Abstract
Understanding the integration of newly recorded species into forest ecosystems is essential for evaluating their ecological impacts on native wildlife diversity. In this study, we examined the spatial and temporal niche dynamics of three sympatric squirrel species within the Labagoumen nature reserve, a temperate forest located in northern China. Particular emphasis was placed on the recently documented Eurasian red squirrel (Sciurus vulgaris) and its potential interactions with two native species: Père David’s rock squirrel (Sciurotamias davidianus) and the Siberian chipmunk (Tamias sibiricus). Using camera trapping data from 91 sites (2019–2024), we examined habitat use, activity rhythms, and niche overlap under contrasting levels of human disturbance. A total of 3419 independent effective photos of squirrels were recorded. S. vulgaris showed a broader spatial distribution and a higher relative abundance index (RAI) in the tourist area, while native species were more abundant in the non-tourist area. All three species showed similar annual activity patterns based on the monthly relative abundance index (MRAI), although native species exhibited an additional activity peak in June–July. Temporal niche overlap (Cih) and the coefficient of overlap (Δ) between S. vulgaris and native species increased during the tourist season, suggesting synchronized activity under high disturbance. In contrast, lower overlap in the non-tourist season indicated stronger temporal partitioning. The daily activity rhythm of S. vulgaris remained stable, while native species displayed more variability, especially in non-tourist areas. S. vulgaris also exhibited a significantly broader spatial niche breadth (Bi), suggesting greater habitat exploitation and adaptability. Non-metric multidimensional scaling (NMDS) revealed no significant spatial segregation among the three species, indicating successful integration of S. vulgaris into the local community. Our findings emphasize the competitive advantage of S. vulgaris and demonstrate how human activities can restructure forest small mammal assemblages by altering spatiotemporal niche partitioning. We recommend long-term ecological monitoring to assess species diversity changes and guide adaptive conservation strategies.
1. Introduction
Niche differentiation is a key ecological mechanism for reducing interspecific competition, maintaining community stability, and promoting multispecies coexistence [1,2]. It can be expressed in multiple dimensions, such as temporal activity, spatial use, and dietary habits [3,4,5], and plays an essential role in structuring species interactions in forest ecosystems. Temporal niche differentiation refers to the differences in activity timing among species, which helps animals avoid competition, thereby improving resource-use efficiency and stabilizing ecosystems [6]. Spatial niche differentiation, on the other hand, reflects variation in the use of physical space [7]. Through mechanisms such as habitat preference and vertical or horizontal segregation, species can minimize spatial overlap and alleviate local resource pressure [8]. Niche differentiation in both time and space is influenced by multiple factors, including food resources, habitat structure, human disturbance, and other ecological or environmental variables [9,10,11,12]. Small forest-dwelling mammals, such as squirrels, are ideal indicators for studying niche use and community dynamics due to their sensitivity to habitat change [13,14,15], manageable activity scales, and ecological importance in seed dispersal and trophic interactions [16,17].
The Eurasian red squirrel (Sciurus vulgaris) is widely distributed across the Eurasian continent and demonstrates strong ecological adaptability, enabling it to occupy various habitat types [18]. Although historically absent from the Beijing region, it has been increasingly reported since 2008 across various forest habitats and administrative regions, including Songshan National Nature Reserve, Baihuashan National Nature Reserve, and Mentougou District [19,20,21,22,23,24]. Its origin and expansion remain unclear, with some studies suggesting human-mediated introduction and potential competition with native squirrel species [22]. Studies from other regions have shown that ecologically flexible or non-native squirrels can rapidly establish and displace native counterparts by occupying broader niches or exploiting disturbed habitats more effectively [25,26,27].
In contrast, Père David’s rock squirrel (Sciurotamias davidianus) and the Siberian chipmunk (Tamias sibiricus) are native to northern China’s forest ecosystems and maintain relatively stable populations with established ecological niches [28,29,30,31,32]. The range expansion of the Eurasian red squirrel may disturb the ecological balance of these native squirrel communities, particularly in areas where habitat overlap occurs or resources are limited. While a few studies have noted its presence and potential impact [24], empirical data on its spatiotemporal niche differentiation and its interactions with native species under different levels of human disturbance remain limited.
In this study, we used camera trapping data from 91 sites in the Labagoumen nature reserve, a forested mountainous region in northern China, to investigate habitat use and spatiotemporal niche dynamics among three sympatric squirrel species. We specifically examined how the newly recorded Eurasian red squirrel interacts with native species across areas experiencing different levels of human disturbance (i.e., tourist vs. non-tourist areas). Our objectives were to quantify species abundance, spatial distribution, temporal activity rhythms, and niche overlap and to assess whether Eurasian red squirrels demonstrate a competitive advantage or have integrated ecologically into the local community. These findings contribute to a deeper understanding of how newly recorded species integrate into existing communities and how anthropogenic factors mediate interspecific interactions. More broadly, they provide important insights into species diversity and community assembly in forest ecosystems under increasing human pressure and support adaptive biodiversity conservation and management strategies tailored to forest-based nature reserves.
2. Materials and Methods
2.1. Study Area
The Labagoumen nature reserve is located in Manchu township, Huairou District, Beijing, at the northernmost tip of the city (Figure 1a). It serves as a critical ecological transition zone between the mountainous regions of North China and the Inner Mongolian grasslands, encompassing a total area of 18,482.5 hectares. The reserve borders Hebei Province to the west, north, and east, while its southern boundary lies within the administrative region of Labagoumen Manchu township. Designated as a nature reserve for forest ecosystems, its primary purpose is the conservation of zonal forest vegetation and associated forest ecosystems. The dominant vegetation type in the reserve is temperate deciduous broadleaf forest. Due to historical human disturbance, the dense original zonal forests are rare, and the current vegetation predominantly consists of large areas of secondary forest. However, the forest coverage remains relatively high at over 60%, with a total forested area of 11,666.5 hectares within the reserve. The vegetation within the reserve is diverse, comprising eight vegetation types and 22 associations. Mongolian oak (Quercus mongolica) forests represent the largest and most widely distributed forest type in the reserve [33,34]. The terrain predominantly consists of mountains, with elevations ranging from 520 to 1705 m. Historically, the area’s remote location, steep terrain, and sparse population have contributed to its relatively intact natural ecology, high forest cover, and abundant biodiversity [33]. The Tang River runs through the reserve, dividing it into eastern and western sections. Due to strong governmental support for the development of the ecotourism area (Figure 1b), the western section has become a major source of income for Labagoumen Manchu township, with tourism revenue now serving as a key economic pillar [35]. Accordingly, this study defines the western part of the reserve as the “tourist area” and the eastern part as the “non-tourist area”.
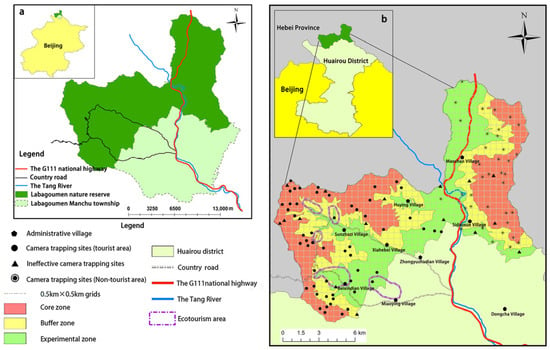
Figure 1.
(a) Location of the Labagoumen nature reserve in Beijing, China; (b) Spatial distribution of camera trapping sites within Labagoumen nature reserve.
According to the Comprehensive Scientific Investigation Report of the Labagoumen nature reserve in Beijing [34], a detailed ecological survey conducted over a decade ago documented 30 mammal species representing 15 families and 6 orders. Among them, only Pére David’s rock squirrel and the Siberian chipmunk were identified from the family Sciuridae. Notably, there were no records of the Eurasian red squirrel at that time.
2.2. Deployment of Camera Trapping
Between July 2019 and August 2024, a total of 106 camera trapping sites were established across the Labagoumen nature reserve, with 64 located in the western (tourist area) and 42 in the eastern (non-tourism area), each equipped with one camera. The selection process involved using ArcMap 10.8 software to divide the reserve into 0.5 km × 0.5 km grids. In the grid, the selection criteria for camera trapping sites included relatively minimal human disturbance, ensuring notable signs of wildlife activity, and having diverse vegetation types such as animal trails, water sources, and ravines. Camera trapping sites were spaced at least 0.3–0.5 km apart to prevent the same animal from being photographed repeatedly in a short timeframe [36,37]. The camera models used for monitoring were Ltl-6210 MC, Ltl-6310 MC, and Ltl-6310 WMC (produced by Zhuhai Ltl Acorn Electronics Co., Ltd., Zhuhai, China). All were positioned 30 to 60 cm above ground level on tree trunks to minimize direct sunlight on lenses. Cameras were set to capture both photos and 10-s videos upon activation, with a 1-s trigger interval, ensuring continuous 24-h monitoring.
