Exploring Correlation between Stand Structural Indices and Parameters across Three Forest Types of the Southeastern Italian Alps
Abstract
:1. Introduction
2. Materials and Methods
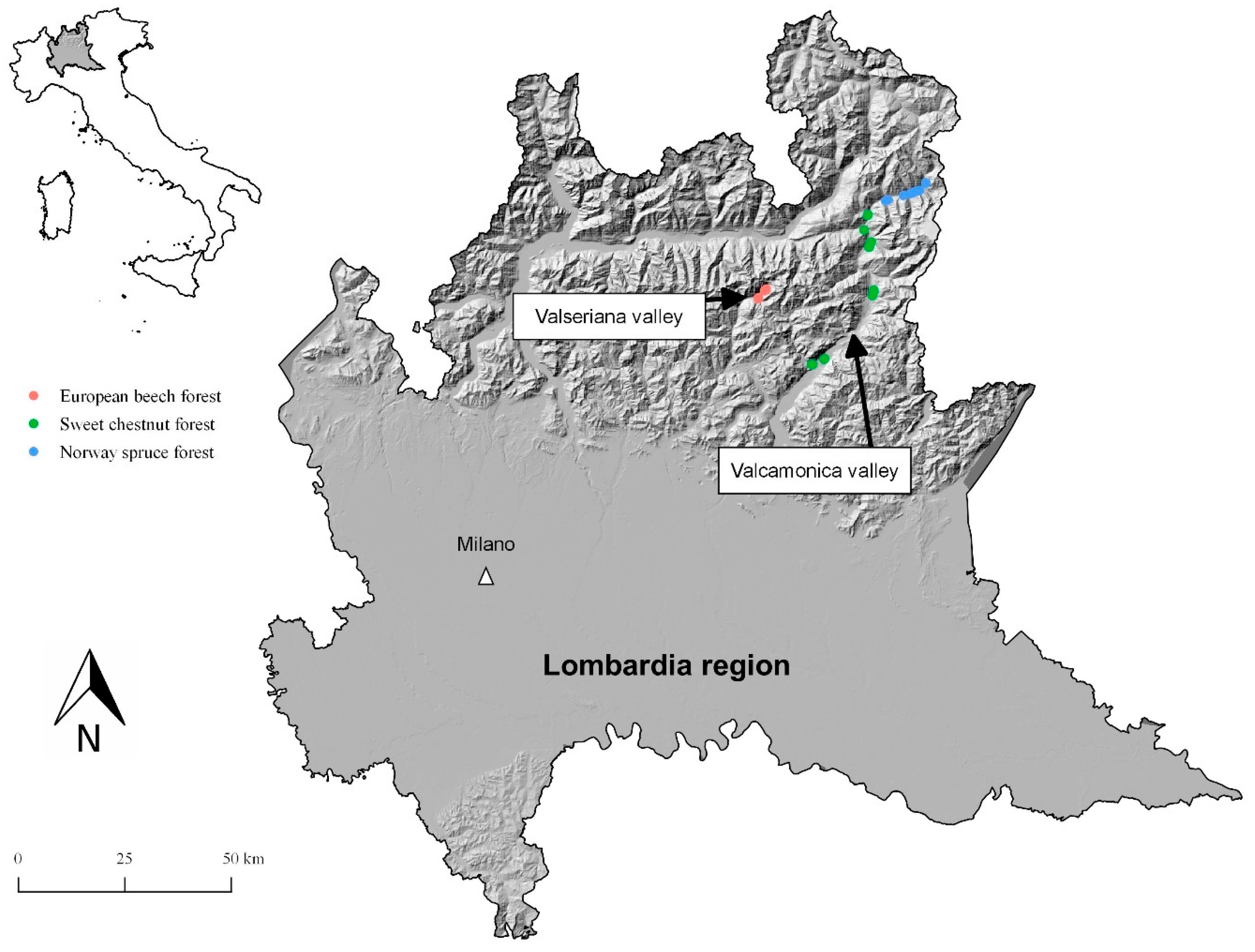
2.1. Study Area
2.2. Sampling Sites and Data Collection
2.3. Stand Structural Indices
2.3.1. Gini Index
2.3.2. Shannon Index
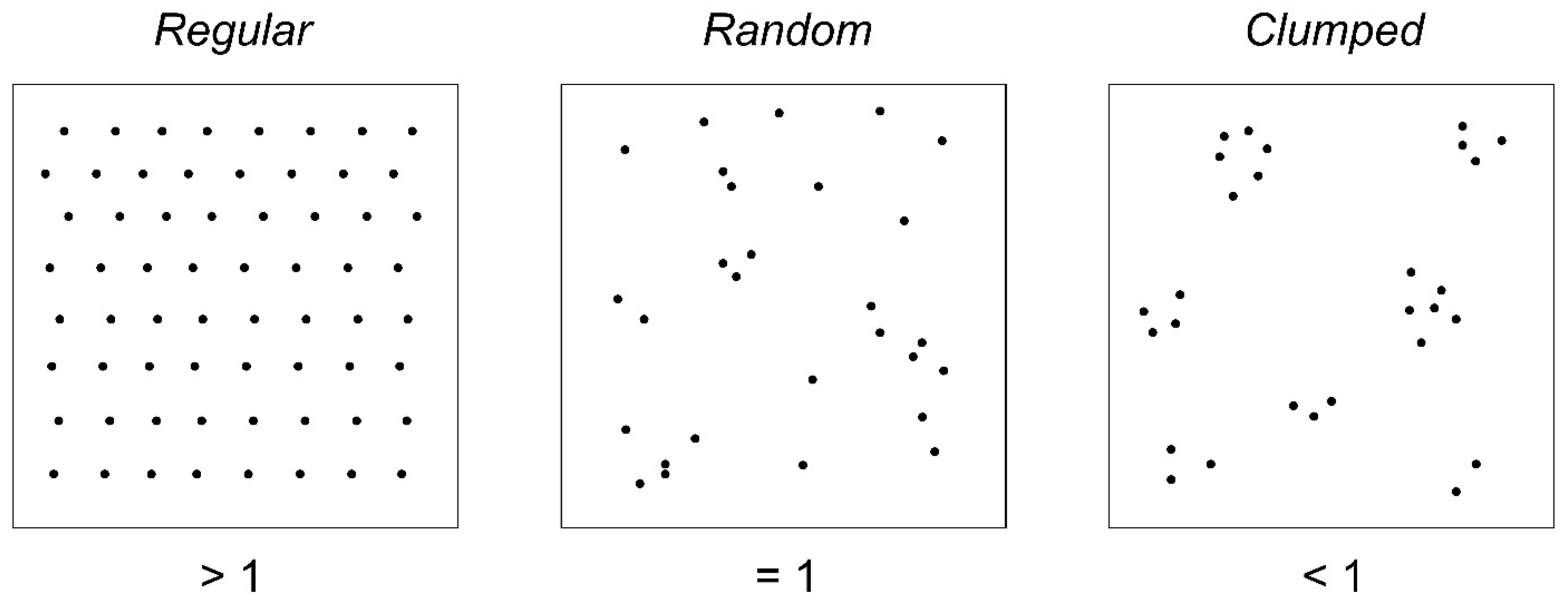
2.3.3. R Aggregation Index
2.3.4. Diameter Dominance Index
2.3.5. Diameter Differentiation Index
2.3.6. Mingling Index
2.3.7. Tree Height Dominance and Differentiation Indices
2.3.8. Indices Means and Edge Correction
2.4. Stand Structural Parameters
2.5. Software Processing and Statistics
3. Results
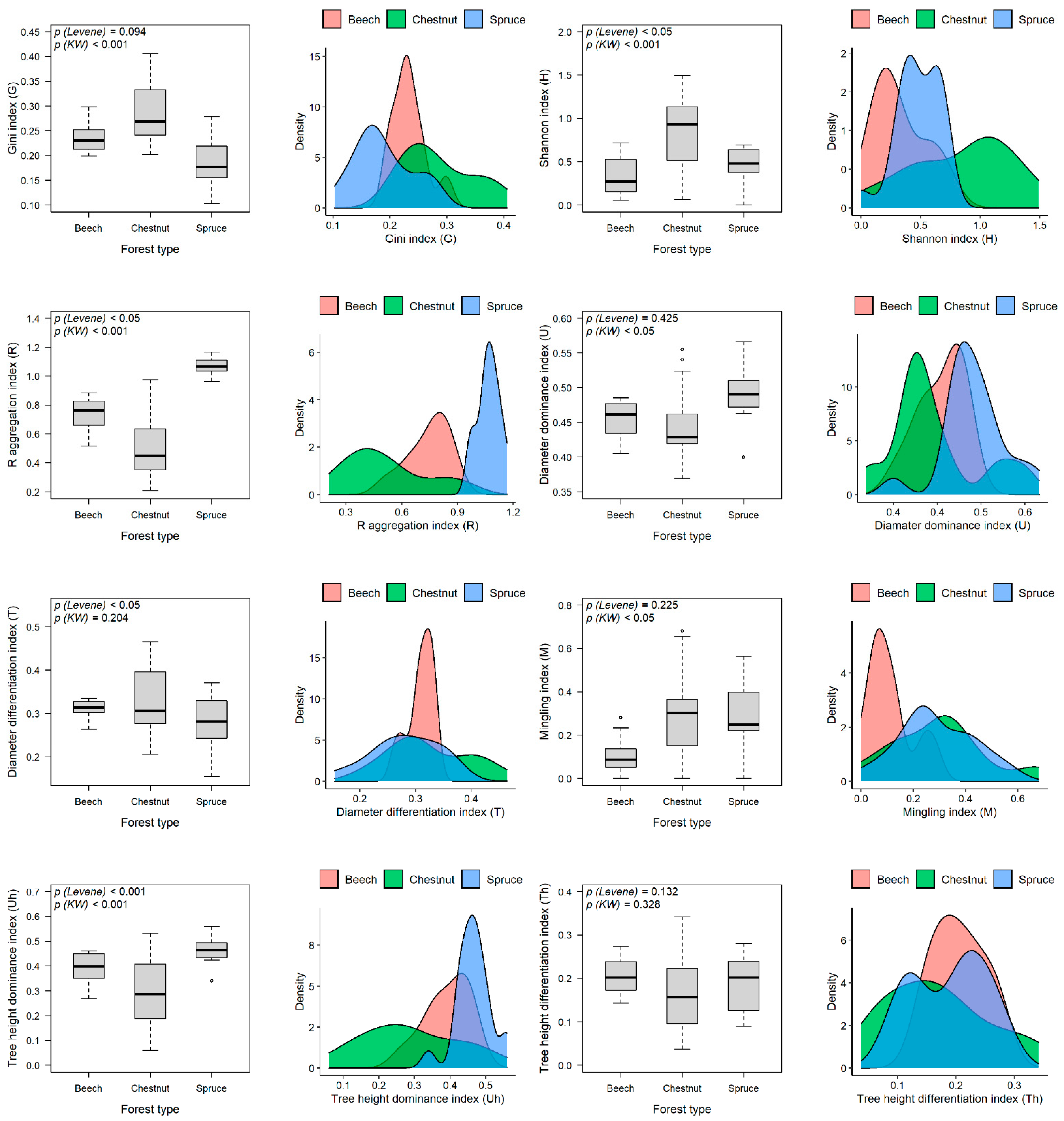
3.1. Stand Structural Indices across Forest Types
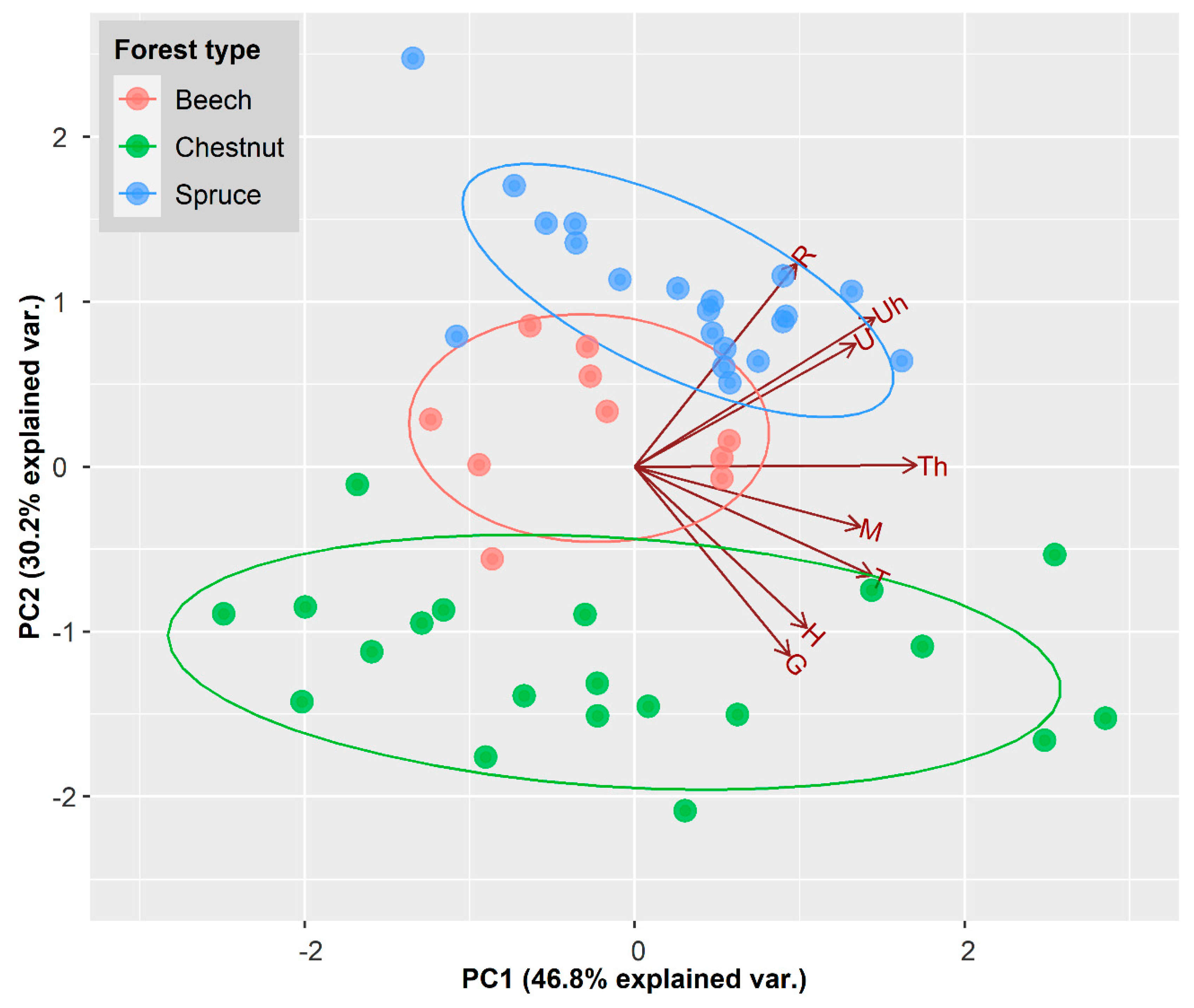
3.2. Correlation among Stand Structural Indices
3.3. Correlation between Stand Structural Indices and Stand Structural Parameters
4. Discussion
4.1. Stand Structural Indices across the Analyzed Forest Types
4.2. Relationship between Stand Structural Indices and Stand Structural Parameters
4.3. Limitation of the Study, Recommendations, and Suggestions for Future Research
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Bagnaresi, U.; Giannini, R.; Grassi, G.; Minotta, G.; Paffetti, D.; Pini Prato, E.; Proietti Placidi, A.M. Stand structure and biodiversity in mixed, uneven-aged coniferous forests in the eastern Alps. Forestry 2002, 75, 357–364. [Google Scholar] [CrossRef] [Green Version]
- Touihri, M.; Villard, M.A.; Charfi, F. Cavity-nesting birds show threshold responses to stand structure in native oak forests of northwestern Tunisia. For. Ecol. Manag. 2014, 325, 1–7. [Google Scholar] [CrossRef]
- Joelsson, K.; Hjältén, J.; Work, T. Uneven-aged silviculture can enhance within stand heterogeneity and beetle diversity. J. Environ. Manag. 2018, 205, 1–8. [Google Scholar] [CrossRef]
