Distribution of Starch in Trunkwood of Catalpa bungei ‘Jinsi’: A Revelation on the Metabolic Process of Energy Storage Substances
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Methods
2.3. Data Analysis
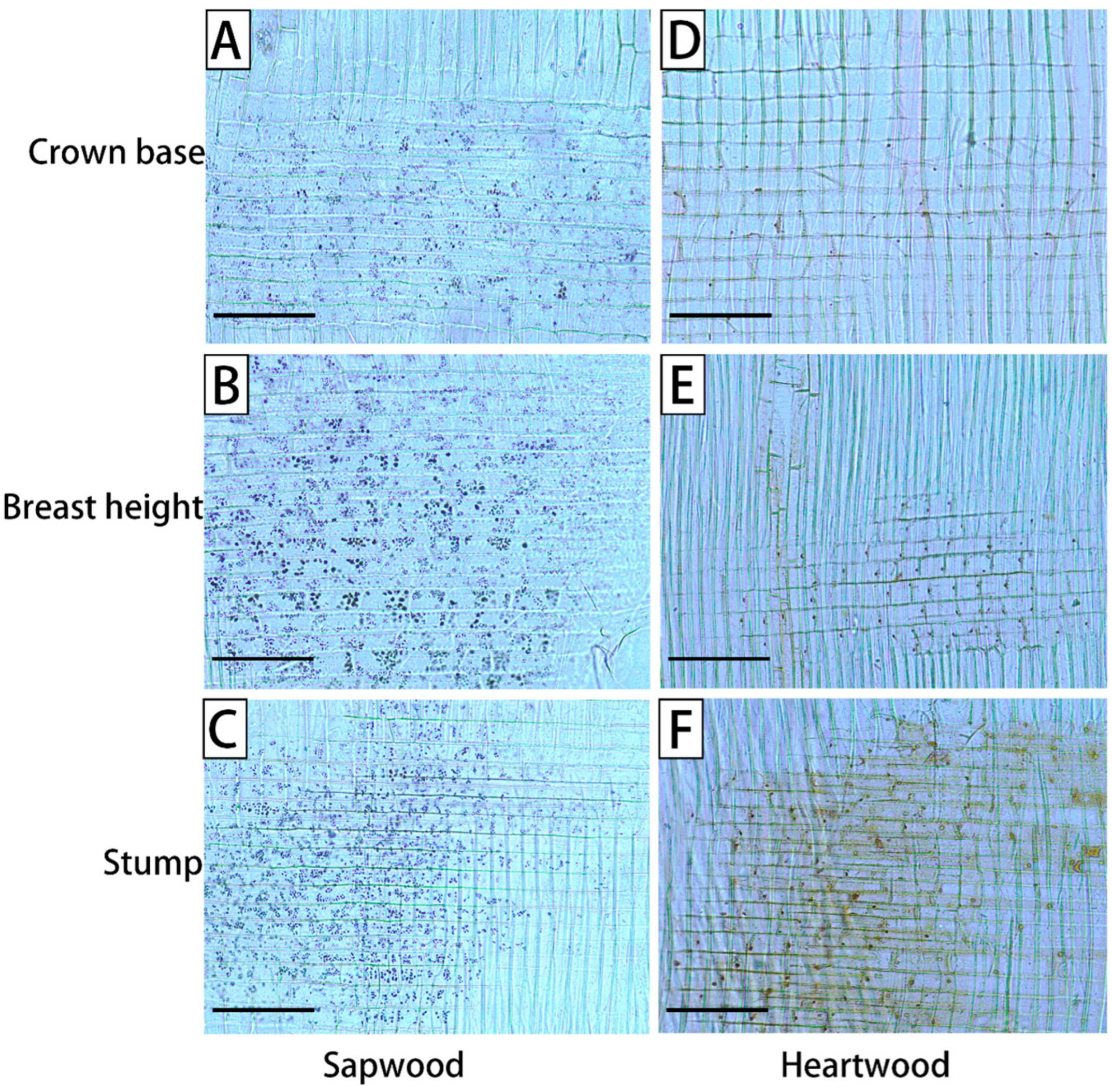
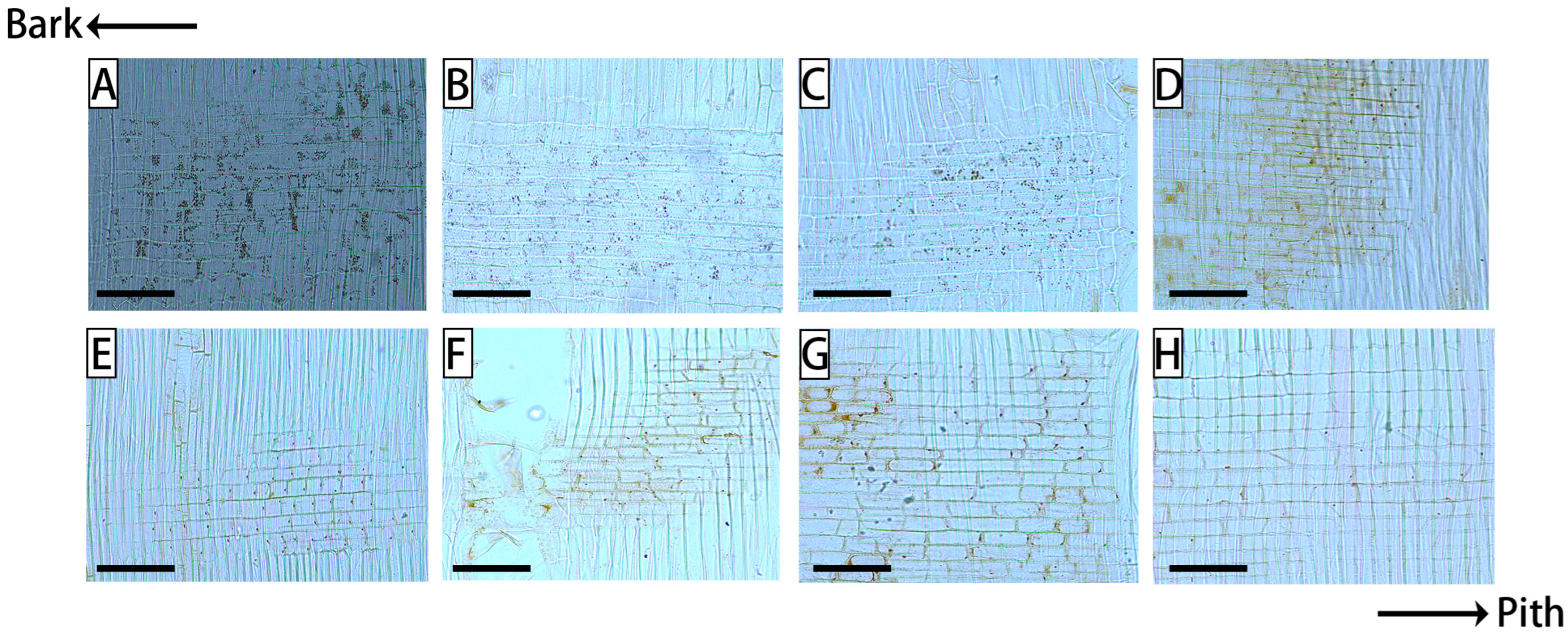
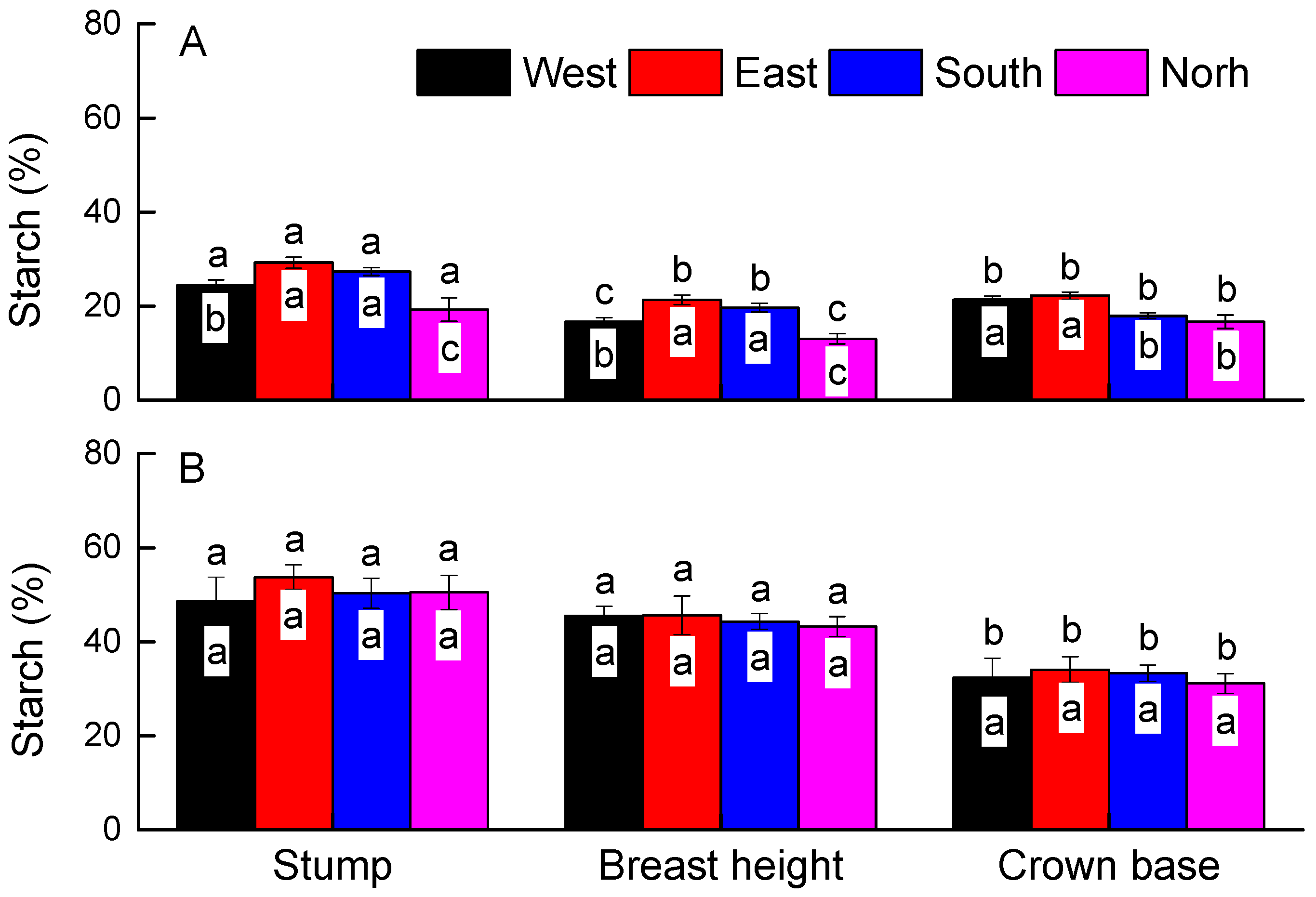
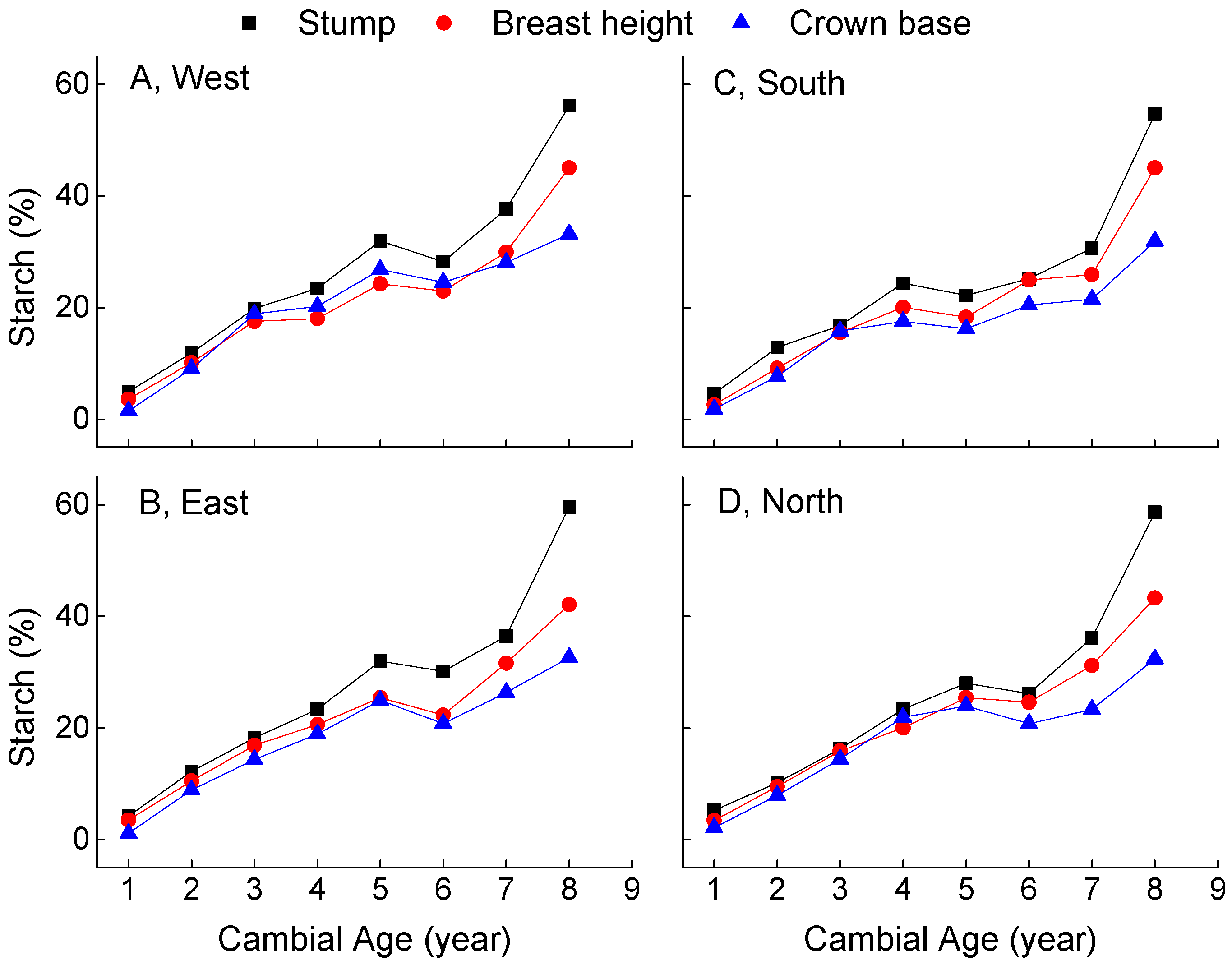
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Moretti, M.M.d.S.; Costa, M.S.; Bai, Y.; Gilbert, R.G.; Rocha, T.d.S. Chapter 9—Structure of starch, focusing on those from underground plant organs. In Starchy Crops Morphology, Extraction, Properties and Applications; Pascoli Cereda, M., François Vilpoux, O., Eds.; Academic Press: Cambridge, MA, USA, 2023; pp. 217–244. [Google Scholar] [CrossRef]
- Li, Z.; Wei, C. Morphology, structure, properties and applications of starch ghost: A review. Int. J. Biol. Macromol. 2020, 163, 2084–2096. [Google Scholar] [CrossRef]
- Herrera-Ramírez, D.; Hartmann, H.; Römermann, C.; Trumbore, S.; Muhr, J.; Santos, L.M.; Brando, P.; Silvério, D.; Huang, J.; Kuhlmann, I.; et al. Anatomical distribution of starch in the stemwood influences carbon dynamics and suggests storage-growth trade-offs in some tropical trees. J. Ecol. 2023, 111, 2532–2548. [Google Scholar] [CrossRef]
- Tanaka, Y.; Konno, N.; Suzuki, T.; Habu, N. Starch-degrading enzymes from the brown-rot fungus Fomitopsis palustris. Protein Expres. Purif. 2020, 170, 105609. [Google Scholar] [CrossRef] [PubMed]
- Cherelli, S.G.; Bellasio, C.; Marcati, C.R.; Rodrigues dos Santos, T.P.; Rodrigues, S.A.; Leonel, M.; Ballarin, A.W. The corewood of 25-year-old Hevea brasiliensis from two rubber plantations has high starch content. Eur. J. Wood Wood Prod. 2023, 81, 847–855. [Google Scholar] [CrossRef]
