Differential Role of Anti-Viral Sensing Pathway for the Production of Type I Interferon β in Dendritic Cells and Macrophages Against Respiratory Syncytial Virus A2 Strain Infection
Abstract
1. Introduction
2. Materials and Methods
2.1. Animals
2.2. Mice Genotyping
2.3. RSV Propagation
2.4. RSV Infection In Vitro and Cytokine Measurements
2.5. Flow Cytometry
2.6. Statistics
3. Results
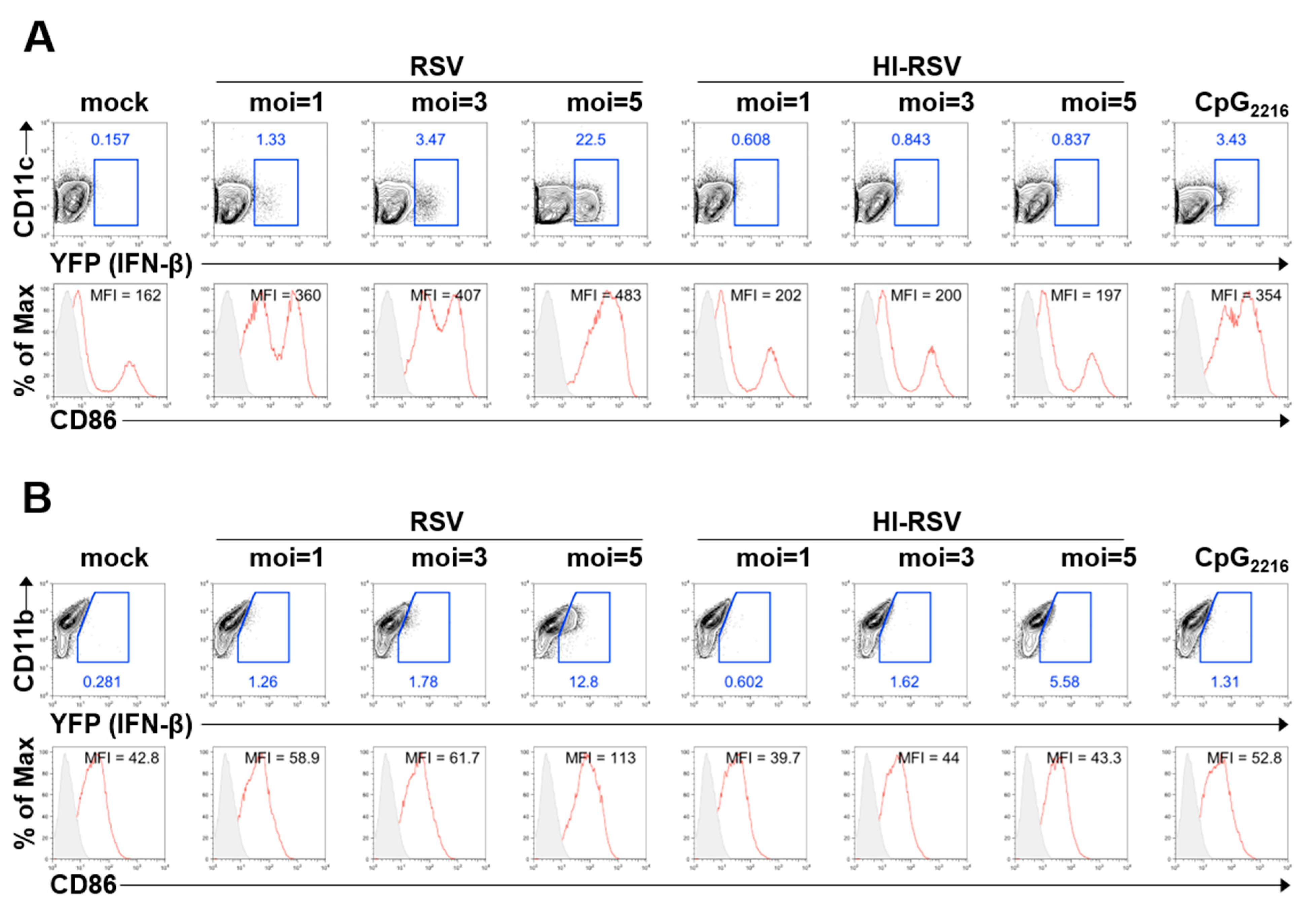
3.1. BM-DCs and BM-DMs Produce IFN-β in Response to RSV A2 Strain Infection
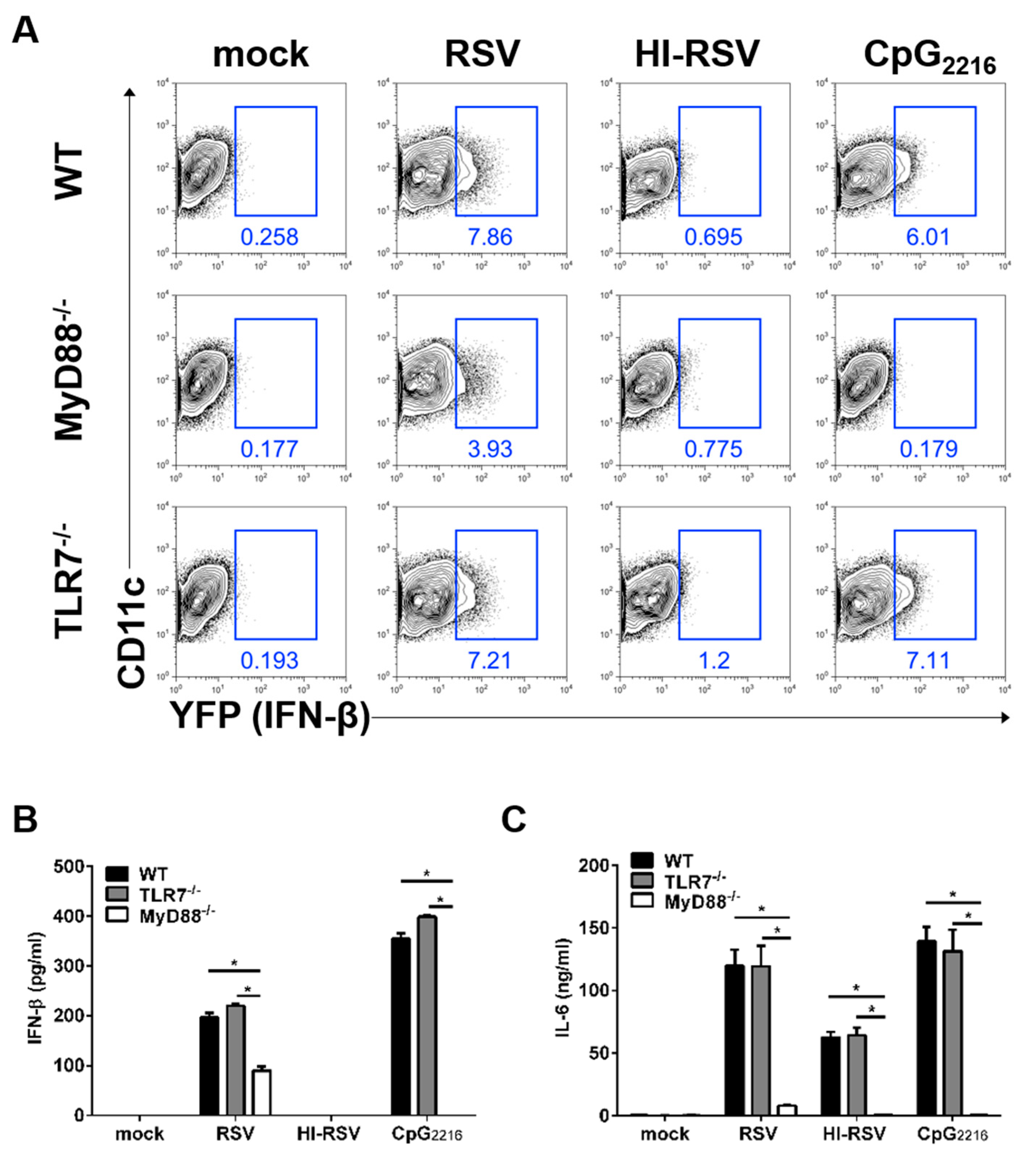
3.2. The MyD88- and TLR7-Mediated Pathways are Required to Induce IFN-β in BM-DCs
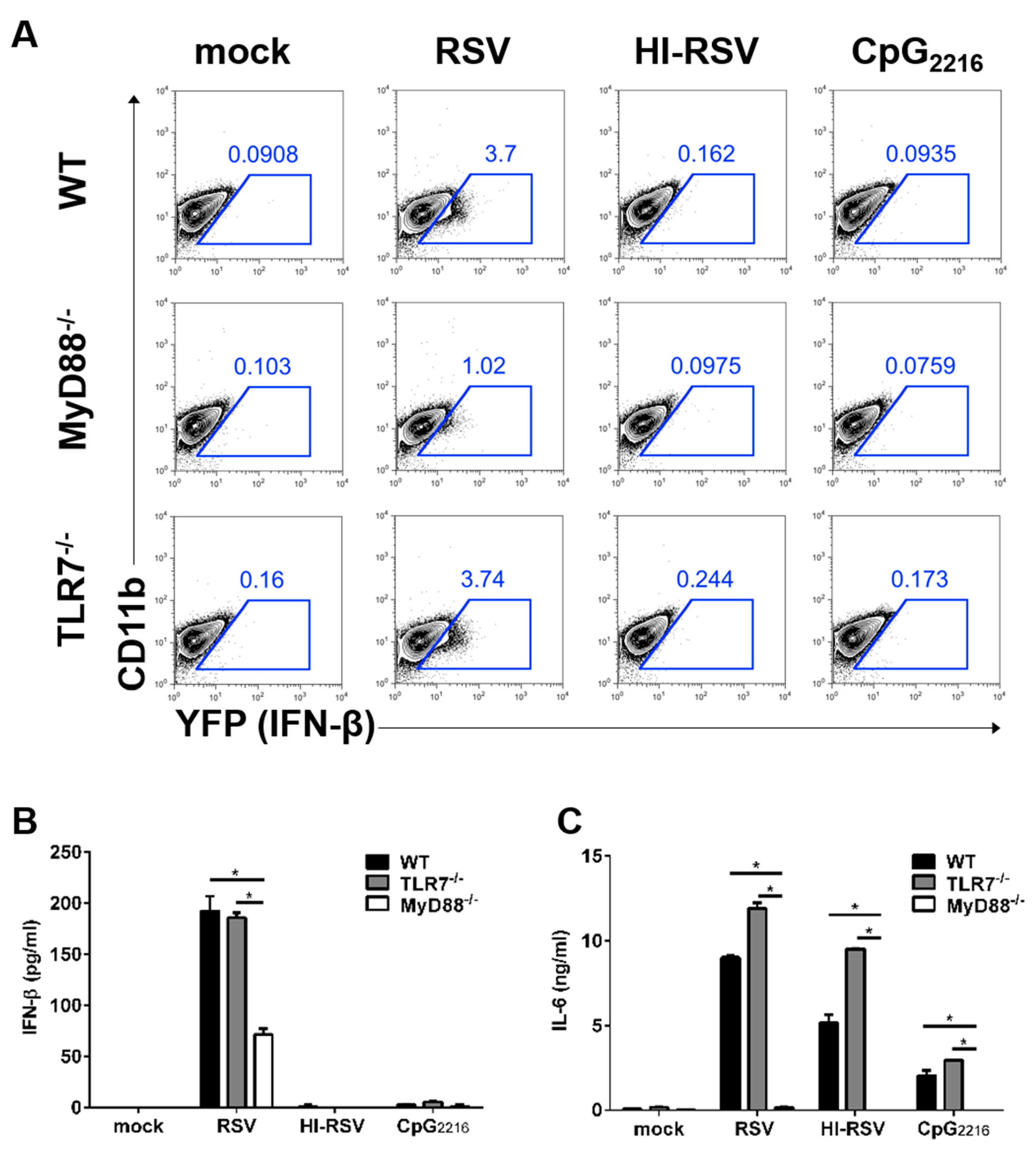
3.3. The MyD88-Mediated Pathways are Required to Induce IFN-β in BM-DMs
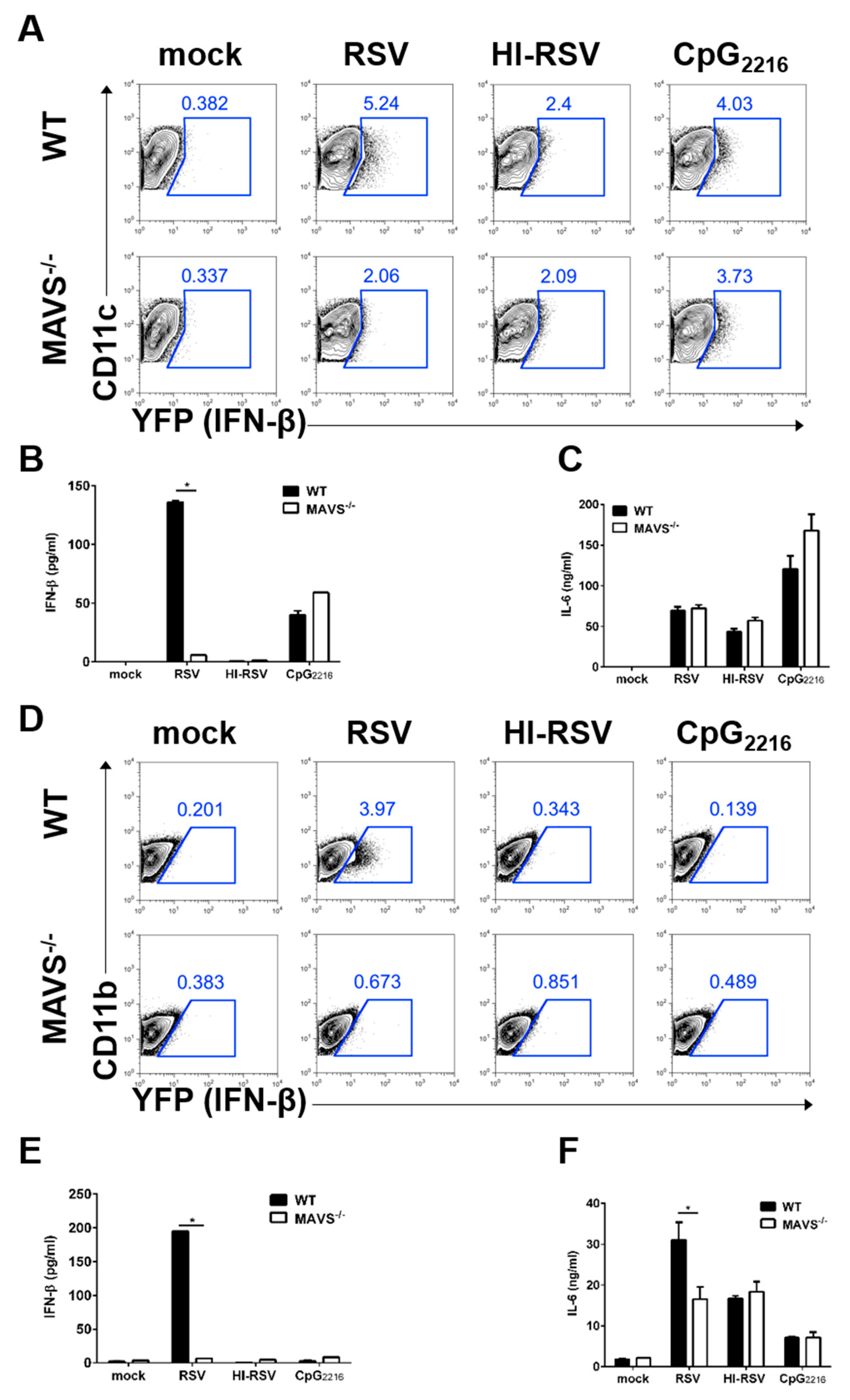
3.4. MAVS-Mediated Pathways are Required to Induce IFN-β in BM-DCs and BM-DMs
4. Discussion
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Collins, P.L.; Graham, B.S. Viral and host factors in human respiratory syncytial virus pathogenesis. J. Virol. 2008, 82, 2040–2055. [Google Scholar] [CrossRef] [PubMed]
- Borchers, A.T.; Chang, C.; Gershwin, M.E.; Gershwin, L.J. Respiratory syncytial virus--a comprehensive review. Clin. Rev. Allergy Immunol. 2013, 45, 331–379. [Google Scholar] [CrossRef] [PubMed]
- Luoto, R.; Jartti, T.; Ruuskanen, O.; Waris, M.; Lehtonen, L.; Heikkinen, T. Review of the clinical significance of respiratory virus infections in newborn infants. Acta Paediatr. 2016, 105, 1132–1139. [Google Scholar] [CrossRef]
- Anderson, L.J.; Dormitzer, P.R.; Nokes, D.J.; Rappuoli, R.; Roca, A.; Graham, B.S. Strategic priorities for respiratory syncytial virus (RSV) vaccine development. Vaccine 2013, 31 (Suppl. 2), B209–B215. [Google Scholar] [CrossRef]
