Global Perspectives on the Medicinal Potential of Pines (Pinus spp.)
Abstract
1. Introduction
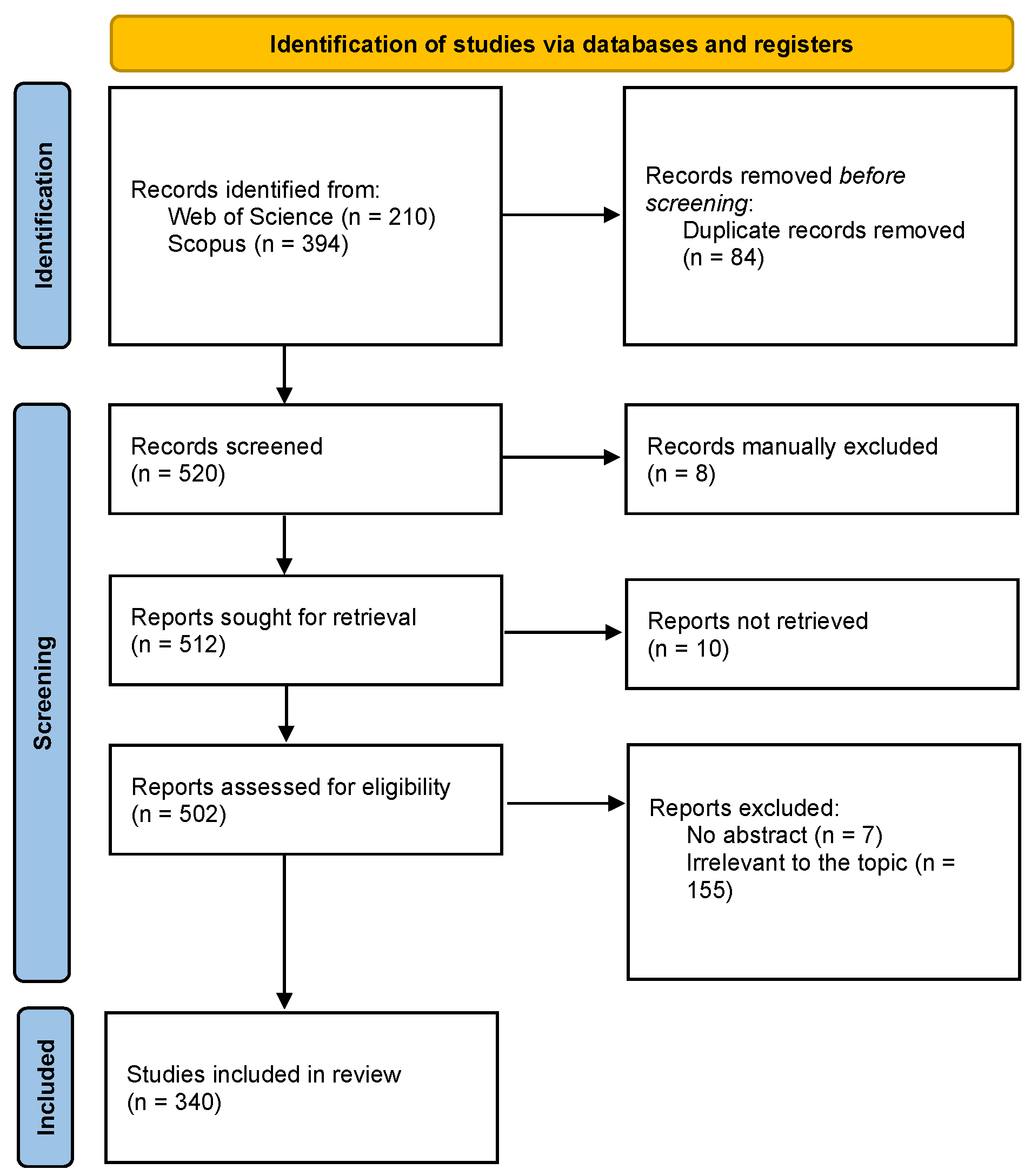
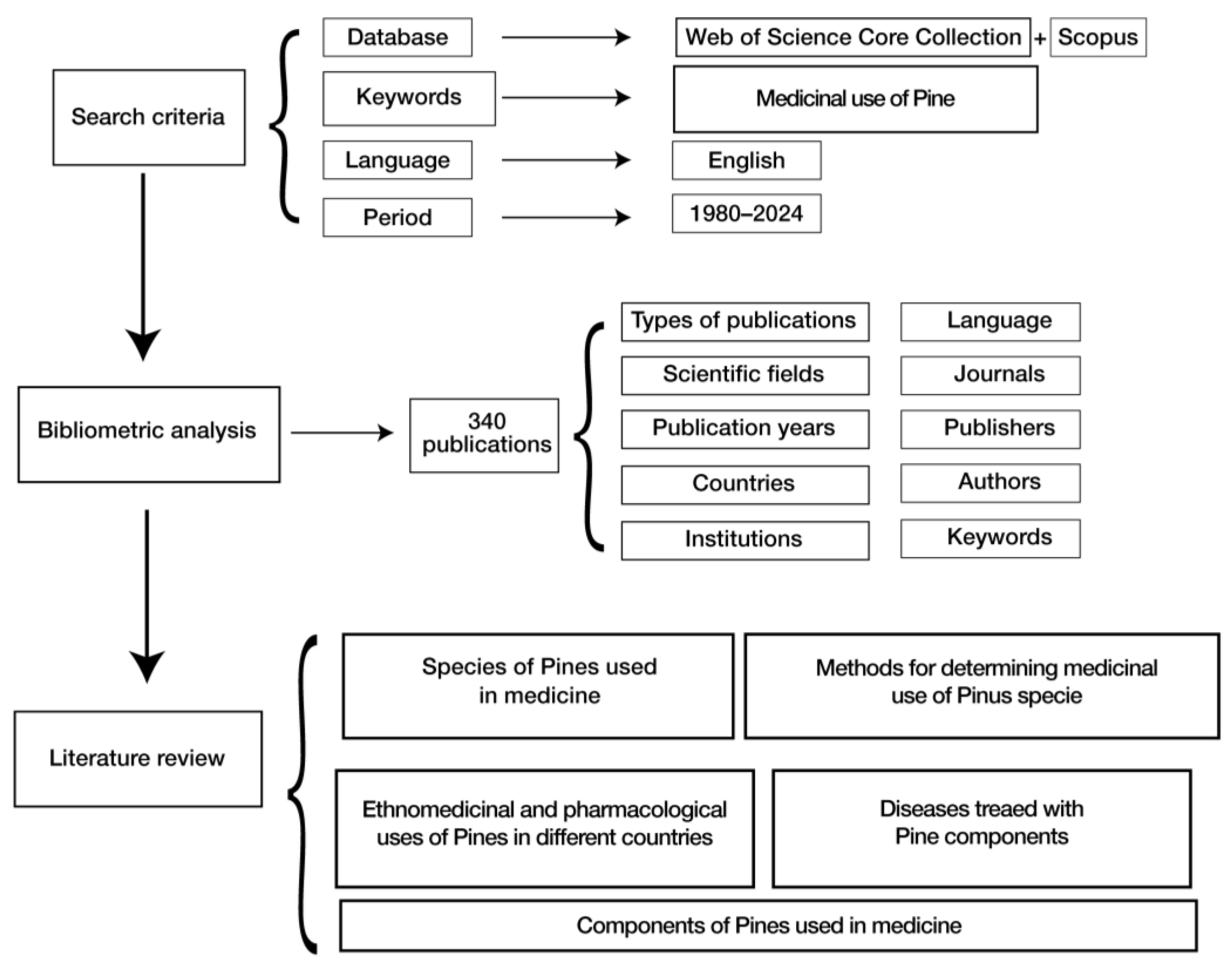
2. Materials and Methods
3. Results
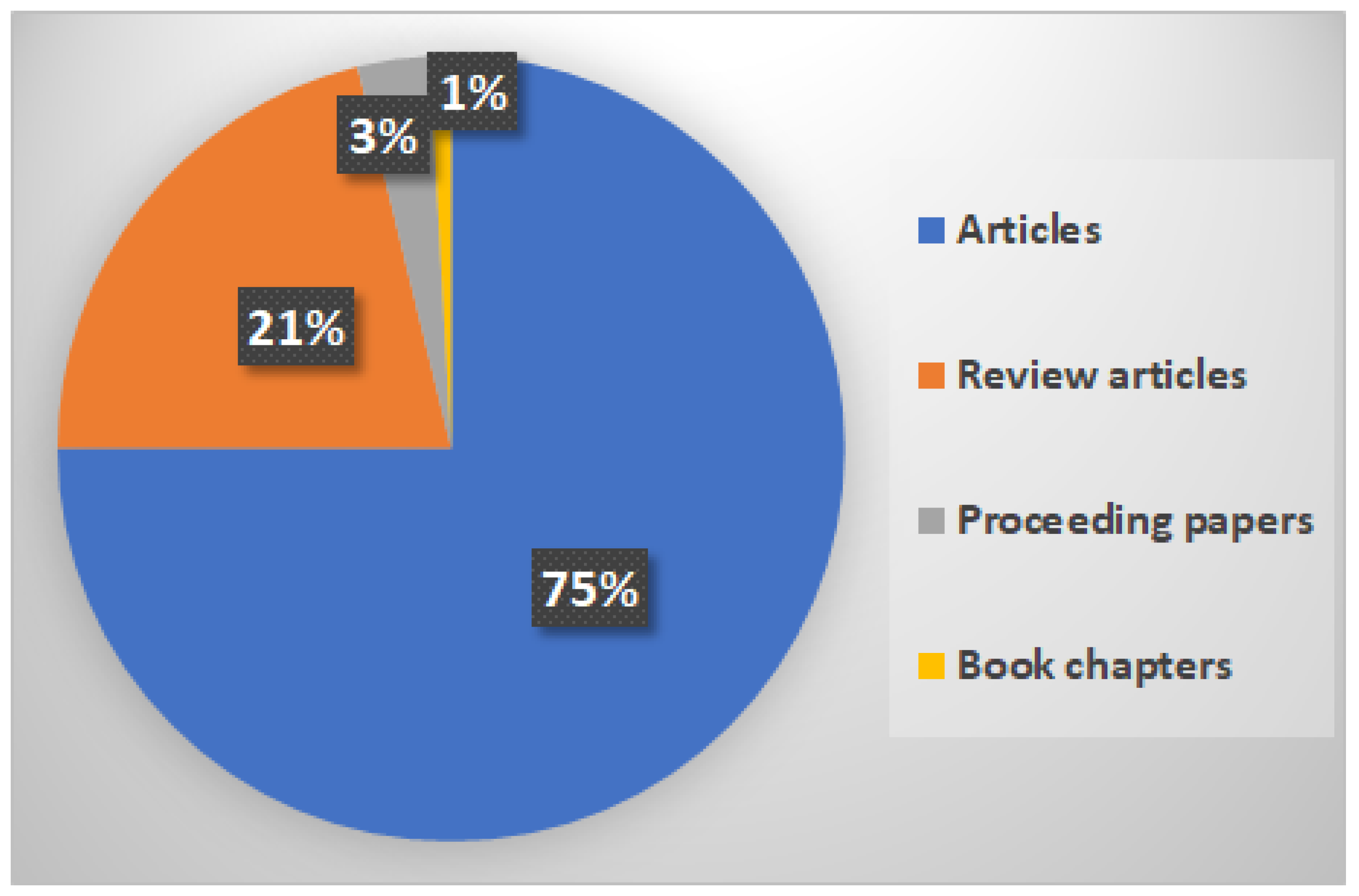
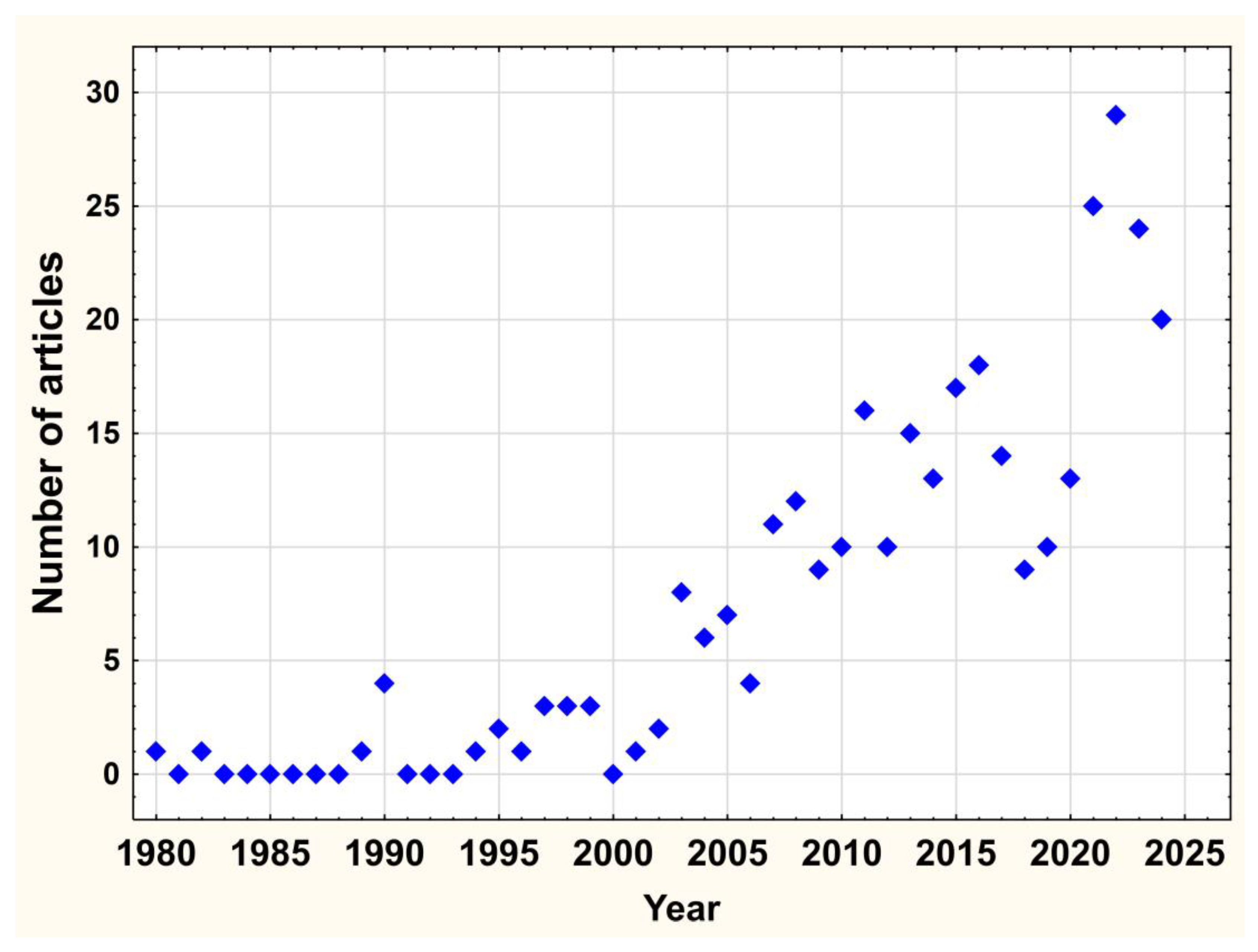
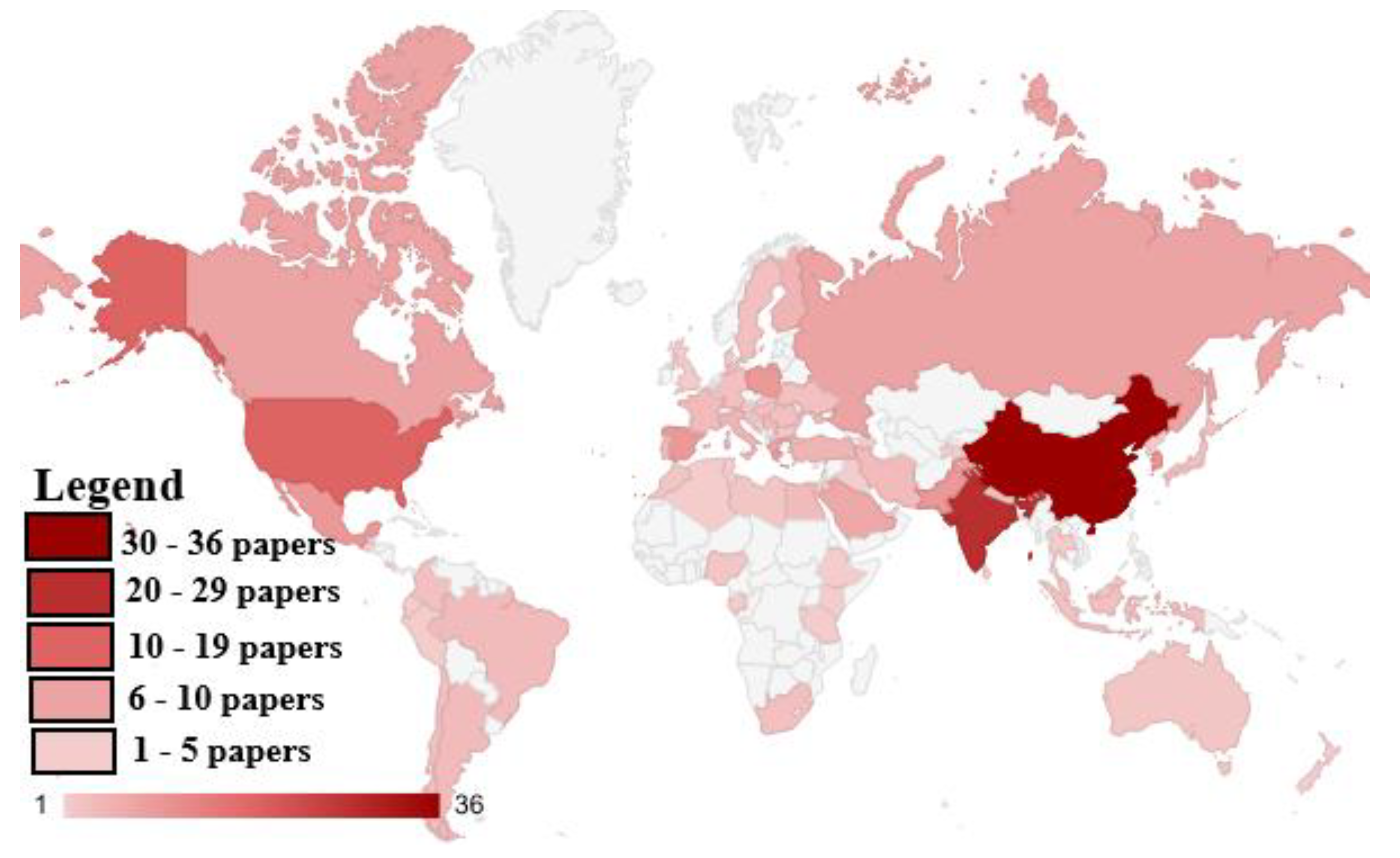
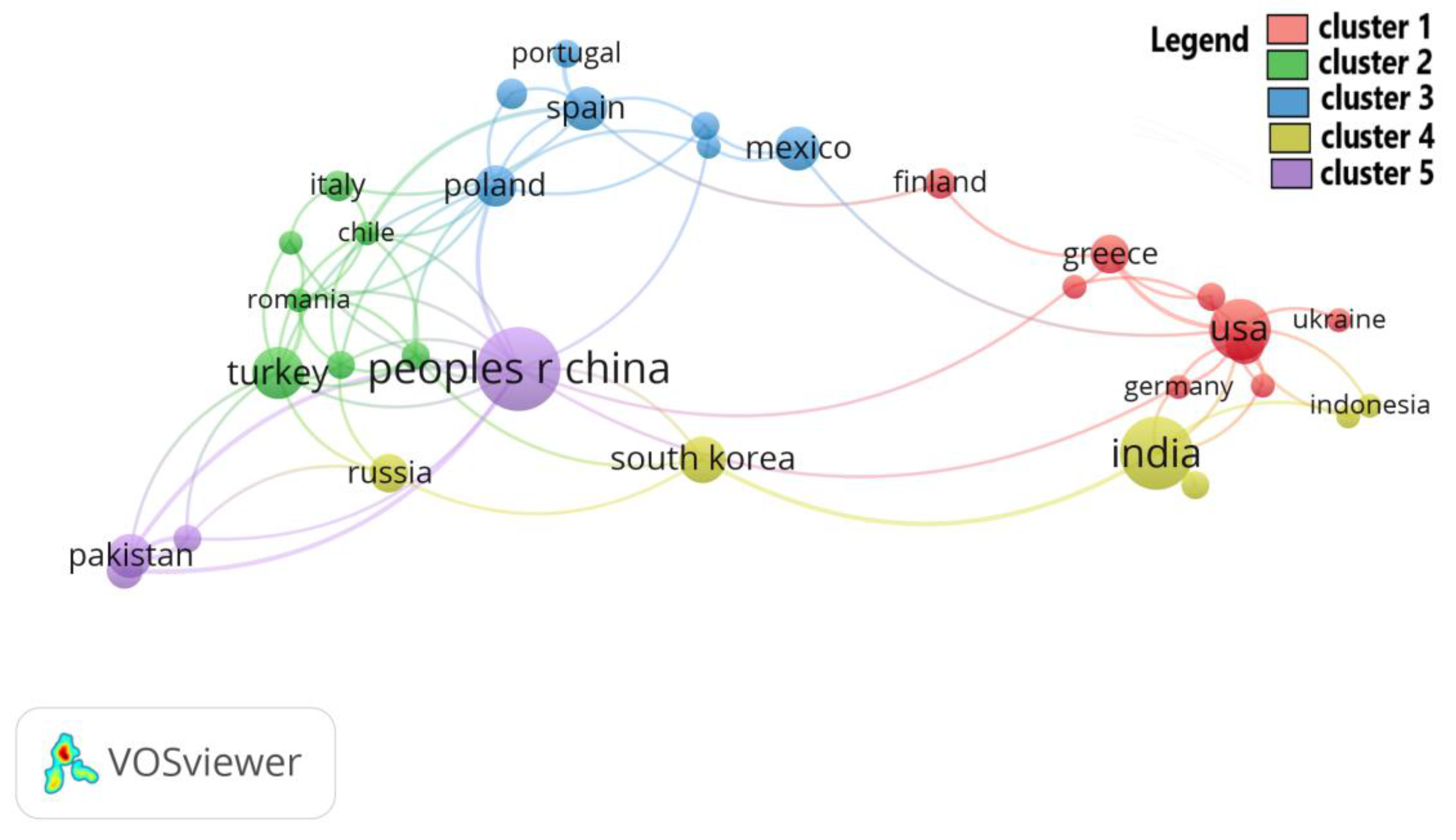
3.1. A Bibliometric Review
3.2. Literature Review
3.2.1. Species of Pines Used in Medicine
3.2.2. Methods for Determining Medicinal Use of Pinus Species
3.2.3. Ethnomedicinal and Pharmacological Uses of Pine in Different Countries
3.2.4. Diseases Treated with Pine Components
Skin Burns and Wound Healing
Antimicrobial Properties
Hepatoprotective Activity
Antidiabetic and Metabolic Effects
Cardiovascular and Antihypertensive Effects
Neurological Disorders
Ethnomedicinal and Traditional Applications
3.2.5. Components of Pines Used in Medicine
Pine Pollen
Leaves
Cones and Needles
Seeds/Nuts
Pine Nodules
Pine Resin and Rosin
Bark
Roots and Other Parts
Novel Compounds
3.2.6. Phytochemical Profile of Pinus Species
4. Discussion
4.1. Bibliometric Review
4.2. Species Diversity and Medicinal Relevance of the Genus Pinus
Taxonomic Breadth and Research Gaps
4.3. Critical Analysis of Experimental Methods in the Evaluation of Medicinal Properties of Pinus Species
4.4. Global Perspectives on the Medicinal and Ethnobotanical Importance of Pine Species
4.5. Therapeutic Significance and Pharmacological Insights into Pinus Species
4.6. Medicinal Potential of Different Pine Components
4.7. Gaps of Our Research and Future Directions of Research
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Şesan, T.E.; Oancea, F.; Toma, C.; Matei, G.-M.; Matei, S.; Chira, F.; Chira, D.; Fodor, E.; Mocan, C.; Ene, M.; et al. Approaches to the study of mycorrhizas in Romania. Symbiosis 2010, 52, 75–85. [Google Scholar] [CrossRef]
- Greene, R.E.; Iglay, R.B.; Evans, K.O.; Miller, D.A.; Wigley, T.B.; Riffell, S.K. A meta-analysis of biodiversity responses to management of southeastern pine forests—Opportunities for open pine conservation. For. Ecol. Manag. 2016, 360, 30–39. [Google Scholar] [CrossRef]
- Crandall, R.M.; Chew, Y.M.; Fill, J.M.; Kreye, J.K.; Varner, J.M.; Kobziar, L.N. Pine trees structure plant biodiversity patterns in savannas. Ecol. Evol. 2024, 14, e70021. [Google Scholar] [CrossRef]
- Martín-Peinado, F.J.; Navarro, F.B.; Jiménez, M.N.; Sierra, M.; Martínez, F.J.; Romero-Freire, A.; Rojo, L.; Fernández-Ondoño, E. Long-term effects of pine plantations on soil quality in southern Spain. Land Degrad. Dev. 2016, 27, 1709–1720. [Google Scholar] [CrossRef]
- Vlad, R.; Constandache, C.; Dincă, L.; Tudose, N.C.; Sidor, C.G.; Popovici, L.; Ispravnic, A. Influence of climatic, site and stand characteristics on some structural parameters of Scots pine (Pinus sylvestris) forests situated on degraded lands from east Romania. Range Manag. Agrofor. 2019, 40, 40–48. [Google Scholar]
- Uribe, S.V.; Estades, C.F.; Radeloff, V.C. Pine plantations and five decades of land use change in central Chile. PLoS ONE 2020, 15, e0230193. [Google Scholar] [CrossRef]
- Silvestru-Grigore, C.V.; Dinulică, F.; Spârchez, G.; Hălălișan, A.F.; Dincă, L.C.; Enescu, R.E.; Crișan, V.E. Radial growth behavior of pines on Romanian degraded lands. Forests 2018, 9, 213. [Google Scholar] [CrossRef]
