Pathological Action, and Treatment Strategies. Front. Microbiol. 2018, 9, 3252. [Google Scholar] [CrossRef] [Green Version]
- Croteau, D.; Bergeron, M.G.; LeBel, M. Pharmacokinetic advantages of erythromycin estolate over ethylsuccinate as determined by high-pressure liquid chromatography. Antimicrob. Agents Chemother. 1988, 32, 561–565. [Google Scholar] [CrossRef] [Green Version]
- Altunaiji, S.; Kukuruzovic, R.; Curtis, N.; Massie, J. Antibiotics for whooping cough (pertussis). Cochrane Database Syst. Rev. 2007, Cd004404. [Google Scholar] [CrossRef]
- Langley, J.M.; Halperin, S.A.; Boucher, F.D.; Smith, B. Azithromycin is as effective as and better tolerated than erythromycin estolate for the treatment of pertussis. Pediatrics. 2004, 114, e96–e101. [Google Scholar] [CrossRef] [Green Version]
- Raynes-Greenow, C.H.; Roberts, C.L.; Bell, J.C.; Peat, B.; Gilbert, G.L. Antibiotics for ureaplasma in the vagina in pregnancy. Cochrane Database Syst. Rev. 2004, Cd003767. [Google Scholar] [CrossRef]
- DeCocker, K. Zika Virus and Pregnancy Concerns. Nurs. Clin. N. Am. 2019, 54, 285–295. [Google Scholar] [CrossRef]
- Grazel, R.; Harris-Haman, P. Zika Virus Infection: A Vector-Borne Threat to Pregnant Women and Infants. Adv. Neonatal Care 2018, 18, 350–359. [Google Scholar] [CrossRef]
- Mota, V.M.R.; Cavalcanti, L.P.G.; Delfino, A.D.S.; Lopes, T.; Pessoa, A.L.S.; Ribeiro, E.M. Abortion in Cases of Zika Virus Congenital Infection. Rev. Bras. Ginecol. Obstet. 2018, 40, 417–424. [Google Scholar] [CrossRef] [Green Version]
- Cui, L.; Zou, P.; Chen, E.; Yao, H.; Zheng, H.; Wang, Q.; Zhu, J.N.; Jiang, S.; Lu, L.; Zhang, J. Visual and Motor Deficits in Grown-up Mice with Congenital Zika Virus Infection. EBioMedicine 2017, 20, 193–201. [Google Scholar] [CrossRef] [Green Version]
- Sakkas, H.; Bozidis, P.; Giannakopoulos, X.; Sofikitis, N.; Papadopoulou, C. An update on sexual transmission of Zika virus. Pathogens 2018, 7, 66. [Google Scholar] [CrossRef] [Green Version]
- Duggal, N.K.; Ritter, J.M.; Pestorius, S.E.; Zaki, S.R.; Davis, B.S.; Chang, G.J.; Bowen, R.A.; Brault, A.C. Frequent Zika Virus Sexual Transmission and Prolonged Viral RNA Shedding in an Immunodeficient Mouse Model. Cell Rep. 2017, 18, 1751–1760. [Google Scholar] [CrossRef] [Green Version]
- Gunawardana, S.A.; Shaw, R.H. Cross-reactive dengue virus-derived monoclonal antibodies to Zika virus envelope protein: Panacea or Pandora’s box? BMC Infect. Dis. 2018, 18, 641. [Google Scholar] [CrossRef] [Green Version]
- Li, M.; Zhao, L.; Zhang, C.; Wang, X.; Hong, W.; Sun, J.; Liu, R.; Wang, J.; Zhang, F.; Jin, X.; et al. Dengue immune sera enhance Zika virus infection in human peripheral blood monocytes through Fc gamma receptors. PLoS ONE 2018, 13, e0200478. [Google Scholar] [CrossRef] [Green Version]
- Durbin, A.P. Dengue Antibody and Zika: Friend or Foe? Trends Immunol. 2016, 37, 635–636. [Google Scholar] [CrossRef] [Green Version]
- Morrone, S.R.; Lok, S.M. Structural perspectives of antibody-dependent enhancement of infection of dengue virus. Curr. Opin. Virol. 2019, 36, 1–8. [Google Scholar] [CrossRef]
- Sapparapu, G.; Fernandez, E.; Kose, N.; Bin, C.; Fox, J.M.; Bombardi, R.G.; Fremont, D.H.; Doranz, B.J.; Diamond, M.S.; Crowe, J.E.; et al. Neutralizing human antibodies prevent Zika virus replication and fetal disease in mice. Nature 2016, 540, 443–447. [Google Scholar] [CrossRef] [Green Version]

© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, X.; Xia, S.; Zou, P.; Lu, L. Erythromycin Estolate Inhibits Zika Virus Infection by Blocking Viral Entry as a Viral Inactivator. Viruses 2019, 11, 1064. https://doi.org/10.3390/v11111064
Wang X, Xia S, Zou P, Lu L. Erythromycin Estolate Inhibits Zika Virus Infection by Blocking Viral Entry as a Viral Inactivator. Viruses. 2019; 11(11):1064. https://doi.org/10.3390/v11111064
Chicago/Turabian StyleWang, Xiaohuan, Shuai Xia, Peng Zou, and Lu Lu. 2019. "Erythromycin Estolate Inhibits Zika Virus Infection by Blocking Viral Entry as a Viral Inactivator" Viruses 11, no. 11: 1064. https://doi.org/10.3390/v11111064
APA StyleWang, X., Xia, S., Zou, P., & Lu, L. (2019). Erythromycin Estolate Inhibits Zika Virus Infection by Blocking Viral Entry as a Viral Inactivator. Viruses, 11(11), 1064. https://doi.org/10.3390/v11111064