- Goritzka, M.; Durant, L.R.; Pereira, C.; Salek-Ardakani, S.; Openshaw, P.J.; Johansson, C. Alpha/beta interferon receptor signaling amplifies early proinflammatory cytokine production in the lung during respiratory syncytial virus infection. J. Virol. 2014, 88, 6128–6136. [Google Scholar] [CrossRef] [PubMed]
- Makris, S.; Paulsen, M.; Johansson, C. Type I interferons as regulators of lung inflammation. Front. Immunol. 2017, 8, 259. [Google Scholar] [CrossRef] [PubMed]
- Kim, T.H.; Lee, H.K. Innate immune recognition of respiratory syncytial virus infection. BMB Rep. 2014, 47, 184–191. [Google Scholar] [CrossRef]
- Lambert, L.; Sagfors, A.M.; Openshaw, P.J.; Culley, F.J. Immunity to RSV in Early-Life. Front. Immunol. 2014, 5, 466. [Google Scholar] [CrossRef]
- Sun, Y.; Lopez, C.B. The innate immune response to RSV: Advances in our understanding of critical viral and host factors. Vaccine 2017, 35, 481–488. [Google Scholar] [CrossRef]
- Lukacs, N.W.; Smit, J.J.; Mukherjee, S.; Morris, S.B.; Nunez, G.; Lindell, D.M. Respiratory virus-induced TLR7 activation controls IL-17-associated increased mucus via IL-23 regulation. J. Immunol. 2010, 185, 2231–2239. [Google Scholar] [CrossRef]
- Goritzka, M.; Makris, S.; Kausar, F.; Durant, L.R.; Pereira, C.; Kumagai, Y.; Culley, F.J.; Mack, M.; Akira, S.; Johansson, C. Alveolar macrophage-derived type I interferons orchestrate innate immunity to RSV through recruitment of antiviral monocytes. J. Exp. Med. 2015, 212, 699–714. [Google Scholar] [CrossRef] [PubMed]
