Abstract
Picea omorika (Pančić) Purk., (Serbian spruce) is a relic, endemic, and vulnerable conifer that remains insufficiently studied to date. To the best of our knowledge, this is the first report on the morpho-anatomical and phytochemical diversity of needles from three populations in Bosnia and Herzegovina. The length of two-year-old needles was measured with a digital caliper. The next six properties were measured based on cross-sections of the needles using a light microscope. An analysis of volatile compounds was carried out using gas chromatography coupled with mass spectrometry (GC-MS) and flame ionization detection (GC-FID). The highest values of needle traits were found in the Viogor population, with the lowest in the Tisovljak population, which was statistically confirmed. There was also a significant difference between needles from Bosnia and Herzegovina and those from Serbia. Bornyl acetate, camphene, limonene, and α-pinene were identified as the major terpene compounds. Multivariate analyses also suggested a tendency toward the separation of the Tisovljak population. A statistical comparison of three Bosnian and Herzegovinian and four Serbian populations (previously studied and published) revealed two distinct groups: (1) three Bosnian populations and the Vranjak population from Serbia, and (2) three populations from Serbia—Štula, Zmajevački Potok, and Mileševka Canyon. The general conclusions are that divergence in needle morpho-anatomy aligns with divergence in needle chemistry and that Bosnian and Herzegovinian populations are distinct from nearly all Serbian populations.
1. Introduction
The Serbian spruce, Picea omorika (Pančić) Purk., is a Balkan endemit and Tertiary relict [1]. Its ancestors, P. palaeomorika and P. omorikoides, inhabited large areas of Northern Europe and Asia. The present-day area of P. omorika is Bosnia and Herzegovina and Serbia, mostly around the middle and lower courses of the river Drina. Several varieties and horticultural forms of this species exist, and among them, var. omorika, with short branches and a columnar shape, is the most famous. According to Mataruga and Milanović [2], populations of P. omorika in Bosnia and Herzegovina were found in 26 small populations and three isolated trees, while 11 populations, reported by Fukarek [3], were missed.
Morpho-anatomical investigations, along with terpene analyses, are crucial for studying species population variability, relatedness, and diversity. In the genus Picea, extensive information is available on the needle shape [1], other morphological [4,5,6] traits, anatomical properties [6,7,8], and terpene composition [9,10,11,12]. A study of several Picea species and the differences between their terpene compounds has also been reported [13]. The relationships among some Picea species have already been confirmed through hybridization [14], phylogenetic [15], and evolutionary studies [16].
Terpene composition analyses are significant because they can quickly and cost-effectively confirm the population divergence obtained through morpho-anatomical analyses. For example, a study of the terpene composition of Pinus nigra populations on the Balkan Peninsula confirmed the phenotypic divergence of these populations, as previously obtained through morpho-anatomical studies [17].
To date, the morpho-anatomy and terpene variability of P. omorika needles have been investigated in several populations in Serbia [18,19,20]. However, to our knowledge, this is the first comparative study of the morpho-anatomical properties and terpene composition at the population level in Bosnia and Herzegovina.
2. Materials and Methods
2.1. Plant Material
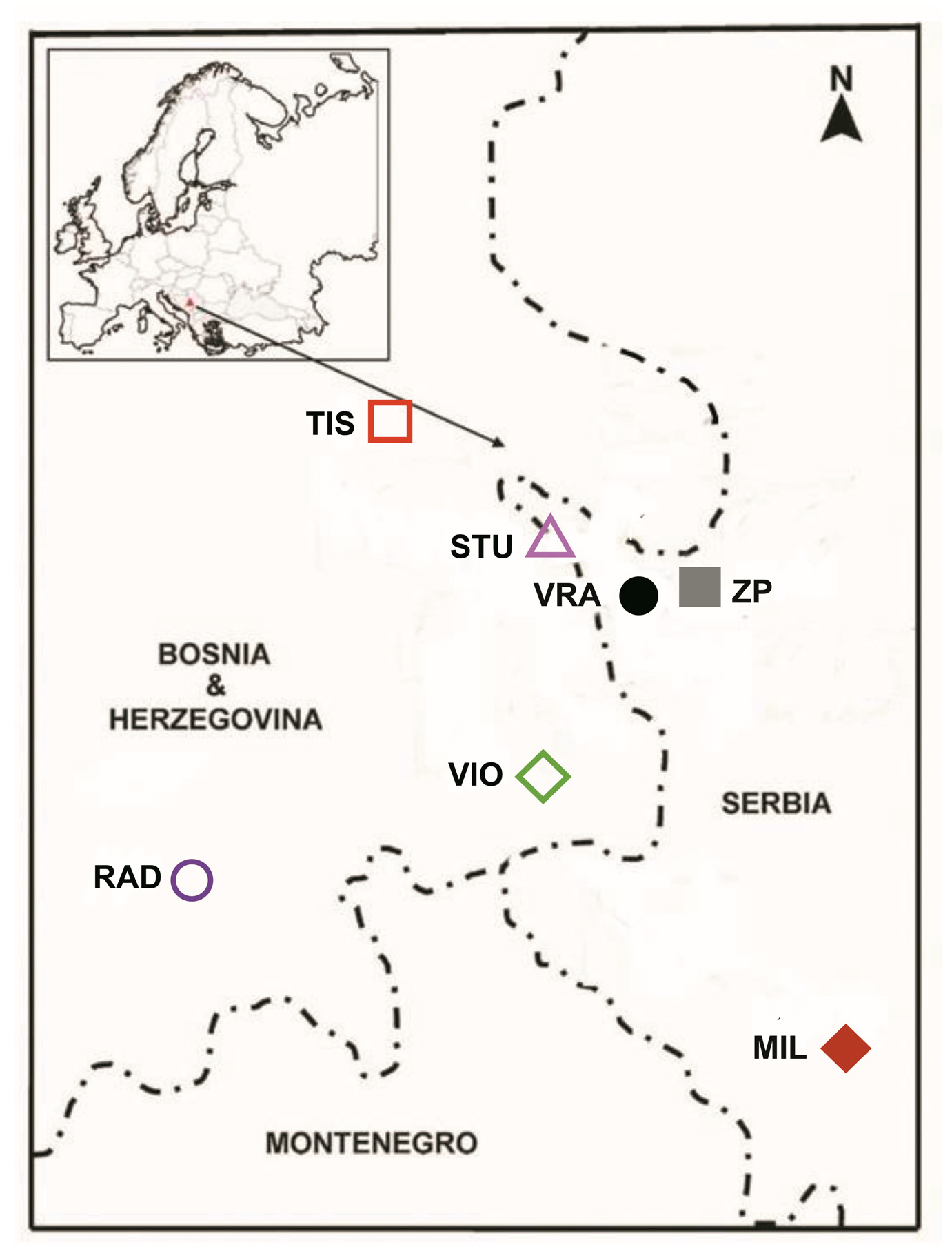
Twigs with two-year-old needles of P. omorika (Pančić) Purk., from the lowest third of adult trees were sampled in the last fall. The origins of twigs were three Bosnian and Herzegovinian populations: Tisovljak (TIS), Viogor (VIO), and Radomišlje (RAD). Ten needles from fifteen trees were sampled at every locality (ca. 450 samples). The locations of these populations could be seen on a map (Figure 1). The main characteristics of localities and the technique of twig transportation were published in a previous article, where n-alkanes were analyzed [21]. Twigs with needles were deep-frozen (−20 °C) until analyses.
