Novel Parvoviruses from Wild and Domestic Animals in Brazil Provide New Insights into Parvovirus Distribution and Diversity
Abstract
1. Introduction
2. Materials and Methods
2.1. Samples
2.2. Preparation of Pools, Viral Genome Sequencing and Assembly
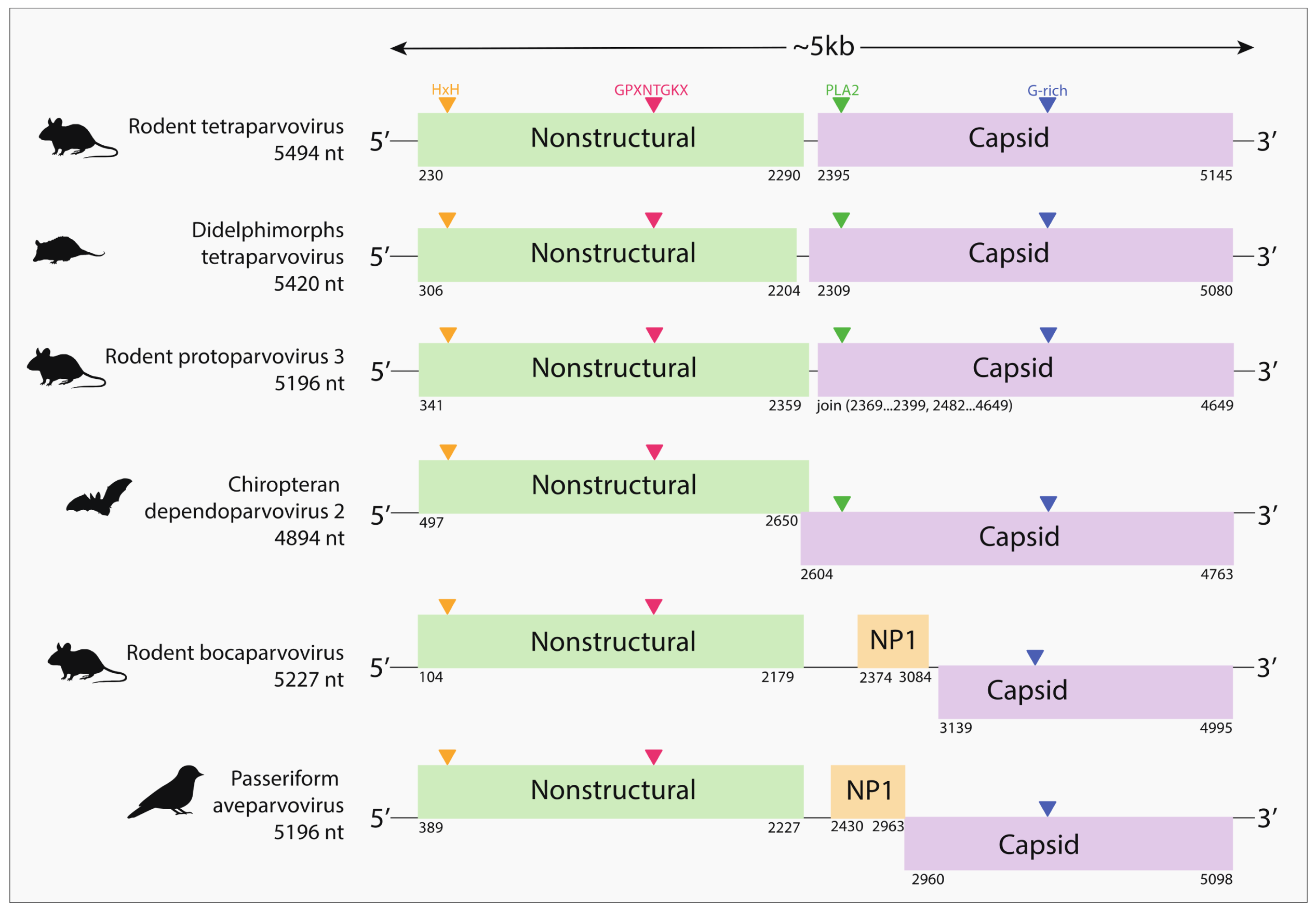
2.3. Genome Characterization
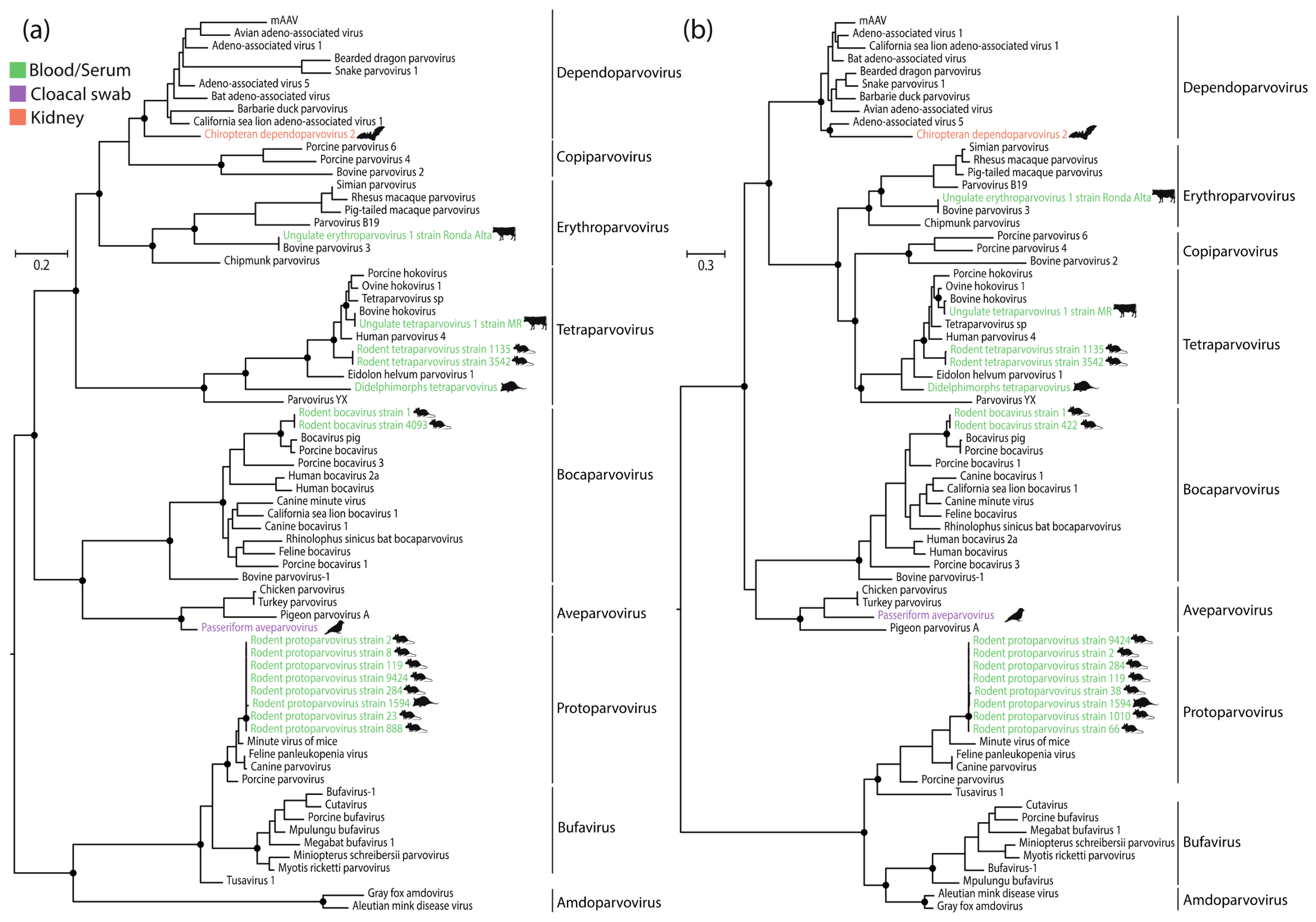
2.4. Phylogenetic Analysis
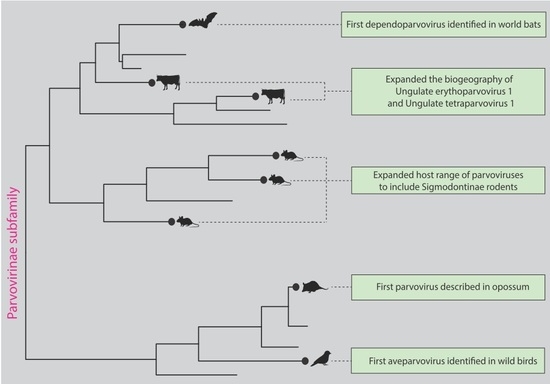
3. Results
4. Discussion
5. Conclusions
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Cotmore, S.F.; Agbandje-McKenna, M.; Chiorini, J.A.; Mukha, D.V.; Pintel, D.J.; Qiu, J.; Soderlund-Venermo, M.; Tattersall, P.; Tijssen, P.; Gatherer, D.; et al. The family Parvoviridae. Arch. Virol. 2014, 159, 1239–1247. [Google Scholar] [CrossRef] [PubMed]
- Qiu, J.; Soderlund-Venermo, M.; Young, N.S. Human parvoviruses. Clin. Microbiol. Rev. 2017, 30, 43–113. [Google Scholar] [CrossRef] [PubMed]
- Miranda, C.; Thompson, G. Canine parvovirus: The worldwide occurrence of antigenic variants. J. Gen. Virol. 2016, 97, 2043–2057. [Google Scholar] [CrossRef] [PubMed]
- Hargitai, R.; Pankovics, P.; Kertesz, A.M.; Biro, H.; Boros, A.; Phan, T.G.; Delwart, E.; Reuter, G. Detection and genetic characterization of a novel parvovirus distantly related to human bufavirus in domestic pigs. Arch. Virol. 2016, 161, 1033–1037. [Google Scholar] [CrossRef] [PubMed]
- Li, L.; Giannitti, F.; Low, J.; Keyes, C.; Ullmann, L.S.; Deng, X.; Aleman, M.; Pesavento, P.A.; Pusterla, N.; Delwart, E. Exploring the virome of diseased horses. J. Gen. Virol. 2015, 96, 2721–2733. [Google Scholar] [CrossRef] [PubMed]
- Souza, W.M.; Romeiro, M.F.; Fumagalli, M.J.; Modha, S.; de Araujo, J.; Queiroz, L.H.; Durigon, E.L.; Figueiredo, L.T.; Murcia, P.R.; Gifford, R.J. Chapparvoviruses occur in at least three vertebrate classes and have a broad biogeographic distribution. J. Gen. Virol. 2017, 98, 225–229. [Google Scholar] [CrossRef] [PubMed]
- Yang, S.; Liu, Z.; Wang, Y.; Li, W.; Fu, X.; Lin, Y.; Shen, Q.; Wang, X.; Wang, H.; Zhang, W. A novel rodent chapparvovirus in feces of wild rats. Virol. J. 2016, 13, 133. [Google Scholar] [CrossRef] [PubMed]
