Abstract
This work presents two protocols for the green in situ synthesis of zinc oxide nanoparticles (ZnO-NP) on cotton with the aim to develop sustainable cotton fabric with an ultraviolet protection factor (UPF). The protocols differed in the order of immersing cotton fabric in reactive solutions of three batches, i.e., precursor (0.1 M zinc acetate dihydrate), reducing agent (aqueous extract of Japanese knotweed leaves) and alkali (wood ash waste). The scanning electron microscope (SEM) results showed that ZnO-NP were successfully synthesised on cotton using both protocols; however, only the protocol where cotton was first immersed in alkali, then in the precursor and, lastly, in the reducing agent enabled very high UPF and higher amount of Zn present on the sample. Due to the different order of cotton fabric immersion in the reactive solutions, dissimilar morphology of the ZnO particles was observed, which resulted in different UV blocking abilities of the samples. The antioxidant analysis (DPPH) showed that the natural reducing agent prepared from Japanese knotweed leaves has very high antioxidant activity, which is attributed to phenolic compounds present in the plant. The reflectance spectroscopy results confirmed that the colour yield and colour of the samples did not influence the UPF value. This protocol is an example of green circular economy where waste materials of invasive alien plant species and pellet heating was used as a natural source of phytochemicals, for the direct synthesis of ZnO-NP to develop cotton fabric with UV-protective properties.
1. Introduction
Zinc oxide (ZnO) exhibits exceptional photocatalytic and broad UV range-absorbing properties, stability under UV radiation and increased temperature [1,2], which is why, in the past years, it has been increasingly studied for textile applications. Researchers have already applied chemically synthesised zinc oxide nanoparticles (ZnO-NP) on different textile substrates and obtained textiles with photocatalytic self-cleaning, antimicrobial, UV blocking and other properties [3,4,5]. While ZnO is typically synthesised chemically and applied on textiles in a separate step (ex situ) [4,6], direct (in situ) synthesis has been widely researched in the past decade. It is a one-step process in which the synthesis and the deposition of nanoparticles on the surface of the substrate is carried out simultaneously [7,8,9]. By reducing the processing steps, energy, time and costs are saved. However, if the in situ synthesis process is fully performed in the presence of a textile substrate (e.g., cellulose), such process has to be carried out carefully, i.e., the synthesis temperatures cannot exceed 150 °C and cannot be performed at a low pH, as the cellulose textile would decompose. There are several hypotheses about the mechanism of ZnO synthesis. Generally, the reduction from zinc salt to ZnO takes place under alkaline conditions, for which NaOH or KOH are most commonly used [10,11,12]. The hydroxyl ions (OH–) contribute to the formation of the intermediate molecule zinc hydroxide (Zn(OH)2), and with increasing the temperature, ZnO particles are formed via dehydration [9,11,13,14,15,16]. While there is a lot of published research regarding the green synthesis of ZnO, we noticed that the majority of them are performed in the presence of classical reducing agents or include the preparation of extracts in organic solvents [10,11] and thus cannot be stated as truly green syntheses. Furthermore, many papers were found where a procedure was named “in situ”, but from the content of the papers, it was discovered that the synthesis was actually not executed directly on the cotton fabric [11,17,18]. In the literature review, we found out that there have been only two studies published on the in situ synthesis of ZnO on textiles without using any standard chemicals [19,20].
In this paper, we present a green in situ synthesis of ZnO-NP on cotton fabric, using Japanese knotweed leaves aqueous extract as the reducing agent. Japanese knotweed is a highly invasive plant that has migrated from Eastern and South-eastern Asia [21] and is recognised as one of the worst invasive alien species globally [22]. Studies have shown that different parts of the plant are comprised of over 100 different compounds [23]. The leaves mainly contain stilbenes, flavonoids, phenolic acids, cartenoids, chlorophylls and triterpenic acid [21,24,25,26]. Due to the broad diversity of chemical compounds present in the Japanese knotweed leaves extract, it is an excellent candidate to be used as a sustainable, cost-effective and an environmentally friendly source of phytochemicals for the functional finishing of textiles. The rhizomes of Japanese knotweed have already been successfully used as source of dyes for textile dyeing [27], pigments for the screen printing of paper and textiles [28] and as a reducing agent for silver nanoparticle formation [29], and the stems of the plant have been successfully used to produce paper [30,31]. However, the potential use of the leaves of Japanese knotweed has not yet been documented. In our research, two protocols using Japanese knotweed leaves as the reducing agent for in situ ZnO synthesis were compared and investigated to determine the most suitable one. The difference between them was the order of the reactive compounds in which cotton fabric was immersed. The formation of ZnO on cotton fabric and its properties were evaluated by analysing the morphology of the samples with a scanning electron microscope (SEM), analysing the chemical composition of the samples with energy-dispersive spectroscopy (EDS), determining the UV protection properties of the fabric with a UV/Vis spectrophotometer and determining the antioxidant activity of the extract and samples with a DPPH assay and the colour measurements with a reflectance spectrophotometer.
2. Materials and Methods
2.1. Materials
Plain-weaved, chemically bleached and mercerised cotton (weight: 110 g/m2, number of warp threads: 60 threads/cm, number of weft threads: 32 threads/cm), (Tekstina d.d., Ajdovščina, Slovenia) and 0.1 M zinc acetate dihydrate (Honeywell, Charlotte, NC, USA) were used in this study.
2.2. Preparation of Natural Extracts
The reducing agent used in the research was prepared as an aqueous extract of Japanese knotweed leaves. The leaves were harvested, cleaned, airdried and ground into powder with a kitchen blender. The extracts were prepared according to procedure described by Verbič et al. 2021 [20] with a slight variation, such as a half-lower concentration of plant material for extract preparation, i.e., 50 g/L. The water extracts were prepared by immersing the ground powder of Japanese knotweed leaves into bidistilled water, heating the mixture until the boiling point and keeping it boiling for 5 min. The mixture was left to cool for two hours and then centrifuged at approx. 4000 rpm for 1 min. Lastly, the supernatant was vacuum-filtered to obtain a pure liquid extract.
The alkali source for the in situ synthesis of ZnO was prepared as an aqueous extract of wood ash waste, as described in Reference [20]. Ash of burnt commercial wood pellets from a household heating system was collected and used without any additional modification or purification. The wood ash extract was prepared by immersing the ash in bidistilled water at a 10 g/L concentration, left aside for 10 min and vacuum-filtered.