Considering the significant human disturbance present in the western ecotourism area of the reserve and its potential long-term impact on wildlife activity patterns [38,39,40], camera trapping sites in this area were strategically deployed primarily within the core and buffer zones, as these two zones prohibit tourist access, to reduce human interference, enhance wildlife detection rates, and capture wildlife in a relatively natural activity state (Figure 1b).
2.3. Data Filtering and Species Classification
After excluding camera trapping sites with missing or damaged data that prevented the retrieval of squirrel images, a total of 91 camera trapping sites (54 in tourist area and 37 in non-tourist area) recorded images of at least one squirrel species. The image data from these 91 camera trapping sites were manually identified and filtered. Independent effective photos were defined using the 30-minute interval method [41]. Excel 2019 was used to compile and analyze information on species, capture time, and camera site. Species identification followed the A Guide to the Mammals of China [42], while classification and nomenclature were based on the “Catalogue of Mammals in China (2021)” [43].
2.4. Data Processing and Analysis
2.4.1. Species Abundance
To assess the activity intensity of the three squirrel species, this study employed the Relative Abundance Index (RAI) to quantify the population size of squirrels captured by camera trapping [44]. Based on the deployment area of the cameras, the RAI values for the Eurasian red squirrel, Pére David’s rock squirrel, and Siberian chipmunk were calculated separately for the entire reserve and for each subregion. The formula is as follows:
In the formula, RAI represents the relative abundance index of a given species, Ti represents the number of independent effective photos of species i under a given analytical factor (e.g., area), and N is the total number of independent effective photos of all squirrel species under the same factor.
2.4.2. Annual Activity Patterns
All data were grouped by month, and the number of independent effective photos of different squirrel species captured in each month was counted. The monthly relative abundance index (MRAI, also referred to as IMRA) was then calculated to reflect the monthly activity levels of each species [45]. The formula is as follows:
In the formula, IMRA denotes the monthly relative abundance index. Sj is the total number of independent effective photos of squirrel species j across all camera trapping sites, and Mkj indicates the number of independent effective photos of species j recorded in the k-th month (k = 1, 2,..., 12). A larger IMRA value suggests a higher level of activity for the species in that month.
2.4.3. Temporal Niche Overlap Index
To quantitatively evaluate the degree of temporal niche overlap between the Eurasian red squirrel and the two native squirrel species, this study defined April to October as the “tourist season” and November to the following March as the “non-tourist season”, based on the operational period of the ecotourism area within the reserve. Following the niche overlap index proposed by Schoener (1968) [46] and relevant studies [47,48,49], the 24-hour day was divided into 12 equal time intervals (each lasting 2 h: 00:00–02:00, 02:00–04:00,…, 22:00–24:00). Based on this division, the proportion of activity frequency of each squirrel species within each time segment across different season and regions was calculated, thereby deriving the temporal niche overlap index. The specific formula is as follows:
In the formula, Cih represents the degree of temporal niche overlap between species i and species h; Nij denotes the number of records (independent effective photos) of species i during time interval j; and Ni is the total number of records of species i across all time intervals. Similarly, Nhj and Nh refer to the number of records of species h during time interval j and its total number of records, respectively. The value of the index ranges from 0 to 1, with higher values indicating greater similarity in the temporal activity patterns between the two species, i.e., a higher degree of overlap. A value of 1 indicates complete overlap in temporal niche use. According to Wathne et al. (2000) [50], Cih > 0.6 indicates a high degree of overlap; 0.6 ≥ Cih ≥ 0.3 indicates a moderate degree of overlap; and Cih < 0.3 suggests a low degree of overlap.
2.4.4. Daily Activity Patterns of Eurasian Red Squirrels with the Two Native Squirrel Species
We then estimated the daily activity patterns of the Eurasian red squirrel and the two native species across different season and spatial zones using Kernel Density Estimation (KDE) [51], following the approach proposed by Ridout and Linkie (2009) [52]. To quantify the degree of overlap in activity patterns, we calculated the coefficient of overlap (∆), which ranges from 0 (no overlap) to 1 (complete overlap). This coefficient was derived from the area under the minimum of two density functions at each time point [53]. We performed 1000 bootstrap iterations to estimate the mean ∆ and its 95% confidence intervals. Based on established thresholds [54], we categorized overlap values into three levels: low (∆ < 0.50), moderate (0.50 ≤ ∆ ≤ 0.80), and high (∆ > 0.80). Given that KDE requires the co-occurrence of species, only camera trapping sites that recorded the activity of at least two squirrel species were included in the analysis; sites that recorded only a single species were excluded [55].
The data were further subdivided by tourist and non-tourist season and stratified by spatial zones (all area, tourist area, and non-tourist area) to examine differences in activity patterns between the Eurasian red squirrel and the two native species under different spatiotemporal combinations. The Watson–Wheeler test of circular homogeneity was applied using the “Watson.two ()” function from the circular package in R to test for differences in daily activity patterns between species [56,57]. As a non-parametric approach, the Watson–Wheeler test evaluates whether two samples of cyclic data differ in a statistically significant way.
2.4.5. Spatial Niche Breadth
To quantitatively analyze the spatial resource utilization breadth of the three squirrel species, we calculated the spatial niche breadth of each species using Levins’ index [58]. This index reflects the degree of spatial habitat utilization and sensitivity of each squirrel species across the infrared camera sites. The formula is as follows:
where Bi is the niche breadth of species i, r is the total number of resource categories (in this study, the total number of camera trapping sites), and Pij is the proportion of independent effective photos of species i recorded at camera trapping site j relative to the total number of independent effective photos of that species. A higher niche breadth indicates a broader distribution and lower habitat sensitivity.
Given the common issues of uneven sample sizes and detection probability in field data, a single point estimate may not fully capture the variability of niche breadth [59]. Therefore, we applied a bootstrap method to estimate the non-parametric distribution of niche breadth. We conducted 1000 bootstrap resampling iterations based on the original sample size, calculating the niche breadth in each iteration to generate a non-parametric distribution and estimate the 95% confidence interval, thereby reflecting the range of variation in spatial niche breadth [60].
To examine whether there were significant differences in spatial niche breadth among the three squirrel species, we developed a custom function in R 4.3.0 to perform bootstrap simulations and statistical tests. We first conducted Shapiro–Wilk tests for normality on the bootstrap samples of each species. The results indicated that none of the three species met the assumption of normality. Therefore, we applied the Wilcoxon rank-sum test to compare the niche breadth distributions between each pair of species and assess the significance of their differences (p < 0.05) [60].
2.4.6. Spatial Niche Differentiation and Community Structure
To assess potential spatial niche differentiation between the Eurasian red squirrel and native species, we used Non-metric Multidimensional Scaling (NMDS) [61] to analyze squirrel distribution patterns within the reserve. NMDS is widely applied in animal ecology, such as in analyzing mesocarnivore distributions via camera trapping data [62] or small mammal community structure [63].
We constructed a species-by-site detection matrix based on independent detections at each camera site, using the Bray–Curtis distance to quantify site similarity. NMDS (k = 2) was performed with the metaMDS() function from the vegan package (version 2.6-4) in R version 4.3.0 (999 iterations), and the results were visualized with ggplot2 (version 3.4.2), including 95% confidence ellipses to show species clustering. To test for significant spatial differences, we applied PERMANOVA based on the Bray–Curtis distance [64,65].
3. Results
3.1. Overview of Camera Trapping Monitoring
From 2019 to 2024, out of 106 camera trapping sites, 91 recorded at least one of the three squirrel species. Over a cumulative total of 45,168 camera-trap days, 3419 independent effective photos of the three squirrel species were obtained.
Among these, the Eurasian red squirrel accounted for 1356 independent effective photos (n = 1356) and showed the widest distribution (site = 80), with 27 sites exclusively recording this species. In contrast, Pére David’s rock squirrel had the highest number of independent effective photos, totaling 1799, and was recorded at 60 sites, including 10 sites where only this species was detected. The least recorded species was the Siberian chipmunk, with 264 independent effective photos across 28 sites, and only one site recorded this species exclusively (Figure 2a,b).