- Francois, S.; Filloux, D.; Roumagnac, P.; Bigot, D.; Gayral, P.; Martin, D.P.; Froissart, R.; Ogliastro, M. Discovery of parvovirus-related sequences in an unexpected broad range of animals. Sci. Rep. 2016, 6, 30880. [Google Scholar] [CrossRef] [PubMed]
- Bonvicino, C.R.; Oliveira, J.A.; D’Andrea, P.S. Guia dos Roedores do Brasil, com Chaves para Gêneros Baseadas em Caracteres Externos, 1st ed.; Centro Pan-Americano de Febre Aftosa-Pan-American Health Organization//World Health Organization: Rio de Janeiro, Brazil, 2008; p. 120. [Google Scholar]
- Ridgely, R.S.; Tudor, G. The Birds of South America; University of Texas Press: Austin, TX, USA, 1994; p. 940. [Google Scholar]
- Sikes, R.S.; Gannon, W.L. Guidelines of the American society of mammalogists for the use of wild mammals in research. J. Mammal. 2011, 92. [Google Scholar] [CrossRef]
- De Souza, W.M.; Fumagalli, M.J.; de Araujo, J.; Sabino-Santos, G., Jr.; Maia, F.G.M.; Romeiro, M.F.; Modha, S.; Nardi, M.S.; Queiroz, L.H.; Durigon, E.L.; et al. Discovery of novel anelloviruses in small mammals expands the host range and diversity of the anelloviridae. Virology 2018, 514, 9–17. [Google Scholar] [CrossRef] [PubMed]
- O’Leary, N.A.; Wright, M.W.; Brister, J.R.; Ciufo, S.; Haddad, D.; McVeigh, R.; Rajput, B.; Robbertse, B.; Smith-White, B.; Ako-Adjei, D.; et al. Reference sequence (RefSeq) database at NCBI: Current status, taxonomic expansion and functional annotation. Nucleic Acids Res. 2016, 44, D733–D745. [Google Scholar] [CrossRef] [PubMed]
- Buchfink, B.; Xie, C.; Huson, D.H. Fast and sensitive protein alignment using diamond. Nat. Methods 2015, 12, 59–60. [Google Scholar] [CrossRef] [PubMed]
- Marchler-Bauer, A.; Derbyshire, M.K.; Gonzales, N.R.; Lu, S.; Chitsaz, F.; Geer, L.Y.; Geer, R.C.; He, J.; Gwadz, M.; Hurwitz, D.I.; et al. Cdd: Ncbi’s conserved domain database. Nucleic Acids Res. 2015, 43, D222–D226. [Google Scholar] [CrossRef] [PubMed]
- Wernersson, R.; Pedersen, A.G. Revtrans: Multiple alignment of coding DNA from aligned amino acid sequences. Nucleic Acids Res. 2003, 31, 3537–3539. [Google Scholar] [CrossRef] [PubMed]
