Fine Root Biomass Mediates Soil Fauna Community in Response to Nitrogen Addition in Poplar Plantations (Populus deltoids) on the East Coast of China
Abstract
:1. Introduction
2. Materials and Methods
2.1. The Experimental Site and Design
2.2. Microclimate
2.3. Measurement of C and N in Litter and Soil Samples
2.4. Estimation of Fine Root Biomass
2.5. Soil Fauna Sampling and Identification
2.6. Statistical Analysis
3. Results
3.1. Microclimate, Litter C and N, Soil Samples, and Fine Root Biomass
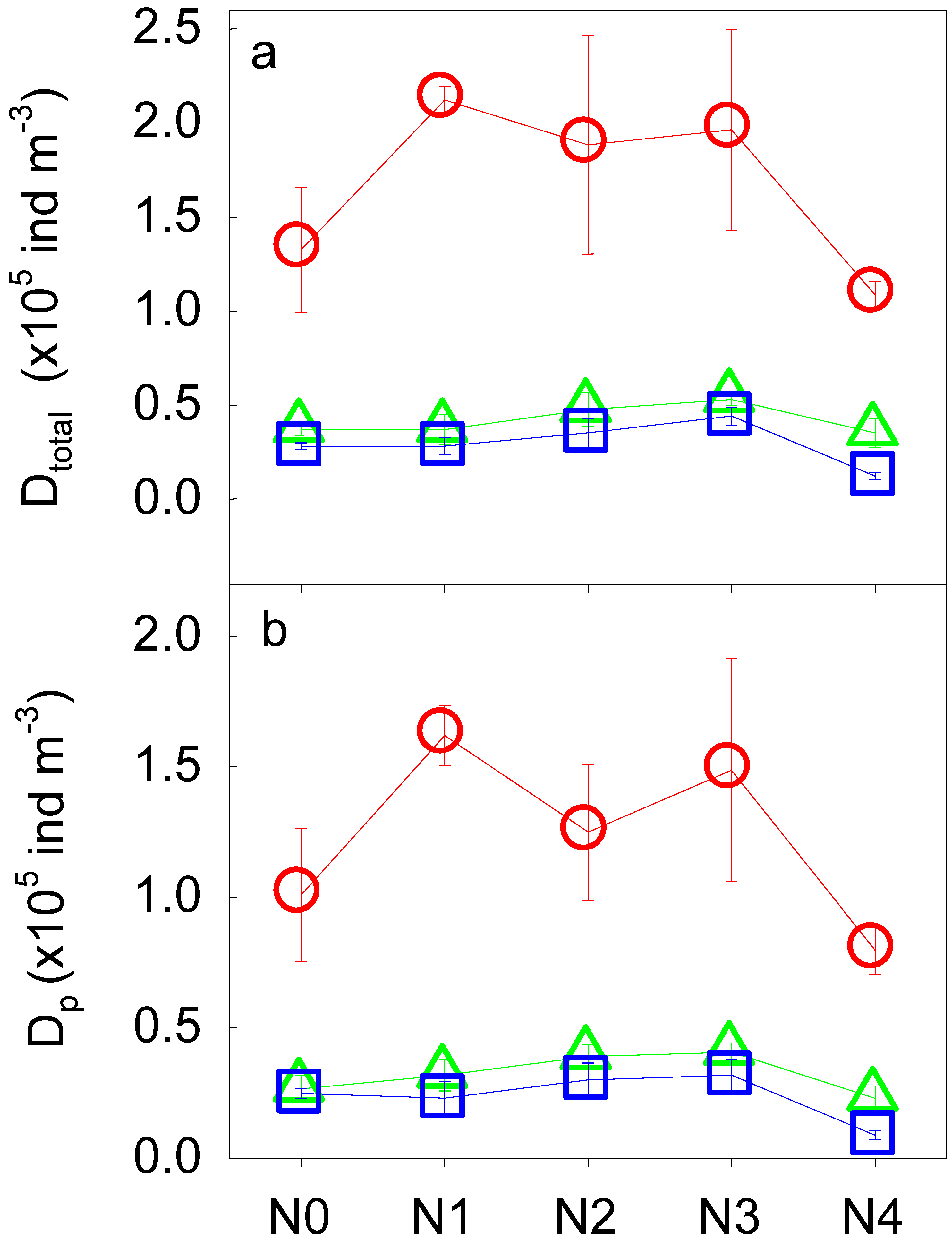
