Abstract
For forests to provide ecosystem services and function optimally, they need to be managed. Forest management measures can cause significant environmental changes, which sometimes appear extreme. The most notable disturbance caused by forest regeneration is the change in canopy cover. Alteration of the canopy cover is followed by the modifications of many microclimatic factors. These changes subsequently affect all the living organisms in the forest. The present study was conducted to determine how the changes caused by modifications of canopy closure by shelterwood regeneration affect the leaf-mining insect community on sessile oak (Quercus petraea (Matt.) Liebl.). We identified that the removal of the canopy significantly affects the microclimate, vegetation, and the leaf miner community. The insolation and temperature increased in the more open areas, while relative air humidity decreased. This affects the characteristics of the young oak plants, which grow taller and produce more leaves in the open-canopy areas. All these changes consequentially affect the leaf miner community. While the species richness and abundance per tree increased with the decrease in canopy closure, the species richness and abundance per leaf decreased. The opening of the canopy positively affected the leaf miners in the end by increasing the diversity and evenness of their community.
1. Introduction
Forests are the most diverse and ecologically important terrestrial habitats on the planet, covering about 30% of the Earth’s land surface [1]. They provide important ecosystem services, such as carbon sequestration, soil and water protection, and consequentially, mitigation of climate change [2]. Most forests require being managed, ideally in accordance with nature, to allow their normal functioning and to maximize the use of these ecosystem services [2,3]. However, regeneration and other forest management techniques might appear drastic and result in major environmental changes [4,5,6]. While these changes can negatively affect some ecosystem components, their long-term impact is generally positive, and without them, many existing forests could not survive [4,5]. The most significant disturbance caused by forest regeneration is the rapid change in canopy cover and light conditions caused by the removal of some or all of the trees in the area under regeneration [5,7]. As a consequence of these modifications, the microclimate in the regenerated areas changes significantly [8,9]. Without the protection of the canopy and/or forest edge, the climate becomes significantly drier and warmer [10]. The canopy is a major driver of the natural regeneration process, influencing the spread of both woody and herbaceous plant communities [11,12,13]. The plants most affected by the changes in canopy conditions are those that develop under the closed canopy of mature trees [11,12,13].
Changes in habitat conditions caused by alterations of canopy closure can have a profound effect on forest insect communities [14,15,16,17]. It can affect them directly by altering their distribution in the forest, or indirectly by affecting the light availability, speed of nutrient cycling, and temperature [18,19,20]. Changes in environmental factors can also affect the insects’ host plants and natural enemies in many ways [21,22,23,24]. In open canopy conditions, insects are more susceptible to predators and adverse abiotic conditions, like high insolation, intense precipitation, and powerful winds, which are typically buffered by the forest canopy [15,24,25,26,27]. Some authors claim that higher canopy closure increases herbivory rates [28], while according to others, the effect depends on the tree species [29].
Previous studies have found that different groups of insects respond differently to changes in the canopy closure [27,28,29,30,31,32]. For example, gallicolous insects and true bugs (Hemiptera, Heteroptera) benefit from a more open canopy [18,28], while ambrosia beetles are negatively affected by this change [33]. As the general trend of the impact of canopy closure on insect communities cannot be identified from the previous work on the topic, we decided to conduct this study, in which we tested the effect of changes in canopy closure caused by shelterwood regeneration on the leaf-mining insect community on sessile oak (Quercus petraea (Matt.) Liebl.).
The shelterwood regeneration method, which was applied in the study areas, incorporates elements of natural regeneration by gradually removing mature trees. In Southeastern Europe, where there is a rich tradition of managing natural forests, the system has been widely adopted [34]. This approach focuses on renewing the stand and planning for future forest composition, with all mature trees removed over a series of two to four fellings that span around 10 years. This contrasts with selective felling, which considers criteria such as size, age, and species of the trees to maintain a specific structure [35,36]. The shelterwood regeneration method gradually prepares young trees for the open canopy conditions, which increases their adaptation and survival rates and conserves diversity [37,38,39].
Leaf miners were used for the experiment as they are susceptible to the effects of environmental changes [16,40,41]. They are a diverse insect guild with members from four orders [41,42], which has been well-studied in Serbia [43,44]. Their larvae live within one leaf their entire life, so they are tied to one location in the forest [40,45]. As they cannot migrate, they are exposed to all the changes the forest is exposed to. They react to those changes directly and indirectly, through the changes in the host plants [25,41,46,47]. They are an ecologically important group because, on one hand, they can cause significant damage to plants, while on the other, they play a crucial role in food webs, serving as prey for birds, parasitoids, and predatory arthropods [24,42,48,49].
Sessile oak was chosen as a host plant because it is widely spread and valued in Europe as it provides many ecological and economic benefits, it reacts to changes in canopy closure and has a rich and diverse insect community [42,43,50,51,52]. Although extensive research was conducted on the effect of forest regeneration on insect communities [16,40,53,54,55], no studies have examined how changes in environmental conditions caused by shelterwood regeneration affect the leaf-mining insect community on young sessile oak plants.
Young forest plants are highly sensitive to environmental changes, particularly those caused by alterations in canopy closure. That is why we investigated how variations in canopy closure affect the young sessile oak plants, their growing environment, and the insects that interact with them. To achieve this, we aimed to determine how the changes in canopy closure affect: (1) the microclimatic conditions, (2) the characteristics of the understory vegetation, and (3) the leaf-mining insect community on young sessile oak plants.
2. Materials and Methods
2.1. Study Sites
The research was conducted in 2020 on three locations in the northeastern part of Serbia (Majdanpek): Ujevac (44°25′ N; 21°52′ E), Crna reka (44°21′ N; 21°55′ E), and Ravna reka (44°26′ N; 21°59′ E). The average annual temperature at the study sites ranged from 9.3 to 10.3 °C, while during the growing season, it was between 15.9 and 17.0 °C. The average annual precipitation varied from 679 to 705 mm, with growing season precipitation averaging between 388 and 403 mm [7]. According to the Thornthwaite climate classification, the studied localities fall under a subhumid moist climate (C2) [7]. In contrast, Lang’s climate classification categorizes them as semi-humid, typical of low-altitude forests [7].
The stand at the locality Ujevac is phytocenologically defined as a community of sessile oak and hairy sedge (Carex pilosa Scop.) (Carici pilosae–Quercetum petraeae). It is situated at 270–290 m above sea level. The slope of the terrain is 20, and the exposition is western. The soil is deep eutric brown, formed on neutral and basic eruptive rocks, and weakly skeletal. The stand at the locality Crna reka is phytocenologically defined as a community of sessile oak and forest fescue (Festuca drymeja Mert. and W.D.J. Koch) (Festuco drymeiae—Quercetum petraeae). It is situated at 450–470 m above sea level. The slope of the terrain is 15° and the exposition is southwest. The soil is loamy ilimerized (luvisol), on a silicate substrate. The stand at the Ravna reka locality is phytocenologically defined as a community of sessile oak with hairy sedge (Carici pilosae—Quercetum petraeae). It is situated at 490–520 m above sea level. The slope of the terrain is 20° and the exposition is south–southwest. The soil is district brown, shallow to medium deep, and formed on gneiss [7].