- Gao, T.; Hedblom, M.; Emilsson, T.; Nielsen, A.B. The role of forest stand structure as biodiversity indicator. For. Ecol. Manag. 2014, 330, 82–93. [Google Scholar] [CrossRef]
- Dănescu, A.; Albrecht, A.T.; Bauhus, J. Structural diversity promotes productivity of mixed, uneven-aged forests in southwestern Germany. Oecologia 2016, 182, 319–333. [Google Scholar] [CrossRef]
- Zhang, Y.; Chen, H.Y.H. Individual size inequality links forest diversity and above-ground biomass. J. Ecol. 2015, 103, 1245–1252. [Google Scholar] [CrossRef]
- Ali, A. Forest stand structure and functioning: Current knowledge and future challenges. Ecol. Indic. 2019, 98, 665–677. [Google Scholar] [CrossRef]
- Ali, A.; Lin, S.L.; He, J.K.; Kong, F.M.; Yu, J.H.; Jiang, H.S. Climate and soils determine aboveground biomass indirectly via species diversity and stand structural complexity in tropical forests. For. Ecol. Manag. 2019, 432, 823–831. [Google Scholar] [CrossRef]
- Vieira, J.; Matos, P.; Mexia, T.; Silva, P.; Lopes, N.; Freitas, C.; Correia, O.; Santos-Reis, M.; Branquinho, C.; Pinho, P. Green spaces are not all the same for the provision of air purification and climate regulation services: The case of urban parks. Environ. Res. 2018, 160, 306–313. [Google Scholar] [CrossRef]
- Cislaghi, A.; Alterio, E.; Fogliata, P.; Rizzi, A.; Lingua, E.; Vacchiano, G.; Bischetti, G.B.; Sitzia, T. Effects of tree spacing and thinning on root reinforcement in mountain forests of the European Southern Alps. For. Ecol. Manag. 2021, 482, 118873. [Google Scholar] [CrossRef]
- Kint, V.; De Wulf, R.; Noël, L. Evaluation of sampling methods for the estimation of structural indices in forest stands. Ecol. Modell. 2004, 180, 461–476. [Google Scholar] [CrossRef]
- Pommerening, A. Approaches to quantifying forest structures. Forestry 2002, 75, 305–324. [Google Scholar] [CrossRef]
- Pommerening, A. Evaluating structural indices by reversing forest structural analysis. For. Ecol. Manag. 2006, 224, 266–277. [Google Scholar] [CrossRef]
