Chemical Composition and Biological Activities of Torreya grandis Kernels: Characteristics of Polymethylene-Interrupted Fatty Acids and Polyphenolic Compounds and Their Potential Health Effects
Abstract
1. Introduction
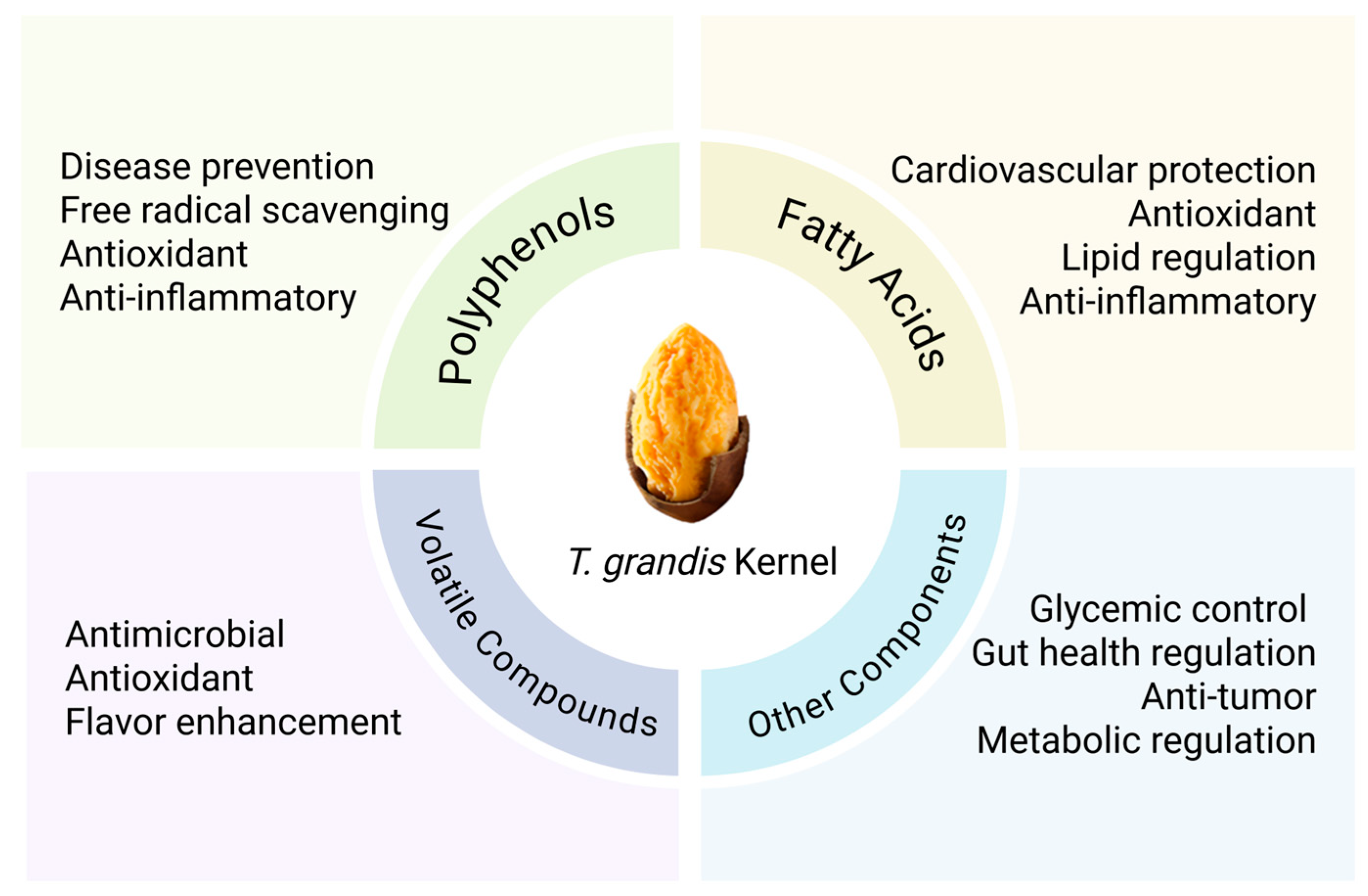
2. Chemical Constituents of T. grandis Kernels
2.1. Fatty Acids
2.2. Polyphenols
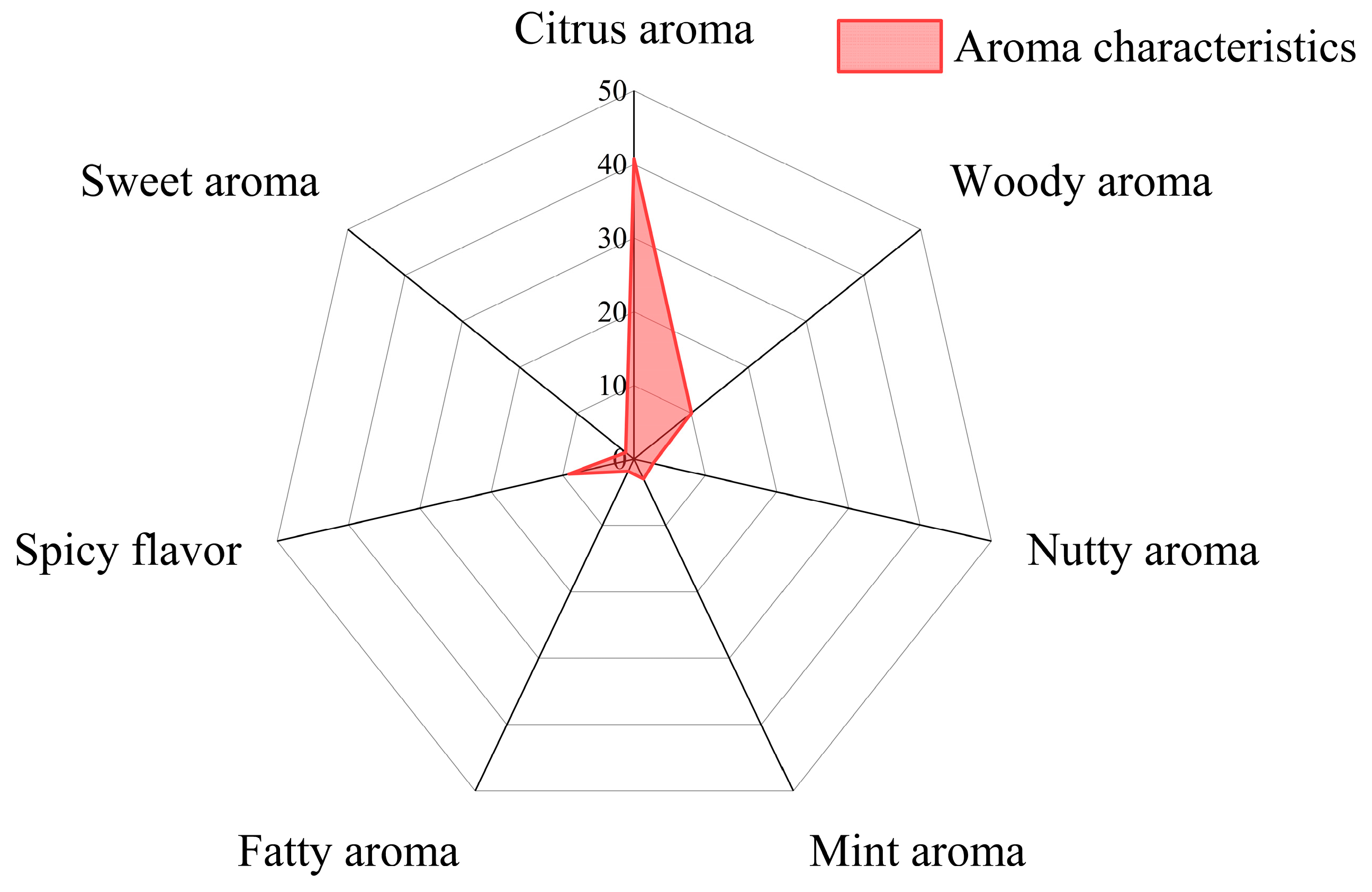
2.3. Volatile Compounds
2.4. Other Constituents
3. Biological Activities of T. grandis Kernels
3.1. Antioxidant Activities
3.2. Anti-Inflammatory Effects
3.3. Cardiovascular and Cerebrovascular Protective Effects
- (1)
- Lack of long-term safety and efficacy evaluation: Current animal studies (such as Xiao et al. [90]) have relatively short durations. While they can reveal short-term effects and partial mechanisms, they are insufficient to simulate scenarios of long-term human consumption or chronic cardiovascular disease prevention/treatment. To date, there are no publicly reported long-term (e.g., 6 months or longer) animal functional studies or chronic toxicity tests following standard guidelines (such as OECD and ICH guidelines) for T. grandis kernels or their extracts. Such long-term studies are crucial for comprehensively evaluating sustained efficacy, identifying potential delayed toxicity or cumulative effects, and determining long-term safe dosage ranges [109]. Given that Xiao et al. [90] mentioned (as described in the anti-inflammatory section) that long-term consumption of T. grandis oil under certain conditions may be associated with liver and intestinal adverse effects, rigorous long-term safety and chronic toxicity assessments are particularly necessary.