2.2. Experimental Design
Three sites with different canopy closure were selected at each location (canopy cover classes). For closed canopy cover class, about 140-year-old stands where no management measures were conducted in the last 30 years were selected. Semi-open canopy cover class was represented by about 140-year-old stands in which the preparation cut of regeneration by shelterwood cutting was conducted in the last 5–10 years when the seed yield was abundant. Open canopy cover class was represented by stands in which the canopy of the about 140-year-old growth forest was removed by final cut of shelterwood cutting in the last 5–10 years after the regeneration has been deemed successful, and oak seedlings higher than the surrounding herbaceous vegetation were identified.
At each site, at each locality, five young (5–7-year-old) sessile oak trees were sampled. All of their leaves were counted twice during the vegetative season (June and August). The leaves with mines on them were separated, and brought to the Entomological Laboratory of Belgrade University, Faculty of Forestry where species causing them were identified based on Hering [56], Patočka and Turčani [57], Laštůvka et al. [58] and Ellis [42]
2.3. Environmental and Plant Characteristics
To analyze the canopy cover, hemispherical canopy photographs were taken at all experimental sites using a Nikon Coolpix 5000 camera (Nikon Corp., Tokyo, Japan) equipped with an FC-E8 hemispherical (“fish-eye” or open-sky) lens. At the experimental sites, photographs were captured on the same day between 9:00 and 12:00 AM, under cloudless sky conditions. During image acquisition, the camera was horizontally and vertically leveled at a height of 1.30 m above ground level. The hemispherical images were processed using the software Gap Light Analyzer (GLA, Version 2.0) [59]. Image processing included registration and classification phases, followed by the calculation of relevant canopy structure parameters. For pixel classification, the threshold value for the image was determined with the aid of the shareware SideLook 1.1 [60] using all the offered color channels. To assess microclimatic conditions, we measured air temperature, relative humidity, and insolation in all experimental sites every two hours from 6 AM to 6 PM over three days in July, the sunniest month. The measurements were taken consecutively from 1–3 July 2020. Since July is the warmest month in the research area, the objective was to collect data on the mentioned climatic elements when differences in various forest canopies are most evident. We utilized a WS-GP1 portable automatic weather station (Delta-T Devices Ltd., Cambridge, UK), which allowed us to collect essential meteorological data at a height of 2 m during the specified time intervals. The cover of herbaceous and woody species was estimated visually from above and recorded in percentages to the nearest 10%, ranging from 10% to 100%, and after that to the nearest 1%, ranging from 1% to 9% [7]. The plant height was measured on all plants present within square sample plots measuring 10 × 10 m (0.01 hectares) on each locality in each canopy cover class, using a measuring tape with an accuracy of 0.5 cm.
2.4. Characteristics of the Leaf Miner Community Analyzed
Leaf mining insect community was quantified by the following parameters at the level of a single tree:
- Species richness (number of species identified) per tree, and the number of species per 100 leaves, calculated as:where si is the number of species found in one sample, li is the number of leaves per sample.
- Total species richness of leaf miners (number of species identified on all trees).
- The abundance of individual species (Ai) per tree, and the number of mines per 100 leaves, calculated as:where ni is the number of mines of one particular species found in one sample and li is the number of leaves per sample.
- The total abundance of leaf miners (Ab) is calculated as a sum of all individual species’ abundance.
- Shannon’s index of diversity (H′), calculated as:where Si is the species richness and pi is the proportion of individuals belonging to the i-th species in the dataset [61].
- Buzas and Gibson’s evenness index (Ev), calculated as:where H is Shannon’s index of diversity and Si is species richness [62].
2.5. Statistical Analysis
As the distribution of the analyzed parameters did not fit the normal distribution (Kolmogorov–Smirnov test), a generalized linear mixed model (GLMM) was used for further analysis, with canopy cover class as a fixed factor and study sites as a random factor. Poisson distribution was assumed for the analysis of the number of miner species or individuals per tree, and gamma distribution was used for the rest of the data. The analyses were conducted with an identity link function, at the confidence level of 95%. An LSD post hoc test was used for comparison among means where the GLMM showed a significant difference. Diversity and evenness indices were calculated using PAST 5.2.2 [63]. Statistical analyses were conducted using IBM SPSS Statistics 26.0 [64].
3. Results
3.1. The Influence of Changes in the Canopy Cover on the Forest Microclimate
Canopy cover differed significantly between the sites at each locality (F = 2517.761, p = 0.000) (Figure 1). The differences in canopy cover caused significant changes in the microclimate. The insolation (F = 1063.681, p = 0.000) and temperature (F = 402.560, p < 0.0001) increased significantly from closed to open canopy conditions, while relative humidity decreased in the same manner (F = 512.479, p < 0.0001) (Figure 1).

Figure 1.
Differences in canopy cover (%) (a) and influence of canopy cover class on insolation (l×/1000) (b), temperature (°C) (c), and relative humidity (%) (d) (mean ± SD) assessed by the GLMM (letters above the columns indicate the grouping calculated by LSD post hoc test).
3.2. The Influence of Canopy Cover on Understory Vegetation
Seven woody and three herbaceous understory plant species were identified as dominant on the study sites in the following order: Quercus petraea (Matt.) Liebl. (59.4%), Rubus hirtus Waldst. and Kit. (12.8%), Tilia tomentosa Moench (8.3%), C. pilosa (5.0%), F. drymeja (4.4%), Fraxinus ornus L. (3.9%), Acer campestre L. (2.2%), Carpinus betulus L. (2.2%), Cornus mas L. (1.1%), and Rosa arvensis Huds. (0.6%) (Figure 2). The total understory plant cover showed a regularity where the highest amount of ground area covered by understory plants was in the open canopy cover class, slightly lower in the area with the semi-open canopy cover class, and significantly lower in the closed canopy cover class (Figure 2 and Table 1). The understory plant ground cover by plant species differed between different canopy cover classes, although this was only significant for some species (Figure 2 and Table 1). Of the identified species, Q. petraea covered the greatest area, and dominated in the open class, compared to both the semi-open and closed canopy classes. From the rest of the identified species, only F. ornus and A. campestre covered a significantly greater ground area in the semi-open compared to the closed and open canopy classes (Figure 2 and Table 1).
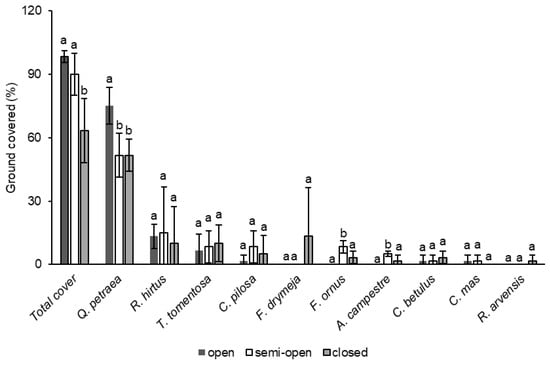
Figure 2.
Differences in total ground cover by understory plants and ground cover by plant species (% of the ground area covered) (mean ± SD) assessed by the GLMM (letters above the columns indicate the grouping calculated by LSD post hoc test).

