Growth Performance of Tamanu (Calophyllum inophyllum L.) in Relation to Peatland Restoration in South Sumatra and Central Kalimantan, Indonesia
Abstract
1. Introduction
2. Materials and Methods
2.1. Research Site and Tree Planting
2.2. Soil Analysis
2.3. Water Level and Rainfall Measurement
2.4. Growth Measurement
2.5. Statistical Analysis
3. Results
3.1. Site Characteristics
3.1.1. Soil Properties
3.1.2. Water Level and Rainfall
3.2. Growth Performance
3.2.1. Survival Rate
3.2.2. Tree Growth
4. Discussion
4.1. Effects of Environmental Factors on Growth Performance
4.2. Implications for Tamanu Restoration Management
4.3. Limitations and Future Research Directions
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Dargie, G.C.; Lewis, S.L.; Lawson, I.T.; Mitchard, E.T.A.; Page, S.E.; Bocko, Y.E.; Ifo, S.A. Age, extent and carbon storage of the central Congo Basin peatland complex. Nature 2017, 542, 86–90. [Google Scholar] [CrossRef] [PubMed]
- Xu, J.; Morris, P.J.; Liu, J.; Holden, J. PEATMAP: Refining estimates of global peatland distribution based on a meta-analysis. CATENA 2018, 160, 134–140. [Google Scholar] [CrossRef]
- Joosten, H. The Global Peatland CO2 Picture: Peatland Status and Drainage Related Emissions in All Countries of the World; Wetlands International: Wageningen, The Netherlands, 2009. [Google Scholar]
- Lehner, B.; Döll, P. Development and validation of a global database of lakes, reservoirs and wetlands. J. Hydrol. 2004, 296, 1–22. [Google Scholar] [CrossRef]
- Leifeld, J.; Menichetti, L. The underappreciated potential of peatlands in global climate change mitigation strategies. Nat. Commun. 2018, 9, 1071. [Google Scholar] [CrossRef]
- Kopansky, D.; Reed, M.; Kaplan, M.; Hughes, J.; Nuutinen, M.; Peters, J.; Barthelmes, A.; Salathe, T.; Miles, L.; Tamelander, J.; et al. Global Peatlands Assessment—The State of the World’s Peatlands: Evidence for Action Toward the Conservation, Restoration, and Sustainable Management of Peatlands; UN Environment Programme: Nairobi, Kenya, 2022. [Google Scholar] [CrossRef]
- Puspitaloka, D.; Kim, Y.S.; Purnomo, H.; Fulé, P.Z. Analysis of challenges, costs, and governance alternative for peatland restoration in Central Kalimantan, Indonesia. Trees For. People 2021, 6, 100131. [Google Scholar] [CrossRef]
- Bonn, A.; Reed, M.S.; Evans, C.D.; Joosten, H.; Bain, C.; Farmer, J.; Emmer, I.; Couwenberg, J.; Moxey, A.; Artz, R.; et al. Investing in nature: Developing ecosystem service markets for peatland restoration. Ecosyst. Serv. 2014, 9, 54–65. [Google Scholar] [CrossRef]
- Yang, H.; Chae, J.; Yang, A.R.; Suwignyo, R.A.; Choi, E. Trends of peatland research based on topic modeling: Toward sustainable management under climate change. Forests 2023, 14, 1818. [Google Scholar] [CrossRef]
- Hodgkins, S.B.; Richardson, C.J.; Dommain, R.; Wang, H.; Glaser, P.H.; Verbeke, B.; Winkler, B.R.; Cobb, A.R.; Rich, V.I.; Missilmani, M.; et al. Tropical peatland carbon storage linked to global latitudinal trends in peat recalcitrance. Nat. Commun. 2018, 9, 3640. [Google Scholar] [CrossRef]