- Kalyaanamoorthy, S.; Minh, B.Q.; Wong, T.K.F.; von Haeseler, A.; Jermiin, L.S. Modelfinder: Fast model selection for accurate phylogenetic estimates. Nat. Methods 2017, 14, 587–589. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, L.T.; Schmidt, H.A.; von Haeseler, A.; Minh, B.Q. Iq-tree: A fast and effective stochastic algorithm for estimating maximum-likelihood phylogenies. Mol. Biol. Evol. 2015, 32, 268–274. [Google Scholar] [CrossRef] [PubMed]
- Muhire, B.M.; Varsani, A.; Martin, D.P. Sdt: A virus classification tool based on pairwise sequence alignment and identity calculation. PLoS ONE 2014, 9, e108277. [Google Scholar] [CrossRef] [PubMed]
- Kivovich, V.; Gilbert, L.; Vuento, M.; Naides, S.J. The putative metal coordination motif in the endonuclease domain of human parvovirus B19 NS1 is critical for NS1 induced s phase arrest and DNA damage. Int. J. Biol. Sci. 2012, 8, 79–92. [Google Scholar] [CrossRef] [PubMed]
- Sasaki, M.; Gonzalez, G.; Wada, Y.; Setiyono, A.; Handharyani, E.; Rahmadani, I.; Taha, S.; Adiani, S.; Latief, M.; Kholilullah, Z.A.; et al. Divergent bufavirus harboured in megabats represents a new lineage of parvoviruses. Sci. Rep. 2016, 6, 24257. [Google Scholar] [CrossRef] [PubMed]
- Walker, S.L.; Wonderling, R.S.; Owens, R.A. Mutational analysis of the adeno-associated virus type 2 Rep68 protein helicase motifs. J. Virol. 1997, 71, 6996–7004. [Google Scholar] [PubMed]
- Castellanos, M.; Perez, R.; Rodriguez-Huete, A.; Grueso, E.; Almendral, J.M.; Mateu, M.G. A slender tract of glycine residues is required for translocation of the VP2 protein N-terminal domain through the parvovirus MVM capsid channel to initiate infection. Biochem. J. 2013, 455, 87–94. [Google Scholar] [CrossRef] [PubMed]
- Ros, C.; Gerber, M.; Kempf, C. Conformational changes in the VP1-unique region of native human parvovirus B19 lead to exposure of internal sequences that play a role in virus neutralization and infectivity. J. Virol. 2006, 80, 12017–12024. [Google Scholar] [CrossRef] [PubMed]