- (2)
- Unclear in vivo processes of active components: Pharmacokinetic data (absorption, distribution, metabolism, excretion) and the bioavailability of key active components (such as specific fatty acids, phytosterols, and their metabolites) remain very limited.
- (3)
- Incomplete risk–benefit assessment: There is a lack of comprehensive risk–benefit analysis based on different doses, populations, and long-term applications.
- (4)
- Absence of human evidence: Most critically, there is a complete absence of well-designed randomized controlled trials in humans to directly verify the cardiovascular and cerebrovascular protective effects and safety of T. grandis kernels or their products.
3.4. Other Biological Activities
4. Factors Influencing Metabolite Composition and Variability in T. grandis Kernels
5. Conclusions and Future Perspectives
Author Contributions
Funding
Conflicts of Interest
References
- Zhu, R.; Gao, N.; Luo, J.; Shi, W. Genome and Transcriptome Analysis of the Torreya grandis WRKY Gene Family During Seed Development. Genes 2024, 15, 267. [Google Scholar] [CrossRef]
- Yan, J.; Zeng, H.; Chen, W.; Zheng, S.; Luo, J.; Jiang, H.; Yang, B.; Farag, M.A.; Lou, H.; Song, L.; et al. Effects of Tree Age on Flavonoids and Antioxiadant Activity in Torreya grandis Nuts Via Integrated Metabolome and Transcriptome Analyses. Food Front. 2023, 4, 358–367. [Google Scholar] [CrossRef]
- Suo, J.; Zhou, Z.; Farag, M.A.; Zhang, Z.; Wu, J.; Hu, Y.; Song, L. Ethylene Mitigates Nut Decay and Improves Nut Quality of Torreya grandis During Postharvest by Changing Microbial Community Composition. Postharvest Biol. Technol. 2025, 219, 113250. [Google Scholar] [CrossRef]
- Quan, W.; Zhang, C.; Wang, Z.; Zeng, M.; Qin, F.; He, Z.; Chen, J. Assessment Antioxidant Properties of Torreya grandis Protein Enzymatic Hydrolysates: Utilization of Industrial by-Products. Food Biosci. 2021, 43, 101325. [Google Scholar] [CrossRef]
- Zongo, A.W.S.; Jin, C.Y.; Hao, G.J.; Yu, N.X.; Zogona, D.; Nie, X.H.; Lu, Y.C.; Ye, Q.; Meng, X.H. Functional Compounds of Torreya grandis Nuts and Their Processing Byproducts: Extraction Process, Health Benefits, and Food Applications—A Comprehensive Review. Food Res. Int. 2024, 197, 115235. [Google Scholar] [CrossRef]
- Shi, L.K.; Zheng, L.; Mao, J.H.; Zhao, C.W.; Huang, J.H.; Liu, R.J.; Chang, M.; Jin, Q.Z.; Wang, X.G. Effects of the Variety and Oil Extraction Method on the Quality, Fatty Acid Composition and Antioxidant Capacity of Torreya grandis Kernel Oils. LWT 2018, 91, 398–405. [Google Scholar] [CrossRef]
- Yu, M.; Zeng, M.; Qin, F.; He, Z.; Chen, J. Physicochemical and Functional Properties of Protein Extracts from Torreya grandis Seeds. Food Chem. 2017, 227, 453–460. [Google Scholar] [CrossRef]
- Chen, W.; Jiang, B.; Zeng, H.; Liu, Z.; Chen, W.; Zheng, S.; Wu, J.; Lou, H. Molecular Regulatory Mechanisms of Staminate Strobilus Development and Dehiscence in Torreya grandis. Plant Physiol. 2024, 195, 534–551. [Google Scholar] [CrossRef]
- Yao, J.; Bai, E.; Duan, Y.; Huang, Y. Ethanol Extracts from Torreya grandis Seed Have Potential to Reduce Hyperuricemia in Mouse Models by Influencing Purine Metabolism. Foods 2024, 13, 840. [Google Scholar] [CrossRef]
- Wang, Y.; Yang, L.; Yao, X.; Wang, K.; Cao, Y.; Zhang, C.; Chang, J.; Ren, H. Oxidation Stability of Seed Oils from Four Woody Oil Plant Species. Cyta J. Food 2024, 22, 2285839. [Google Scholar] [CrossRef]
- Chen, H.; Yue, X.; Yang, J.; Lv, C.; Dong, S.; Luo, X.; Sun, Z.; Zhang, Y.; Li, B.; Zhang, F.; et al. Pyrolysis Molecule of Torreya grandis Bark for Potential Biomedicine. Saudi J. Biol. Sci. 2019, 26, 808–815. [Google Scholar] [CrossRef]
- Zhang, Z.; Chen, W.; Tao, L.; Wei, X.; Gao, L.; Gao, Y.; Suo, J.; Yu, W.; Hu, Y.; Yang, B.; et al. Ethylene Treatment Promotes Umami Taste-Active Amino Acids Accumulation of Torreya grandis Nuts Post-Harvest by Comparative Chemical and Transcript Analyses. Food Chem. 2023, 408, 135214. [Google Scholar] [CrossRef]
- Hu, Y.; Suo, J.; Jiang, G.; Shen, J.; Cheng, H.; Lou, H.; Yu, W.; Wu, J.; Song, L. The Effect of Ethylene on Squalene and Beta-Sitosterol Biosynthesis and Its Key Gene Network Analysis in Torreya grandis Nuts During Post-Ripening Process. Food Chem. 2022, 368, 130819. [Google Scholar] [CrossRef]
- Miao, Z.-P.; Niu, X.-N.; Wang, R.-B.; Huang, L.; Ma, B.-B.; Li, J.-H.; Hong, X. Study of the Genus Torreya (Taxaceae) Based on Chloroplast Genomes. Front. Biosci. 2022, 27, 9. [Google Scholar] [CrossRef]
- Zhang, F.; Kong, C.; Ma, Z.; Chen, W.; Li, Y.; Lou, H.; Wu, J. Molecular Characterization and Transcriptional Regulation Analysis of the Torreya grandis Squalene Synthase Gene Involved in Sitosterol Biosynthesis and Drought Response. Front. Plant Sci. 2023, 14, 1136643. [Google Scholar] [CrossRef]
- Wang, X.; Guo, R.; Yu, Z.; Zikela, L.; Li, J.; Li, S.; Han, Q. Torreya grandis Kernel Oil Alleviates Loperamide-Induced Slow Transit Constipation Via up-Regulating the Colonic Expressions of Occludin/Claudin-1/Zo-1 and 5-Ht3r/5-Ht4r in Balb/C Mice. Mol. Nutr. Food Res. 2024, 68, e2300615. [Google Scholar] [CrossRef]
- Jiang, M.; Wang, J.; Zhang, H. Characterization of the Complete Plastome Sequence of Torreya grandis var. jiulongshanensis (Taxaceae), a Rare and Endangered Plant Species Endemic to Zhejiang Province, China. Mitochondrial DNA Part B 2020, 5, 834–836. [Google Scholar] [CrossRef]