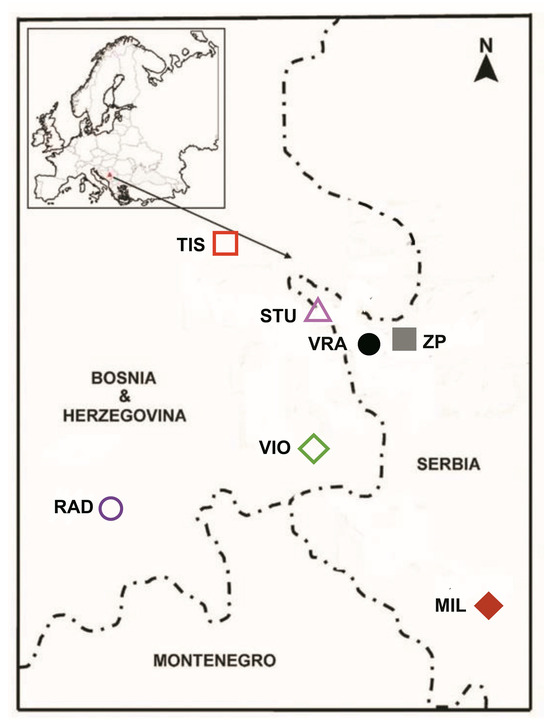
Figure 1.
Locations of populations of Picea omorika (Pančić) Purk., from Bosnia and Herzegovina (TIS, VIO, RAD), as well as from Serbia (STU, VRA, ZP, MIL).
2.2. Morpho-Anatomical Measurements of Needles
Two-year-old needles were cut with a razor blade on the central part of the needles [22]. The needle length was measured with a digital caliper. The other characteristics (needle height, needle width, cuticle + epidermis width, hypodermis width, number of resin ducts, and resin duct diameter) were measured based on cross sections of needles using a Leica Gallen III light microscope equipped with a CCD Camera model Topica TP/5001. Measurements were made using the software Toup View version 3.7.
2.3. Isolation of Volatile Compounds
Volatile compounds were isolated simultaneously with hydrodistillation and extraction from 3–5 g of Serbian spruce needles using 5 mL of dichloromethane in a Likens–Nickerson apparatus for 2 h [23]. The obtained DCM extract was further analyzed on a GC-FID/MS instrument.
2.4. GC-FID-MS Analysis
The analysis of volatile compounds was carried out using gas chromatography coupled with mass spectrometry (GC-MS) and flame ionization detection (GC-FID) on an Agilent 7890A system equipped with an inert 5975C XL EI/CI mass spectrometer. A semi-polar HP-5MS capillary column (30 m × 0.25 mm, film thickness 0.25 μm) was used for compound separation. Helium served as the carrier gas under constant pressure conditions (16.255 psi). The oven temperature program ranged from 60 °C to 300 °C, increasing at a rate of 3 °C per minute, with a final hold of 10 min. Sample injection (1 μL) was performed automatically (Agilent 7683B Series Injector, Agilent Technologies, Santa Clara, CA, USA) in split mode (10:1), with the injector temperature set at 300 °C and the detector at 300 °C. Mass spectra were acquired in electron ionization (EI) mode over a scan range of 40–600 m/z, with a source temperature of 230 °C, a quadrupole temperature of 150 °C, and a solvent delay of 3 min.
The identification of components was based on a comparison of mass spectra and retention indices (RIs), calculated relative to a series of n-alkanes, C8–C32. The identification process involved matching experimental spectra with commercial libraries (Wiley 7, NIST 17, and retention-time-locked Adams 4) using an Automated Mass Spectral Deconvolution and Identification System (AMDIS 32 v2.73) and NIST search software (v2.3). The relative percentages of the detected compounds were determined from the GC-FID chromatograms.
2.5. Statistical Analyses
Calculations of mean values (X) and standard deviations (SD) of the populations, one-way analyses of variance (ANOVAs), principal-component analysis (PCA), discriminant analysis (CDA), and cluster analysis (UPGA) were carried out using Statgraphics Plus software (version 5.0; Statistical Graphics Corporation, Warrenton, VA, USA) and STATISTICA 8 software (Statsoft, Inc., Tulsa, OK, USA).
3. Results
3.1. Morpho-Anatomical Characteristics of Needles and Population Variability
The results of descriptive statistics, ANOVA, and the LSD test are presented in Table 1. The lowest mean needle length was found in population TIS, with the highest in population VIO. This population also had the highest mean values of needle width, needle height (thickness), and number of resin ducts. The hypodermis width and resin duct diameter did not vary among populations. (ns). Strong differences among populations (***) were found for almost all properties. The smallest differences were found for the hypodermis width (*) (Table 1).

Table 1.
Morpho-anatomical characteristics of the studied P. omorika (Pančić) Purk., needles from Bosnia and Herzegovina: descriptive statistics, results of ANOVA, and LSD test.