- Huang, Q.; Deng, X.; Yan, Z.; Cheng, F.; Luo, Y.; Shen, W.; Lei-Butters, D.C.; Chen, A.Y.; Li, Y.; Tang, L.; et al. Establishment of a reverse genetics system for studying human bocavirus in human airway epithelia. PLoS Pathog. 2012, 8, e1002899. [Google Scholar] [CrossRef] [PubMed]
- Sun, Y.; Chen, A.Y.; Cheng, F.; Guan, W.; Johnson, F.B.; Qiu, J. Molecular characterization of infectious clones of the minute virus of canines reveals unique features of bocaviruses. J. Virol. 2009, 83, 3956–3967. [Google Scholar] [CrossRef] [PubMed]
- Zou, W.; Cheng, F.; Shen, W.; Engelhardt, J.F.; Yan, Z.; Qiu, J. Nonstructural protein NP1 of human bocavirus 1 plays a critical role in the expression of viral capsid proteins. J. Virol. 2016, 90, 4658–4669. [Google Scholar] [CrossRef] [PubMed]
- Zhang, R.; Fang, L.; Wu, W.; Zhao, F.; Song, T.; Xie, L.; Li, Y.; Chen, H.; Xiao, S. Porcine bocavirus NP1 protein suppresses type I IFN production by interfering with IRF3 DNA-binding activity. Virus Genes 2016, 52, 797–805. [Google Scholar] [CrossRef] [PubMed]
- Olival, K.J.; Hosseini, P.R.; Zambrana-Torrelio, C.; Ross, N.; Bogich, T.L.; Daszak, P. Host and viral traits predict zoonotic spillover from mammals. Nature 2017, 546, 646–650. [Google Scholar] [CrossRef] [PubMed]
- Kailasan, S.; Agbandje-McKenna, M.; Parrish, C.R. Parvovirus family conundrum: What makes a killer? Annu. Rev. Virol. 2015, 2, 425–450. [Google Scholar] [CrossRef] [PubMed]
- Souza, C.K.; Streck, A.F.; Goncalves, K.R.; Pinto, L.D.; Ravazzolo, A.P.; de Barcellos, D.E.; Canal, C.W. Phylogenetic characterization of the first ungulate tetraparvovirus 2 detected in pigs in brazil. Braz. J. Microbiol. 2016, 47, 513–517. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Lau, S.K.; Woo, P.C.; Tse, H.; Fu, C.T.; Au, W.K.; Chen, X.C.; Tsoi, H.W.; Tsang, T.H.; Chan, J.S.; Tsang, D.N.; et al. Identification of novel porcine and bovine parvoviruses closely related to human parvovirus 4. J. Gen. Virol. 2008, 89, 1840–1848. [Google Scholar] [CrossRef] [PubMed]