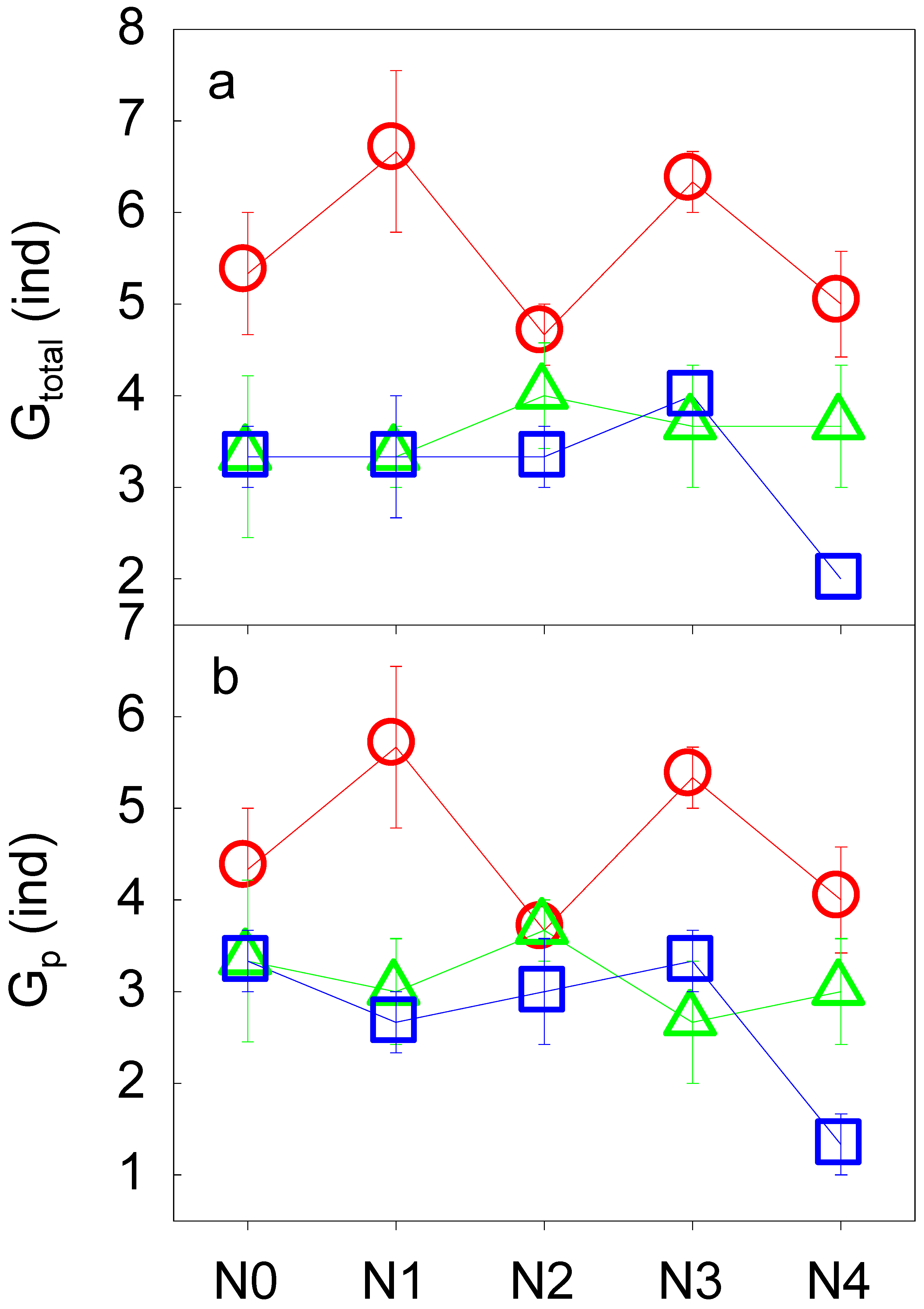
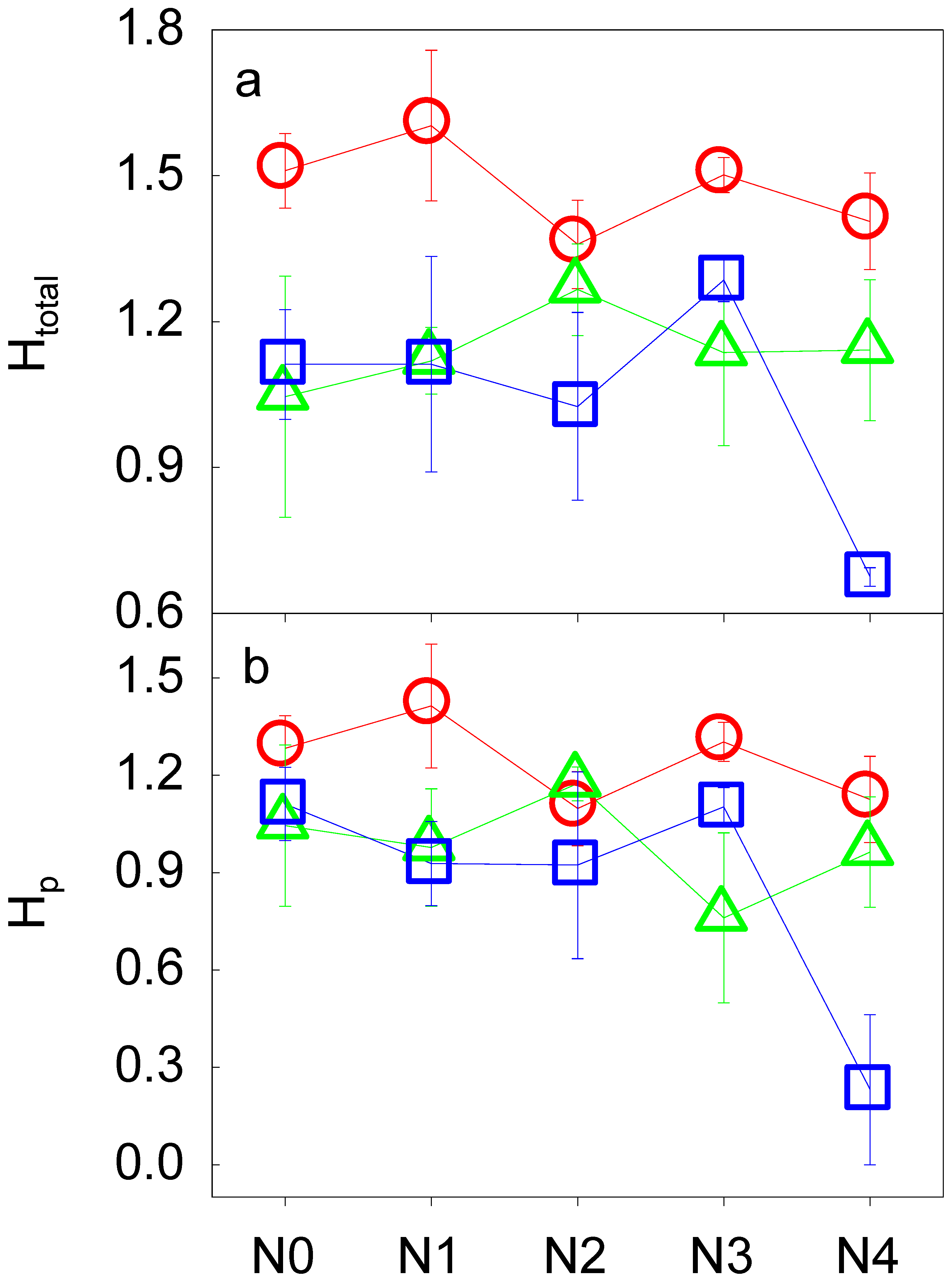
3.2. Soil Fauna Community as Affected by N Addition
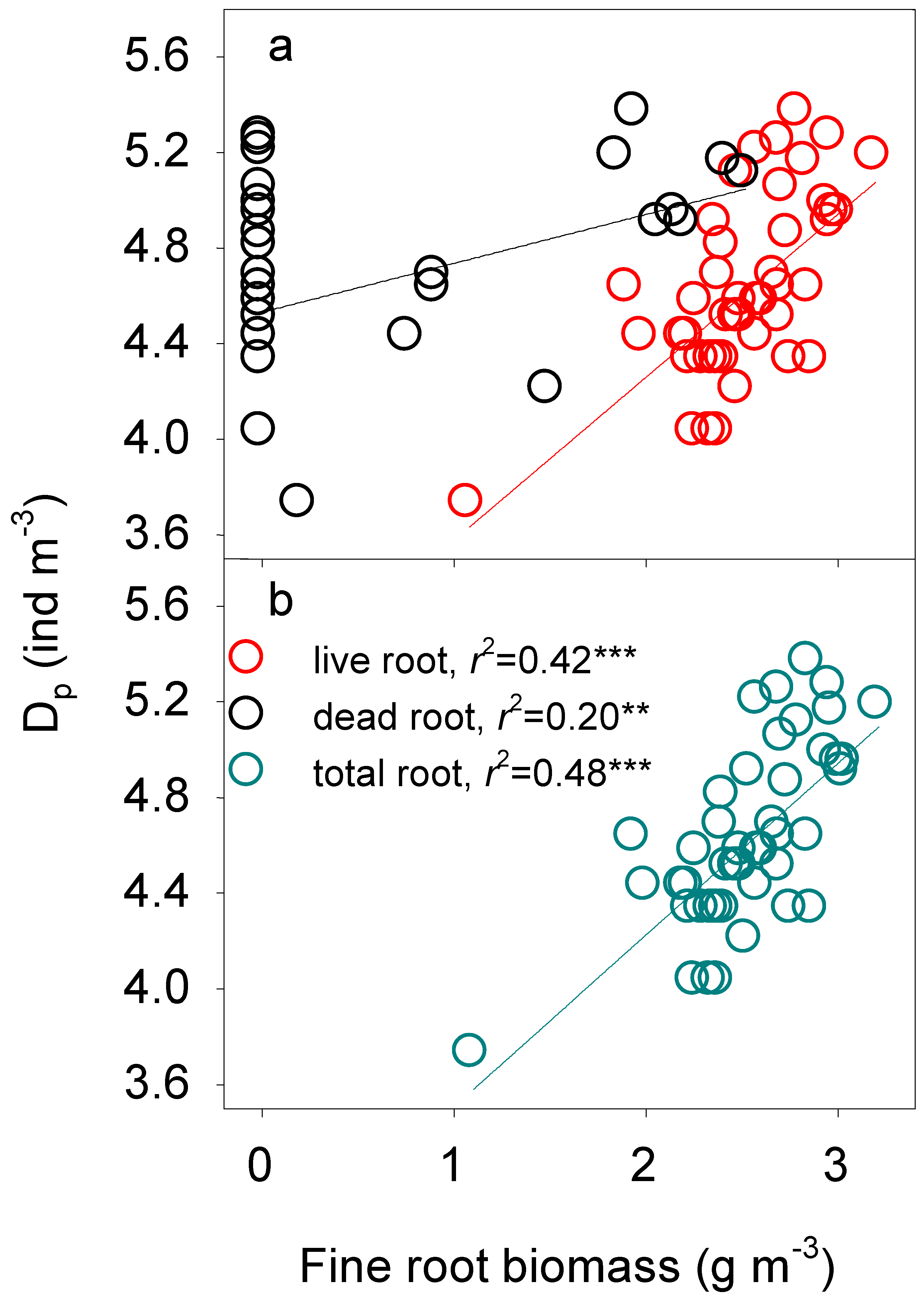
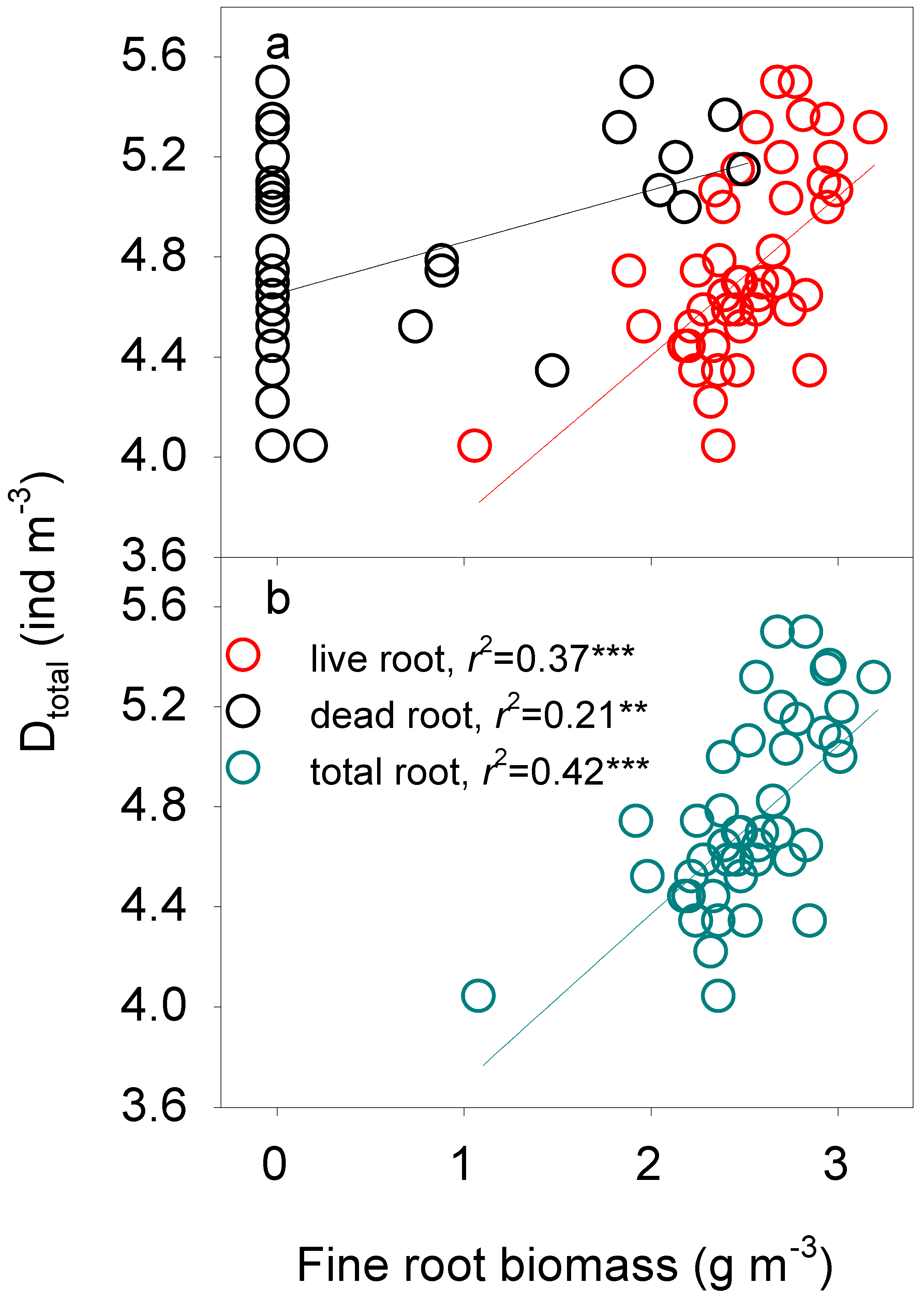
3.3. Fine Root Biomass Regulation of Soil Fauna Density
4. Discussion
4.1. N-addition Impacts on Soil Fauna Density
4.2. N-addition Impacts on the Number of Groups and Diversity of Soil Fauna
4.3. Increasing N-Addition Thresholds along Soil Profile
4.4. Regulation of Fine Root Biomass on Soil Fauna