- Ribeiro, K.; Pacheco, F.S.; Ferreira, J.W.; de Sousa-Neto, E.R.; Hastie, A.; Krieger Filho, G.C.; Alvalá, P.C.; Forti, M.C.; Ometto, J.P.; Forti, M.C.; et al. Tropical peatlands and their contribution to the global carbon cycle and climate change. Glob. Change Biol. 2021, 27, 489–505. [Google Scholar] [CrossRef]
- Anda, M.; Ritung, S.; Suryani, E.; Sukarman, M.; Hikmat, M.; Yatno, E.; Mulyani, A.; Subandiono, R.E.; Suratman; Husnain. Revisiting tropical peatlands in Indonesia: Semi-detailed mapping, extent and depth distribution assessment. Geoderma 2021, 402, 115235. [Google Scholar] [CrossRef]
- Asyhari, A.; Gangga, A.; Putra, C.A.S.; Ritonga, R.P.; Candra, R.A.; Anshari, G.Z.; Bowen, J.C.; Perryman, C.R.; Novita, N.; Perryman, C.R.; et al. Quantifying the fluxes of carbon loss from an undrained tropical peatland ecosystem in Indonesia. Sci. Rep. 2024, 14, 11459. [Google Scholar] [CrossRef]
- Lestari, N.S.; Rochmayanto, Y.; Salminah, M.; Novita, N.; Asyhari, A.; Gangga, A.; Ritonga, R.; Yeo, S.; Albar, I.; Yeo, S.; et al. Opportunities and risk management of peat restoration in Indonesia: Lessons learned from peat restoration actors. Restor. Ecol. 2024, 32, e14054. [Google Scholar] [CrossRef]
- Prananto, J.A.; Minasny, B.; Comeau, L.P.; Rudiyanto, R.; Grace, P. Drainage increases CO2 and N2O emissions from tropical peat soils. Glob. Change Biol. 2020, 26, 4583–4600. [Google Scholar] [CrossRef] [PubMed]
- Kunarso, A.; Bonner, M.T.L.; Blanch, E.W.; Grover, S. Differences in tropical peat soil physical and chemical properties under different land uses: A systematic review and meta-analysis. J. Soil Sci. Plant Nutr. 2022, 22, 4063–4083. [Google Scholar] [CrossRef]
- Sinclair, A.L.; Graham, L.L.B.; Putra, E.I.; Saharjo, B.H.; Applegate, G.; Grover, S.P.; Cochrane, M.A. Effects of distance from canal and degradation history on peat bulk density in a degraded tropical peatland. Sci. Total Environ. 2020, 699, 134199. [Google Scholar] [CrossRef]
- Kurnianto, S.; Selker, J.; Boone Kauffman, J.; Murdiyarso, D.; Peterson, J.T. The influence of land-cover changes on the variability of saturated hydraulic conductivity in tropical peatlands. Mitigation Adapt. Strateg. Glob. Change 2019, 24, 535–555. [Google Scholar] [CrossRef]
- Badan Restorasi Gambut (BRG). Rencana Kontijensi Badan Restorasi Gambut: Perubahan (Contingency Planning of Peatland Restoration Agency); Badan Restorasi Gambut: Jakarta, Indonesia, 2016. (In Indonesian)
- Ulya, N.A.; Martin, E.; Rahmat, M.; Premono, B.T.; Malau, L.R.E.; Waluyo, E.A.; Imanullah, A.; Lukman, A.H.; Asmaliyah; Armansyah; et al. Enabling factors of NTFP business development for ecosystem restoration: The case of Tamanu oil in Indonesian degraded peatland. Sustainability 2022, 14, 10681. [Google Scholar] [CrossRef]
- Maulidya, A.; Suwignyo, R.A.; Priadi, D.P.; Baral, H.; Choi, E.; Adriansyah, F.; Yang, H. Survival and growth performance of Calophyllum inophyllum L. seedlings in peat soil and at different levels of groundwater. Land 2024, 13, 879. [Google Scholar] [CrossRef]
- Leksono, B.; Windyarini, E.; Hasnah, T.M.; Maimunah, S.; Artati, Y.; Baral, H. Tamanu (Calophyllum inophyllum) growth performance on different types of degraded peatlands in Central Kalimantan. IOP Conf. Ser. Earth Environ. Sci. 2021, 914, 012009. [Google Scholar] [CrossRef]
