Simultaneous Immunization with Multivalent Norovirus VLPs Induces Better Protective Immune Responses to Norovirus than Sequential Immunization
Abstract
:1. Introduction
2. Materials and Methods
2.1. Recombinant Proteins Production and Purification
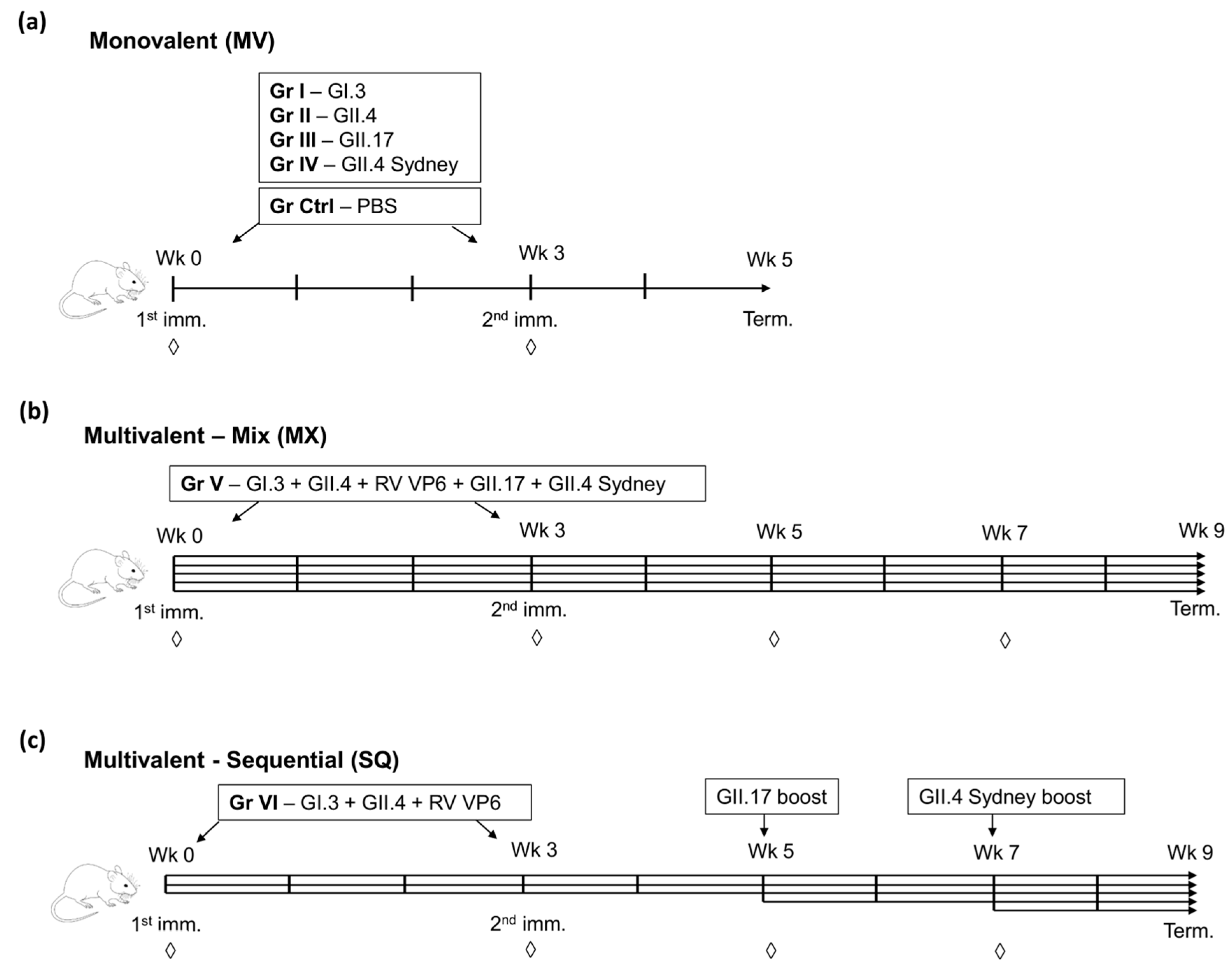
2.2. Mice Immunization
2.3. NoV-Specific ELISA
2.4. Blocking Assay
2.5. Statistics
3. Results
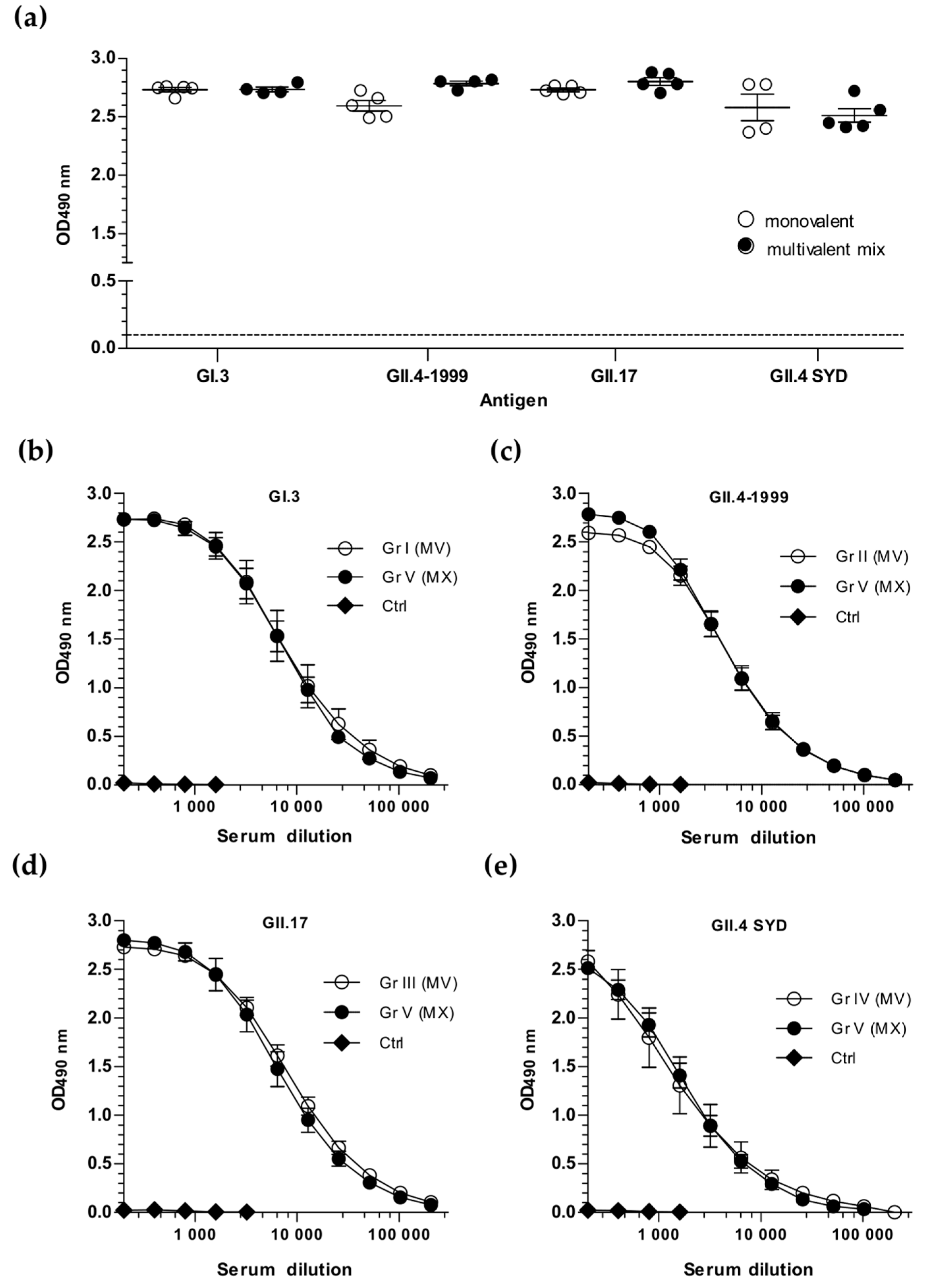
3.1. Simultaneous Immunization with Multivalent VLP Mixture Formulation
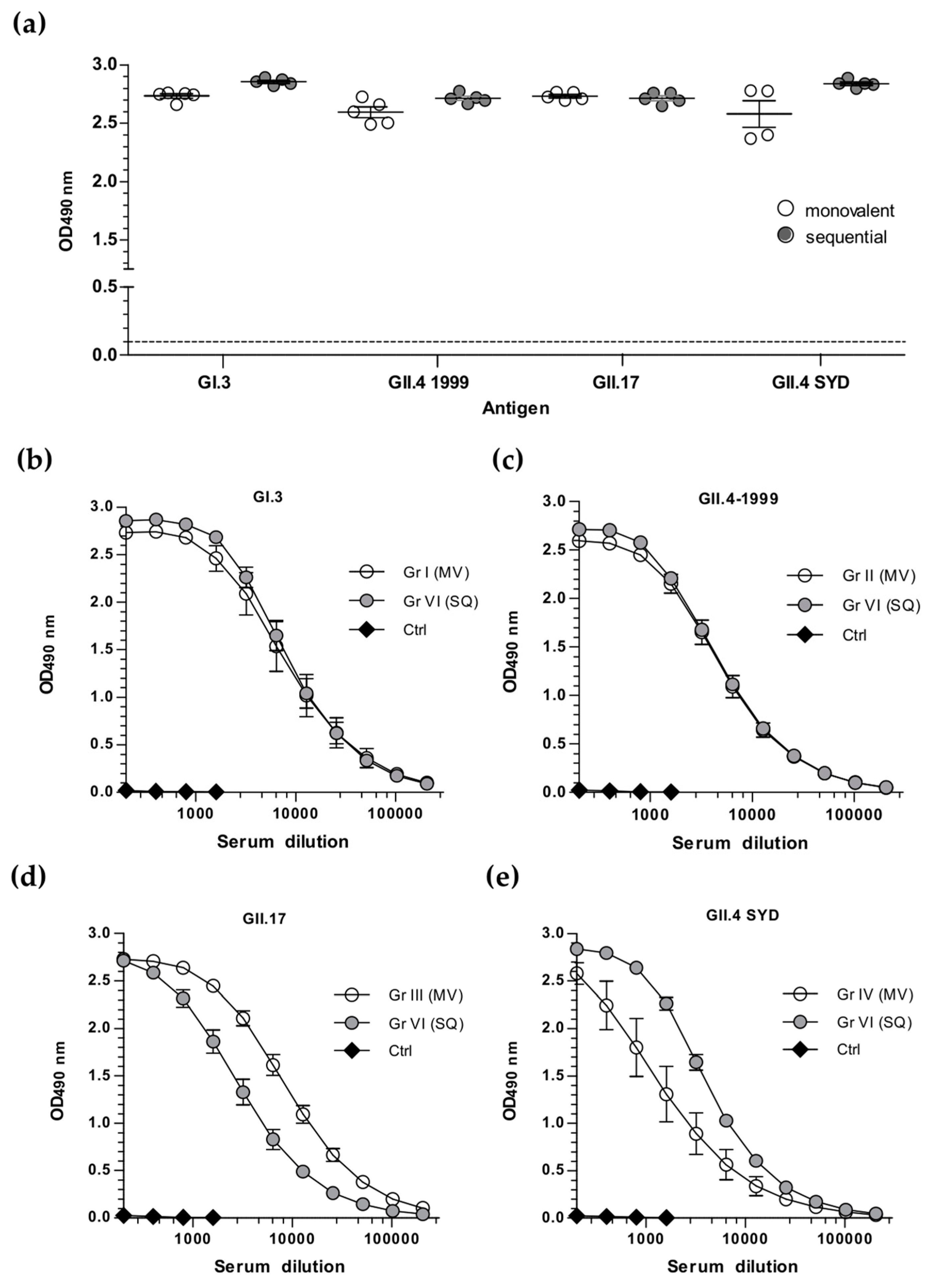
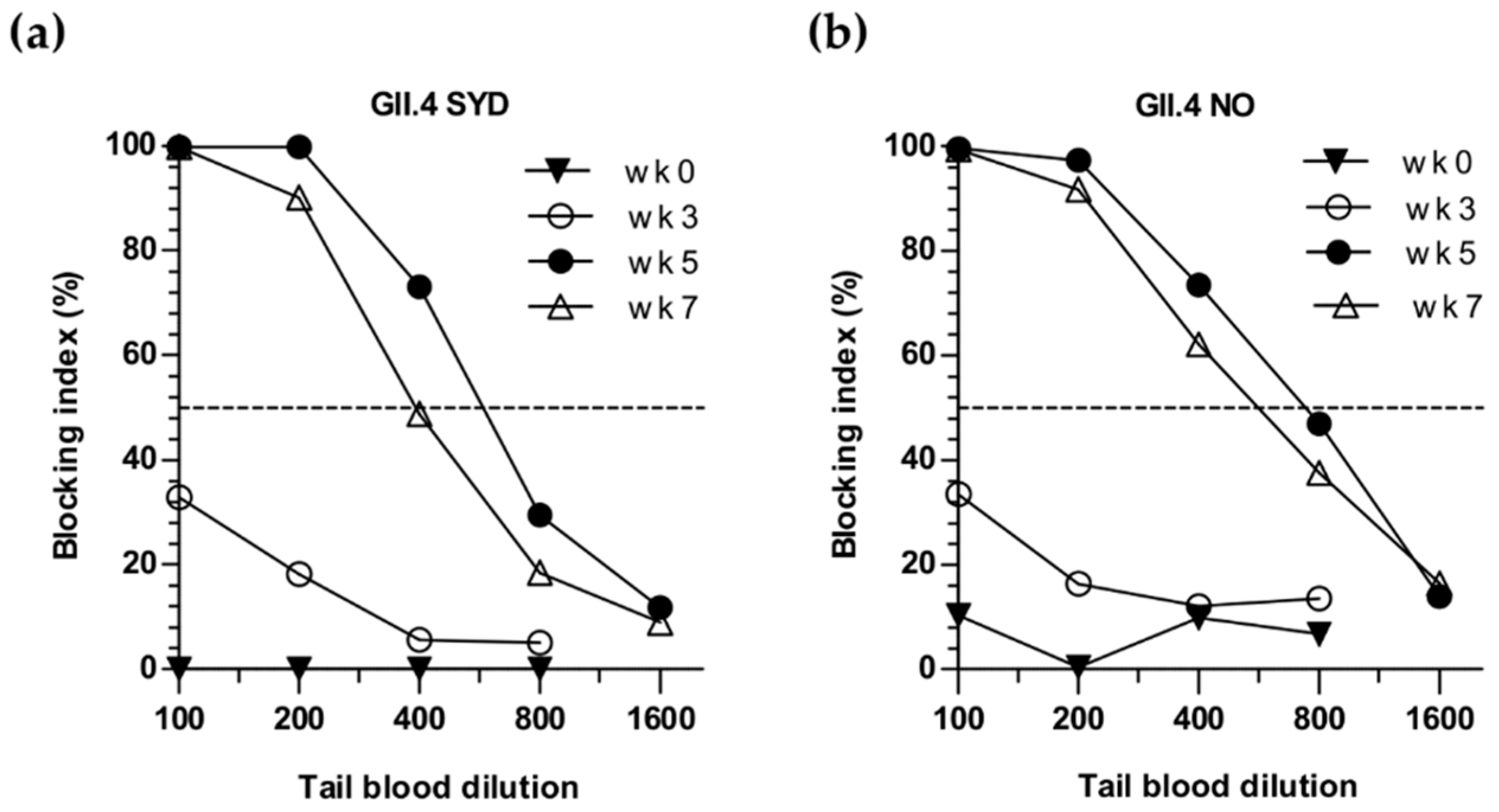
3.2. Sequential Immunization with Genetically Distant and Closely Related NoV VLPs
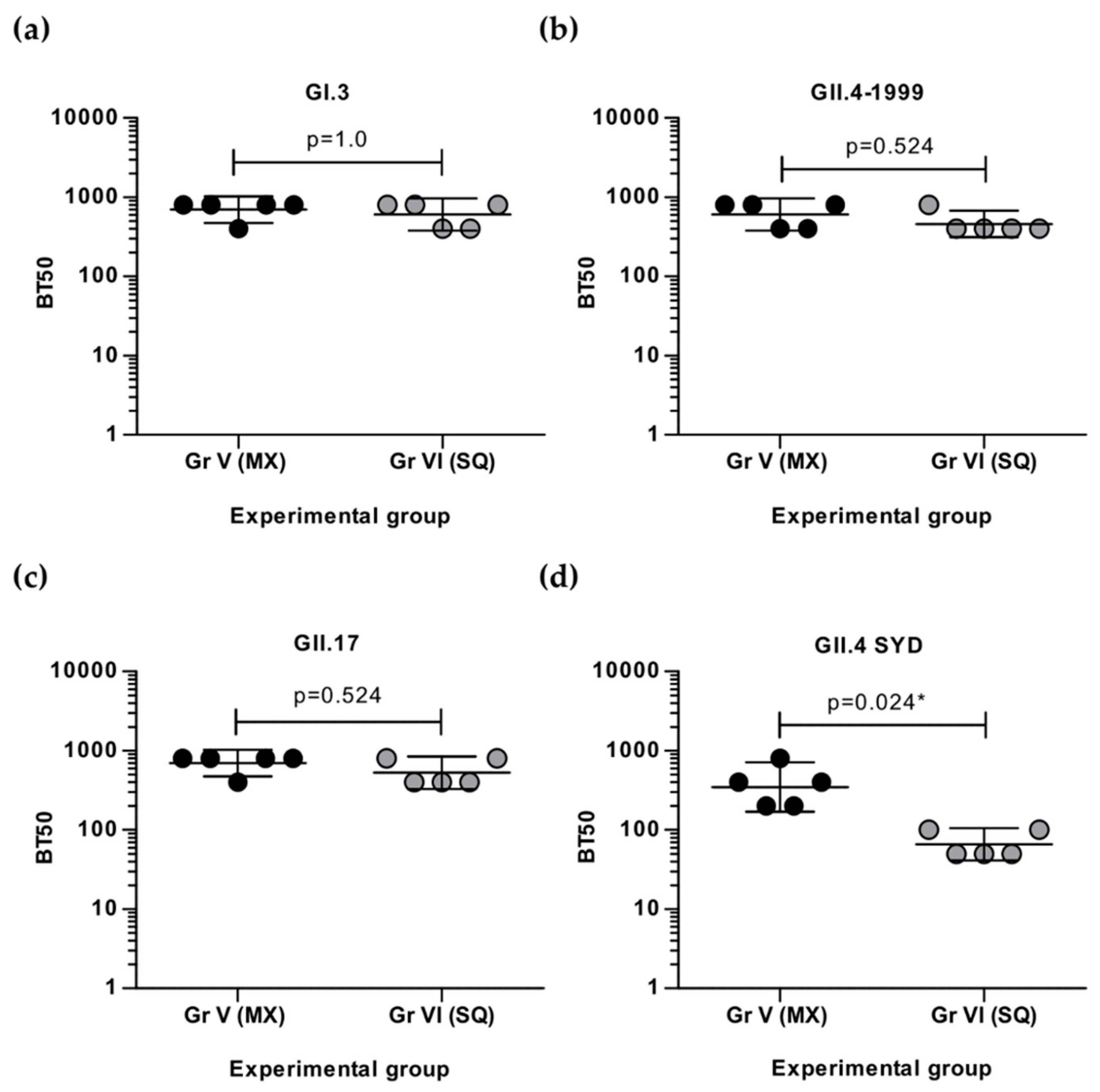
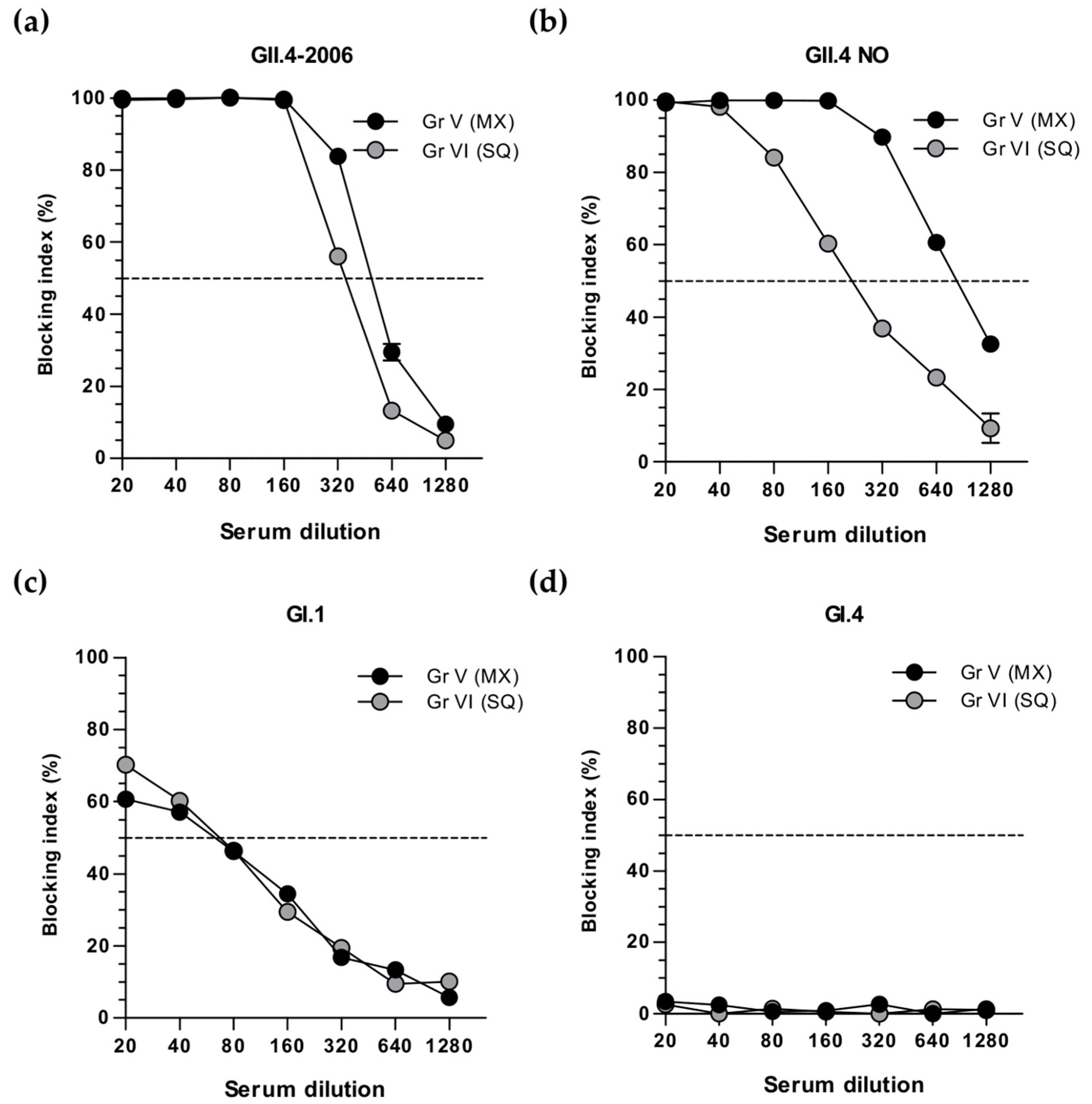
3.3. Simultaneous and Sequential Immunizations Induce Different Level of Blocking Antibodies
3.4. Cross-Protective Blocking Antibody Responses
4. Discussion
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Lopman, B.A.; Steele, D.; Kirkwood, C.D.; Parashar, U.D. The Vast and Varied Global Burden of Norovirus: Prospects for Prevention and Control. PLoS Med. 2016, 13, e1001999. [Google Scholar] [CrossRef] [PubMed]
- Cortes-Penfield, N.W.; Ramani, S.; Estes, M.K.; Atmar, R.L. Prospects and Challenges in the Development of a Norovirus Vaccine. Clin. Ther. 2017, 39, 1537–1549. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jiang, X.; Wang, M.; Graham, D.Y.; Estes, M.K. Expression, self-assembly, and antigenicity of the Norwalk virus capsid protein. J. Virol. 1992, 66, 6527–6532. [Google Scholar] [PubMed]
- Malm, M.; Diessner, A.; Tamminen, K.; Liebscher, M.; Vesikari, T.; Blazevic, V. Rotavirus VP6 as an Adjuvant for Bivalent Norovirus Vaccine Produced in Nicotiana benthamiana. Pharmaceutics 2019, 11, 229. [Google Scholar] [CrossRef]
- Blazevic, V.; Lappalainen, S.; Nurminen, K.; Huhti, L.; Vesikari, T. Norovirus VLPs and rotavirus VP6 protein as combined vaccine for childhood gastroenteritis. Vaccine 2011, 29, 8126–8133. [Google Scholar] [CrossRef]