Table 1.
Effect of canopy cover class on the ground cover area covered by understory vegetation assessed by the GLMM (bold values indicate statistically significant values).
The young oak plants that grew in the closed canopy conditions differed from the plants that grew in the semi-open or open canopy conditions. The height of the plants (F = 63.308, p < 0.0001), as well as the number of leaves (F = 111.763, p < 0.0001), differed significantly between different canopy cover classes. The plants’ height, as well as the number of leaves, was highest in the open canopy cover class, lower in the semi-open, and lowest in the closed canopy cover class (Figure 3).
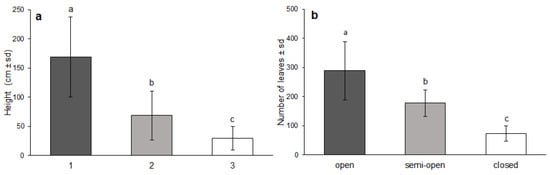
Figure 3.
Influence of canopy cover class on the height (a) and number of leaves (b) of young sessile oak trees (mean ± SD) assessed by the GLMM (letters above the columns indicate the grouping calculated by LSD post hoc test).
3.3. The Influence of Canopy Closure on the Leaf Miner Community on Young Sessile Oak Plants
A total of 12 leaf miner species were identified on the leaves of the studied plants (Table 2). The most frequent and abundant of all the identified species were Profenusa pygmaea (Klug, 1816), Phyllonorycter quercifoliella (Zeller, 1839), and Tischeria ekebladella (Bjerkander, 1795).

Table 2.
Leaf miner species identified on the young sessile oak plants in different canopy cover classes.
Canopy cover had a significant effect on the number of leaf miner species per tree (F = 4.695, p = 0.012), as well as per leaf (F = 15.644, p = 0.000). While the number of species per tree increased with the decrease in canopy cover, the number of species per leaf decreased (Figure 4a,b). The situation regarding the number of mines was the same. Canopy cover had a significant effect on the number of leaf mines per tree (F = 7.310, p = 0.001) as well as per leaf (F = 10.263, p = 0.000), and the number of mines varied in the same order (Figure 4c,d). Canopy cover also significantly affected the Shannon diversity index (F = 16.052, p = 0.000) and the Buzas and Gibson’s evenness index (F = 15.270, p < 0.0001). Both of these indices increased in the direction from closed to open canopy cover classes (Figure 4e,f).
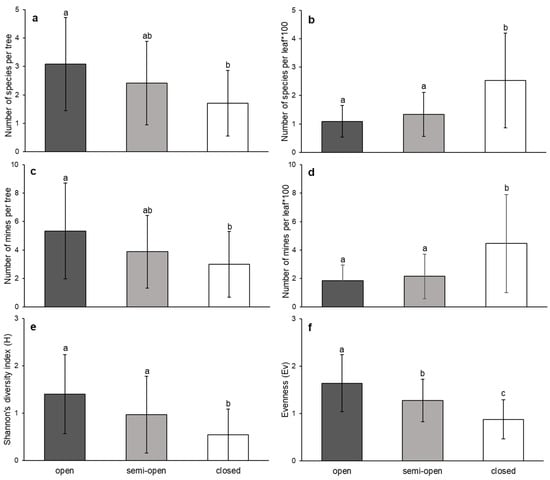
Figure 4.
The influence of canopy cover class on the sessile oak leaf miner community (mean ± SD), represented by the number of species per tree (a), number of species per leaf × 100 (b), number of mines per tree (c), number of mines per leaf × 100 (d), Shannon’s diversity index (e), and evenness (f), assessed by the GLMM (letters above the columns indicate the grouping calculated by LSD post hoc test).
4. Discussion
In this study, we found that shelterwood regeneration has a significant impact on the sessile oak forest microclimate, vegetation, and its leaf-mining insect community. The reduction in the canopy cover caused by the combined preparation–seed cut increased insolation by more than 17 times, while the final cut increased it by more than 133 times. These changes affected the vegetation characteristics both directly and indirectly by altering the microclimate characteristics, such as increasing the temperature and airflow and reducing air humidity. This consequentially contributed to plant growth as light intensity positively affected the rate of photosynthesis, biomass accumulation, and structural development [65,66]. On the other hand, they could negatively affect plants by increasing drought stress, negatively affecting soil microorganisms, and increasing the risk of pest outbreaks [67,68,69]. In the mosaic of different microclimate conditions, the areas with closed canopy may have served as climate buffers, preventing warm, dry air from entering other parts of the forest, thus mitigating drought stress and providing refuge for sensitive species [70,71,72]. These newly created conditions promote the growth of most plant species, which is why shelterwood cutting is widely used in forestry to support natural regeneration [7,73,74].
After the combined preparation–seed cut and especially final-cut understory plant cover increased. Heliophilous plant species, including sessile oak, were particularly promoted. Besides the sessile oak cover, F. Ornus and A. campestre were significantly affected by the changes in canopy closure, although they were not present in the open canopy conditions where Q. petraea dominated. The oak plants that were exposed to more light also grew taller and produced greater leaf mass, as a consequence of enhanced photosynthesis due to more available light [7,75]. In contrast, shaded plants developed larger leaves to optimize light capture at the cost of reduced overall biomass [76].
The changes in forest cover also affected the leaf miner community. All the studied parameters per tree were significantly higher in open or semi-open conditions, compared to the closed canopy cover class. Leaf miners, like most herbivorous insects, prefer areas with higher insolation due to the associated increase in host plant quantity, quality, and microclimatic benefits for their development [77,78]. The change in canopy cover affected leaf miners both directly by modifying the microclimate and indirectly by altering host plant characteristics. The increased temperature most likely benefited the leaf miners as it accelerates insect metabolism and development, increasing their voltinism, and subsequently their survival rates [78,79,80]. Changes in microclimate could have affected the interactions of leaf miners with competitive herbivorous insect species, as well as their natural enemies [24,81,82]. Higher temperatures and insolation also affect leaf miners indirectly through their effects on host plants, by affecting their phenology, size, leaf mass, etc. [15,16,78,80]. Plants in open-canopy areas were significantly taller and had more leaves which emitted stronger chemical cues, making them easier to detect by the leaf miners [16,40]. This likely explains why all insect community parameters per tree were significantly higher in the open and semi-open cover classes. In contrast, shade-grown plants were smaller, produced fewer leaves, and emitted weaker chemical signals, making them less attractive. On the other side, sun-exposed plants produce more protective chemicals such as tannins and flavonoids, enhancing herbivore resistance, whereas shaded plants allocate fewer resources to chemical defense, making them more vulnerable [83,84,85]. Additionally, some leaf miner species prefer shaded environments where plants produce larger, more hydrated leaves which provide better feeding conditions for future larvae [86,87]. Overstory trees in those shaded areas also serve as reservoirs for colonizing species, as they can maintain a sustainable and more diverse population of leaf miners [16,88].