- Hirano, T.; Jauhiainen, J.; Inoue, T.; Takahashi, H. Controls on the carbon balance of tropical peatlands. Ecosystems 2009, 12, 873–887. [Google Scholar] [CrossRef]
- Busman, N.A.; Melling, L.; Goh, K.J.; Imran, Y.; Sangok, F.E.; Watanabe, A. Soil CO2 and CH4 fluxes from different forest types in tropical peat swamp forest. Sci. Total Environ. 2023, 858, 159973. [Google Scholar] [CrossRef]
- Irfan, M.; Kurniawati, N.; Ariani, M.; Sulaiman, A.; Iskandar, I. Study of groundwater level and its correlation to soil moisture on peatlands in south Sumatra. J. Phys. Conf. Ser. 2020, 1568, 012028. [Google Scholar] [CrossRef]
- Khakim, M.Y.N.; Bama, A.A.; Tsuji, T. Spatiotemporal variations of soil moisture and groundwater level in a south Sumatra peatland, Indonesia during 2015–2018. Geogr. Environ. Sustain. 2022, 15, 58–70. [Google Scholar] [CrossRef]
- Tata, H.L.; Pradjadinata, S. Native species for degraded peat swamp forest rehabilitation. J. Trop. Silvic. 2016, 7, S80–S82. [Google Scholar] [CrossRef]
- Maimunah, S.; Rahman, S.A.; Samsudin, Y.B.; Artati, Y.; Simamora, T.I.; Andini, S.; Lee, S.M.; Baral, H.; Baral, H. Assessment of suitability of tree species for bioenergy production on burned and degraded peatlands in Central Kalimantan, Indonesia. Land 2018, 7, 115. [Google Scholar] [CrossRef]
- Leksono, B.; Sumedi, N.; Seol, M. Nyamplung (Calophyllum inophyllum)-based agroforestry for landscape restoration and rural livelihoods in Indonesia. In Proceedings of the XV World Forestry Congress, Seoul, Republic of Korea, 2–6 May 2022; FAO: Rome, Italy, 2022. [Google Scholar]
- Korea Forest Research Institute. Forest Biomass and Soil Carbon Survey and Analysis Standards; Report No. TRKO201800030681; Korea Forest Research Institute: Seoul, Republic of Korea, 2007.
- Kauffman, J.B.; Arifanti, V.B.; Basuki, I.; Kurnianto, S.; Novita, N.; Murdiyarso, D.; Donato, D.C.; Warren, M.W. Protocols for the Measurement, Monitoring, and Reporting of Structure, Biomass, Carbon Stocks and Greenhouse Gas Emissions in Tropical Peat Swamp Forests; Center for International Forestry Research: Bogor, Indonesia, 2017. [Google Scholar]
- LVS EN ISO 10390:2022; Soil, Treated Biowaste and Sludge–Determination of pH. Latvian Standards: Riga, Latvia, 2022.
- O’Dell, J.W. Determination of Total Kjeldahl Nitrogen by Semi-Automated Colorimetry; Method 351.2; U.S. Environmental Protection Agency: Cincinnati, OH, USA, 1993.
- FAO. Standard Operating Procedure for Soil Available Phosphorus: Bray I and Bray II Method; Report No. GLOSOLAN-SOP-09; Food and Agriculture Organization of the United Nations: Rome, Italy, 2021. [Google Scholar]
- Walkley, A.; Black, I.A. An examination of the Degtjareff method for determining soil organic matter, and a proposed modification of the chromic acid titration method. Soil Sci. 1934, 37, 29–38. [Google Scholar] [CrossRef]
- BS 1377-2; Methods of Test for Soils for Civil Engineering Purposes. Classification Tests; British Standard: London, UK, 1990.