2.3. In Situ Synthesis
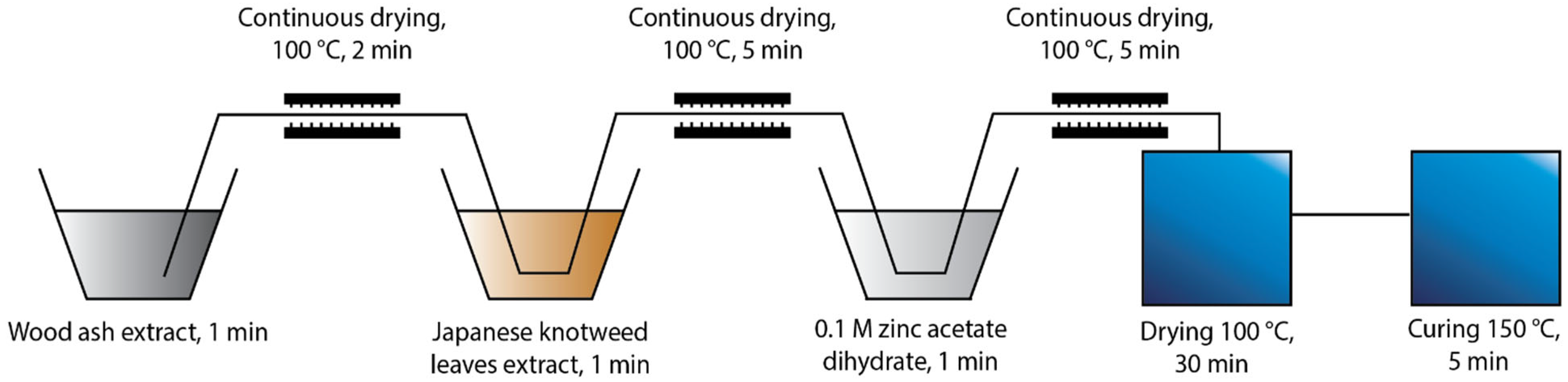
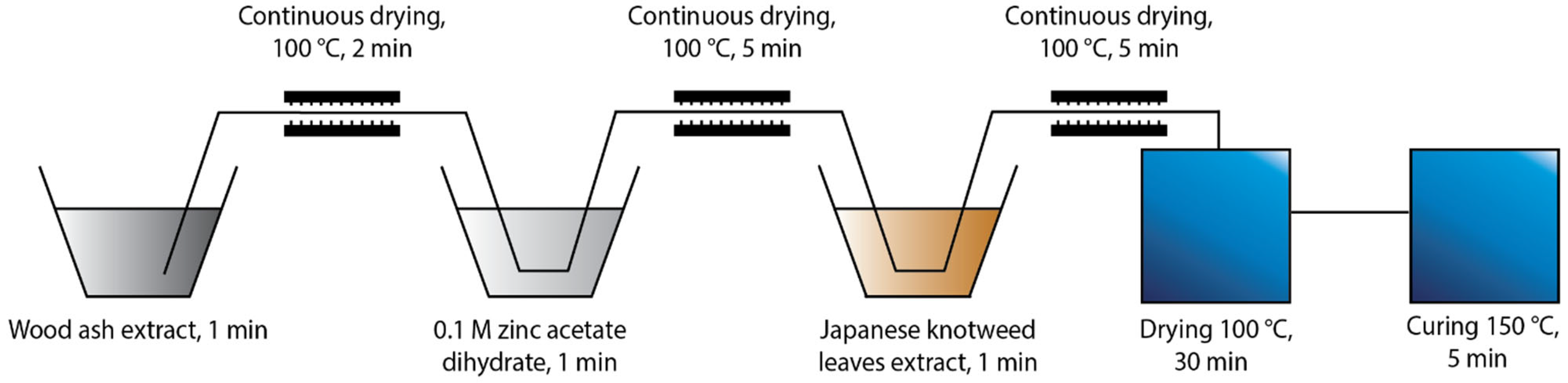
Two different protocols of in situ synthesis were used, which differed in the order of immersing the cotton fabric into the synthesis solutions. The first protocol “Ash + Extr + 0.1 ZnAc” (Figure 1) included the immersion of the cotton sample in the wood ash extract, followed by Japanese knotweed leaves extract and, finally, 0.1 M zinc acetate dihydrate. The second protocol “Ash + 0.1 ZnAc + Extr” (Figure 2) included the immersion of cotton fabric in the wood ash extract, followed by 0.1 M zinc acetate dihydrate and, lastly, Japanese knotweed leaves extract. All cotton samples were immersed in each synthesis solution for 1 min and, afterwards, dried in a continuous dryer at 100 °C. Finally, all samples were dried in a laboratory oven at 100 °C for 30 min and cured at 150 °C for 5 min.
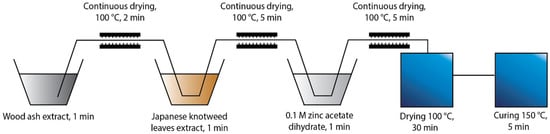
Figure 1.
Schematic presentation of the Ash + Extr + 0.1 ZnAc protocol of the in situ synthesis of ZnO-NP on cotton.
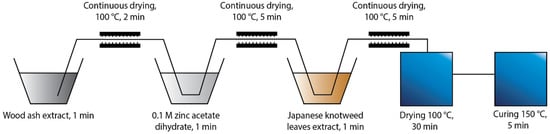
Figure 2.
Schematic presentation of the Ash + 0.1 ZnAc + Extr protocol of the in situ synthesis of ZnO-NP on cotton.
2.4. UV Protection Factor Measurements
The ultraviolet protection factor (UPF) of the untreated and functionalized cotton samples was determined according to the AATCC TM 183 standard. Measurements were performed using a Varian CARY 1E UV/Vis spectrophotometer (Varian, Melbourne, VIC, Australia). The transmissions of ultraviolet radiation through the samples were measured in a spectral range between 280 and 400 nm, and the UPF was calculated according to the following equation:
where Eλ is the relative eurythermal spectral effectiveness, Sλ is the solar spectral irradiance, Tλ is the spectral transmittance of the specimen and Δλ is the measured wavelength interval in nm. The UPF rating was calculated from six obtained measurements of UV transmission of each sample, and the UVR protection categories were determined from the calculated UPF values according to the Australian/New Zealand Standard: Sun protective clothing—Evaluation and classification [32].
2.5. Scanning Electron Microscopy (SEM)
The morphology of the untreated and functionalized cotton samples was observed using a JSM-6060 LV (JEOL, Tokyo, Japan) scanning electron microscope. Prior to the SEM analysis, the samples were coated with a layer of gold with a Jeol JFC-1300 auto fine coater for 60 s at 30 mA. The thickness of the gold coating was 19.3 nm.