- Xu, F.; Pan, Y.; Wang, M.; Wu, X.; Tian, L.; Baloch, A.R.; Zeng, Q. First detection of ungulate tetraparvovirus 1 (bovine hokovirus 1) in domestic yaks in northwestern china. Arch. Virol. 2016, 161, 177–180. [Google Scholar] [CrossRef] [PubMed]
- Katzourakis, A.; Gifford, R.J. Endogenous viral elements in animal genomes. PLoS Genet. 2010, 6, e1001191. [Google Scholar] [CrossRef] [PubMed]
- Liu, H.; Fu, Y.; Xie, J.; Cheng, J.; Ghabrial, S.A.; Li, G.; Peng, Y.; Yi, X.; Jiang, D. Widespread endogenization of densoviruses and parvoviruses in animal and human genomes. J. Virol. 2011, 85, 9863–9876. [Google Scholar] [CrossRef] [PubMed]
- Lau, S.K.P.; Ahmed, S.S.; Tsoi, H.W.; Yeung, H.C.; Li, K.S.M.; Fan, R.Y.Y.; Zhao, P.S.H.; Lau, C.C.C.; Lam, C.S.F.; Choi, K.K.F.; et al. Bats host diverse parvoviruses as possible origin of mammalian dependoparvoviruses and source for bat-swine interspecies transmission. J. Gen. Virol. 2017, 98, 3046–3059. [Google Scholar] [CrossRef] [PubMed]
- Hu, D.; Zhu, C.; Wang, Y.; Ai, L.; Yang, L.; Ye, F.; Ding, C.; Chen, J.; He, B.; Zhu, J.; et al. Virome analysis for identification of novel mammalian viruses in bats from southeast china. Sci. Rep. 2017, 7, 10917. [Google Scholar] [CrossRef] [PubMed]
- Marusak, R.A.; Guy, J.S.; Abdul-Aziz, T.A.; West, M.A.; Fletcher, O.J.; Day, J.M.; Zsak, L.; Barnes, H.J. Parvovirus-associated cerebellar hypoplasia and hydrocephalus in day old broiler chickens. Avian Dis. 2010, 54, 156–160. [Google Scholar] [CrossRef] [PubMed]
- Day, J.M.; Zsak, L. Determination and analysis of the full-length chicken parvovirus genome. Virology 2010, 399, 59–64. [Google Scholar] [CrossRef] [PubMed]
- Allander, T.; Emerson, S.U.; Engle, R.E.; Purcell, R.H.; Bukh, J. A virus discovery method incorporating DNase treatment and its application to the identification of two bovine parvovirus species. Proc. Natl. Acad. Sci. USA 2001, 98, 11609–11614. [Google Scholar] [CrossRef] [PubMed]
- Janus, L.M.; Mahler, M.; Kohl, W.; Smoczek, A.; Hedrich, H.J.; Bleich, A. Minute virus of mice: Antibody response, viral shedding and persistence of viral DNA in multiple strains of mice. Comp. Med. 2008, 58, 360–368. [Google Scholar] [PubMed]