- ISO. Determination of Effective Cation Exchange Capacity and Base Saturation Level Using Barium Chloride Solution; International Organization for Standardization: Geneva, Switzerland, 2018. [Google Scholar]
- USBR. Procedure for Using Piezometers to Monitor Water Pressure in a Rock Mass; Materials Engineering and Research Laboratory, Technical Service Center: Denver, CO, USA, 2009. [Google Scholar]
- Saloko, S.A.; Elfatma, O.; Santi, I.S.; Hidayat, A.; Ridho, M.; Siregar, A.K.; Rangkuti, R.R. Practical rain measurement. IOP Conf. Ser. Earth Environ. Sci. 2023, 1230, 012135. [Google Scholar] [CrossRef]
- South, D.B.; Harris, S.W.; Burnett, J.P.; Hainds, M.J.; Gjerstad, D.H. Effect of container type and seedling size on survival and early height growth of Pinus palustris seedlings in Alabama, USA. For. Ecol. Manag. 2005, 204, 385–398. [Google Scholar] [CrossRef]
- Pinto, J.R.; Marshall, J.D.; Dumroese, R.K.; Davis, A.S.; Cobos, D.R. Establishment and growth of container seedlings for reforestation: A function of stocktype and edaphic conditions. Forest Ecol. Manag. 2011, 261, 1876–1884. [Google Scholar] [CrossRef]
- SAS Institute Inc. SAS/STAT 9.4 User’s Guide; SAS Institute Inc.: Cary, NC, USA, 2013. [Google Scholar]
- BMKG. Peta Rata-Rata Curah Hujan dan Hari Hujan Periode 1991–2020; Badan Meteorologi, Klimatologi, dan Geofisika: Jakarta, Indonesia, 2021. [Google Scholar]
- Agus, F.; Hairiah, K.; Mulyani, A. Measuring Carbon Stock in Peat Soils: Practical Guidelines; World Agroforestry Centre: Bogor, Indonesia, 2010. [Google Scholar]
- Könönen, M.; Jauhiainen, J.; Laiho, R.; Kusin, K.; Vasander, H. Physical and chemical properties of tropical peat under stabilised land uses. Mires Peat 2015, 16, 8. [Google Scholar] [CrossRef]