- Lucero, Y.; Vidal, R.; O’Ryan, G.M. Norovirus vaccines under development. Vaccine 2017, 36, 5435–5441. [Google Scholar] [CrossRef]
- Chhabra, P.; de Graaf, M.; Parra, G.I.; Chan, M.C.; Green, K.; Martella, V.; Wang, Q.; White, P.A.; Katayama, K.; Vennema, H.; et al. Updated classification of norovirus genogroups and genotypes. J. Gen. Virol. 2019, 100, 1393–1406. [Google Scholar] [CrossRef]
- Vega, E.; Barclay, L.; Gregoricus, N.; Shirley, S.H.; Lee, D.; Vinje, J. Genotypic and epidemiologic trends of norovirus outbreaks in the United States, 2009 to 2013. J. Clin. Microbiol. 2014, 52, 147–155. [Google Scholar] [CrossRef]
- Uusi-Kerttula, H.; Tamminen, K.; Malm, M.; Vesikari, T.; Blazevic, V. Comparison of human saliva and synthetic histo-blood group antigens usage as ligands in norovirus-like particle binding and blocking assays. Microbes. Infect. 2014, 16, 472–480. [Google Scholar] [CrossRef]
- Harrington, P.R.; Lindesmith, L.; Yount, B.; Moe, C.L.; Baric, R.S. Binding of Norwalk virus-like particles to ABH histo-blood group antigens is blocked by antisera from infected human volunteers or experimentally vaccinated mice. J. Virol. 2002, 76, 12335–12343. [Google Scholar] [CrossRef]
- Tan, M.; Jiang, X. Norovirus and its histo-blood group antigen receptors: An answer to a historical puzzle. Trends Microbiol. 2005, 13, 285–293. [Google Scholar] [CrossRef] [PubMed]
- Reeck, A.; Kavanagh, O.; Estes, M.K.; Opekun, A.R.; Gilger, M.A.; Graham, D.Y.; Atmar, R.L. Serological correlate of protection against norovirus-induced gastroenteritis. J. Infect. Dis. 2010, 202, 1212–1218. [Google Scholar] [CrossRef] [PubMed]
- Malm, M.; Tamminen, K.; Blazevic, V. Assessment of Functional Norovirus Antibody Responses by Blocking Assay in Mice. Methods Mol. Biol. 2016, 1403, 259–268. [Google Scholar] [CrossRef] [PubMed]