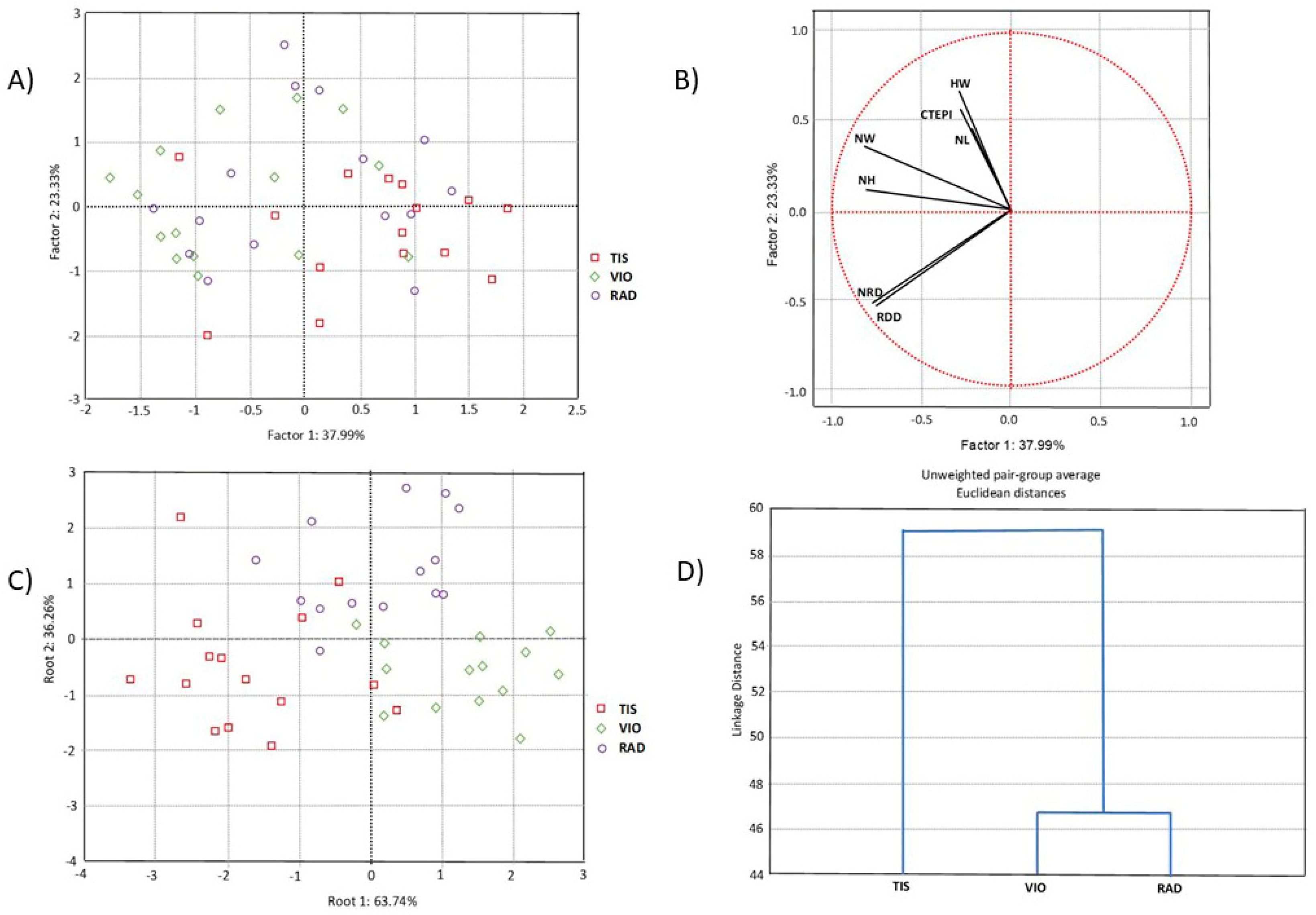
Multivariate statistical analyses were performed on all (seven) needle properties. PCA was performed to determine the overall morpho-anatomical variation of 45 individuals from the three studied populations of P. omorika from Bosnia and Herzegovina. The first two principal component axes represented 61.32% of the total variation, of which the first axis accounted for 37.99% (Figure 2A). However, the scatter plot in the projection of the first two axes revealed an overlap of all populations. CDA was performed to check the hypothesis that the analyzed sample was composed of discrete groups that are morpho-anatomically differentiated from each other. The CDA based on three populations of P. omorika from Bosnia and Herzegovina showed that the first two discriminant functions participated in 100.0% of the total discrimination, of which the first function was represented by 63.74% (Table 2, Figure 2C). The first function was mainly determined by the characteristics NL, NW, NRD, and RDD, while NH, NW, CTEPI, and HW considerably affected the second function (Table 3). The scatter plot obtained through CDA suggested the differentiation of the TIS population in relation to the remaining two analyzed populations (VIO and RAD; Figure 2C). CA separated the TIS population from the VIO and RAD populations (Figure 2D), in agreement with the CDA.
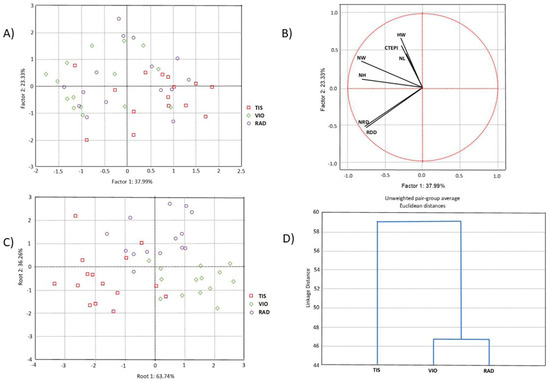
Figure 2.
Multivariate statistical analyses based on the needle morpho-anatomical properties of 45 individuals from three populations of P. omorika from Bosnia and Herzegovina: (A) PCA analysis; (B) projection of seven needle properties; (C) CDA analysis; (D) UPGA cluster analysis.

Table 2.
Standardized coefficients for the first two canonical axes (Cas) of variation in seven needle morpho-anatomical properties from two discriminant functional analyses. Significant coefficients are in boldface.

Table 3.
Standardized coefficients for the first six canonical axes (Cas) of variation in seven needle morpho-anatomical properties from two discriminant functional analyses. Significant coefficients are in boldface.
Furthermore, we compared the obtained results with previously published data of P. omorika needles from Serbian populations [20]. Bosnian populations have lower mean values of NL and CTEPI, but higher mean values of NW, NH, HW, and RDD, while NRD values are approximately equal.
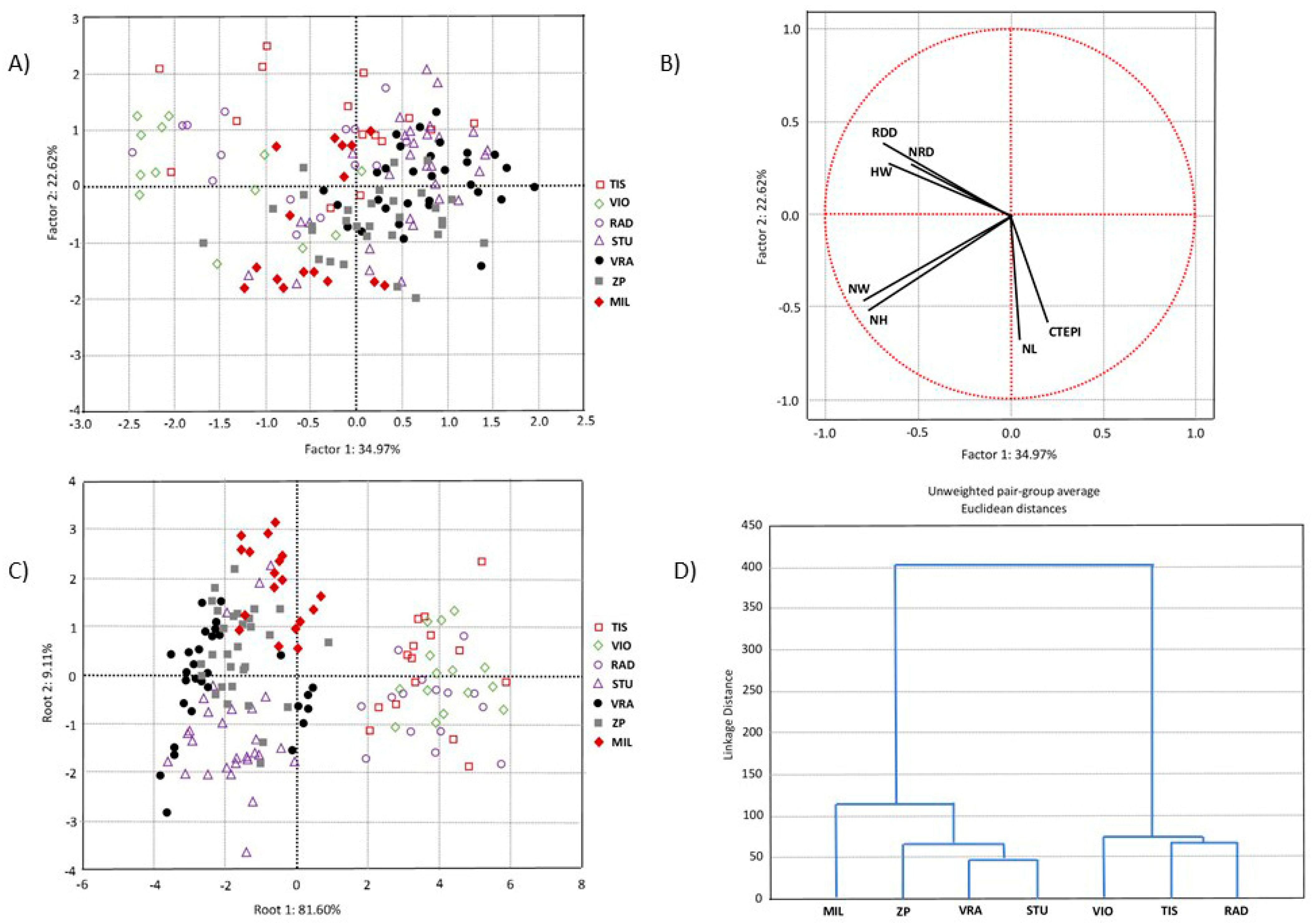
PCA was performed to determine the overall morpho-anatomical variation of 153 individuals from three populations of P. omorika from Bosnia and Herzegovina and four populations from Serbia. The first two principal component axes explained 57.60% of the total variation, of which the first axis accounted for 34.97% (Figure 3A). The scatter plot in the projection of the first two axes suggested a slight tendency for separation between the populations from Bosnia and Herzegovina on the one hand and the populations from Serbia on the other. However, this separation was not sharp, since a considerable number of individuals