Rapid Viral Diagnosis of Orthopoxviruses by Electron Microscopy: Optional or a Must?
Abstract
1. Introduction
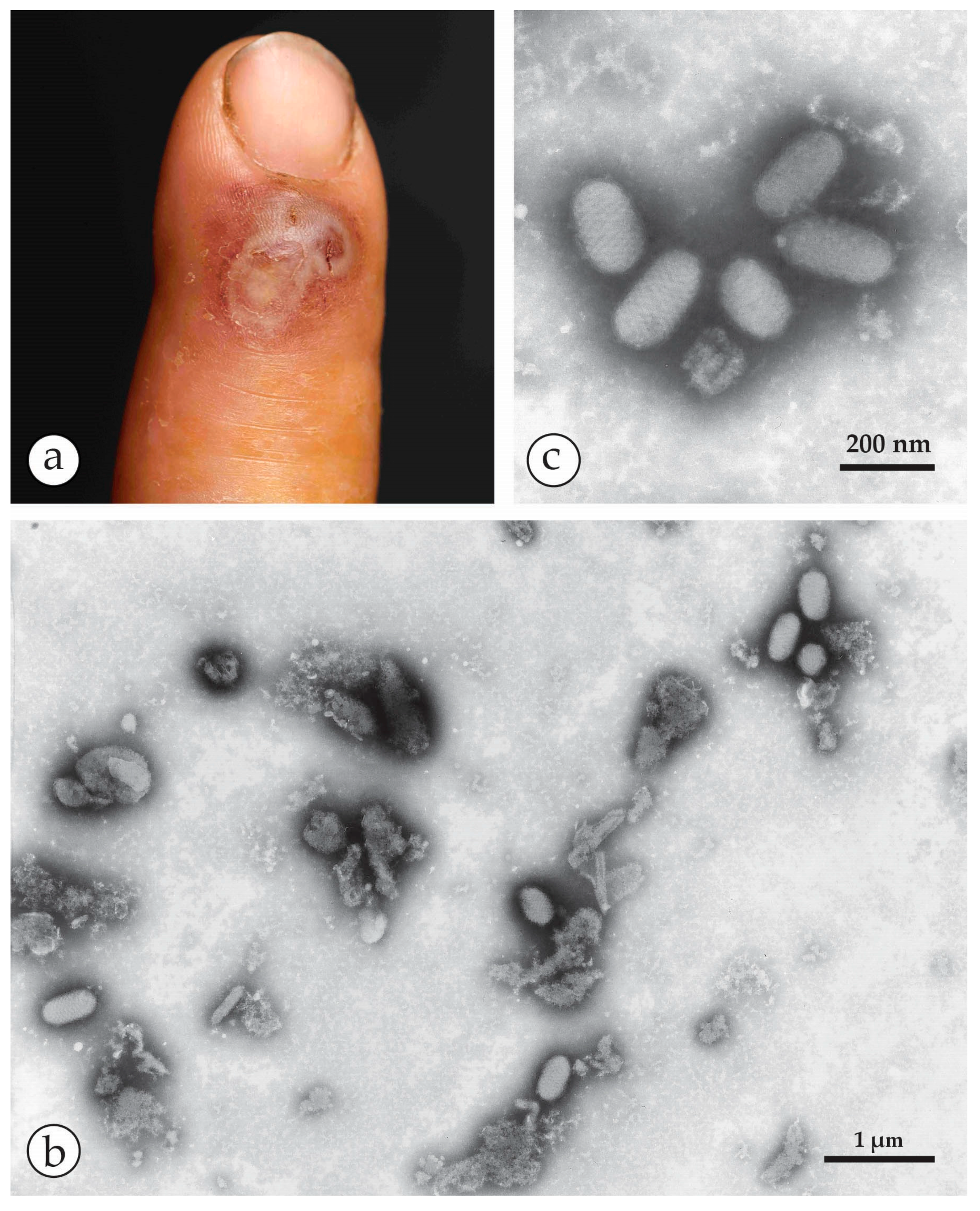
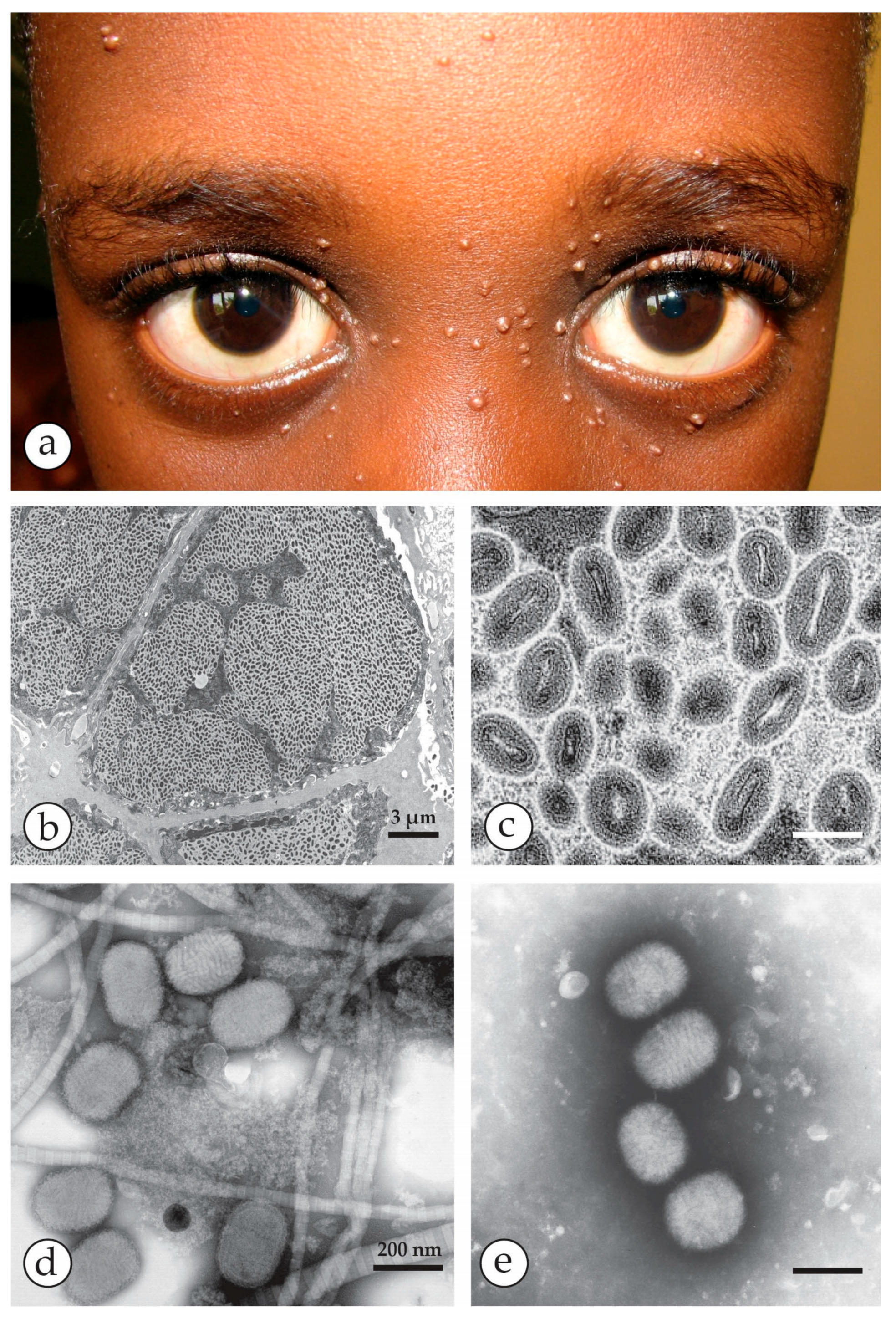
2. Orthopoxviruses (OPV), Herpesviruses and Other Agents
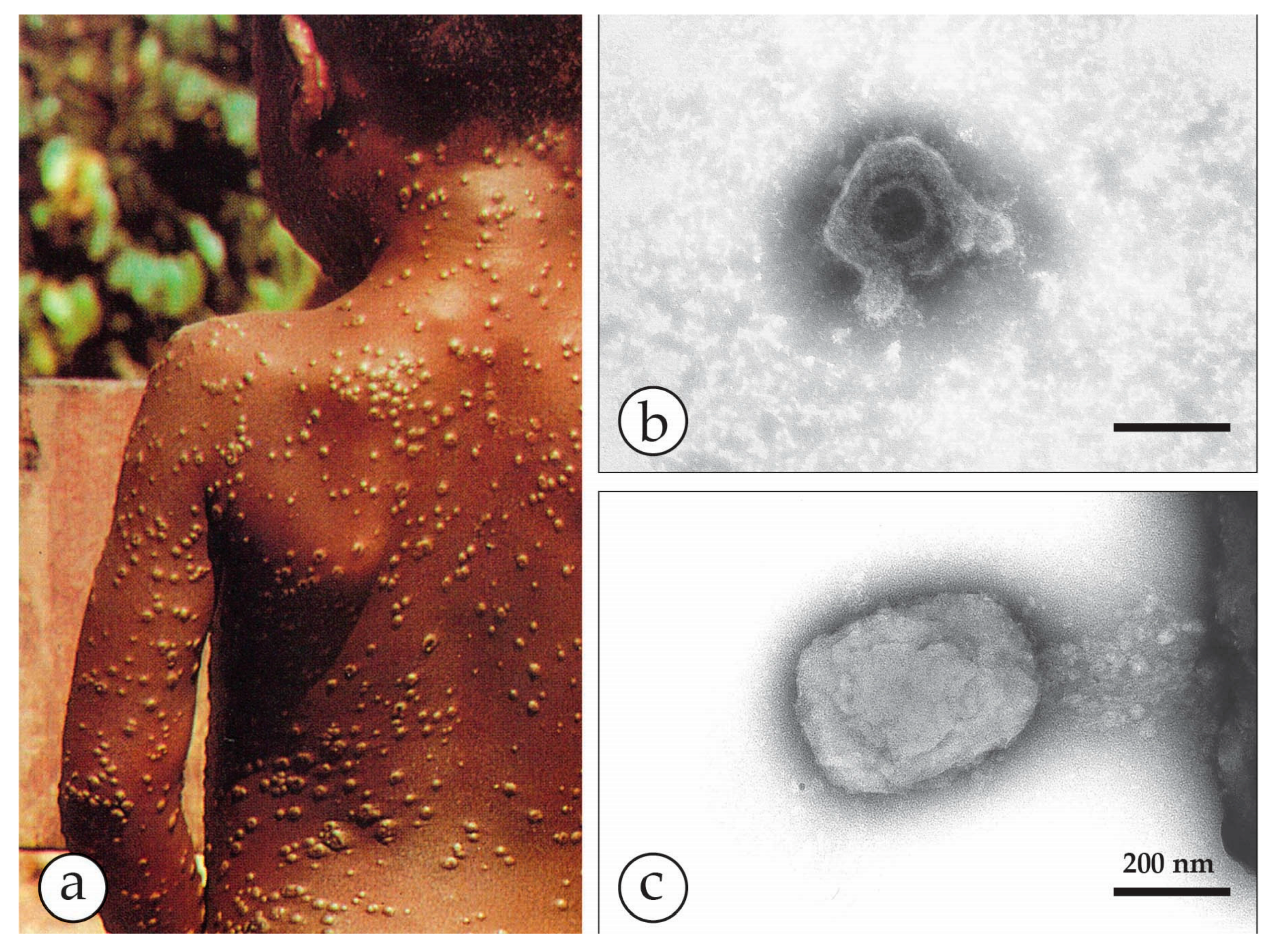
3. Smallpox (Variola)—The Disease
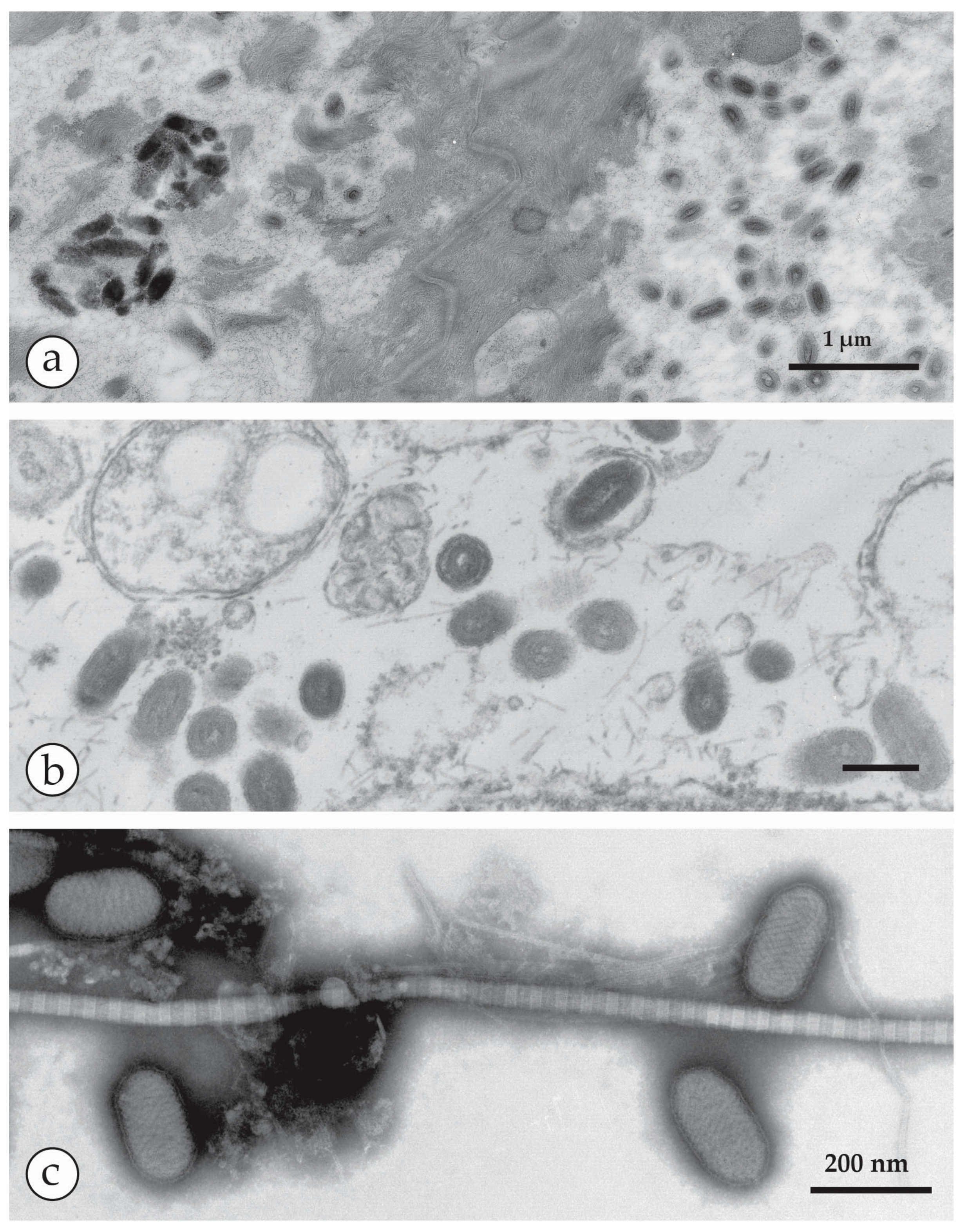
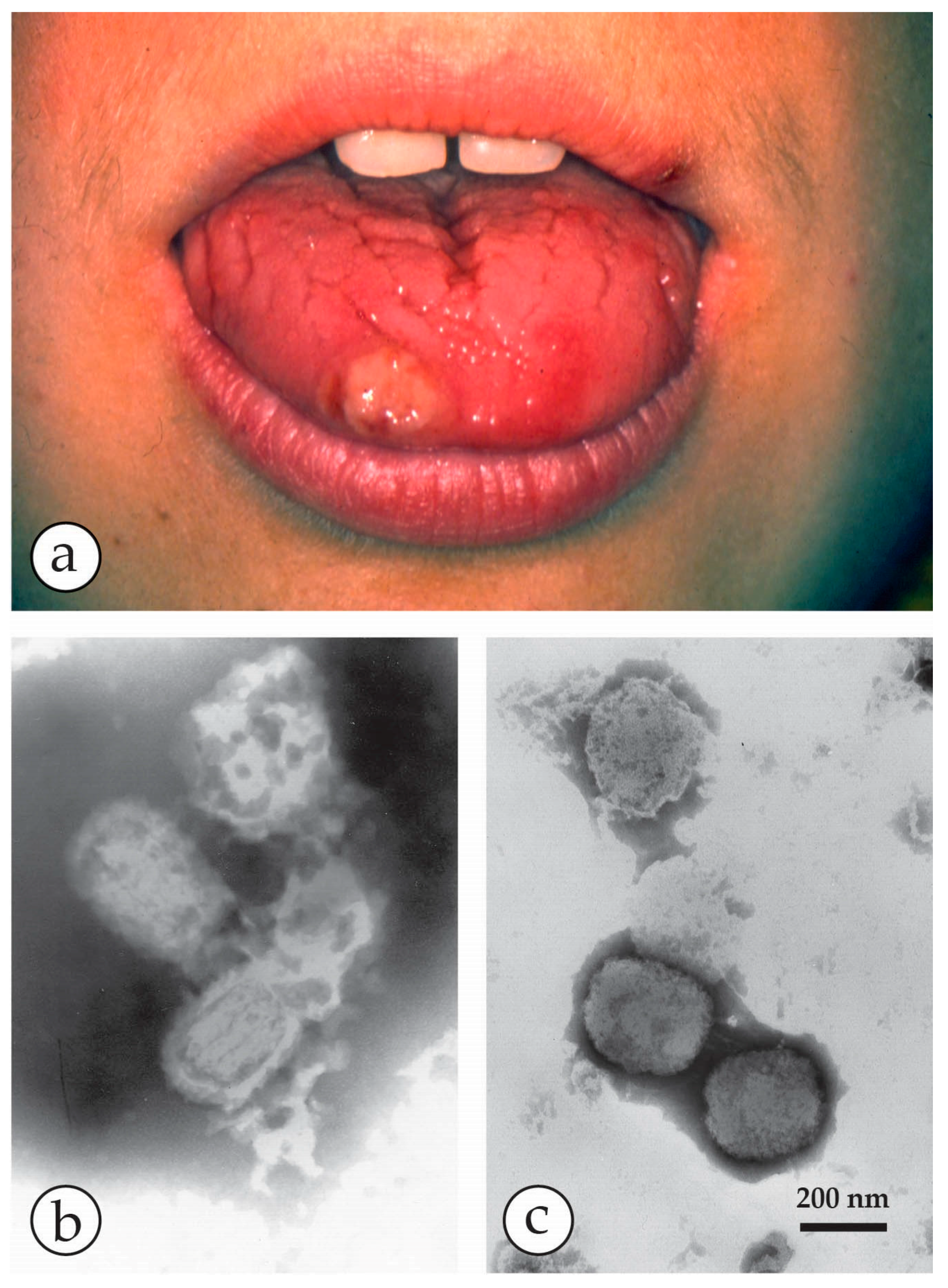
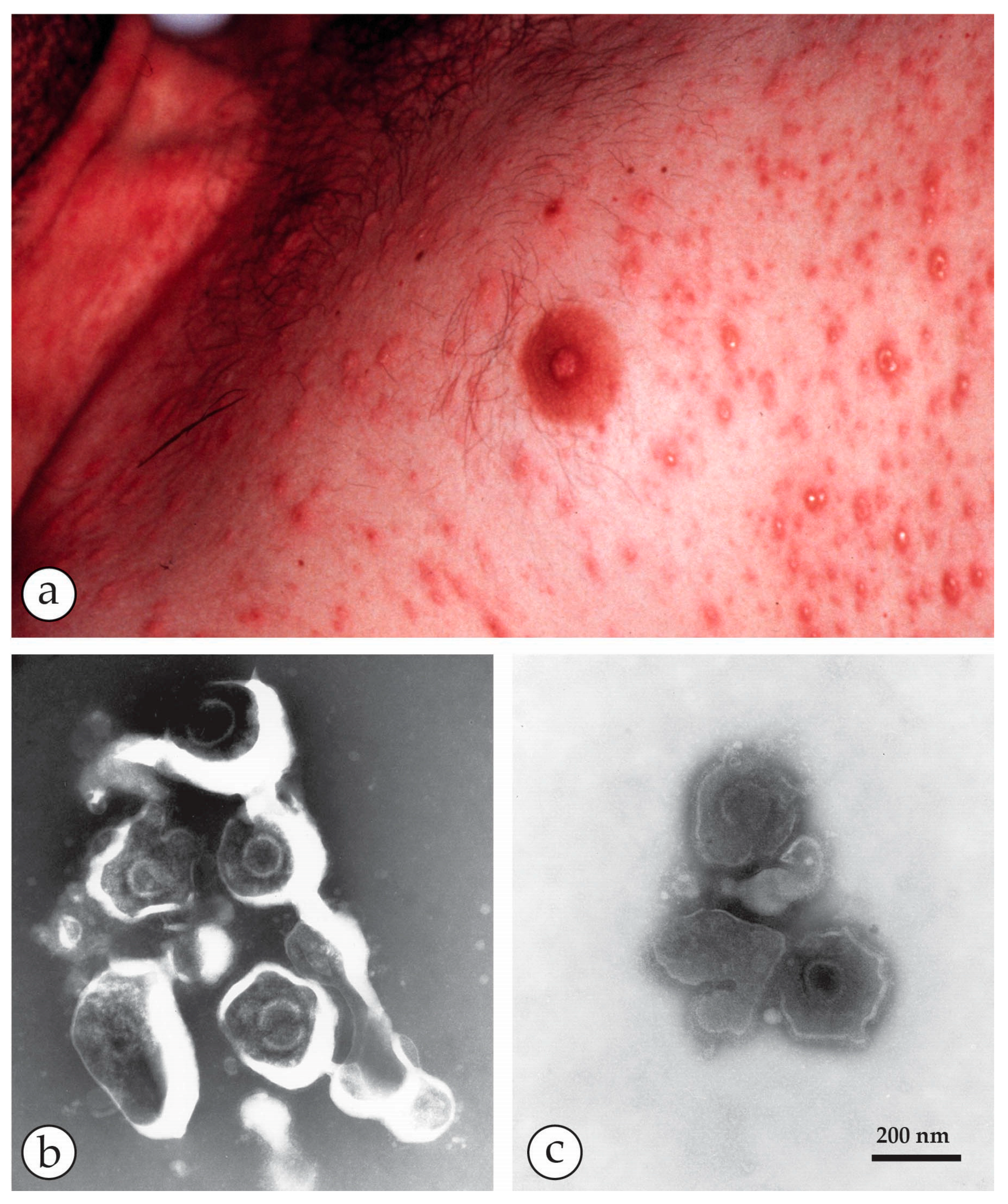
4. Diagnosis of Vesicular Skin Rashes
