Hemodynamic and Pulmonary Permeability Characterization of Hantavirus Cardiopulmonary Syndrome by Transpulmonary Thermodilution
Abstract
1. Introduction
2. Methods
2.1. Study Design and Patients
2.2. Methodological Aspects of Transpulmonary Thermodilution and Arterial Pulse Contour Analysis
2.3. Variables of Interest
- -
- “Classic hemodynamic”: HR, mean systemic arterial pressure (MAP), central venous pressure (CVP), CI, SI, and systemic vascular resistance index (SVRi), which is the ratio between the difference of MAP and CVP, and CI times 80;
- -
- Myocardial contractility: GEF, CFI, and dPmax;
- -
- Volumetric preload: ITBVi and GEDVi;
- -
- Fluid responsiveness predictor: SVV;
- -
- Pulmonary edema: EVLWi and PVPI.
2.4. Statistical Analysis
3. Results
4. Discussion
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Mertz, G.J.; Hielle, B.L.; Bryan, R.T. Hantavirus infection. Dis. Month 1998, 44, 85–138. [Google Scholar] [CrossRef]
- Lefkowitz, E.J.; Dempsey, D.M.; Hendrickson, R.C.; Orton, R.J.; Siddell, S.G.; Smith, D.B. Virus taxonomy: The database of the International Committee on Taxonomy of Viruses (ICTV). Nucleic. Acids. Res. 2018, 46, D708–D717. [Google Scholar] [CrossRef] [PubMed]
- Manigold, T.; Vial, P. Human hantavirus infections: Epidemiology, clinical features, pathogenesis and immunology. Swiss Med. Wkly. 2014, 144, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Medina, R.A.; Torres-Perez, F.; Galeno, H.; Navarrete, M.; Vial, P.A.; Palma, R.E.; Ferres, M.; Cook, J.A.; Hjelle, B. Ecology, Genetic Diversity, and Phylogeographic Structure of Andes Virus in Humans and Rodents in Chile. J. Vir. 2009, 83, 2446–2459. [Google Scholar] [CrossRef] [PubMed]
- Hart, C.A.; Bennett, M. Hantavirus infections: Epidemiology and pathogenesis. Microbes. Infect. 1999, 1, 1229–1237. [Google Scholar] [CrossRef]
- Sotomayor, V.; Aguilera, X. Epidemiología de la infección humana por hantavirus en Chile. Rev. Chil. Infectología. 2000, 17, 220–2327. [Google Scholar] [CrossRef]
- Padula, P.J.; Edelstein, A.; Miguel, S.D.; López, N.M.; Rossi, C.M.; Rabinovich, R.D. Hantavirus pulmonary syndrome outbreak in Argentina: Molecular evidence for person-to-person transmission of Andes virus. Virology 1998, 241, 323–330. [Google Scholar] [CrossRef]