- Kunarso, A.; Farquharson, R.; Rachmanadi, D.; Hearn, K.; Blanch, E.W.; Grover, S. Land use change alters carbon composition and degree of decomposition of tropical peat soils. Mires Peat 2024, 30, 6. [Google Scholar] [CrossRef]
- Yin, T.; Feng, M.; Qiu, C.; Peng, S. Biological nitrogen fixation and nitrogen accumulation in peatlands. Front. Earth Sci. 2022, 10, 670867. [Google Scholar] [CrossRef]
- Tang, J.; Sun, B.; Cheng, R.; Shi, Z.; Luo, D.; Liu, S.; Centritto, M. Effects of soil nitrogen (N) deficiency on photosynthetic N-use efficiency in N-fixing and non-N-fixing tree seedlings in subtropical China. Sci. Rep. 2019, 9, 4604. [Google Scholar] [CrossRef] [PubMed]
- Tcherkez, G.; Carroll, A.; Abadie, C.; Mainguet, S.; Davanture, M.; Zivy, M. Protein synthesis increases with photosynthesis via the stimulation of translation initiation. Plant Sci. 2020, 291, 110352. [Google Scholar] [CrossRef]
- Guo, W.; Nazim, H.; Liang, Z.; Yang, D. Magnesium deficiency in plants: An urgent problem. Crop J. 2016, 4, 83–91. [Google Scholar] [CrossRef]
- Ishfaq, M.; Wang, Y.; Yan, M.; Wang, Z.; Wu, L.; Li, C.; Li, X. Physiological essence of magnesium in plants and its widespread deficiency in the farming system of China. Front. Plant Sci. 2022, 13, 802274. [Google Scholar] [CrossRef]
- Jing, T.; Li, J.; He, Y.; Shankar, A.; Saxena, A.; Tiwari, A.; Maturi, K.C.; Solanki, M.K.; Singh, V.; Eissa, M.A.; et al. Role of calcium nutrition in plant Physiology: Advances in research and insights into acidic soil conditions—A comprehensive review. Plant Physiol. Biochem. 2024, 210, 108602. [Google Scholar] [CrossRef]
- Hashim, S.A.; Teh, C.B.S.; Ahmed, O.H. Influence of water table depths, nutrients leaching losses, subsidence of tropical peat soil and oil palm (Elaeis guineensis Jacq.) seedling growth. Malays. J. Soil Sci. 2019, 23, 13. [Google Scholar]