4.1. Unique Advantages of DEM in Rapid Viral Diagnosis
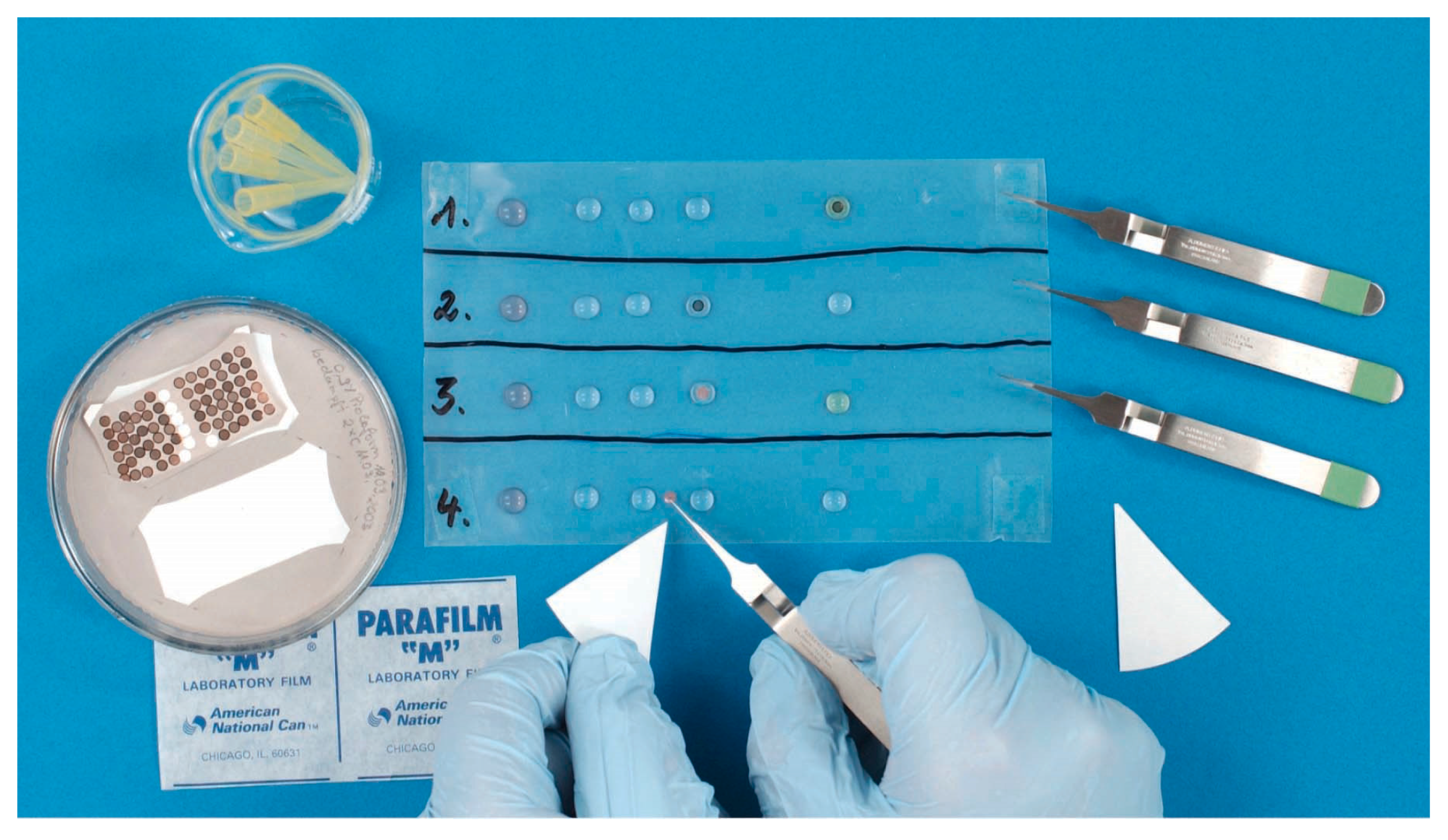
4.2. Specimen Collection and Preparation
Collecting Diagnostic Fluids from Vesicles
- Aspirate fluid from three lesions into a small tuberculin-type syringe with a fine needle (29 gauge is ideal). Carefully re-cap the syringe and place it into a transport container. Cave: Recapping syringes is strictly forbidden in the US for safety reasons (unless it is one-handed or done with a device to hold the cap).