- Proenca-Modena, J.L.; Gagliardi, T.B.; Paula, F.E.; Iwamoto, M.A.; Criado, M.F.; Camara, A.A.; Acrani, G.O.; Cintra, O.A.; Cervi, M.C.; Arruda, L.K.; et al. Detection of human bocavirus mrna in respiratory secretions correlates with high viral load and concurrent diarrhea. PLoS ONE 2011, 6, e21083. [Google Scholar] [CrossRef] [PubMed]
- Lau, S.K.; Yeung, H.C.; Li, K.S.; Lam, C.S.; Cai, J.P.; Yuen, M.C.; Wang, M.; Zheng, B.J.; Woo, P.C.; Yuen, K.Y. Identification and genomic characterization of a novel rat bocavirus from brown rats in china. Infect. Genet. Evol. 2017, 47, 68–76. [Google Scholar] [CrossRef] [PubMed]
- Allison, A.B.; Kohler, D.J.; Fox, K.A.; Brown, J.D.; Gerhold, R.W.; Shearn-Bochsler, V.I.; Dubovi, E.J.; Parrish, C.R.; Holmes, E.C. Frequent cross-species transmission of parvoviruses among diverse carnivore hosts. J. Virol. 2013, 87, 2342–2347. [Google Scholar] [CrossRef] [PubMed]
- Parrish, C.R.; Holmes, E.C.; Morens, D.M.; Park, E.C.; Burke, D.S.; Calisher, C.H.; Laughlin, C.A.; Saif, L.J.; Daszak, P. Cross-species virus transmission and the emergence of new epidemic diseases. Microbiol. Mol. Biol. Rev. 2008, 72, 457–470. [Google Scholar] [CrossRef] [PubMed]
- Canuti, M.; Whitney, H.G.; Lang, A.S. Amdoparvoviruses in small mammals: Expanding our understanding of parvovirus diversity, distribution and pathology. Front. Microbiol. 2015, 6, 1119. [Google Scholar] [CrossRef] [PubMed]
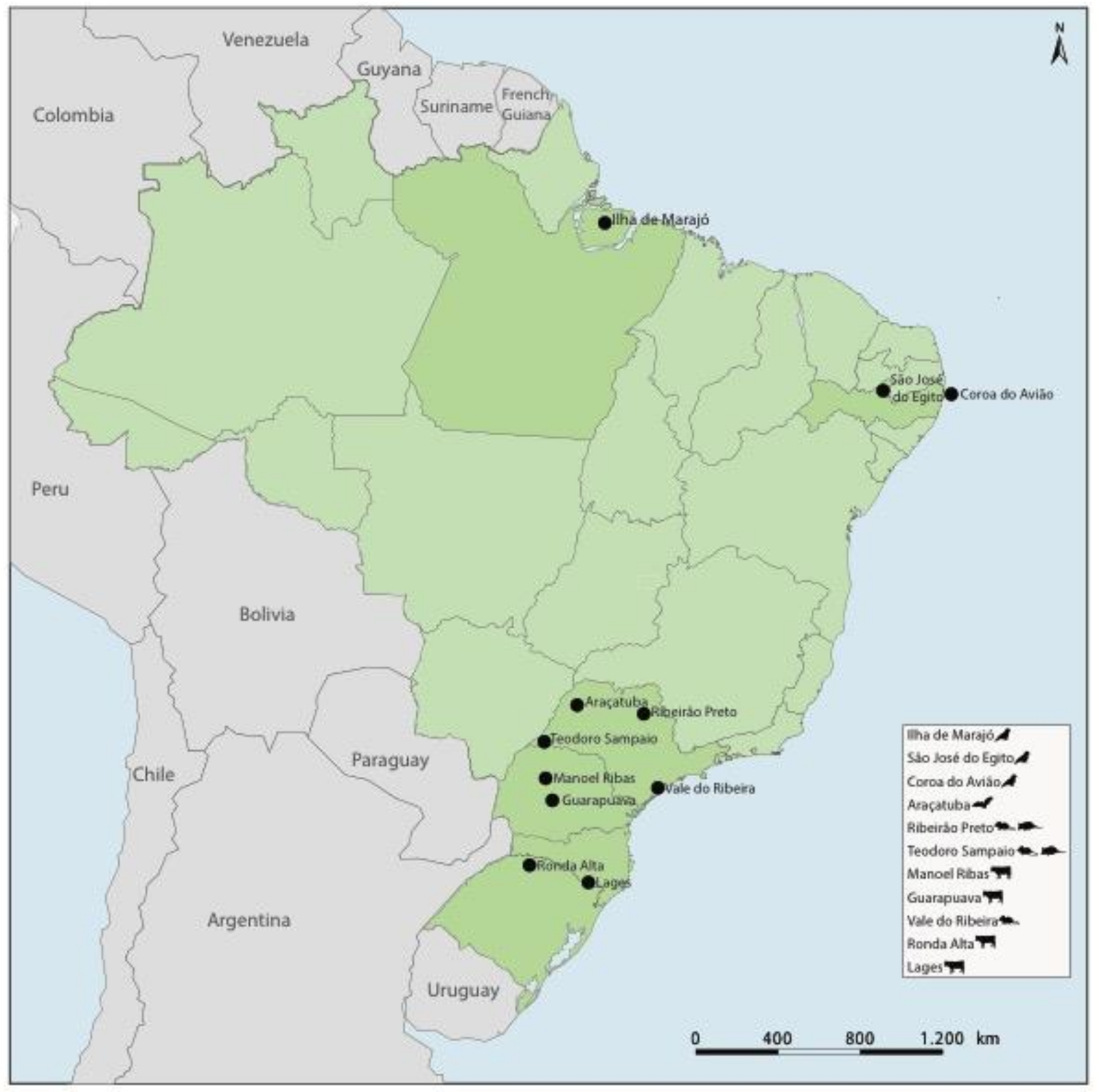
| Genus | Viral Species | Strain | Genome | Size (nt) | Host Species | Sample | Samples Per Pool | Location | Date | GenBank |
|---|---|---|---|---|---|---|---|---|---|---|
| Tetraparvovirus | Rodent tetraparvovirus | 1135 | Nearly complete | 5494 | Necromys lasiurus | Blood | 59 | Ribeirão Preto, SP | 2008 | MG745669 |
| Tetraparvovirus | Rodent tetraparvovirus | 3542 | Nearly complete | 5494 | Necromys lasiurus | Blood | 52 | Ribeirão Preto, SP | 2009 | MG745670 |
| Tetraparvovirus | Didelphimorphs tetraparvovirus | 4113 | Nearly complete | 5420 | Didelphis albiventris | Serum | 14 | Teodoro Sampaio, SP | 2009 | MG745671 |