- Demoor, T.; Petersen, B.C.; Morris, S.; Mukherjee, S.; Ptaschinski, C.; De Almeida Nagata, D.E.; Kawai, T.; Ito, T.; Akira, S.; Kunkel, S.L.; et al. IPS-1 signaling has a nonredundant role in mediating antiviral responses and the clearance of respiratory syncytial virus. J. Immunol. 2012, 189, 5942–5953. [Google Scholar] [CrossRef] [PubMed]
- Scheu, S.; Dresing, P.; Locksley, R.M. Visualization of IFNbeta production by plasmacytoid versus conventional dendritic cells under specific stimulation conditions in vivo. Proc. Natl. Acad. Sci. USA 2008, 105, 20416–20421. [Google Scholar] [CrossRef] [PubMed]
- Adachi, O.; Kawai, T.; Takeda, K.; Matsumoto, M.; Tsutsui, H.; Sakagami, M.; Nakanishi, K.; Akira, S. Targeted disruption of the MyD88 gene results in loss of IL-1- and IL-18-mediated function. Immunity 1998, 9, 143–150. [Google Scholar] [CrossRef]
- Lund, J.M.; Alexopoulou, L.; Sato, A.; Karow, M.; Adams, N.C.; Gale, N.W.; Iwasaki, A.; Flavell, R.A. Recognition of single-stranded RNA viruses by Toll-like receptor 7. Proc. Natl. Acad. Sci. USA 2004, 101, 5598–5603. [Google Scholar] [CrossRef] [PubMed]
- Sun, Q.; Sun, L.; Liu, H.H.; Chen, X.; Seth, R.B.; Forman, J.; Chen, Z.J. The specific and essential role of MAVS in antiviral innate immune responses. Immunity 2006, 24, 633–642. [Google Scholar] [CrossRef] [PubMed]
- Shim, Y.R.; Lee, H.K. Caspase-1 independent viral clearance and adaptive immunity against mucosal respiratory syncytial virus infection. Immune Netw. 2015, 15, 73–82. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.; Joo, D.H.; Lee, J.B.; Shim, B.S.; Cheon, I.S.; Jang, J.E.; Song, H.H.; Kim, K.H.; Song, M.K.; Chang, J. Dual Role of Respiratory Syncytial Virus Glycoprotein Fragment as a Mucosal Immunogen and Chemotactic Adjuvant. PLoS ONE 2012, 7. [Google Scholar] [CrossRef]