- Open the surface of a vesicle using a sterile (injection) needle. Collect fluid into a fine glass tube by capillary attraction and close the tube at both ends with dental wax. The wax is removed later in a Laminar Flow Safety Cabinet and the fluid is carefully expelled onto a sheet of ParafilmTM using a small rubber bulb on the “clean” end of the capillary.
- Open the vesicle as in 2 above. Press the middle of a sterile glass light microscopic slide onto the fluid, remove the slide, let it dry, and mark it on the reverse where the droplet has dried to make finding the sample easier. Place the slide in a Petri dish for transport. To protect the dried fluid during transport, two matchsticks are placed as spacers at the ends of the sample slide which is then covered with another plain slide. Both slides are then bound together using an elastic band at each end.
- With an open ulcerated lesion or when the base of a vesicle is uncovered, touch a microscope grid briefly onto the base holding it with fine-pointed forceps. Place the grid with specimen side uppermost onto a filter-paper disc placed in a Petri dish. Cover before transporting to the laboratory.
4.3. Specimen Support Grids for DEM
4.4. Negative Staining of a DEM Sample
- To avoid cross-contamination use a different pair of forceps for each specimen. Always disinfect and clean the forceps carefully immediately afterwards.
- The washing steps are helpful in getting an even distribution of stain on the grid. However, each washing step will appreciably reduce the number of particles on the grid.
- Before staining with UAc, the grid with adherent specimen material must be washed on 3–5 droplets of distilled water to remove interfering phosphate ions. Successful NS with PTA does not require extensive washing; washing on a single droplet will suffice.
- While checking the first grid in the TEM, the remaining specimen, the washing and stain droplets are left on the ParafilmTM, protected from dust and drying in the wet chamber. This helps to shorten preparation time in case further preparations, possibly stained differently, are needed.
- Viruses and other biologicals will concentrate at the interface between the air and the sample fluid [103]. Letting the sample droplet remain untouched for a few minutes (or even longer, e.g., overnight at 4 °C in the refrigerator, if found necessary) before the grid is placed onto it for adsorption can help with low-concentration samples.
- With sample volumes below 5 µL the sample may be placed directly onto the grid´s surface for adsorption.
- While PTA staining tends to make biological structures more labile and porous, UAc is both a stain and a fixative [104]. When one stain does not result in a satisfactory preparation, the other usually does. Therefore, with unknown samples it may be advisable to use both stains in parallel. Preparations stained with either stain tend to deteriorate in the course of a fortnight. A loss of fine structure and the appearance of larger stain crystals (“grain”) can be avoided by storing “valuable” grids in vacuo in a desiccator containing some phospho-pentoxide as a desiccant. As well as these two common stains, a number of alternatives, e.g., ammonium molybdate, sodium silico-tungstate and uranyl formate have also been used successfully [90,98,105]. All three excel by a very fine “grain” and ammonium molybdate in addition by a well-balanced contrast.
4.5. Biological Safety in DEM
5. Limitations of DEM
5.1. Cost and Availability of a Specific DEM Laboratory
5.2. Too Few Particles in the Specimen
5.3. Lack of Dedicated and Experienced Staff for DEM
5.4. Low Sample Through-Put by DEM
6. Conclusions
7. What is the Future for DEM?
Acknowledgments
Author Contributions
Conflicts of Interest
Abbreviations
| BPXV | Buffalopox virus |
| CAM | Chorio-Allantoic Membrane of fertile hen’s egg, used for isolating pox and herpes viruses |
| CDC | Centers for Disease Control & Prevention, Atlanta, GA, USA |
| CMLV | Camelpox virus |
| CPXV | Cowpox virus |
| DEM | Diagnostic Electron Microscopy |
| DNA | DeoxyriboNucleic Acid |
| ELISA | Enzyme-Linked Immunosorbent Assay (often abbreviated to EIA) |
| EM | Electron Microscope or Electron Microscopy |
| GLP | Good Laboratory Practice |
| HSV | Herpes Simplex Virus |
| MC | Molluscum Contagiosum |
| MCV | Molluscipox Virus |
| MERS | Middle East Respiratory Syndrome |
| MPXV | Monkeypox virus |
| NAT | Nucleic Acid Amplification Techniques |
| NS | Negative Staining |
| NS-EM | Negative Staining Electron Microscopy |
| OPD | Out-Patients Department |
| OPV | OrthoPoxVirus |
| PCR | Polymerase Chain Reaction |
| PPV | ParaPoxVirus |
| PTA | Potassium phosphoTungstic Acid |
| SARS | Severe Acute Respiratory Syndrome |
| TEM | Transmission Electron Microscopy |
| TS | Thin Section |
| TS-EM | Thin-Section Electron microscopy |
| UAc | Uranyl Acetate |
| VACV | Vaccinia virus, used for prophylaxis against smallpox |
| VARV | Variola virus, cause of Smallpox |
| VZV | Varicella Zoster Virus |
References
- Fenner, F.; Henderson, D.A.; Arita, I.; Jezek, L.; Ladnyi, I.D. Smallpox and Its Eradication; World Health Organization: Geneva, Switzerland, 1988. [Google Scholar]
- Essbauer, S.; Pfeffer, M.; Meyer, H. Zoonotic poxviruses. Vet. Microbiol. 2010, 140, 229–236. [Google Scholar] [CrossRef] [PubMed]
- Morens, D.M.; Folkers, G.K.; Fauci, A.S. The challenge of emerging and re-emerging infectious diseases. Nature 2004, 430, 242–249. [Google Scholar] [CrossRef] [PubMed]
- Shchelkunov, S.N. An increasing danger of zoonotic orthopoxvirus infections. PLoS Pathog. 2013, 9, e1003756. [Google Scholar] [CrossRef] [PubMed]
- Olson, V.; Shchelkunov, S. Are we prepared in case of a possible smallpox-like disease emergence? Viruses 2017, 9, 242. [Google Scholar] [CrossRef]
- Alibek, K. Smallpox: A disease and a weapon. Int. J. Infect. Dis. 2004, 8 (Suppl. 2). [Google Scholar] [CrossRef] [PubMed]
- Brumfiel, G. Russia’s bioweapons labs: Still out in the cold. Nature 2003, 423, 678–680. [Google Scholar] [CrossRef] [PubMed]
- Food and Drug Administration (FDA). FDA Review of the 2014 Discovery of Vials Labeled “Variola” and Other Vials Discovered in an FDA-Occupied Building on the NIH Campus. Available online: https://www.fda.gov/downloads/aboutfda/reportsmanualsforms/reports/ucm532877.pdf (accessed on 22 December 2017).
- World Health Organization (WHO). WHO Advisory Committee on Variola Virus Research: Report of the Eighteenth Meeting, Geneva, Switzerland, 2017. Available online: http://www.who.int/csr/resources/publications/smallpox/variola-research-2016/en/ (accessed on 12 January 2018).
- Henderson, D.A.; Inglesby, T.V.; Bartlett, J.G.; Ascher, M.S.; Eitzen, E.; Jahrling, P.B.; Hauer, J.; Layton, M.; McDade, J.; Osterholm, M.T.; et al. Smallpox as a biological weapon: medical and public health management. Working Group on Civilian Biodefense. JAMA 1999, 281, 2127–2137. [Google Scholar] [CrossRef] [PubMed]
- Henderson, D.A.; Arita, I. The smallpox threat: A time to reconsider global policy. Biosecur. Bioterror. 2014, 12, 117–121. [Google Scholar] [CrossRef] [PubMed]
- LeDuc, J.W.; Jahrling, P.B. Strengthening national preparedness for smallpox: An update. Emerg. Infect. Dis. 2001, 7, 155–157. [Google Scholar] [CrossRef] [PubMed]
- International Committee on Taxonomy of Viruses (ICTV). Available online: http://ictvonline.org/taxonomyRelease.asp (accessed on 13 October 2017).