- Yamamoto, Y.; Yoshida, T.; Yanagidate, I.; Polnaya, F.; Siahaya, W.; Jong, F.; Pasolon, Y.; Miyazaki, A.; Hamanishi, T.; Hirao, K. Studies on growth characteristics and starch productivity of the sago palm (Metroxylon sagu Rottb.) folk varieties in Seram and Ambon Islands, Maluku, Indonesia. Trop. Agric. Dev. 2020, 64, 125–134. [Google Scholar] [CrossRef]
- Herrera-Ramírez, D.; Sierra, C.A.; Römermann, C.; Muhr, J.; Trumbore, S.; Silvério, D.; Brando, P.M.; Hartmann, H. Starch and lipid storage strategies in tropical trees relate to growth and mortality. N. Phytol. 2021, 230, 139–154. [Google Scholar] [CrossRef] [PubMed]
- Islam, M.A.; Begum, S.; Nakaba, S.; Funada, R. Distribution and pattern of availability of storage starch and cell death of ray parenchyma cells of a conifer tree (Larix kaempferi). Res. J. Recent Sci. 2012, 1, 28–37. [Google Scholar] [CrossRef]
- Dietze, M.C.; Sala, A.; Carbone, M.S.; Czimczik, C.I.; Mantooth, J.A.; Richardson, A.D.; Vargas, R. Nonstructural carbon in woody plants. Plant Biol. 2013, 65, 667–687. [Google Scholar] [CrossRef]
- Woodruff, D.; Meinzer, F. Water stress, shoot growth and storage of non-structural carbohydrates along a tree height gradient in a tall conifer. Plant Cell Environ. 2011, 34, 1920–1930. [Google Scholar] [CrossRef]
- Smith, M.G.; Miller, R.E.; Arndt, S.K.; Kasel, S.; Bennett, L.T. Whole-tree distribution and temporal variation of non-structural carbohydrates in broadleaf evergreen trees. Tree Physiol. 2018, 38, 570–581. [Google Scholar] [CrossRef]
- Cambou, A.; Thaler, P.; Clément-Vidal, A.; Barthès, B.; Charbonnier, F.; Van den Meersche, K.; María Elena, A.; Avelino, J.; Davrieux, F.; Labouisse, J.-P.; et al. Concurrent starch accumulation in stump and high fruit production in coffee (Coffea arabica). Tree Physiol. 2021, 41, 2308–2325. [Google Scholar] [CrossRef] [PubMed]
