Influence of Mechanical Wounding and Compartmentalization Mechanism on the Suppression of Invasive Plant Species Using the Example of Cherry Laurel (Prunus laurocerasus)
Abstract
:1. Introduction
- plucking, where the entire younger plant can be removed along with the root system;
- digging, where, especially in certain species, we want to remove the entire root system from which a new shoot might emerge;
- removal by pruning, which is the simplest method but usually less effective as the plant often sprouts even more vegetative buds, so it is crucial to repeat the annual pruning [16] and;
- various types of bark and cambium removal, known as girdling, applied mainly to species with thicker stems (trunks).
2. Materials and Methods
2.1. Plant Species Studied
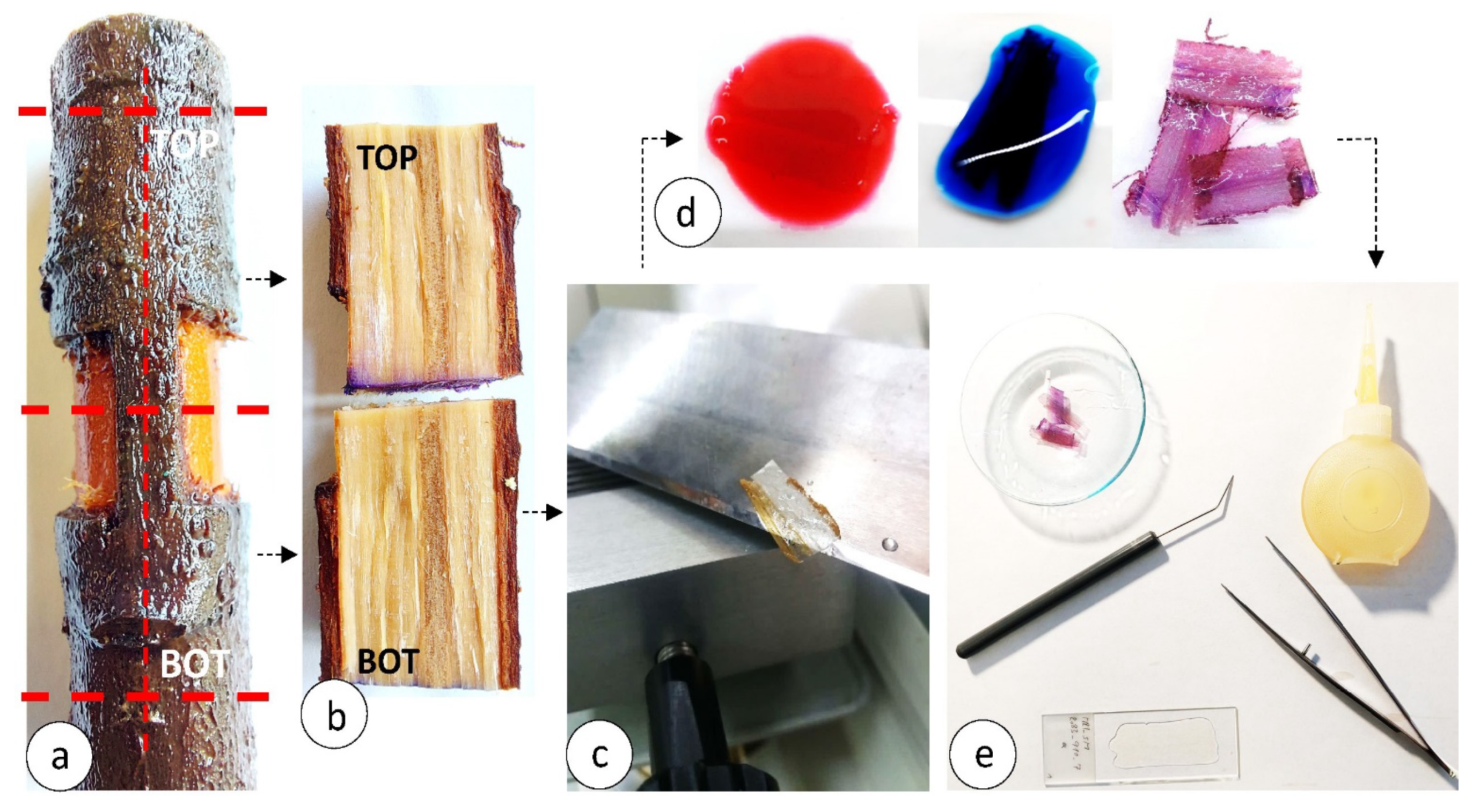
2.2. Seedling Selection, Wounding and Sampling
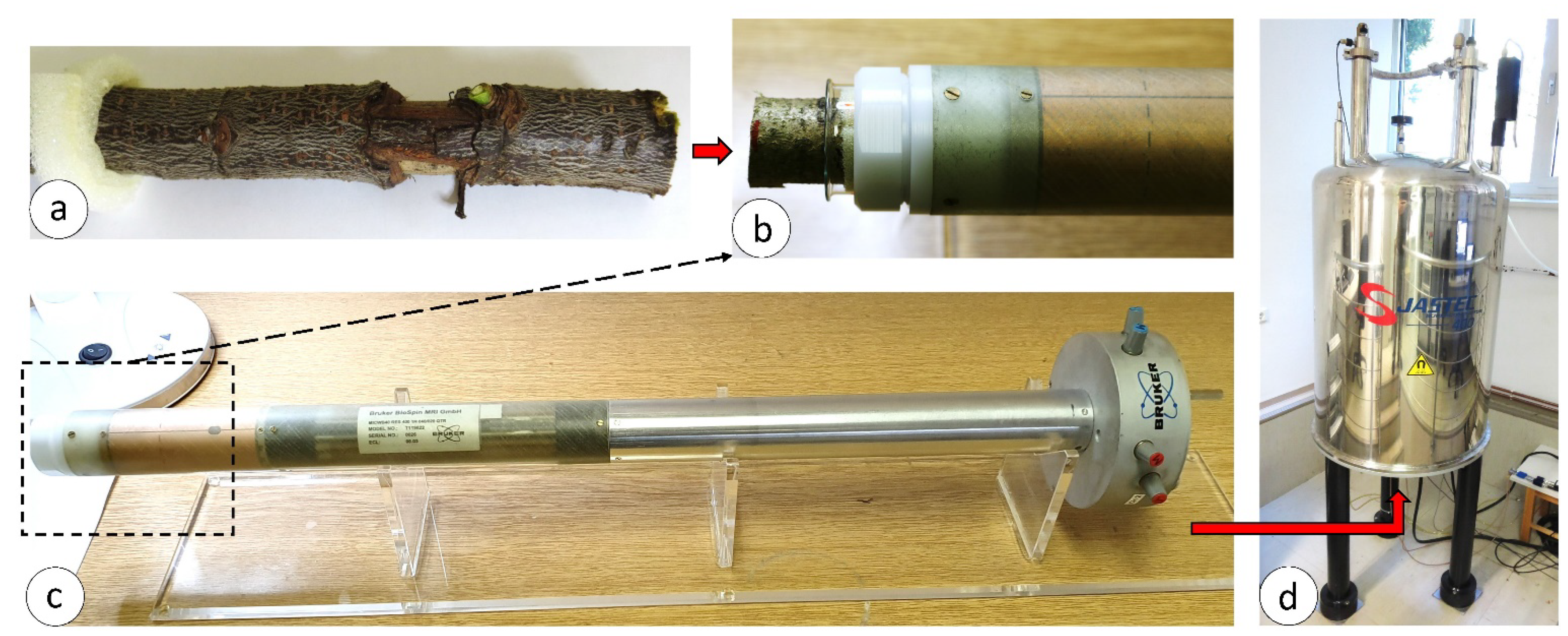
2.3. Magnetic Resonance Imaging and Wood Moisture Determination
2.4. Wood Anatomical Investigation
3. Results and Discussion
3.1. Morphological and Water Distribution Changes after Injury