- Chaparro, J.; Vega, J.; Terry, W.; Vera, J.L.; Barra, B.; Meyer, R.; Peters, C.J.; Khan, A.S.; Ksiazek, T.G. Assessment of person-to-person transmission of hantavirus pulmonary syndrome in a Chilean hospital setting. J. Hosp. Infect. 1998, 40, 281–285. [Google Scholar] [CrossRef]
- Ferrés, M.; Vial, P.; Marco, C.; Yan, L.; Godoy, P.; Castillo, C.; Hjelle, B.; Delgado, I.; Lee, S.; Mertz, G.J.; et al. Prospective evaluation of household contacts of persons with hantavirus cardiopulmonary syndrome in Chile. J. Infect. Dis. 2007, 195, 1563–1571. [Google Scholar] [CrossRef]
- Martinez-Valdebenito, C.; Calvo, M.; Vial, C.; Mansilla, R.; Marco, C.; Palma, R.E.; Vial, P.A.; Valdivieso, F.; Mertz, G.; Ferrés, M. Person-to-person household and nosocomial transmission of andes hantavirus, Southern Chile, 2011. Emerg. Infect. Dis. 2014, 20, 1629–1636. [Google Scholar] [CrossRef]
- Vial, P.A.; Valdivieso, F.; Mertz, G.; Castillo, C.; Belmar, E.; Delgado, I.; Tapia, M.; Ferrés, M. Incubation period of hantavirus cardiopulmonary syndrome. Emerg. Infect. Dis. 2006, 12, 1271–1273. [Google Scholar] [CrossRef]
- Núñez, J.J.; Fritz, C.L.; Knust, B.; Buttke, D.; Enge, B.; Novak, M.G.; Kramer, V.; Osadebe, L.; Messenger, S.; Albariño, C.G.; et al. Hantavirus infections among overnight visitors to Yosemite National Park, California, USA, 2012. Emerg. Infect. Dis. 2014, 20, 386–393. [Google Scholar] [CrossRef]
- Mertz, G.J.; Hjelle, B.; Crowley, M.; Iwamoto, G.; Tomicic, V.; Vial, P.A. Diagnosis and treatment of new world hantavirus infections. Curr. Opin. Infect. Dis. 2006, 19, 437–442. [Google Scholar] [CrossRef]
- Llah, S.T.; Mir, S.; Sharif, S.; Khan, S.; Mir, M.A. Hantavirus induced cardiopulmonary syndrome: A public health concern. J. Med. Virol. 2018, 90, 1003–1009. [Google Scholar] [CrossRef]
- Gavrilovskaya, I.N.; Shepley, M.; Shaw, R.; Ginsberg, M.H.; Mackow, E.R. Beta3 Integrins mediate the cellular entry of hantaviruses that cause respiratory failure. Proc. Natl. Acad. Sci. USA 1998, 95, 7074–7079. [Google Scholar] [CrossRef]
- Jangra, R.K.; Herbert, A.S.; Li, R.; Jae, L.T.; Kleinfelter, L.M.; Slough, M.M.; Barker, S.L.; Guardado-Calvo, P.; Román-Sosa, G.; Dieterle, M.E.; et al. Protocadherin-1 is essential for cell entry by New World hantaviruses. Nature 2018, 563, 559–563. [Google Scholar] [CrossRef]
- Kilpatrick, E.D.; Terajima, M.; Koster, F.T.; Catalina, M.D.; Cruz, J.; Ennis, F.A. Role of specific CD8+ T cells in the severity of a fulminant zoonotic viral hemorrhagic fever, hantavirus pulmonary syndrome. J. Immunol. 2004, 172, 3297–3304. [Google Scholar] [CrossRef]