- McKimm-Breschkin, J.L. A simplified plaque assay for respiratory syncytial virus--direct visualization of plaques without immunostaining. J. Virol. Methods 2004, 120, 113–117. [Google Scholar] [CrossRef]
- Ichinohe, T.; Lee, H.K.; Ogura, Y.; Flavell, R.; Iwasaki, A. Inflammasome recognition of influenza virus is essential for adaptive immune responses. J. Exp. Med. 2009, 206, 79–87. [Google Scholar] [CrossRef] [PubMed]
- Hillyer, P.; Mane, V.P.; Chen, A.; Dos Santos, M.B.; Schramm, L.M.; Shepard, R.E.; Luongo, C.; Le Nouen, C.; Huang, L.; Yan, L.; et al. Respiratory syncytial virus infection induces a subset of types I and III interferons in human dendritic cells. Virology 2017, 504, 63–72. [Google Scholar] [CrossRef] [PubMed]
- Bhoj, V.G.; Sun, Q.; Bhoj, E.J.; Somers, C.; Chen, X.; Torres, J.P.; Mejias, A.; Gomez, A.M.; Jafri, H.; Ramilo, O.; et al. MAVS and MyD88 are essential for innate immunity but not cytotoxic T lymphocyte response against respiratory syncytial virus. Proc. Natl. Acad. Sci. USA 2008, 105, 14046–14051. [Google Scholar] [CrossRef] [PubMed]
- Kim, T.H.; Lee, H.K. Differential roles of lung dendritic cell subsets against respiratory virus infection. Immune Netw. 2014, 14, 128–137. [Google Scholar] [CrossRef] [PubMed]
- Munir, S.; Le Nouen, C.; Luongo, C.; Buchholz, U.J.; Collins, P.L.; Bukreyev, A. Nonstructural proteins 1 and 2 of respiratory syncytial virus suppress maturation of human dendritic cells. J. Virol. 2008, 82, 8780–8796. [Google Scholar] [CrossRef] [PubMed]