- Gao, Y.; Wang, C.; Wu, T.; Ma, Z.; Chen, W.; Chang, H.; Jing, Y.; Tao, H.; Yu, W.; Jiang, H.; et al. Multiplex Approach of Metabolite and Transcript Profiling Identify a Biosynthetic Mechanism for Kayaflavone Biosynthesis in Torreya grandis. Ind. Crops Prod. 2024, 214, 118482. [Google Scholar] [CrossRef]
- Suo, J.; Liu, Y.; Yan, J.; Li, Q.; Chen, W.; Liu, Z.; Zhang, Z.; Hu, Y.; Yu, W.; Yan, J.; et al. Sucrose Promotes Cone Enlargement Via the TgNGA1-TgWRKY47-TgEXPA2 Module in Torreya grandis. New Phytol. 2024, 243, 1823–1839. [Google Scholar] [CrossRef]
- Suo, J.; Zhong, J.; Yang, M.; Li, Q.; Hu, Y.; Yu, W.; Yan, J.; Wu, J. The Role and Mechanism of TgCWIN2-Mediated Changes of Photo-Assimilates in Modulating Early Development of Torreya grandis Seeds. Plant Physiol. Biochem. 2024, 216, 109188. [Google Scholar] [CrossRef]
- Durrani, R.; Meiyun, Y.; Yang, B.; Durand, E.; Delavault, A.; Bowen, H.; Weiwei, H.; Yiyang, L.; Lili, S.; Fei, G. Identification of Novel Bioactive Proteins and Their Produced Oligopeptides from Torreya grandis Nuts Using Proteomic Based Prediction. Food Chem. 2023, 405, 134843. [Google Scholar] [CrossRef]
- Chen, W.; Yan, J.; Guan, Y.; Lou, H.; Wu, J. Genome-Wide Identification of WOX Gene Family and Its Expression Pattern in Rapid Expansion of Torreya grandis Ovulate and Staminate Strobili. Sci. Hortic. 2024, 330, 113050. [Google Scholar] [CrossRef]
- Shen, H.; Hou, Y.; Wang, X.; Li, Y.; Wu, J.; Lou, H. Genome-Wide Identification, Expression Analysis under Abiotic Stress and Co-Expression Analysis of MATE Gene Family in Torreya grandis. Int. J. Mol. Sci. 2024, 25, 3859. [Google Scholar] [CrossRef]
- Lou, H.; Zheng, S.; Chen, W.; Yu, W.; Jiang, H.; Farag, M.A.; Xiao, J.; Wu, J.; Song, L. Transcriptome-Referenced Association Study Provides Insights into the Regulation of Oil and Fatty Acid Biosynthesis in Torreya grandis Kernel. J. Adv. Res. 2024, 62, 1–14. [Google Scholar] [CrossRef]
- Miu, Z.-P.; Zhang, J.-M.; Li, J.-H.; Hong, X.; Pan, T. The Complete Chloroplast Genome Sequence of an Conifer Plant Torreya grandis (Pinales, Taxaceae). Mitochondrial DNA Part B 2018, 3, 1152–1153. [Google Scholar] [CrossRef]
- Hu, Y.; Zhang, Z.; Hua, B.; Tao, L.; Chen, W.; Gao, Y.; Suo, J.; Yu, W.; Wu, J.; Song, L. The Interaction of Temperature and Relative Humidity Affects the Main Aromatic Components in Postharvest Torreya grandis Nuts. Food Chem. 2022, 368, 130836. [Google Scholar] [CrossRef]
- Suo, J.; Tong, K.; Wu, J.; Ding, M.; Chen, W.; Yang, Y.; Lou, H.; Hu, Y.; Yu, W.; Song, L. Comparative Transcriptome Analysis Reveals Key Genes in the Regulation of Squalene and Beta-Sitosterol Biosynthesis in Torreya grandis. Ind. Crops Prod. 2019, 131, 182–193. [Google Scholar] [CrossRef]
- Ni, L.; Shi, W. Composition and Free Radical Scavenging Activity of Kernel Oil from Torreya grandis, Carya cathayensis and Myrica rubra. Iran. J. Pharm. Res. 2014, 13, 221–226. [Google Scholar]
- Rauf, S.; Jamil, N.; Tariq, S.A.; Khan, M.; Kausar, M.; Kaya, Y. Progress in Modification of Sunflower Oil to Expand Its Industrial Value. J. Sci. Food Agric. 2017, 97, 1997–2006. [Google Scholar] [CrossRef]
- Jiang, W.; Liu, K.; Huan, W.; Wu, X.; Zhu, M.; Tao, H.; Song, L.; Gao, F. Specific Extraction of Bioactive Flavonoids from Torreya grandis Pomace Using Magnetic Nanoparticles Modified with a ChCl/Acetamide Deep Eutectic Solvent. LWT 2024, 211, 116914. [Google Scholar] [CrossRef]
- Zhang, C.; Liu, H.K.; Zhang, H.; Dang, W.Y.; Zhou, C.H.; Zhang, M. Comparative De Novo Transcriptome Analysis of Two Cultivars with Contrasting Content of Oil and Fatty Acids During Kernel Development in Torreya grandis. Front. Plant Sci. 2022, 13, 909759. [Google Scholar] [CrossRef]
- Ding, M.Z.; Lou, H.Q.; Chen, W.C.; Zhou, Y.; Zhang, Z.H.; Xiao, M.H.; Wang, Z.Q.; Yang, Y.; Yang, L.; Zhang, F.C.; et al. Comparative Transcriptome Analysis of the Genes Involved in Lipid Biosynthesis Pathway and Regulation of Oil Body Formation in Torreya grandis Kernels. Ind. Crops Prod. 2020, 145, 112051. [Google Scholar] [CrossRef]
- Bell, A.E.; Culp, P.A. Reduction in Saturated Fat Intake for Cardiovascular Disease. Am. Fam. Physician 2022, 105, 35029948. [Google Scholar]
- He, M.; Qin, C.X.; Wang, X.; Ding, N.Z. Plant Unsaturated Fatty Acids: Biosynthesis and Regulation. Front. Plant Sci. 2020, 11, 390. [Google Scholar] [CrossRef]
- Beld, J.; Lee, D.J.; Burkart, M.D. Fatty Acid Biosynthesis Revisited: Structure Elucidation and Metabolic Engineering. Mol. Biosyst. 2015, 11, 38–59. [Google Scholar] [CrossRef]
- Czumaj, A.; Sledzinski, T. Biological Role of Unsaturated Fatty Acid Desaturases in Health and Disease. Nutrients 2020, 12, 356. [Google Scholar] [CrossRef]
- Lee, J.M.; Lee, H.; Kang, S.; Park, W.J. Fatty Acid Desaturases, Polyunsaturated Fatty Acid Regulation, and Biotechnological Advances. Nutrients 2016, 8, 23. [Google Scholar] [CrossRef]
- Sayanova, O.; Haslam, R.; Caleron, M.V.; Napier, J.A. Cloning and Characterization of Unusual Fatty Acid Desaturases from Anemone leveillei: Identification of an Acyl-Coenzyme A C20 Δ5-Desaturase Responsible for the Synthesis of Sciadonic Acid. Plant Physiol. 2007, 144, 455–467. [Google Scholar] [CrossRef]