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Penuelas, J.; Poulter, B.; Sardans, J.; Ciais, P.; van der Velde, M.; Bopp, L.; Boucher, O.; Godderis, Y.; Hinsinger, P.; Llusia, J.; et al. Human-induced nitrogen-phosphorus imbalances alter natural and managed ecosystems across the globe. Nat. Commun. 2013, 4, 2934. [Google Scholar] [CrossRef] [PubMed]
- Niu, S.; Classen, A.T.; Dukes, J.S.; Kardol, P.; Liu, L.; Luo, Y.; Rustad, L.; Sun, J.; Tang, J.; Templer, P.H. Global patterns and substrate-based mechanisms of the terrestrial nitrogen cycle. Ecol. Lett. 2016, 19, 697–709. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Schwede, D.B.; Simpson, D.; Tan, J.; Fu, J.S.; Dentener, F.; Du, E.; deVries, W. Spatial variation of modelled total, dry and wet nitrogen deposition to forests at global scale. Environ. Pollut. 2018, 243, 1287–1301. [Google Scholar] [CrossRef]
- Xu, G.-L.; Mo, J.-M.; Zhou, G.-Y.; Fu, S.-L. Preliminary response of soil fauna to simulated N deposition in three typical subtropical forests. Pedosphere 2006, 16, 596–601. [Google Scholar] [CrossRef]