- Damon, I.K. Poxviruses. In Fields Virology, 6th ed.; Fields, B.N., Knipe, D.M., Howley, P.M., Eds.; Wolters Kluwer/Lippincott, Williams & Wilkins Health: Philadelphia, PA, USA, 2013. [Google Scholar]
- Carroll, D.S.; Emerson, G.L.; Li, Y.; Sammons, S.; Olson, V.; Frace, M.; Nakazawa, Y.; Czerny, C.P.; Tryland, M.; Kolodziejek, J.; et al. Chasing Jenner’s vaccine: Revisiting cowpox virus classification. PLoS ONE 2011, 6. [Google Scholar] [CrossRef] [PubMed]
- Esparza, J.; Schrick, L.; Damaso, C.R.; Nitsche, A. Equination (inoculation of horsepox): An early alternative to vaccination (inoculation of cowpox) and the potential role of horsepox virus in the origin of the smallpox vaccine. Vaccine 2017, 35, 7222–7230. [Google Scholar] [CrossRef] [PubMed]
- Abrahao, J.S.; Campos, R.K.; Trindade, G.S.; da Fonseca, F.G.; Ferreira, P.C.; Kroon, E.G. Outbreak of severe zoonotic vaccinia virus infection, Southeastern Brazil. Emerg. Infect. Dis. 2015, 21, 695–698. [Google Scholar] [CrossRef] [PubMed]
- Assis, F.L.; Borges, I.A.; Ferreira, P.C.; Bonjardim, C.A.; Trindade, G.S.; Lobato, Z.I.; Guedes, M.I.; Mesquita, V.; Kroon, E.G.; Abrahao, J.S. Group 2 vaccinia virus, Brazil. Emerg. Infect. Dis. 2012, 18, 2035–2038. [Google Scholar] [CrossRef] [PubMed]
- Gurav, Y.K.; Raut, C.G.; Yadav, P.D.; Tandale, B.V.; Sivaram, A.; Pore, M.D.; Basu, A.; Mourya, D.T.; Mishra, A.C. Buffalopox outbreak in humans and animals in Western Maharashtra, India. Prev. Vet. Med. 2011, 100, 242–247. [Google Scholar] [CrossRef] [PubMed]
- Kroon, E.G.; Mota, B.E.; Abrahao, J.S.; da Fonseca, F.G.; Trindade, G.S. Zoonotic Brazilian Vaccinia virus: from field to therapy. Antivir. Res. 2011, 92, 150–163. [Google Scholar] [CrossRef] [PubMed]
- Leite, J.A.; Drumond, B.P.; Trindade, G.S.; Lobato, Z.I.; da Fonseca, F.G.; dos, S.J.; Madureira, M.C.; Guedes, M.I.; Ferreira, J.M.; Bonjardim, C.A.; et al. Passatempo virus, a vaccinia virus strain, Brazil. Emerg. Infect. Dis. 2005, 11, 1935–1938. [Google Scholar] [CrossRef] [PubMed]
- Moussatche, N.; Damaso, C.R.; McFadden, G. When good vaccines go wild: Feral Orthopoxvirus in developing countries and beyond. J. Infect. Dev. Ctries 2008, 2, 156–173. [Google Scholar] [CrossRef] [PubMed]
- Zafar, A.; Swanepoel, R.; Hewson, R.; Nizam, M.; Ahmed, A.; Husain, A.; Grobbelaar, A.; Bewley, K.; Mioulet, V.; Dowsett, B.; et al. Nosocomial buffalopoxvirus infection, Karachi, Pakistan. Emerg. Infect. Dis. 2007, 13, 902–904. [Google Scholar] [CrossRef] [PubMed]
- Franke, A.; Pfaff, F.; Jenckel, M.; Hoffmann, B.; Hoper, D.; Antwerpen, M.; Meyer, H.; Beer, M.; Hoffmann, D. Classification of cowpox viruses into several distinct clades and identification of a novel lineage. Viruses 2017, 9. [Google Scholar] [CrossRef] [PubMed]
- McCollum, A.M.; Damon, I.K. Human monkeypox. Clin. Infect. Dis. 2014, 58, 260–267. [Google Scholar] [CrossRef] [PubMed]
- Bhanuprakash, V.; Prabhu, M.; Venkatesan, G.; Balamurugan, V.; Hosamani, M.; Pathak, K.M.; Singh, R.K. Camelpox: Epidemiology, diagnosis and control measures. Expert Rev. Anti. Infect. Ther. 2010, 8, 1187–1201. [Google Scholar] [CrossRef] [PubMed]
- Prabhu, M.; Yogisharadhya, R.; Pavulraj, S.; Suresh, C.; Sathish, G.; Singh, R.K. Camelpox and buffalopox: Two emerging and re-emerging orthopox viral diseases of India. Adv. Anim. Vet. Sci. 2015, 3, 527–541. [Google Scholar] [CrossRef]
- Qin, L.; Favis, N.; Famulski, J.; Evans, D.H. Evolution of and evolutionary relationships between extant vaccinia virus strains. J. Virol. 2015, 89, 1809–1824. [Google Scholar] [CrossRef] [PubMed]
- Young, G.E.; Hidalgo, C.M.; Sullivan-Frohm, A.; Schult, C.; Davis, S.; Kelly-Cirino, C.; Egan, C.; Wilkins, K.; Emerson, G.L.; Noyes, K.; et al. Secondary and tertiary transmission of vaccinia virus from US military service member. Emerg. Infect. Dis. 2011, 17, 718–721. [Google Scholar] [CrossRef] [PubMed]
- Höpken, W.; Willers, H.; Knocke, K.W.; Olberding, P.; Liess, B.; Petzold, K.; Laufs, R.; Raub, H.W. Two cases of smallpox in Hanover (1967 and 1972): Clinical features, epidemiology, and laboratory diagnosis. Dtsch. Med. Wochenschr. 1973, 98, 587–593. [Google Scholar] [CrossRef] [PubMed]
- Riedel, S. Edward Jenner and the history of smallpox and vaccination. Proc. (Bayl. Univ. Med. Cent.) 2005, 18, 21–25. [Google Scholar] [CrossRef] [PubMed]
- Mauldin, M.R.; Antwerpen, M.; Emerson, G.L.; Li, Y.; Zoeller, G.; Carroll, D.S.; Meyer, H. Cowpox virus: What’s in a Name? Viruses 2017, 9, 101. [Google Scholar] [CrossRef] [PubMed]
- Pfeiff, B.; Pullmann, H.; Eis-Hubinger, A.M.; Gerritzen, A.; Schneweis, K.E.; Mayr, A. Letale Tierpockeninfektion bei einem Atopiker unter dem Bild einer Variola vera. Hautarzt 1991, 42, 293–297. [Google Scholar] [PubMed]
- Kurth, A.; Wibbelt, G.; Gerber, H.P.; Petschaelis, A.; Pauli, G.; Nitsche, A. Rat-to-elephant-to-human transmission of cowpox virus. Emerg. Infect. Dis. 2008, 14, 670–671. [Google Scholar] [CrossRef] [PubMed]
- Kastenmayer, R.J.; Maruri-Avidal, L.; Americo, J.L.; Earl, P.L.; Weisberg, A.S.; Moss, B. Elimination of A-type inclusion formation enhances cowpox virus replication in mice: Implications for orthopoxvirus evolution. Virology 2014, 452–453, 59–66. [Google Scholar] [CrossRef] [PubMed]
- CDC. Update: Multistate outbreak of monkeypox—Illinois, Indiana, Kansas, Missouri, Ohio, and Wisconsin, 2003. MMWR Morb. Mortal. Wkly. Rep. 2003, 52, 561–564. [Google Scholar]
- Reed, K.D.; Melski, J.W.; Graham, M.B.; Regnery, R.L.; Sotir, M.J.; Wegner, M.V.; Kazmierczak, J.J.; Stratman, E.J.; Li, Y.; Fairley, J.A.; et al. The detection of monkeypox in humans in the Western Hemisphere. N. Engl. J. Med. 2004, 350, 342–350. [Google Scholar] [CrossRef] [PubMed]
- Rimoin, A.W.; Mulembakani, P.M.; Johnston, S.C.; Lloyd Smith, J.O.; Kisalu, N.K.; Kinkela, T.L.; Blumberg, S.; Thomassen, H.A.; Pike, B.L.; Fair, J.N.; et al. Major increase in human monkeypox incidence 30 years after smallpox vaccination campaigns cease in the Democratic Republic of Congo. Proc. Natl. Acad. Sci. USA 2010, 107, 16262–16267. [Google Scholar] [CrossRef] [PubMed]
- Meyer, H.; Perrichot, M.; Stemmler, M.; Emmerich, P.; Schmitz, H.; Varaine, F.; Shungu, R.; Tshioko, F.; Formenty, P. Outbreaks of disease suspected of being due to human monkeypox virus infection in the Democratic Republic of Congo in 2001. J. Clin. Microbiol. 2002, 40, 2919–2921. [Google Scholar] [CrossRef] [PubMed]
- Nitsche, A.; Gelderblom, H.R.; Eisendle, K.; Romani, N.; Pauli, G. Pitfalls in diagnosing human poxvirus infections. J. Clin. Virol. 2007, 38, 165–168. [Google Scholar] [CrossRef] [PubMed]
- Dhar, A.D.; Werchniak, A.E.; Li, Y.; Brennick, J.B.; Goldsmith, C.S.; Kline, R.; Damon, I.; Klaus, S.N. Tanapox infection in a college student. N. Engl. J. Med. 2004, 350, 361–366. [Google Scholar] [CrossRef] [PubMed]
- Friederichs, S.; Krebs, S.; Blum, H.; Lang, H.; Buttner, M. Parapoxvirus (PPV) of red deer reveals subclinical infection and confirms a unique species. J. Gen. Virol. 2015, 96, 1446–1462. [Google Scholar] [CrossRef] [PubMed]
- Wibbelt, G.; Tausch, S.H.; Dabrowski, P.W.; Kershaw, O.; Nitsche, A.; Schrick, L. Berlin Squirrelpox Virus, a New Poxvirus in Red Squirrels, Berlin, Germany. Emerg. Infect. Dis. 2017, 23, 1726–1729. [Google Scholar] [CrossRef] [PubMed]
- Gelderblom, H.R.; Bannert, N.; Muss, W.; Madeley, C.R. Routine, rapid, noninvasive diagnosis of viral skin exanthems. Br. J. Dermatol. 2006, 154, 558–560. [Google Scholar] [CrossRef] [PubMed]
- Gelderblom, H.R. Elektronenmikroskopie in der Erregerdiagnostik. In Lexikon der Infektionskrankheiten des Menschen, 4th ed.; Darai, G., Handermann, M., Sonntag, H.G., Zöller, L., Eds.; Springer: Heidelberg, Germany; New York, NY, USA; Tokyo, Japan, 2012. [Google Scholar]
- Almeida, J.D. Uses and abuses of diagnostic electron microscopy. Curr. Top. Microbiol. Immunol. 1983, 104, 147–158. [Google Scholar] [PubMed]
- Gelderblom, H.R.; Hazelton, P.R. Specimen collection for electron microscopy. Emerg. Infect. Dis. 2000, 6, 433–434. [Google Scholar] [CrossRef] [PubMed]
- Williams, M.G.; Almeida, J.D.; Howatson, A.F. Electron microscope studies on viral skin lesions. A simple and rapid method of identifying virus particles. Arch. Dermatol. 1962, 86, 290–297. [Google Scholar] [CrossRef] [PubMed]
- Laue, M.; Bannert, N. Detection limit of negative staining electron microscopy for the diagnosis of bioterrorism-related micro-organisms. J. Appl. Microbiol. 2010, 109, 1159–1168. [Google Scholar] [CrossRef] [PubMed]
- Ruska, H. Über das virus der varicellen und des zoster. Klin. Wochenschr. 1943, 22, 703–704. [Google Scholar] [CrossRef]
- Nagler, F.P.O.; Rake, G. The use of the electron microscope in diagnosis of variola, vaccinia, and varicella. J. Bacteriol. 1948, 55, 45–51. [Google Scholar] [PubMed]
- Van Rooyen, C.E.; Scott, G.D. Smallpox diagnosis with special reference to electron microscopy. Can. J. Publ. Health 1948, 39, 467–477. [Google Scholar]
- Brenner, S.; Horne, R.W. A negative staining method for high resolution electron microscopy of viruses. Biochim. Biophys. Acta 1959, 34, 103–110. [Google Scholar] [CrossRef]
- Peters, D.; Nielsen, G.; Bayer, M.E. Variola. Reliability of the rapid electron-microscopic diagnosis. Dtsch. Med. Wochenschr. 1962, 87, 2240–2246. [Google Scholar] [CrossRef] [PubMed]
- Nagington, J. Electron microscopy in differential diagnosis of poxvirus infections. Br. Med. J. 1964, 2, 1499–1500. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Cruickshank, J.G.; Bedson, H.S.; Watson, D.H. Electron microscopy in the rapid diagnosis of smallpox. Lancet 1966, 2, 527–530. [Google Scholar] [CrossRef]
- Long, G.W.; Nobel, J., Jr.; Murphy, F.A.; Herrmann, K.L.; Lourie, B. Experience with electron microscopy in the differential diagnosis of smallpox. Appl. Microbiol. 1970, 20, 497–504. [Google Scholar] [PubMed]