- Bakri, B.; Imanudin, M.S.I.; Napoleon, A.; Syazili, A.A.S.; Prayitno, M.B.P.; Hermawan, A.; Yang, H. Peatland degradation level and restoration model of Perigi Village in Ogan Komering Ilir, South Sumatra, Indonesia. J. Degrad. Min. Lands Manag. 2025, 12, 8117–8126. [Google Scholar] [CrossRef]
- Jaenicke, J.; Rieley, J.O.; Mott, C.; Kimman, P.; Siegert, F. Determination of the amount of carbon stored in Indonesian peatlands. Geoderma 2008, 147, 151–158. [Google Scholar] [CrossRef]
- Wösten, J.H.M.; Clymans, E.; Page, S.E.; Rieley, J.O.; Limin, S.H. Peat–water interrelationships in a tropical peatland ecosystem in Southeast Asia. CATENA 2008, 73, 212–224. [Google Scholar] [CrossRef]
- Hooijer, A.; Page, S.; Jauhiainen, J.; Lee, W.A.; Lu, X.X.; Idris, A.; Anshari, G. Subsidence and carbon loss in drained tropical peatlands. Biogeosciences 2012, 9, 1053–1071. [Google Scholar] [CrossRef]
- Ritzema, H.; Limin, S.; Kusin, K.; Jauhiainen, J.; Wösten, H. Canal blocking strategies for hydrological restoration of degraded tropical peatlands in Central Kalimantan, Indonesia. CATENA 2014, 114, 11–20. [Google Scholar] [CrossRef]
- Manghwar, H.; Hussain, A.; Alam, I.; Khoso, M.A.; Ali, Q.; Liu, F. Waterlogging stress in plants: Unraveling the mechanisms and impacts on growth, development, and productivity. Environ. Exp. Bot. 2024, 224, 105824. [Google Scholar] [CrossRef]
- Permatasari, I.; Fatonah, S.; Iriani, D. Respons pertumbuhan anakan nyamplung (Calophyllum inophyllum L.) pada kondisi penggenangan dengan media tanah mineral dan tanah gambut. J. Online Mhs. JOM Bid. Mat. Ilmu Pengetah. Alam 2014, 1, 450. [Google Scholar]
- Sari, A.; Fatonah, S.; Iriani, D. Respons Anakan Tumbuhan Nyamplung (Calophyllum inophyllum L.) Pada Berbagai Periode Penggenangan. Ph.D. Thesis, Riau University, Pekanbaru, Indonesia, 2015. [Google Scholar]