- Zhang, Q.; Zhang, Y.; Peng, S.; Yirdaw, E.; Wu, N. Spatial structure of alpine trees in mountain baima xueshan on the southeast tibetan plateau. Silva Fenn. 2009, 43, 197–208. [Google Scholar] [CrossRef] [Green Version]
- Sterba, H. Diversity indices based on angle count sampling and their interrelationships when used in forest inventories. Forestry 2008, 81, 587–597. [Google Scholar] [CrossRef] [Green Version]
- Pommerening, A.; Grabarnik, P. Individual-Based Methods in Forest Ecology and Management; Springer Nature Switzerland: Cham, Switzerland, 2019. [Google Scholar]
- Pach, M.; Podlaski, R. Tree diameter structural diversity in Central European forests with Abies alba and Fagus sylvatica: Managed versus unmanaged forest stands. Ecol. Res. 2015, 30, 367–384. [Google Scholar] [CrossRef] [Green Version]
- Barbeito, I.; Cañellas, I.; Montes, F. Evaluating the behaviour of vertical structure indices in Scots pine forests. Ann. For. Sci. 2009, 66, 710. [Google Scholar] [CrossRef] [Green Version]
- Bravo, F.; Guerra, B. Forest structure and diameter growth in maritime pine in a Mediterranean area. In Continuous Cover Forestry Assessment, Analysis, Scenarios; Gadow, K., Nagel, J., Saborowski, J., Eds.; Kluwer Academic Publishers: Dordrecht, The Netherlands, 2002; pp. 123–134. [Google Scholar]
- Pastorella, F.; Paletto, A. Stand structure indices as tools to support forest management: An application in Trentino forests (Italy). J. For. Sci. 2013, 59, 159–168. [Google Scholar] [CrossRef] [Green Version]
- Keren, S.; Svoboda, M.; Janda, P.; Nagel, T.A. Relationships between Structural Indices and Conventional Stand Attributes in an Old-Growth Forest in Southeast Europe. Forests 2020, 11, 4. [Google Scholar] [CrossRef] [Green Version]
- Schall, P.; Schulze, E.D.; Fischer, M.; Ayasse, M.; Ammer, C. Relations between forest management, stand structure and productivity across different types of Central European forests. Basic Appl. Ecol. 2018, 32, 39–52. [Google Scholar] [CrossRef]