- Sasse, J.; Gelderblom, H.R. Lessons learnt from the German smallpox outbreaks after World War II. Bundesgesundheitsblatt Gesundh. Gesundh. 2015, 58, 730–737. [Google Scholar] [CrossRef] [PubMed][Green Version]
- von Borries, B.; Ruska, E.; Ruska, H. Bakterien und Virus in Übermikroskopischer Aufnahme. Klin. Wochenschr. 1938, 17, 921–925. [Google Scholar] [CrossRef]
- Utagawa, E.T.; Nakazawa, E.; Matsuo, K.; Oishi, I.; Takeda, N.; Miyamura, T. Application of an automated specimen search system installed in a transmission electron microscope for the detection of caliciviruses in clinical specimens. J. Virol. Methods 2002, 100, 49–56. [Google Scholar] [CrossRef]
- Kylberg, G. Automatic Virus Identification Using TEM: Image Segmentation and Texture Analysis. Ph.D. Thesis, Acta Universitatis Upsaliensis: Uppsala, Sweden, 2014. [Google Scholar]
- Ong, H.; Chandran, V. Identification of gastroenteric viruses by electron microscopy using higher order spectral features. J. Clin. Virol. 2005, 34, 195–206. [Google Scholar] [CrossRef] [PubMed]
- Schroeder, J.A. Ultrastructural pathology today-paradigm change and the impact of microwave technology and telemicroscopy. In Diagnostic Electron Microscopy. A Practical Guide to Interpretation and Technique; Stirling, J.W., Curry, A., Eyden, B., Eds.; Wiley and Sons Ltd.: Chichester, UK, 2013. [Google Scholar]
- Miller, S.E.; Howell, D.N. Concerted use of immunologic and ultrastructural analyses in diagnostic medicine: Immunoelectron microscopy and correlative microscopy. Immunol. Investig. 1997, 26, 29–38. [Google Scholar] [CrossRef]
- Laue, M. Electron microscopy of viruses. In Virus Cell Culture—A Practical Approach, Cann, A., Ed.; Oxford University Press: Oxford, UK, 1999. [Google Scholar]
- Lavazza, A.; Tittarelli, C.; Cerioli, M. The use of convalescent sera in immune-electron microscopy to detect non-suspected/new viral agents. Viruses 2015, 7, 2683–2703. [Google Scholar] [CrossRef] [PubMed]
- Almeida, J.D. A classification of virus particles based on morphology. Can. Med. Assoc. J. 1963, 89, 787–798. [Google Scholar] [PubMed]
- Biel, S.S.; Gelderblom, H.R. Diagnostic electron microscopy is still a timely and rewarding method. J. Clin. Virol. 1999, 13, 105–119. [Google Scholar] [CrossRef]
- Biel, S.S.; Madeley, D. Diagnostic virology—The need for electron microscopy: A discussion paper. J. Clin. Virol. 2001, 22, 1–9. [Google Scholar] [CrossRef]
- Curry, A.; Appleton, H.; Dowsett, B. Application of transmission electron microscopy to the clinical study of viral and bacterial infections: Present and future. Micron 2006, 37, 91–106. [Google Scholar] [CrossRef] [PubMed]
- Field, A.M. Diagnostic virology using electron microscopic techniques. Adv. Virus Res. 1982, 27, 1–69. [Google Scholar] [PubMed]
- Gelderblom, H.R.; Renz, H.; Ozel, M. Negative staining in diagnostic virology. Micron. Microsc. Acta 1991, 22, 435–447. [Google Scholar] [CrossRef]
- Gentile, M.; Gelderblom, H.R. Electron microscopy in rapid viral diagnosis: An update. N. Microbiol. 2014, 37, 403–422. [Google Scholar]
- Goldsmith, C.S.; Ksiazek, T.G.; Rollin, P.E.; Comer, J.A.; Nicholson, W.L.; Peret, T.C.; Erdman, D.D.; Bellini, W.J.; Harcourt, B.H.; Rota, P.A.; et al. Cell culture and electron microscopy for identifying viruses in diseases of unknown cause. Emerg. Infect. Dis. 2013, 19, 886–891. [Google Scholar] [CrossRef] [PubMed]
- Goldsmith, C.S. Morphologic differentiation of viruses beyond the family level. Viruses 2014, 6, 4902–4913. [Google Scholar] [CrossRef] [PubMed]
- Hazelton, P.R.; Gelderblom, H.R. Electron microscopy for rapid diagnosis of infectious agents in emergent situations. Emerg. Infect. Dis. 2003, 9, 294–303. [Google Scholar] [CrossRef] [PubMed]
- Hsiung, G.-D.; Fong, C.F.Y.; Landry, M.L.; Fong, C.K.Y.; Landry, M.L. Hsiung’s Diagnostic Virology: As Illustrated by Light and Electron Microscopy, 4th ed.; Yale University Press: New Haven, CT, USA, 1994. [Google Scholar]
- Kjeldsberg, E. Application of electron microscopy in viral diagnosis. Pathol. Res. Pract. 1980, 167, 3–21. [Google Scholar] [CrossRef]
- Madeley, C.R. Viruses associated with acute diarrhoeal disease. In Principles and Practice of Clinical Virology, 3rd ed.; Zuckermann, A.J., Banatvala, J.E., Pattison, J.R., Eds.; Wiley: Chichester, UK, 1995. [Google Scholar]
- Madeley, C.R.; Field, A.M. Virus Morphology, 2nd ed.; Churchill Livingstone: London, UK, 1988. [Google Scholar]
- Palmer, E.L.; Martin, M.L. Electron Microscopy in Viral Diagnosis; CRC Press Inc: Boca Raton, FL, USA, 1988. [Google Scholar]
- Roingeard, P. Viral detection by electron microscopy: Past, present and future. Biol. Cell 2008, 100, 491–501. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Hung, T.; Song, J.; He, J. Electron microscopy: Essentials for viral structure, morphogenesis and rapid diagnosis. Sci. China Life Sci. 2013, 56, 421–430. [Google Scholar] [CrossRef] [PubMed]
- Doane, F.W.; Anderson, N. Electron Microscopy in Diagnostic Virology: A Practical Guide and Atlas; Cambridge University Press: Cambridge, UK, 1987. [Google Scholar]
- Goldsmith, C.S.; Miller, S.E. Modern uses of electron microscopy for detection of viruses. Clin. Microbiol. Rev. 2009, 22, 552–563. [Google Scholar] [CrossRef] [PubMed]
- Madeley, C.R. Diagnosing smallpox in possible bioterrorist attack. Lancet 2003, 361, 97–98. [Google Scholar] [CrossRef]
- Stern, D.; Olson, V.A.; Smith, S.K.; Pietraszczyk, M.; Miller, L.; Miethe, P.; Dorner, B.G.; Nitsche, A. Rapid and sensitive point-of-care detection of Orthopoxviruses by ABICAP immunofiltration. Virol. J. 2016, 13. [Google Scholar] [CrossRef] [PubMed]
- Dumont, C.; Irenge, L.M.; Magazani, E.K.; Garin, D.; Muyembe, J.J.; Bentahir, M.; Gala, J.L. Simple technique for in field samples collection in the cases of skin rash illness and subsequent PCR detection of orthopoxviruses and varicella zoster virus. PLoS ONE 2014, 9, e96930. [Google Scholar] [CrossRef] [PubMed]
- Laue, M.; Niederwohrmeier, B.; Bannert, N. Rapid diagnostic thin section electron microscopy of bacterial endospores. J. Microbiol. Methods 2007, 70, 45–54. [Google Scholar] [CrossRef] [PubMed]
- Laue, M. Electron microscopy of viruses. Methods Cell Biol. 2010, 96. [Google Scholar] [CrossRef]
- Schroeder, J.A.; Gelderblom, H.R.; Hauroeder, B.; Schmetz, C.; Milios, J.; Hofstaedter, F. Microwave-assisted tissue processing for same-day EM-diagnosis of potential bioterrorism and clinical samples. Micron 2006, 37, 577–590. [Google Scholar] [CrossRef] [PubMed]
- Peters, D.; Mueller, G. The fine structure of the dna-containing core of vaccinia virus. Virology 1963, 21, 267–269. [Google Scholar] [CrossRef]
- Remy, W.; Gelderblom, H. Hetero-inoculation of vaccinia on the tongue tip. Hautarzt 1974, 25, 148–149. [Google Scholar] [PubMed]
- Sommer, J.R. To Cationize Glass. J. Cell Biol. 1977, 75, A245. [Google Scholar]
- Aebi, U.; Pollard, T.D. A glow discharge unit to render electron microscope grids and other surfaces hydrophilic. J. Electron. Microsc. Tech. 1987, 7, 29–33. [Google Scholar] [CrossRef] [PubMed]
- Consultant Laboratory for Diagnostic Electron Microscopy of Infectious Pathogens. Available online: www.rki.de/cl-em (accessed on 20 November 2017).
- Gelderblom, H.R.; Möller, L.; Laue, M. External Quality Assurance (EQA) in Diagnostic Electron Microscopy (DEM) of Infectious Diseases: Aims and Roots, Results and Perspectives. Available online: www.rki.de/cl-em, urn:nbn:de:0257-10055788 (accessed on 12 December 2017).
- Hayat, M.A.; Miller, S.E. Negative Staining; McGraw-Hill: New York, NY, USA, 1990. [Google Scholar]
- Harris, J.R.; Horne, R.W. Negative staining. In Electron Microscopy in Biology: A Practical Approach; Harris, J.R., Ed.; IRL Press at Oxford University Press: Oxford, UK, 1991. [Google Scholar]
- Miller, S.E. Bioterrorism and electron microscopic differentiation of poxviruses from herpesviruses: Dos and don’ts. Ultrastruct Pathol. 2003, 27, 133–140. [Google Scholar] [CrossRef] [PubMed]
- Kurth, A.; Nitsche, A. Detection of human-pathogenic poxviruses. Methods Mol. Biol. 2011, 665, 257–278. [Google Scholar] [PubMed]
- Centers for Disease Control and Prevention (CDC). Negative Staining Electron Microscope Protocol for Rash Illness. Available online: https://www.cdc.gov/smallpox/lab-personnel/specimen-collection/negative-stain.html (accessed on 23 November 2017).
- Johnson, R.P.; Gregory, D.W. Viruses accumulate spontaneously near droplet surfaces: A method to concentrate viruses for electron microscopy. J. Microsc. 1993, 171, 125–136. [Google Scholar] [CrossRef] [PubMed]
- Terzakis, J.A. Uranyl acetate, a stain and a fixative. J. Ultrastruct. Res. 1968, 22, 168–184. [Google Scholar] [CrossRef]
- Harris, J.R. Negative Staining and Cryoelectron Microscopy: The Thin Film Techniques; BIOS Scientific: Milton Park, UK, 1997. [Google Scholar]
- Luftig, R. An accurate measurement of the catalase crystal period and its use as an internal marker for electron microscopy. J. Ultrastruct. Res. 1967, 20, 91–102. [Google Scholar] [CrossRef]
- Centers for Disease Control and Prevention (CDC). Biosafety in Microbiological and Biomedical Laboratories (BMBL) 5th Edition. Available online: https://www.cdc.gov/biosafety/publications/bmbl5/index.htm (accessed on 20 December 2017).
- Möller, L.; Schunadel, L.; Nitsche, A.; Schwebke, I.; Hanisch, M.; Laue, M. Evaluation of virus inactivation by formaldehyde to enhance biosafety of diagnostic electron microscopy. Viruses 2015, 7, 666–679. [Google Scholar] [CrossRef] [PubMed]
- Laboratory Response Network (LRN). Available online: https://emergency.cdc.gov/lrn/index.asp (accessed on 20 December 2017).