from the Serbian and Bosnian populations overlapped. The CDA based on three populations of P. omorika from Bosnia and Herzegovina and four populations from Serbia showed that the first two discriminant functions participated in 90.71% of the total discrimination, of which the first function was represented by 81.60% (Table 3, Figure 3C). Only one characteristic, i.e., HW, had a significant impact on the first function, while the second function was mainly determined by the characteristic NH (Table 3). The scatter plot obtained through CDA indicated a clear differentiation between the populations from Bosnia and Herzegovina on the one hand and the populations from Serbia on the other (Figure 3C). CA clearly separated Bosnian and Serbian populations (Figure 3D), in agreement with the CDA.
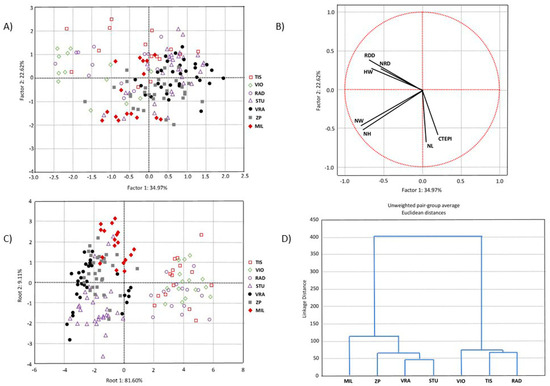
Figure 3.
Multivariate statistical analyses based on seven needle morpho-anatomical properties of 153 individuals from three populations of Picea omorika from Bosnia and Herzegovina and four populations from Serbia: (A) PCA analysis; (B) projection of seven needle properties; (C) CDA analysis; (D) UPGA cluster analysis.
The presented results showed that there is significant diversity between P. omorika needles from Bosnia and Herzegovina and Serbia. Bosnian populations had a higher mean value in all examined needle properties (except for needle length and number of resin ducts). As it was suggested earlier [20], population Štula (STU), positioned on the border between these two states, showed great similarity with Serbian populations.
3.2. Terpene Composition of Needles and Population Variability
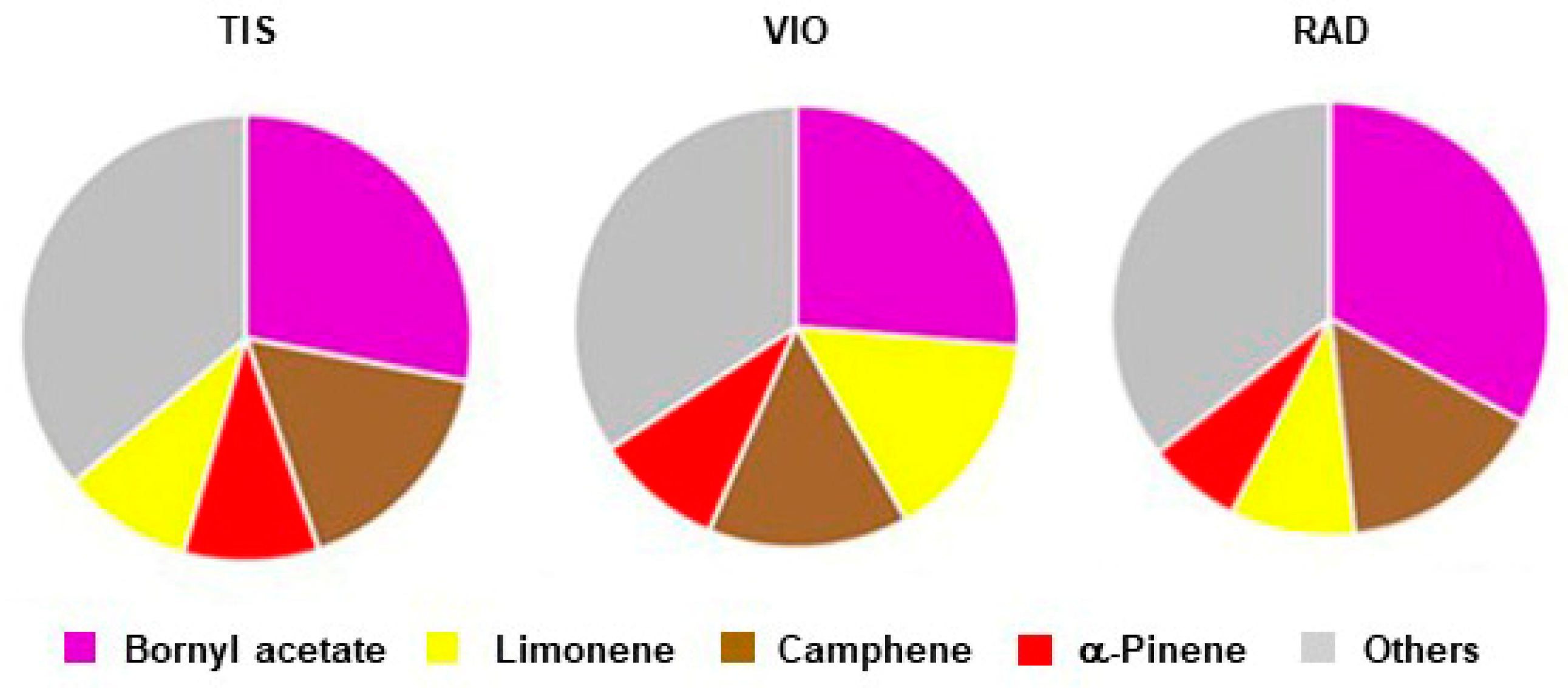
The terpene composition of three populations of P. omorika from Bosnia and Herzegovina is given in Table 4. Out of 128 compounds, 112 were identified. In the overall terpene profile, monoterpenes were the dominate terpene classes, comprising 89.1% of the essential oil. One oxygenated monoterpene, bornyl acetate (56), and three monoterpene hydrocarbons, camphene (7), limonene (18), and α-pinene (6), were the most abundant, with average contents of 29.3%, 15.6%, 11.4%, and 8.7%, respectively. Together, they comprised 64.7% of the total terpene extract. The average profile of major terpene compounds was as follows: bornyl acetate >> camphene > limonene > α-pinene (symbols denote differences according to Petrakis et al. [24]). Population TIS had the most abundant camphene and α-pinene (Table 4, Figure 4), while population VIO had the most abundant limonene. Population RAD had the most abundant bornyl acetate and the less abundant α-pinene (Figure 4). In addition to α-pinene, 21 compounds also had medium-to high amounts (0.5–10%) [24]: hexanal (1), trans-hex-2-enal (2), santene (4), tricyclene (5), β-pinene (10), myrcene (11), α-phellandrene (14), terpinolene (22), n.i. 1 (23), n-nonanal (27), camphene hydrate (34), borneol (37), citronellol (48), geraniol (52), geranyl acetate (67), δ-cadinene (84), τ-cadinol (95), α-cadinol (97), octadec-9-enal (111), phytol (117), and nonacosan-10-ol (128).