- Nurminen, K.; Blazevic, V.; Huhti, L.; Rasanen, S.; Koho, T.; Hytonen, V.P.; Vesikari, T. Prevalence of norovirus GII-4 antibodies in Finnish children. J. Med. Virol. 2011, 83, 525–531. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ramani, S.; Estes, M.K.; Atmar, R.L. Correlates of Protection against Norovirus Infection and Disease-Where Are We Now, Where Do We Go? PLoS Pathog. 2016, 12, e1005334. [Google Scholar] [CrossRef]
- Malm, M.; Uusi-Kerttula, H.; Vesikari, T.; Blazevic, V. High serum levels of norovirus genotype-specific blocking antibodies correlate with protection from infection in children. J. Infect. Dis. 2014, 210, 1755–1762. [Google Scholar] [CrossRef]
- Malm, M.; Tamminen, K.; Lappalainen, S.; Uusi-Kerttula, H.; Vesikari, T.; Blazevic, V. Genotype considerations for virus-like particle-based bivalent norovirus vaccine composition. Clin. Vaccine Immunol. 2015, 22, 656–663. [Google Scholar] [CrossRef]
- Hansman, G.S.; Natori, K.; Shirato-Horikoshi, H.; Ogawa, S.; Oka, T.; Katayama, K.; Tanaka, T.; Miyoshi, T.; Sakae, K.; Kobayashi, S.; et al. Genetic and antigenic diversity among noroviruses. J. Gen. Virol. 2006, 87, 909–919. [Google Scholar] [CrossRef]
- Blazevic, V.; Malm, M.; Vesikari, T. Induction of homologous and cross-reactive GII.4-specific blocking antibodies in children after GII.4 New Orleans norovirus infection. J. Med. Virol. 2015, 87, 1656–1661. [Google Scholar] [CrossRef]
- Tamminen, K.; Malm, M.; Vesikari, T.; Blazevic, V. Immunological Cross-Reactivity of an Ancestral and the Most Recent Pandemic Norovirus GII.4 Variant. Viruses 2019, 11, 91. [Google Scholar] [CrossRef]
- Blazevic, V.; Malm, M.; Honkanen, H.; Knip, M.; Hyoty, H.; Vesikari, T. Development and maturation of norovirus antibodies in childhood. Microbes. Infect. 2015, 18, 263–269. [Google Scholar] [CrossRef] [PubMed]