| Aveparvovirus | Passeriform aveparvovirus | 29 | Nearly complete | 5368 | Coryphospingus pileatus | Cloacal Swab | 4 | São José do Egito, PE | 2010 | MG745672 |
| Bocaparvovirus | Rodent bocaparvovirus | 1 | Nearly complete | 5227 | Necromys lasiurus | Blood | 58 | Ribeirão Preto, SP | 2008 | MG745673 |
| Protoparvovirus | Rodent protoparvovirus | 9424 | Nearly complete | 5219 | Necromys lasiurus | Blood | 58 | Ribeirão Preto, SP | 2008 | MG745674 |
| Protoparvovirus | Rodent protoparvovirus | 284 | Nearly complete | 5196 | Akodon montensis | Blood | 41 | Ribeirão Preto, SP | 2009 | MG745675 |
| Protoparvovirus | Rodent protoparvovirus | 119 | Nearly complete | 4998 | Calomys tener | Blood | 38 | Ribeirão Preto, SP | 2008 | MG745676 |
| Dependoparvovirus | Chiropteran dependoparvovirus 2 | 246 | Nearly complete | 4894 | Desmodus rotundus | Kidney | 8 | Araçatuba, SP | 2010 | MG745677 |
| Protoparvovirus | Rodent protoparvovirus | 2 | Nearly complete | 4898 | Necromys lasiurus | Blood | 59 | Ribeirão Preto, SP | 2008 | MG745678 |
| Tetraparvovirus | Ungulate tetraparvovirus | MR | Nearly complete | 5368 | Bos taurus | Blood | 15 | Manoel Ribas, PR | 2016 | MG745679 |
| Erythroparvovirus | Ungulate erythroparvovirus 1 | Ronda Alta | Nearly complete | 5220 | Bos taurus | Blood | 6 | Ronda Alta, RS | 2016 | MG745680 |
| Protoparvovirus | Rodent protoparvovirus | 1594 | Partial | 2255 | Didelphis albiventris | Blood | 32 | Ribeirão Preto, SP | 2012–2013 | MG745681 |
| Bocaparvovirus | Rodent bocaparvovirus | 4093 | Partial | 2844 | Necromys lasiurus | Blood | 52 | Ribeirão Preto, SP | 2009 | MG745682 |
| Protoparvovirus | Rodent protoparvovirus | 8 | Partial | 1679 | Calomys tener | Blood | 34 | Ribeirão Preto, SP | 2009, 2012–2013 | MG745683 |
| Protoparvovirus | Rodent protoparvovirus | 888 | Partial | 1606 | Oligoryzomys nigripes | Blood | 20 | Ribeirão Preto, SP | 2012–2013 | MG745684 |
| Protoparvovirus | Rodent protoparvovirus | 23 | Partial | 1566 | Akodon montensis | Blood | 55 | Ribeirão Preto, SP | 2008 | MG745685 |
| Bocaparvovirus | Rodent bocaparvovirus | 422 | Partial | 1362 | Necromys lasiurus | Blood | 52 | Ribeirão Preto, SP | 2009 | MG745686 |