- Ravi, L.I.; Li, L.; Sutejo, R.; Chen, H.; Wong, P.S.; Tan, B.H.; Sugrue, R.J. A systems-based approach to analyse the host response in murine lung macrophages challenged with respiratory syncytial virus. BMC Genom. 2013, 14, 190. [Google Scholar] [CrossRef] [PubMed]
- Mufson, M.A.; Orvell, C.; Rafnar, B.; Norrby, E. Two distinct subtypes of human respiratory syncytial virus. J. Gen. Virol. 1985, 66, 2111–2124. [Google Scholar] [CrossRef]
- Walsh, E.E.; Hall, C.B.; Schlesinger, J.J.; Brandriss, M.W.; Hildreth, S.; Paradiso, P. Comparison of Antigenic Sites of Subtype-Specific Respiratory Syncytial Virus Attachment Proteins. J. Gen. Virol. 1989, 70, 2953–2961. [Google Scholar] [CrossRef]
- Johnson, T.R.; Graham, B.S. Contribution of respiratory syncytial virus G antigenicity to vaccine-enhanced illness and the implications for severe disease during primary respiratory syncytial virus infection. Pediatr. Infect. Dis. J. 2004, 23, S46–S57. [Google Scholar] [CrossRef]
- Bohmwald, K.; Espinoza, J.A.; Rey-Jurado, E.; Gomez, R.S.; Gonzalez, P.A.; Bueno, S.M.; Riedel, C.A.; Kalergis, A.M. Human Respiratory Syncytial Virus: Infection and Pathology. Semin. Respir. Crit. Care Med. 2016, 37, 522–537. [Google Scholar] [CrossRef]
- Fonseca, A.; Scott, N.; Strickland, D.; Everard, M. Persistence of respiratory syncytial virus replication in lung dendritic cells. Eur. Respir. Soc. 2015. [Google Scholar] [CrossRef]
- Rivera-Toledo, E.; Gomez, B. Respiratory syncytial virus persistence in macrophages alters the profile of cellular gene expression. Viruses 2012, 4, 3270–3280. [Google Scholar] [CrossRef] [PubMed]
- Rudd, B.D.; Schaller, M.A.; Smit, J.J.; Kunkel, S.L.; Neupane, R.; Kelley, L.; Berlin, A.A.; Lukacs, N.W. MyD88-mediated instructive signals in dendritic cells regulate pulmonary immune responses during respiratory virus infection. J. Immunol. 2007, 178, 5820–5827. [Google Scholar] [CrossRef] [PubMed]