- Laue, M.; Möller, L. The VirusExplorer DEM—A Database for Diagnostic Electron Microscopy of Viruses; Zenodo: Berlin, Germany, 2016. [Google Scholar]
- Gelderblom, H.R.; Reupke, H. Rapid Viral Diagnosis Using the Airfuge. Abstract of the Fourth International Congress for Virology; Internat. Virology IV: The Hague, The Netherlands, 1978. [Google Scholar]
- Hammond, G.W.; Hazelton, P.R.; Chuang, I.; Klisko, B. Improved detection of viruses by electron microscopy after direct ultracentrifuge preparation of specimens. J. Clin. Microbiol. 1981, 14, 210–221. [Google Scholar] [PubMed]
- Derrick, K.S. Quantitative assay for plant viruses using serologically specific electron microscopy. Virology 1973, 56, 652–653. [Google Scholar] [CrossRef]
- Kurth, A.; Achenbach, J.; Miller, L.; Mackay, I.M.; Pauli, G.; Nitsche, A. Orthopoxvirus detection in environmental specimens during suspected bioterror attacks: Inhibitory influences of common household products. Appl. Environ. Microbiol. 2008, 74, 32–37. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Ruska, H. Versuch zu einer Ordnung der Virusarten. Arch. Ges Virusforsch. 1943, 2, 480–498. [Google Scholar] [CrossRef]
- Kruger, D.H.; Schneck, P.; Gelderblom, H.R. Helmut Ruska and the visualisation of viruses. Lancet 2000, 355, 1713–1717. [Google Scholar] [CrossRef]
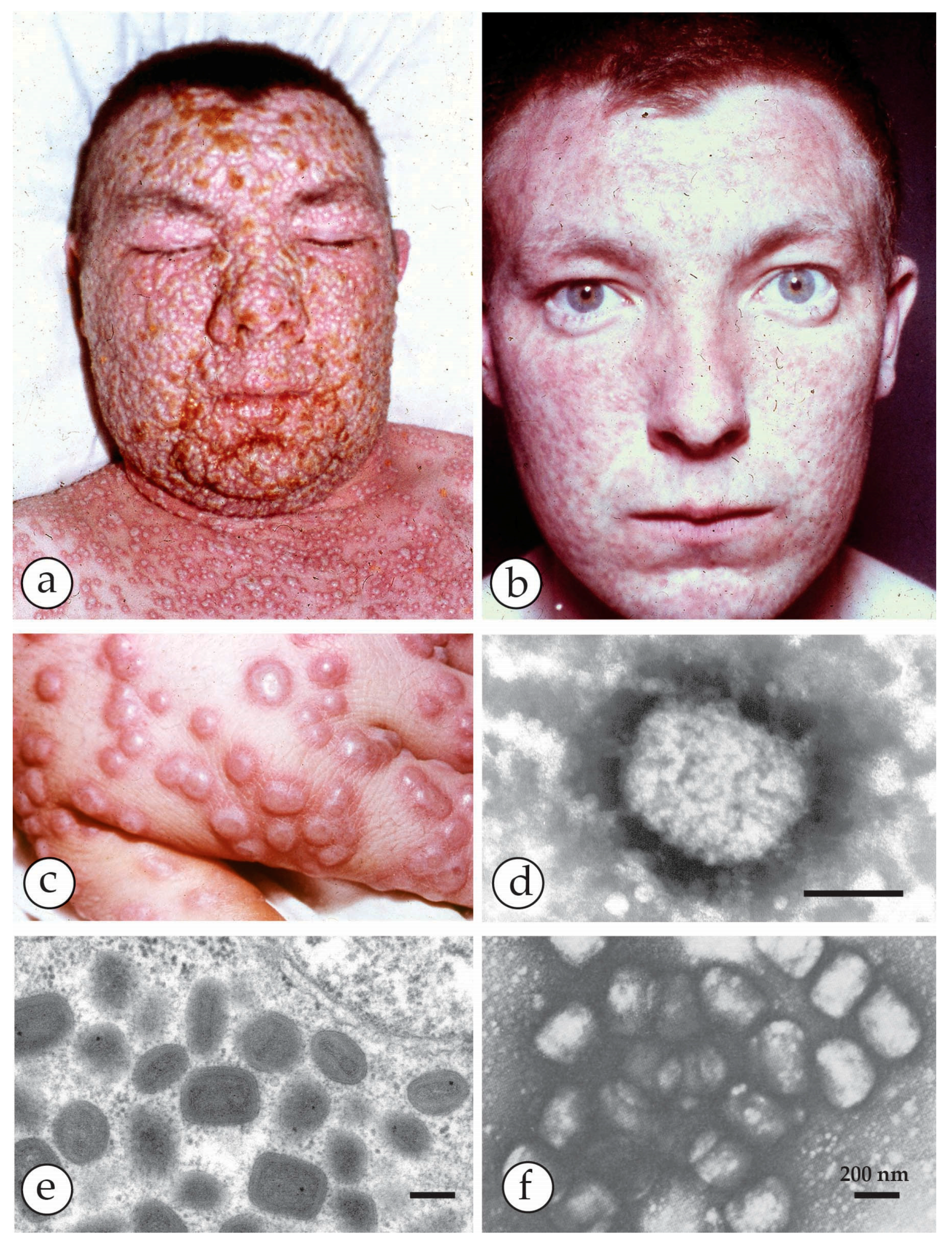
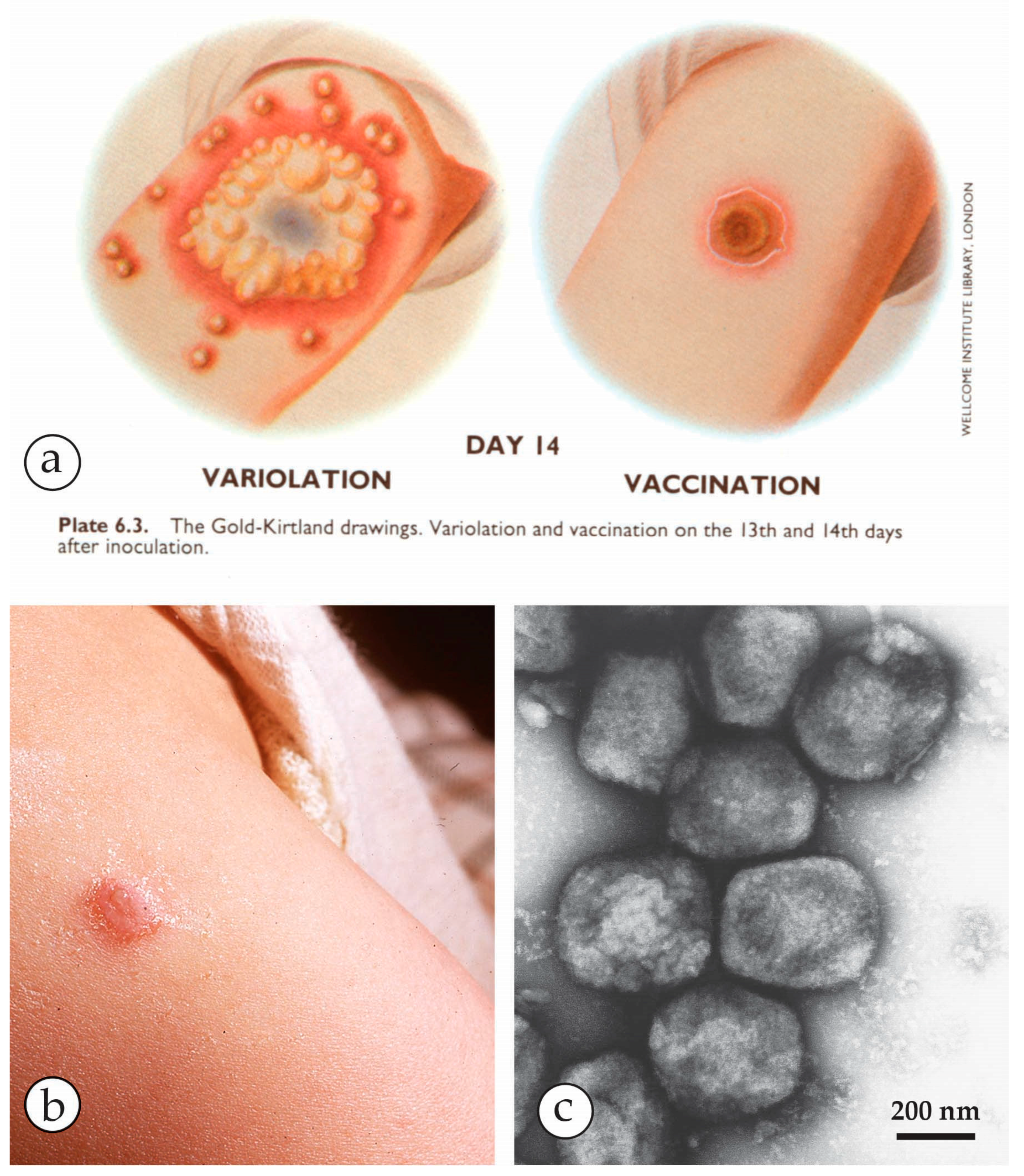
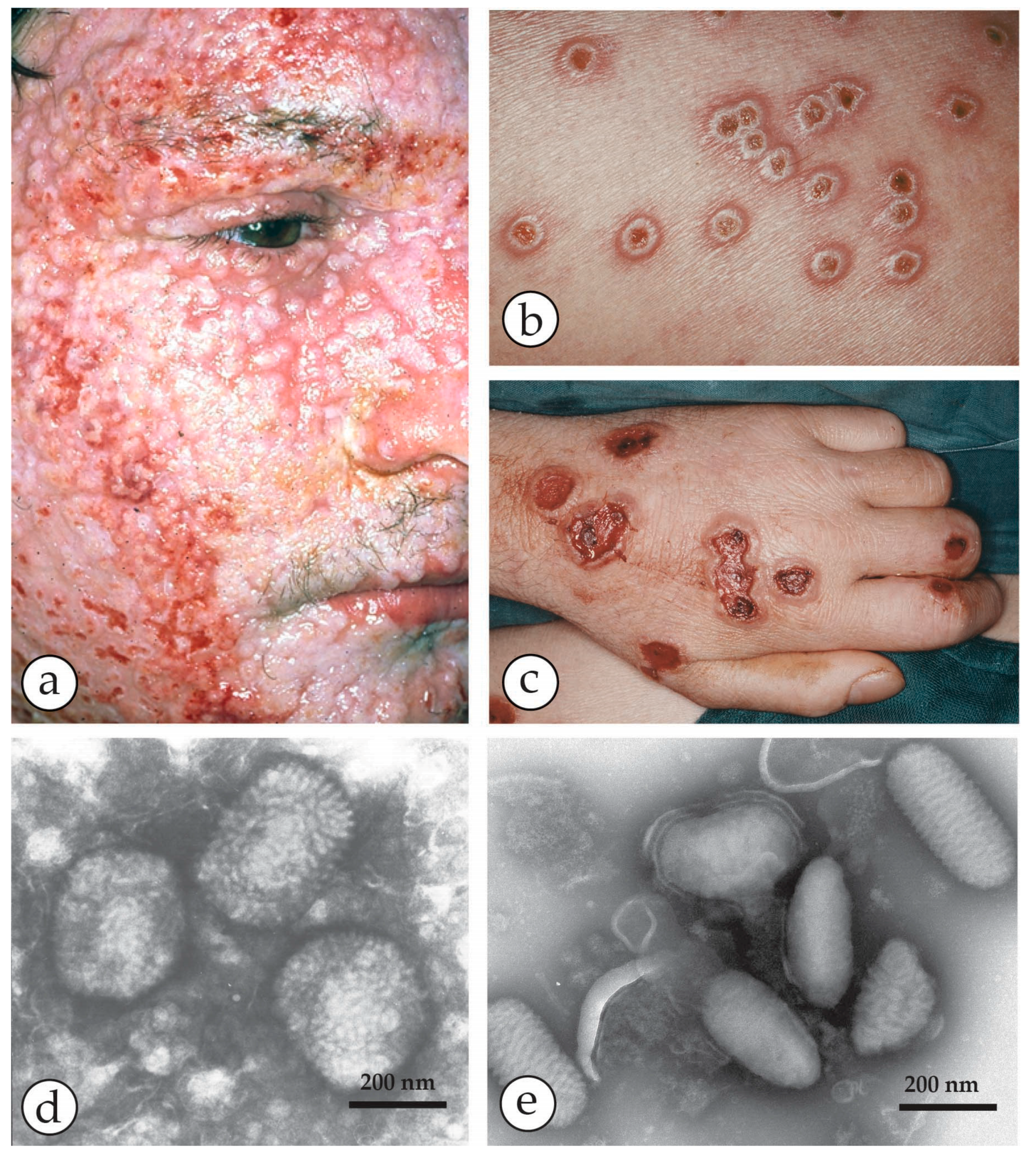

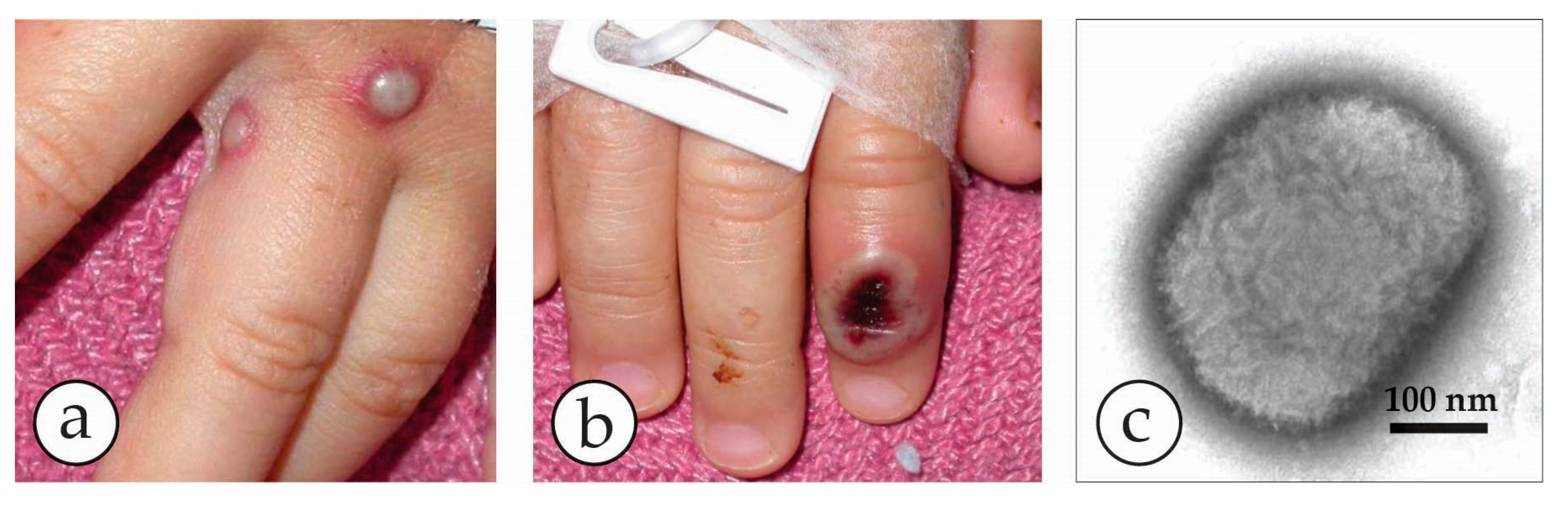
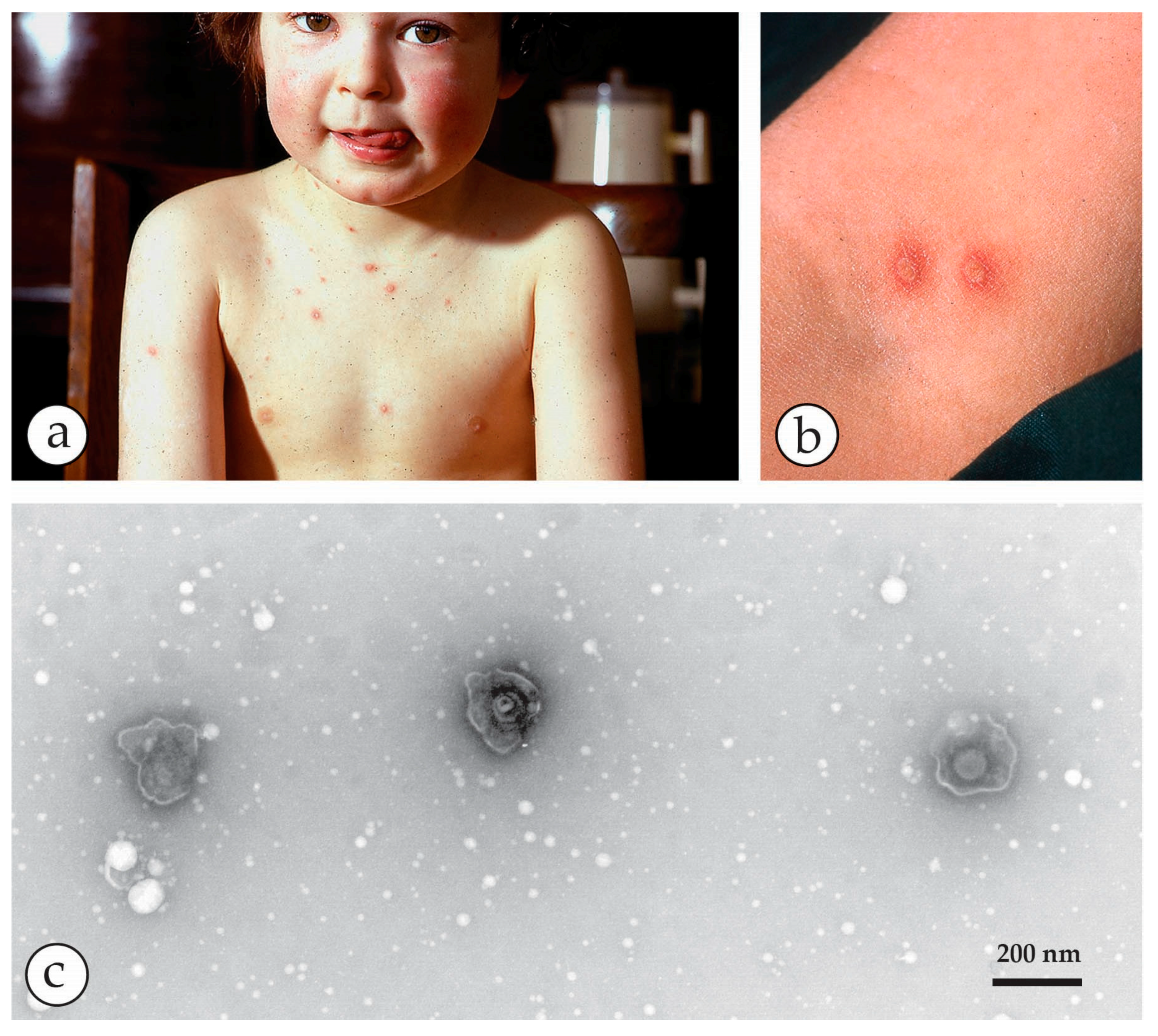
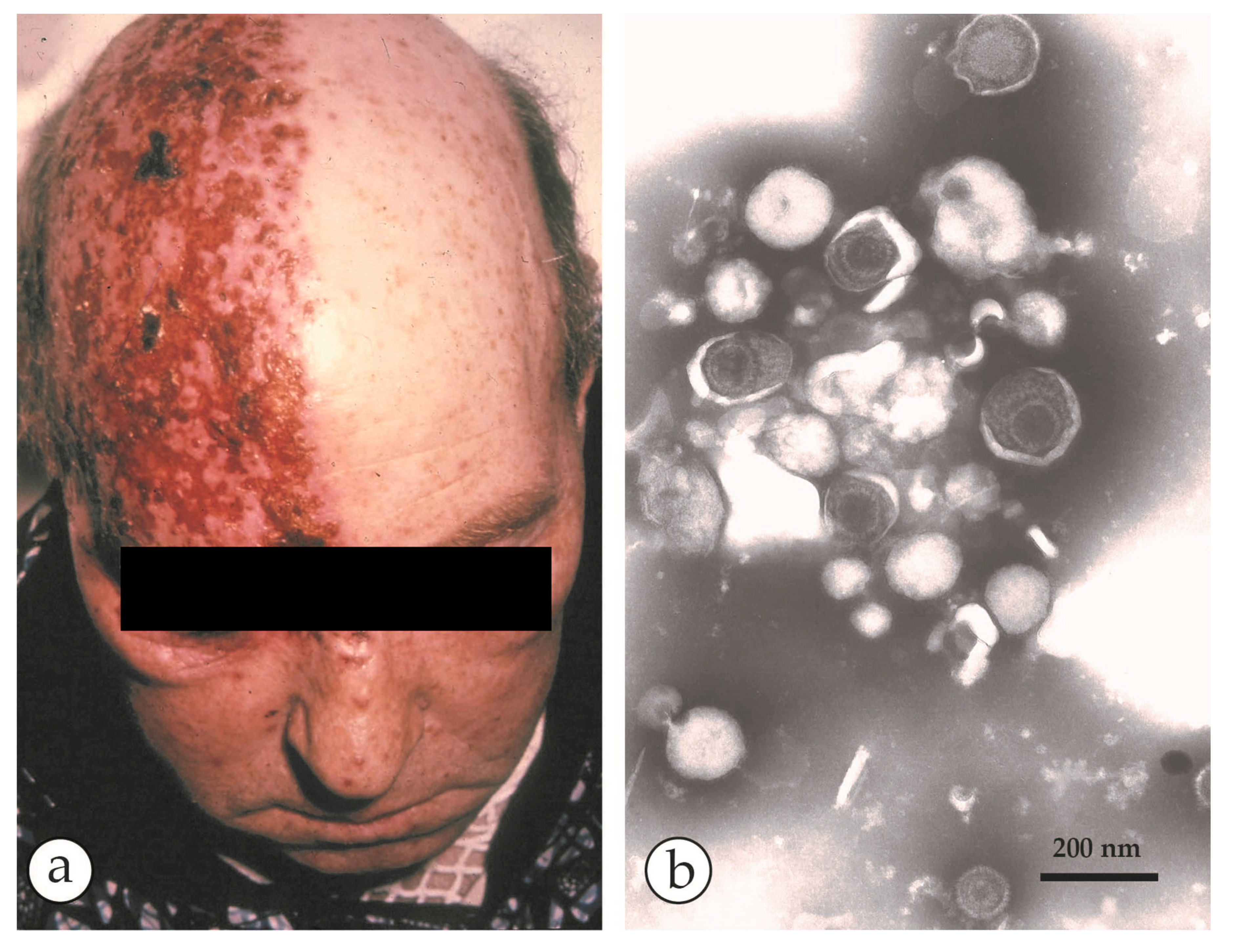
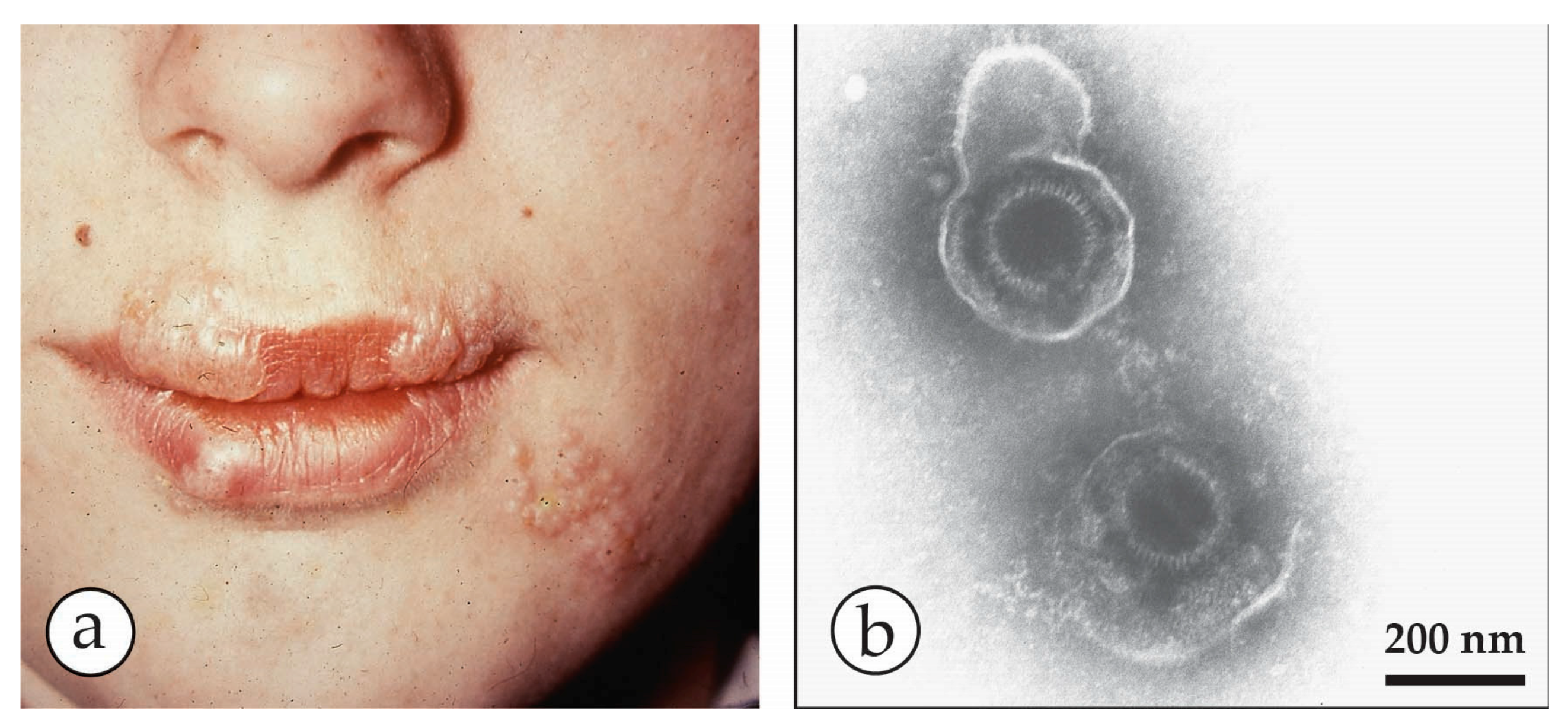
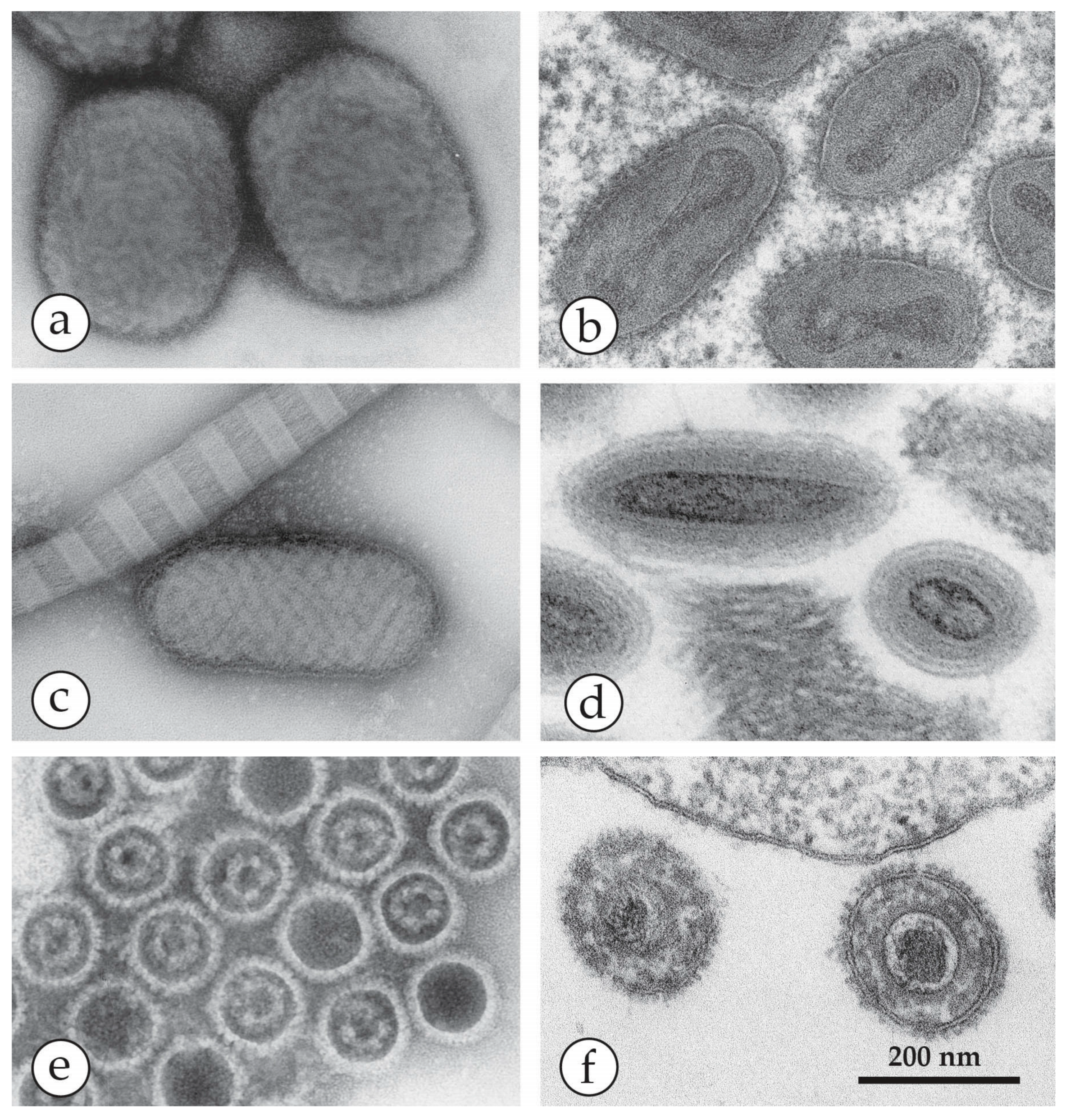
| Micro-Organism, Agent or Condition | Disease | Lesion Appearance * |
|---|---|---|
| Orthopoxviridae (OPV) Variola major virus Variola minor = Alastrim Man is only known host, currently eradicated as a human disease | Smallpox Alastrim | Generalised vesicular rash with large deep-seated vesicles all at the same stage, dimpled at the centre developing into pustules and crusting over later. Centrifugal distribution, including soles and palms. May be modified by previous vaccination |
| Other OPV: Vaccinia virus, buffalopox, cowpox, monkeypox, camelpox | Various animal diseases, occasionally transmitted to man | Usually single vesicular or papular lesion, developing into ulcer, and crusting later Lesions larger, up to 1cm in diameter, may not be clearly vesicular |