- Peel, M.C.; Finlayson, B.L.; McMahon, T.A. Updated world map of the Koppen-Geiger climate classificatio. Hydrol. Earth Syst. Sci. 2007, 11, 1633–1644. [Google Scholar] [CrossRef] [Green Version]
- Gini, C. Variabilità e Mutuabilità. Contributo allo Studio delle Distribuzioni e delle Relazioni Statistiche; Cuppini: Bologna, Italy, 1912. [Google Scholar]
- Lorenz, M. Methods of Measuring the Concentration of Wealth. Publ. Am. Stat. Assoc. 1905, 9, 209–219. [Google Scholar] [CrossRef]
- Gastwirth, J.L. The Estimation of the Lorenz Curve and Gini Index. Rev. Econ. Stat. 1972, 54, 306–316. [Google Scholar] [CrossRef]
- Cordonnier, T.; Kunstler, G. The Gini index brings asymmetric competition to light. Perspect. Plant Ecol. Evol. Syst. 2015, 17, 107–115. [Google Scholar] [CrossRef]
- Katholnig, L. Growth Dominance and Gini-Index in Even-Aged and in Uneven-Aged Forests; University of Natural Resources and Applied Life Sciences: Vienna, Austria, 2012. [Google Scholar]
- Mendes, R.S.; Evangelista, L.R.; Thomaz, S.M.; Agostinho, A.A.; Gomes, L.C. A unified index to measure ecological diversity and species rarity. Ecography 2008, 31, 450–456. [Google Scholar] [CrossRef]
- Spellerberg, I.F.; Fedor, P.J. A tribute to Claude-Shannon (1916–2001) and a plea for more rigorous use of species richness, species diversity and the “Shannon-Wiener” Index. Glob. Ecol. Biogeogr. 2003, 12, 177–179. [Google Scholar] [CrossRef] [Green Version]
- Spatharis, S.; Roelke, D.L.; Dimitrakopoulos, P.G.; Kokkoris, G.D. Analyzing the (mis)behavior of Shannon index in eutrophication studies using field and simulated phytoplankton assemblages. Ecol. Indic. 2011, 11, 697–703. [Google Scholar] [CrossRef]
- Nagendra, H. Opposite trends in response for the Shannon and Simpson indices of landscape diversity. Appl. Geogr. 2002, 22, 175–186. [Google Scholar] [CrossRef]
- Clark, P.J.; Evans, F.C. Distance to Nearest Neighbor as a Measure of Spatial Relationships in Populations. Ecology 1954, 35, 445–453. [Google Scholar] [CrossRef]