2.6. Energy-Dispersive Spectroscopy (EDS)
EDS was performed with an environmental SEM Quanta 650 operated in low-vacuum mode at 70 Pa; therefore, no coating was needed. It has a tungsten filament, excellent analytical current of up to 2000 nA and the latest state-of-the-art Oxford Live EDS Ultim max 40 mm2 SDD detector.
2.7. Colour Measurements
A reflectance spectrophotometer Spectraflash 600 PLUS-CT (Datacolor, Lawrenceville, NJ, USA) was used for measuring the CIE L*a*b* values of the samples. The colour strength (K/S values) from the reflectance measurement was calculated based on Equation (2):
where R represents the reflectance, K means the absorbance and S represents the scattering of the sample.
2.8. Antioxidant Activity (DPPH Assay)
The antioxidant activity of cotton samples was analysed with the 1,1-diphenyl-2-picrylhyrazyl (DPPH) free radical scavenging method [33,34,35], as described by Verbič et al. 2021 [20]. The antioxidant activity was calculated according to the Equation (3) below:
where Ac is the absorbance of the blank DPPH solution and As is the absorbance of the DPPH solution in contact with the functionalised cotton fabric or extract [33].
3. Results and Discussion
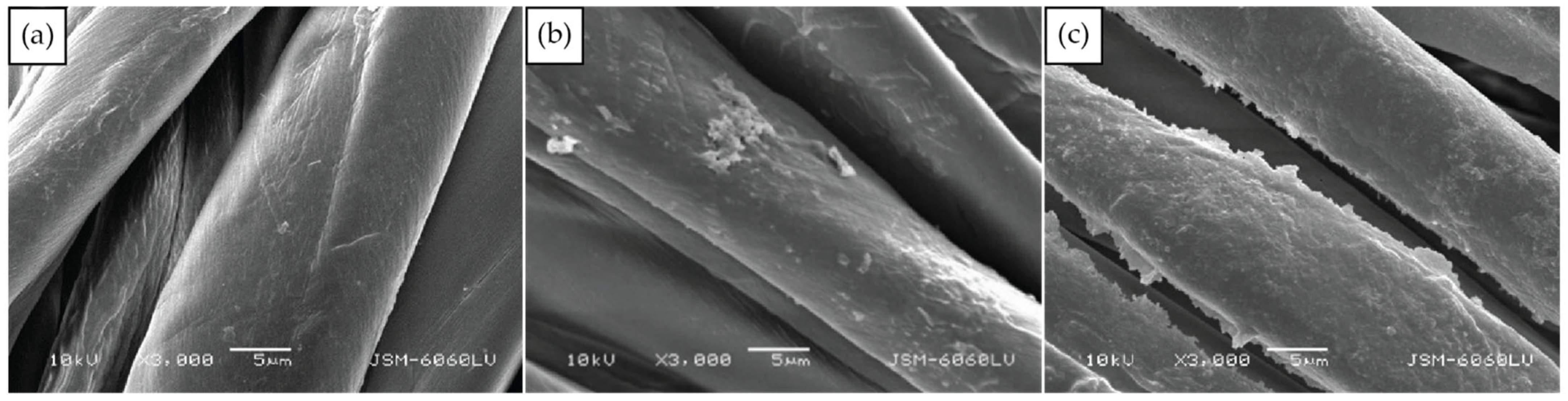
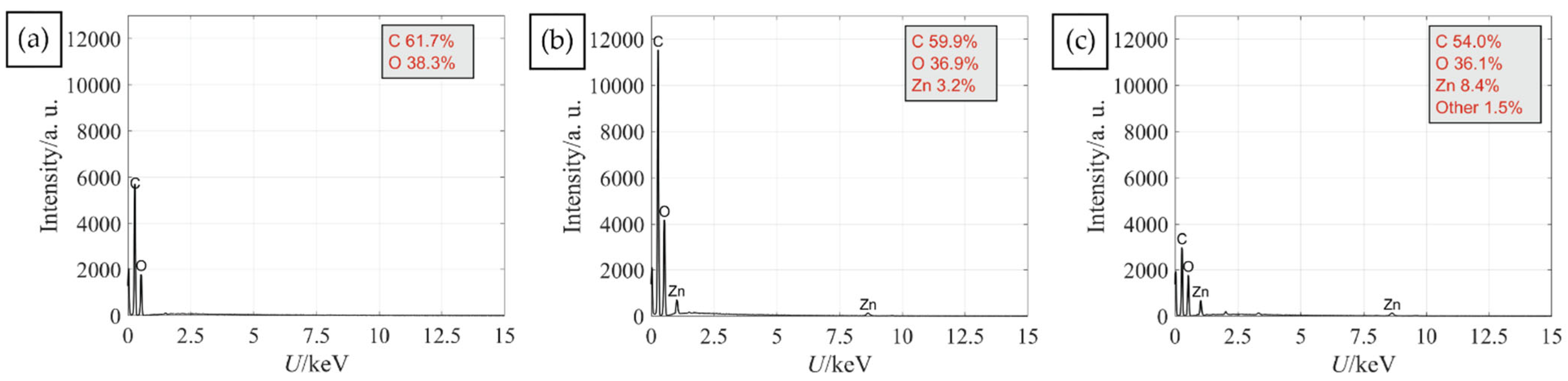
To enable protection against UV radiation the functionalisation of cotton was performed using completely green in situ synthesis of a ZnO protocol with the use of plant waste materials. Two protocols differing in the order of immersing the cotton fabric in synthesis solutions were compared. The first protocol (Ash + Extr + 0.1 ZnAc) consisted of the immersion of fabric in wood ash extract, then immersion in the plant extract and, lastly, in the zinc precursor. The second protocol (Ash + 0.1 ZnAc + Extr) consisted of the immersion of fabric in wood ash extract, then in the zinc precursor and, lastly, in the plant extract. The morphology of the fibres and formation of ZnO was firstly analysed by SEM (Figure 3) to examine if the sequence of synthesis solutions influenced the morphology of the synthesised particles. In Figure 3a, the SEM image of untreated cotton is presented. Protocol Ash + Extr + 0.1 ZnAc produced visibly smaller amounts of ZnO particles on the cotton fibres surface (Figure 3b) than protocol Ash + 0.1 ZnAc + Extr (Figure 3c). Additionally, the ZnO particles produced with the protocol Ash + Extr + 0.1 ZnAc formed an uneven coating due to the particle agglomeration and formation of separate clusters. When the Ash + 0.1 ZnAc + Extr protocol was used, the cotton fibres became completely and uniformly coated with ZnO-NP. Larger layers of ZnO were also visible between the fibres. The coating consisted of evenly distributed small particles, and no large agglomerates were noticed. Additionally, the EDS analysis was performed to evaluate the elemental composition and weight percentage of untreated cotton and cotton samples functionalised with protocols Ash + Extr + 0.1 ZnAc and Ash + 0.1 ZnAc + Extr. The results are presented in Figure 4. The presence of Zn was confirmed for samples where the in situ synthesis of ZnO was performed, with higher amounts on the sample Ash + 0.1 ZnAc + Extr (8.4%) than on Ash + Extr + 0.1 ZnAc (3.2%).