| Parapoxviridae (PPV) of goat, sheep: orf; cattle: paravaccinia, pseudocowpox | Animal diseases transmitted to man as Orf, Pseudocowpox (Milker’s nodules) | Large (up to 1 cm) nodular with little vesicular fluid, developing into an ulcer, crusting later May not be clearly vesicular |
| Molluscipoxvirus (MCV) specific for man. There is also animal specific MCV | Molluscum contagiosum warty lesions–may be multiple, may be passed as a sexually transmitted disease. Auto-inoculation may spread the lesions | Solid, firm, wart-like tumours: dome-shaped or flat. Pearly or flesh-coloured nodules with a depression on the top. Not clearly vesicular but contain waxy sacs packed with virus particles |
| Herpes varicella zoster (VZV) | Chickenpox, usually in childhood or Shingles (herpes zoster = recrudescence of previous varicella) | Generalised or scanty vesicular rash, mostly on head and trunk becoming pustular and crusting later. Lesions smaller, frailer and less deep-seated than smallpox, can be easily ruptured. Differ in size and stage of development Shingles: similar lesions but confined to the distribution of one or more sensory nerves. |
| Herpes simplex virus HSV-1, HSV-2 | “Cold sores”, usually limited to a few localised lesions, usually on the upper lip. Very occasionally a herpes encephalitis. Herpes simplex may be sexually transmitted | Limited recurrent vesicular lesions with prodromal tingling Lesions smaller and less deep-seated than smallpox, crusting later. |
| Enterovirus and other small spherical RNA-containing viruses | Hand-foot-and-mouth disease | Aphthous oral ulcers and small vesicular lesions 2–4 mm in diameter on the hands and feet, can also generalize. |
| Anthrax, Bacillus anthracis | Cutaneous Anthrax | Single or small number of large vesicles later, surrounding a dark central crust (“Malignant pustule”) |
| Treponema pallidum | Primary and secondary syphilis | Single red papule 0.5 to 2 cm in diameter developing into ulcer with an indurated margin and exudate |
| Drug-induced rashes | A variety of rashes: exanthematous pustulosis, Erythema multiforme | No specific micro-organisms present |
| Scabies and insect bites | Variety of single or multiple quasi-vesicular lesions | No specific micro-organisms present |