- Kullman, L.; Öberg, L. Accelerated Pine (Pinus sylvestris) Rise in the Alpine Treeline Ecotone of the Swedish Scandes—Demographic Data 1973–2024. Eur. J. Appl. Sci. 2025, 13, 447–472. [Google Scholar] [CrossRef]
- Biocca, M.; Gallo, P.; Sperandio, G. Technical and economic analysis of Stone pine (Pinus pinea L.) maintenance in urban areas. Trees For. People 2021, 6, 100162. [Google Scholar] [CrossRef]
- Petrova, S. The Added Value of Urban Trees (Tilia tomentosa Moench, Fraxinus excelsior L. and Pinus nigra J.F. Arnold) in Terms of Air Pollutant Removal. Forests 2024, 15, 1034. [Google Scholar] [CrossRef]
- Bolea, V.; Popa, A. Tree by tree for more beautiful cities and a cleaner environment. Rev. Silvic. Cinegetică 2025, 55, 67–80. [Google Scholar]
- Mihalciuc, V.; Danci, V.; Bujila, M.; Chira, D. The frequency and intensity of bark beetle infestations recorded in Romanian forests affected by windfall in 1995. In Proceedings: Ecology, Survey and Management of Forest Insects, Krakow, Poland, 1–5 September 2002; Gen. Tech. Rep., NE-311; McManus, M.L., Liebhold, A.M., Eds.; US Dept. of Agriculture, Forest Service, Northeastern Research Station: Newtown Square, PA, USA, 2003; pp. 162–164. [Google Scholar]
- Fernández-Fernández, M.; Naves, P.; Witzell, J.; Musolin, D.L.; Selikhovkin, A.V.; Paraschiv, M.; Chira, D.; Martínez-Álvarez, P.; Martín-García, J.; Muñoz-Adalia, E.J.; et al. Pine pitch canker and insects: Relationships and implications for disease spread in Europe. Forests 2019, 10, 627. [Google Scholar] [CrossRef]
- Senf, C.; Seidl, R. Persistent impacts of the 2018 drought on forest disturbance regimes in Europe. Biogeosciences 2021, 18, 5223–5230. [Google Scholar] [CrossRef]
- Liu, Q.; Peng, C.; Schneider, R.; Cyr, D.; McDowell, N.G.; Kneeshaw, D. Drought-induced increase in tree mortality and corresponding decrease in the carbon sink capacity of Canada’s boreal forests from 1970 to 2020. Glob. Change Biol. 2023, 29, 2274–2285. [Google Scholar] [CrossRef]
- Parks, S.A.; Hefty, K.L.; Rushing, J.F.; Goeking, S.A.; Tomback, D.F.; Hood, S.M.; Toney, J.C.; Harrell, D.L.; Lindstrom, J.; Naficy, C.E.; et al. Whitebark pine in the United States projected to experience an 80% reduction in climatically suitable area by the mid-21st century. Environ. Res. Lett. 2025, 20, 104012. [Google Scholar] [CrossRef]
- Mao, G.X.; Zheng, L.D.; Cao, Y.B.; Chen, Z.M.; Lv, Y.D.; Wang, Y.Z.; Hu, X.-L.; Wang, G.-F.; Yan, J. Antiaging effect of pine pollen in human diploid fibroblasts and in a mouse model induced by D-galactose. Oxidative Med. Cell. Longev. 2012, 2012, 750963. [Google Scholar] [CrossRef]
- Drehsen, G. From ancient pine bark uses to Pycnogenol. In Antioxidant Food Supplements in Human Health; Academic Press: New York, NY, USA, 1999; pp. 311–322. [Google Scholar]
- Liang, Z.; Yan, J.; Zhao, S.; He, L.; Zhao, X.; Cai, L.; You, C.; Wang, F. Efficient Extraction, Chemical Characterization, and Bioactivity of Essential Oil from Pine Needles. Phytochem. Anal. 2025, 36, 1539–1559. [Google Scholar] [CrossRef]
- Liu, J.; Yuan, X.; Wei, Y.; Yuan, W.; Wang, Z.; Ding, C. Extraction, purification, structural characterization, bioactivities and application of polysaccharides from different parts of pine. Fitoterapia 2025, 183, 106569. [Google Scholar] [CrossRef]
- Fierascu, R.C.; Fierascu, I.C.; Dinu-Pirvu, C.E.; Fierascu, I.; Paunescu, A. The application of essential oils as a next-generation of pesticides: Recent developments and future perspectives. Z. Für. Naturforschung. C 2020, 75, 183–204. [Google Scholar] [CrossRef]