Figure 3.
SEM images of untreated cotton sample (a) and cotton samples functionalised with protocol Ash + Extr + 0.1 ZnAc (b) and Ash + 0.1 ZnAc + Extr (c).
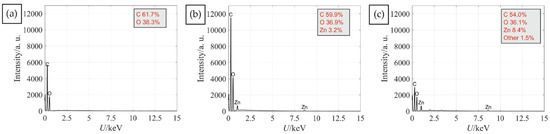
Figure 4.
EDS spectra of untreated cotton sample (a) and cotton samples functionalised with protocol Ash + Extr + 0.1 ZnAc (b) and Ash + 0.1 ZnAc + Extr (c).
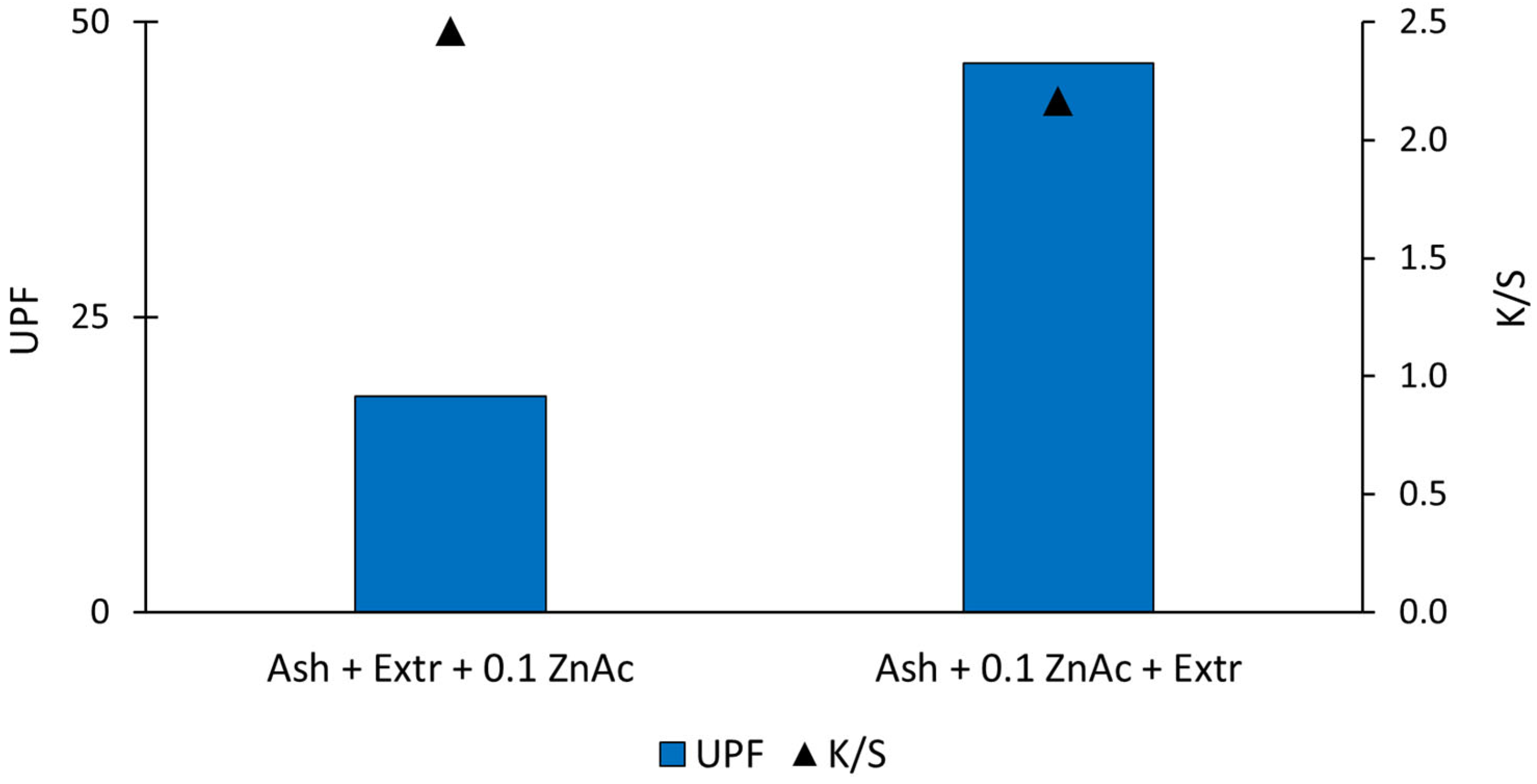
The UV blocking ability of the functionalised samples was analysed further. For that purpose, UV/Vis spectroscopy was used, and the ultraviolet protection factor (UPF) was determined according to the AS/NZ 4399:2017 standard [32]. The standard classified the UV protective effectiveness of textiles into three categories. The values were in the range of 15–50, and the higher the value, the better the UV protection. UPF values from 15 to 30 were classified as the minimum, from 30 to 50 as good and above 50 as excellent UV protection. In Table 1, the UPF values of the untreated and functionalised samples are shown. The determined UPF of untreated cotton fabric was very low (value 3.9), which is insufficient UV protection according to the AS/NZ 4399:2017 standard. The UPF increased for both functionalised cotton samples. When protocol Ash + Extr + 0.1 ZnAc was used, the cotton samples exhibited only minimal UV protection with a UPF value of 18.33. Protocol Ash + 0.1 ZnAc + Extr resulted in a significantly increased UPF value (UPF = 46.57), which is very good UV protection. The results are in accordance with the SEM images and EDS results above, where we observed that the sequence of the synthesis solutions influenced the amount and morphology of the synthesised particles. The sample prepared using the Ash + 0.1 ZnAc + Extr protocol resulted in a higher content of zinc present on the cotton sample and a consequently higher UPF value. Using the Ash + Extr + 0.1 ZnAc protocol, the particles were clustered and agglomerated, which influenced the UV blocking ability significantly. The functional properties of cotton fabric depend on the size of the ZnO particles [36]. ZnO particles of smaller sizes offer a greater protection against harmful UV radiation than larger or agglomerated particles [37]. Using the protocol where immersion in the wood ash extract was followed by the Japanese knotweed extract and, lastly, zinc precursor resulted in agglomerated ZnO particles and a consequently low UV protective ability. With the Ash + 0.1 ZnAc + Extr protocol, the particles were smaller and more evenly distributed all over the fibre’s surface; therefore, the fabric had very good UV protection.