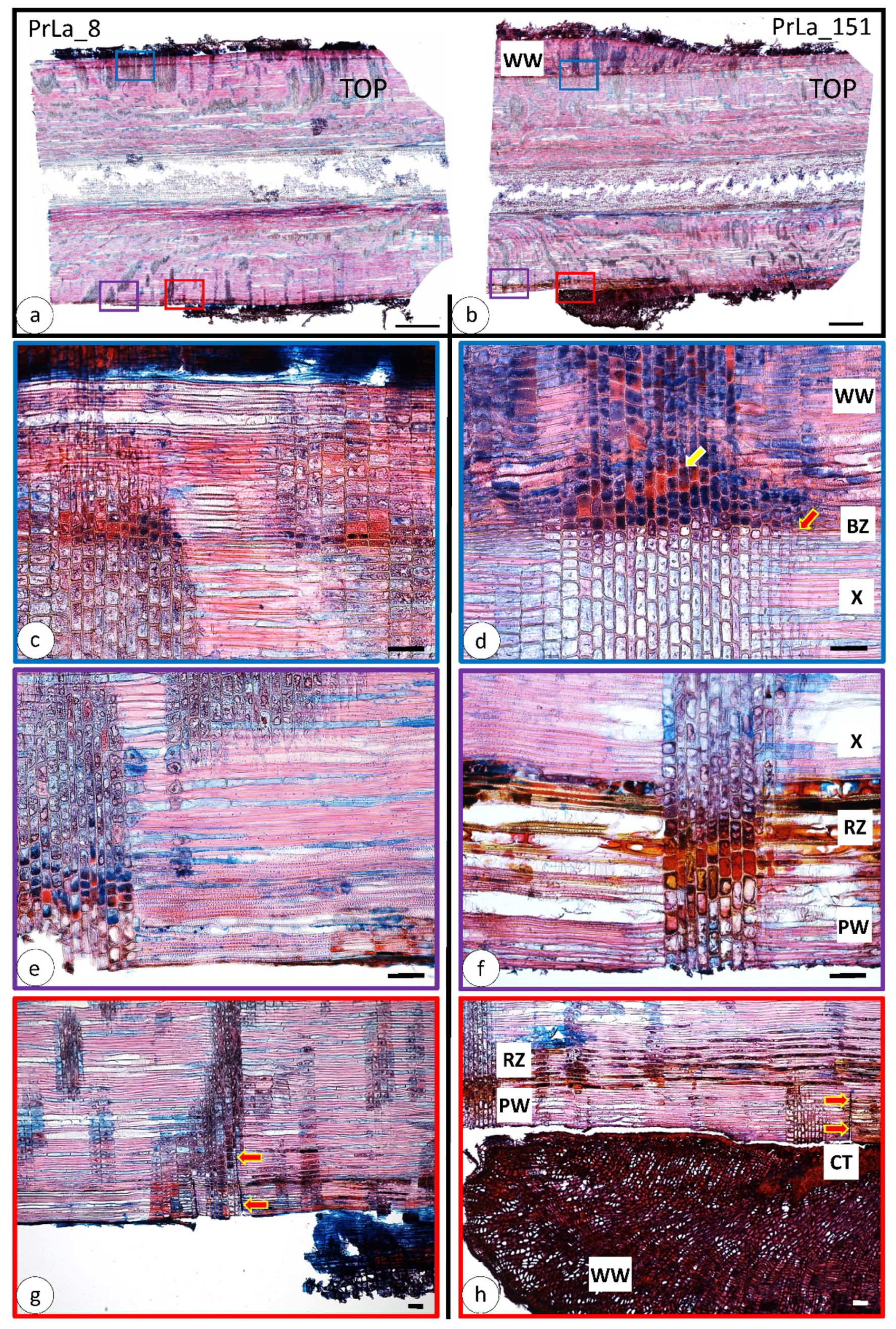
3.2. Wood Anatomical Changes Visible on Radial Section
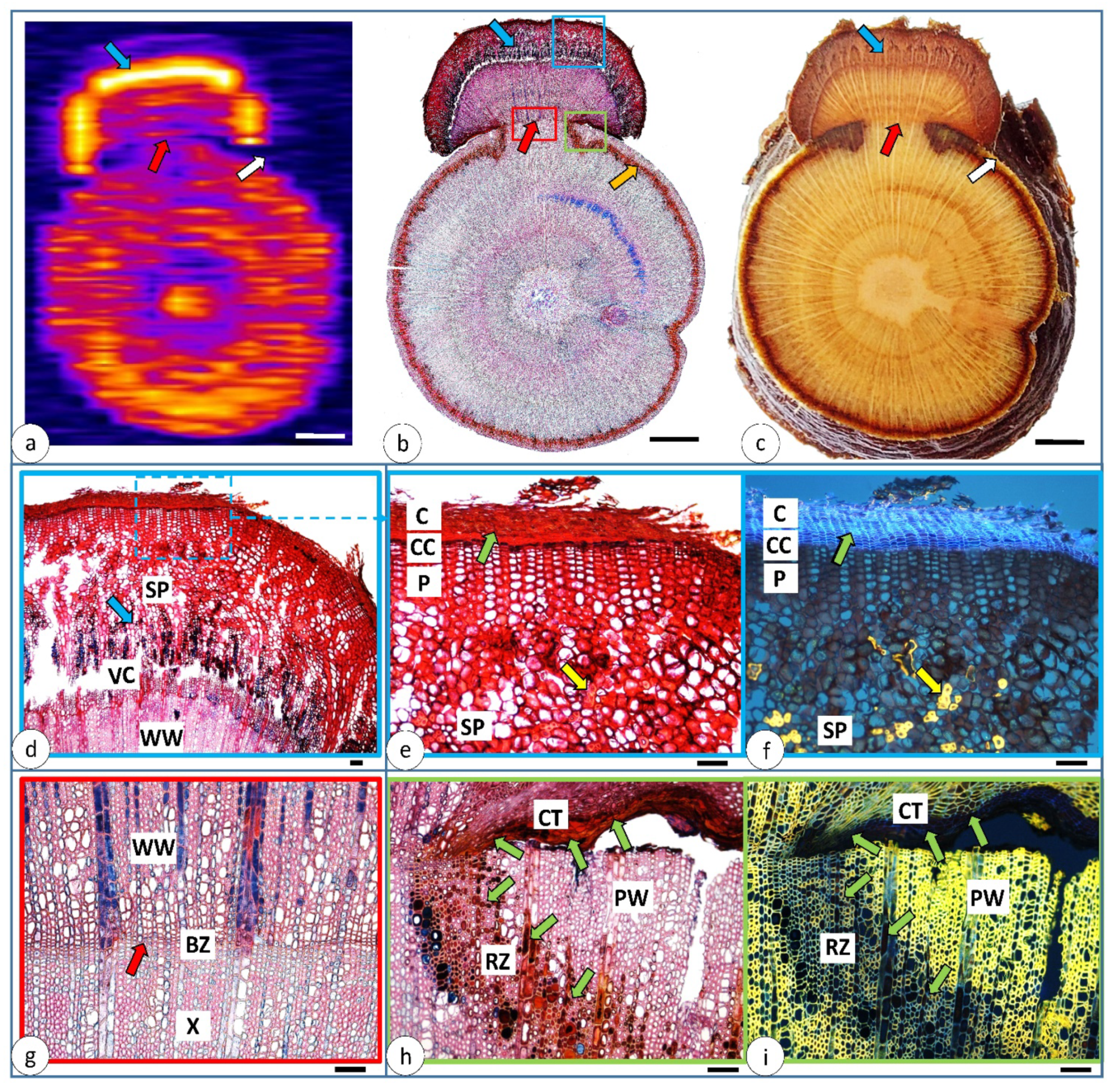
3.3. Changes 151 Days after Injury—The Cross-Section
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Appendix A
Appendix A.1. Average Moisture Content
| PrLa | mgreen [g] | m0 [g] | u [%] |
|---|---|---|---|
| 1 | 3.191 | 1.869 | 70.7 |
| 2 | 2.724 | 1.574 | 73.0 |
| 3 | 2.536 | 1.429 | 77.5 |
| 4 | 3.409 | 1.863 | 83.0 |
| 5 | 2.658 | 1.433 | 85.5 |
| 6 | 2.480 | 1.360 | 82.4 |
| 7 | 6.083 | 3.559 | 70.9 |
| 8 | 5.778 | 3.363 | 71.8 |
| 9 | 6.792 | 3.908 | 73.8 |
| Aver. MC: 76.5 |
Appendix A.2. Correction Factor
| MR Image | |||
|---|---|---|---|
| days after injury | 8 | 76 | 151 |
| sample | PrLa8 | PrLa76 | PrLa151 |
| PIV | 68 | 50 | 83 |
| CF | 1.13 | 1.53 | 0.92 |
References
- Jogan, N.; Kos, I. Poti vnosa, prenosa in širjenja tujerodnih vrst. CRP NeoBiota—Končno Poročilo 2012, 31–42. Available online: http://www.bioportal.si/neobiota.php (accessed on 10 June 2021).