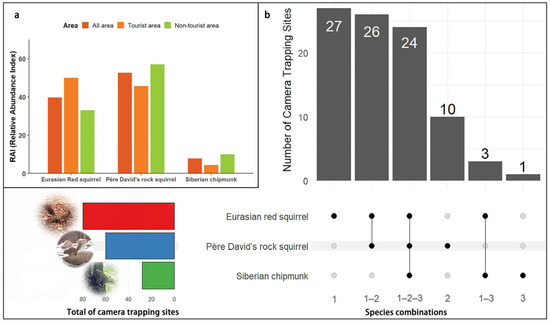
Figure 2.
(a) The relative abundance index (RAI) of three squirrel species in the Labagoumen nature reserve; (b) Site occupancy intersections of squirrels in the Labagoumen nature reserve.
Across the entire reserve, RAI values ranked as follows: Pére David’s rock squirrel > Eurasian red squirrel > Siberian chipmunk. In tourist areas, the Eurasian red squirrel had the highest RAI, whereas in non-tourist areas, Pére David’s rock squirrel dominated (Table 1). These patterns suggest that the Eurasian red squirrel may prefer tourist areas, while the native squirrel species may be better adapted to non-tourist areas with lower levels of human disturbance.

Table 1.
Distribution and RAI of three squirrel species in different areas.
3.2. Analysis of Annual Activity Patterns
The monthly relative abundance index (MRAI) indicates that the overall activity rhythms of the three squirrel species are generally similar, with particularly consistent monthly variation trends observed between the native rock squirrel and the Siberian chipmunk. However, compared to the northern red squirrel, the native squirrels exhibited an additional activity peak during June–July. All three species showed higher activity frequencies in April, followed by synchronized declines during April–May, September–October, and November–December. Consistent increases were observed from February to April and from October to November (Figure 3).
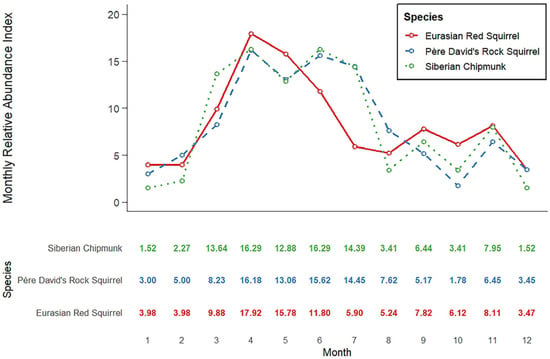
Figure 3.
Annual activity patterns of three squirrel species at Labagoumen nature reserve.
The MRAI values of the Eurasian red squirrel were generally higher than those of the native species during spring (March–May), autumn (September–November), and winter (December to the following February) but lower during summer (June–August). The three squirrel species exhibited considerable consistency in their annual activity patterns, indicating that their rhythms may be shaped by common seasonal environmental drivers.
3.3. Analysis of the Temporal Niche Overlap Index
Based on temporal niche overlap indices (Cih), this study quantifies the activity time overlap between the Eurasian red squirrel and two native species across different seasons and areas. All three species showed high temporal overlap (Cih > 0.6), with the Eurasian red squirrel generally aligning more closely with Pére David’s rock squirrel than with the Siberian chipmunk, indicating stronger temporal consistency.
Across all seasons and areas, Cih values between the Eurasian red squirrel and Pére David’s rock squirrel and Siberian chipmunk were 0.95 and 0.94, respectively, reflecting highly similar activity rhythms. Considering seasonal effects, overlap in the tourist season remained high across all areas combined (0.91 and 0.89), as well as within tourist areas (0.92 and 0.86) and non-tourist areas (0.89 and 0.87), suggesting synchronized activity as a potential shared response to human disturbance, though possibly increasing interspecific competition.
By contrast, in the non-tourist season, overlap was considerably lower across all areas (0.72 and 0.64) and further decreased within tourist areas (0.73 and 0.65) and non-tourist areas (0.69 and 0.63), representing the lowest observed values.
In summary, temporal overlap between the Eurasian red squirrel and native species varies seasonally and spatially. High overlap under disturbance may reflect competition or adaptive synchrony, while reduced overlap in quieter periods suggests divergent resource use strategies.
3.4. Daily Activity Rhythms of Three Squirrel Species
According to the daily activity density curves of the three squirrel species (Figure 4a), all three squirrel species exhibited predominantly diurnal activity patterns. The activity of the Eurasian red squirrel showed a unimodal pattern across all years and all areas, with a peak between 05:00 and 08:00. The Pére David’s rock squirrel was mainly active from 05:00 to 09:00, with a secondary peak observed in the evening (16:00–18:00). The Siberian chipmunk showed peak activity between 04:00 and 09:00, with an additional tendency toward evening activity from 15:00 to 17:00. Across all years and the entire study area (all area), Eurasian red squirrels and Pére David’s rock squirrels showed a high activity overlap (Δ = 0.947, 95% CI: 0.925–0.969; Figure 4b) with no significant difference in rhythms (p > 0.10; Table 2).
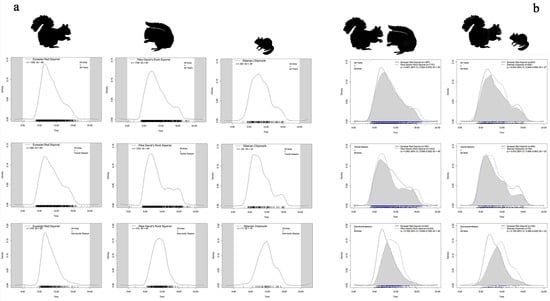
Figure 4.
(a) Daily activity density curves of the three squirrel species across all area and in different season; (b) Overlap in daily activity rhythms between Eurasian red squirrels and the two native species across all areas and different seasons.

Table 2.
Overlap analysis of daily activity patterns between red squirrels and two native squirrel species across different seasons and areas.
During the tourist season, the activity peaks of all three species tended to converge. Eurasian red squirrels remained active in the early morning (05:00–08:00); Pére David’s rock squirrels were active from 05:00 to 09:00 and again from 12:00 to 18:00; and Siberian chipmunks were active from 05:00 to 11:00 (Figure 4a). Overlap between red and rock squirrels slightly declined (Δ = 0.893), with a significant difference in activity patterns (p < 0.001) (Figure 4b; Table 2).
In the non-tourist season, red squirrels maintained a stable early rhythm (05:00–08:00), while activity peaks for Pére David’s rock squirrels and Siberian chipmunks shifted later (07:00–11:00 and 07:00–12:00) (Figure 4a). Overlap dropped to moderate levels (Δ = 0.709 and Δ = 0.708), with highly significant rhythm differences (p < 0.001), indicating increased temporal differentiation in the absence of human disturbance (Figure 4b; Table 2).
3.5. Overlap Between Eurasian Red Squirrels and Native Species Across Seasons and Areas
Further spatiotemporal analysis revealed that during the tourist season in the tourist area, Eurasian red squirrels and Pére David’s rock squirrels exhibited a high degree of overlap in daily activity rhythms (Δ = 0.906) (Figure 5), though their activity patterns differed significantly (p < 0.05). In the non-tourist area, the difference was even more pronounced (Δ = 0.892, p < 0.01) (Table 2). During the non-tourist season in the tourist area, their overlap was the lowest (Δ = 0.696), with highly significant differences in activity rhythms (p < 0.001), indicating clear temporal separation. In the non-tourist area during the non-tourist season, the overlap slightly increased (Δ = 0.744), but the difference in activity rhythms remained highly significant (p < 0.001) (Table 2), showing evident temporal segregation (Figure 5).
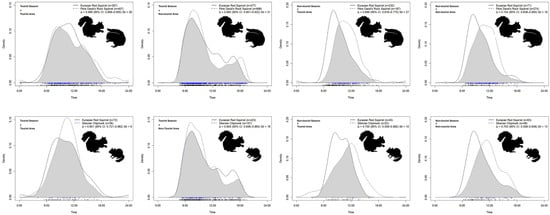
Figure 5.
Overlap in daily activity rhythms between Eurasian red squirrels and the two native squirrel species across different seasons and areas.
In comparison, further spatiotemporal analysis of Eurasian red squirrels and Siberian chipmunks revealed that in the tourist area during the tourist season, the two species showed a high degree of overlap in daily activity rhythms (Δ = 0.851), with no significant difference in rhythm (p > 0.10). In the non-tourist area during the same season, their activity times were even more synchronized, with no significant difference in rhythms (p > 0.05) (Figure 5). However, during the non-tourist season, whether in the tourist or non-tourist area, the overlap between them decreased to moderate levels (Δ ≈ 0.70), and their activity rhythms showed significant differences (p < 0.01 and p < 0.001) (Table 2), indicating clear temporal separation between the two species (Figure 5).