- Blazevic, V.; Malm, M.; Salminen, M.; Oikarinen, S.; Hyoty, H.; Veijola, R.; Vesikari, T. Multiple consecutive norovirus infections in the first 2 years of life. Eur. J. Pediatr. 2015, 174, 1679–1683. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Saito, M.; Goel-Apaza, S.; Espetia, S.; Velasquez, D.; Cabrera, L.; Loli, S.; Crabtree, J.E.; Black, R.E.; Kosek, M.; Checkley, W.; et al. Multiple norovirus infections in a birth cohort in a Peruvian Periurban community. Clin. Infect. Dis. 2014, 58, 483–491. [Google Scholar] [CrossRef] [PubMed]
- Zheng, D.P.; Widdowson, M.A.; Glass, R.I.; Vinje, J. Molecular epidemiology of genogroup II-genotype 4 noroviruses in the United States between 1994 and 2006. J. Clin. Microbiol. 2010, 48, 168–177. [Google Scholar] [CrossRef] [PubMed]
- Eden, J.S.; Tanaka, M.M.; Boni, M.F.; Rawlinson, W.D.; White, P.A. Recombination within the pandemic norovirus GII.4 lineage. J. Virol. 2013, 87, 6270–6282. [Google Scholar] [CrossRef] [PubMed]
- Hasing, M.E.; Lee, B.E.; Qiu, Y.Y.; Xia, M.; Pabbaraju, K.; Wong, A.; Tipples, G.; Jiang, X.; Pang, X.L.L. Changes in norovirus genotype diversity in gastroenteritis outbreaks in Alberta, Canada: 2012-2018. BMC Infect. Dis. 2019, 19, 177. [Google Scholar] [CrossRef] [PubMed]
- De Graaf, M.; Van Beek, J.; Vennema, H.; Podkolzin, A.T.; Hewitt, J.; Bucardo, F.; Templeton, K.; Mans, J.; Nordgren, J.; Reuter, G.; et al. Emergence of a novel GII.17 norovirus - End of the GII.4 era? Euro. Surveill. 2015, 20, 21178. [Google Scholar] [CrossRef]
- Malm, M.; Tamminen, K.; Vesikari, T.; Blazevic, V. Norovirus GII.17 Virus-Like Particles Bind to Different Histo-Blood Group Antigens and Cross-React with Genogroup II-Specific Mouse Sera. Viral Immunol. 2018, 31, 649–657. [Google Scholar] [CrossRef]
- Dai, Y.C.; Xia, M.; Huang, Q.; Tan, M.; Qin, L.; Zhuang, Y.L.; Long, Y.; Li, J.D.; Jiang, X.; Zhang, X.F. Characterization of Antigenic Relatedness between GII.4 and GII.17 Noroviruses by Use of Serum Samples from Norovirus-Infected Patients. J. Clin. Microbiol. 2017, 55, 3366–3373. [Google Scholar] [CrossRef] [Green Version]