Table 1.
Ultraviolet protection factor (UPF), transmission of UVA and UVB radiation (T(UVA) and T(UVB)), UVA and UVB blocking and the protection category of the cotton samples.
Since we used an aqueous extract of natural origin (plant), which also yielded colour and dyed the cotton fabric during the synthesis process, we analysed if there was a correlation between the UPF and colour. It is known that dyeing textiles also influences their ability to block UV radiation [38]. Wong et al. [39] researched the influence of fabric colour on UV protective properties and found that it depends on the dye structure that is used to functionalise fabric. Additionally, UV protection is affected by fabric construction [40]. Although we used the same fabric and natural extracts in both in situ synthesis protocols, we analysed the colour yield (K/S) and colour (CIE L*a*b*) to see if there was any correlation to the UV protective properties of the samples or if the samples in fact possessed a UV blocking ability due to the presence of ZnO-NP. In Figure 5, the K/S values of the functionalised samples are shown. The K/S value of the sample where ZnO-NP were synthesised using the Ash + Extr + 0.1 ZnAc protocol was 2.46, and the K/S value of the sample where ZnO-NP were synthesised using the Ash + 0.1 ZnAc + Extr protocol was 2.17. Comparing the UPF values and K/S values, it is evident that the values were not correlated, since the higher K/S value was calculated for the sample with the lower UPF. The K/S value presents the colour strength of the sample, and a high value means that the sample contains the highest amount of the colourant [27]. Our results showed that, while the colour strength of the two samples was almost the same, there was a big difference in the UV protection factor of the samples. From the results, we can conclude that the high UPF value was really due to the synthesised ZnO-NP particles and not due to the colour from the natural extract.
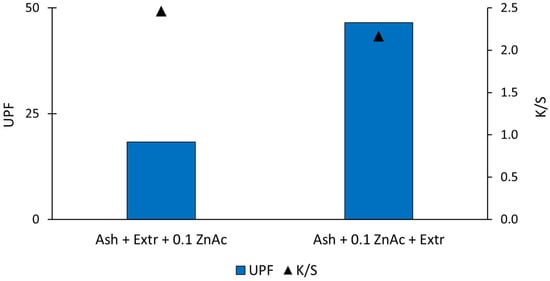
Figure 5.
UPF and K/S values of the functionalised cotton samples.
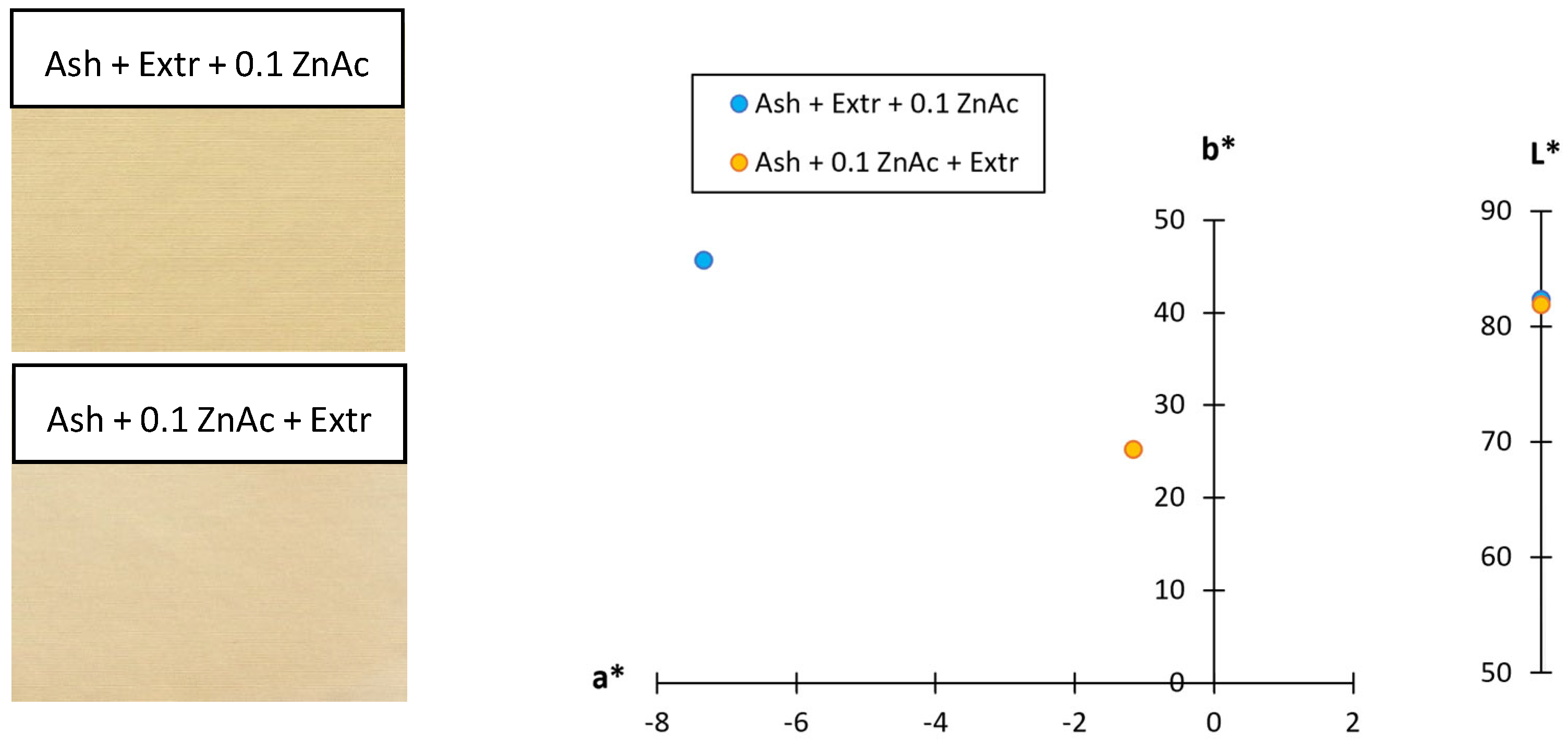
To additionally confirm that the colour of the sample did not influence the UPF value, the CIE L*a*b* values were also determined (Figure 6). Comparing the two protocols, the lightness of both samples was very similar (L* = 82.36 and 81.94), while there was only a difference observed in the values of the green–red and blue–yellow colour coordinates, meaning the hue of the samples was different. When the cotton sample was functionalised using the Ash + Extr + 0.1 ZnAc protocol, the sample was greener (a* = −7.33) and bluer (b* = 45.63). When the Ash + 0.1 ZnAc + Extr protocol was used, the values of the a* and b* coordinates were still on the green and blue side of the axis, but the values were lower (a* = −1.16, b* = 25.24). This means that, whereas the lightness of the sample was almost the same, using the Ash + Extr + 0.1 ZnAc protocol resulted in a more pronounced green and blue hue of the sample than using the Ash + 0.1 ZnAc + Extr protocol. This is in accordance with K/S results described before, as the colour yield of this sample was also higher and again confirmed that the UV blocking ability of the sample was due to the synthesised ZnO-NP and not from the colour of the sample.