3.6. Differences in the Spatial Niche Breadth
Based on Levins’ niche breadth index, we quantitatively evaluated the spatial niche breadth of the three squirrel species. Using bootstrap resampling (n = 1000), we calculated the spatial niche breadth and its 95% confidence interval across all years and the entire study area. While this index is related to the number of camera trap sites where each species was recorded, it also incorporates the evenness of their distribution across sites, allowing species with similar site counts to differ in niche breadth if their spatial distributions vary in aggregation or spread. The results showed that the red squirrel had the widest spatial niche breadth at 26.4, followed by the rock squirrel at 13.1, and the Siberian chipmunk had the narrowest at 7.63 (Figure 6a).
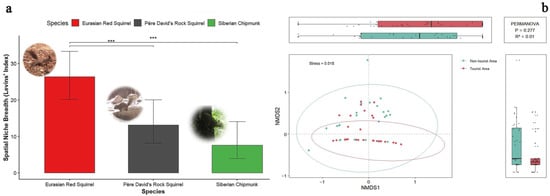
Figure 6.
(a) Comparative spatial niche breadths of three squirrel species in the Labagoumen nature reserve; (b) NMDS ordination of squirrel community composition between the tourist and non-tourist area. *** indicates that the spatial niche breadth of the Eurasian red squirrel is significantly greater than that of the other two squirrel species (p < 0.001).
Further bootstrap estimation of pairwise niche breadth differences revealed that the 95% confidence interval for the difference between Eurasian red squirrels and Pére David’s rock squirrels was 3.51–20.03 and between Eurasian red squirrels and Siberian chipmunk it was 9.95–26.42. As both intervals did not include 0, this indicates that the red squirrel’s spatial niche breadth was significantly greater than that of the Pére David’s rock squirrel and Siberian chipmunk. Non-parametric comparison using the Wilcoxon rank-sum test further confirmed that the spatial niche breadth of the Eurasian red squirrel was significantly greater than that of the Pére David’s rock squirrel and Siberian chipmunk (p < 0.001) (Table 3). As a newly recorded species in the Labagoumen nature reserve, the Eurasian red squirrel exhibited a notably broader spatial niche, suggesting stronger ecological adaptability in habitat use.

Table 3.
Statistical test results of spatial niche breadth differences between the Eurasian red squirrel and native squirrel species.
3.7. Analysis of the Spatial Community Structure
The NMDS analysis yielded a stress value of 0.018, indicating an excellent representation of the Bray–Curtis dissimilarities in two-dimensional space. Points representing sites from the tourist and non-tourist areas showed a tendency for separation, suggesting potential differences in community structure between the two disturbance regimes. However, PERMANOVA revealed no statistically significant difference in community composition between the two areas (df = 1, pseudo-F = 1.286, R2 = 0.014, p = 0.277) (Figure 6b).
The lack of a statistical difference in species composition or abundance may suggest that the Eurasian red squirrel, as a newly expanding species, has successfully integrated into the local ecosystem.
4. Discussion
4.1. Competitive Advantages of Eurasian Red Squirrels in Forest Ecosystems
Despite being a newly recorded species in the Labagoumen nature reserve, the Eurasian red squirrel exhibited the broadest spatial distribution and the greatest spatial niche breadth among the three sympatric squirrel species. Its daily activity rhythm remained relatively stable across gradients of human disturbance. Moreover, its relative abundance index (RAI) was comparable to that of the native Père David’s rock squirrel and significantly higher than that of the Siberian chipmunk, suggesting strong ecological competitiveness within the local forest mammal community.
Previous studies have shown that some species, including squirrels, can rapidly establish populations and exert invasion-like ecological effects even when introduced in small numbers [15,66,67]. In contrast, effective limitations on spread typically occur only when native species are significantly larger in body size, such as the suppression of invasive American mink (Neovison vison) by the larger Eurasian otter (Lutra lutra) [68,69].
We hypothesize that the strong ecological competitiveness of the Eurasian red squirrel in this reserve may be attributed to the following three factors:
(1) Body Size Advantage
The adult Eurasian red squirrel has a head-body length of 178–260 mm and a weight range of 260–480 g, making it generally larger than Pére David’s rock squirrel (190–250 mm; 219–400 g) and significantly larger than Siberian chipmunk (120–165 mm; 78–102 g) [42,70]. Larger body size is often associated with broader ecological niches and enhanced competitive capacity [71,72]. In squirrels, size correlates with bite force and dietary breadth, facilitating access to harder food resources like nuts and cones [73,74].
(2) Habitat Adaptability
Detected at 80 of 91 camera trap sites, the Eurasian red squirrel showed the highest site occupancy and niche breadth, suggesting high spatial adaptability. Known to inhabit a wide variety of forest types—including coniferous, deciduous, mixed, and even urban woodlands—the Eurasian red squirrel is considered ecologically flexible [75,76,77]. In contrast, the Pére David’s rock squirrel prefers mountainous or rocky habitats, often inhabiting rock crevices and tree root hollows [78], while the Siberian chipmunk is a typical forest-associated rodent species, commonly found in shrubland habitats and tree cavities within forested and hilly regions [42,79]. The high adaptability and behavioral flexibility of the Eurasian red squirrel may contribute to its ability to swiftly identify suitable habitats and effectively exploit resources within forest environments, thereby enhancing its population growth and competitive advantage.
(3) Dietary Breadth and Resource Use
Differences in habitat around camera trapping sites likely influence the availability of food resources. The Eurasian red squirrel has a highly generalized diet, consuming seeds, nuts, leaves, mushrooms, and even tree bark [80], which is considerably more diverse than that of the rock squirrel, which mainly feeds on nuts and fruits [81], or the Siberian chipmunk, whose small body size constrains it to a relatively narrow diet [82]. This dietary flexibility likely confers greater ecological adaptability and facilitates competitive success in varied environments.
In summary, the Eurasian red squirrel exhibits significant competitive advantages across multiple dimensions—including body size, spatial use, dietary flexibility, and niche breadth—which likely contribute to its rapid expansion within the nature reserve. These traits may pose potential interspecific competitive pressures on native species such as the Pére David’s rock squirrel and Siberian chipmunk and merit attention from conservation managers.
4.2. Interactive Effects of Human Disturbance and Competitive Exclusion
In ecological communities, dominant species often suppress subordinates by monopolizing key resources or core habitats, thereby reducing interspecific niche overlap [83,84]. In our study, we found that during the non-tourist season, the temporal niche overlap between Eurasian red squirrels and native squirrels generally declined, with the ∆ dropping from high to moderate. This suggests that Eurasian red squirrels may competitively exclude native species by constraining their niche use, likely due to advantages in resource acquisition. This pattern resembles the displacement of Eurasian red squirrels by the Eastern gray squirrel (Sciurus carolinensis) in Europe [85]. Similarly, Eurasian red squirrels have been reported to reduce Siberian chipmunk habitat use in Italy [86], consistent with our findings that Siberian chipmunks showed the narrowest distribution and lowest RAI.
Eurasian red squirrels also appear more tolerant of human disturbance and tend to behave more boldly in areas with frequent human activity. Such activity during the tourist season may increase food availability [87,88], potentially enhancing Eurasian red squirrel population performance. In our data, Eurasian red squirrels showed higher RAI values in the tourist area than in the non-tourist area, whereas native species exhibited the opposite trend. These results suggest that human disturbance may intensify interspecific interactions and shift species relationships. Eurasian red squirrels may be gaining a local competitive advantage through the combined effects of competitive exclusion and human activity, potentially reshaping the activity patterns and spatial distributions of native squirrels.
4.3. Temporal Niche Shifts of Native Squirrels in Response to the Eurasian Red Squirrel
Temporal niche differentiation is a key mechanism that facilitates the coexistence of closely related species by mitigating interspecific competition [89,90,91]. For instance, in the Mentougou District of Beijing, when the native Pére David’s rock squirrel is dominant, it exhibits a diurnal activity pattern, while the Eurasian red squirrel adopts a more cathemeral pattern [24]. In contrast, our research in the Labagoumen nature reserve shows that Eurasian red squirrels are no longer at a numerical disadvantage. Both Eurasian red squirrels and Pére David’s rock squirrels demonstrate typical diurnal activity, and the Eurasian red squirrels’ activity peaks remain relatively stable across both the tourist and non-tourist season.