- Faria, J.M.; Barbosa, P.; Vieira, P.; Vicente, C.S.; Figueiredo, A.C.; Mota, M. Phytochemicals as biopesticides against the pinewood nematode Bursaphelenchus xylophilus: A review on essential oils and their volatiles. Plants 2021, 10, 2614. [Google Scholar] [CrossRef]
- Park, J.H.; Park, S.H.; Lee, H.J.; Kang, J.W.; Lee, K.M.; Yeon, P.S. Study on NVOCs concentration characteristics by season, time and climatic factors: Focused on Pinus densiflora forest in National Center for Forest Therapy. J. People Plants Environ. 2018, 21, 403–409. [Google Scholar] [CrossRef]
- Bratu, I.; Dincă, L.; Constandache, C.; Murariu, G. Resilience and decline: The impact of climatic variability on temperate oak forests. Climate 2025, 13, 119. [Google Scholar] [CrossRef]
- Dincă, L.; Crisan, V.; Ienaşoiu, G.; Murariu, G.; Drăşovean, R. Environmental indicator plants in mountain forests: A review. Plants 2024, 13, 3358. [Google Scholar] [CrossRef] [PubMed]
- Murariu, G.; Stanciu, S.; Dincă, L.; Munteanu, D. GIS applications in monitoring and managing heavy metal contamination of water resources. Appl. Sci. 2025, 15, 10332. [Google Scholar] [CrossRef]
- Dincă, L.; Murariu, G.; Lupoae, M. Understanding the ecosystem services of riparian forests: Patterns, gaps, and global trends. Forests 2025, 16, 947. [Google Scholar] [CrossRef]
- Slepetiene, A.; Belova, O.; Fastovetska, K.; Dinca, L.; Murariu, G. Managing boreal birch forests for climate change mitigation. Land 2025, 14, 1909. [Google Scholar] [CrossRef]
- He, T.; Vaidya, B.N.; Perry, Z.D.; Parajuli, P.; Joshee, N. Paulownia as a medicinal tree: Traditional uses and current advances. Eur. J. Med. Plants 2016, 14, 1–15. [Google Scholar] [CrossRef]
- Omar, S.; Lemonnier, B.; Jones, N.; Ficker, C.; Smith, M.; Neema, C.; Towers, G.; Goel, K.; Arnason, J. Antimicrobial activity of extracts of eastern North American hardwood trees and relation to traditional medicine. J. Ethnopharmacol. 2000, 73, 161–170. [Google Scholar] [CrossRef]
- Padayachee, B.; Baijnath, H. An overview of the medicinal importance of Moringaceae. J. Med. Plants Res. 2012, 6, 5831–5839. [Google Scholar]
- Birkle, C.; Pendlebury, D.A.; Schnell, J.; Adams, J. Web of Science as a data source for research on scientific and scholarly activity. Quant. Sci. Stud. 2020, 1, 363–376. [Google Scholar] [CrossRef]
- Page, M.J.; McKenzie, J.E.; Bossuyt, P.M.; Boutron, I.; Hoffmann, T.C.; Mulrow, C.D.; Shamseer, L.; Tetzlaff, J.M.; Akl, E.A.; Brennan, S.E.; et al. The PRISMA 2020 statement: An updated guideline for reporting systematic reviews. BMJ 2021, 372, n71. [Google Scholar] [CrossRef]
- Clarivate. Web of Science Core Collection. Available online: https://clarivate.com/products/scientific-and-academic-research/research-discovery-and-workflow-solutions/webofscience-platform/web-of-science-core-collection/ (accessed on 20 June 2025).
- Elsevier. Scopus. Available online: https://www.elsevier.com/products/scopus (accessed on 20 June 2025).
- Microsoft Corporation. Microsoft Excel. Available online: https://www.microsoft.com/en-us/microsoft-365/excel?legRedir=true&CorrelationId=3bb60ab0-fe13-41a4-812b-2627667cf346 (accessed on 26 June 2025).
- Google. Geochart. Available online: https://developers.google.com/chart/interactive/docs/gallery/geochart (accessed on 23 June 2025).
- VOSviewer. Available online: https://www.vosviewer.com/ (accessed on 22 June 2025).