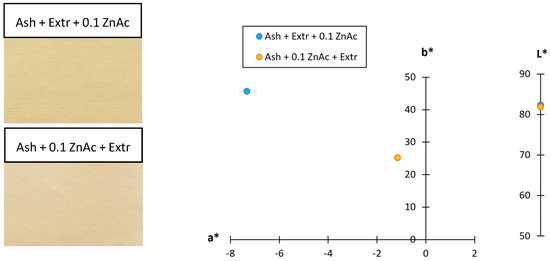
Figure 6.
Scanned functionalised cotton samples and their CIE L*a*b* colour coordinates.
There have been many studies published regarding the green synthesis of ZnO and other metal nanoparticles, which describe the importance of phenolic compounds in natural extracts and their reducing ability [41,42,43,44,45]. Phenolic compounds have high antioxidant activity, and since antioxidants are great reducers of metal ions, it has already been discussed that a high phenolic content is essential for the reduction process [46].
In a literature review, we found that a Japanese knotweed extract exhibit high values of phenolic compounds [24,25,26,47], and hence, a DPPH analysis was performed to examine the antioxidant activity of our samples (Table 2). The analysis was performed on the liquid Japanese knotweed leaves extract, and the results showed very high antioxidant activity (81.36%). While the untreated cotton sample had almost no antioxidant activity (0.31%), the activity increased for both functionalised cotton samples. When protocol Ash + Extr + 0.1 ZnAc was used, the value increased to 21.14%, while, for the Ash + 0.1 ZnAc + Extr protocol, the value increased to 25.08%. However, the values were lower than the extract alone. The decrease occurred because the phenolic compounds were consumed in the synthesis process for the formation of ZnO and adsorption on cotton fabric, which has already been observed in research synthesising ZnO-NP alone [48] and on cotton [20]. While, according to the UPF and SEM results, it would be expected that the sample prepared using the Ash + 0.1 ZnAc + Extr protocol would have a lower antioxidant ability due to the more consumed phenolic compounds in the synthesis process, more synthesised ZnO-NP and, consequently, higher UV blocking ability, there was only a slight difference between the two samples. This could be due to the fact that similar amounts of phenolics were, in fact, consumed in both protocols, but the first protocol (Ash + Extr + 0.1 ZnAc) produced more agglomerated particles, and that was why the fabric exhibited a lower UV blocking ability. The SEM, EDS and UPF results were also in accordance with the colour measurements discussed before, as the functionalised cotton samples had similar K/S values, and therefore, a similar amount of extract was adsorbed on cotton. From the results, we can conclude that the second protocol (Ash + 0.1 ZnAc + Extr) was more appropriate for the synthesis of ZnO directly on cotton fabric, since, regardless of the same amount of extract adsorbed and similar values of antioxidant activity, the second protocol (Ash + 0.1 ZnAc + Extr) still produced smaller and more evenly distributed ZnO, which results in cotton fabric with a higher UV blocking ability.

Table 2.
Average antioxidant activity (%) of the untreated cotton sample, Japanese knotweed extract and cotton samples functionalised using Ash + Extr + 0.1 ZnAc and Ash + 0.1 ZnAc + Extr protocols.
4. Conclusions
In this study, ZnO-NP were synthesised directly on cotton fabric using a green and circular economy approach using 0.1 M zinc acetate dihydrate as a zinc precursor, Japanese knotweed leaves aqueous extract as a reducing agent and wood ash waste aqueous extract as the source of alkali. Two different in situ synthesis processes were compared to determine the more appropriate one. The results showed that the synthesis protocol where the immersion of cotton fabric in wood ash extract was followed by immersion in the zinc precursor and, finally, immersion in the natural extract resulted in uniformly coated fibres with a higher amount of ZnO-NP, which enabled great protection against UV radiation (UPF = 46.57). The colour measurements showed that the synthesis protocol influenced the hue of the samples, while the lightness of the samples did not change. With both protocols, similar K/S values were obtained, which means that both samples adsorbed similar amounts of the natural extract, which was also confirmed with the DPPH assay. However, the UPF value of the sample prepared with the Ash + Extr + 0.1 ZnO protocol was much lower than in the case of the sample Ash + 0.1 ZnAc + Extr. These results showed that the high UPF value was not due to the colour of the samples or presence of phytochemicals on the samples but from the successful formation of evenly distributed ZnO-NP on the fibres, as also confirmed by EDS. This green in situ synthesis simultaneously addressed multiple problems: besides using the biowaste of Japanese knotweed, which is typically burned to decrease the plant overpopulation, and using ashes of burned wooden pellets that are usually discarded, the process was performed at room temperature and with short treatment times, being cost- and energy-efficient. Furthermore, the chemical reducing and stabilising agents that are typically used in the synthesis process were replaced with natural extracts.
Author Contributions
Conceptualisation, M.G.; Formal analysis, A.V., K.B. and G.P.; Funding acquisition, M.G.; Investigation, A.V.; Methodology, A.V.; Supervision, M.G.; Visualisation, K.B.; Writing—original draft, A.V. and M.G. and Writing—review and editing, A.V., K.B., G.P. and M.G. All authors have read and agreed to the published version of the manuscript.
Funding
This work was financially supported by the Slovenian Research Agency (Programme P2-0213 Textiles and Ecology, Project J2-1720, and a grant for the doctoral student (A.V.)).