- LoBue, A.D.; Lindesmith, L.; Yount, B.; Harrington, P.R.; Thompson, J.M.; Johnston, R.E.; Moe, C.L.; Baric, R.S. Multivalent norovirus vaccines induce strong mucosal and systemic blocking antibodies against multiple strains. Vaccine 2006, 24, 5220–5234. [Google Scholar] [CrossRef]
- Treanor, J.J.; Atmar, R.L.; Frey, S.E.; Gormley, R.; Chen, W.H.; Ferreira, J.; Goodwin, R.; Borkowski, A.; Clemens, R.; Mendelman, P.M. A Novel Intramuscular Bivalent Norovirus Virus-Like Particle Vaccine Candidate-Reactogenicity, Safety, and Immunogenicity in a Phase 1 Trial in Healthy Adults. J. Infect. Dis. 2014, 210, 1763–1771. [Google Scholar] [CrossRef] [PubMed]
- Tamminen, K.; Lappalainen, S.; Huhti, L.; Vesikari, T.; Blazevic, V. Trivalent combination vaccine induces broad heterologous immune responses to norovirus and rotavirus in mice. PLoS ONE 2013, 8, e70409. [Google Scholar] [CrossRef] [PubMed]
- Clark, A.; van Zandvoort, K.; Flasche, S.; Sanderson, C.; Bines, J.; Tate, J.; Parashar, U.; Jit, M. Efficacy of live oral rotavirus vaccines by duration of follow-up: A meta-regression of randomised controlled trials. Lancet Infect. Dis. 2019, 19, 717–727. [Google Scholar] [CrossRef]
- Ward, R.L.; McNeal, M.M. VP6: A candidate rotavirus vaccine. J. Infect. Dis. 2010, 202 (Suppl. 1), S101–S107. [Google Scholar] [CrossRef]
- Lappalainen, S.; Pastor, A.R.; Tamminen, K.; Lopez-Guerrero, V.; Esquivel-Guadarrama, F.; Palomares, L.A.; Vesikari, T.; Blazevic, V. Immune responses elicited against rotavirus middle layer protein VP6 inhibit viral replication in vitro and in vivo. Hum. Vaccin. Immunother. 2014, 10, 2039–2047. [Google Scholar] [CrossRef]
- Huhti, L.; Blazevic, V.; Nurminen, K.; Koho, T.; Hytonen, V.P.; Vesikari, T. A comparison of methods for purification and concentration of norovirus GII-4 capsid virus-like particles. Arch. Virol. 2010, 155, 1855–1858. [Google Scholar] [CrossRef] [Green Version]