- Cyr, S.L.; Angers, I.; Guillot, L.; Stoica-Popescu, I.; Lussier, M.; Qureshi, S.; Burt, D.S.; Ward, B.J. TLR4 and MyD88 control protection and pulmonary granulocytic recruitment in a murine intranasal RSV immunization and challenge model. Vaccine 2009, 27, 421–430. [Google Scholar] [CrossRef] [PubMed]
- Openshaw, P.J.M.; Chiu, C.; Culley, F.J.; Johansson, C. Protective and Harmful Immunity to RSV Infection. Annu. Rev. Immunol. 2017, 35, 501–532. [Google Scholar] [CrossRef] [PubMed]
- Sparrer, K.M.; Gack, M.U. Intracellular detection of viral nucleic acids. Curr. Opin. Microbiol. 2015, 26, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Oh, D.S.; Oh, J.E.; Jung, H.E.; Lee, H.K. Transient Depletion of CD169(+) Cells Contributes to Impaired Early Protection and Effector CD8(+) T Cell Recruitment against Mucosal Respiratory Syncytial Virus Infection. Front. Immunol. 2017, 8, 819. [Google Scholar] [CrossRef]
- Morris, S.; Swanson, M.S.; Lieberman, A.; Reed, M.; Yue, Z.; Lindell, D.M.; Lukacs, N.W. Autophagy-mediated dendritic cell activation is essential for innate cytokine production and APC function with respiratory syncytial virus responses. J. Immunol. 2011, 187, 3953–3961. [Google Scholar] [CrossRef]
- Boyapalle, S.; Wong, T.; Garay, J.; Teng, M.; San Juan-Vergara, H.; Mohapatra, S.; Mohapatra, S. Respiratory syncytial virus NS1 protein colocalizes with mitochondrial antiviral signaling protein MAVS following infection. PLoS ONE 2012, 7, e29386. [Google Scholar] [CrossRef]