Unlike the Eurasian red squirrel’s relatively fixed activity rhythm, native Pére David’s rock squirrels and Siberian chipmunks exhibit greater flexibility in their temporal activity patterns, especially during the non-tourist season. During this period, both species show marked temporal segregation from Eurasian red squirrels, suggesting a pronounced strategy of temporal avoidance. This adjustment is likely a response to seasonal resource constraints. The non-tourist season in the Labagoumen nature reserve coincides with a colder winter and early spring months, during which forest resource availability in northern China is generally limited. Animals often undergo marked seasonal shifts in diet and activity during these times [55,92]. Under such conditions of scarcity, interspecific competition intensifies, posing a potential threat to native populations [93].
According to niche differentiation theory, sympatric species can reduce competitive pressure and promote coexistence by minimizing overlap along ecological dimensions such as time and space [94]. The observed seasonal adjustments in activity rhythms by native squirrels in this study may reflect an adaptive response to heightened resource competition. By temporally segregating their activity from that of the competitively advantaged Eurasian red squirrels, native species may reduce direct encounters and resource overlap, thereby improving their chances of survival under challenging conditions.
4.4. Niche Compression
It is noteworthy that the temporal segregation in daily activity rhythms among squirrel species was more evident in the non-tourist season and non-tourist area, rather than in periods or regions with high levels of human disturbance. This observation contradicts the common hypothesis that human disturbance exacerbates interspecific competition and promotes greater temporal niche separation [95,96].
We hypothesize that this pattern may be due to intensified human activity during the tourist season, which effectively compresses the temporal and spatial niches available to wildlife [97,98]. In other words, increased human presence may force different species into overlapping activity windows, particularly constraining the activity of native Pére David’s rock squirrels and Siberian chipmunks to time periods dominated by Eurasian red squirrels. This phenomenon may represent a case of niche compression [99,100], where competitive subordinates are restricted in their temporal flexibility.
In contrast, during the non-tourist season, when human disturbance is relatively low, native species such as rock squirrels and chipmunks have greater freedom to adjust their activity rhythms. This results in more pronounced temporal segregation from red squirrels, which may help reduce interspecific competition and facilitate more stable coexistence.
While these findings provide important insights into potential mechanisms of coexistence and competition, our current study design did not include replicated control sites (with only native species) or experimental sites (with both Eurasian red squirrels and native species). Such a replicated control-impact framework would offer stronger causal inference regarding the effects of red squirrel presence on the niches of native species. Owing to logistical constraints in our long-term monitoring program and the strict traffic restrictions in China during the COVID-19 pandemic, this was not feasible at the time. Nevertheless, our dataset offers valuable baseline information on spatial and temporal niche patterns following the recent colonization of Eurasian red squirrels in the reserve. Future research adopting a replicated control–impact design will be important for rigorously testing interspecific interaction hypotheses and informing targeted conservation strategies.
5. Conclusions
This study evaluated the spatial distribution and abundance, activity rhythms, and spatiotemporal niche dynamics of three sympatric squirrel species in the forest ecosystem of the Labagoumen nature reserve, a temperate montane forest in northern China. The Eurasian red squirrel (Sciurus vulgaris), newly recorded in the area, exhibited the broadest spatial distribution and a notable association with tourist-affected areas, suggesting higher ecological adaptability or tolerance to human disturbance. In contrast, the native Pére David’s rock squirrel (Sciurotamias davidianus) and Siberian chipmunk (Tamias sibiricus) were more abundant in the non-tourist area. Although all three species showed diurnal activity with overlapping rhythms, native squirrels exhibited greater temporal divergence from the Eurasian red squirrel in non-tourist seasons, likely reflecting temporal partitioning to avoid competition.
The temporal niche overlap index (Cih) and coefficient of overlap (Δ) were higher during the tourist season, indicating increased synchrony possibly due to shared responses to human disturbance or intensified competition, while reduced overlap in undisturbed conditions suggested greater temporal niche differentiation. These findings suggest that the continued expansion of Eurasian red squirrels is already exerting competitive pressure on native squirrel species.
Human disturbance not only affects the spatial distribution of sympatric squirrels but also acts as a key underlying driver of their temporal coexistence. The competitive advantage of the Eurasian red squirrel and its expanding presence emphasize the need for long-term monitoring of newly recorded species in forest ecosystems. Our findings provide insights for biodiversity conservation and offer a reference for adaptive management and habitat modeling in forest-based nature reserves.
Author Contributions
Conceptualization, W.Z. and X.L.; Formal analysis, W.Z. and X.L.; Funding acquisition, G.C.; Investigation, W.Z.; Methodology, W.Z. and X.L.; Software, W.Z. and T.Z.; Validation, G.C.; Resources, G.C.; Data curation, W.Z., X.L., and T.Z.; Writing—original draft, W.Z. and X.L.; Writing—review and editing, W.Z., X.L., and T.Z.; Supervision, G.C.; Project administration, G.C. All authors have read and agreed to the published version of the manuscript.
Funding
This research was supported by the National Natural Science Foundation of China (Grant No. 32171545, Quantitative analysis of forest ecosystem integrity and authenticity).
Data Availability Statement
Acknowledgments
We extend our sincere gratitude to the leadership of Labagoumen Manchu Township People’s Government in Beijing and the staff of Labagoumen Nature Reserve Management Center for their invaluable support during the survey period. Additionally, we appreciate the participation and assistance of the undergraduate student Jiaze Huai from Beijing Forestry University and the master’s student Ning Xu from Beijing Normal University in conducting this survey.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Salinas-Ramos, V.B.; Ancillotto, L.; Cistrone, L.; Nastasi, C.; Bosso, L.; Smeraldo, S.; Sánchez Cordero, V.; Russo, D. Artificial illumination influences niche segregation in bats. Environ. Pollut. 2021, 284, 117187. [Google Scholar] [CrossRef] [PubMed]
- Chu, C.J.; Wang, Y.S.; Liu, Y.; Jiang, L.; He, F.L. Advances in species coexistence theory. Biodivers. Sci. 2017, 25, 345–354. [Google Scholar] [CrossRef]
- Kronfeld-Schor, N.; Dayan, T. Partitioning of time as an ecological resource. Annu. Rev. Ecol. Evol. Syst. 2003, 34, 153–181. [Google Scholar] [CrossRef]
- Zhou, T.X.; Yang, H.L.; Zhang, G.Q.; Yang, J.; Feng, X.; Hu, Q.; Cheng, Y.H.; Zhang, J.D.; Wang, B.; Zhou, C.Q. Temporal and spatial niche differentiation among three alpine Galliformes with sympatric distribution in the Wolong National Nature Reserve, Sichuan Province. Biodivers. Sci. 2022, 30, 22026. [Google Scholar] [CrossRef]
- Rodríguez-Luna, C.R.; Servin, J.; Valenzuela-Galván, D.; List, R. A matter of time: Temporal partitioning facilitates coexistence between coyotes (Canis latrans) and gray foxes (Urocyon cinereoargenteus) in temperate forests of Mexico. Mamm. Biol. 2024, 104, 363–377. [Google Scholar] [CrossRef]
- Schoener, T.W. Resource partitioning in ecological communities. Science 1974, 185, 27–39. [Google Scholar] [CrossRef]