Table 4.
Terpene compositions of three Bosnian and Herzegovinian populations of P. omorika (in %).
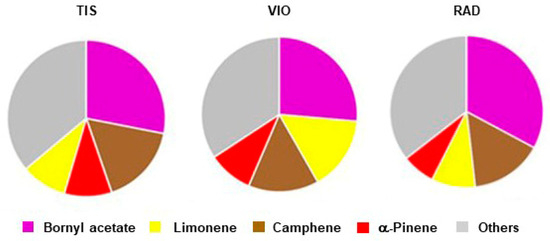
Figure 4.
Main terpenes in three populations of P. omorika from Bosnia and Herzegovina (TIS, VIO, RAD).
Out of 112 detected compounds, seven distributions that correspond to the normal (χ2, p ≥ 0.05) were selected for multivariate statistical analyses: tricyclene, α-pinene, camphene, β-pinene, bornyl acetate, δ-cadinene, and τ-cadinol.
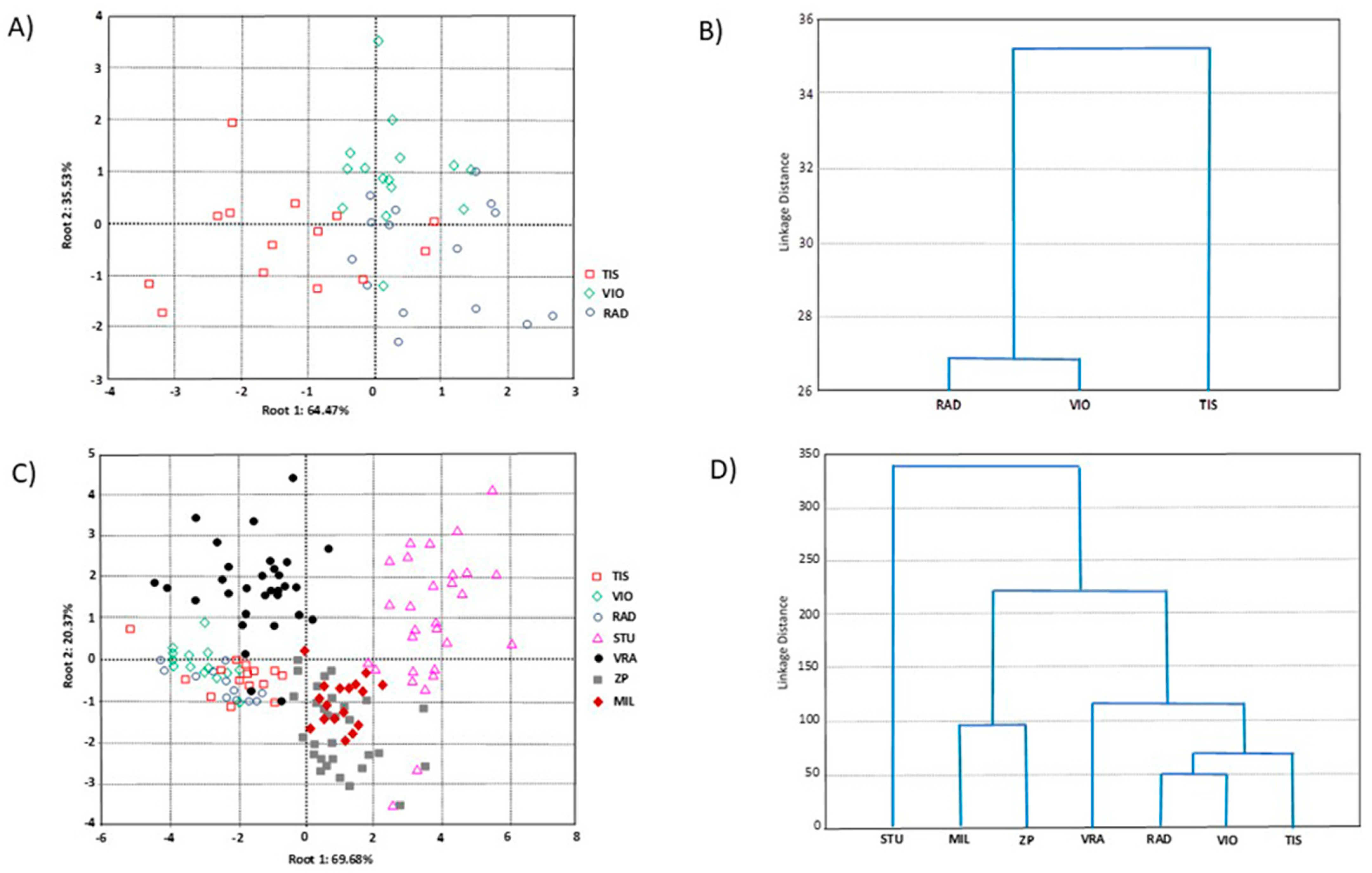
The principal component analysis (PCA) based on three populations of P. omorika from Bosnia and Herzegovina revealed overlap among all populations, so it was not presented. On the other hand, the canonical discriminant analysis (CDA) showed that the first two axes accounted for 100.00% of the total discrimination, with the first axis (CA1) contributing 64.47% (Table 5). Four compounds, i.e., τ-cadinol, δ-cadinene, tricyclene, and bornyl acetate, had a significant impact on CA1, while τ-cadinol and bornyl acetate also significantly influenced CA2 (Table 5). The scatter plot obtained from this CDA (Figure 5A) suggested a tendency for the separation of the TIS population (whose individuals predominantly showed negative values for CA1) from the VIO and RAD populations (whose individuals mainly showed positive values for CA1). The agglomerative hierarchical clustering (AHC) clearly separated the TIS population from the VIO and RAD populations (Figure 5B), in agreement with the CDA results.