- Lijuan, Y.; Lingyu, M.; Xiaomei, J.; Yonggang, Z.; Yupei, W.; Yuan, C.; Lihong, Y.; Juan, G. Positional differences in the micro- and ultra-structural variations of ray parenchyma cells during the transformation from sapwood to heartwood. Front. Plant Sci. 2024, 15, 1431818. [Google Scholar] [CrossRef]
- Von Arx, G.; Arzac, A.; Fonti, P.; Frank, D.; Zweifel, R.; Rigling, A.; Galiano, L.; Gessler, A.; Olano, J.M. Responses of sapwood ray parenchyma and non-structural carbohydrates of Pinus sylvestris to drought and long-term irrigation. Funct. Ecol. 2017, 31, 1371–1382. [Google Scholar] [CrossRef]
- Ingel, B.; Reyes, C.; Massonnet, M.; Boudreau, B.; Sun, Y.; Sun, Q.; McElrone, A.; Cantu, D.; Roper, C. Xylella fastidiosa causes transcriptional shifts that precede tylose formation and starch depletion in xylem. Mol. Plant Pathol. 2020, 22, 175–188. [Google Scholar] [CrossRef]
- Pratt, R.B.; Tobin, M.; Jacobsen, A.; Traugh, C.; Guzman, M.; Hayes, C.; Toschi, H.; MacKinnon, E.; Percolla, M.; Clem, M.; et al. Starch storage capacity of sapwood is related to dehydration avoidance during drought. Am. J. Bot. 2020, 108, 91–101. [Google Scholar] [CrossRef] [PubMed]
- Ma, M.; Xu, Z.; Li, P.; Sui, Z.; Corke, H. Removal of starch granule-associated proteins affects amyloglucosidase hydrolysis of rice starch granules. Carbohyd. Polym. 2020, 247, 116674. [Google Scholar] [CrossRef]
- Sandmann, M.; Rading, M. Starch granules in algal cells play an inherent role to shape the popular SSC signal in flow cytometry. BMC Res. Notes 2024, 17, 327. [Google Scholar] [CrossRef] [PubMed]
- Cho, Y.G.; Kang, K.K. Functional analysis of starch metabolism in plants. Plants 2020, 9, 1152. [Google Scholar] [CrossRef]