- Prieto, C.; Dahners, H.W. Resource utilization and environmental and spatio-temporal overlap of a hilltopping lycaenid butterfly community in the Colombian Andes. J. Insect Sci. 2009, 9, 16. [Google Scholar] [CrossRef]
- Pynne, J.T.; Stober, J.M.; Edelman, A.J. Eastern Fox Squirrel (Sciurus niger) occupancy in fragmented montane longleaf pine forests. Southeast. Nat. 2020, 19, 403–417. [Google Scholar] [CrossRef]
- Bonnot, N.C.; Couriot, O.; Berger, A.; Cagnacci, F.; Ciuti, S.; De Groeve, J.E.; Gehr, B.; Heurich, M.; Kjellander, P.; Kröschel, M.; et al. Fear of the dark? Contrasting impacts of humans versus lynx on diel activity of roe deer across Europe. J. Anim. Ecol. 2020, 89, 132–145. [Google Scholar] [CrossRef]
- Murru, V.; Farris, E.; Santo, A.; Grillo, O.; Piazza, C.; Gaio, A.; Bacchetta, G.; Thompson, J.D. Niche Differentiation at Multiple Spatial Scales on Large and Small Mediterranean Islands for the Endemic Silene velutina Pourr. ex Loisel. (Caryophyllaceae). Plants 2021, 10, 2298. [Google Scholar] [CrossRef]
- Bell, O.; Jones, M.E.; Ruiz-Aravena, M.; Hamilton, D.G.; Comte, S.; Hamer, R.; Hamede, R.K.; Newton, J.; Bearhop, S.; McDonald, R.A. Human habitat modification, not apex scavenger decline, drives isotopic niche variation in a carnivore community. Oecologia 2024, 204, 943–957. [Google Scholar] [CrossRef]
- Říha, M.; Vejřík, L.; Rabaneda-Bueno, R.; Jarić, I.; Prchalová, M.; Vejříková, I.; Šmejkal, M.; Blabolil, P.; Čech, M.; Draštík, V.; et al. Ecosystem, spatial and trophic dimensions of niche partitioning among freshwater fish predators. Mov. Ecol. 2025, 13, 36. [Google Scholar] [CrossRef]
- Vaniscotte, A.; Pleydell, D.; Raoul, F.; Quéré, J.P.; Jiamin, Q.; Wang, Q.; Tiaoying, L.; Bernard, N.; Coeurdassier, M.; Delattre, P.; et al. Modelling and spatial discrimination of small mammal assemblages: An example from western Sichuan (China). Ecol. Model. 2009, 220, 1218–1231. [Google Scholar] [CrossRef] [PubMed]
- Rocha, R.G.; Leite, Y.L.R.; Costa, L.P.; Rojas, D. Independent reversals to terrestriality in squirrels (Rodentia: Sciuridae) support ecologically mediated modes of adaptation. J. Evol. Biol. 2016, 29, 2471–2479. [Google Scholar] [CrossRef] [PubMed]
- Mikhalap, S.G.; Istomin, A.V. Modeling Spatial Niches of Small Mammals Using the Example of Myodes glareolus in Mosaic Southern Taiga Forests. Biol. Bull. 2023, 50, S156–S164. [Google Scholar] [CrossRef]
- Kelt, D.A.; Coppeto, S.A.; Van Vuren, D.H.; Sullivan, J.; Wilson, J.A.; Reid, N.M. Niche conservatism versus niche differentiation in sympatric chipmunks in the northern Sierra Nevada. J. Mammal. 2023, 104, 979–992. [Google Scholar] [CrossRef]
- Rossi, A.J.; Klinger, R.C.; Hellwig, E.C.; Van Vuren, D.H. Niches of three sympatric montane ground-dwelling squirrels: Relative importance of climate, topography, and landcover. Ecol. Evol. 2023, 13, e9949. [Google Scholar] [CrossRef]
- Fingland, K.; Ward, S.J.; Bates, A.J.; Bremner-Harrison, S. A systematic review into the suitability of urban refugia for the Eurasian red squirrel Sciurus vulgaris. Mammal. Rev. 2021, 52, 26–38. [Google Scholar] [CrossRef]
- Deng, T. A new record for the Sciurus vulgaris chiliensis in Beijing. Chin. J. Zool. 2008, 43, 37. [Google Scholar] [CrossRef]
- Du, L.H. Comprehensive Scientific Survey Report of Beijing Songshan Nature Reserve; China Foresty Publishing House: Beijing, China, 2012; pp. 105–116. [Google Scholar]
- Shen, J.Y.; Cui, G.F.; Liu, R.Z.; Qu, H.; Huang, S.X.; Wu, J.G.; Fan, Y.Q. Distribution of wildlife along tourism routes in Beijing Songshan National Nature Reserve. J. Beijing For. Univ. 2016, 38, 71–80. [Google Scholar] [CrossRef]
- Zhang, Y.S.; Jiang, J.; Jiang, W.J.; Wang, D.; Fan, Y.Q.; Tang, X.M.; Bao, W.D. Activity Patterns of Mammals in Beijing Songshan National Nature Reserve. Sichuan J. Zool. 2017, 36, 460–467. [Google Scholar] [CrossRef]
- Liu, L.L.; Zhao, Y.J.; Wang, Q.C.; Cui, G.F.; Yang, N.; Zhen, C.Y.; Liu, D. Preliminary investigation of wildlife using camera traps along tourism routes in Beijing Baihua Mountain National Nature Reserve. Acta Ecol. Sin. 2018, 38, 8324–8335. Available online: https://www.ecologica.cn/stxb/ch/reader/view_abstract.aspx?file_no=stxb201709191671 (accessed on 14 August 2025).
- Ji, S.N.; Deng, H.Q.; Fan, P.L.; Liu, R.S.; Zhao, Z.P.; Xiao, N.W.; Liu, X.C. The monitoring research of Sciurinae species in Mentougou district, Beijing. Chin. J. Zool. 2020, 55, 289–296. [Google Scholar] [CrossRef]
- Lioy, S.; Mori, E.; Wauters, L.A.; Bertolino, S. Weight operated see-saw feeding hoppers are not selective for red squirrels when greys are present. Mamm. Biol. 2016, 81, 365–371. [Google Scholar] [CrossRef]
- Bertolino, S.; Di Montezemolo, N.C.; Preatoni, D.G.; Wauters, L.A.; Martinoli, A. A grey future for Europe: Sciurus carolinensis is replacing native red squirrels in Italy. Biol. Invasions 2014, 16, 53–62. [Google Scholar] [CrossRef]
- Mazzamuto, M.V.; Bisi, F.; Wauters, L.A.; Preatoni, D.G.; Martinoli, A. Interspecific competittion between alien Pallas’s squirrels and Eurasian red squirrels reduces density of the native species. Biol. Invasions 2017, 19, 723–735. [Google Scholar] [CrossRef]
- Chen, W.; Gao, W. Report on Mammal Survey of Beijing Songshan Nature Reserve. J. Beijing Teach. Coll (Nat. Sci. Ed.) 1991, 1, 64–69. [Google Scholar] [CrossRef]
- Li, L.J.; Li, S.F.; Zhuang, G.L.; Cao, D.Q.; Chu, M.W.; Liu, X.F. Structure of rodent community in Lingshan Mountain area of Mentougou in Beijing. Cap. J. Public Health 2008, 2, 6–8. [Google Scholar] [CrossRef]
- Liu, F.; Li, D.Q.; Wu, J.G. Using infrared camera to survey wildlife in Beijing Songshan National Nature Reserve. Acta Ecol. Sin. 2012, 32, 730–739. [Google Scholar] [CrossRef]
- Tang, X.M.; Zhang, D.H.; Ma, Z.H.; Wu, T.L.; Zhang, Y.S.; Bao, W.D. Camera trapping survey on ground-dwelling birds and mammals of spring and winter in Beijing Wulingshan Nature Reserve. Chin. J. Zool. 2016, 51, 751–760. [Google Scholar] [CrossRef]
- Lan, H.; Jin, K. Infrared-triggered camera technology application in the investigation of mammals in Beijing Wulingshan National Nature Reserve. Acta Theriol. Sin. 2016, 36, 322–329. [Google Scholar] [CrossRef]
- Cui, G.F.; Li, J.Q.; Niu, S.K.; Hu, D.F.; Lu, D.Z.; Wang, J.Z.; Meng, Z.X.; Li, D.; Yu, B.J.; Yu, B.J.; et al. The establishment and the functional areas’ division of Labagoumen reserve in Beijing. J. Beijing For. Univ. 2000, 22, 40–45. [Google Scholar] [CrossRef]
- Cui, G.F.; Xing, S.H. Comprehensive Scientific Investigation Report of Labagoumen Nature Reserve in Beijing; China Foresty Publishing House: Beijing, China, 2009; pp. 1–74. [Google Scholar]
- Li, Z.M.; Li, J.Q. Function of Eco-tourism to the Economy Development of Labagoumen Nature Reserve. Sci. Technol. Eng. 2007, 4, 567–571. Available online: http://www.stae.com.cn/jsygc/article/abstract/200704143?st=search (accessed on 14 August 2025).
- Azevedo, F.C.; Lemos, F.G.; Freitas-Junior, M.C.; Rocha, D.G.; Azevedo, F.C.C. Puma activity patterns and temporal overlap with prey in a human-modified landscape at southeastern brazil. J. Zool. 2018, 305, 246–255. [Google Scholar] [CrossRef]
- Liu, P.; Fu, M.X.; Qi, D.W.; Song, X.Q.; Wei, W.; Yang, W.J.; Chen, Y.X.; Zhou, Y.S.; Liu, J.B.; Ma, R.; et al. Camera-trapping survey of wild mammals and birds in Daxiangling Nature Reserve. Biodivers. Sci. 2020, 28, 905–912. [Google Scholar] [CrossRef]
- Gaynor, K.M.; Hojnowski, C.E.; Carter, N.H.; Brashares, J.S. The influence of human disturbance on wildlife nocturnality. Science 2018, 360, 1232–1235. [Google Scholar] [CrossRef] [PubMed]
- Hu, Y.F.; Li, B.Q.; Liang, D.; Li, X.Q.; Liu, L.X.; Yang, J.W.; Luo, X. Effect of anthropogenic disturbance on Lady Amherst’s pheasant (Chrysolophus amherstiae) activity. Biodivers. Sci. 2022, 30, 21484. [Google Scholar] [CrossRef]
- Liu, X.; Qi, X.H.; Cheng, Y.; Zhang, H.G.; Cai, B. Activity Rhythm of Lophura nycthemera and Muntiacus reevesi in Wuyishan National Park and Its Influencing Factors. J. Hainan Norm. Univ. Nat. Sci. (Nat. Sci.) 2023, 36, 65–72. Available online: https://hsxblk.hainnu.edu.cn/ch/reader/key_query.aspx (accessed on 14 August 2025).