- Szmyt, J.; Korzeniewicz, R. Spatial diversity of planted and untended silver birch (Betula pendula L.) stands. For. Res. Pap. 2013, 73, 323–330. [Google Scholar] [CrossRef]
- Szmyt, J. Spatial statistics in ecological analysis: From indices to functions. Silva Fenn. 2014, 48, 1–31. [Google Scholar] [CrossRef] [Green Version]
- Gadow, K.; Hui, G. Characterizing Forest Spatial Structure and Diversity. In Proceedings of the IUFRO International Workshop on Sustainable Forestry in Temperate Regions, Lund, Sweden, 7–9 April 2002. [Google Scholar]
- Aguirre, O.; Hui, G.; von Gadow, K.; Jiménez, J. An analysis of spatial forest structure using neighbourhood-based variables. For. Ecol. Manag. 2003, 183, 137–145. [Google Scholar] [CrossRef]
- Kaplan, E.L.; Meier, P. Nonparametric Estimation from Incomplete Observations. J. Am. Stat. Assoc. 1958, 53, 457–481. [Google Scholar] [CrossRef]
- Tarmu, T.; Laarmann, D.; Kiviste, A. Mean height or dominant height—What to prefer for modelling the site index of Estonian forests? For. Stud. 2020, 72, 121–138. [Google Scholar] [CrossRef]
- Tabacchi, G.; Di Cosmo, L.; Gasparini, P.; Morelli, S. Stima del Volume e della Fitomassa delle Principali Specie Forestali Italiane. Equazioni di Previsione, Tavole del Volume e Tavole della Fitomassa Arborea Epigea; Consiglio per la Ricerca e la sperimentazione in Agricoltura, Unità di Ricerca per il Monitoraggio e la Pianificazione Forestale: Trento, Italy, 2011. [Google Scholar]
- R Core Team. A Language and Environment for Statistical Computing; Foundation for Statistical Computing: Vienna, Austria, 2018. [Google Scholar]
- Handcock, M.S.; Morris, M. Relative Distribution Methods in the Social Sciences; Springer: New York, NY, USA, 1999. [Google Scholar]
- Handcock, M.S. Relative Distribution Methods; R Package Version 1.6-6. 2016. Available online: https://journals.sagepub.com/doi/10.1111/0081-1750.00042 (accessed on 11 October 2021).