Table 5.
Standardized coefficients for the first two canonical axes (Cas) of variation for seven terpene compounds from two discriminant functional analyses. Significant coefficients are in boldface.
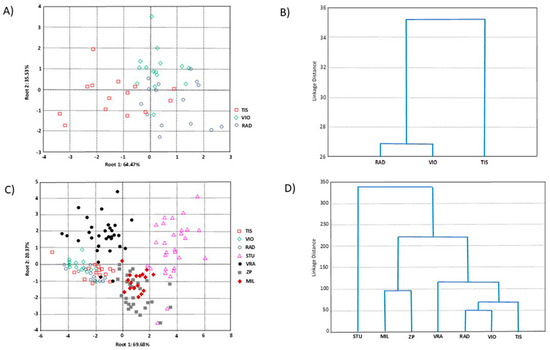
Figure 5.
Multivariate statistical analyses based on seven terpene compounds of 45 individuals from three populations of P. omorika from Bosnia and Herzegovina: (A) CDA analysis; (B) UPGA analysis, as well as on 153 individuals from seven populations of P. omorika from Bosnia and Herzegovina and Serbia; (C) CDA analysis; (D) UPGA cluster analysis.
The PCA, based on three populations of P. omorika from Bosnia and Herzegovina and four previously investigated populations from Serbia [19], again revealed overlap among all populations, so it was not presented. However, the CDA showed that the first two axes accounted for 90.04% of the total discrimination, with the first axis contributing 69.68% (Table 6). Five compounds, i.e., α-pinene, bornyl acetate, τ-cadinol, tricyclene, and δ-cadinene, had a significant impact on CA1, while δ-cadinene and bornyl acetate also significantly influenced CA2 (Table 6). The scatter plot (Figure 5C) suggested a tendency for the formation of two distinct population groups. Specifically, two groups of populations were separated along the CA1, which explained the highest percentage of discrimination. The first group consisted of individuals belonging to populations from Bosnia and Herzegovina (TIS, RAD, and VIO) and Serbia (VRA), showing negative values for CA1, while the second group, with positive values for CA1, included individuals from the remaining Serbian populations (STU, ZP, and MIL). Within the first group, some separation of the Serbian population (VRA) from the Bosnian populations (TIS, RAD, and VIO) was evident, but this trend along the CA2 was weaker than the main trends already described in the CDA.

Table 6.
Standardized coefficients for the first five canonical axes (Cas) of variation for seven terpene compounds from two discriminant functional analyses. Significant coefficients are in boldface.
Similarly, within the second group, which included only Serbian populations, some separation of the STU population from the ZP and MIL populations along CA2 was evident. The AHC results largely confirmed the CDA findings (Figure 5D) but separated the Serbian population into three clusters: (1) STU; (2) ZP and MIL; and (3) VRA along with the Bosnian populations.
4. Discussion
4.1. Morpho-Anatomy of Serbian Spruce Needles
Bosnian and Herzegovinian populations of P. omorika have a lower mean value of needle length (NL, 13.3 mm) in comparison with Serbian ones (13.6 mm) [20], but higher in comparison with the result of Mileševka Canyon (9.9 mm) [25]. Bosnian P. omorika needles are still shorter than those of Picea sitchensis [4]. In the case of needle width (NW), Bosnian-population needles have a higher mean value (1.58 mm) than needles from Serbia (1.49 mm) [20] and artificial populations (as well as needle height (thickness), NH (0.88 and 0.80 mm, respectively). In some artificial sites [26,27], needles were longer (16.4 mm and 14.0 mm, respectively) and shorter (1.49 mm and 1.4 mm, resp.), as well as thinner [28,29] (0.94 mm and 0.90 mm, resp.). P. glechnii [8] has smaller needles than P. omorika from Bosnia and Herzegovina and Serbia. Serbian populations have higher mean values than the Bosnian population of CT + EPI (22.8 µm and 18.9 µm, resp.), but lower for HW (17.5 µm and 26.8 µm, resp.). The CT + EPI mean values of the presented results are higher than at Mt. Tara [30] and one of the artificial sites [29]. In the case of NRD, both man values are the same (0.74 and 0.80, resp.). The third resin duct was first reported in 1995 [28] in a very polluted area. Variability in NRD could also be explained by discontinuous (intermittent) resin ducts, which are well known in some North American spruces (P. glauca, P. engelmanni, P. mexicana, P. pungens, and P. sitchensis) [6]. The RDD (resin duct diameter) is lower in Serbia than in Bosnia and Herzegovina but higher than in one natural population [30] (51,8 µm, 61.2 µm, 37 µm, and respectively). P. sitchensis has a higher RDD (70 µm [7] in comparison with P. omorika from Serbia [20] and Bosnia and Herzegovina (51.8 µm and 61.5 µm, respectively).
In population studies of the morpho-anatomy of Picea abies needles, significant differentiations were observed both between mountain regions and among populations, as well as within populations [31]. NL was strongly influenced by genetic factors; the needle width was determined by both genetic and environmental factors, while for other needle traits, the environmental component of variability prevailed.
4.2. Terpene Composition of Serbian Spruce Needles
The terpene profile of Picea species is a key determinant of their ecological fitness and physiological resilience. Individual terpenes play distinct yet interconnected roles in defense, metabolism, and environmental adaptation. Monoterpenes such as α-pinene, β-pinene, and 3-carene function as volatile chemical defenses, deterring herbivores and inhibiting pathogenic fungi while mediating plant–plant and plant–microbe interactions [32,33]. Sesquiterpenes, including longifolene and β-caryophyllene, are often inducible upon biotic stress and contribute to both direct toxicity and signaling in defense-related responses [34]. Diterpenes like abietic and levopimaric acids, though less volatile, form critical components of the oleoresin, creating physical and chemical barriers against invading organisms and mitigating abiotic stresses, such as drought [35]. Profiling these compounds is vital for understanding the metabolic plasticity of Picea, their adaptive strategies in boreal ecosystems, and the molecular basis of resistance to environmental challenges. Recent advances in metabolomics and genomic tools have revealed the complexity of terpene biosynthesis in conifers, including highly specialized terpene synthase gene families and regulatory networks underlying species-specific chemical defenses [36]. This knowledge is increasingly important for forest-management strategies, breeding programs, conservation, and the sustainable utilization of conifer resources.
Bosnian and Herzegovinian populations have a higher total number of essential oil compounds (128 compounds) compared to Serbian populations (78) [19]. Bosnian and Herzegovinian populations also contain a greater proportion of total monoterpenes (89.1% on average) than the Serbian populations (74.3% on average), except for the population from Mileševka Canyon (MIL) (88.5%) [19].
However, comparing Serbian and Bosnian and Herzegovinian populations via multivariate statistical analyses, population Štula (STU) is found to be the most distant. Furthermore, population Vranjak (VRA) is a Serbian population that is the most similar to Bosnian and Herzegovinian populations.
The needle leaf oil of Bosnian P. omorika populations has the following average terpene profile, bornyl acetate >> camphene > limonene > α-pinene, while in Serbian populations, the average terpene profile was bornyl acetate >> camphene > α-pinene. The main terpene profiles of other sites of Serbian spruce also had bornyl acetate, limonene, camphene, and α-pinene, sometimes in a slightly different arrangement [13,37,38].