- Moore, A.; Ankney, E.; Swor, K.; Poudel, A.; Satyal, P.; Setzer, W.N. Leaf essential oil compositions and enantiomeric distributions of monoterpenoids in Pinus species: Pinus albicaulis, Pinus flexilis, Pinus lambertiana, Pinus monticola, and Pinus sabiniana. Molecules 2025, 30, 244. [Google Scholar] [CrossRef] [PubMed]
- Ioannou, E.; Koutsaviti, A.; Tzakou, O.; Roussis, V. The genus Pinus: A comparative study on the needle essential oil composition of 46 pine species. Phytochem. Rev. 2014, 13, 741–768. [Google Scholar] [CrossRef]
- Delgado-Alvarado, E.A.; Almaraz-Abarca, N.; Herrera-Arrieta, Y.; Torres-Ricario, R.; González-Valdez, L.S.; Ávila-Reyes, J.A.; Naranjo-Jimenez, N. Phenolics content, antioxidant potential, α-glucosidase and α-amylase inhibitory activities of four foliar extracts from Pinus species. Farmacia 2022, 70, 214–221. [Google Scholar] [CrossRef]
- Yang, X.; Ding, Y.; Sun, Z.H.; Zhang, D.M. Studies on chemical constituents of Pinus armandii. Yao Xue Xue Bao (Acta Pharm. Sin.) 2005, 40, 435–437. [Google Scholar]
- Koutsaviti, A.; Toutoungy, S.; Saliba, R.; Loupassaki, S.; Tzakou, O.; Roussis, V.; Ioannou, E. Antioxidant potential of pine needles: A systematic study on the essential oils and extracts of 46 species of the genus Pinus. Foods 2021, 10, 142. [Google Scholar] [CrossRef]
- Rosales Castro, M.; Pérez López, M.E.; Ponce Rodríguez, M.D.C. Propiedades antirradicales libres y antibacterianas de extractos de corteza de pino. Madera Y Bosques 2006, 12, 37–49. [Google Scholar] [CrossRef]
- Georges, P.; Legault, J.; Lavoie, S.; Grenon, C.; Pichette, A. Diterpenoids from the buds of Pinus banksiana Lamb. Molecules 2012, 17, 9716–9727. [Google Scholar] [CrossRef]
- Elangovan, B. A review on pharmacological studies of natural flavanone: Pinobanksin. 3 Biotech 2024, 14, 111. [Google Scholar] [CrossRef]
- Haagen-Smit, A.J.; Wang, T.H.; Mirov, N.T. Composition of gum turpentines of Pinus aristata, P. balfouriana, P. flexilis, and P. parviflora. J. Am. Pharm. Assoc. (Sci. Ed.) 1950, 39, 254–259. [Google Scholar] [CrossRef]
- Stekrova, M.; Matouskova, M.; Vyskocilova, E.; Cerveny, L. Selective preparation of campholenic aldehyde over heterogenized methyltrioxorhenium. Res. Chem. Intermed. 2015, 41, 9003–9013. [Google Scholar] [CrossRef]
- Süntar, I.; Tumen, I.; Ustün, O.; Keleş, H.; Küpeli Akkol, E. Appraisal on the wound healing and anti-inflammatory activities of the essential oils obtained from the cones and needles of Pinus species by in vivo and in vitro experimental models. J. Ethnopharmacol. 2012, 139, 533–540. [Google Scholar] [CrossRef]
- Asmaa, B.H.; Ream, N. In vitro screening of the pancreatic cholesterol esterase inhibitory activity of some medicinal plants grown in Syria. Int. J. Pharmacogn. Phytochem. Res. 2016, 8, 1432–1436. [Google Scholar]
- Mehrzadi, S.; Ghaznavi, H.; Tajallizadehkhoob, Y.; Fakhrzadeh, H. Effects of Pinus eldarica Medw. nut extract on blood glucose and cholesterol levels in hypercholesterolemic alloxan-induced diabetic rats. J. Med. Plants 2013, 12, 68–74. [Google Scholar]
- Kamal, R.M.; Sabry, M.M.; Younis, I.Y.; El-Halawany, A.M.; Hifnawy, M.S. The in vitro evaluation of cholinesterase inhibition and the antioxidant effect of Cupressus arizonica Greene, Cupressus lusitanica Mill. and Pinus canariensis C. Sm. aerial parts. Trop. J. Nat. Prod. Res. 2024, 8, 6192–6196. [Google Scholar]
- Sinyeue, C.; Maerker, L.; Guentas, L.; Medevielle, V.; Bregier, F.; Chaleix, V.; Sol, V.; Lebouvier, N. Polyphenol content, antioxidant, and antibiotic activities of Pinus caribaea Morelet forestry coproducts. Nat. Prod. Commun. 2023, 18, 1934578X231211958. [Google Scholar]
- Nikolić, B.; Todosijević, M.; Ratknić, M.; Đorđević, I.; Stanković, J.; Cvetković, M.; Marin, P.; Tešević, V. Terpenes and n-alkanes in needles of Pinus cembra. Nat. Prod. Commun. 2018, 13, 1934578X1801300828. [Google Scholar]
- del Castillo, R.F.; Acosta, S. Ethnobotanical notes on Pinus strobus var. chiapensis. An. Inst. Biología. Ser. Botánica 2002, 73, 319–327. [Google Scholar]
- Swor, K.; Satyal, P.; Poudel, A.; Setzer, W.N. Gymnosperms of Idaho: Chemical compositions and enantiomeric distributions of essential oils of Abies lasiocarpa, Picea engelmannii, Pinus contorta, Pseudotsuga menziesii, and Thuja plicata. Molecules 2023, 28, 2477. [Google Scholar] [CrossRef]
- Merah, S.; Dahmane, D.; Krimat, S.; Metidji, H.; Nouasri, A.; Lamari, L.; Dob, T. Chemical analysis of phenolic compounds and determination of antioxidant, antimicrobial and cytotoxic activities of organic extracts of Pinus coulteri. Bangladesh J. Pharmacol. 2018, 13, 120–129. [Google Scholar] [CrossRef]
- Rosales-Castro, M.; González-Laredo, R.F.; Rocha-Guzmán, N.E.; Gallegos-Infante, J.A.; Peralta-Cruz, J.; Karchesy, J.J. Evaluación química y capacidad antioxidante de extractos polifenólicos de cortezas de Pinus cooperi, P. engelmannii, P. leiophylla y P. teocote. Madera Y Bosques 2009, 15, 87–105. [Google Scholar] [CrossRef]
- Sa, H.N.; Thuy, T.T.; Tam, N.T.; Anh, N.T.H.; Quan, T.D.; Thien, D.D.; Adorisio, S.; Sung, T.V.; Delfino, D.V. Chemical constituents of Pinus dalatensis Ferré wood and their effect on proliferation of acute myeloid leukemia cells. Lett. Org. Chem. 2018, 15, 641–652. [Google Scholar] [CrossRef]
- Yue, R.; Li, B.; Shen, Y.; Zeng, H.; Yuan, H.; He, Y.; Zhang, W. 6-C-methyl flavonoids isolated from Pinus densata inhibit the proliferation and promote the apoptosis of the HL-60 human promyelocytic leukaemia cell line. Planta Medica 2013, 79, 1024–1030. [Google Scholar] [CrossRef] [PubMed]
- Kim, E.; Yang, S.; Jeon, B.B.; Song, E.; Lee, H. Terpene compound composition and antioxidant activity of essential oils from needles of Pinus densiflora, Pinus koraiensis, Abies holophylla, and Juniperus chinensis by harvest period. Forests 2024, 15, 566. [Google Scholar] [CrossRef]
- Hu, C.L.; Xiong, J.; Gao, L.X.; Li, J.; Zeng, H.; Zou, Y.; Hu, J.F. Diterpenoids from the shed trunk barks of the endangered plant Pinus dabeshanensis and their PTP1B inhibitory effects. RSC Adv. 2016, 6, 60467–60478. [Google Scholar] [CrossRef]
- Peng, C.; An, D.; Ding, W.X.; Zhu, Y.X.; Ye, L.; Li, J. Fungichromin production by Streptomyces sp. WP-1, an endophyte from Pinus dabeshanensis, and its antifungal activity against Fusarium oxysporum. Appl. Microbiol. Biotechnol. 2020, 104, 10437–10449. [Google Scholar] [CrossRef]
- Mickles, A.J.; Chou, C.; Deleger, J.N.; Swords, E.F.; Schlarman, M.S.; Braude, S. Antimicrobial activity of bark from four North American tree species. Wilderness Environ. Med. 2024, 35, 439–442. [Google Scholar] [CrossRef]
- Poulson, A.; Wilson, T.M.; Packer, C.; Carlson, R.E.; Buch, R.M. Essential oils of trunk, limbs, needles, and seed cones of Pinus edulis (Pinaceae) from Utah. Phytologia 2020, 102, 200–207. [Google Scholar]
- Leandro, L.F.; Cardoso, M.J.O.; Silva, S.D.C.; Souza, M.G.M.; Veneziani, R.C.S.; Ambrosio, S.R.; Martins, C.H.G. Antibacterial activity of Pinus elliottii and its major compound, dehydroabietic acid, against multidrug-resistant strains. J. Med. Microbiol. 2014, 63, 1649–1653. [Google Scholar] [CrossRef]
- Satoh, K.; Kihara, T.; Ida, Y.; Sakagami, H.; Koyama, N.; Premanathan, M.; Hata, N. Radical modulation activity of pine cone extracts of Pinus elliottii var. elliottii. Anticancer Res. 1999, 19, 357–364. [Google Scholar]
- Tran, H.T.; Nguyen, T.H.; Trinh, X.T.; Nguyen, Q.H.; Paoli, M.; Bighelli, A.; Casanova, J. Chemical composition of the needle and cone essential oils of Pinus fenzeliana (Pinaceae) from Vietnam. Acad. J. Biol. 2023, 45, 127–135. [Google Scholar] [CrossRef]
- Ansari, P.; Samia, J.F.; Khan, J.T.; Rafi, M.R.; Rahman, M.S.; Rahman, A.B.; Seidel, V. Protective effects of medicinal plant-based foods against diabetes: A review on pharmacology, phytochemistry, and molecular mechanisms. Nutrients 2023, 15, 3266. [Google Scholar] [CrossRef]
- Singh, G.; Kumar, D.; Dash, A.K. Pinus gerardiana Wallich ex D. Don—A review. Phytomed. Plus 2021, 1, 100024. [Google Scholar] [CrossRef]
- Amrati, F.E.Z.; Bourhia, M.; Slighoua, M.; Salamatullah, A.M.; Alzahrani, A.; Ullah, R.; Bousta, D. Traditional medicinal knowledge of plants used for cancer treatment by communities of mountainous areas of Fez-Meknes-Morocco. Saudi Pharm. J. 2021, 29, 1185–1204. [Google Scholar] [CrossRef] [PubMed]
- Basholli-Salihu, M.; Schuster, R.; Hajdari, A.; Mulla, D.; Viernstein, H.; Mustafa, B.; Mueller, M. Phytochemical composition, anti-inflammatory activity and cytotoxic effects of essential oils from three Pinus spp. Pharm. Biol. 2017, 55, 1553–1560. [Google Scholar]
- Xie, Q.; Liu, Z.; Li, Z. Chemical composition and antioxidant activity of essential oil of six Pinus taxa native to China. Molecules 2015, 20, 9380–9392. [Google Scholar] [CrossRef] [PubMed]
- Khedhri, S.; Polito, F.; Caputo, L.; Khamassi, M.; Hamrouni, L.; Nazzaro, F.; Amri, I. Chemical composition, phytotoxic and antibiofilm activity of Pinus canariensis, P. jeffreyi and P. taeda essential oils. J. Essent. Oil Bear. Plants 2024, 27, 482–497. [Google Scholar] [CrossRef]
- Weerapreeyakul, N.; Machana, S.; Barusrux, S. Synergistic effects of melphalan and Pinus kesiya Royle ex Gordon (Simaosong) extracts on apoptosis induction in human cancer cells. Chin. Med. 2016, 11, 29. [Google Scholar] [CrossRef]
- Hong, S.H.; Lee, H.; Lee, H.J.; Kim, B.; Nam, M.H.; Shim, B.S.; Kim, S.H. Ethanol extract of Pinus koraiensis leaf ameliorates alcoholic fatty liver via the activation of LKB1–AMPK signaling in vitro and in vivo. Phytother. Res. 2017, 31, 783–791. [Google Scholar] [CrossRef]