- Fatonah, S.; Permatasari, I.; Iriani, D. Growth of Tamanu Seedlings (Calophyllum inophyllum L.) in flooding on Several Types of Soil. Bull. Sci. Res. 2023, 5, 42–48. [Google Scholar] [CrossRef]

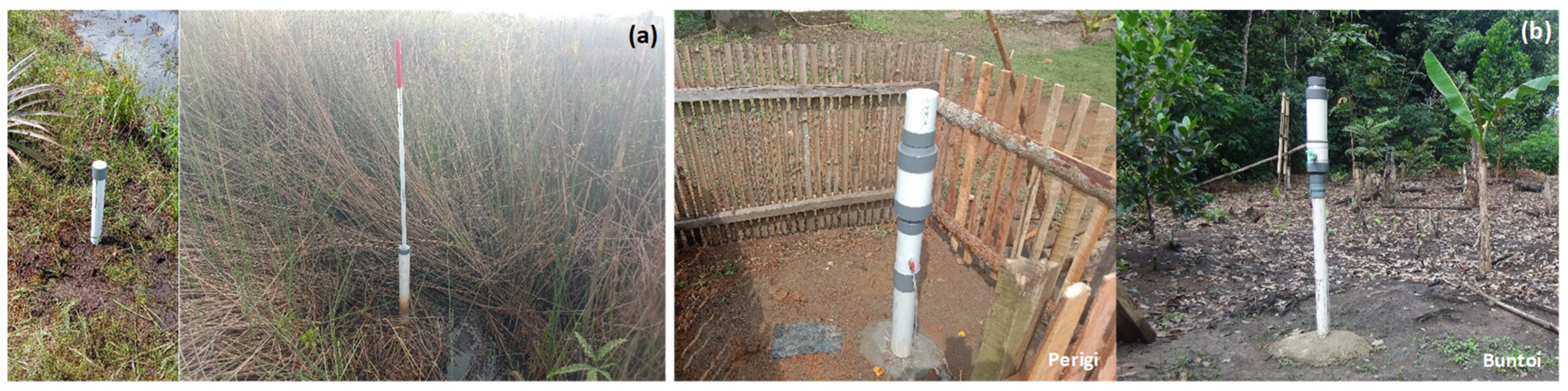

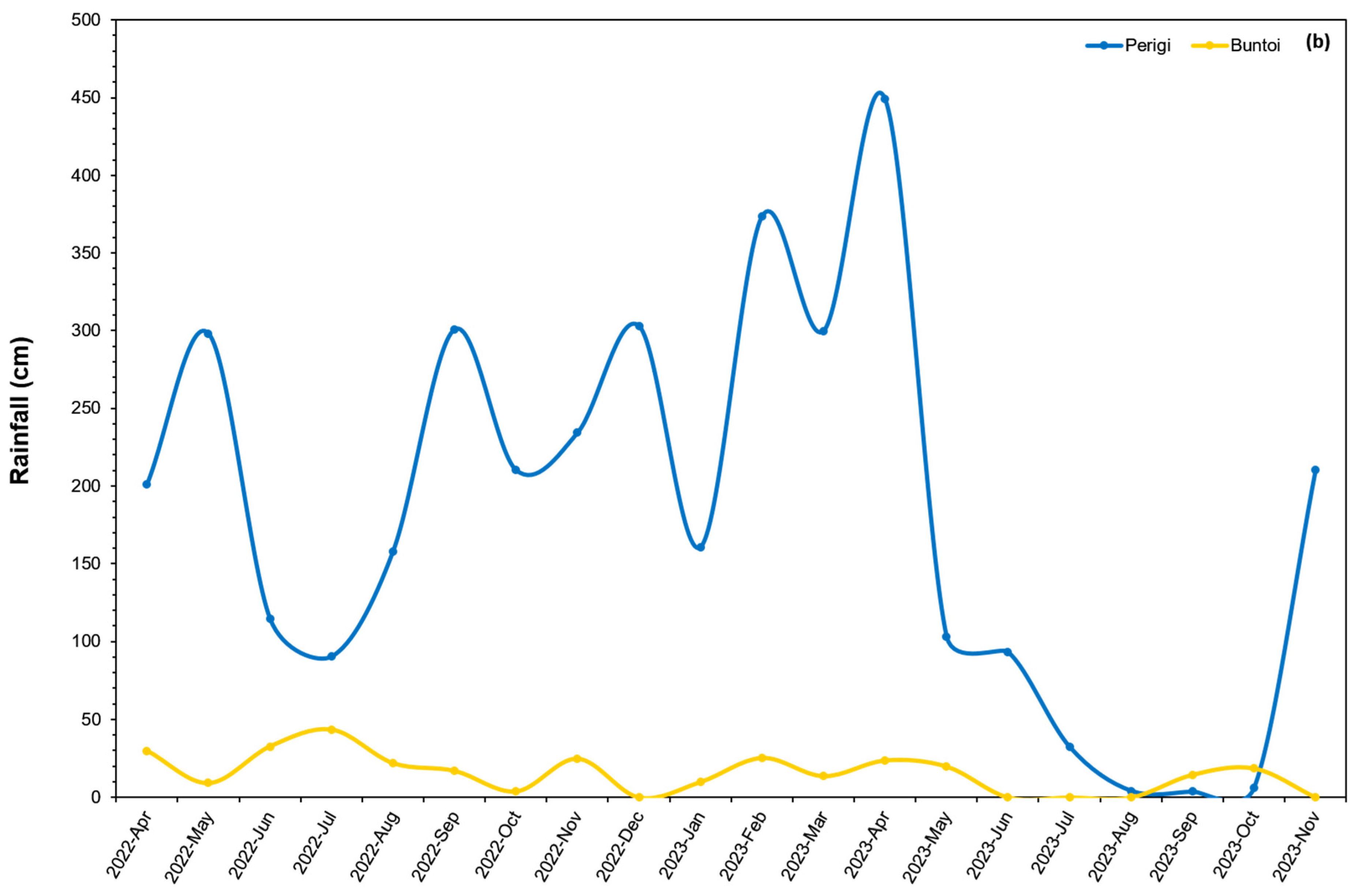
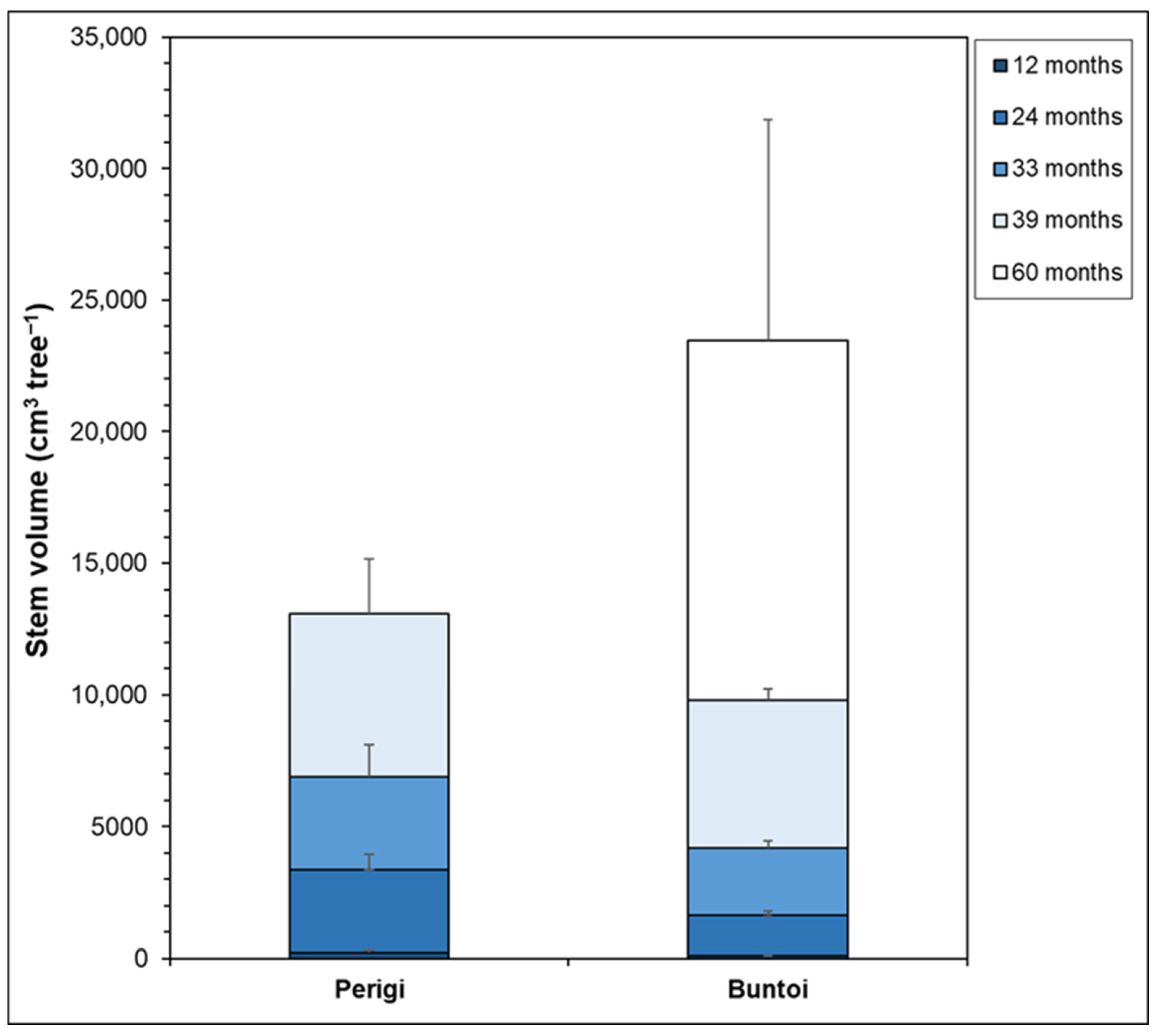
| Site Characteristic | Perigi | Buntoi |
|---|---|---|
| Area (ha) | 0.5 | 2 |
| Location | 3°06′14.0″ S, 105°03′46.6″ E | 2°49′40.6″ S, 114°08′57.5″ E |
| Average air temperature (℃) | 25.1 ± 0.6 | 32.3 ± 1.7 |
| Average air humidity (%) | 90.9 ± 1.1 | 87.4 ± 3.4 |
| Altitude (m) | 9 | 6–8 |
| Type of peatland | Topogenous | Topogenous |
| Average peat soil depth (cm) | 128.5 ± 45.9 (2019) | 61.3 ± 18.9 (2018) |
| Soil Properties | Perigi | Buntoi | p-Value | |
|---|---|---|---|---|
| pH | 0.17 | 0.07 | 0.0018 ** | |
| Total nitrogen (%) | 0.20 | 0.01 | 0.0189 * | |
| Available phosphorus (mg kg−1) | 9.89 | 1.41 | 0.0927 | |
| Organic carbon (%) | 0.61 | 0.45 | <0.0001 *** | |
| Cation Exchange Capacity (cmolc kg−1) | 2.92 | 1.51 | <0.0001 *** | |
| Exchangeable cations (cmolc kg−1) | Ca2+ | 1.62 | 0.06 | 0.0737 |
| Mg2+ | 0.95 | 0.07 | 0.0807 | |
| K+ | 0.02 | 0.03 | 0.0654 | |
| Na+ | 0.02 | 0.01 | 0.0580 | |
| Perigi | Buntoi | ||
|---|---|---|---|
| Period after planting | 6 months (May 2019) | 0 *** | 1.83 |
| 12 months (December 2019) | 0 *** | 0.35 | |
| 24 months (December 2020) | 0 ** | .20 | |
| 33 months (September 2021) | 0.53 *** | 0.20 | |
| 39 months (March 2022) | 0.83 *** | 0.20 | |
| 47 months (November 2022) | 1.27 | 0.20 *** | |
| 60 months (December 2023) | 0 | 0.35 *** | |
| Period (P) | 266.40 *** | ||
| Region (R) | 1715.82 *** | ||
| R | 1272.52 *** | ||
| Growth Performance | Period After Planting | Perigi | Buntoi | p-Value |