- Malm, M.; Heinimäki, S.; Vesikari, T.; Blazevic, V. Rotavirus capsid VP6 tubular and spherical nanostructures act as local adjuvants when co-delivered with norovirus VLPs. Clin. Exp. Immunol. 2017, 189, 331–341. [Google Scholar] [CrossRef] [Green Version]
- Lindesmith, L.C.; Debbink, K.; Swanstrom, J.; Vinje, J.; Costantini, V.; Baric, R.S.; Donaldson, E.F. Monoclonal antibody-based antigenic mapping of norovirus GII.4-2002. J. Virol. 2012, 86, 873–883. [Google Scholar] [CrossRef]
- Malm, M.; Tamminen, K.; Heinimaki, S.; Vesikari, T.; Blazevic, V. Functionality and avidity of norovirus-specific antibodies and T cells induced by GII.4 virus-like particles alone or co-administered with different genotypes. Vaccine 2018, 36, 484–490. [Google Scholar] [CrossRef] [Green Version]
- Sato, S.; Hisaie, K.; Kurokawa, S.; Suzuki, A.; Sakon, N.; Uchida, Y.; Yuki, Y.; Kiyono, H. Human Norovirus Propagation in Human Induced Pluripotent Stem Cell-Derived Intestinal Epithelial Cells. Cell. Mol. Gastroenterol. Hepatol. 2019, 7, 686–688.e5. [Google Scholar] [CrossRef]
- Malm, M.; Tamminen, K.; Lappalainen, S.; Vesikari, T.; Blazevic, V. Rotavirus Recombinant VP6 Nanotubes Act as an Immunomodulator and Delivery Vehicle for Norovirus Virus-Like Particles. J. Immunol. Res. 2016, 2016, 9171632. [Google Scholar] [CrossRef] [PubMed]
- Puustinen, L.; Blazevic, V.; Huhti, L.; Szakal, E.D.; Halkosalo, A.; Salminen, M.; Vesikari, T. Norovirus genotypes in endemic acute gastroenteritis of infants and children in Finland between 1994 and 2007. Epidemiol. Infect. 2012, 140, 268–275. [Google Scholar] [CrossRef] [PubMed]