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Kołodziejczak-Radzimska, A.; Jesionowski, T. Zinc oxide—From synthesis to application: A review. Materials 2014, 7, 2833–2881. [Google Scholar] [CrossRef] [Green Version]
- Sirelkhatim, A.; Mahmud, S.; Seeni, A.; Kaus, N.H.M.; Ann, L.C.; Bakhori, S.K.M.; Hasan, H.; Mohamad, D. Review on zinc oxide nanoparticles: Antibacterial activity and toxicity mechanism. Nano-Micro Lett. 2015, 7, 219–242. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Verbič, A.; Gorjanc, M.; Simončič, B. Zinc oxide for functional textile coatings: Recent advances. Coatings 2019, 9, 550. [Google Scholar] [CrossRef] [Green Version]
- Shubha, P.; Gowda, M.L.; Namratha, K.; Shyamsunder, S.; Manjunatha, H.B.; Byrappa, K. Ex-situ fabrication of ZnO nanoparticles coated silk fiber for surgical applications. Mater. Chem. Phys. 2019, 231, 21–26. [Google Scholar] [CrossRef]
- Malis, D.; Jeršek, B.; Tomšič, B.; Štular, D.; Golja, B.; Kapun, G.; Simončič, B. Antibacterial activity and biodegradation of cellulose fiber blends with incorporated ZnO. Materials 2019, 12, 3399. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Khatami, M.; Varma, R.S.; Zafarnia, N.; Yaghoobi, H.; Sarani, M.; Kumar, V.G. Applications of green synthesized Ag, ZnO and Ag/ZnO nanoparticles for making clinical antimicrobial wound-healing bandages. Sustain. Chem. Pharm. 2018, 10, 9–15. [Google Scholar] [CrossRef]
- Khosravian, S.; Montazer, M.; Malek, R.M.; Harifi, T. In situ synthesis of nano ZnO on starch sized cotton introducing nano photo active fabric optimized with response surface methodology. Carbohydr. Polym. 2015, 132, 126–133. [Google Scholar] [CrossRef]
- Montazer, M.; Maali Amiri, M. ZnO nano reactor on textiles and polymers: Ex situ and in situ synthesis, application, and characterization. J. Phys. Chem. B 2014, 118, 1453–1470. [Google Scholar] [CrossRef]
- Verbič, A.; Šala, M.; Gorjanc, M. The Influence of in situ Synthesis Parameters on the Formation of ZnO Nanoparticles and the UPF Value of Cotton Fabric. Tekstilec 2018, 61, 280–288. [Google Scholar] [CrossRef]
- Ghayempour, S.; Montazer, M. Ultrasound irradiation based in-situ synthesis of star-like Tragacanth gum/zinc oxide nanoparticles on cotton fabric. Ultrason. Sonochem. 2017, 34, 458–465. [Google Scholar] [CrossRef]
- El-Naggar, M.E.; Shaarawy, S.; Hebeish, A.A. Multifunctional properties of cotton fabrics coated with in situ synthesis of zinc oxide nanoparticles capped with date seed extract. Carbohydr. Polym. 2018, 181, 307–316. [Google Scholar] [CrossRef] [PubMed]
- Ravichandran, V.; Sumitha, S.; Ning, C.Y.; Xian, O.Y.; Yu, U.K.; Paliwal, N.; Shah, S.A.A.; Tripathy, M. Durian waste mediated green synthesis of zinc oxide nanoparticles and evaluation of their antibacterial, antioxidant, cytotoxicity and photocatalytic activity. Green Chem. Lett. Rev. 2020, 13, 102–116. [Google Scholar] [CrossRef]
- Xu, S.; Wang, Z.L. One-dimensional ZnO nanostructures: Solution growth and functional properties. Nano Res. 2011, 4, 1013–1098. [Google Scholar] [CrossRef] [Green Version]
- Dhandapani, P.; Siddarth, A.S.; Kamalasekaran, S.; Maruthamuthu, S.; Rajagopal, G. Bio-approach: Ureolytic bacteria mediated synthesis of ZnO nanocrystals on cotton fabric and evaluation of their antibacterial properties. Carbohydr. Polym. 2014, 103, 448–455. [Google Scholar] [CrossRef]
- Sundrarajan, M.; Ambika, S.; Bharathi, K. Plant-extract mediated synthesis of ZnO nanoparticles using Pongamia pinnata and their activity against pathogenic bacteria. Adv. Powder Technol. 2015, 26, 1294–1299. [Google Scholar] [CrossRef]
- Yuvakkumar, R.; Suresh, J.; Nathanael, A.J.; Sundrarajan, M.; Hong, S.I. Novel green synthetic strategy to prepare ZnO nanocrystals using rambutan (Nephelium lappaceum L.) peel extract and its antibacterial applications. Mater. Sci. Eng. C 2014, 41, 17–27. [Google Scholar] [CrossRef]
- Zhang, G.; Morikawa, H.; Chen, Y.; Miura, M. In-situ synthesis of ZnO nanoparticles on bamboo pulp fabric. Mater. Lett. 2013, 97, 184–186. [Google Scholar] [CrossRef]
- Shaheen, T.I.; El-Naggar, M.E.; Abdelgawad, A.M.; Hebeish, A. Durable antibacterial and UV protections of in situ synthesized zinc oxide nanoparticles onto cotton fabrics. Int. J. Biol. Macromol. 2016, 83, 426–432. [Google Scholar] [CrossRef] [PubMed]
- Aladpoosh, R.; Montazer, M. The role of cellulosic chains of cotton in biosynthesis of ZnO nanorods producing multifunctional properties: Mechanism, characterizations and features. Carbohydr. Polym. 2015, 126, 122–129. [Google Scholar] [CrossRef]
- Verbič, A.; Šala, M.; Jerman, I.; Gorjanc, M. Novel green in situ synthesis of ZnO nanoparticles on cotton using pomegranate peel extract. Materials 2021, 14, 4472. [Google Scholar] [CrossRef]
- Patocka, J.; Navratilova, Z. Biologically active compounds of knotweed (Reynoutria spp.). Mil. Med. Sci. Lett. 2017, 86, 17–31. [Google Scholar] [CrossRef] [Green Version]
- Lowe, S.; Browne, M.; Boudjelas, S.; De Poorter, M. 100 of the World’s Worst Invasive Alien Species: A Selection from the Global Invasive Species; Invasive Species Specialist Group (ISSG): Auckland, New Zealand, 2000. [Google Scholar]
- Nawrot-Hadzik, I.; Ślusarczyk, S.; Granica, S.; Hadzik, J.; Matkowski, A. Phytochemical diversity in rhizomes of three Reynoutria species and their antioxidant activity correlations elucidated by LC-ESI-MS/MS analysis. Molecules 2019, 24, 1136. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bensa, M.; Glavnik, V.; Vovk, I. Leaves of invasive plants—Japanese, Bohemian and giant knotweed—The promising new source of flavan-3-ols and proanthocyanidins. Plants 2020, 9, 118. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lachowicz, S.; Oszmiański, J.; Wojdyło, A.; Cebulak, T.; Hirnle, L.; Siewiński, M. UPLC-PDA-Q/TOF-MS identification of bioactive compounds and online UPLC-ABTS assay in Fallopia japonica Houtt and Fallopia sachalinensis (F. Schmidt) leaves and rhizomes grown in Poland. Eur. Food Res. Technol. 2018, 245, 691–706. [Google Scholar] [CrossRef] [Green Version]
- Lachowicz, S.; Oszmiański, J. Profile of bioactive compounds in the morphological parts of wild Fallopia japonica (Houtt) and Fallopia sachalinensis (F. Schmidt) and their antioxidative activity. Molecules 2019, 24, 1436. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gorjanc, M.; Savić, A.; Topalić-Trivunović, L.; Mozetič, M.; Zaplotnik, R.; Vesel, A.; Grujić, D. Dyeing of plasma treated cotton and bamboo rayon with Fallopia japonica extract. Cellulose 2016, 23, 2221–2228. [Google Scholar] [CrossRef]
- Klančnik, M. Screen printing with natural dye extract from Japanese knotweed rhizome. Fibers Polym. 2021, 22, 2498–2506. [Google Scholar] [CrossRef]
- Čuk, N.; Šala, M.; Gorjanc, M. Development of antibacterial and UV protective cotton fabrics using plant food waste and alien invasive plant extracts as reducing agents for the in-situ synthesis of silver nanoparticles. Cellulose 2021, 28, 3215–3233. [Google Scholar] [CrossRef]
- Možina, K.; Bračko, S.; Kovačević, D.; Blaznik, B.; Možina, K. Legibility of Prints on Paper Made from Japanese Knotweed. BioResources 2020, 15, 3999–4015. [Google Scholar] [CrossRef]
- Starešinič, M.; Boh Podgornik, B.; Javoršek, D.; Leskovšek, M.; Možina, K. Fibers Obtained from Invasive Alien Plant Species as a Base Material for Paper Production. Forests 2021, 12, 527. [Google Scholar] [CrossRef]
- AS/NZS 4399; Sun Protective Clothing—Evaluation and Classification; Standards Australia/Standards New Zealand: Wellington, New Zealand, 2017.