According to von Ruddlof [39], bornyl acetate is the highest compound in P. omorika as well, with the profile bornyl acetate >>> limonene > α-pinene > camphene. In Picea mariana and P. rubens (section Eupicea) terpene profiles were similar to Serbian populations, but with more abundant bornyl acetate (bornyl acetate >>> camphene > α-pinene), while in P. breweriana (section Omorika), myrcene was the most abundant (myrcene > bornyl acetate > α-pinene > β-pinene) [39]. The terpene profile of P. orientalis (section Eupicea) was bornyl acetate >>> camphene and limonene/β-phellandrene [13]. The most abundant compounds of P. jezoensis (section Casicta) were camphene = limonene/β-phellandrene > myrcene > bornyl acetate, while in P. sitchensis (section Casicta), the terpene profile was unique (myrcene > piperitone > camphor > limonene/β-phellandrene) [13].
The significant variability in and diversity of P. omorika populations can undoubtedly be explained by historical changes in ancient times ([19] and references cited therein). The Dinaric Alps are geomorphologically complex due to tectonic disturbances during the Pliocene and the formation of ecological niches during the Pleistocene glaciation, factors that have contributed to the biodiversity and divergences found in some P. omorika populations.
5. Conclusions
The differences and diversity observed among the analyzed populations are important for understanding the variability of this relic and endemic species. This article presents several new terpene compounds of Picea omorika that were identified.
Almost complete separation of the populations of Bosnia and Herzegovina from the Serbian ones was confirmed in terms of the morpho-anatomy. Multivariate terpene analyses did not show differentiation between populations of the left and right coast of the river Drina, which is the natural border between the countries Bosnia and Herzegovina and Serbia. Population VRA from Serbia is closer to Bosnian and Herzegovinian populations, while population STU, which lays on the border between two countries, was the most distant.
Extending our analyses to all P. omorika populations in Bosnia and Herzegovina could provide a more comprehensive understanding of the morpho-anatomical characteristics and terpene profile in needles. This would help clarify the phenotypic diversification of P. omorika throughout its natural distribution.
Author Contributions
B.M.N.—Conceptualization, Resources, Visualization, Writing and editing; Z.S.M.—Conceptualization, Formal analysis, Resources; Writing—original draft; D.B.—Visualization, Resources, M.M.T.—Data curation, Methodology, J.S.N.—Project administration, Data curation; S.I.—Data curation, Methodology; V.V.T.—Resources, Conceptualization, Supervision. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the Ministry of Science, Technological Development and Innovation of the Republic of Serbia, grant numbers 451-03-136/2025-03/200027, 451-03-137/2025-03/200124, 451-03-136/2025-03/200026, and 451-03-136/2025-03/200168.
Data Availability Statement
The data presented in this study are available on reasonable request from the corresponding author.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Vidaković, M.; Franjić, J. Golosjemenjače; Šumarski Fakultet Sveučilišta u Zagrebu, Hrvatske Šume, Akademija Šumarskih Znanosti: Zagreb, Croatia, 2004. [Google Scholar]
- Mataruga, M.; Milanović, Đ. Natural populations of Serbian spruce in the Republic of Srpska (Bosnia and Herzegovina). Glas. Šumarskog Fak. Univ. U Banjoj Luci 2020, 30, 77–113. [Google Scholar] [CrossRef]
- Fukarek, P. Staništa Pančićeve omorike nakon šumskih požara u 1946/47 godini. Šumarski List. 1951, 75, 61–75. [Google Scholar]
- Chandler, J.W.; Dale, J.E. Needle growth in Sitka spruce (Picea sitchensis): Effects of nutrient deficiency and needle position within shoots. Tree Physiol. 1990, 6, 41–56. [Google Scholar] [CrossRef]
- Vidaković, M. Četinjače. In Morfologija i Varijabilnost. Zagreb; JAZU i Sveučilišna Naklada Liber: Zagreb, Croatia, 1982. [Google Scholar]
- Weng, C.; Jackson, S.T. Species differentiation of North American spruce (Picea) based on morphological and anatomical characteristics of needles. Can. J. Bot. 2000, 78, 1367–1383. [Google Scholar] [CrossRef]
- Marco, H.F. The anatomy of spruce needles. J. Agric. Res. 1939, 58, 357–368. [Google Scholar]
- Ishii, H.; Ooishi, M.; Maruyama, Y.; Koike, T. Acclimation of shoot and needle morphology and photosynthesis of two Picea species to differences in soil nutrient availability. Tree Physiol. 2003, 23, 453–461. [Google Scholar] [CrossRef]
- Garneau, F.X.; Collin, G.; Gagnon, H.; Pichette, A. Chemical composition of the hydrosol and the essential oil of three different species of the Pinaceae family: Picea glauca (Moench) Voss., Picea mariana (Mill.) B.S.P., and Abies balsamea (L.) Mill. J. Essent. Oil-Bear. Plants 2012, 15, 227–236. [Google Scholar] [CrossRef]
- Wajs-Bonikowska, A.; Szoka, Ł.; Karna, E.; Wiktorowska-Owczarek, A.; Sienkiewicz, M. Composition and biological activity of Picea pungens and Picea orientalis seed and cone essential oils. Chem. Biodiv. 2017, 14, e1600264. [Google Scholar] [CrossRef]
- Champagne, E.; Bonin, M.; Royo, A.; Tremblay, J.-P.; Raymond, P. Predicting terpene content in dried conifer shoots using near infrared spectroscopy. J. Near Infrared Spec. 2020, 28, 308–314. [Google Scholar] [CrossRef]
- Tang, M.; Ai, Y.; Song, N.; Geng, L.; Ren, L.; Zhu, S.; Ai, Y.; Cai, S.; Zhang, Z.; Zhang, L.; et al. Chemical composition and hypnotic effect of Picea mariana essential oils. J. Essent. Oil Bear. Plants 2023, 26, 362–377. [Google Scholar] [CrossRef]
- Stevanović Janežić, T.; Isajev, V.; Lange, W. Relations of needle terpene compositions for selected species from genus Picea. J. Serb. Chem. Soc. 1994, 59, 359–365. [Google Scholar] [CrossRef]
- Ledig, F.T.; Hodgskiss, P.D.; Krutovskii, K.V.; Neale, D.B.; Eguiluz Piedra, T. Relationships among the spruces (Picea, Pinaceae) of southwestern North America. Syst. Bot. 2004, 29, 275–295. [Google Scholar] [CrossRef]