Leaf miner diversity and evenness were significantly higher in the areas with open and semi-open canopies compared to areas with closed canopies. From an ecological perspective, this is viewed as positive as increased biodiversity implies a more resilient ecosystem [89,90,91]. However, this study refers to only one insect guild on one host plant species, and the study was performed in a single forest location, so the results are hard to generalize in this context without analyzing other ecosystem members on a greater multitude of ecologically different localities, which is why it should serve as a reference point for future research. Nevertheless, shelterwood regeneration created a mosaic of diverse habitats with natural, reduced, and removed canopy cover [92,93,94,95]. The formation of these microhabitats allowed the survival of both sciophilous and heliophilous plant species and both the forest specialist and open-area insect species which feed on them in a relatively small area [39,94,95,96,97]. These mixed habitats accommodate greater forest species richness and diversity compared to both clear-cutting and selective cutting, which tend to homogenize environmental conditions over large areas [97,98,99]. The diverse forest structure can also boost carbon sequestration in the long term while maintaining ecological stability, as the forest is not completely removed at any moment, which is a significant advantage compared to clear-cutting [5,100,101]. Forestry practitioners understand this, which is why forest regeneration methods incorporate diversity conservation as an important factor in recent years by leaving retention trees or groups of trees when applying clear-cutting, or reducing the area under regeneration when applying shelterwood regeneration [16,55,102]. If forests like the one in this study were left to regenerate passively, similarly to protected virgin oak forests, then shrub species, lime, hornbeam, or even invasive species like ghetto palm would probably dominate these forests [103,104,105]. In the long term (over a century), large forest complexes might gradually develop into mixed oak forests, but many of their ecological functions, including biodiversity conservation, would be lost [106]. This would result in significant ecological and economic losses [51,107].
5. Conclusions
Shelterwood regeneration of sessile oak forests causes changes in canopy cover which affect the forest microclimate by increasing insolation and temperature, and decreasing air humidity. This subsequently influences the plants in the regenerated areas, as well as the insect communities feeding on them. The increase in light availability contributes to an increase in the plants’ height and production of increased leaf mass as a consequence of enhanced photosynthesis.
Sessile oak leaf miner species richness and abundance per tree, as well as diversity and evenness, were significantly higher in the open or semi-open canopy cover classes, while species richness and abundance per leaf decreased in the open or semi-open canopy cover classes. Leaf miners benefited from the increased insolation due to the increase in host plant quantity, quality, and microclimatic benefits for their development. The parameters calculated per leaf were higher in the shaded environment, probably due to the increased production of protective chemicals of the sun-exposed plants; the characteristics of the shaded plants which produce larger, more hydrated leaves; and overstory trees which can sustain an abundant leaf miner population and act as population reservoirs.
Either way, the shelterwood forest regeneration had a positive influence on the sessile oak and other understory plants as well as on the leaf miner community. It created a mosaic of diverse habitats that accommodated both young and mature sessile oak plants. This affected the oak age diversity positively, while also increasing the leaf miner species richness and diversity, allowing long-term forest stability and sustainability. This way, many other ecological functions to which oak forests contribute were enabled, while simultaneously allowing for economic profitability. As the study was performed in a single forest location, which had a single insect guild feeding on it, the extension of results to other forest formations, climatic zones, or insect groups can be constrained, which is why additional temporal, spatial, and species replications need to be conducted to confirm these conclusions.
Author Contributions
Conceptualization, J.D.; methodology, J.D., Č.M. and B.K.; validation, J.D., Č.M. and B.K.; formal analysis, J.D. and B.K.; investigation, J.D. and B.K.; resources, J.D., Č.M. and B.K.; data curation, J.D. and B.K.; writing—original draft preparation, J.D.; writing—review and editing, J.D. and B.K.; visualization, J.D.; supervision, J.D., Č.M. and B.K.; project administration, J.D.; funding acquisition, J.D., Č.M. and B.K. All authors have read and agreed to the published version of the manuscript.
Funding
We would like to thank the Serbian Ministry of Education, Science and Technological Development, grant number 451-03-137/2025-03/200169, for financing scientific research at the University of Belgrade, Faculty of Forestry.
Data Availability Statement
The datasets generated and/or analyzed during the current study are available from the corresponding author on reasonable request.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Fornoff, F.; Staab, M.; Zhu, C.D.; Klein, A.M. Multi-Trophic Communities Re-Establish with Canopy Cover and Microclimate in a Subtropical Forest Biodiversity Experiment. Oecologia 2021, 196, 289–301. [Google Scholar] [CrossRef] [PubMed]
- FAO; UNEP. The State of the World’s Forests 2020; De Gruyter: Rome, Italy, 2020; ISBN 978-92-5-132419-6. [Google Scholar]