- Thai, T.H.; Hien, N.T.; Ha, C.T.T.; Loc, P.K.; Dai, D.N.; Ogunwande, I.A. Chemical constituents of essential oils from three Vietnamese species of Pinus. Boletín Latinoam. Y Caribe Plantas Med. Y Aromáticas 2018, 17, 53–60. [Google Scholar]
- Vo, N.T.T.; Do, M.Q.; Van Pham, V. Green chemistry synthesis and Escherichia coli antibacterial activity of silver and zinc oxide nanoparticles. J. Aust. Ceram. Soc. 2023, 59, 1205–1212. [Google Scholar] [CrossRef]
- Loader, N.J.; Street-Perrott, F.A.; Daley, T.J.; Hughes, P.D.M.; Kimak, A.; Levanic, T.; Leuenberger, M. Simultaneous determination of stable carbon, oxygen, and hydrogen isotopes in cellulose. Anal. Chem. 2015, 87, 376–380. [Google Scholar] [CrossRef] [PubMed]
- Minami, T.; Wada, S.I.; Tokuda, H.; Tanabe, G.; Muraoka, O.; Tanaka, R. Potential antitumor-promoting diterpenes from the cones of Pinus luchuensis. J. Nat. Prod. 2002, 65, 1921–1923. [Google Scholar] [CrossRef] [PubMed]
- Takashi, H.; Shoei, T. The antioxidant activity of phenolic compounds isolated from the bark of Ryukyu pine trees (Pinus luchuensis Mayr.). Curr. Bioact. Compd. 2022, 18, 62–67. [Google Scholar] [CrossRef]
- Cui, W.; Huang, J.; Niu, X.; Shang, H.; Sha, Z.; Miao, Y.; Zhu, R. Screening active fractions from Pinus massoniana pollen for inhibiting ALV-J replication and their structure–activity relationship investigation. Vet. Microbiol. 2021, 252, 108908. [Google Scholar] [CrossRef]
- Wardani, G.; Ernawati; Eraiko, K.; Sudjarwo, S.A. The role of antioxidant activity of chitosan–Pinus merkusii extract nanoparticle against lead acetate-induced toxicity in rat pancreas. Vet. Med. Int. 2019, 2019, 9874601. [Google Scholar] [CrossRef]
- Liu, T.W.; Hsiao, S.W.; Lin, C.T.; Hsiao, G.; Lee, C.K. Anti-aging constituents from Pinus morrisonicola leaves. Molecules 2023, 28, 5063. [Google Scholar] [CrossRef]
- Hou, C.W.; Zhao, B.Y.; Liu, S.L.; Chen, Y.S. Anti-inflammatory effect of medicinal fungus Antrodia cinnamomea cultivated on Pinus morrisonicola Hayata. Food Technol. Biotechnol. 2024, 62, 292–301. [Google Scholar] [CrossRef]
- Karapandzova, M.; Stefkov, G.; Cvetkovikj, I.; Stanoeva, J.P.; Stefova, M.; Kulevanova, S. Flavonoids and other phenolic compounds in needles of Pinus peuce and other pine species from the Macedonian flora. Nat. Prod. Commun. 2015, 10, 1934578X1501000647. [Google Scholar] [CrossRef]
- Nikolić, B.M.; Ballian, D.; Mitić, Z.S. Autochthonous conifers of family Pinaceae in Europe: Broad review of morpho-anatomical and phytochemical properties of needles and genetic investigations. Forests 2024, 15, 989. [Google Scholar] [CrossRef]
- Arı, S.; Kargıoğlu, M.; Temel, M.; Konuk, M. Traditional tar production from the Anatolian black pine [Pinus nigra Arn. subsp. pallasiana (Lamb.) Holmboe var. pallasiana] and its usages in Afyonkarahisar, Central Western Turkey. J. Ethnobiol. Ethnomedicine 2014, 10, 29. [Google Scholar]
- Gülçin, İ.; Büyükokuroğlu, M.E.; Oktay, M.; Küfrevioğlu, Ö.İ. Antioxidant and analgesic activities of turpentine of Pinus nigra Arn. subsp. pallasiana (Lamb.). Holmboe. J. Ethnopharmacol. 2003, 86, 51–58. [Google Scholar] [CrossRef]
- Chalchat, J.C.; Gorunovic, M.S. Chemotaxonomy of pines native to the Balkans (III): Variations in the composition of essential oils of Pinus nigra Arnold ssp. dalmatica according to age of specimens. Pharmazie 1995, 50, 575–576. [Google Scholar]
- Rubio, J.; Calderón, J.S.; Flores, A.; Castro, C.; Céspedes, C.L. Trypanocidal activity of oleoresin and terpenoids isolated from Pinus oocarpa. Z. Für. Naturforschung. C 2005, 60, 711–716. [Google Scholar] [CrossRef]
- Sarria-Villa, R.A.; Gallo-Corredor, J.A.; Benítez-Benítez, R. Characterization and determination of the quality of rosins and turpentines extracted from Pinus oocarpa and Pinus patula resin. Heliyon 2021, 7, e07750. [Google Scholar] [CrossRef]
- Clark, S.P.; Bollag, W.B.; Westlund, K.N.; Ma, F.; Falls, G.; Xie, D.; Bhattacharyya, M.H. Pine oil effects on chemical and thermal injury in mice and cultured mouse dorsal root ganglion neurons. Phytother. Res. 2014, 28, 252–260. [Google Scholar] [CrossRef]
- Tamura, Y.; Lai, K.; Bradley, G. A soluble factor induced by an extract from Pinus parviflora Sieb. Zucc. can inhibit the replication of human immunodeficiency virus in vitro. Proc. Natl. Acad. Sci. USA 1991, 88, 2249–2253. [Google Scholar] [CrossRef]
- Arhima, M.H.; Gulati, O.P.; Sharma, S.C. The effect of Pycnogenol on fluoride-induced rat kidney lysosomal damage in vitro. Phytother. Res. 2004, 18, 244–246. [Google Scholar] [CrossRef]
- Ankney, E.; Swor, K.; Satyal, P.; Setzer, W.N. Essential oil compositions of Pinus species (P. contorta subsp. contorta, P. ponderosa var. ponderosa, and P. flexilis); enantiomeric distribution of terpenoids in Pinus species. Molecules 2022, 27, 5658. [Google Scholar]
- Venkatesan, T.; Choi, Y.W.; Mun, S.P.; Kim, Y.K. Pinus radiata bark extract induces caspase-independent apoptosis-like cell death in MCF-7 human breast cancer cells. Cell Biol. Toxicol. 2016, 32, 451–464. [Google Scholar] [CrossRef]
- Simard, F.; Legault, J.; Lavoie, S.; Mshvildadze, V.; Pichette, A. Isolation and identification of cytotoxic compounds from the wood of Pinus resinosa. Phytother. Res. 2008, 22, 919–922. [Google Scholar] [CrossRef]
- Legault, J.; Girard-Lalancette, K.; Dufour, D.; Pichette, A. Antioxidant potential of bark extracts from boreal forest conifers. Antioxidants 2013, 2, 77–89. [Google Scholar] [CrossRef] [PubMed]
- Taha, A.S.; Abo-Elgat, W.; Salem, M.; Salim, E. Pinus rigida wood extract as an antifungal activity for a model paper and leather comparable to historical manuscripts: An experimental research. Egypt. J. Chem. 2025, 68, 29–41. [Google Scholar] [CrossRef]
- Kaushik, D.; Kumar, A.; Kaushik, P.; Rana, A.C. Analgesic and anti-inflammatory activity of Pinus roxburghii Sarg. Adv. Pharmacol. Pharm. Sci. 2012, 2012, 245431. [Google Scholar]
- Aman, Z.; Afzal, A.; Masood, F.; Mahmood, A.; Anwar, W. Pharmacognostic studies and antimicrobial investigation of bark, roots, and cones of Pinus roxburghii Sargent. Pak. J. Phytopathol. 2023, 35, 1–15. [Google Scholar] [CrossRef]
- Lantto, T.A.; Dorman, H.D.; Shikov, A.N.; Pozharitskaya, O.N.; Makarov, V.G.; Tikhonov, V.P.; Raasmaja, A. Chemical composition, antioxidative activity and cell viability effects of a Siberian pine (Pinus sibirica Du Tour) extract. Food Chem. 2009, 112, 936–943. [Google Scholar] [CrossRef]
- Averina, E.S.; Seewald, G.; Müller, R.H.; Radnaeva, L.D.; Popov, D.V. Nanostructured lipid carriers (NLC) on the basis of Siberian pine (Pinus sibirica) seed oil. Die Pharm.—Int. J. Pharm. Sci. 2010, 65, 25–31. [Google Scholar]
- Akbulut, S.; Zengin, Z. Ethnobotanical survey of wild plants used in Gümüşhane province (Turkey). Boletín Latinoam. Y Caribe Plantas Med. Y Aromáticas 2023, 22, 160–172. [Google Scholar] [CrossRef]
- Liang, S.B.; Liang, N.; Bu, F.L.; Lai, B.Y.; Zhang, Y.P.; Cao, H.J.; Liu, J.P. The potential effects and use of Chinese herbal medicine pine pollen (Pinus pollen): A bibliometric analysis of pharmacological and clinical studies. World J. Tradit. Chin. Med. 2020, 6, 163–170. [Google Scholar] [CrossRef]
- Cheng, Y.; Wang, Z.; Quan, W.; Xue, C.; Qu, T.; Wang, T.; He, Z. Pine pollen: A review of its chemical composition, health effects, processing, and food applications. Trends Food Sci. Technol. 2023, 138, 599–614. [Google Scholar] [CrossRef]
- Adams, J.; Gibson, K.E.; Martin, E.M.; Almeida, G.; Ricke, S.C.; Frederick, N.; Carrier, D.J. Characterization and variation of essential oil from Pinus taeda and antimicrobial effects against antibiotic-resistant and -susceptible Staphylococcus aureus. For. Prod. J. 2014, 64, 161–165. [Google Scholar] [CrossRef]
- Kuo, P.C.; Li, Y.C.; Kusuma, A.M.; Tzen, J.T.; Hwang, T.L.; Ye, G.H.; Wang, S.Y. Anti-inflammatory principles from the needles of Pinus taiwanensis Hayata and in silico studies of their potential anti-aging effects. Antioxidants 2021, 10, 598. [Google Scholar] [CrossRef] [PubMed]
- Chamawan, P.; Thisayakorn, K.; Phornchirasilp, S. Effects of pine pollen extract in relieving hot flushes in sex hormone-deficient rats. Thai J. Pharmacol. 2017, 39, 19–37. [Google Scholar]
- Park, J.S.; Lee, G.H. Volatile compounds and antimicrobial and antioxidant activities of the essential oils of the needles of Pinus densiflora and Pinus thunbergii. J. Sci. Food Agric. 2011, 91, 703–709. [Google Scholar] [CrossRef]
- Silva, K.R.D.; Damasceno, J.L.; Inácio, M.D.O.; Abrão, F.; Ferreira, N.H.; Tavares, D.C.; Martins, C.H.G. Antibacterial and cytotoxic activities of Pinus tropicalis and Pinus elliottii resins and of the diterpene dehydroabietic acid against bacteria that cause dental caries. Front. Microbiol. 2019, 10, 987. [Google Scholar] [CrossRef]
- Stewart, C.D.; Jones, C.D.; Setzer, W.N. Essential oil compositions of Juniperus virginiana and Pinus virginiana, two important trees in Cherokee traditional medicine. Am. J. Essent. Oil Nat. Prod. 2014, 2, 17–24. [Google Scholar]
- Ahmad, K.; Ahmad, M.; Weckerle, C. Ethnoveterinary medicinal plant knowledge and practice among the tribal communities of Thakht-e-Sulaiman hills, West Pakistan. J. Ethnopharmacol. 2015, 170, 275–283. [Google Scholar] [CrossRef]
- Thai, T.H.; Cuong, N.T.; Hien, N.T.; Paoli, M.; Casanova, J.; Tomi, F. Chemical composition of needle and twig essential oils from Pinus krempfii Lecomte, an endemic species to Vietnam. J. Essent. Oil Res. 2021, 33, 63–68. [Google Scholar] [CrossRef]