|---|---|---|---|---|
| Root collar diameter (mm) | 12 months | 1.99 | 0.49 | 0.0002 *** |
| 24 months | 3.58 | 2.23 | 0.0027 ** | |
| 33 months | 4.83 | 1.38 | 0.0013 ** | |
| 39 months | 5.74 | 1.38 | 0.0440 * | |
| 60 months | - | 5.43 | - | |
| Height (cm) | 12 months | 6.54 | 2.05 | 0.1786 |
| 24 months | 10.26 | 6.69 | 0.8865 | |
| 33 months | 10.55 | 4.80 | 0.0003 *** | |
| 39 months | 9.14 | 6.74 | <0.0001 *** | |
| 60 months | - | 10.02 | - | |
| H/RCD ratio | 12 months | 4.35 | 1.37 | 0.0059 ** |
| 24 months | 2.02 | 2.04 | 0.0001 *** | |
| 33 months | 2.39 | 1.13 | <0.0001 *** | |
| 39 months | 1.23 | 1.00 | <0.0001 *** | |
| 60 months | - | 1.72 | - |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yang, A.-R.; Choi, E.; Yang, H.; Cha, J.; Wahyuni, N. Growth Performance of Tamanu (Calophyllum inophyllum L.) in Relation to Peatland Restoration in South Sumatra and Central Kalimantan, Indonesia. Forests 2025, 16, 1740. https://doi.org/10.3390/f16111740
Yang A-R, Choi E, Yang H, Cha J, Wahyuni N. Growth Performance of Tamanu (Calophyllum inophyllum L.) in Relation to Peatland Restoration in South Sumatra and Central Kalimantan, Indonesia. Forests. 2025; 16(11):1740. https://doi.org/10.3390/f16111740
Chicago/Turabian StyleYang, A-Ram, Eunho Choi, Hyunyoung Yang, Jumi Cha, and Novisari Wahyuni. 2025. "Growth Performance of Tamanu (Calophyllum inophyllum L.) in Relation to Peatland Restoration in South Sumatra and Central Kalimantan, Indonesia" Forests 16, no. 11: 1740. https://doi.org/10.3390/f16111740
APA StyleYang, A.-R., Choi, E., Yang, H., Cha, J., & Wahyuni, N. (2025). Growth Performance of Tamanu (Calophyllum inophyllum L.) in Relation to Peatland Restoration in South Sumatra and Central Kalimantan, Indonesia. Forests, 16(11), 1740. https://doi.org/10.3390/f16111740