- Kim, L.; Liebowitz, D.; Lin, K.; Kasparek, K.; Pasetti, M.F.; Garg, S.J.; Gottlieb, K.; Trager, G.; Tucker, S.N. Safety and immunogenicity of an oral tablet norovirus vaccine, a phase I randomized, placebo-controlled trial. JCI Insight 2018, 3, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Leroux-Roels, G.; Cramer, J.P.; Mendelman, P.M.; Sherwood, J.; Clemens, R.; Aerssens, A.; De Coster, I.; Borkowski, A.; Baehner, F.; Van Damme, P. Safety and Immunogenicity of Different Formulations of Norovirus Vaccine Candidate in Healthy Adults: A Randomized, Controlled, Double-Blind Clinical Trial. J. Infect. Dis. 2018, 217, 597–607. [Google Scholar] [CrossRef]
- Malm, M.; Tamminen, K.; Vesikari, T.; Blazevic, V. Comparison of Intramuscular, Intranasal and Combined Administration of Norovirus Virus-Like Particle Subunit Vaccine Candidate for Induction of Protective Immune Responses in Mice. J. Clin. Cell. Immunol. 2015, 6, 1–7. [Google Scholar] [CrossRef]
- Malm, M.; Hyoty, H.; Knip, M.; Vesikari, T.; Blazevic, V. Development of T cell immunity to norovirus and rotavirus in children under five years of age. Sci. Rep. 2019, 9, 3199. [Google Scholar] [CrossRef] [Green Version]
- Tamminen, K.; Huhti, L.; Vesikari, T.; Blazevic, V. Pre-existing immunity to norovirus GII-4 virus-like particles does not impair de novo immune responses to norovirus GII-12 genotype. Viral Immunol. 2013, 26, 167–170. [Google Scholar] [CrossRef]
- Kim, J.H.; Skountzou, I.; Compans, R.; Jacob, J. Original antigenic sin responses to influenza viruses. J. Immunol. 2009, 183, 3294–3301. [Google Scholar] [CrossRef]
- Lessler, J.; Riley, S.; Read, J.M.; Wang, S.; Zhu, H.; Smith, G.J.; Guan, Y.; Jiang, C.Q.; Cummings, D.A. Evidence for antigenic seniority in influenza A (H3N2) antibody responses in southern China. PLoS Pathog. 2012, 8, e1002802. [Google Scholar] [CrossRef]
- Smith, D.J.; Forrest, S.; Ackley, D.H.; Perelson, A.S. Variable efficacy of repeated annual influenza vaccination. Proc. Natl. Acad. Sci. USA 1999, 96, 14001–14006. [Google Scholar] [CrossRef] [Green Version]