- Oksanen, J.; Blanchet, G.F.; Friendly, M.; Kindt, R.; Legendre, P.; McGlinn, D.; Minchin, P.R.; O’Hara, R.B.; Simpson, G.L.; Solymos, P.; et al. Vegan: Community Ecology Package; R Package Version 2.5-7. 2020. Available online: https://cran.r-project.org/web/packages/vegan/index.html (accessed on 11 October 2021).
- Baddeley, A.; Turner, R. Spatstat: An R Package for Analyzing Spatial Point Patterns. J. Stat. Soft. 2005, 12, 1–42. [Google Scholar] [CrossRef] [Green Version]
- Baddeley, A.; Rubak, E.; Turner, R. Spatial Point Patterns: Methodology and Applications with R; Chapman and Hall/CRC Press: London, UK, 2015. [Google Scholar]
- Sitzia, T.; Trentanovi, G.; Dainese, M.; Gobbo, G.; Lingua, E.; Sommacal, M. Stand structure and plant species diversity in managed and abandoned silver fir mature woodlands. For. Ecol. Manag. 2012, 270, 232–238. [Google Scholar] [CrossRef]
- Sterba, H.; Zingg, A. Abstandsabhängige und abstandsunabhängige Bestandesstrukurbeschreibung. Allg. Forst Jagdztg. 2006, 177, 169–176. [Google Scholar]
- Sitzia, T.; Barcaccia, G.; Lucchin, M. Genetic diversity and stand structure of neighboring white willow (Salix alba L.) populations along fragmented riparian corridors: A case study. Silv. Gen. 2018, 67, 79–88. [Google Scholar] [CrossRef] [Green Version]
- Nocentini, S. Structure and management of beech (Fagus sylvatica L.) forests in Italy. IForest 2009, 2, 105–113. [Google Scholar] [CrossRef] [Green Version]
- Szmyt, J. Spatial structure of managed beech-dominated forest: Applicability of nearest neighbors indices. Dendrobiology 2012, 68, 69–76. [Google Scholar]
- Crecente-Campo, F.; Pommerening, A.; Rodríguez-Soalleiro, R. Impacts of thinning on structure, growth and risk of crown fire in a Pinus sylvestris L. plantation in northern Spain. For. Ecol. Manag. 2009, 257, 1945–1954. [Google Scholar] [CrossRef]
- Mason, W.L.; Connolly, T.; Pommerening, A.; Edwards, C. Spatial structure of semi-natural and plantation stands of Scots pine (Pinus sylvestris L.) in northern Scotland. Forestry 2007, 80, 567–586. [Google Scholar] [CrossRef] [Green Version]
- Deans, A.M.; Malcolm, J.R.; Smith, S.M.; Carleton, T.J. A comparison of forest structure among old-growth, variable retention harvested, and clearcut peatland black spruce (Picea mariana) forests in boreal northeastern Ontario. For. Chron. 2003, 79, 579–589. [Google Scholar] [CrossRef] [Green Version]
- Pirotti, F.; Grigolato, S.; Lingua, E.; Sitzia, T.; Tarolli, P. Laser scanner applications in forest and environmental sciences. Ital. J. Remote Sens. 2012, 44, 109–123. [Google Scholar] [CrossRef]
- Marchi, N.; Pirotti, F.; Lingua, E. Airborne and Terrestrial Laser Scanning Data for the Assessment of Standing and Lying Deadwood: Current Situation and New Perspectives. Remote Sens. 2018, 10, 1356. [Google Scholar] [CrossRef] [Green Version]
- Peck, J.E.; Zenner, E.K.; Brang, P.; Zingg, A. Tree size distribution and abundance explain structural complexity differentially within stands of even-aged and uneven-aged structure types. Eur. J. For. Res. 2014, 133, 335–346. [Google Scholar] [CrossRef]
- Lilleleht, A.; Sims, A.; Pommerening, A. Spatial Forest structure reconstruction as a strategy for mitigating edge-bias in circular monitoring plots. For. Ecol. Manag. 2014, 316, 47–53. [Google Scholar] [CrossRef]