- Furze, M.E.; Huggett, B.A.; Chamberlain, C.J.; Wieringa, M.M.; Aubrecht, D.M.; Carbone, M.S.; Walker, J.C.; Xu, X.; Czimczik, C.I.; Richardson, A.D. Seasonal fluctuation of nonstructural carbohydrates reveals the metabolic availability of stemwood reserves in temperate trees with contrasting wood anatomy. Tree Physiol. 2020, 40, 1355–1365. [Google Scholar] [CrossRef]
- Zhang, H.; Wang, C.; Wang, X. Spatial variations in non-structural carbohydrates in stems of twelve temperate tree species. Trees-Struct. Funct. 2014, 28, 77–89. [Google Scholar] [CrossRef]
- Guo, P.; Zhao, X.; Yang, Z.; Wang, Y.; Li, H.; Zhang, L. Water, starch, and nuclear behavior in ray parenchyma during heartwood formation of Catalpa bungei ‘Jinsi’. Heliyon 2024, 10, e27231. [Google Scholar] [CrossRef] [PubMed]
- Qiao, L.; Zhang, Q.; Li, J.; Guan, Z.; He, Q. Nutrient stoichiometry and tree development: Insights from a 5-year study on Catalpa bungei fertilization. Forests 2024, 15, 1836. [Google Scholar] [CrossRef]
- Pasolon, Y.B.; Hayati, N.; Rembon, F.S.; Baka, L.R.; Cahyono, E. Prediction of distributed material based on disk measurements: An application on predicting sago starch of a tree trunk. Proceedings of International Conference on Mathematical, Computational and Statistical Sciences, Dubai, United Arab Emirates, 22–24 February 2015; pp. 466–469. [Google Scholar]
- Reghu, C.P.; Patel, J.D. Distribution of starch and lipids in reaction wood of some angiosperm trees. Nord. J. Bot. 1984, 4, 357–363. [Google Scholar] [CrossRef]
- Qi, Y.; Li, Z.; Zhang, Y.; Zheng, Y.; Zhang, X.; Duan, X.; Yang, F.; Zhang, W. Application of area analysis in mineral content analysis under microscope. Adv. Geosci. 2021, 11, 1400–1406. [Google Scholar] [CrossRef]