| Protoparvovirus | Rodent protoparvovirus | 1010 | Partial | 1283 | Oligoryzomys nigripes | Blood | 20 | Ribeirão Preto, SP | 2012–2013 | MG745687 |
| Protoparvovirus | Rodent protoparvovirus | 66 | Partial | 1099 | Akodon montensis | Blood | 55 | Ribeirão Preto, SP | 2008 | MG745688 |
| Protoparvovirus | Rodent protoparvovirus | 38 | Partial | 1067 | Calomys tener | Blood | 34 | Ribeirão Preto, SP | 2009,2012-2013 | MG745689 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
De Souza, W.M.; Dennis, T.; Fumagalli, M.J.; Araujo, J.; Sabino-Santos, G., Jr; Maia, F.G.M.; Acrani, G.O.; Carrasco, A.D.O.T.; Romeiro, M.F.; Modha, S.; et al. Novel Parvoviruses from Wild and Domestic Animals in Brazil Provide New Insights into Parvovirus Distribution and Diversity. Viruses 2018, 10, 143. https://doi.org/10.3390/v10040143
De Souza WM, Dennis T, Fumagalli MJ, Araujo J, Sabino-Santos G Jr, Maia FGM, Acrani GO, Carrasco ADOT, Romeiro MF, Modha S, et al. Novel Parvoviruses from Wild and Domestic Animals in Brazil Provide New Insights into Parvovirus Distribution and Diversity. Viruses. 2018; 10(4):143. https://doi.org/10.3390/v10040143
Chicago/Turabian StyleDe Souza, William Marciel, Tristan Dennis, Marcílio Jorge Fumagalli, Jansen Araujo, Gilberto Sabino-Santos, Jr, Felipe Gonçalves Motta Maia, Gustavo Olszanski Acrani, Adriano De Oliveira Torres Carrasco, Marilia Farignoli Romeiro, Sejal Modha, and et al. 2018. "Novel Parvoviruses from Wild and Domestic Animals in Brazil Provide New Insights into Parvovirus Distribution and Diversity" Viruses 10, no. 4: 143. https://doi.org/10.3390/v10040143
APA StyleDe Souza, W. M., Dennis, T., Fumagalli, M. J., Araujo, J., Sabino-Santos, G., Jr, Maia, F. G. M., Acrani, G. O., Carrasco, A. D. O. T., Romeiro, M. F., Modha, S., Vieira, L. C., Ometto, T., Queiroz, L. H., Durigon, E. L., Nunes, M. R. T., Figueiredo, L. T. M., & Gifford, R. J. (2018). Novel Parvoviruses from Wild and Domestic Animals in Brazil Provide New Insights into Parvovirus Distribution and Diversity. Viruses, 10(4), 143. https://doi.org/10.3390/v10040143