- Meesapyodsuk, D.; Qiu, X. The Front-End Desaturase: Structure, Function, Evolution and Biotechnological Use. Lipids 2012, 47, 227–237. [Google Scholar] [CrossRef]
- Nakamura, M.T.; Nara, T.Y. Structure, Function, and Dietary Regulation of Δ6, Δ5, and Δ9 Desaturases. Annu. Rev. Nutr. 2004, 24, 345–376. [Google Scholar] [CrossRef]
- Song, L.L.; Wen, S.S.; Ye, Q.; Lou, H.Q.; Gao, Y.D.; Bajpai, V.K.; Carpena, M.; Prieto, M.A.; Simal-Gandara, J.; Xiao, J.B.; et al. Advances on Delta 5-Unsaturated-Polymethylene-Interrupted Fatty Acids: Resources, Biosynthesis, and Benefits. Crit. Rev. Food Sci. Nutr. 2021, 63, 767–789. [Google Scholar] [CrossRef]
- Wolff, R.L.; Pédrono, F.; Pasquier, E.; Marpeau, A.M. General Characteristics of Pinus spp. Seed Fatty Acid Compositions, and Importance of Δ5-Olefinic Acids in the Taxonomy and Phylogeny of the Genus. Lipids 2000, 35, 1–22. [Google Scholar] [CrossRef] [PubMed]
- Lou, H.Q.; Song, L.L.; Li, X.L.; Zi, H.L.; Chen, W.J.; Gao, Y.D.; Zheng, S.; Fei, Z.J.; Sun, X.P.; Wu, J.S. The Torreya grandis Genome Illuminates the Origin and Evolution of Gymnosperm-Specific Sciadonic Acid Biosynthesis. Nat. Commun. 2023, 14, 1315. [Google Scholar] [CrossRef] [PubMed]
- Zhou, L.X.; Wu, Q.F.; Yang, Y.D.; Li, Q.H.; Li, R.; Ye, J.Q. Regulation of Oil Biosynthesis and Genetic Improvement in Plants: Advances and Prospects. Genes 2024, 15, 1125. [Google Scholar] [CrossRef]
- Huang, A.H.C. Plant Lipid Droplets and Their Associated Proteins: Potential for Rapid Advances. Plant Physiol. 2018, 176, 1894–1918. [Google Scholar] [CrossRef]
- Christie, W.W. Gas Chromatography Mass Spectrometry Methods for Structural Analysis of Fatty Acids. Lipids 1998, 33, 343–353. [Google Scholar] [CrossRef]
- Harayama, T.; Riezman, H. Understanding the Diversity of Membrane Lipid Composition. Nat. Rev. Mol. Cell Biol. 2018, 19, 281–296. [Google Scholar] [CrossRef]
- Gabbs, M.; Leng, S.; Devassy, J.G.; Monirujjaman, M.; Aukema, H.M. Advances in Our Understanding of Oxylipins Derived from Dietary PUFAs. Adv. Nutr. 2015, 6, 513–540. [Google Scholar] [CrossRef]
- Ahmadian, M.; Suh, J.M.; Hah, N.; Liddle, C.; Atkins, A.R.; Downes, M.; Evans, R.M. PPARγ Signaling and Metabolism: The Good, the Bad and the Future. Nat. Med. 2013, 19, 557–566. [Google Scholar] [CrossRef]
- Issemann, I.; Green, S. Activation of a Member of the Steroid Hormone Receptor Superfamily by Peroxisome Proliferators. Nature 1990, 347, 645–650. [Google Scholar] [CrossRef]
- Varga, T.; Czimmerer, Z.; Nagy, L. PPARs Are a Unique Set of Fatty Acid Regulated Transcription Factors Controlling Both Lipid Metabolism and Inflammation. Biochim. Biophys. Acta Mol. Basis Dis. 2011, 1812, 1007–1022. [Google Scholar] [CrossRef]
- Wakutsu, M.; Tsunoda, N.; Muraki, E.; Kasono, K. Participation of Peroxisome Proliferator-Activated Receptors (PPARs) in Glucose and Lipid Metabolism under Fish Oil Diet. Chem. Phys. Lipids 2009, 160, S46–S47. [Google Scholar] [CrossRef]
- Baldelli, S.; Aiello, G.; Mansilla Di Martino, E.; Campaci, D.; Muthanna, F.M.S.; Lombardo, M. The Role of Adipose Tissue and Nutrition in the Regulation of Adiponectin. Nutrients 2024, 16, 2436. [Google Scholar] [CrossRef]
- Fang, H.; Judd, R.L. Adiponectin Regulation and Function. Compr. Physiol. 2018, 8, 1031–1063. [Google Scholar] [CrossRef]
- Chemat, F.; Rombaut, N.; Sicaire, A.G.; Meullemiestre, A.; Fabiano-Tixier, A.S.; Abert-Vian, M. Ultrasound Assisted Extraction of Food and Natural Products. Mechanisms, Techniques, Combinations, Protocols and Applications. A Review. Ultrason. Sonochem. 2017, 34, 540–560. [Google Scholar] [CrossRef]
- Dong, D.D.; Wang, H.F.; Xu, F.; Xu, C.; Shao, X.F.; Li, H.S. Supercritical Carbon Dioxide Extraction, Fatty Acid Composition, Oxidative Stability, and Antioxidant Effect of Torreya grandis Seed Oil. J. Am. Oil Chem. Soc. 2014, 91, 817–825. [Google Scholar] [CrossRef]
- Fantino, V.M.; Bodoira, R.M.; Penci, M.C.; Ribotta, P.D.; Martínez, M.L. Effect of Screw-Press Extraction Process Parameters on the Recovery and Quality of Pistachio Oil. Grasas Aceites 2020, 71, e360. [Google Scholar] [CrossRef]
- Danlami, J.M.; Arsad, A.; Ahmad Zaini, M.A.; Sulaiman, H. A Comparative Study of Various Oil Extraction Techniques from Plants. Rev. Chem. Eng. 2014, 30, 605–626. [Google Scholar] [CrossRef]
- Shanmugam, V. Green Extraction of Natural Products: Theory and Practice. Green. Process Synth. 2015, 4, 453–454. [Google Scholar] [CrossRef]
- Jiang, L.H.; Hua, D.; Wang, Z.; Xu, S.Y. Aqueous Enzymatic Extraction of Peanut Oil and Protein Hydrolysates. Food Bioprod. Process 2010, 88, 233–238. [Google Scholar] [CrossRef]
- Rosenthal, A.; Pyle, D.L.; Niranjan, K. Aqueous and Enzymatic Processes for Edible Oil Extraction. Enzyme Microb. Technol. 1996, 19, 402–420. [Google Scholar] [CrossRef]
- Hrncic, M.K.; Cör, D.; Verboten, M.T.; Knez, Z. Application of Supercritical and Subcritical Fluids in Food Processing. Food Qual. Saf. 2018, 2, 59–67. [Google Scholar] [CrossRef]
- Liu, H.M.; Wang, F.Y.; Li, H.Y.; Wang, X.D.; Qin, G.Y. Subcritical Butane and Propane Extraction of Oil from Rice Bran. BioResources 2015, 10, 4652–4662. [Google Scholar] [CrossRef]
- Nüchter, M.; Ondruschka, B.; Fischer, B.; Tied, A.; Lautenschläger, W. Microwave-Assisted Extraction of Natural Products. Chem. Ing. Tech. 2005, 77, 171–175. [Google Scholar] [CrossRef]