- Gorbunova, E.; Gavrilovskaya, I.N.; Mackow, E.R. Pathogenic hantaviruses Andes virus and Hantaan virus induce adherens junction disassembly by directing vascular endothelial cadherin internalization in human endothelial cells. J. Virol. 2010, 84, 7405–7411. [Google Scholar] [CrossRef]
- Li, Y.; Wang, W.; Wang, J.P.; Pan, L.; Zhang, Y.; Yu, H.T.; Bai, X.F. Elevated vascular endothelial growth factor levels induce hyperpermeability of endothelial cells in hantavi rus infection. J. Inte. Med. Res. 2012, 40, 1812–1821. [Google Scholar]
- Shrivastava-Ranjan, P.; Rollin, P.E.; Spiropoulou, C.F. Andes virus disrupts the endothelial cell barrier by induction of vascular endothelial growth factor and downregulation of VE-cadherin. J. Virol. 2010, 84, 11227–11234. [Google Scholar] [CrossRef]
- Gorbunova, E.E.; Simons, M.J.; Gavrilovskaya, I.N.; Mackow, E.R. The andes virus nucleocapsid protein directs basal endothelial cell permeability by activating RhoA. mBio 2016, 7, 1–12. [Google Scholar] [CrossRef]
- Bryan, B.A.; Dennstedt, E.; Mitchell, D.C.; Walshe, T.E.; Noma, K.; Loureiro, R.; D’Amore, P.A. RhoA/ROCK signaling is essential for multiple aspects of VEGF-mediated angiogenesis. FASEB J. 2010, 24, 3186–3195. [Google Scholar] [CrossRef]
- Szulcek, R.; Beckers, C.M.; Hodzic, J.; de Wit, J.; Chen, Z.; Grob, T.; Musters, J.P.; Minshall, R.D.; van Hinsbergh, W.M.; van Nieuw Amerongen, G.P. Localized RhoA GTPase activity regulates dynamics of endothelial monolayer integrity. Cardiovascular. Res. 2013, 99, 471–482. [Google Scholar] [CrossRef]
- Duchin, J.; Koster, F.; Peters, C.; Simpson, G.; Tempest, B.; Zaki, S.R.; Ksiazek, T.G.; Rollin, P.E.; Nichol, S.; Umland, E.T.; et al. Hantavirus Pulmonary Syndrome: A Clinical Description of 17 Patients with newly recognize disease. N. Engl. J. Med. 1994, 330, 949–955. [Google Scholar] [CrossRef]
- Wernly, J.A.; Dietl, C.A.; Tabe, C.E.; Pett, S.B.; Crandall, C.; Milligan, K.; Crowley, M.R. Extracorporeal membrane oxygenation support improves survival of patients with Hantavirus cardiopulmonary syndrome refractory to medical treatment. Eur. J. Cardiothorac. Surg. 2011, 40, 1334–1340. [Google Scholar] [CrossRef]