- Spann, K.M.; Tran, K.C.; Collins, P.L. Effects of nonstructural proteins NS1 and NS2 of human respiratory syncytial virus on interferon regulatory factor 3, NF-kappaB, and proinflammatory cytokines. J. Virol. 2005, 79, 5353–5362. [Google Scholar] [CrossRef]
- Runkel, L.; Pfeffer, L.; Lewerenz, M.; Monneron, D.; Yang, C.H.; Murti, A.; Pellegrini, S.; Goelz, S.; Uze, G.; Mogensen, K. Differences in activity between alpha and beta type I interferons explored by mutational analysis. J. Biol. Chem. 1998, 273, 8003–8008. [Google Scholar] [CrossRef]
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Oh, D.S.; Kim, T.H.; Lee, H.K. Differential Role of Anti-Viral Sensing Pathway for the Production of Type I Interferon β in Dendritic Cells and Macrophages Against Respiratory Syncytial Virus A2 Strain Infection. Viruses 2019, 11, 62. https://doi.org/10.3390/v11010062
Oh DS, Kim TH, Lee HK. Differential Role of Anti-Viral Sensing Pathway for the Production of Type I Interferon β in Dendritic Cells and Macrophages Against Respiratory Syncytial Virus A2 Strain Infection. Viruses. 2019; 11(1):62. https://doi.org/10.3390/v11010062
Chicago/Turabian StyleOh, Dong Sun, Tae Hoon Kim, and Heung Kyu Lee. 2019. "Differential Role of Anti-Viral Sensing Pathway for the Production of Type I Interferon β in Dendritic Cells and Macrophages Against Respiratory Syncytial Virus A2 Strain Infection" Viruses 11, no. 1: 62. https://doi.org/10.3390/v11010062
APA StyleOh, D. S., Kim, T. H., & Lee, H. K. (2019). Differential Role of Anti-Viral Sensing Pathway for the Production of Type I Interferon β in Dendritic Cells and Macrophages Against Respiratory Syncytial Virus A2 Strain Infection. Viruses, 11(1), 62. https://doi.org/10.3390/v11010062