- Skowronski, D.M.; Chambers, C.; De Serres, G.; Sabaiduc, S.; Winter, A.L.; Dickinson, J.A.; Gubbay, J.B.; Fonseca, K.; Drews, S.J.; Charest, H.; et al. Serial Vaccination and the Antigenic Distance Hypothesis: Effects on Influenza Vaccine Effectiveness During A(H3N2) Epidemics in Canada, 2010–2011 to 2014–2015. J. Infect. Dis. 2017, 215, 1059–1099. [Google Scholar] [CrossRef] [PubMed]
- Malm, M.; Tamminen, K.; Vesikari, T.; Blazevic, V. Type-specific and cross-reactive antibodies and T cell responses in norovirus VLP immunized mice are targeted both to conserved and variable domains of capsid VP1 protein. Mol. Immunol. 2016, 78, 27–37. [Google Scholar] [CrossRef] [PubMed]
- Siebenga, J.J.; Vennema, H.; Zheng, D.P.; Vinje, J.; Lee, B.E.; Pang, X.L.; Ho, E.C.; Lim, W.; Choudekar, A.; Broor, S.; et al. Norovirus illness is a global problem: Emergence and spread of norovirus GII.4 variants, 2001-2007. J. Infect. Dis. 2009, 200, 802–812. [Google Scholar] [CrossRef] [PubMed]


© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Malm, M.; Vesikari, T.; Blazevic, V. Simultaneous Immunization with Multivalent Norovirus VLPs Induces Better Protective Immune Responses to Norovirus than Sequential Immunization. Viruses 2019, 11, 1018. https://doi.org/10.3390/v11111018
Malm M, Vesikari T, Blazevic V. Simultaneous Immunization with Multivalent Norovirus VLPs Induces Better Protective Immune Responses to Norovirus than Sequential Immunization. Viruses. 2019; 11(11):1018. https://doi.org/10.3390/v11111018
Chicago/Turabian StyleMalm, Maria, Timo Vesikari, and Vesna Blazevic. 2019. "Simultaneous Immunization with Multivalent Norovirus VLPs Induces Better Protective Immune Responses to Norovirus than Sequential Immunization" Viruses 11, no. 11: 1018. https://doi.org/10.3390/v11111018
APA StyleMalm, M., Vesikari, T., & Blazevic, V. (2019). Simultaneous Immunization with Multivalent Norovirus VLPs Induces Better Protective Immune Responses to Norovirus than Sequential Immunization. Viruses, 11(11), 1018. https://doi.org/10.3390/v11111018