- Lei, T.; Li, Y.; Li, D.M.; Liu, G.M.; Liu, J.K.; Wang, F. A novel phenolic compound from Pinus yunnanensis. J. Asian Nat. Prod. Res. 2011, 13, 425–429. [Google Scholar] [CrossRef]
- Mehmood, S.; Abbasi, A.M.; Hussain, H.; Khan, S.A.; Almutairi, H.H.; Ismail, A.M.; Saikhan, M.S.A.; El-Beltagi, H.S. Phytochemical profile, antioxidant and antibacterial activities analysis of crude extract and essential oil of Pinus roxburghii and Pinus wallichiana: In vitro and in silico analyses. Cogent Food Agric. 2024, 10, 2403648. [Google Scholar] [CrossRef]
- Acra, A.M.; Uota, S.; Yoshihara, M.; Murakami, Y.; Sakagami, H. Potential medicinal efficacy of alkaline extract of pine seed shell: Anti-UVC activity and macrophage activation. In Vivo 2024, 38, 2629–2638. [Google Scholar] [CrossRef]
- Park, J.Y.; Lee, Y.K.; Lee, D.S.; Yoo, J.E.; Shin, M.S.; Yamabe, N.; Kim, S.N.; Lee, S.; Kim, K.H.; Lee, H.J.; et al. Abietic acid isolated from pine resin (Resina Pini) enhances angiogenesis in HUVECs and accelerates cutaneous wound healing in mice. J. Ethnopharmacol. 2017, 203, 279–287. [Google Scholar] [CrossRef] [PubMed]
- Kwak, C.S.; Moon, S.C.; Lee, M.S. Antioxidant, antimutagenic, and antitumor effects of pine needles (Pinus densiflora). Nutr. Cancer 2006, 56, 162–171. [Google Scholar] [CrossRef] [PubMed]
- He, X.Y.; Sun, X.Y.; Yu, Z.Y. Effective components and pharmacological function of pine pollen. J. Northeast. For. Univ. 2007, 35, 78–80. [Google Scholar]
- Jin, X.; Cong, T.; Zhao, L.; Ma, L.; Li, R.; Zhao, P.; Guo, C. The protective effects of Masson pine pollen aqueous extract on CCl4-induced oxidative damage of human hepatic cells. Int. J. Clin. Exp. Med. 2015, 8, 17773. [Google Scholar]
- Lee, K.H.; Kim, A.J.; Choi, E.M. Antioxidant and antiinflammatory activity of pine pollen extract in vitro. Phytother. Res. 2009, 23, 41–48. [Google Scholar] [CrossRef]
- Fan, B.L.; Liu, L.G. Study on the effect of broken pine pollen on blood lipid and its mechanism in rats. Occup. Health 2005, 6, 809–811. [Google Scholar]
- Gerginova, D.; Dimova, D.; Simova, S. Preliminary NMR and chemometric study of pine jams used as medicinal remedies. Bulg. Chem. Commun. 2017, 49, 215–220. [Google Scholar]
- Pak, R.N.; Tusupbekova, M.M.; Batralieva, A.K.; Zhaugasheva, S.K.; Snopkova, V.A.; Rakhimov, K.D.; Adekenov, S.M. Wound-healing and antimicrobial activity of the neutral fraction of pine gallipot. Pharm. Chem. J. 2003, 37, 318–320. [Google Scholar] [CrossRef]
- Rakhimov, K.D.; Pak, R.N.; Tekhneryadnov, A.V.; Adekenov, S.M.; Kul’zhanov, Z.K.; Kim, T.D.; Koishibaev, B.G. Wound-healing activity of bialm ointment. Pharm. Chem. J. 2000, 34, 511–513. [Google Scholar] [CrossRef]
- Lardos, A.; Prieto-Garcia, J.; Heinrich, M. Resins and gums in historical iatrosophia texts from Cyprus–a botanical and medico-pharmacological approach. Front. Pharmacol. 2011, 2, 32. [Google Scholar] [CrossRef] [PubMed]
- Mirković, S.; Martinović, M.; Tadić, V.M.; Nešić, I.; Jovanović, A.S.; Žugić, A. Antimicrobial and antioxidant activity of essential oils from selected Pinus species from Bosnia and Herzegovina. Antibiotics 2025, 14, 677. [Google Scholar] [CrossRef] [PubMed]
- Boulâacheb, N. La Résine de Pinus halepensis Mill. Usage traditionnel par la population de la Petite Kabylie (Algérie, Nord Afrique). Acta Hortic. 2009, 853, 435–438. [Google Scholar] [CrossRef]
- Salhi, N.; El Guourrami, O.; Balahbib, A.; Rouas, L.; Moussaid, S.; Moutawalli, A.; Benkhouili, F.Z.; Ameggouz, M.; Ullah, R.; Alotaibi, A.; et al. Application of Aleppo pine extract for skin burn treatment. J. Appl. Biomater. Funct. Mater. 2024, 22, 22808000241236020. [Google Scholar] [CrossRef]
- Wang, C.; Shang, H.; Cui, W.; Zhou, F.; Zhang, S.; Wang, X.; Gao, P.; Wei, K.; Zhu, R. Pine pollen polysaccharides promote cell proliferation and accelerate wound healing by activating the JAK2–STAT3 signaling pathway. Int. J. Biol. Macromol. 2022, 210, 579–587. [Google Scholar] [CrossRef]
- Fujita, T.; Sezik, E.; Tabata, M.; Yesilada, E.; Honda, G.; Takeda, Y.; Tanaka, T.; Takaishi, Y. Traditional medicine in Turkey VII. Folk medicine in middle and west Black Sea regions. Econ. Bot. 1995, 49, 406–422. [Google Scholar] [CrossRef]
- Cisár, P.; Jány, R.; Waczulíková, I.; Sumegová, K.; Muchová, J.; Vojtaššák, J.; Ďuraćková, Z.; Lisý, M.; Rohdewald, P. Effect of pine bark extract (Pycnogenol®) on symptoms of knee osteoarthritis. Phytother. Res. 2008, 22, 1087–1092. [Google Scholar] [CrossRef]
- Yeşilada, E.; Honda, G.; Sezik, E.; Tabata, M.; Goto, K.; Ikeshiro, Y. Traditional medicine in Turkey IV. Folk medicine in the Mediterranean subdivision. J. Ethnopharmacol. 1993, 39, 31–38. [Google Scholar] [CrossRef]
- Gök, H.N.; Hina, G.Ü.L.; Gülfraz, M.; Asad, M.J.; Öztürk, N.; Şanal, F.; Orhan, I.E. Preclinical study on the hepatoprotective effect of pollen extract of Pinus brutia Ten. (Red Pine) in mice and phenolic acid analysis. Turk. J. Pharm. Sci. 2021, 18, 319. [Google Scholar] [CrossRef]
- Daoudi, N.E.; Bouhrim, M.; Bnouham, M. Review on hepatoprotective effects of some medicinal plant oils. Lett. Drug Des. Discov. 2021, 18, 239–248. [Google Scholar] [CrossRef]
- Hosseini, S.A.; Vali, M.; Haghighi-Zade, M.H.; Siahpoosh, A.; Malihi, R. The effect of Chilgoza pine nut (Pinus gerardiana Wall.) on blood glucose and oxidative stress in diabetic rats. Diabetes Metab. Syndr. Obes. Targets Ther. 2020, 13, 2399–2408. [Google Scholar] [CrossRef]
- Zulfqar, F.; Akhtar, M.F.; Saleem, A.; Akhtar, B.; Sharif, A.; Saleem, U. Chemical characterization, antioxidant evaluation, and antidiabetic potential of Pinus gerardiana (pine nuts) extracts. J. Food Biochem. 2020, 44, e13199. [Google Scholar] [CrossRef]
- Cheong, H.S.; Lim, D.Y. Pine needle extracts inhibit contractile responses of the isolated rat aortic strips. Nat. Prod. Sci. 2010, 16, 123–132. [Google Scholar]
- Nagata, K.; Sakagami, H.; Harada, H.; Nonoyama, M.; Ishihama, A.; Konno, K. Inhibition of influenza virus infection by pine cone antitumor substances. Antivir. Res. 1990, 13, 11–21. [Google Scholar] [CrossRef] [PubMed]
- Gullilat, H.; Kumari, R.; Chandan, G.; Saini, A.K.; Malik, T.; Saini, R.V. Immunomodulatory potential of the ethyl acetate fraction of Pinus roxburghii from the Himalayan region of India towards Ehrlich ascites carcinoma. S. Afr. J. Bot. 2022, 149, 878–886. [Google Scholar] [CrossRef]
- Kayıpmaz, A.E.; Erdem, R.; Yılmaz, C.; Deniz, E.E.; Kavalcı, C.; Özdemir, A.; Güler, I.; Caferoğlu, E.; Kalyoncu, F.S.; Güven, Ö. The effect of Pycnogenol® on spatial learning and memory in rats with experimental closed head injury. Haseki. Tıp Bul. 2017, 55, 101. [Google Scholar] [CrossRef]
- Simpson, T.; Kure, C.; Stough, C. Assessing the efficacy and mechanisms of Pycnogenol® on cognitive aging from in vitro, animal, and human studies. Front. Pharmacol. 2019, 10, 694. [Google Scholar] [CrossRef]
- Xia, R.; Ji, C.; Zhang, L. Neuroprotective effects of Pycnogenol against oxygen–glucose deprivation/reoxygenation-induced injury in primary rat astrocytes via NF-κB and ERK1/2 MAPK pathways. Cell Physiol. Biochem. 2017, 42, 987–998. [Google Scholar] [CrossRef]
- Uhlenhut, K.; Högger, P. Facilitated cellular uptake and suppression of inducible nitric oxide synthase by a metabolite of maritime pine bark extract (Pycnogenol). Free Radic. Biol. Med. 2012, 53, 305–313. [Google Scholar] [CrossRef]
- El Omari, N.; Guaouguaou, F.E.; El Menyiy, N.; Benali, T.; Aanniz, T.; Chamkhi, I.; Balahbib, A.; Taha, D.; Shariati, M.A.; Zengin, G.; et al. Phytochemical and biological activities of Pinus halepensis Mill., and their ethnomedicinal use. J. Ethnopharmacol. 2021, 268, 113661. [Google Scholar] [CrossRef]
- Manzione, M.G.; Vitalini, S.; Sharopov, F.; Capó, X.; Iriti, M.; Martorell, M.; Pezzani, R. Pinus mugo Turra and its therapeutic potential: A narrative review. Nat. Prod. Commun. 2024, 19, 1934578X241265934. [Google Scholar] [CrossRef]
- Nikolic, M.V.; Jakovljevic, V.L.; Bradic, J.V.; Tomovic, M.T.; Petrovic, B.P.; Petrovic, A.M. Korean and Siberian pine: Review of chemical composition and pharmacological profile. Acta Pol. Pharm. 2022, 79, 785–797. [Google Scholar] [CrossRef] [PubMed]
- Shah, A.; Rahim, S.; Shamshad, I. A review on botany, ethnobotany, phytochemistry, ethnopharmacology and conservation status of Pinus gerardiana Wall. ex D. Don—The “elixir of life”. Genet. Resour. Crop Evol. 2025, 72, 5053–5069. [Google Scholar] [CrossRef]
- Schumann, M.; Günzel, D.; Buergel, N.; Richter, J.F.; Troeger, H.; May, C.; Fromm, A.; Sorgenfrei, D.; Daum, S.; Bojarski, C.; et al. Cell polarity-determining proteins Par-3 and PP-1 are involved in epithelial tight junction defects in coeliac disease. Gut 2012, 61, 220–228. [Google Scholar] [CrossRef]
- Guo, M.Q.; Hu, X.; Wang, C.; Ai, L. Polysaccharides: Structure and solubility. Solubility Polysacch. 2017, 2, 8–21. [Google Scholar]
- Kohlert, C.; Van Rensen, I.; Marz, R.; Schindler, G.; Graefe, E.U.; Veit, M. Bioavailability and pharmacokinetics of natural volatile terpenes in animals and humans. Planta Med. 2000, 66, 495–505. [Google Scholar] [CrossRef]
- Schafer, D.; Schafer, W. Pharmakologische Untersuchungen zur broncholytischen und sekretolytisch-expektorierenden Wirksamkeit einer Salbe auf Basis von Menthol, Campher und ätherischen Ölen. Arzneim.-Forsch. 1981, 31, 82–86. [Google Scholar]