- O’Brien, T.G.; Kinnaird, M.F.; Wibisono, H.T. Crouching tigers, hidden prey: Sumatran tiger and prey populations in a tropical forest landscape. Anim. Conserv. 2003, 6, 131–139. [Google Scholar] [CrossRef]
- Smith, A.T.; Xie, Y. A Guide to the Mammals of China; Hunan Education Press: Changsha, China, 2009; pp. 31–68. [Google Scholar]
- Wei, F.W.; Yang, Q.S.; Wu, Y.; Jiang, X.L.; Liu, S.Y.; Li, B.G.; Yang, G.; Li, M.; Zhou, J.; Li, S.; et al. Catalogue of mammals in China (2021). Acta Theriol. Sin. 2021, 41, 487–501. [Google Scholar] [CrossRef]
- Chen, L.J.; Xiao, W.H.; Xiao, Z.S. Limitations of relative abundance indices calculated from camera-trapping data. Biodivers. Sci. 2019, 27, 243–248. [Google Scholar] [CrossRef]
- Wu, P.F.; Liu, X.H.; Cai, Q.; He, X.B.; Melissa, S.; Zhu, Y.; Shao, X.M. The application of infrared camera in mammal research in Guanyinshan Nature Reserve, Shaanxi. Acta Theriol. Sin. 2012, 32, 67–71. [Google Scholar] [CrossRef]
- Schoener, T.W. The Anolis Lizards of Bimini: Resource Partitioning in a Complex Fauna. Ecology 1968, 49, 704–726. [Google Scholar] [CrossRef]
- Sun, R.Y. Principles of Animal Ecology, 3rd ed.; Beijing Normal University Press: Beijing, China, 2001; pp. 334–336. [Google Scholar]
- Yang, C.W.; Ma, J.Z.; Jin, J.L.; Liu, Z. Research on Autumn Time Niche of Five Rodents in Forests Ecosystem. Chin. J. Zool. 2008, 43, 64–69. [Google Scholar] [CrossRef]
- Chen, C.; Hou, R.; Wu, W.; Zhang, Z.H.; Gu, X.D.; Qi, D.W. Study on the Temporal Niche Characteristics and Overlaps of Carnivores and Their Preys Using Infrared Cameras in Xinlong County. Sichuan J. Zool. 2021, 40, 15–22. [Google Scholar] [CrossRef]
- Wathne, J.A.; Haug, T.; Lydersen, C. Prey preference andniche overlap of ringed seals (Phoca hispida) and harp seals (Phoca groenlandica) in the Barents Sea. Mar. Ecol. Prog. Ser. 2000, 194, 233–239. [Google Scholar] [CrossRef]
- Rowcliffe, M. Activity: Animal Activity Statistics. 2016. Available online: https://CRAN.R-project.org/package=activity (accessed on 10 July 2025).
- Ridout, M.S.; Linkie, M. Estimating overlap of daily activity patterns from camera trap data. J. Agric. Biol. Environ. Stat. 2009, 14, 322–337. [Google Scholar] [CrossRef]
- Meredith, M.; Ridout, M. Overlap: Estimates of Coefficient of Overlapping for Animal Activity Patterns. 2014. Available online: https://CRAN.R-project.org/package=overlap (accessed on 7 August 2025).
- Lynam, A.J.; Jenks, K.E.; Tantipisanuh, N.; Chutipong, W.; Ngoprasert, D.; Steinmetz, R.; Sukmasuang, R.; Jr, L.I.G.; Cutter, P.; Kitamura, S.; et al. Terrestrial Activity patterns of wild cats from camera-trapping. Raffles Bull. Zool. 2013, 61, 407–415. [Google Scholar]
- Liu, P.; Liu, Z.S.; Cao, H.; Li, Z.Z.; Zhang, Z.R.; Teng, L.W. Comparing the activity pattern of red deer (Cervus alashanicus) and blue sheep (Pseudois nayaur) using camera-traps in Helan Mountains. Acta Ecol. Sin. 2019, 39, 9365–9372. [Google Scholar] [CrossRef]
- Rao, J.S.; SenGupta, A. Topics in Circular Statistics; Section 7.5; World Scientific Press: Singapore, 2001. [Google Scholar]
- Agnostinelli, C.; Lund, U. Circular Statistics. Package ‘Circular’. 2017. Available online: https://cran.r-project.org/web/packages/circular/circular.pdf (accessed on 2 July 2025).
- Levins, R. Evolution in Changing Environments: Some Theoretical Explorations. (MPB-2); Princeton University Press: Princeton, NJ, USA, 1968. [Google Scholar] [CrossRef]
- Pla, L. Bootstrap Confidence intervals for the Shannon biodiversity index: A simulation study. J. Agric. Biol. Environ. Stat. 2004, 9, 42–56. [Google Scholar] [CrossRef]
- Zhao, L.; Cheng, J.X.; Xu, M.Q.; Li, M. Bootstrap Method and its Application in Biology. Sichuan J. Zool. 2010, 29, 638–641. [Google Scholar]
- Clarke, K.R. Non-parametric multivariate analyses of changes in community structure. Aust. J. Ecol. 1993, 18, 117–143. [Google Scholar] [CrossRef]
- Bandyopadhyay, M.; Burton, A.C.; Gupta, S.K.; Krishnamurthy, R. Understanding the distribution and fine-scale habitat selection of mesocarnivores along a habitat quality gradient in western Himalaya. PeerJ 2022, 10, e13993. [Google Scholar] [CrossRef] [PubMed]
- Cully, J.F.; Collinge, S.K.; VanNimwegen, R.E.; Ray, C.; Johnson, W.C.; Thiagarajan, B.; Conlin, D.B.; Holmes, B.E. Spatial variation in keystone effects: Small mammal diversity associated with black-tailed prairie dog colonies. Ecography 2010, 33, 667–677. [Google Scholar] [CrossRef]
- Anderson, M.J. PERMANOVA: A FORTRAN Computer Program for Permutational Multivariate Analysis of Variance. Ph.D. Thesis, Department of Statistics, University of Auckland, Auckland, New Zealand, 2005. [Google Scholar]
- Oksanen, J. 2015 Multivariate Analysis of Ecological Commu-Nities in R: Vegan Tutorial. R Package Version 1.7. Available online: https://cran.r-project.org/web/packages/vegan/ (accessed on 10 May 2025).