- Vial, P.A.; Valdivieso, F.; Calvo, M.; Rioseco, M.L.; Riquelme, R.; Araneda, A.; Tomicic, V.; Graf, J.; Paredes, L.; Florenzano, M.; et al. A non-randomized multicentre trial of human immune plasma for treatment of hantavirus cardiopulmonary syndrome by ANDV. Antivir. Ther. 2014, 20, 377–386. [Google Scholar] [CrossRef]
- Vial, P.A.; Valdivieso, F.; Ferres, M.; Riquelme, R.; Rioseco, M.L.; Calvo, M.; Castillo, C.; Díaz, R.; Scholz, L.; Cuiza, A.; et al. High-dose intravenous methylprednisolone for hantavirus cardiopulmonary syndrome in Chile: A double-blind, randomized controlled clinical trial. Clin. Infect. Dis. 2013, 57, 943–951. [Google Scholar] [CrossRef]
- Crowley, M.R.; Katz, R.W.; Kessler, R.; Simpson, S.Q.; Levy, H.; Hallin, G.W.; Cappon, J.; Krahling, J.B.; Wernly, J. Successful treatment of adults with severe Hantavirus pulmonary syndrome with extracorporeal membrane oxygenation. Crit. Care Med. 1998, 26, 409–414. [Google Scholar] [CrossRef]
- Monnet, X.; Anguel, N.; Osman, D.; Hamzaoui, O.; Richard, C.; Teboul, J.L. Assessing pulmonary permeability by transpulmonary thermodilution allows differentiation of hydrostatic pulmonary edema from ALI/ARDS. Intensive Care Med. 2007, 33, 448–453. [Google Scholar] [CrossRef]
- Katzenelson, R.; Perel, A.; Berkenstadt, H.; Preisman, S.; Koganm, S.; Sternik, L.; Segal, E. Accuracy of transpulmonary thermodilution versus gravimetric measurement of extravascular lung water. Crit. Care Med. 2004, 32, 1550–1554. [Google Scholar] [CrossRef]
- Sakka, S.G.; Klein, M.; Reinhart, K.; Meier-Hellmann, A. Prognostic value of extravascular lung water in critically ill patients. Chest 2002, 122, 2080–2086. [Google Scholar] [CrossRef]
- Tagami, T.; Ong, M.E.H. Extravascular lung water measurements in acute respiratory distress syndrome: why, how, and when? Curr. Opin. Crit. Care 2018, 24, 209–215. [Google Scholar] [CrossRef]
- Monnet, X.; Teboul, J.L. Transpulmonary thermodilution: advantages and limits. Crit. Care 2017, 21, 147. [Google Scholar] [CrossRef]
- Berkenstadt, H.; Margalit, N.; Hadani, M.; Friedman, Z.; Segal, E.; Villa, Y.; Perel, A. Stroke volume variation as a predictor of fluid responsiveness in patients undergoing brain surgery. Anesth. Analg. 2001, 92, 984–989. [Google Scholar] [CrossRef]
- Monge Garcia, M.I.; Jian, Z.; Settels, J.J.; Hunley, C.; Cecconi, M.; Hatib, F.; Pinsky, M.R. Performance comparison of ventricular and arterial dP/dt max for assessing left ventricular systolic function during different experimental loading and contractile conditions. Crit. Care. 2018, 22, 325. [Google Scholar] [CrossRef]