- Ostad, S.N.; Vazirian, M.; Pahlevani, R.; Hadjiakhondi, A.; Hamedani, M.P.; Almasian, A.; Manayi, A. Toxicity evaluation of aromatic water of Pinus eldarica Medw. in acute and sub-chronic toxicity experiments. Prog. Nutr. 2018, 20, 68–74. [Google Scholar]
- Sahin, H.T.; Yalcin, O.U. Conifer cones: An alternative raw material for industry. Br. J. Pharm. Res. 2017, 17, 1–9. Available online: https://journaljpri.com/index.php/JPRI/article/view/162/324 (accessed on 6 June 2025). [CrossRef]
- Semeniuc, C.A.; Rotar, A.; Stan, L.; Pop, C.R.; Socaci, S.; Mireşan, V.; Muste, S. Characterization of pine bud syrup and its effect on physicochemical and sensory properties of kefir. J. Food 2016, 14, 213–218. [Google Scholar] [CrossRef]
- Singh, L.; Dixit, P.; Upadhyay, A.K.; Srivastava, R.P.; Pandey, S.; Verma, P.C.; Saxena, G. Assessment of total phenolic and flavonoid contents and potential biological efficacy of few Pinus species growing in Northern Himalayas. Agric. Conspec. Sci. 2020, 85, 335–344. [Google Scholar]
- Alami, K.; Mousavi, S.Y. Afghan Chehelghoza (Pinus gerardiana L.) pine nut diet enhances the learning and memory in male rats. Nutr. Diet. Suppl. 2020, 12, 277–288. [Google Scholar] [CrossRef]
- Bhardwaj, K.; Sharma, R.; Cruz-Martins, N.; Valko, M.; Upadhyay, N.K.; Kuča, K.; Bhardwaj, P. Studies of phytochemicals, antioxidant, and antibacterial activities of Pinus gerardiana and Pinus roxburghii seed extracts. BioMed Res. Int. 2022, 2022, e5938610. [Google Scholar] [CrossRef] [PubMed]
- Kadri, N.; Khettal, B.; Adjebli, A.; Cresteil, T.; Yahiaoui-Zaidi, R.; Barragan-Montero, V.; Montero, J.L. Antiangiogenic activity of neutral lipids, glycolipids, and phospholipids fractions of Pinus halepensis Mill. seeds. Ind. Crops Prod. 2014, 54, 6–12. [Google Scholar] [CrossRef]
- Li, X.; Horishita, T.; Toyohira, Y.; Shao, H.; Bai, J.; Bo, H.; Song, X.; Ishikane, S.; Yoshinaga, Y.; Satoh, N.; et al. Inhibitory effects of pine nodule extract and its component, SJ-2, on acetylcholine-induced catecholamine secretion and synthesis in bovine adrenal medullary cells. J. Pharmacol. Sci. 2017, 133, 268–275. [Google Scholar] [CrossRef] [PubMed]
- Gonzalez, M.A.; Perez-Guaita, D.; Correa-Royero, J.; Zapata, B.; Agudelo, L.; Mesa-Arango, A.; Betancur-Galvis, L. Synthesis and biological evaluation of dehydroabietic acid derivatives. Eur. J. Med. Chem. 2010, 45, 811–816. [Google Scholar] [CrossRef]
- Gao, Y.; Liu, Z.; Fang, X.; Lin, C.; Pan, J.; Shen, L.; Chen, J.; Chen, L.; Liu, J. Abietic acid attenuates allergic airway inflammation in a mouse allergic asthma model. Int. Immunopharmacol. 2016, 38, 261–266. [Google Scholar] [CrossRef]
- El-Hallouty, S.M.; Soliman, A.A.; Nassrallah, A.; Salamatullah, A.; Alkaltham, M.S.; Kamal, K.Y.; Hanafy, E.A.; Gaballa, H.S.; Aboul-Soud, M.A. Crude methanol extract of rosin gum exhibits specific cytotoxicity against human breast cancer cells via apoptosis induction. Anti-Cancer Agents Med. Chem. 2020, 20, 1028–1036. [Google Scholar] [CrossRef]
- Pipingas, A.; Silberstein, R.B.; Vitetta, L.; Van Rooy, C.; Harris, E.V.; Young, J.M.; Frampton, C.M.; Sali, A.; Nastasi, J. Improved cognitive performance after dietary supplementation with a Pinus radiata bark extract formulation. Phytother. Res. 2008, 22, 1168–1174. [Google Scholar] [CrossRef]
- Rihn, B.; Saliou, C.; Bottin, M.C.; Keith, G.; Packer, L. From ancient remedies to modern therapeutics: Pine bark uses in skin disorders revisited. Phytother. Res. 2001, 15, 76–78. [Google Scholar] [CrossRef]
- Kızılarslan, Ç.; Sevgi, E. Ethnobotanical uses of genus Pinus L. (Pinaceae) in Turkey. Indian J. Trad. Med. 2013, 12, 209–220. [Google Scholar]
- Chen, J.Q.; Zhang, L.Z.; Ma, J.; Li, C.J.; Zang, Y.D.; Sun, H.; Zhang, D.M. Three undescribed diterpenoids from Pini Lignum Nodi with hepatoprotective activities. J. Asian Nat. Prod. Res. 2025, 27, 854–863. [Google Scholar] [CrossRef]
- Achim, F.; Dinca, L.; Chira, D.; Raducu, R.; Chirca, A.; Murariu, G. Sustainable management of willow forest landscapes: A review of ecosystem functions and conservation strategies. Land 2025, 14, 1593. [Google Scholar] [CrossRef]
- Dincă, L.; Coca, A.; Tudose, N.C.; Marin, M.; Murariu, G.; Munteanu, D. The role of trees in sand dune rehabilitation: Insights from global experiences. Appl. Sci. 2025, 15, 7358. [Google Scholar] [CrossRef]
- Enescu, C.M.; Mihalache, M.; Ilie, L.; Dincă, L.; Constandache, C.; Murariu, G. Agricultural benefits of shelterbelts and windbreaks: A bibliometric analysis. Agriculture 2025, 15, 1204. [Google Scholar] [CrossRef]
- Bratu, I.; Dincă, L.; Schiteanu, I.; Mocanu, G.; Murariu, G.; Stanciu, M.; Zhiyanski, M. Sports in natural forests: A systematic review of environmental impact and compatibility for readability. Sports 2025, 13, 250. [Google Scholar] [CrossRef] [PubMed]
- Murariu, G.; Dincă, L.; Munteanu, D. Trends and applications of principal component analysis in forestry research: A literature and bibliometric review. Forests 2025, 16, 1155. [Google Scholar] [CrossRef]
- Dincă, L.; Crișan, V.; Murariu, G.; Țupu, E. Oxalis acetosella in forests: A systematic bibliometric study over the last 47 years. Sci. Pap. Ser. B Hortic. 2025, 69, 756–761. [Google Scholar]
- Budău, R.; Timofte, C.S.C.; Mirisan, L.V.; Bei, M.; Dincă, L.; Murariu, G.; Racz, K.A. Living landmarks: A review of monumental trees and their role in ecosystems. Plants 2025, 14, 2075. [Google Scholar] [CrossRef]
- Dincă, L.; Constandache, C.; Postolache, R.; Murariu, G.; Țupu, E. Timber harvesting in mountainous regions: A comprehensive review. Forests 2025, 16, 495. [Google Scholar] [CrossRef]
- Timis-Gansac, V.; Dincă, L.; Tudose, N.C.; Constandache, C.; Murariu, G.; Cheregi, G.; Moțiu, P.T.; Derecichei, L.M. Community-based conservation in mountain forests: Patterns, challenges, and policy implications. Trees For. People 2025, 22, 101041. [Google Scholar] [CrossRef]
- Dincă, L.; Lupoae, M.; Murariu, G.; Țupu, E.; Munteanu, D. Conservation biodiversity in silver fir (Abies alba Mill.) forests: A bibliometric review. Int. J. Conserv. Sci. 2025, 16, 1529–1544. [Google Scholar] [CrossRef]
- Mihalache, A.L.; Marin, M.; Davidescu, Ș.O.; Ungurean, C.; Adorjani, A.; Tudose, N.C.; Davidescu, A.A.; Clinciu, I. Physical status of torrent control structures in Romania. Environ. Eng. Manag. J. 2020, 19, 861–872. [Google Scholar] [CrossRef]
- Marin, M.; Clinciu, I.; Tudose, N.C.; Ungurean, C.; Mihalache, A.L.; Mărțoiu, N.E.; Tudose, O.N. Assessment of seasonal surface runoff under climate and land use change scenarios for a small forested watershed: Upper Tarlung Watershed (Romania). Water 2022, 14, 2860. [Google Scholar] [CrossRef]
- Davidescu, Ș.O.; Clinciu, I.; Tudose, N.C.; Ungurean, C. An evaluating methodology for hydrotechnical torrent-control structures condition. Ann. For. Res. 2012, 55, 125–143. [Google Scholar] [CrossRef]
- Tudose, N.C.; Petritan, I.C.; Toiu, F.L.; Petritan, A.-M.; Marin, M. Relation between topography and gap characteristics in a mixed sessile oak–beech old-growth forest. Forests 2023, 14, 188. [Google Scholar] [CrossRef]
- Marin, M.; Tudose, N.C.; Ungurean, C.; Mihalache, A.L. Application of life cycle assessment for torrent control structures: A review. Land 2024, 13, 1956. [Google Scholar] [CrossRef]
- Vasile, D.; Petritan, A.-M.; Tudose, N.C.; Toiu, F.L.; Scarlatescu, V.; Petritan, I.C. Structure and spatial distribution of dead wood in two temperate old-growth mixed European beech forests. Not. Bot. Horti Agrobot. Cluj-Napoca 2017, 45, 639–645. [Google Scholar] [CrossRef]
- Mustățea, M.; Clius, M.; Tudose, N.C.; Cheval, S. An enhanced Machado Index of naturalness. Catena 2022, 212, 106091. [Google Scholar] [CrossRef]
- Oprică, R.; Tudose, N.C.; Davidescu, Ș.O.; Zup, M.; Marin, M.; Comanici, A.N.; Crit, M.N.; Pitar, D. Gender inequalities in Transylvania’s largest peri-urban forest usage. Ann. For. Res. 2022, 65, 57–69. [Google Scholar] [CrossRef]
- Budeanu, M.; Popescu, F.; Beșliu, E.; Apostol, N.E. Diallel crossing (10 × 10) in Swiss stone pine. Juvenile-adult correlations and genetic gain for predicting forward selection. Ann. For. Res. 2024, 67, 109–120. [Google Scholar] [CrossRef]
- Budeanu, M.; Beșliu, E.; Pepelea, D. Testing the radial increment and climate–growth relationship between Swiss stone pine European provenances in the Romanian Carpathians. Forests 2025, 16, 391. [Google Scholar] [CrossRef]
- Beșliu, E.; Curtu, A.L.; Apostol, E.N.; Budeanu, M. Using adapted and productive European beech (Fagus sylvatica L.) provenances as future solutions for sustainable forest management in Romania. Land 2024, 13, 183. [Google Scholar] [CrossRef]
- Budeanu, M.; Șofletea, N.; Petrițan, I.C. Among-population variation in quality traits in two Romanian provenance trials with Picea abies L. Balt. For. 2014, 20, 37–47. [Google Scholar]
- Șofletea, N.; Curtu, A.L.; Daia, M.L.; Budeanu, M. The dynamics and variability of radial growth in provenance trials of Norway spruce (Picea abies (L.) Karst.) within and beyond the hot margins of its natural range. Not. Bot. Horti Agrobot. Cluj-Napoca 2015, 43, 265–271. [Google Scholar] [CrossRef]
- Tudor, C.; Constandache, C.; Dincă, L.; Murariu, G.; Badea, N.O.; Tudose, N.C.; Marin, M. Pine afforestation on degraded lands: A global review of carbon sequestration potential. Front. For. Glob. Change 2025, 8, 1648094. [Google Scholar] [CrossRef]
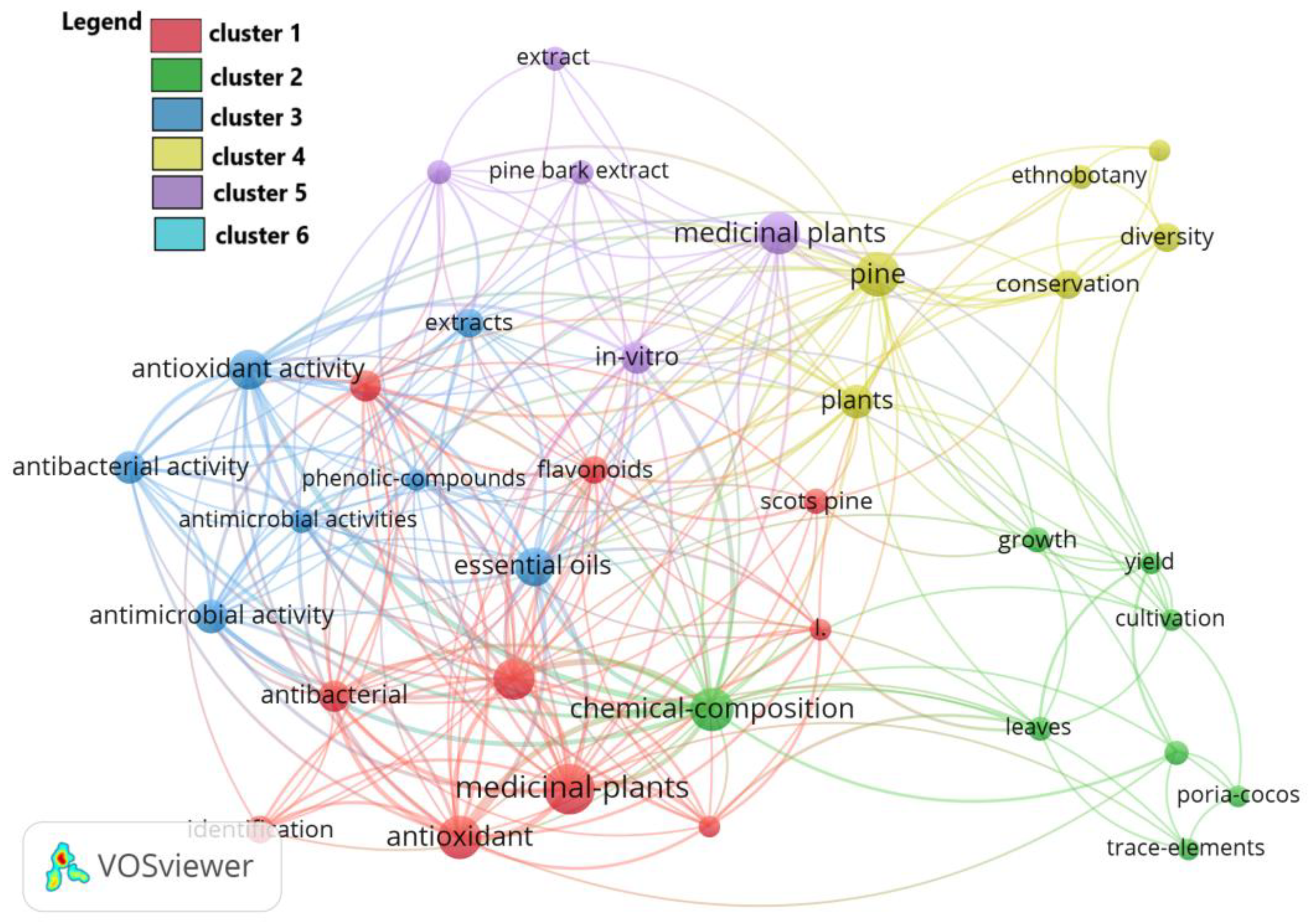
| Crt. No. | Keyword | Occurrences | Total Link Strength |
|---|---|---|---|
| 1 | chemical-composition | 19 | 66 |
| 2 | medicinal-plants | 26 | 59 |
| 3 | essential oil | 17 | 56 |