- Karthäuser, J.; Treu, A.; Larnøy, E.; Militz, H.; Alfredsen, G. Resistance against fungal decay of Scots pine sapwood modified with phenol-formaldehyde resins with substitution of phenol by lignin pyrolysis products. Holzforschung 2024, 78, 231–243. [Google Scholar] [CrossRef]
- Yang, J.; Wang, S.; Huang, Z.; Guo, P. The complete chloroplast genome sequence of Catalpa bungei (Bignoniaceae): A high-quality timber species from China. Mitochondrial DNA B 2020, 5, 3854–3855. [Google Scholar] [CrossRef] [PubMed]
- You, J.; Jing, Z.; Tai, D.; He, W.; Zhang, H.; Hse, C.; Lee, d.; Samimoto, M. Studies on active antifungal substrates in natural durable species: III. Extraction, separation, purification and toxicity experiment of effective antifungal of heartwood of Catalpa bungei. J. Nanjing For. Univ. (Nat. Sci. Ed.) 1990, 14, 61–67. [Google Scholar]
- Simpson, L.; Barton, A. Time dependence of starch levels in the sapwood of Eucalyptus diversicolor (Karri) as: Standing trees, stored saw-logs, ringbarked trees and trees felled without lopping. Holzforschung 1991, 45, 253–257. [Google Scholar] [CrossRef]
- Galibina, N.; Nikerova, K.; Moshchenskaya, Y.L.; Ershova, M. Physiological, biochemical and molecular genetic aspects of heartwood formation mechanisms. Trans. KarRC RAS 2020, 11, 20–37. [Google Scholar] [CrossRef]
- Yang, X.; Yu, X.; Liu, Y.; Shi, Z.; Li, L.; Xie, S.; Zhu, G.; Zhao, P. Comparative metabolomics analysis reveals the color variation between heartwood and sapwood of Chinese fir (Cunninghamia lanceolata (Lamb.) Hook. Ind. Crop. Prod. 2021, 169, 113656. [Google Scholar] [CrossRef]
- Zhang, H.; Wang, C.; Wang, X.; Cheng, F. Spatial variation of non-structural carbohydrates in Betula platyphylla and Tilia amurensis stems. Chin. J. Appl. Ecol. 2013, 24, 3050–3056. [Google Scholar]