- Romero, C.; Andresen, M.; Díaz, O.; Tomicic, V.; Baraona, F.; Mercado, M.; Pérez, C.; Downey, P.; Dougnac, A. Síndrome cardiopulmonary por Hantavirus: utilidad de la monitorización con el Sistema PiCCO. Rev. Med. Chil. 2003, 131, 1173–1178. [Google Scholar] [CrossRef]
- Hallin, G.W.; Simpson, S.Q.; Crowell, R.E.; James, D.S.; Koster, F.T.; Mertz, G.J.; Levy, H. Cardiopulmonary manifestations of hantavirus pulmonary syndrome. Crit. Care Med. 1996, 24, 252–258. [Google Scholar] [CrossRef]
- Jardin, F.; Vieillard-Baron, A. Right ventricular function and positive pressure ventilation in clinical practice: from hemodynamic subsets to respirator settings. Intensive Care Med. 2003, 29, 1426–1434. [Google Scholar] [CrossRef]
- Wellhöfer, H.; Zeravik, J.; Perker, M.; Blümel, G.; Zimmermann, G.; Pfeiffer, U.J. PEEP-Induced Changes of Pulmonary Capillary Wedge Pressure, Prepulmonary and Total Intrathoracic Blood Volume in Anesthetized Dogs. In Practical Applications of Fiberoptics in Critical Care Monitoring; Lewis, F.R., Pfeiffer, U.J., Eds.; Springer: Berlin/Heidelberg, Germany, 1990; pp. 32–41. [Google Scholar]
- Luecke, T.; Roth, H.; Herrmann, P.; Joachim, A.; Weisser, G.; Pelosi, P.; Quintel, M. PEEP decreases atelectasis and extravascular lung water but not lung tissue volume in surfactant-washout lung injury. Intensive Care Med. 2003, 29, 2026–2033. [Google Scholar] [CrossRef]
- Dongaonkar, R.M.; Stewart, R.H.; Geissler, H.J.; Laine, G.A. Myocardial microvascular permeability, interstitial oedema, and compromised cardiac function. Cardiovasc. Res. 2010, 87, 331–339. [Google Scholar] [CrossRef]
- Saggioro, F.P.; Rossi, M.A.; Duarte, M.I.; Martin, C.C.; Alves, V.A.; Moreli, M.L.; Figueiredo, L.T.; Moreira, J.E.; Borges, A.A.; Neder, L. Hantavirus infection induces a typical myocarditis that may be responsible for myocardial depression and shock in hantavirus pulmonary syndrome. J. Infect. Dis. 2007, 195, 1541–1549. [Google Scholar] [CrossRef]
- Rezoagli, E.; Fumagalli, R.; Bellani, G. Definition and epidemiology of acute respiratory distress syndrome. Ann. Transl. Med. 2017, 5, 282. [Google Scholar] [CrossRef]
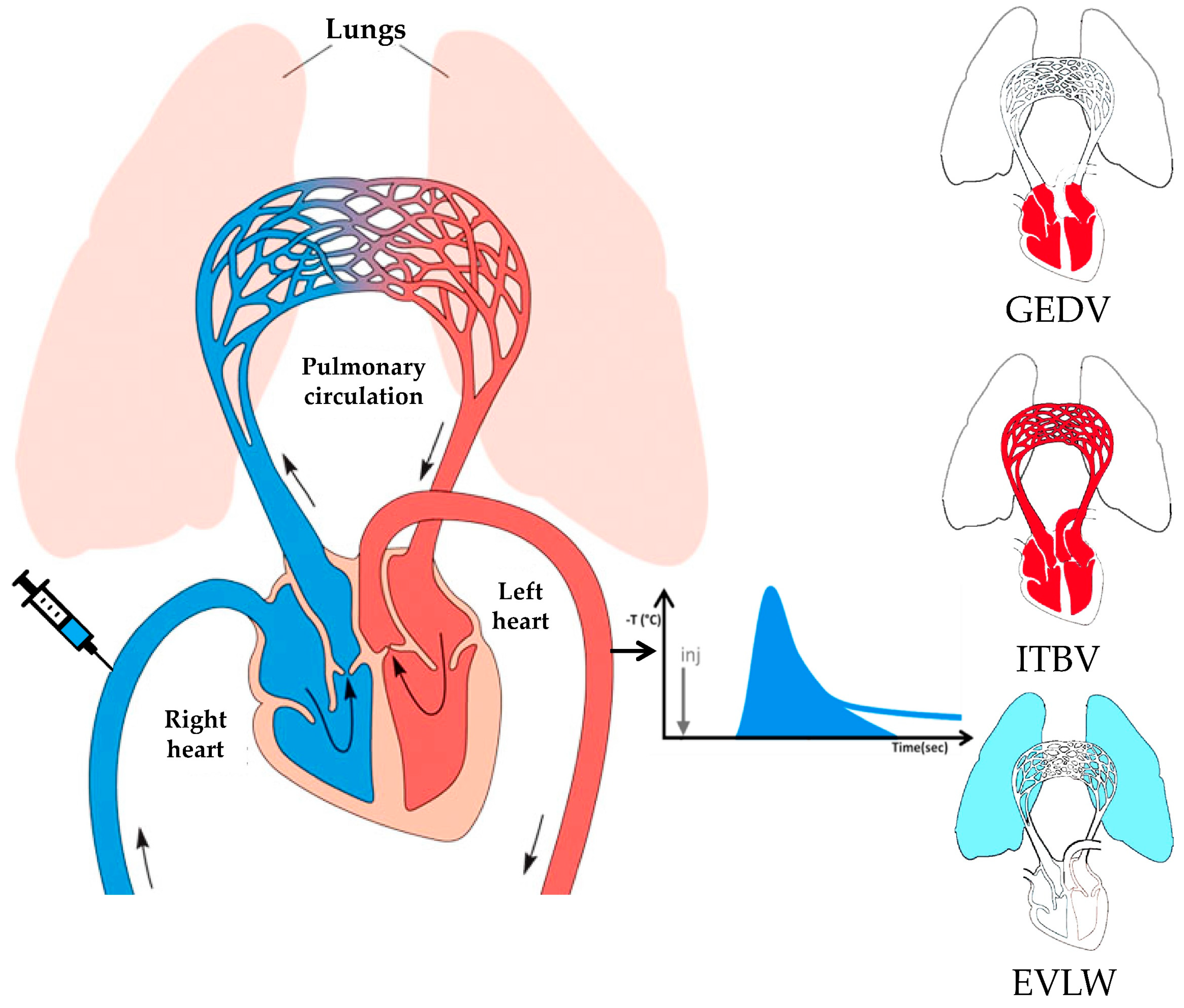
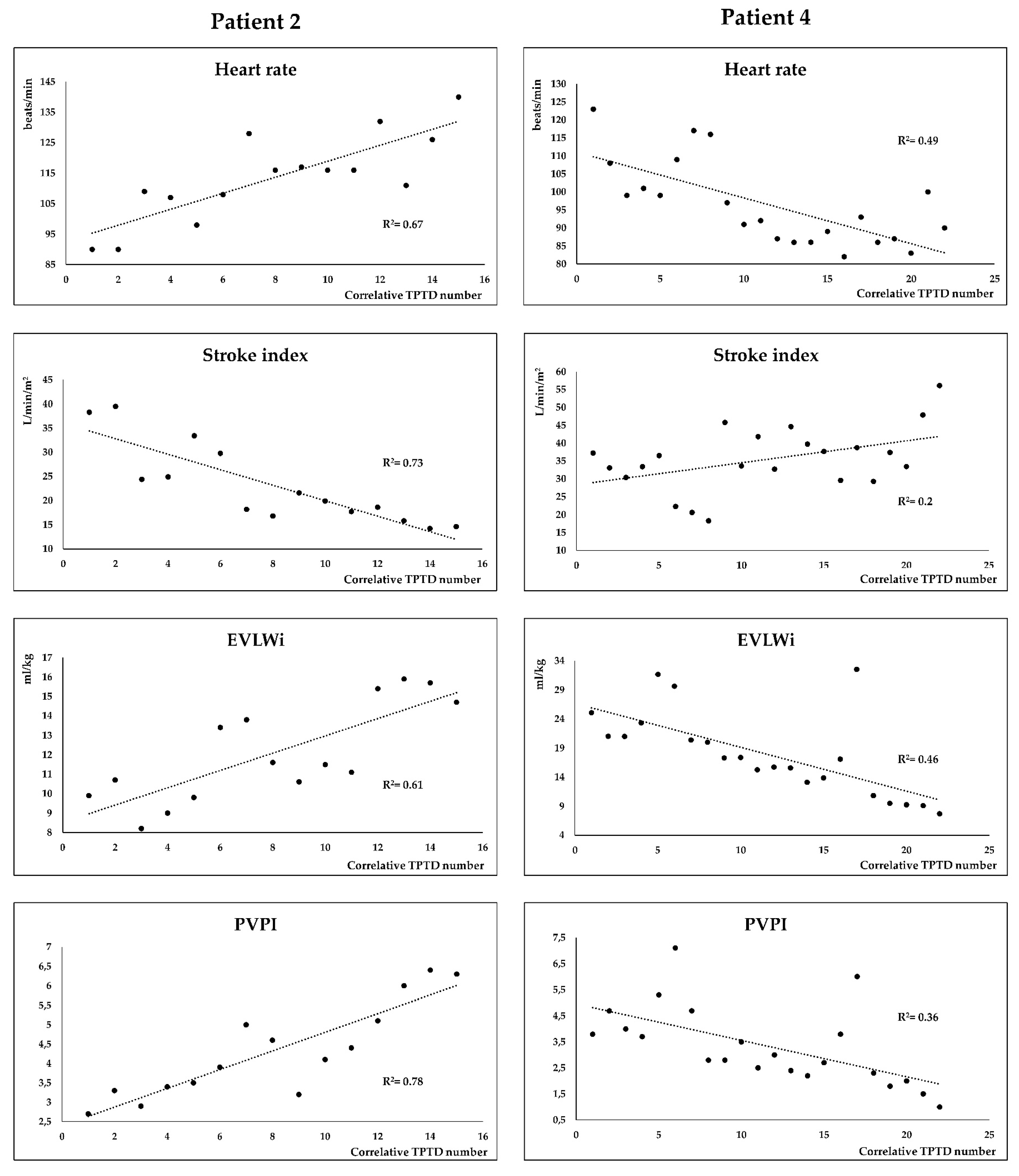
| Characteristic | Value |
|---|---|
| Male, N (%) | 7 (64) |