- Smith, D.E.; Klein, A.S. Phylogenetic inferences on the relationship of North American and European Picea species based on nuclear ribosomal 18S sequences and the internal transcribed spacer 1 region. Mol. Phylogenet. Evol. 1994, 3, 17–26. [Google Scholar] [CrossRef]
- Campbell, C.S.; Wright, W.A.; Cox, M.; Vining, T.F.; Major, C.S.; Arsenault, M.P. Internal transcribed spacer 1 (ITS1) in Picea (Pinaceae). Sequence divergence and structure. Mol. Phylogenet. Evol. 2005, 35, 165–185. [Google Scholar] [CrossRef] [PubMed]
- Mitić, Z.S.; Nikolić, B.M.; Stojković, J.P.; Jevtović, S.Č.; Stojanović, G.S.; Zlatković, B.K.; Marin, P.D. Morpho-anatomical characteristics and volatile profiles of Pinus nigra J. F. Arnold from the Balkan Peninsula and Southern Carpathians. Forests 2024, 15, 739. [Google Scholar] [CrossRef]
- Nikolić, B.; Tešević, V.; Bajić, D.; Bojović, S.; Marin, P.D. Needle essential oil composition of Picea omorika var. vukomanii. Chem. Nat. Compd. 2008, 44, 526–527. [Google Scholar] [CrossRef]
- Nikolić, B.; Tešević, V.; Đorđević, I.; Marin, P.D.; Bojović, S. Essential oil variability in natural populations of Picea omorika, a rare European conifer. Chem. Biodiv. 2009, 6, 193–203. [Google Scholar] [CrossRef]
- Nikolić, B.; Bojović, S.; Marin, P.D. Variability of needle morpho-anatomical characters of Picea omorika from natural populations in Serbia. Plant Biosyst. 2015, 149, 61–67. [Google Scholar] [CrossRef]
- Nikolić, B.M.; Ballian, D.A.; Đorđević, I.Z.; Rajčević, N.F.; Todosijević, M.M.; Stanković Jeremić, J.M.; Mitić, Z.S.; Bojović, S.R.; Tešević, V.V. n-Alkanes variability in natural populations of Picea omorika (Pančić) Purk. from Bosnia and Herzegovina. Biochem. Syst. Ecol. 2023, 106, 104544. [Google Scholar] [CrossRef]
- Blaženčić, J. Praktikum iz Anatomije Biljaka sa Osnovama Mikroskopske Tehnike; Naučna knjiga: Belgrade, Serbia, 1979. [Google Scholar]
- Liao, P.H.; Yang, H.H.; Wu, P.C.; Abu Bakar, N.H.; Urban, P.L. On-line coupling of simultaneous distillation-extraction using the Likens-Nickerson apparatus with gas chromatography. Anal. Chem. 2020, 92, 1228–1235. [Google Scholar] [CrossRef]
- Petrakis, P.V.; Tsitsimpikou, C.; Tzakou, O.; Couladis, M.; Vagias, C.; Roussis, V. Needle volatiles from five Pinus species growing in Greece. Flavour Fragr. J. 2001, 16, 249. [Google Scholar] [CrossRef]
- Pavlović, B.P.; Matović, M. Vukoman’s spruce-new variety of Serbian spruce in the Mileševka kanyon—Picea omorika var. vukomanii. Arch. Biol. Sci. 1994, 3–4, 27–28. [Google Scholar]
- Milovanović, J.; Ivetić, V.; Vilotić, D.; Šijačić-Nikolić, M. Morfo-anatomske karakteristike četina različitih fenogrupa omorike. Acta Herbol. 2005, 14, 41–49. [Google Scholar]
- Isajev, V.; Šijačić, M.; Vilotić, D. Promenljivost nekih svojstava dvogodišnjih četina omorike iz šest populacija sa različitim stepenom aerozagadenja. Šumarstvo 1999, 2–3, 147–157. [Google Scholar]
- Ilijin-Jug, M. Histološko-Citološke i Morfološke Promene Kod Vrste Picea Omorica na Području Pančeva u Hortikulturi, Izazvane Aerozagadenjem. Master’s Thesis, Biološki fakultet, University of Belgrade, Belgrade, Serbia, 1995; p. 153. [Google Scholar]
- Radotić, S.; Topuzović, M. Uporedna analiza anatomskih karakteristika listova vrsta Picea omorica (Pančić) Purk. i Picea excelsa (Lam.) Link. (Pinaceae) sa istog staništa. In Proceedings of the Simpozijum Proučavanje Biljnog i Životinjskog Sveta sa Aspekta Problema Zaš Tite i Unapredenja Životne Sredine-100 Godina bez Pančića 1888–1988, Kragujevac, Serbia, 29–30 September 1988; pp. 83–89. [Google Scholar]
- Vilotić, D. Anatomska grada stabala omorike Picea omorika (Pančić) Purkyne sa područja Nacionalnog parka Tara. In Nacionalnog Vegetacija Parka Tara; Gajić, M., Kojić, M., Karadžić, D., Vasiljević, M., Stanić, M., Eds.; Šumarski Fakultet Beograd: Belgrade, Serbia, 1994; pp. 33–49. [Google Scholar]
- Popović, V.; Nikolić, B.; Lučić, A.; Rakonjac, L.; Šešlija Jovanović, D.; Miljković, D. Morpho anatomical trait variability of the Norway spruce (Picea abies (L.) Karst.) needles in natural populations along elevational diversity gradient. Trees 2022, 36, 1131–1147. [Google Scholar] [CrossRef]
- Raffa, K.F.; Aukema, B.H.; Bentz, B.J.; Carroll, A.L.; Hicke, J.A.; Turner, M.G.; Romme, W.H. Cross-scale drivers of natural disturbances prone to anthropogenic amplification: The dynamics of bark beetle eruptions. BioScience 2013, 58, 501–517. [Google Scholar] [CrossRef]
- Kännaste, A.; Copolovici, L.; Niinemets, Ü. Gas chromatography–mass spectrometry method for determination of biogenic volatile organic compounds emitted by plants. Methods Mol. Biol. 2013, 1009, 161–178. [Google Scholar] [CrossRef]
- Zhao, T.; Krokene, P.; Hu, J.; Christiansen, E.; Björklund, N.; Långström, B.; Solheim, H.; Borg-Karlson, A.K. Induced terpene accumulation in Norway spruce inhibits bark beetle colonization in a dose-dependent manner. PLoS ONE 2011, 6, e26649. [Google Scholar] [CrossRef]
- Keeling, C.I.; Bohlmann, J. Genes, enzymes and chemicals of terpenoid diversity in the constitutive and induced defence of conifers against insects and pathogens. New Phytol. 2006, 170, 657–675. [Google Scholar] [CrossRef]
- Hall, D.E.; Robert, J.A.; Keeling, C.I.; Domanski, D.; Quesada, A.L.; Jancsik, S.; Kuzyk, M.A.; Hamberger, B.; Borchers, C.H.; Bohlmann, J. An integrated genomic, proteomic, and biochemical analysis of 3-carene biosynthesis in Sitka spruce (Picea sitchensis). Plant Physiol. 2011, 157, 1034–1050. [Google Scholar] [CrossRef]
- Janežić, T.Š.; Isajev, V. Needle oil terpenes of Serbian spruce from three localities. Holz Roh Werkst. 1993, 51, 283–286. [Google Scholar] [CrossRef]
- Chalchat, J.C.; Gorunovic, M.S. Chemotaxonomy of pines native to the Balkans (IV): Variations in the composition of essential oils of Pinus omorika Pancic according to plant part and age of specimens. Pharmazie 1995, 50, 640–641. [Google Scholar]
- Von Ruddlof, E. Volatile leaf oil analysis in chemosystematic studies of North American conifers. Biochem. Syst. Ecol. 1975, 2, 131–167. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).