| 4 | antioxidant | 19 | 54 |
| 5 | antioxidant activity | 16 | 49 |
| 6 | pine | 20 | 38 |
| 7 | antimicrobial activity | 12 | 36 |
| 8 | antibacterial | 10 | 31 |
| 9 | constituents | 10 | 31 |
| 10 | in vitro | 10 | 28 |
| 11 | antibacterial activity | 11 | 27 |
| 12 | plants | 12 | 27 |
| 13 | extracts | 9 | 23 |
| 14 | flavonoides | 8 | 23 |
| Cur. No. | Species | Country | Citing Article |
|---|---|---|---|
| 1 | Pinus albicaulis Engelm. | USA | Moore et al., 2025 [39] |
| 2 | Pinus aristata Engelm. | Greece | Ioannou et al., 2014 [40] |
| 3 | Pinus arizonica Engelm. | Mexico | Delgado-Alvarado et al., 2022 [41] |
| 4 | Pinus armandii Franch. | China | Yang et al., 2005 [42] |
| 5 | Pinus attenuata Lemmon | Greece | Koutsaviti et al., 2021 [43] |
| 6 | Pinus ayacahuite Ehrenb. ex Schltdl. | Mexico | Rosales Castro et al., 2006 [44] |
| 7 | Pinus banksiana Lamb. | Canada, India | Georges et al., 2012 [45]; Elangovan, 2024 [46] |
| 8 | Pinus balfouriana Balf. | USA | Haagen-Smit et al., 1950 [47]; Stekrova et al., 2015 [48] |
| 9 | Pinus brutia Ten. | Turkey; Syria | Süntar et al., 2012 [49]; Asmaa et al., 2016 [50]; Mehrzadi et al. [51] |
| 10 | Pinus canariensis C. Sm. | Egypt | Kamal et al., 2024 [52] |
| 11 | Pinus caribaea Morelet | New Caledonia, France | Sinyeue et al., 2023 [53] |
| 12 | Pinus cembra L. | Slovakia | Nikolić et al., 2018 [54] |
| 13 | Pinus cembroides Zucc. | Mexico | Delgado-Alvarado et al., 2022 [41] |
| 14 | Pinus chiapensis | Mexico | del Castillo & Acosta, 2002 [55] |
| 15 | Pinus contorta Douglas | USA | Swor et al., 2023 [56] |
| 16 | Pinus coulteri D.Don | Algeria | Merah et al., 2018 [57] |
| 17 | Pinus cooperi Blanco | Mexico | Rosales-Castro et al., 2009 [58] |
| 18 | Pinus culminicola Andresen & Beaman | Greece | Ioannou et al., 2014 [40] |
| 19 | Pinus dalatensis Ferré | Vietnam | Sa et al., 2018 [59] |
| 20 | Pinus densata Mast. | China | Yue et al., 2013 [60] |
| 21 | Pinus densiflora Siebold & Zucc. | Korea | Kim et al., 2024 [61] |
| 22 | Pinus dabeshanensis W.C.Cheng & Y.W.Law | China | Hu et al., 2016 [62]; Peng et al., 2020 [63] |
| 23 | Pinus durangensis Martínez | Mexico | Delgado-Alvarado et al., 2022 [41] |
| 24 | Pinus echinata Mill. | USA | Mickles et al., 2024 [64] |
| 25 | Pinus edulis | USA | Poulson et al., 2020 [65] |
| 26 | Pinus elliottii Engelm. | Brazil, Japan | Leandro et al., 2014 [66]; Satoh et al., 1999 [67] |
| 27 | Pinus engelmannii Carr. | Mexico | Rosales-Castro et al., 2009 [58] |
| 28 | Pinus fenzeliana Hand.-Mazz. | Vietnam | Tran et al., 2023 [68] |
| 29 | Pinus gerardiana Wall. ex D. Don | Bangladesh | Ansari et al., 2023 [69] |
| 30 | Pinus greggii Engelm. ex Parl. | India | Singh et al., 2020 [70] |
| 31 | Pinus halepensis Mill. | Turkey, Marocco | Süntar et al., 2012 [49]; Amrati et al., 2021 [71] |
| 32 | Pinus heldreichii Christ | Austria | Basholli-Salihu et al., 2017 [72] |
| 33 | Pinus henryi Mast. | China | Xie et al., 2015 [73] |
| 34 | Pinus jeffreyi Balf. | Tunisia, Italy | Khedhri et al., 2024 [74] |
| 35 | Pinus kesiya Royle ex Gordon | India | Weerapreeyakul et al., 2016 [75] |
| 36 | Pinus koraiensis Siebold & Zucc. | Korea | Hong et al., 2017 [76] |
| 37 | Pinus krempfii Lecomte | Vietnam | Thai et al., 2021 [77] |
| 38 | Pinus lambertiana Douglas | USA | Moore et al., 2025 [39] |
| 39 | Pinus latteri Mason | Vietnam | Vo et al., 2023 [78] |
| 40 | Pinus leiophylla Schiede ex Schltdl. & Cham. | Mexico | Rosales-Castro et al., 2009 [58] |
| 41 | Pinus longaeva Bailey | UK | Loader et al., 2015 [79] |
| 42 | Pinus luchuensis Mayr. | Japan | Minami et al., 2002 [80]; Takashi, & Shoei, 2022 [81] |
| 43 | Pinus massoniana Lamb. | China | Cui et al., 2021 [82] |
| 44 | Pinus merkusii Jungh. & de Vriese | Indonesia | Wardani et al., 2019 [83] |
| 45 | Pinus monophyla Torr. & Frém. | Greece | Ioannou et al., 2014 [40] |
| 46 | Pinus monticola Douglas ex D. Don | USA | Moore et al., 2025 [39] |
| 47 | Pinus morrisonicola Hayata | Taiwan | Liu et al., 2023 [84]; Hou et al., 2024 [85] |
| 48 | Pinus mugo Turra | Austria, R. Macedonia; Serbia, Bosnia and Herzegovina, Slovenia | Basholli-Salihu et al., 2017 [72]; Karapandzova et al., 2018 [86]; Nikolić et al., 2024 [87] |
| 49 | Pinus muricata D.Don | Greece | Ioannou et al., 2014 [40] |
| 50 | Pinus nigra | Turkey | Süntar et al., 2012 [49]; Arı et al., 2014 [88]; Gülçin et al., 2003 [89]; Chalchat et al., 1995 [90] |
| 51 | Pinus oocarpa Schiede ex Schltdl. | Germany, Colombia | Rubio et al., 2005 [91]; Sarria-Villa et al., 2021 [92] |
| 52 | Pinus palustris Mill. | USA | Clark et al., 2014 [93] |
| 53 | Pinus patula Schiede ex Schltdl. & Cham. | Colombia | Sarria-Villa et al., 2021 [92] |
| 54 | Pinus parviflora Siebold & Zucc. | USA | Tamura et al., 1991 [94] |
| 55 | Pinus peuce Griseb. | Austria | Basholli-Salihu et al., 2017 [72] |
| 56 | Pinus pinaster Aiton | Ireland | Arhima et al., 2004 [95] |
| 57 | Pinus pinea L. | Turkey | Süntar et al., 2012 [49] |
| 58 | Pinus ponderosa Douglas ex C.Lawson | USA | Ankney et al., 2022 [96] |
| 59 | Pinus pumila (Pall.) Regel | Greece | Ioannou et al., 2014 [40] |
| 60 | Pinus radiata D.Don | South Korea | Venkatesan et al., 2016 [97] |
| 61 | Pinus resinosa Sol. ex Aiton | Canada | Simard et al., 2008 [98]; Legault et al., 2013 [99] |
| 62 | Pinus rigida Mill. | Egypt | Taha et al., 2025 [100] |
| 63 | Pinus roxburghii Sarg. | India; Australia | Kaushik et al., 2012 [101]; Aman et al., 2023 [102] |
| 64 | Pinus sibirica Du Tour | Finland, Russia | Lantto et al., 2009 [103]; Averina et al., 2010 [104] |
| 65 | Pinus strobus L. | Greece | Ioannou et al., 2014 [40] |
| 66 | Pinus sylvestris L. | Turkey | Akbulut and Zengin, 2023 [105] |
| 67 | Pinus tabuliformis Carriere | China | Liang et al., 2020 [106]; Cheng et al., 2023 [107] |
| 68 | Pinus taeda L. | Adams et al., 2014 [108] | |
| 69 | Pinus taiwanensis Hayata | Taiwan | Kuo et al., 2021 [109] |
| 70 | Pinus tecunumanii Schwerdtf. ex Eguiluz & Perry | Thailand | Chamawan et al., 2017 [110] |
| 71 | Pinus teocote Schiede ex Schltdl. & Cham. | Mexico | Rosales-Castro et al., 2009 [58] |
| 72 | Pinus thunbergii Parl. | South Korea | Park & Lee, 2011 [111] |
| 73 | Pinus tropicalis Morelet | Brazil | Silva et al., 2019 [112] |
| 74 | Pinus virginiana Mill. | USA | Stewart et al., 2014 [113] |
| 75 | Pinus wallichiana A.B. Jacks. | Pakistan | Ahmad et al., 2015 [114] |
| 76 | Pinus wangii subsp. kwangtungensis | Vietnam | Thai et al., 2018 [115] |
| 77 | Pinus yunnanensis Franch. | China | Lei et al., 2011 [116]; Mehmood et al., 2024 [117] |
| Species | Major Compound Classes | Representative Bioactive Constituents | Reported Biological Relevance |
|---|---|---|---|
| Pinus massoniana | Diterpenoids, flavonoids, polysaccharides | Abietic acid, neoabietic acid, pinusins A–C, catechin, PPPS (PPP-1, -2, -3) | Wound healing, hepatoprotection, antioxidant, antiviral (ALV-J), anti-inflammatory |
| Pinus densiflora | Phenolics, flavonoids, lignans, terpenoids | Catechin, taxifolin, pinosylvin, α-pinene, β-pinene, limonene | Antioxidant, antimutagenic, anticancer, anti-inflammatory |
| Pinus pinaster | Procyanidins, catechins, phenolic acids, terpenoids | Procyanidin B1/B3, catechin, caffeic/ferulic acids, α-pinene | Antioxidant, anti-inflammatory, cardioprotective, neuroprotective |
| Pinus halepensis | Essential oils, resin acids, phenolics | α-pinene, β-caryophyllene, limonene, abietic acid, gallic acid | Wound healing, antimicrobial, anti-inflammatory |
| Pinus brutia | Essential oils, resin diterpenoids, flavonoids | α-pinene, β-pinene, abietic acid derivatives | Wound healing, antihyaluronidase, antimicrobial |
| Pinus sibirica | Phenolics, tannins, proanthocyanidins, fatty acids | Gallic acid, cinnamic acid derivatives, flavan-3-ols, linoleic/oleic acids | Antioxidant, anti-inflammatory, nutritional; nanocarrier formulations |
| Pinus gerardiana | Fatty acids, phenolics, sterols | Gallic acid, ellagic acid, pinolenic acid, β-sitosterol | Antidiabetic, hypolipidemic, antioxidant, tonic/aphrodisiac traditional uses |
| Pinus koraiensis | Fatty acids, terpenoids, phenolics | Pinolenic acid, linoleic acid, catechins, α-pinene | Anti-obesity, hepatoprotective, anti-inflammatory |
| Pinus tabuliformis | Terpenoids, flavonoids, lignans | Abietic acid, taxifolin, pinosylvin | Anti-inflammatory, antioxidant, hepatoprotective |
| Pinus pinea | Essential oils, fatty acids, terpenoids | α-pinene, β-pinene, limonene, tocopherols, oleic/pinolenic acids | Wound healing, antimicrobial, circulatory benefits |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Munteanu, D.; Murariu, G.; Lupoae, M.; Dinca, L.; Chira, D.; Popa, A.-S. Global Perspectives on the Medicinal Potential of Pines (Pinus spp.). Forests 2025, 16, 1772. https://doi.org/10.3390/f16121772
Munteanu D, Murariu G, Lupoae M, Dinca L, Chira D, Popa A-S. Global Perspectives on the Medicinal Potential of Pines (Pinus spp.). Forests. 2025; 16(12):1772. https://doi.org/10.3390/f16121772
Chicago/Turabian StyleMunteanu, Dan, Gabriel Murariu, Mariana Lupoae, Lucian Dinca, Danut Chira, and Andy-Stefan Popa. 2025. "Global Perspectives on the Medicinal Potential of Pines (Pinus spp.)" Forests 16, no. 12: 1772. https://doi.org/10.3390/f16121772
APA StyleMunteanu, D., Murariu, G., Lupoae, M., Dinca, L., Chira, D., & Popa, A.-S. (2025). Global Perspectives on the Medicinal Potential of Pines (Pinus spp.). Forests, 16(12), 1772. https://doi.org/10.3390/f16121772