- European Commission: Directorate-General for Environment. Guidelines on Closer-to-Nature Forest Management; Publications Office of the European Union: Luxembourg, 2023. [Google Scholar]
- Du Bus De Warnaffe, G.; Angerand, S. Forest Management and Climate Change: A New Approach to the French Strategy; Fern: Brussels, Belgium, 2020. [Google Scholar]
- Ruiz-Peinado, R.; Bravo-Oviedo, A.; López-Senespleda, E.; Bravo, F.; Del Río, M. Forest Management and Carbon Sequestration in the Mediterranean Region: A Review. For. Syst. 2017, 26, 10. [Google Scholar] [CrossRef]
- Hämäläinen, A.; Runnel, K.; Ranius, T.; Strengbom, J. Diversity of Forest Structures Important for Biodiversity Is Determined by the Combined Effects of Productivity, Stand Age, and Management. Ambio 2024, 53, 718–729. [Google Scholar] [CrossRef]
- Kanjevac, B.; Krstić, M.; Babić, V.; Govedar, Z. Regeneration Dynamics and Development of Seedlings in Sessile Oak Forests in Relation to the Light Availability and Competing Vegetation. Forests 2021, 12, 384. [Google Scholar] [CrossRef]
- Máliš, F.; Ujházy, K.; Hederová, L.; Ujházyová, M.; Csölleová, L.; Coomes, D.A.; Zellweger, F. Microclimate Variation and Recovery Time in Managed and Old-Growth Temperate Forests. Agric. For. Meteorol. 2023, 342, 109722. [Google Scholar] [CrossRef]
- Horváth, C.V.; Kovács, B.; Tinya, F.; Schadeck Locatelli, J.; Németh, C.; Crecco, L.; Illés, G.; Csépányi, P.; Ódor, P. A Matter of Size and Shape: Microclimatic Changes Induced by Experimental Gap Openings in a Sessile Oak–Hornbeam Forest. Sci. Total Environ. 2023, 873, 162302. [Google Scholar] [CrossRef]
- De Frenne, P.; Zellweger, F.; Rodríguez-Sánchez, F.; Scheffers, B.R.; Hylander, K.; Luoto, M.; Vellend, M.; Verheyen, K.; Lenoir, J. Global Buffering of Temperatures Under Forest Canopies. Nat. Ecol. Evol. 2019, 3, 744–749. [Google Scholar] [CrossRef]
- Kanjevac, B.; Babić, V.; Stajić, S.; Martać, N.; Pavlović, B.; Furtula, D.; Čokeša, V. Key Drivers Affecting the Spatial Heterogeneity of the Regeneration Process in Old-Growth Beech Forests in Southeastern Europe. Front. For. Glob. Change 2023, 6, 1304037. [Google Scholar] [CrossRef]
- Bolton, N.W.; D’Amato, A.W. Herbaceous Vegetation Responses to Gap Size Within Natural Disturbance-Based Silvicultural Systems in Northeastern Minnesota, USA. Forests 2019, 10, 111. [Google Scholar] [CrossRef]
- Tinya, F.; Csépányi, P.; Horváth, C.V.; Kovács, B.; Németh, C.; Ódor, P. Fine-Scale Interventions Can Reinforce the Forest Character of the Understory Vegetation—The Effects of Different Artificial Gaps in an Oak-Dominated Forest. For. Ecol. Manag. 2025, 578, 122471. [Google Scholar] [CrossRef]
- Horák, J.; Marković, Č.; Dobrosavljević, J.; Rada, P.; Mladenović, S.; Kohutka, A.; Míkovcová, A.; Pech, P.; Drábek, O.; Tejnecký, V. Influence of Forest Structure and Soil Chemistry on Ants, Land Snails, and Beetles in Balkan Floodplain Forests: Examining Species Richness and Habitat Preferences. J. Soil Water Conserv. 2024, 79, 303–306. [Google Scholar] [CrossRef]
- Dobrosavljević, J.; Marković, Č.; Marjanović, M.; Milanović, S. Pedunculate Oak Leaf Miners’ Community: Urban vs. Rural Habitat. Forests 2020, 11, 1300. [Google Scholar] [CrossRef]
- Marković, Č.; Dobrosavljević, J.; Vujičić, P.; Cebeci, H.H. Impact of Regeneration by Shelterwood Cutting on the Pedunculate Oak (Quercus robur) Leaf Mining Insect Community. Biologia 2021, 76, 1197–1203. [Google Scholar] [CrossRef]
- Valdés-Correcher, E.; Moreira, X.; Augusto, L.; Barbaro, L.; Bouget, C.; Bouriaud, O.; Branco, M.; Centenaro, G.; Csóka, G.; Damestoy, T.; et al. Search for Top-down and Bottom-up Drivers of Latitudinal Trends in Insect Herbivory in Oak Trees in Europe. Glob. Ecol. Biogeogr. 2021, 30, 651–665. [Google Scholar] [CrossRef]
- Achury, R.; Staab, M.; Blüthgen, N.; Weisser, W.W. Forest Gaps Increase True Bug Diversity by Recruiting Open Land Species. Oecologia 2023, 202, 299–312. [Google Scholar] [CrossRef]
- Preisser, E.; Smith, D.C.; Lowman, M.D. Canopy and Ground Level Insect Distribution in a Temperate Forest. Selbyana 1998, 19, 141–146. [Google Scholar]
- Thomson, L.J.; Macfadyen, S.; Hoffmann, A.A. Predicting the Effects of Climate Change on Natural Enemies of Agricultural Pests. Biol. Control 2010, 52, 296–306. [Google Scholar] [CrossRef]
- Ellsworth, D.S.; Reich, P.B. Canopy Structure and Vertical Patterns of Photosynthesis and Related Leaf Traits in a Deciduous Forest. Oecologia 1993, 96, 169–178. [Google Scholar] [CrossRef]
- Caldwell, E.; Read, J.; Sanson, G.D. Which Leaf Mechanical Traits Correlate with Insect Herbivory Among Feeding Guilds? Ann. Bot. 2016, 117, 349–361. [Google Scholar] [CrossRef]
- Klapwijk, M.J.; Bylund, H.; Schroeder, M.; Björkman, C. Forest Management and Natural Biocontrol of Insect Pests. Forestry 2016, 89, 253–262. [Google Scholar] [CrossRef]
- Schillé, L.; Valdés-Correcher, E.; Archaux, F.; Bălăcenoiu, F.; Bjørn, M.C.; Bogdziewicz, M.; Boivin, T.; Branco, M.; Damestoy, T.; de Groot, M.; et al. Decomposing Drivers in Avian Insectivory: Large-Scale Effects of Climate, Habitat and Bird Diversity. J. Biogeogr. 2024, 51, 1079–1094. [Google Scholar] [CrossRef]
- Dobrosavljević, J.; Marković, Č.; Marjanović, M. The Effect of Urban–Rural Gradient on Black Poplar Endophagous Herbivorous Insects. Arthropod-Plant Interact. 2023, 17, 341–350. [Google Scholar] [CrossRef]
- Lenk, A.; Richter, R.; Kretz, L.; Wirth, C. Effects of Canopy Gaps on Microclimate, Soil Biological Activity and Their Relationship in a European Mixed Floodplain Forest. Sci. Total Environ. 2024, 941, 173572. [Google Scholar] [CrossRef] [PubMed]
- Perlík, M.; Kraus, D.; Bußler, H.; Neudam, L.; Pietsch, S.; Mergner, U.; Seidel, D.; Sebek, P.; Thorn, S. Canopy Openness as the Main Driver of Aculeate Hymenoptera and Saproxylic Beetle Diversity Following Natural Disturbances and Salvage Logging. For. Ecol. Manag. 2023, 540, 121033. [Google Scholar] [CrossRef]