- Richardson, D.M.; Hui, C.; Nuñez, M.A.; Pauchard, A. Tree invasions: Patterns, processes, challenges and opportunities. Biol. Invasions 2014, 16, 473–481. [Google Scholar] [CrossRef]
- Brus, R.; Pötzelsberger, E.; Lapin, K.; Brundu, G.; Orazio, C.; Straigyte, L.; Hasenauer, H. Extent, distribution and origin of non-native forest tree species in Europe. Scand. J. For. Res. 2019, 34, 533–544. [Google Scholar] [CrossRef]
- Castro-Díez, P.; Vaz, A.S.; Silva, J.S.; van Loo, M.; Alonso, Á.; Aponte, C.; Bayón, Á.; Bellingham, P.J.; Chiuffo, M.C.; DiManno, N.; et al. Global effects of non-native tree species on multiple ecosystem services. Biol. Rev. 2019, 94, 1477–1501. [Google Scholar] [CrossRef] [PubMed]
- Sádlo, J.; Vítková, M.; Pergl, J.; Pyšek, P. Towards site-specific management of invasive alien trees based on the assessment of their impacts: The case of Robinia pseudoacacia. Neobita 2017, 35, 1–34. [Google Scholar] [CrossRef] [Green Version]
- Swearingen, J.; Slattery, B.; Reshetiloff, K.; Zwicker, S. Plant Invaders of Natural Areas, 5th ed.; National Park Service, US Fish and Wildlife Service: Washington, DC, USA, 2014.
- Regulation (EU) No 1143/2014 of the European Parliament and of the Council of 22 October 2014 on the Prevention and Management of the Introduction and Spread of Invasive Alien Species. 2014, pp. 35–55. Available online: https://eur-lex.europa.eu/legal-content/EN/TXT/?uri=celex%3A32014R1143 (accessed on 18 June 2021).
- Roženbergar, D.; Nagel, T.A.; Urbas, B.; Marion, L.; Brus, R. Nekateri ukrepi za omejevanje širjenja visokega pajesena (Ailanthus altissima (Mill.) Swingle) in smernice za gozdnogojitveno ukrepanje ob vdoru potencialno invazivnih tujerodnih drevesnih vrst v ohranjene gozdove v Sloveniji. Gozdarski Vestn. 2017, 1, 3–20. [Google Scholar]
- Gorišek, Ž.; Plavčak, D.; Straže, A.; Merela, M. Technological properties and usability of chinese sumac wood in comparison with ash wood. Les/Wood 2018, 67, 29–44. [Google Scholar] [CrossRef] [Green Version]
- Plavčak, D.; Gorišek, Ž.; Straže, A.; Merela, M. Drying characteristics of wood of invasive tree species growing in an urban environment. Les/Wood 2019, 68, 31–43. [Google Scholar] [CrossRef] [Green Version]
- Merela, M.; Thaler, N.; Balzano, A.; Plavčak, D. Optimal Surface Preparation for Wood Anatomy Research of Invasive Species by Scanning Electron Microscopy. Drv. Ind. 2020, 71, 117–127. [Google Scholar] [CrossRef]
- Merhar, M.; Gornik Bučar, D.; Merela, M. Machinability Research of the Most Common Invasive Tree Species in Slovenia. Forests 2020, 11, 752. [Google Scholar] [CrossRef]
- Gornik Bučar, D.; Prislan, P.; Smolnikar, P.; Stare, D.; Krajnc, N. Uporabnost lesnih ostankov tujerodnih invazivnih drevesnih vrst za proizvodnjo peletov. Les/Wood 2021, 70, 45–58. [Google Scholar] [CrossRef]
- Horvat, M.; Iskra, J.; Pavlič, M.; Žigon, J.; Merela, M. Wood dyes from invasive alien plants. Les/Wood 2020, 69, 37–48. [Google Scholar] [CrossRef]
- Gornik Bučar, D.; Gospodarič, B.; Smolnikar, P.; Stare, D.; Krajnc, N.; Prislan, P. Invasive species as raw material for pellets production. In Proceedings of the 70th Anniversary of Drvna Industrija Journal, Zagreb, Croatia, 12–13 December 2019; pp. 61–68. [Google Scholar]
- Burch, P.L.; Zedaker, S.M. Removing the invasive tree Ailanthus Altissima and restoring natural cover. J. Arboric. 2003, 29, 18–24. [Google Scholar]
- Moore, G.M. Ring-Barking and Girdling: How Much Vascular Connection Do You Need between Roots and Crown? In Proceedings of the 14th National Street Tree Symposium; University of Adelaide Australia: Adelaide, Australia, 2013; pp. 87–96. [Google Scholar]
- Kowarik, I.; Säumel, I. Biological flora of Central Europe: Ailanthus altissima (Mill.) Swingle. Perspect. Plant. Ecol. Evol. Syst. 2007, 8, 207–237. [Google Scholar] [CrossRef]