| Contact dermatitis | Symptomatic toxic-dermatitis | No specific micro-organisms present |
| Genus | Members, Species | Disease in Healthy Men | Natural Host | Appearance in DEM and Size |
|---|---|---|---|---|
| Orthopoxvirus (OPV) | Variola virus (VARV) | Smallpox Variola major Variola minor | Man only | Brick-shaped virions, 250–350 nm × 200 nm with an irregular array of 10–15 nm surface “protrusions” (threads). |
| Vaccinia virus (VACV) | Vaccination = local, self-limiting lesion | endemic in cattle and buffaloes in India and Brazil | ||
| Cowpox virus (CPXV) | Scanty vesicular rash developing into ulcer | Rodents transmitting CPXV to cattle, cats and other mammals | ||
| Monkeypox virus (MPXV) | Vesicular rash, similar to smallpox | Squirrels, non-human primates | ||
| other OPV: camelpox, buffalopox | Single or multiple vesicular-pustular lesions developing into ulcer | Various * | ||
| mousepox (ectromelia) and several others, some unclassified | no known disease in man | |||
| Parapoxvirus (PPV) | Orf (ecthyma contagiosum) | Single tender nodule developing into ulcer 10–15 mm in size | Sheep, goats | Oval virions: 250–300 nm × 150–180 nm with long, spiral surface threads |
| Pseudocowpox, Bovine papular stomatitis | see Orf | Cattle | ||
| Molluscipoxvirus (MCV) | Molluscum contagiosum virus (MCV) | Single or multiple papules developing into pink fleshy “warts”, often with umbilicated centre | Man only | Brick-shaped virion, short threads: Very similar to OPV |
| Yatapoxvirus | Tanapox | Single or multiple firm nodules | Non-human primates | Brick-shaped, very similar to OPV |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gelderblom, H.R.; Madeley, D. Rapid Viral Diagnosis of Orthopoxviruses by Electron Microscopy: Optional or a Must? Viruses 2018, 10, 142. https://doi.org/10.3390/v10040142
Gelderblom HR, Madeley D. Rapid Viral Diagnosis of Orthopoxviruses by Electron Microscopy: Optional or a Must? Viruses. 2018; 10(4):142. https://doi.org/10.3390/v10040142
Chicago/Turabian StyleGelderblom, Hans R., and Dick Madeley. 2018. "Rapid Viral Diagnosis of Orthopoxviruses by Electron Microscopy: Optional or a Must?" Viruses 10, no. 4: 142. https://doi.org/10.3390/v10040142
APA StyleGelderblom, H. R., & Madeley, D. (2018). Rapid Viral Diagnosis of Orthopoxviruses by Electron Microscopy: Optional or a Must? Viruses, 10(4), 142. https://doi.org/10.3390/v10040142