- Piispanen, R.; Saranpää, P. Variation of non-structural carbohydrates in silver birch (Betula pendula Roth) wood. Trees-Struct. Funct. 2001, 15, 444–451. [Google Scholar] [CrossRef]
- Cui, Z.; Hu, H.; Li, X.; Liu, X.; Zhang, Q.; Hong, Z.; Zhang, N.; Lin, W.; Xu, D. Physiological and biochemical mechanisms of drought regulating the size and color of heartwood in Dalbergia odorifera. Tree Physiol. 2025, 45, tpae157. [Google Scholar] [CrossRef] [PubMed]
- Dong, H.-J.; Wang, X.-C.; Yuan, D.-Y.; Liu, D.; Liu, Y.-L.; Sang, Y.; Wang, X.-C. Radial distribution differences of non-structural carbohydrates in stems of tree species of different wood in a temperate forest. Chin. J. Plant Ecol. 2022, 46, 722–734. [Google Scholar] [CrossRef]
- Millard, P.; Sommerkorn, M.; Grelet, G.A.J.T.N.p. Environmental change and carbon limitation in trees: A biochemical, ecophysiological and ecosystem appraisal. New Phytol. 2007, 175, 11–28. [Google Scholar] [CrossRef]
- Gérard, B.; Bréda, N. Radial distribution of carbohydrate reserves in the trunk of declining European beech trees (Fagus sylvatica L.). Ann. Forest Sci. 2014, 71, 675–682. [Google Scholar] [CrossRef]
- Michelot-Antalik, A.; Granda, E.; Fresneau, C.; Damesin, C. Evidence of a seasonal trade-off between growth and starch storage in declining beeches: Assessment through stem radial increment, non-structural carbohydrates and intra-ring δ13C. Tree Physiol. 2019, 39, 831–844. [Google Scholar] [CrossRef]
- Richardson, A.; Carbone, M.; Czimczik, C.; Hollinger, D.; Murakami, P.; Schaberg, P.; Xu, X. Seasonal dynamics and age of stemwood nonstructural carbohydrates in temperate forest trees. New Phytol. 2012, 197, 850–861. [Google Scholar] [CrossRef]