| Age, years, median (range) | 29 (15–59) |
| SOFA score, points, median (range) | 9 (4–14) |
| APACHE II score, points, median (range) | 10 (5–30) |
| Invasive mechanical ventilation, N (%) | 8 (73) |
| Inotropic drugs, N (%) | 8 (73) |
| VA-ECMO, N (%) | 3 (27) |
| ICU-LOS, days, median (range) | 6 (4–18) |
| Hospital-LOS, days, median (range) | 12 (4–87) |
| Transpulmonary Thermodilution Variables | Median (IQR) | Reference Range |
|---|---|---|
| Classic hemodynamic | ||
| Heart rate, beats/min | 99 (90–109) | 60–100 |
| Mean arterial pressure, mmHg | 82 (75–89) | 70–90 |
| Central venous pressure, mmHg | 8 (4–11) | 6–12 |
| Cardiac index, L/min/m2 | 3.1 (2.5–3.8) | 3.0–5.0 |
| Stroke index, mL/m2 | 34 (26–41) | 40–60 |
| Systemic vascular resistance index, dyn·sec·cm−5·m−2 | 1880 (1546–2364) | 1700–2400 |
| Myocardial contractility | ||
| Global ejection fraction, % | 24 (21–27) | 25–35 |
| Cardiac function index, 1/min | 5.6 (5.0–6.3) | 4.5–6.5 |
| dPmax, mmHg/s | 950 (762–1094) | 900–1200 |
| Cardiac preload | ||
| Intrathoracic blood volume index, mL/m2 | 667 (553–790) | 850–1000 |
| Global end diastolic volume index, mL/m2 | 538 (442–635) | 680–800 |
| Fluid responsiveness predictor | ||
| Stroke volume variation, % | 13 (8–17) | ≤10 |
| Pulmonary edema | ||
| Extravascular lung water index, mL/Kg | 13.1 (10.2–17.3) | 3.0–7.0 |
| Pulmonary vascular permeability index, dimensionless | 3.2 (2.7–4.7) | 1.0–3.0 |
| Transpulmonary Thermodilution Variables | EVLWi | p Value | PVPI | p Value |
|---|---|---|---|---|
| Classic hemodynamic | ||||
| Heart rate, beats/min | 0.20 | 0.02 | 0.31 | <0.01 |
| Mean arterial pressure, mmHg | −0.27 | <0.01 | −0.10 | 0.26 |
| Central venous pressure, mmHg | 0.23 | 0.01 | 0.26 | <0.01 |
| Cardiac index, L/min/m2 | 0.02 | 0.79 | −0.49 | <0.01 |
| Stroke index, mL/m2 | 0.06 | 0.51 | −0.12 | 0.17 |
| Systemic vascular resistance index, dyn·sec·cm−5·m2 | −0.18 | 0.04 | 0.38 | <0.01 |
| Myocardial contractility | ||||
| Global ejection fraction, % | −0.36 | <0.01 | −0.40 | <0.01 |
| Cardiac function index, 1/min | −0.25 | <0.01 | −0.20 | 0.02 |
| dPmax, mmHg/s | 0.12 | 0.18 | 0.16 | 0.08 |
| Cardiac preload | ||||
| Intrathoracic blood volume index, mL/m2 | 0.21 | 0.01 | −0.48 | <0.01 |
| Global end diastolic volume index, mL/m2 | 0.21 | 0.01 | −0.48 | <0.01 |
| Fluid responsiveness predictor | ||||