- Esclapez, M.D.; García-Pérez, J.V.; Mulet, A.; Cárcel, J.A. Ultrasound-Assisted Extraction of Natural Products. Food Eng. Rev. 2011, 3, 108–120. [Google Scholar] [CrossRef]
- He, Z.Y.; Zhu, H.D.; Li, W.L.; Zeng, M.M.; Wu, S.F.; Chen, S.W.; Qin, F.; Chen, J. Chemical Components of Cold Pressed Kernel Oils from Different Torreya grandis Cultivars. Food Chem. 2016, 209, 196–202. [Google Scholar] [CrossRef]
- Hussain, T.; Tan, B.; Yin, Y.; Blachier, F.; Tossou, M.C.B.; Rahu, N. Oxidative Stress and Inflammation: What Polyphenols Can Do for Us? Oxid. Med. Cell Longev. 2016, 2016, 7432797. [Google Scholar] [CrossRef]
- Yan, J.; Zeng, H.; Chen, W.; Luo, J.; Kong, C.; Lou, H.; Wu, J. New Insights into the Carotenoid Biosynthesis in Torreya grandis Kernels. Hortic. Plant J. 2023, 9, 1108–1118. [Google Scholar] [CrossRef]
- Zhu, M.-F.; Tu, Z.-C.; Zhang, L.; Liao, H. Antioxidant, Metabolic Enzymes Inhibitory Ability of Torreya grandis Kernels, and Phytochemical Profiling Identified by HPLC-QTOF-MS/MS. J. Food Biochem. 2019, 43, e13043. [Google Scholar] [CrossRef]
- Manach, C.; Scalbert, A.; Morand, C.; Rémésy, C.; Jiménez, L. Polyphenols: Food Sources and Bioavailability. Am. J. Clin. Nutr. 2004, 79, 727–747. [Google Scholar] [CrossRef]
- Aura, A.-M. Microbial Metabolism of Dietary Phenolic Compounds in the Colon. Phytochem. Rev. 2008, 7, 407–429. [Google Scholar] [CrossRef]
- Crozier, A.; Jaganath, I.B.; Clifford, M.N. Dietary Phenolics: Chemistry, Bioavailability and Effects on Health. Nat. Prod. Rep. 2009, 26, 1001–1043. [Google Scholar] [CrossRef] [PubMed]
- Kay, C.D. The Future of Flavonoid Research. Br. J. Nutr. 2010, 104, S91–S95. [Google Scholar] [CrossRef]
- Selma, M.V.; Espín, J.C.; Tomás-Barberán, F.A. Interaction between Phenolics and Gut Microbiota: Role in Human Health. J. Agric. Food Chem. 2009, 57, 6485–6501. [Google Scholar] [CrossRef]
- Cardona, F.; Andrés-Lacueva, C.; Tulipani, S.; Tinahones, F.J.; Queipo-Ortuño, M.I. Benefits of Polyphenols on Gut Microbiota and Implications in Human Health. J. Nutr. Biochem. 2013, 24, 1415–1422. [Google Scholar] [CrossRef]
- He, X.Q.; Yang, F.; Huang, X.A. Proceedings of Chemistry, Pharmacology, Pharmacokinetics and Synthesis of Biflavonoids. Molecules 2021, 26, 6088. [Google Scholar] [CrossRef]
- Gao, Y.; Hu, Y.; Shen, J.; Meng, X.; Suo, J.; Zhang, Z.; Song, L.; Wu, J. Acceleration of Aril Cracking by Ethylene in Torreya grandis During Nut Maturation. Front. Plant Sci. 2021, 12, 761139. [Google Scholar] [CrossRef]
- Wen, S.S.; Lu, Y.C.; Yu, N.X.; Nie, X.H.; Meng, X.H. Microwave Pre-Treatment Aqueous Enzymatic Extraction (MPAEE): A Case Study on the Torreya grandis Seed Kernels Oil. J. Food Process Preserv. 2022, 46, e17115. [Google Scholar] [CrossRef]
- Suo, J.; Ma, Z.; Zhao, B.; Ma, S.; Zhang, Z.; Hu, Y.; Yang, B.; Yu, W.; Wu, J.; Song, L. Metabolomics Reveal Changes in Flavor Quality and Bioactive Components in Post-Ripening Torreya grandis Nuts and the Underlying Mechanism. Food Chem. 2023, 406, 134987. [Google Scholar] [CrossRef]
- Xiang, J.; Huang, Y.; Guan, S.; Shang, Y.; Bao, L.; Yan, X.; Hassan, M.; Xu, L.; Zhao, C. A Sustainable Way to Determine the Water Content in Torreya grandis Kernels Based on Near-Infrared Spectroscopy. Sustainability 2023, 15, 12423. [Google Scholar] [CrossRef]
- Yu, M.; Zhang, C.; Zeng, M.M.; He, Z.Y.; Chen, J. Research Progress on Oil and Protein in Torreya grandis Nuts. Food Sci. 2016, 37, 252–256. [Google Scholar]
- Choi, Y.; Kim, J.; Lee, H.S.; Kim, C.I.; Hwang, I.K.; Park, H.K.; Oh, C.H. Selenium Content in Representative Korean Foods. J. Food Compos. Anal. 2009, 22, 117–122. [Google Scholar] [CrossRef]
- Suo, J.; Gao, Y.; Zhang, H.; Wang, G.; Cheng, H.; Hu, Y.; Lou, H.; Yu, W.; Dai, W.; Song, L.; et al. New Insights into the Accumulation of Vitamin B(3) in Torreya grandis Nuts Via Ethylene Induced Key Gene Expression. Food Chem. 2022, 371, 131050. [Google Scholar] [CrossRef]
- Song, L.; Meng, X.; Yang, L.; Ma, Z.; Zhou, M.; Yu, C.; Zhang, Z.; Yu, W.; Wu, J.; Lou, H. Identification of Key Genes and Enzymes Contributing to Nutrition Conversion of Torreya grandis Nuts During Post-Ripening Process. Food Chem. 2022, 384, 132454. [Google Scholar] [CrossRef]
- Wang, Y.P.; Yao, X.H.; Yang, L.; Fei, X.Q.; Cao, Y.Q.; Wang, K.L.; Guo, S.H. Effects of Harvest Time on the Yield, Quality and Active Substance of Torreya grandis Nut and Its Oil. J. Oleo Sci. 2021, 70, 175–184. [Google Scholar] [CrossRef]
- Tian, X.; Mu, W.B.; Zhang, J.Y. Research Progress on Chemical Constituents and Pharmacological Activities of Different Parts of Torreya grandis. Nat. Prod. Res. Dev. 2021, 33, 691–715. [Google Scholar]
- Zhang, Z.Y.; Jin, H.B.; Suo, J.W.; Yu, W.Y.; Zhou, M.Y.; Dai, W.S.; Song, L.L.; Hu, Y.Y.; Wu, J.S. Effect of Temperature and Humidity on Oil Quality of Harvested Torreya grandis cv. Merrillii Nuts During the After-Ripening Stage. Front. Plant Sci. 2020, 11, 573681. [Google Scholar] [CrossRef]
- Ni, Q.; Gao, Q.; Yu, W.; Liu, X.; Xu, G.; Zhang, Y. Supercritical Carbon Dioxide Extraction of Oils from Two Torreya grandis Varieties Seeds and Their Physicochemical and Antioxidant Properties. LWT 2015, 60, 1226–1234. [Google Scholar] [CrossRef]