- Valdés-Correcher, E.; Popova, A.; Galmán, A.; Prinzing, A.; Selikhovkin, A.V.; Howe, A.G.; Mrazova, A.; Dulaurent, A.; Hampe, A.; Tack, A.J.M.; et al. Herbivory on the Pedunculate Oak along an Urbanization Gradient in Europe: Effects of Impervious Surface, Local Tree Cover, and Insect Feeding Guild. Ecol. Evol. 2022, 12, e8709. [Google Scholar] [CrossRef]
- Takafumi, H.; Kawase, S.; Nakamura, M.; Hiura, T. Herbivory in Canopy Gaps Created by a Typhoon Varies by Understory Plant Leaf Phenology. Ecol. Entomol. 2010, 35, 576–585. [Google Scholar] [CrossRef]
- Wildermuth, B.; Penanhoat, A.; Sennhenn-Reulen, H.; Matevski, D.; Drescher, J.; Aubry-Kientz, M.; Seidel, D.; Schuldt, A. Canopy Structure Influences Arthropod Communities Within and Beyond Tree Identity Effects: Insights from Combining LiDAR Data, Insecticidal Fogging and Machine Learning Regression Modelling. Ecol. Indic. 2024, 160, 111901. [Google Scholar] [CrossRef]
- Parker, G.G.; Fitzjarrald, D.R.; Gonçalves Sampaio, I.C. Consequences of Environmental Heterogeneity for the Photosynthetic Light Environment of a Tropical Forest. Agric. For. Meteorol. 2019, 278, 107661. [Google Scholar] [CrossRef]
- Parker, G.G.; Lowman, M.D.; Nadkarni, N.M. Structure and Microclimate of Forest Canopies. In Forest Canopies; Lowman, M.D., Nadkari, N.M., Eds.; Academic Press: San Diego, CA, USA, 1995; pp. 73–106. [Google Scholar]
- Holuša, J.; Fiala, T.; Foit, J. Ambrosia Beetles Prefer Closed Canopies: A Case Study in Oak Forests in Central Europe. Forests 2021, 12, 1223. [Google Scholar] [CrossRef]
- O’Hara, K.L.; Ina, A.B.; Diaci, J.; Anić, I.; Boydak, M.; Curovic, M.; Govedar, Z.; Grigoriadis, N.; Ivojevic, S.; Keren, S.; et al. Culture and Silviculture: Origins and Evolution of Silviculture in Southeast Europe. Int. For. Rev. 2018, 20, 130–143. [Google Scholar] [CrossRef]
- Brockway, D.G.; Outcalt, K.W. Influence of Selection Systems and Shelterwood Methods on Understory Plant Communities of Longleaf Pine Forests in Flatwoods and Uplands. For. Ecol. Manag. 2015, 357, 138–150. [Google Scholar] [CrossRef]
- Day, K.; Koot, C.; Wiensczyk, A. The Shelterwood Silvicultural System in British Columbia—A Practitioner’s Guide Part 3: Operational Implementation. J. Ecosyst. Manag. 2011, 12, 95–106. [Google Scholar] [CrossRef]
- Agestam, E.; Ekö, P.M.; Nilsson, U.; Welander, N.T. The Effects of Shelterwood Density and Site Preparation on Natural Regeneration of Fagus Sylvatica in Southern Sweden. For. Ecol. Manag. 2003, 176, 61–73. [Google Scholar] [CrossRef]
- Nolet, P.; Kneeshaw, D.; Messier, C.; Béland, M. Comparing the Effects of Even- and Uneven-aged Silviculture on Ecological Diversity and Processes: A Review. Ecol. Evol. 2018, 8, 1217–1226. [Google Scholar] [CrossRef] [PubMed]
- Nyland, R.D. The Shelterwood Method: Adapting to Diverse Management Objectives. J. For. 2010, 108, 419–420. [Google Scholar] [CrossRef]
- Atkinson, B.; Bailey, S.; Vaughan, I.P.; Memmott, J. A Comparison of Clearfelling and Gradual Thinning of Plantations for the Restoration of Insect Herbivores and Woodland Plants. J. Appl. Ecol. 2015, 52, 1538–1546. [Google Scholar] [CrossRef]
- Hering, E.M. Biology of the Leaf Miners; Dr. W. Junk: Hague, The Netherlands, 1951. [Google Scholar]
- Ellis, W.N. Leafminers and Plant Galls of Europe. Available online: http://www.bladmineerders.nl/ (accessed on 17 October 2023).
- Dobrosavljević, J.; Marković, Č. First Findings of Deciduous Woody Plant Leaf Miners in Serbia. J. Entomol. Res. Soc. 2024, 26, 183–207. [Google Scholar] [CrossRef]
- Dobrosavljević, J.; Marković, Č.; Bojić, S. Overview of Leaf Miner Fauna in Serbia. In Proceedings of the VIII International Agriculture Symposium “AGROSYM 2017”, Jahorina, Bosnia and Herzegovina, 5–8 October 2017; pp. 1490–1498. [Google Scholar]
- Jones, E.L.; Leather, S.R. Invertebrates in Urban Areas: A Review. Eur. J. Entomol. 2012, 109, 463–478. [Google Scholar] [CrossRef]
- Maldonado-López, Y.; Cuevas-Reyes, P.; González-Rodríguez, A.; Pérez-López, G.; Acosta-Gómez, C.; Oyama, K. Relationships among Plant Genetics, Phytochemistry and Herbivory Patterns in Quercus castanea Across a Fragmented Landscape. Ecol. Res. 2015, 30, 133–143. [Google Scholar] [CrossRef]
- Rickman, J.K.; Connor, E.F. The Effect of Urbanization on the Quality of Remnant Habitats for Leaf-Mining Lepidoptera on Quercus agrifolia. Ecography 2003, 26, 777–787. [Google Scholar] [CrossRef]
- Grabenweger, G.; Kehrli, P.; Schlick-Steiner, B.; Steiner, F.; Stolz, M.; Bacher, S. Predator Complex of the Horse Chestnut Leafminer Cameraria ohridella: Identification and Impact Assessment. J. Appl. Entomol. 2005, 129, 353–362. [Google Scholar] [CrossRef]
- Cebeci, H.H.; Markovic, C.; Grabenweger, G.; Ayberk, H.; Dobrosavljevic, J.; Goltas, M.; Stojanovic, A.; Ale, A.; Bacchetta, C.; Cazenave, J. Preliminary Notes on Pupal Parasitism Rates of the Horse Chestnut Leafminer, Cameraria ohridella (Lepidoptera gracillariidae) in Belgrade and Istanbul. Fresen. Environ. Bull. 2018, 27, 7122–7124. [Google Scholar]
- Černý, J.; Špulák, O.; Kománek, M.; Žižková, E.; Sýkora, P. Sessile Oak (Quercus petraea [Matt.] Liebl.) and Its Adaptation Strategies in the Context of Global Climate Change: A Review. Cent. Eur. For. J. 2024, 70, 77–94. [Google Scholar] [CrossRef]
- Eaton, E.; Caudullo, G.; Oliveira, S.; de Rigo, D. Quercus Robur and Quercus Petraea in Europe: Distribution, Habitat, Usage and Threats. In European Atlas of Forest Tree Species; San-Miguel-Ayanz, J., de Rigo, D., Caudullo, G., Houston Durrant, T., Mauri, A., Eds.; Publication Office of the European Union: Luxembourg, 2016; pp. 160–163. ISBN 978-92-79-52833-0. [Google Scholar]
- Marković, Č.; Dobrosavljević, J. Review of Scolytinae (Coleoptera, Curculionidae) of Serbia. J. Entomol. Res. Soc. 2023, 25, 545–561. [Google Scholar] [CrossRef]