- Deng, L.; Ren, S.; Lv, J.; Wang, Y.; Ren, H.; Zhao, R. Growth characteristics and variation of heartwood and sapwood of Catalpa bungei. China Wood Ind. 2019, 33, 9–13. [Google Scholar] [CrossRef]
- Burtin, P.; Jay-Allemand, C.; Charpentier, J.-P.; Janin, G. Natural wood colouring process in Juglans sp. (J. nigra, J. regia and hybrid J. nigra 23 × J. regia) depends on native phenolic compounds accumulated in the transition zone between sapwood and heartwood. Trees-Struct. Funct. 1998, 12, 258–264. [Google Scholar] [CrossRef]
- Magel, E.A. Biochemistry and physiology of heartwood formation. Exptl. Biol. Rev. 2000, 363–376. [Google Scholar]
- Marler, T. Axial and radial spatial patterns of non-structural carbohydrates in Cycas micronesica stems. Plants 2018, 7, 49. [Google Scholar] [CrossRef] [PubMed]
- Marcin, N.; Pazdrowski, W.; Szymański, M. Dynamics of heartwood formation and axial and radial distribution of sapwood and heartwood in stems of European larch (Larix decidua Mill.). J. For. Sci. 2008, 54, 409–417. [Google Scholar] [CrossRef]
- Goodwin, P.; Campbell, L. Plant structure and function. In The Scientific Basis of Modern Agriculture, 2nd ed.; Campbell, K.O., Bowyer, J.W., Eds.; Oxford University Press: Oxford, UK, 1992; pp. 111–130. [Google Scholar]
- Fu, Z.; Cui, Z. Effects of environmental factors and management measures on heartwood formation. World For. Res. 2024, 37, 43–48. [Google Scholar]
- Li, G.; Zhang, M.; Chen, F.; Huang, C.; Dong, X.; Chen, L.; Lin, L. A review on artificially induced heartwood formation and its chemical composition in Dalbergia odorifera. Mol. Plant Breed. 2024, 1–17. [Google Scholar]
| Tree Number | Tree Diameter at Breast Height (cm) | Tree Height (m) | Crown Base Height (m) | Crown Width (m) |