- Singh, A.; Sheikh, J. Cleaner functional dyeing of wool using Kigelia Africana natural dye and Terminalia chebula bio-mordant. Sustain. Chem. Pharm. 2020, 17, 100286. [Google Scholar] [CrossRef]
- Jadav, K.M.; Gowda, N. Antibacterial and antioxidant properties of silk fabric dyed with Cichorium intybus root extract. Int. J. Pharm. 2017, 4, 299–304. [Google Scholar]
- Turkoğlu, G.C.; Sarıışık, A.M.; Erkan, G.; Kayalar, H.; Kontart, O.; Öztuna, S. Determination of antioxidant capacity of capsule loaded textiles. Indian J. Fibre Text. Res. 2017, 42, 189–195. [Google Scholar]
- Kert, M.; Jazbec, K.; Černe, L.; Jerman, I.; Gorjanc, M. The influence of nano-ZnO application methods on UV protective properties of cotton. Acta Chim. Slov. 2014, 61, 587–594. [Google Scholar] [PubMed]
- Yadav, A.; Prasad, V.; Kathe, A.A.; Raj, S.; Yadav, D.; Sundaramoorthy, C.; Vigneshwaran, N. Functional finishing in cotton fabrics using zinc oxide nanoparticles. Bull. Mater. Sci. 2006, 29, 641–645. [Google Scholar] [CrossRef]
- Gorjanc, M.; Mozetič, M.; Vesel, A.; Zaplotnik, R. Natural dyeing and UV protection of plasma treated cotton. Eur. Phys. J. D 2018, 72, 1–6. [Google Scholar] [CrossRef]
- Wong, W.Y.; Lam, J.K.C.; Kan, C.W.; Postle, R. Influence of reactive dyes on ultraviolet protection of cotton knitted fabrics with different fabric constructions. Text. Res. J. 2016, 86, 512–532. [Google Scholar] [CrossRef]
- Urbas, R.; Kostanjšek, K.; Dimitrovski, K. Impact of structure and yarn colour on UV properties and air permeability of multilayer cotton woven fabrics. Text. Res. J. 2011, 81, 1916–1925. [Google Scholar] [CrossRef]
- Nadagouda, M.N.; Iyanna, N.; Lalley, J.; Han, C.; Dionysiou, D.D.; Varma, R.S. Synthesis of silver and gold nanoparticles using antioxidants from blackberry, blueberry, pomegranate, and turmeric extracts. ACS Sustain. Chem. Eng. 2014, 2, 1717–1723. [Google Scholar] [CrossRef]
- Hashemi, S.; Asrar, Z.; Pourseyedi, S.; Nadernejad, N. Green synthesis of ZnO nanoparticles by Olive (Olea europaea). IET Nanobiotechnol. 2016, 10, 400–404. [Google Scholar] [CrossRef]
- Senthilkumar, S.R.; Sivakumar, T. Green tea (Camellia sinensis) mediated synthesis of zinc oxide (ZnO) nanoparticles and studies on their antimicrobial activities. Int. J. Pharm. Pharm. Sci. 2014, 6, 461–465. [Google Scholar]
- Selim, Y.A.; Azb, M.A.; Ragab, I.; Abd El-Azim, M.H. Green synthesis of zinc oxide nanoparticles using aqueous extract of Deverra tortuosa and their cytotoxic activities. Sci. Rep. 2020, 10, 3445. [Google Scholar] [CrossRef] [Green Version]
- Gour, A.; Jain, N.K. Advances in green synthesis of nanoparticles. Artif. Cells Nanomed. Biotechnol. 2019, 47, 844–851. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Majhi, K.; Let, M.; Kabiraj, A.; Sarkar, S.; Halder, U.; Dutta, B.; Biswas, R.; Bandopadhyay, R. Metal recovery using nanobiotechnology. In Nanobiotechnology, 1st ed.; Ghosh, S., Webster, T., Eds.; Elsevier: Amsterdam, The Netherlands, 2021; pp. 283–301. [Google Scholar]
- Božin, B.; Gavrilović, M.; Kladar, N.; Rat, M.; Anačkov, G.; Gavarić, N. Highly invasive alien plant Reynoutria japonica Houtt. represents a novel source for pharmaceutical industry–Evidence from phenolic profile and biological activity. J. Serb. Chem. Soc. 2017, 82, 803–813. [Google Scholar] [CrossRef]
- Mahendra, C.; Chandra, M.N.; Murali, M.; Abhilash, M.R.; Singh, S.B.; Satish, S.; Sudarshana, M.S. Phyto-fabricated ZnO nanoparticles from Canthium dicoccum (L.) for Antimicrobial, Anti-tuberculosis and Antioxidant activity. Process Biochem. 2020, 89, 220–226. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).