- Yao, L.; Sun, J.; Liang, Y.; Feng, T.; Wang, H.; Sun, M.; Yu, W. Volatile Fingerprints of Torreya grandis Hydrosols under Different Downstream Processes Using HS-GC–IMS and the Enhanced Stability and Bioactivity of Hydrosols by High Pressure Homogenization. Food Control 2022, 139, 109058. [Google Scholar] [CrossRef]
- Xiao, J.B.; Dong, J.; Song, L.L.; Wang, D.Q. Effects and Mechanisms of Torreya grandis Seed Oil on Lipid Metabolism in SD Rats Fed a High-Fat Diet. China Oils Fats 2022, 47, 71–77. [Google Scholar]
- Zhang, F.; Ma, Z.; Qiao, Y.; Wang, Z.; Chen, W.; Zheng, S.; Yu, C.; Song, L.; Lou, H.; Wu, J. Transcriptome Sequencing and Metabolomics Analyses Provide Insights into the Flavonoid Biosynthesis in Torreya grandis Kernels. Food Chem. 2022, 374, 131558. [Google Scholar] [CrossRef]
- Liu, R.H. Potential Synergy of Phytochemicals in Cancer Prevention: Mechanism of Action. J. Nutr. 2004, 134, 3479S–3485S. [Google Scholar] [CrossRef]
- Wang, S.; Meckling, K.A.; Marcone, M.F.; Kakuda, Y.; Tsao, R. Synergistic, Additive, and Antagonistic Effects of Food Mixtures on Total Antioxidant Capacities. J. Agric. Food Chem. 2011, 59, 960–968. [Google Scholar] [CrossRef]
- Holst, B.; Williamson, G. Nutrients and Phytochemicals: From Bioavailability to Bioefficacy Beyond Antioxidants. Curr. Opin. Biotechnol. 2008, 19, 73–82. [Google Scholar] [CrossRef]
- Wu, J.S.; Huang, J.D.; Hong, Y.W.; Zhang, H.Z.; Ding, M.Z.; Lou, H.Q.; Hu, Y.Y.; Yu, W.W.; Song, L.L. De Novo Transcriptome Sequencing of Torreya grandis Reveals Gene Regulation in Sciadonic Acid Biosynthesis Pathway. Ind. Crops Prod. 2018, 120, 47–60. [Google Scholar] [CrossRef]
- Zhou, X.R.; Shang, J.; Qin, M.Y.; Wang, J.H.; Jiang, B.; Yang, H.; Zhang, Y. Fractionated Antioxidant and Anti-Inflammatory Kernel Oil from Torreya fargesii. Molecules 2019, 24, 3402. [Google Scholar] [CrossRef]
- Wagner, H. Synergy Research: Approaching a New Generation of Phytopharmaceuticals. Fitoterapia 2011, 82, 34–37. [Google Scholar] [CrossRef]
- Ou, X.; Chen, X.; Fang, Z.; Zhao, J. Proanthocyanidin B2 Alleviates PG.LPS-Induced RAW264.7 Cellular Inflammation and Oxidative Stress Via PI3K/Akt/NFκB Pathway. Cytotechnology 2025, 77, 77–87. [Google Scholar]
- Gilbert, B.; Alves, L.F. Synergy in Plant Medicines. Curr. Med. Chem. 2003, 10, 13–20. [Google Scholar] [CrossRef]
- Yahfoufi, N.; Alsadi, N.; Jambi, M.; Matar, C. The Immunomodulatory and Anti-Inflammatory Role of Polyphenols. Nutrients 2018, 10, 1618. [Google Scholar] [CrossRef]
- Chandra Dash, U.; Bhol, N.K.; Swain, S.K.; Samal, R.R.; Nayak, P.K.; Raina, V.; Panda, S.K.; Kerry, R.G.; Duttaroy, A.K.; Jena, A.B. Oxidative Stress and Inflammation in the Pathogenesis of Neurological Disorders: Mechanisms and Implications. Acta Pharm. Sin. B 2025, 15, 15–34. [Google Scholar] [CrossRef] [PubMed]
- Wagner, H.; Ulrich-Merzenich, G. Synergy Research: Approaching a New Generation of Phytopharmaceuticals. Phytomedicine 2009, 16, 97–110. [Google Scholar] [CrossRef] [PubMed]
- Atanasov, A.G.; Zotchev, S.B.; Dirsch, V.M.; Supuran, C.T. Natural Products in Drug Discovery: Advances and Opportunities. Nat. Rev. Drug Discov. 2021, 20, 200–216. [Google Scholar] [CrossRef] [PubMed]
- Scholz, S.; Williamson, G. Interactions Affecting the Bioavailability of Dietary Polyphenols in vivo. Int. J. Vitam. Nutr. Res. 2007, 77, 224–235. [Google Scholar] [CrossRef]
- Li, Y.; Xu, S.; Mihaylova, M.M.; Zheng, B.; Hou, X.; Jiang, B.; Park, O.; Luo, Z.; Lefai, E.; Shyy, J.Y.; et al. AMPK Phosphorylates and Inhibits SREBP Activity to Attenuate Hepatic Steatosis and Atherosclerosis in Diet-Induced Insulin-Resistant Mice. Cell Metab. 2011, 13, 376–388. [Google Scholar] [CrossRef]
- Li, Y.; Li, S.; Lin, S.J.; Zhang, J.J.; Zhao, C.N.; Li, H.B. Microwave-Assisted Extraction of Natural Antioxidants from the Exotic Gordonia axillaris Fruit: Optimization and Identification of Phenolic Compounds. Molecules 2017, 22, 1481. [Google Scholar] [CrossRef]
- Gligor, O.; Mocan, A.; Moldovan, C.; Locatelli, M.; Crisan, G.; Ferreira, I. Enzyme-Assisted Extractions of Polyphenols—A Comprehensive Review. Trends Food Sci. Technol. 2019, 88, 302–315. [Google Scholar] [CrossRef]
- Gylling, H.; Plat, J.; Turley, S.; Ginsberg, H.N.; Ellegård, L.; Jessup, W.; Jones, P.J.; Lütjohann, D.; Maerz, W.; Masana, L.; et al. Plant Sterols and Plant Stanols in the Management of Dyslipidaemia and Prevention of Cardiovascular Disease. Atherosclerosis 2014, 232, 346–360. [Google Scholar] [CrossRef]
- Ekor, M. The Growing Use of Herbal Medicines: Issues Relating to Adverse Reactions and Challenges in Monitoring Safety. Front. Pharmacol. 2014, 4, 177. [Google Scholar] [CrossRef]
- Rinninella, E.; Raoul, P.; Cintoni, M.; Franceschi, F.; Miggiano, G.A.D.; Gasbarrini, A.; Mele, M.C. What Is the Healthy Gut Microbiota Composition? A Changing Ecosystem across Age, Environment, Diet, and Diseases. Microorganisms 2019, 7, 14. [Google Scholar] [CrossRef]
- Mu, K.W.; Kitts, D.D. Intestinal Polyphenol Antioxidant Activity Involves Redox Signaling Mechanisms Facilitated by Aquaporin Activity. Redox Biol. 2023, 68, 102948. [Google Scholar] [CrossRef] [PubMed]
- Yan, J.; Chen, W.; Zeng, H.; Cheng, H.; Suo, J.; Yu, C.; Yang, B.; Lou, H.; Song, L.; Wu, J. Unraveling the Malate Biosynthesis During Development of Torreya grandis Nuts. Curr. Res. Food Sci. 2022, 5, 2309–2315. [Google Scholar] [CrossRef]