- Noel, A.R.A. The Girdled Tree. Bot. Rev. 1970, 36, 162–195. [Google Scholar] [CrossRef]
- Drescher, A.; Ließ, N. Control of alien woody species in the Danube National Park (Austria) the Example of Ailanthus altissima. BfN-Skripten 2006, 184. Available online: https://www.researchgate.net/profile/Anton-Drescher/publication/320357724_Control_of_alien_woody_species_in_the_Danube_National_Park_Austria_The_example_of_Ailanthus_altissima/links/59dfe095a6fdcca98420e68a/Control-of-alien-woody-species-in-the-Danube-National-Park-Austria-The-example-of-Ailanthus-altissima.pdf (accessed on 10 June 2021).
- De Schepper, V.; Steppe, K. Tree girdling: A tool to improve our understanding of coupled sugar and water transport. Acta Hortic. 2013, 990, 313–320. [Google Scholar] [CrossRef] [Green Version]
- Nyssen, B.; Schmidt, U.E.; Muys, B.; van der Lei, P.B.; Pyttel, P. The history of introduced tree species in Europe in a nutshell. In Introduced Tree Species in European Forests: Opportunities and Challenges; Krumm, F., Vítková, L., Eds.; European Forest Institute: Freiburg, Germany, 2016; p. 423. [Google Scholar]
- Rudolf, S.; Brus, R. Razširjenost in invazivnost robinije (Robinia pseudoacacia L.) v severovzhodni Sloveniji. Gozdarski Vestn. 2006, 64, 134–140. [Google Scholar]
- De Groot, M.; Ogris, N.; Kobler, A. The effects of a large-scale ice storm event on the drivers of bark beetle outbreaks and associated management practices. For. Ecol. Manag. 2018, 408, 195–201. [Google Scholar] [CrossRef]
- Life Artemis Alien Species in Slovenija. Available online: https://www.tujerodne-vrste.info/en/alien-species-slovenia/alien-plants/ (accessed on 18 June 2021).
- Rusterholz, H.P.; Schneuwly, J.; Baur, B. Invasion of the alien shrub Prunus laurocerasus in suburban deciduous forests: Effects on native vegetation and soil properties. Acta Oecol. 2018, 92, 44–51. [Google Scholar] [CrossRef]
- Applause APPLAUSE—Alien Plant Species from Harmful to Useful with Citizens’ Led Activities. Available online: https://www.uia-initiative.eu/en/uia-cities/ljubljana. (accessed on 18 June 2021).
- Plavčak, D.; Balzano, A.; Krže, L.; Merela, M. Wood Response Investigation of Four Selected Non-Native Woody Plants to Various Mechanical Injuries. In Proceedings of the 9th Hardwood Proceedings Part I; University of Sopron: Sopron, Hungary, 2020; pp. 212–217. [Google Scholar]
- Merela, M.; Oven, P.; Sepe, A.; Serša, I. Three-dimensional in vivo magnetic resonance microscopy of beech (Fagus sylvatica L.) wood. Magn. Reson. Mater. Phys. Biol. Med. 2005, 18, 171–174. [Google Scholar] [CrossRef] [PubMed]
- Mikac, U.; Merela, M.; Oven, P.; Sepe, A.; Serša, I. MR Study of Water Distribution in a Beech (Fagus sylvatica) Branch Using Relaxometry Methods. Molecules 2021, 26, 4305. [Google Scholar] [CrossRef] [PubMed]
- Oven, P.; Merela, M.; Mikac, U.; Serša, I. 3D magnetic resonance microscopy of a wounded beech branch. Holzforschung 2008, 62, 322–328. [Google Scholar] [CrossRef]
- Oven, P.; Merela, M.; Mikac, U.; Serša, I. Application of 3D magnetic resonance microscopy to the anatomy of woody tissues. IAWA J. 2011, 32, 401–414. [Google Scholar] [CrossRef] [Green Version]
- Merela, M.; Oven, P.; Serša, I.; Mikac, U. A single point NMR method for an instantaneous determination of the moisture content of wood. Holzforschung 2009, 63, 348–351. [Google Scholar] [CrossRef]
- Hall, L.D.; Rajanayagam, V. Evaluation of the distribution of water in wood by use of three dimensional proton NMR volume imaging. Wood Sci. Technol. 1986, 20, 329–333. [Google Scholar] [CrossRef]
- SIST EN 13183-1: Moisture Content of a Piece Of Sawn Timber—Part 1: Determination by oven Dry Method. 2002. Available online: https://standards.iteh.ai/catalog/standards/cen/ebaf1b83-ed78-4bfe-838f-b02fef750459/en-13183-1-2002 (accessed on 18 June 2021).