|---|---|---|---|---|
| 1 | 18.6 | 13.1 | 3.8 | 4.6 |
| 2 | 17.8 | 14.6 | 3.6 | 4.2 |
| 3 | 18.4 | 13.4 | 3.3 | 4.8 |
| Position | Heartwood Diameter (cm) | Sapwood Width (mm) |
|---|---|---|
| Stump | 14.1 ± 2.7 | 10 ± 2.6 |
| Breast height | 10.0 ± 1.8 | 10.7 ± 5.6 |
| Crown base | 10.0 ± 1.4 | 9.0 ± 2.8 |
| Factor | Degrees of Freedom | F Value | p Value |
|---|---|---|---|
| Height position | 2 | 91.097 | <0.01 |
| Direction | 3 | 5.147 | 0.02 |
| Heartwood/sapwood | 1 | 624.966 | <0.01 |
| Height position × Direction | 2 | 25.388 | 0.100 |
| Height position × Heartwood/sapwood | 2 | 25.388 | <0.01 |
| Direction × Heartwood/sapwood | 3 | 1.087 | 0.354 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhao, X.; Liu, F.; Guo, P.; Feng, Q.; Wang, D.; Hao, Z. Distribution of Starch in Trunkwood of Catalpa bungei ‘Jinsi’: A Revelation on the Metabolic Process of Energy Storage Substances. Forests 2025, 16, 242. https://doi.org/10.3390/f16020242
Zhao X, Liu F, Guo P, Feng Q, Wang D, Hao Z. Distribution of Starch in Trunkwood of Catalpa bungei ‘Jinsi’: A Revelation on the Metabolic Process of Energy Storage Substances. Forests. 2025; 16(2):242. https://doi.org/10.3390/f16020242
Chicago/Turabian StyleZhao, Xiping, Fei Liu, Pingping Guo, Qi Feng, Dongfang Wang, and Ziyuan Hao. 2025. "Distribution of Starch in Trunkwood of Catalpa bungei ‘Jinsi’: A Revelation on the Metabolic Process of Energy Storage Substances" Forests 16, no. 2: 242. https://doi.org/10.3390/f16020242
APA StyleZhao, X., Liu, F., Guo, P., Feng, Q., Wang, D., & Hao, Z. (2025). Distribution of Starch in Trunkwood of Catalpa bungei ‘Jinsi’: A Revelation on the Metabolic Process of Energy Storage Substances. Forests, 16(2), 242. https://doi.org/10.3390/f16020242