- Guan, S.; Shang, Y.; Zhao, C. Storage Time Detection of Torreya grandis Kernels Using Near Infrared Spectroscopy. Sustainability 2023, 15, 7757. [Google Scholar] [CrossRef]
- Phang, S.J.; Teh, H.X.; Looi, M.L.; Arumugam, B.; Fauzi, M.B.; Kuppusamy, U.R. Phlorotannins from Brown Algae: A Review on Their Antioxidant Mechanisms and Applications in Oxidative Stress-Mediated Diseases. J. Appl. Phycol. 2023, 35, 867–892. [Google Scholar] [CrossRef]
- Meng, X.; Ding, J.; Ye, Q. Silver Ion Assisted Urea Complexation for the Enrichment of Sciadonic Acid from Torreya fargesii Kernel Oil. Biochem. Eng. J. 2023, 197, 108970. [Google Scholar] [CrossRef]
- Chen, S.J.; Huang, W.C.; Yang, T.T.; Lu, J.H.; Chuang, L.T. Incorporation of Sciadonic Acid into Cellular Phospholipids Reduces Pro-Inflammatory Mediators in Murine Macrophages through NF-κB and MAPK Signaling Pathways. Food Chem. Toxicol. 2012, 50, 3687–3695. [Google Scholar] [CrossRef]
- Chen, S.J.; Chuang, L.T.; Liao, J.S.; Huang, W.C.; Lin, H.H. Phospholipid Incorporation of Non-Methylene-Interrupted Fatty Acids (NMIFA) in Murine Microglial BV-2 Cells Reduces Pro-Inflammatory Mediator Production. Inflammation 2015, 38, 2133–2145. [Google Scholar] [CrossRef]
- Park, H.G.; Zhang, J.Y.; Foster, C.; Sudilovsky, D.; Schwed, D.A.; Mecenas, J.; Devapatla, S.; Lawrence, P.; Kothapalli, K.S.D.; Brenna, J.T. A Rare Eicosanoid Precursor Analogue, Sciadonic Acid (5Z,11Z,14Z-20:3), Detected in vivo in Hormone Positive Breast Cancer Tissue. Prostaglandins Leukot. Essent. Fatty Acids 2018, 134, 1–6. [Google Scholar] [CrossRef]
- Chen, S.J.; Hsu, C.P.; Li, C.W.; Lu, J.H.; Chuang, L.T. Pinolenic Acid Inhibits Human Breast Cancer MDA-MB-231 Cell Metastasis in vitro. Food Chem. 2011, 126, 1708–1715. [Google Scholar] [CrossRef]
- Moreira, M.M.; Barrosoa, M.F.; Boeykens, A.; Withouck, H.; Morais, S.; Delerue-Matos, C. Valorization of Apple Tree Wood Residues by Polyphenols Extraction: Comparison between Conventional and Microwave-Assisted Extraction. Ind. Crops Prod. 2017, 104, 210–220. [Google Scholar] [CrossRef]
- Skrovankova, S.; Sumczynski, D.; Mlcek, J.; Jurikova, T.; Sochor, J. Bioactive Compounds and Antioxidant Activity in Different Types of Berries. Int. J. Mol. Sci. 2015, 16, 24673–24706. [Google Scholar] [CrossRef] [PubMed]
- King, A.J.F. The Use of Animal Models in Diabetes Research. Br. J. Pharmacol. 2012, 166, 877–894. [Google Scholar] [CrossRef] [PubMed]
- Ihara, E.; Manabe, N.; Ohkubo, H.; Ogasawara, N.; Ogino, H.; Kakimoto, K.; Kanazawa, M.; Kawahara, H.; Kusano, C.; Kuribayashi, S.; et al. Evidence-Based Clinical Guidelines for Chronic Constipation 2023. Digestion 2025, 106, 62–89. [Google Scholar] [CrossRef]
- Li, X.F.; Gao, H.Y.; Chen, H.J.; Fang, X.J.; Ge, L.M. Research Progress on Bioactive Components and Antioxidant Activity of Torreya grandis Nuts. Food Sci. 2012, 33, 341–345. [Google Scholar]
- Shi, L.K.; Mao, J.H.; Zheng, L.; Zhao, C.W.; Jin, Q.Z.; Wang, X.G. Chemical Characterization and Free Radical Scavenging Capacity of Oils Obtained from Torreya grandis Fort. Ex. Lindl. and Torreya grandis Fort. var. merrillii: A Comparative Study Using Chemometrics. Ind. Crops Prod. 2018, 115, 250–260. [Google Scholar] [CrossRef]
- Lou, H.Q.; Yang, Y.; Zheng, S.; Ma, Z.M.; Chen, W.C.; Yu, C.L.; Song, L.L.; Wu, J.S. Identification of Key Genes Contributing to Amino Acid Biosynthesis in Torreya grandis Using Transcriptome and Metabolome Analysis. Food Chem. 2022, 379, 132078. [Google Scholar] [CrossRef]
- Hao, Q.C.; Xie, J.Q.; Dai, W.S.; Li, K.Y.; Yu, C.L.; Yu, W.W. Effects of Foliar Fertilization on Fruit Quality During Fruit Filling Stage of Torreya grandis. J. Zhejiang A F Univ. 2024, 41, 457–466. [Google Scholar]
- Tan, Y.M.; Ou, Q.; Huang, X.; Wang, Y.J.; Kou, Y.X. Alien Species Introduction and Demographic Changes Contributed to the Population Genetic Structure of the Nut-Yielding Conifer Torreya grandis (Taxaceae). Forests 2024, 15, 1451. [Google Scholar] [CrossRef]
- Sun, X.H.; Zhou, J.; Hu, C.X.; Lyu, H.F.; Chu, K.J.; Wang, G.F. Effects of Different Altitudes on Appearance Characters and Nutritional Quality of Torreya grandis Seeds. J. Fruit. Sci. 2019, 36, 476–485. [Google Scholar]
- Guan, F.; Wang, R.; Yi, Z.; Luo, P.; Liu, W.; Xie, Y.; Liu, Z.; Xia, Z.; Zhang, H.; Cheng, Q. Tissue Macrophages: Origin, Heterogenity, Biological Functions, Diseases and Therapeutic Targets. Signal Transduct. Target. Ther. 2025, 10, 93. [Google Scholar]



| Fatty Acid Name | Formula | Content (%) |
|---|---|---|
| Oleic Acid | C18:1 | 31.32–37.87 |
| Linoleic Acid | C18:2 | 35.22–37.74 |
| Palmitic Acid | C16:0 | 13.05–20.73 |
| Sciadonic acid | C20:3 Δ5,11,14 | 9.13–12.96 |
| Hexadecatrienoic Acid | C16:3 | 9.53 |
| Heptadecanoic Acid | C17:0 | 1.78–6.31 |
| Eicosatrienoic Acid | C20:3 | 1.66–6.31 |
| Stearic Acid | C18:0 | 2.76–3.54 |
| Arachidic Acid | C20:0 | 1.62 |
| Ethyl linoleate | C18:2n−6 | 1.14 |
| Eicosadienoic Acid | C20:2 | 0.22–0.88 |
| Eicosenoic Acid | C20:1 | 0.16–0.50 |
| Myristic Acid | C14:0 | Not Detected |
| Palmitoleic Acid | C16:1 | Not Detected |
| Extraction Method | Principle | Advantages | Disadvantages |
|---|---|---|---|