- Bertolino, S. Animal trade and non-indigenous species introduction: The world-wide spread of squirrels. Divers. Distrib. 2009, 15, 701–708. [Google Scholar] [CrossRef]
- Banks, P.B.; Hughes, N.K. A review of the evidence for potential impacts of black rats (Rattus rattus) on wildlife and humans in Australia. Wildl. Res. 2012, 39, 78–88. [Google Scholar] [CrossRef]
- Bonesi, L.; MacDonald, D.W. Impact of Released Eurasian Otters on a Population of American Mink: A Test Using an Experimental Approach. Oikos 2004, 106, 9–18. Available online: http://www.jstor.org/stable/3548390 (accessed on 2 July 2025). [CrossRef]
- Bonesi, L.; Chanin, P.; Macdonald, D.W. Competition between Eurasian otter Lutra lutra and American mink Mustela vison probed by niche shift. Oikos 2004, 106, 19–26. [Google Scholar] [CrossRef]
- Chen, W.; Gao, W.; Fu, B.Q. The Mammal Fauna of Beijing; Beijing Publishing House: Beijing, China, 2002; pp. 164–178. [Google Scholar]
- Costa-Pereira, R.; Araújo, M.S.; Souza, F.L.; Ingram, T. Competition and resource breadth shape niche variation and overlap in multiple trophic dimensions. Proc. Biol. Scie 2019, 286, 20190369. [Google Scholar] [CrossRef]
- Wen, Z.; Feijó, A.; Cheng, J.; Du, Y.; Ge, D.; Xia, L.; Yang, Q. Explaining mammalian abundance and elevational range size with body mass and niche characteristics. J. Mammal. 2021, 102, 13–27. [Google Scholar] [CrossRef]
- Poorboy, D.M.; Calède, J.J.M.; Chavez, A.S. Craniodental divergence associated with bite force between hybridizing pine squirrels (Tamiasciurus). PLoS ONE 2023, 18, e0284094. [Google Scholar] [CrossRef]
- Speakman, J.R. Body size, energy metabolism and lifespan. J. Exp. Biol. 2005, 208, 1717–1730. [Google Scholar] [CrossRef] [PubMed]
- Gurnell, J.; Wauters, L.A.; Lurz, P.W.W.; Tosi, G. Alien species and interspecific competition: Effects of introduced eastern grey squirrels on red squirrel population dynamics. J. Anim. Ecol. 2004, 73, 26–35. [Google Scholar] [CrossRef]
- Chow, P.K.Y.; Uchida, K.; von Bayern, A.M.P.; Koizumi, I. Characteristics of urban environments and novel problem-solving performance in Eurasian red squirrels. Proc. Biol. Sci. 2021, 288, 20202832. [Google Scholar] [CrossRef] [PubMed]
- Mazzamuto, M.V.; Merrick, M.J.; Bisi, F.; Koprowski, J.L.; Wauters, L.; Martinoli, A. Timing of Resource Availability Drives Divergent Social Systems and Home Range Dynamics in Ecologically Similar Tree Squirrels. Front. Ecol. Evol. 2020, 8, 174. [Google Scholar] [CrossRef]
- Huang, W.J.; Chen, Y.X.; Wen, Y.X. Rodents of China; Fudan University Press: Shanghai, China, 1995; pp. 86–89. [Google Scholar]
- Yang, C.W. Coexistence Mechanisms of Five Rodent Species in Major Forest Regions of Northeast China. Ph.D. Thesis, Northeast Forestry University, Harbin, China, 2007. [Google Scholar]
- Li, J.S. Study on Nutritional Adaptation Strategies of the Red Squirrel (Sciurus Vulgaris Mantchuricus). Ph.D. Thesis, Northeast Forestry University, Harbin, China, 2001. [Google Scholar]
- Wang, W.; Hu, J.C. Study of Spatial Memory in Seed-caching Sciurotamias davidianus. Sichuan J. Zool. 2013, 32, 658–663. Available online: http://www.scdwzz.com/thesisDetails?columnId=29112746&Fpath=home&index=0&lang=zh (accessed on 14 August 2025).
- Wei, S.; Liang, Z.L.; Teng, W.; Jing, Y.; Yu, M.C.; Rong, K. Food Composition and Feeding Preference of Chipmunks in a Broadleaved Red Pine Forest. Chin. J. Wildl. 2023, 44, 506–514. [Google Scholar] [CrossRef]
- Lucherini, M.; Reppucci, J.I.; Walker, R.S.; Villalba, M.L.; Wurstten, A.; Gallardo, G.; Iriarte, A.; Villalobos, R.; Perovic, P. Activity Pattern Segregation of Carnivores in the High Andes. J. Mammal. 2009, 90, 1404–1409. [Google Scholar] [CrossRef]
- Shi, X.G.; Shi, X.Y.; Hu, Q.; Feng, X.; Jin, S.L.; Cheng, Y.H.; Zhang, J.; Yao, M.; Li, S. Spatiotemporal relationships between snow leopard (Panthera uncia) and red fox (Vulpes vulpes) in Qionglai Mountains, Sichuan Province. Acta Theriol. Sin. 2021, 41, 115–127. [Google Scholar] [CrossRef]
- Wauters, L.A.; Lurz, P.W.W.; Santicchia, F.; Romeo, C.; Ferrari, N.; Martinoli, A.; Gurnell, J. Interactions between native and invasive species: A systematic review of the red squirrel-gray squirrel paradigm. Front. Ecol. Evol. 2023, 11, 1083008. [Google Scholar] [CrossRef]
- Mori, E.; Zozzoli, R.; Mazza, G. Coming in like a wrecking-ball: Are native Eurasian red squirrels displacing invasive Siberian chipmunks? A study from an urban park. Urban. Ecosyst. 2018, 21, 975–981. [Google Scholar] [CrossRef]
- Woolway, E.E.; Goodenough, A.E. Effects of visitor numbers on captive European red squirrels (Sciurus vulgaris) and impacts on visitor experience. Zoo Biol. 2017, 36, 112–119. [Google Scholar] [CrossRef]
- Krauze-Gryz, D.; Gryz, J.; Brach, M. Spatial organization, behaviour and feeding habits of red squirrels: Differences between an urban park and an urban forest. J. Zool. 2021, 315, 69–78. [Google Scholar] [CrossRef]
- Di Bitetti, M.S.; De Angelo, C.; Di Blanco, Y.E.; Paviolo, A. Niche partitioning and species coexistence in a Neotropical felid assemblage. Acta Oecol. 2010, 36, 403–412. [Google Scholar] [CrossRef]
- Sun, N.; Cao, G.; Li, G.; Liu, Z.; Quan, R.C. Macaca leonina has a wider niche breadth than sympatric M. mulatta in a fragmented tropical forest in southwest China. Am. J. Primatol. 2020, 82, e23100. [Google Scholar] [CrossRef] [PubMed]
- Yan, M.X.; Sun, N.; Gu, B.J.; He, R.C.; Liu, Y. Spatio-Temporal Niche Differentiation of Sympatric Green Peafowl (Pavo muticus) and Silver Pheasant (Lophura nycthemera). Sichuan J. Zool. 2021, 40, 150–158. [Google Scholar] [CrossRef]
- Li, J.S.; Ma, J.Z.; Song, Y.L.; Zeng, Z.G. Comparative Studies on the Seasonal Changes of Some Eco-physiological Indices of Red Squirrels, Sciurus vulgaris mantchuricus, in Northeast Forestry Region, China. Acta Ecol. Sin. 2002, 22, 1995–2000. [Google Scholar]
- Viviano, A.; Mori, E.; Fattorini, N.; Mazza, G.; Lazzeri, L.; Panichi, A.; Strianese, L.; Mohamed, W.F. Spatiotemporal Overlap between the european brown hare and its potential predators and competitors. Animals 2021, 11, 562. [Google Scholar] [CrossRef]
- Nagy-Reis, M.B.; Iwakami, V.H.; Estevo, C.A.; Setz, E.Z.F. Temporal and dietary segregation in a neotropical small-felid assemblage and its relation to prey activity. Mamm. Biol. 2019, 95, 1–8. [Google Scholar] [CrossRef]
- Cui, P.; Kang, M.J.; Deng, W.H. Foraging habitat selection by sympatric Temminck’s tragopan and blood pheasant during breeding season in southwestern China. Biodivers. Sci. 2008, 16, 143–149. [Google Scholar] [CrossRef]
- Wang, L.; Lu, Y.; Cai, Y.; Ji, L.; Pang, D.; Zhou, M.; Cheng, Y.; Pu, F.; Zhang, B. The Spatial Relationship Between Two Sympatric Pheasant Species and Various Human Disturbance Activities. Animals 2025, 15, 95. [Google Scholar] [CrossRef]
- Parsons, A.W.; Rota, C.T.; Forrester, T.; Baker-Whatton, M.C.; McShea, W.J.; Schuttler, S.G.; Millspaugh, J.J.; Kays, R. Urbanization focuses carnivore activity in remaining natural habitats, increasing species interactions. J. Appl. Ecol. 2019, 56, 1894–1904. [Google Scholar] [CrossRef]
- Gilbert, N.A.; Stenglein, J.L.; Pauli, J.N.; Zuckerberg, B. Human disturbance compresses the spatiotemporal niche. Proc. Natl. Acad. Sci. USA 2022, 119, e2206339119. [Google Scholar] [CrossRef]
- Sévêque, A.; Gentle, L.K.; López-Bao, J.V.; Yarnell, R.W.; Uzal, A. Human disturbance has contrasting effects on niche partitioning within carnivore communities. Biol.Rev. Camb. Philos. Soc. 2020, 95, 1689–1705. [Google Scholar] [CrossRef]
- Wilson, M.W.; Ridlon, A.D.; Gaynor, K.M.; Gaines, S.D.; Stier, A.C.; Halpern, B.S. Ecological impacts of human-induced animal behaviour change. Ecol. Lett. 2020, 23, 1522–1536. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