- Pommerening, A.; Stoyan, D. Reconstructing spatial tree point patterns from nearest neighbour summary statistics measured in small subwindows. Can. J. For. Res. 2008, 38, 1110–1122. [Google Scholar] [CrossRef] [Green Version]
- Pommerening, A.; Stoyan, D. Edge-correction needs in estimating indices of spatial forest structure. Can. J. For. Res. 2006, 36, 1723–1739. [Google Scholar] [CrossRef] [Green Version]
- Sterba, J.; Sterba, H. Semilogarithmische Stammzahlverteilungen und Gini-Index—Strukturdiversität in “Gleichgewichtsverteilungen”. Austrian J. For. Sci. 2018, 135, 19–31. [Google Scholar]

| 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | 10 | 11 | 12 | 13 | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1. Gini index (G) | 1.000 | ||||||||||||
| 2. Shannon index (H) | 0.709 | 1.000 | |||||||||||
| 3. R aggregation index (R) | 0.382 | 0.430 | 1.000 | ||||||||||
| 4. Diameter dominance index (U) | 0.394 | 0.321 | 0.806 | 1.000 | |||||||||
| 5. Diameter differentiation index (T) | 0.515 | 0.467 | 0.467 | 0.600 | 1.000 | ||||||||
| 6. Mingling index (M) | 0.636 | 0.891 | 0.309 | 0.358 | 0.721 | 1.000 | |||||||
| 7. Tree height dominance index (Uh) | 0.164 | 0.152 | 0.758 | 0.624 | 0.139 | −0.030 | 1.000 | ||||||
| 8. Tree height differentiation index (Th) | 0.515 | 0.418 | 0.600 | 0.527 | 0.624 | 0.418 | 0.673 | 1.000 | |||||
| 9. Number of trees (N) | 0.018 | 0.442 | −0.188 | −0.273 | −0.079 | 0.491 | −0.442 | −0.394 | 1.000 | ||||
| 10. Basal area per hectare (B) | 0.122 | 0.632 | 0.182 | 0.024 | −0.049 | 0.517 | −0.164 | −0.328 | 0.833 | 1.000 | |||
| 11. Mean diameter (d) | 0.127 | 0.067 | 0.406 | 0.212 | −0.176 | −0.224 | 0.152 | −0.236 | −0.224 | 0.249 | 1.000 | ||
| 12. Tree dominant height (Dh) | 0.377 | 0.840 | 0.340 | 0.204 | 0.321 | 0.735 | 0.241 | 0.253 | 0.488 | 0.690 | 0.056 | 1.000 | |
| 13. Stand volume (V) | 0.103 | 0.636 | 0.152 | 0.006 | −0.030 | 0.539 | −0.188 | −0.321 | 0.855 | 0.997 | 0.200 | 0.704 | 1.000 |
| 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | 10 | 11 | 12 | 13 | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1. Gini index (G) | 1.000 | ||||||||||||
| 2. Shannon index (H) | 0.696 | 1.000 | |||||||||||
| 3. R aggregation index (R) | 0.636 | 0.538 | 1.000 | ||||||||||
| 4. Diameter dominance index (U) | 0.495 | 0.374 | 0.695 | 1.000 | |||||||||
| 5. Diameter differentiation index (T) | 0.744 | 0.516 | 0.746 | 0.765 | 1.000 | ||||||||
| 6. Mingling index (M) | 0.725 | 0.911 | 0.672 | 0.642 | 0.719 | 1.000 | |||||||
| 7. Tree height dominance index (Uh) | 0.789 | 0.782 | 0.761 | 0.606 | 0.848 | 0.889 | 1.000 | ||||||
| 8. Tree height differentiation index (Th) | 0.830 | 0.752 | 0.798 | 0.586 | 0.845 | 0.839 | 0.970 | 1.000 | |||||
| 9. Number of trees (N) | −0.696 | −0.550 | −0.649 | −0.457 | −0.797 | −0.642 | −0.851 | −0.810 | 1.000 | ||||
| 10. Basal area per hectare (B) | −0.002 | −0.312 | 0.349 | 0.176 | 0.204 | −0.099 | −0.020 | 0.037 | 0.030 | 1.000 | |||
| 11. Mean diameter (d) | 0.648 | 0.337 | 0.767 | 0.549 | 0.777 | 0.526 | 0.738 | 0.746 | −0.847 | 0.441 | 1.000 | ||
| 12. Tree dominant height (Dh) | 0.248 | 0.116 | 0.440 | 0.275 | 0.286 | 0.253 | 0.329 | 0.301 | −0.472 | 0.482 | 0.709 | 1.000 | |
| 13. Stand volume (V) | 0.101 | −0.206 | 0.394 | 0.192 | 0.230 | −0.002 | 0.087 | 0.120 | −0.135 | 0.929 | 0.582 | 0.744 | 1.000 |
| 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | 10 | 11 | 12 | 13 | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1. Gini index (G) | 1.000 | ||||||||||||