| Pressing | Physical/mechanical pressure squeezes oil out. Includes cold and hot pressing. | Cold pressing: Simple, relatively low-cost [55]; solvent-free, good quality [57]. Hot pressing: Higher yield than cold pressing [57]. | Cold pressing: Low yield, high residual oil in cake [55,57]. Hot pressing: High temperature can damage nutrients/quality [57]. |
| Solvent Extraction | Uses organic solvents to dissolve and extract oil and then removes solvent by distillation. | High yield, mature technology, suitable for industrial scale [55,58]. | Risk of solvent residue [58,59]; high safety requirements [55]; high-temp. desolventizing affects quality [58]; environmental pollution [55]. |
| SFE-CO2 | Uses supercritical CO2 as a selective solvent; separates oil by reducing pressure. | No solvent residue, pure product [56,59]; low temp., protects active compounds, high-quality [55,56]; environmentally friendly [55,56]. | High equipment and operating costs [55,56]; relatively complex operation [55]. |
| Aqueous Enzymatic Extraction | Uses enzymes in water to break down cell structures and release oil. | Solvent-free, eco-friendly, high safety [55,60]; mild conditions protect active compounds [60]; can co-extract oil and protein [60]. | Often low yield [55,61]; high enzyme cost [60]; long reaction time [61]; emulsification makes separation difficult [55,60]; complex process [61]. |
| Subcritical Fluid Extraction | Uses subcritical low-carbon alkanes (e.g., butane, propane) as solvent; separates by depressurization. | Lower P/T requirements than SFE-CO2, lower investment [62]; higher efficiency [62,63]; low-residue (testing needed) [63]; lower operating temp [62]. | Very high safety risks (flammable solvents) [55,62,63]. |
| Microwave-Assisted Extraction | Microwaves rapidly heat polar molecules, rupturing cells and speeding up oil release into solvent. Often used with other methods. | Significantly shorter time, higher efficiency [55,64]; less solvent needed [64]; lower energy consumption [55]. | Risk of local overheating damaging sensitive compounds [55,64]; uniformity challenge [64]; scale-up difficulties [55]. |
| Ultrasound-Assisted Extraction | Ultrasound effects (cavitation, vibration) break cell walls, improving solvent penetration and mass transfer. Often used with other methods. | Higher efficiency, shorter time [55,65]; lower temp. operation protects sensitive compounds [65]; simpler equipment, easier operation/scale-up [55]. | High intensity may affect oil stability [55,65]; noise, complex optimization [65]; equipment heating [55]. |
| Compound Name | Formula | Content (%) | Reference |
|---|---|---|---|
| D-Limonene | C10H16 | 31.37–46.16 | [31] |
| Carveol | C10H16O | 1.67–4.36 | [31] |
| α-Pinene | C10H16 | 1.63–5.78 | [31] |
| L-Limonene | C10H16 | 1.99 | [69] |
| 2,4-Decadienal | C10H16O | 0.60 | [69] |
| Octadecanal | C18H36O | 1.22 | [69] |
| Terpinolene | C10H16 | 1.60–4.97 | [12] |
| Guaiene | C15H24 | 1.91–4.14 | [12] |
| 1,2,3-Trimethoxy-5-methylbenzene | C10H14O3 | 5.50–12.74 | [12] |
| 2,5-Dimethylpyrazine | C6H8N2 | 1.46–3.90 | [12] |
| Maltol | C6H6O3 | 0.83–2.04 | [12] |
| Sample Type | Parameter | Value | Reference |
|---|---|---|---|
| Kernel extract | DPPH (IC50) Hydroxyl radical (IC50) | 120 μg/mL >100 μg/mL | [29] |
| [29] | |||
| Kernel extract | DPPH (IC50) ABTS (IC50) | 0.08 mg/mL 0.07 mg/mL | [52] |
| [52] | |||
| Kernel extract (70% ethanol) | ABTS (IC50) DPPH (IC50) Total phenolic content Total flavonoid content | 0.70 mg/mL 11.48 mg/mL 5.86 mg/g 7.78 mg/g | [41] |
| [41] | |||
| [41] | |||
| [41] | |||
| Kernel oil (Zhejiang No. 1) | DPPH (IC50) | 123.76 ± 0.13 μg/mL | [38] |
| Homogenized hydrosol | ABTS scavenging rate | 84% | [50] |
| DPPH scavenging rate | 58% | [50] | |
| Hydroxyl radical scavenging rate | 60% | [50] | |
| Kernel oil (2% in high-fat diet) | SOD activity GSH-Px activity T-AOC activity MDA level | ↑ * ↑ * ↑ * ↓ * | [10] |
| [10] | |||
| [10] | |||
| [10] | |||
| Kernel oil | Total phenolic content | 11.3 mg/kg | [10] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liu, R.; Zhou, B.; Che, K.; Gao, W.; Luo, H.; Yang, J.; Chen, Z.; Hu, W. Chemical Composition and Biological Activities of Torreya grandis Kernels: Characteristics of Polymethylene-Interrupted Fatty Acids and Polyphenolic Compounds and Their Potential Health Effects. Forests 2025, 16, 737. https://doi.org/10.3390/f16050737
Liu R, Zhou B, Che K, Gao W, Luo H, Yang J, Chen Z, Hu W. Chemical Composition and Biological Activities of Torreya grandis Kernels: Characteristics of Polymethylene-Interrupted Fatty Acids and Polyphenolic Compounds and Their Potential Health Effects. Forests. 2025; 16(5):737. https://doi.org/10.3390/f16050737
Chicago/Turabian StyleLiu, Ran, Baogang Zhou, Kundian Che, Wei Gao, Haoyuan Luo, Jialin Yang, Zhanjun Chen, and Wenzhong Hu. 2025. "Chemical Composition and Biological Activities of Torreya grandis Kernels: Characteristics of Polymethylene-Interrupted Fatty Acids and Polyphenolic Compounds and Their Potential Health Effects" Forests 16, no. 5: 737. https://doi.org/10.3390/f16050737
APA StyleLiu, R., Zhou, B., Che, K., Gao, W., Luo, H., Yang, J., Chen, Z., & Hu, W. (2025). Chemical Composition and Biological Activities of Torreya grandis Kernels: Characteristics of Polymethylene-Interrupted Fatty Acids and Polyphenolic Compounds and Their Potential Health Effects. Forests, 16(5), 737. https://doi.org/10.3390/f16050737