- Yu, X.-D.; Luo, T.-H.; Zhou, H.-Z. Distribution of Carabid Beetles among Regenerating and Natural Forest Types in Southwestern China. For. Ecol. Manag. 2006, 231, 169–177. [Google Scholar] [CrossRef]
- Forrester, G.J. The Population Ecology of Acorn Weevils and Their Influence on Natural Regeneration of Oak. Ph.D. Thesis, University of London, Imperial College, London, UK, 1990. [Google Scholar]
- Siira-Pietikäinen, A.; Haimi, J.; Siitonen, J. Short-Term Responses of Soil Macroarthropod Community to Clear Felling and Alternative Forest Regeneration Methods. For. Ecol. Manag. 2003, 172, 339–353. [Google Scholar] [CrossRef]
- Hering, E.M. Bestimmungstabellen der Blattminen von Europa Einschliesslich des Mittelmeerbeckens und Der Kanarishen Inseln, Band I, II und III.; Dr. W. Junk: Hague, The Netherlands, 1957; ISBN 9789061939818. [Google Scholar]
- Patočka, J.; Turčani, M. Lepidoptera Pupae. Central European Species; BRILL: Stenstrup, The Netherlands, 2005; ISBN 9788788757477. [Google Scholar]
- Laštůvka, A.; Zdeněk, L.; Liška, J.; Šumpich, J. Motýli a Housenky Střední Evropy V., Drobní Motýli I.; Academia: Prague, Czech Republic, 2018; ISBN 978-80-200-2852-5. [Google Scholar]
- Frazer, G.W.; Canham, C.D.; Lertzman, K.P. Version 2.0: Imaging to Extract Canopy Structure and Gap Light Transmission Indices from True-Colour Fisheye, Users Manual and Program Documentation; SCIRP: Wuhan, China, 1999. [Google Scholar]
- Nobis, M.; Hunziker, U. Automatic Thresholding for Hemispherical Canopy-Photographs Based on Edge Detection. Agric. For. Meteorol. 2005, 128, 243–250. [Google Scholar] [CrossRef]
- Chao, A.; Chiu, C.H.; Jost, L. Unifying Species Diversity, Phylogenetic Diversity, Functional Diversity, and Related Similarity and Differentiation Measures Through Hill Numbers. Annu. Rev. Ecol. Evol. Syst. 2014, 45, 297–324. [Google Scholar] [CrossRef]
- Buzas, M.A.; Hayek, L.-A.C. Biodiversity Resolution: An Integrated Approach. Biodivers. Lett. 1996, 3, 40–43. [Google Scholar] [CrossRef]
- Hammer, D.A.T.; Ryan, P.D.; Hammer, Ø.; Harper, D.A.T. Past: Paleontological Statistics Software Package for Education and Data Analysis. Palaeontol. Electron. 2001, 4, 9. [Google Scholar]
- IBM Corp. IBM SPSS Statistics for Windows; Version 26.0; IBM Corp: New York, NY, USA, 2019. [Google Scholar]
- Niinemets, Ü.; Valladares, F. Tolerance to Shade, Drought, and Waterlogging of Temperate Northern Hemisphere Trees and Shrubs. Ecol. Monogr. 2006, 76, 521–547. [Google Scholar] [CrossRef]
- Lichtenthaler, H.K.; Babani, F.; Langsdorf, G. Chlorophyll Fluorescence Imaging of Photosynthetic Activity in Sun and Shade Leaves of Trees. Photosynth. Res. 2007, 93, 235–244. [Google Scholar] [CrossRef]
- Huber, A.E.; Bauerle, T.L. Long-Distance Plant Signaling Pathways in Response to Multiple Stressors: The Gap in Knowledge. J. Exp. Bot. 2016, 67, 2063–2079. [Google Scholar] [CrossRef] [PubMed]
- Al-Khayri, J.M.; Rashmi, R.; Toppo, V.; Chole, P.B.; Banadka, A.; Sudheer, W.N.; Nagella, P.; Shehata, W.F.; Al-Mssallem, M.Q.; Alessa, F.M.; et al. Plant Secondary Metabolites: The Weapons for Biotic Stress Management. Metabolites 2023, 13, 716. [Google Scholar] [CrossRef]
- Pereira, A. Plant Abiotic Stress Challenges from the Changing Environment. Front. Plant Sci. 2016, 7, 1123. [Google Scholar] [CrossRef] [PubMed]
- Keppel, G.; Anderson, S.; Williams, C.; Kleindorfer, S.; O’Connell, C. Microhabitats and Canopy Cover Moderate High Summer Temperatures in a Fragmented Mediterranean Landscape. PLoS ONE 2017, 12, e0183106. [Google Scholar] [CrossRef]
- Richter, R.; Ballasus, H.; Engelmann, R.A.; Zielhofer, C.; Sanaei, A.; Wirth, C. Tree Species Matter for Forest Microclimate Regulation During the Drought Year 2018: Disentangling Environmental Drivers and Biotic Drivers. Sci. Rep. 2022, 12, 17559. [Google Scholar] [CrossRef]
- Wolf, J.; Asch, J.; Tian, F.; Georgiou, K.; Ahlström, A. Canopy Responses of Swedish Primary and Secondary Forests to the 2018 Drought. Environ. Res. Lett. 2023, 18, 064044. [Google Scholar] [CrossRef]
- Kohler, M.; Pyttel, P.; Kuehne, C.; Modrow, T.; Bauhus, J. On the Knowns and Unknowns of Natural Regeneration of Silviculturally Managed Sessile Oak (Quercus petraea (Matt.) Liebl.) Forests—A Literature Review. Ann. Sci. 2020, 77, 101. [Google Scholar] [CrossRef]
- Modrow, T.; Kuehne, C.; Saha, S.; Bauhus, J.; Pyttel, P.L. Photosynthetic Performance, Height Growth, and Dominance of Naturally Regenerated Sessile Oak (Quercus petraea [Mattuschka] Liebl.) Seedlings in Small-Scale Canopy Openings of Varying Sizes. Eur. J. For. Res. 2020, 139, 41–52. [Google Scholar] [CrossRef]
- Jarvis, P.G. The Adaptability to Light Intensity of Seedlings of Quercus petraea (Matt.) Liebl. J. Ecol. 1964, 52, 545–571. [Google Scholar] [CrossRef]
- Leuschner, C.; Ellenberg, H. Ecology of Central European Forests; Springer International Publishing: Cham, Switzerland, 2017; ISBN 978-3-319-43040-9. [Google Scholar]
- Kingsolver, J.G.; Huey, R.B. Size, Temperature, and Fitness: Three Rules. Evol. Ecol. Res. 2008, 10, 251–268. [Google Scholar]
- Bale, J.S.; Masters, G.J.; Hodkinson, I.D.; Awmack, C.; Bezemer, T.M.; Brown, V.K.; Butterfield, J.; Buse, A.; Coulson, J.C.; Farrar, J.; et al. Herbivory in Global Climate Change Research: Direct Effects of Rising Temperature on Insect Herbivores. Glob. Change Biol. 2002, 8, 1–16. [Google Scholar] [CrossRef]
- Altermatt, F. Climatic Warming Increases Voltinism in European Butterflies and Moths. Proc. R. Soc. B Biol. Sci. 2010, 277, 1281–1287. [Google Scholar] [CrossRef]
- Jaworski, T.; Hilszczański, J. The Effect of Temperature and Humidity Changes on Insects Development Their Impact on Forest Ecosystems in the Expected Climate Change. For. Res. Pap. 2013, 74, 345–355. [Google Scholar] [CrossRef]
- Rasmann, S.; Erwin, A.C.; Halitschke, R.; Agrawal, A.A. Direct and Indirect Root Defences of Milkweed (Asclepias syriaca): Trophic Cascades, Trade-Offs and Novel Methods for Studying Subterranean Herbivory. J. Ecol. 2011, 99, 16–25. [Google Scholar] [CrossRef]