- Prislan, P.; Merela, M.; Zupančič, M.; Krže, L.; Čufar, K. Uporaba izbranih svetlobno mikroskopskih tehnik za raziskave lesa in skorje. Les/Wood 2009, 5, 222–229. [Google Scholar]
- Čufar, K.; Merela, M.; Erič, M. A Roman barge in the Ljubljanica river (Slovenia): Wood identification, dendrochronological dating and wood preservation research. J. Archaeol. Sci. 2014, 44, 128–135. [Google Scholar] [CrossRef]
- Shigo, A.L.; Marx, H.G. Compartmentalization of Decay in Trees; US Department of Agriculture, Forest Service: Washington, DC, USA, 1977; 73p.
- Rayner, A.D.; Boddy, L. Fungal Decomposition of Wood—Its Biology and Ecology, 1st ed.; Wiley: Wiltshire, UK, 1988. [Google Scholar]
- Merela, M.; Oven, P.; Serša, I.; Mikac, U. Response of beech trunk tissues to mechanical wouding. In Rational Use of Wood in the Context of Sustainable Forest Management; Humar, M., Krajger, H., Eds.; Studia forestalia Slovenica: Ljubljana, Slovenia, 2009; pp. 89–97. [Google Scholar]
- Oven, P.; Zupančič, M.; Merela, M.; Torelli, N. Zgradba reakcijskih con pri bukvi (Fagus sylvatica L.) Anatomy of reaction zones in beech (Fagus sylvatica L.). Zb Gozdarstva Lesar. 2004, 73, 51–62. [Google Scholar]
- Oven, P. Odziv drevesnih tkiv na poškodbe in infekcijo. Zb Gozdarstva Lesar. 1998, 55, 113–133. [Google Scholar]
- Torelli, N.; Oven, P.; Zupančič, M. Nastanek in značilnosti barierne cone ter lesa nastalega po ranitvi. Zb Gozdarstva Lesar. 1990, 36, 3–16. [Google Scholar]


Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Plavčak, D.; Mikac, U.; Merela, M. Influence of Mechanical Wounding and Compartmentalization Mechanism on the Suppression of Invasive Plant Species Using the Example of Cherry Laurel (Prunus laurocerasus). Forests 2021, 12, 1646. https://doi.org/10.3390/f12121646
Plavčak D, Mikac U, Merela M. Influence of Mechanical Wounding and Compartmentalization Mechanism on the Suppression of Invasive Plant Species Using the Example of Cherry Laurel (Prunus laurocerasus). Forests. 2021; 12(12):1646. https://doi.org/10.3390/f12121646
Chicago/Turabian StylePlavčak, Denis, Urša Mikac, and Maks Merela. 2021. "Influence of Mechanical Wounding and Compartmentalization Mechanism on the Suppression of Invasive Plant Species Using the Example of Cherry Laurel (Prunus laurocerasus)" Forests 12, no. 12: 1646. https://doi.org/10.3390/f12121646
APA StylePlavčak, D., Mikac, U., & Merela, M. (2021). Influence of Mechanical Wounding and Compartmentalization Mechanism on the Suppression of Invasive Plant Species Using the Example of Cherry Laurel (Prunus laurocerasus). Forests, 12(12), 1646. https://doi.org/10.3390/f12121646