| 2. Shannon index (H) | −0.080 | 1.000 | |||||||||||
| 3. R aggregation index (R) | 0.250 | −0.072 | 1.000 | ||||||||||
| 4. Diameter dominance index (U) | 0.220 | −0.041 | −0.153 | 1.000 | |||||||||
| 5. Diameter differentiation index (T) | 0.832 | 0.150 | 0.129 | 0.208 | 1.000 | ||||||||
| 6. Mingling index (M) | −0.259 | 0.892 | −0.066 | −0.092 | 0.006 | 1.000 | |||||||
| 7. Tree height dominance index (Uh) | 0.287 | 0.208 | −0.129 | 0.205 | 0.417 | 0.299 | 1.000 | ||||||
| 8. Tree height differentiation index (Th) | 0.741 | 0.075 | −0.045 | 0.158 | 0.899 | 0.009 | 0.495 | 1.000 | |||||
| 9. Number of trees (N) | 0.115 | 0.183 | 0.084 | −0.159 | 0.074 | −0.014 | −0.139 | 0.026 | 1.000 | ||||
| 10. Basal area per hectare (B) | 0.100 | 0.322 | 0.196 | −0.181 | 0.140 | 0.248 | −0.111 | −0.056 | 0.396 | 1.000 | |||
| 11. Mean diameter (d) | −0.158 | −0.153 | −0.044 | 0.167 | −0.069 | 0.054 | 0.000 | −0.087 | −0.890 | −0.096 | 1.000 | ||
| 12. Tree dominant height (Dh) | 0.286 | −0.015 | 0.256 | 0.364 | 0.290 | 0.011 | 0.288 | 0.188 | −0.621 | −0.017 | 0.697 | 1.000 | |
| 13. Stand volume (V) | 0.074 | 0.051 | 0.286 | 0.000 | 0.186 | 0.044 | 0.170 | 0.020 | 0.177 | 0.689 | 0.060 | 0.318 | 1.000 |
| 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | 10 | 11 | 12 | 13 | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1. Gini index (G) | 1.000 | ||||||||||||
| 2. Shannon index (H) | 0.477 | 1.000 | |||||||||||
| 3. R aggregation index (R) | −0.403 | −0.161 | 1.000 | ||||||||||
| 4. Diameter dominance index (U) | −0.038 | 0.024 | 0.649 | 1.000 | |||||||||
| 5. Diameter differentiation index (T) | 0.688 | 0.317 | 0.022 | 0.314 | 1.000 | ||||||||
| 6. Mingling index (M) | 0.182 | 0.842 | 0.214 | 0.300 | 0.307 | 1.000 | |||||||
| 7. Tree height dominance index (Uh) | −0.150 | 0.076 | 0.756 | 0.761 | 0.253 | 0.387 | 1.000 | ||||||
| 8. Tree height differentiation index (Th) | 0.432 | 0.284 | 0.347 | 0.508 | 0.772 | 0.381 | 0.649 | 1.000 | |||||
| 9. Number of trees (N) | 0.055 | −0.205 | −0.549 | −0.539 | −0.196 | −0.470 | −0.602 | −0.357 | 1.000 | ||||
| 10. Basal area per hectare (B) | −0.391 | −0.121 | 0.579 | 0.283 | −0.059 | 0.161 | 0.302 | −0.031 | −0.200 | 1.000 | |||
| 11. Mean diameter (d) | −0.214 | 0.076 | 0.695 | 0.591 | 0.120 | 0.417 | 0.604 | 0.250 | −0.877 | 0.591 | 1.000 | ||
| 12. Tree dominant height (Dh) | −0.436 | −0.171 | 0.825 | 0.598 | −0.060 | 0.173 | 0.688 | 0.222 | −0.542 | 0.665 | 0.757 | 1.000 | |
| 13. Stand volume (V) | −0.218 | −0.094 | 0.460 | 0.297 | 0.088 | 0.104 | 0.291 | 0.035 | −0.173 | 0.863 | 0.533 | 0.634 | 1.000 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Alterio, E.; Cislaghi, A.; Bischetti, G.B.; Sitzia, T. Exploring Correlation between Stand Structural Indices and Parameters across Three Forest Types of the Southeastern Italian Alps. Forests 2021, 12, 1645. https://doi.org/10.3390/f12121645
Alterio E, Cislaghi A, Bischetti GB, Sitzia T. Exploring Correlation between Stand Structural Indices and Parameters across Three Forest Types of the Southeastern Italian Alps. Forests. 2021; 12(12):1645. https://doi.org/10.3390/f12121645
Chicago/Turabian StyleAlterio, Edoardo, Alessio Cislaghi, Gian Battista Bischetti, and Tommaso Sitzia. 2021. "Exploring Correlation between Stand Structural Indices and Parameters across Three Forest Types of the Southeastern Italian Alps" Forests 12, no. 12: 1645. https://doi.org/10.3390/f12121645