- Yarnes, C.T.; Boecklen, W.J. Abiotic Mosaics Affect Seasonal Variation of Plant Resources and Influence the Performance and Mortality of a Leaf-Miner in Gambel’s Oak (Quercus gambelii, Nutt.). Ecol. Res. 2006, 21, 157–163. [Google Scholar] [CrossRef]
- Close, D.C.; McArthur, C. Rethinking the Role of Many Plant Phenolics: Protection from Photodamage Not Herbivores? Oikos 2002, 99, 166–172. [Google Scholar] [CrossRef]
- Barbehenn, R.V.; Constabel, C.P. Tannins in Plant–Herbivore Interactions. Phytochemistry 2011, 72, 1551–1565. [Google Scholar] [CrossRef]
- Barton, K.E.; Hanley, M.E. Seedling-Herbivore Interactions: Insights into Plant Defence and Regeneration Patterns. Ann. Bot. 2013, 112, 643–650. [Google Scholar] [CrossRef]
- Forkner, R.E.; Marquis, R.J.; Lill, J.T. Feeny Revisited: Condensed Tannins as Anti-Herbivore Defences in Leaf-Chewing Herbivore Communities of Quercus. Ecol. Entomol. 2004, 29, 174–187. [Google Scholar] [CrossRef]
- Ermolaev, I.V.; Zorin, D.A. Distribution of the Lime Leafminer Phyllonorycter issikii (Lepidoptera, Gracillariidae) in Natural Stands. Entomol. Rev. 2011, 91, 1088–1091. [Google Scholar] [CrossRef]
- Gripenberg, S.; Ovaskainen, O.; Elly, M.; Roslin, T. Spatial Population Structure of a Specialist Leaf-Mining Moth. J. Anim. Ecol. 2008, 77, 757–767. [Google Scholar] [CrossRef] [PubMed]
- Isbell, F.; Craven, D.; Connolly, J.; Loreau, M.; Schmid, B.; Beierkuhnlein, C.; Bezemer, T.M.; Bonin, C.; Bruelheide, H.; de Luca, E.; et al. Biodiversity Increases the Resistance of Ecosystem Productivity to Climate Extremes. Nature 2015, 526, 574–577. [Google Scholar] [CrossRef]
- Vasiliev, D. The Role of Biodiversity in Ecosystem Resilience. IOP Conf. Ser. Earth Environ. Sci. 2022, 1072, 012012. [Google Scholar] [CrossRef]
- Oliver, T.H.; Heard, M.S.; Isaac, N.J.B.; Roy, D.B.; Procter, D.; Eigenbrod, F.; Freckleton, R.; Hector, A.; Orme, C.D.L.; Petchey, O.L.; et al. Biodiversity and Resilience of Ecosystem Functions. Trends Ecol. Evol. 2015, 30, 673–684. [Google Scholar] [CrossRef] [PubMed]
- Goodale, E.; Lalbhai, P.; Goodale, U.M.; Ashton, P.M.S. The Relationship Between Shelterwood Cuts and Crown Thinnings and the Abundance and Distribution of Birds in a Southern New England Forest. For. Ecol. Manag. 2009, 258, 314–322. [Google Scholar] [CrossRef]
- Koivula, M.; Felton, A.; Jönsson, M.; Löfroth, T.; Schei, F.H.; Siitonen, J.; Sjögren, J. Biodiversity. In Continuous Cover Forestry in Boreal Nordic Countries; Springer: Cham, Switzerland, 2025; pp. 195–220. [Google Scholar]
- Pourmajidian, M.R.; Jalilvand, H.; Fallah, A.; Hosseini, S.A.; Parsakhoo, A.; Vosoughian, A.; Rahmani, A. Effect of Shelterwood Cutting Method on Forest Regeneration and Stand Structure in a Hyrcanian Forest Ecosystem. J. For. Res. 2010, 21, 265–272. [Google Scholar] [CrossRef]
- Munck, I.A.; Yamasaki, M.; Janelle, J. Silvicultural Treatments Improve Pest and Disease Conditions of White Pine (Pinus strobus) Residual Trees and Regeneration. Front. For. Glob. Change 2023, 6, 1239835. [Google Scholar] [CrossRef]
- Leidinger, J.; Blaschke, M.; Ehrhardt, M.; Fischer, A.; Gossner, M.M.; Jung, K.; Kienlein, S.; Kózak, J.; Michler, B.; Mosandl, R.; et al. Shifting Tree Species Composition Affects Biodiversity of Multiple Taxa in Central European Forests. For. Ecol. Manag. 2021, 498, 119552. [Google Scholar] [CrossRef]
- Hannerz, M.; Hånell, B. Effects on the Flora in Norway Spruce Forests Following Clearcutting and Shelterwood Cutting. Ecol. Manag. 1997, 90, 29–49. [Google Scholar] [CrossRef]
- Nasiri, M.; Parsakhoo, A. Shelterwood Cutting System for Forest Management. J. Appl. Biol. Sci. 2012, 6, 57–60. [Google Scholar]
- Uhl, B.; Schall, P.; Bässler, C. Achieving Structural Heterogeneity and High Multi-Taxon Biodiversity in Managed Forest Ecosystems: A European Review. Biodivers. Conserv. 2024, 1–32. [Google Scholar] [CrossRef]
- Chen, K.; Li, T.; Yang, M.; Zhou, X.; Peng, C. The Effects of Environmental Factors and Plant Diversity on Forest Carbon Sequestration Vary Between Eastern and Western Regions of China. J. Clean. Prod. 2024, 437, 140371. [Google Scholar] [CrossRef]
- Ali, A.; Lin, S.L.; He, J.K.; Kong, F.M.; Yu, J.H.; Jiang, H.S. Climate and Soils Determine Aboveground Biomass Indirectly via Species Diversity and Stand Structural Complexity in Tropical Forests. For. Ecol. Manag. 2019, 432, 823–831. [Google Scholar] [CrossRef]
- Chaudhary, A.; Burivalova, Z.; Koh, L.P.; Hellweg, S. Impact of Forest Management on Species Richness: Global Meta-Analysis and Economic Trade-Offs. Sci. Rep. 2016, 6, 23954. [Google Scholar] [CrossRef]
- Lapin, K.; Oettel, J.; Steiner, H.; Langmaier, M.; Sustic, D.; Starlinger, F.; Kindermann, G.; Frank, G. Invasive Alien Plant Species in Unmanaged Forest Reserves, Austria. NeoBiota 2019, 48, 71–96. [Google Scholar] [CrossRef]
- Bobiec, A.; Jaszcz, E.; Wojtunik, K. Oak (Quercus Robur L.) Regeneration as a Response to Natural Dynamics of Stands in European Hemiboreal Zone. Eur. J. For. Res. 2011, 130, 785–797. [Google Scholar] [CrossRef]
- Povak, N.A.; Lorimer, C.G.; Guries, R.P. Altering Successional Trends in Oak Forests: 19 Year Experimental Results of Low- and Moderate-Intensity Silvicultural Treatments. Can. J. For. Res. 2008, 38, 2880–2895. [Google Scholar] [CrossRef]
- Maleki, K.; Zeller, L.; Pretzsch, H. Oak Often Needs to Be Promoted in Mixed Beech-Oak Stands—The Structural Processes behind Competition and Silvicultural Management in Mixed Stands of European Beech and Sessile Oak. iForest 2020, 13, 80–88. [Google Scholar] [CrossRef]
- Mölder, A.; Sennhenn-Reulen, H.; Fischer, C.; Rumpf, H.; Schönfelder, E.; Stockmann, J.; Nagel, R.-V. Success Factors for High-Quality Oak Forest (Quercus robur, Q. petraea) Regeneration. For. Ecosyst. 2019, 6, 49. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).