| Stroke volume variation, % | 0.10 | 0.28 | 0.22 | 0.01 |
| Hemodynamic Variables | EVLWi < 15 mL/kg | EVLWi ≥ 15 mL/kg | p-Value |
|---|---|---|---|
| Classic hemodynamic | |||
| Heart rate, beats/min | 96 (18) | 103 (14) | 0.02 |
| Mean arterial pressure, mmHg | 84 (10) | 79 (9) | <0.01 |
| Central venous pressure, mmHg | 6 (4) | 10 (5) | <0.01 |
| Cardiac index, L/min/m2 | 3.2 (0.9) | 3.1 (0.9) | 0.74 |
| Stroke index, mL/m2 | 34 (9) | 35 (30) | 0.75 |
| Systemic vascular resistance index, dyn·sec·cm−5·m2 | 2093 (702) | 1915 (620) | 0.15 |
| Myocardial contractility | |||
| Global ejection fraction, % | 25 (4) | 22 (5) | <0.01 |
| Cardiac function index, 1/min | 5.9 (1.1) | 5.4 (0.9) | 0.01 |
| dPmax, mmHg/s | 925 (223) | 1007 (304) | 0.12 |
| Cardiac preload | |||
| Intrathoracic blood volume index, mL/m2 | 680 (172) | 735 (222) | 0.12 |
| Global end diastolic volume index, mL/m2 | 546 (138) | 588 (177) | 0.14 |
| Fluid responsiveness predictor | |||
| Stroke volume variation, % | 13 (6) | 14 (7) | 0.68 |
| Pulmonary edema | |||
| Extravascular lung water index, mL/Kg | 11.1 (2.2) | 20.0 (4.3) | <0.01 |
| Pulmonary vascular permeability index, dimensionless | 3.1 (0.9) | 5.1 (1.7) | <0.01 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
López, R.; Pérez-Araos, R.; Salazar, Á.; Ulloa, A.L.; Vial, C.; Vial, P.A.; Graf, J. Hemodynamic and Pulmonary Permeability Characterization of Hantavirus Cardiopulmonary Syndrome by Transpulmonary Thermodilution. Viruses 2019, 11, 900. https://doi.org/10.3390/v11100900
López R, Pérez-Araos R, Salazar Á, Ulloa AL, Vial C, Vial PA, Graf J. Hemodynamic and Pulmonary Permeability Characterization of Hantavirus Cardiopulmonary Syndrome by Transpulmonary Thermodilution. Viruses. 2019; 11(10):900. https://doi.org/10.3390/v11100900
Chicago/Turabian StyleLópez, René, Rodrigo Pérez-Araos, Álvaro Salazar, Ana L. Ulloa, Cecilia Vial, Pablo A. Vial, and Jerónimo Graf. 2019. "Hemodynamic and Pulmonary Permeability Characterization of Hantavirus Cardiopulmonary Syndrome by Transpulmonary Thermodilution" Viruses 11, no. 10: 900. https://doi.org/10.3390/v11100900
APA StyleLópez, R., Pérez-Araos, R., Salazar, Á., Ulloa, A. L., Vial, C., Vial, P. A., & Graf, J. (2019). Hemodynamic and Pulmonary Permeability Characterization of Hantavirus Cardiopulmonary Syndrome by Transpulmonary Thermodilution. Viruses, 11(10), 900. https://doi.org/10.3390/v11100900