- Sun, F.; Tariq, A.; Chen, H.; He, Q.; Guan, Y.; Pan, K.; Chen, S.; Li, J.; Zhao, C.; Wang, H.; et al. Effect of nitrogen and phosphorus application on agricultural soil food webs. Arch. Agron. Soil Sci. 2017, 63, 1176–1186. [Google Scholar] [CrossRef]
- Xin, W.D.; Yin, X.Q.; Song, B. Contribution of soil fauna to litter decomposition in Songnen sandy lands in northeastern China. J. Arid Environ. 2012, 77, 90–95. [Google Scholar] [CrossRef]
- Oliverio, A.M.; Gan, H.; Wickings, K.; Fierer, N. A DNA metabarcoding approach to characterize soil arthropod communities. Soil. Biol. Biochem. 2018, 125, 37–43. [Google Scholar] [CrossRef]
- Li, X.; Yin, X.; Wang, Z.; Fan, W. Litter mass loss and nutrient release influenced by soil fauna of Betula ermanii forest floor of the Changbai Mountains, China. Appl. Soil Ecol. 2015, 95, 15–22. [Google Scholar] [CrossRef]
- Lu, P.; Dai, N.H.; Sun, X.Y.; Zhang, G.H.; Xu, D.R.; Zhan, Y.H. Composition and structure of soil fauna community in the Dexing Copper Mine tailings pool after revegetation. Turk. J. Zool. 2018, 42, 307–315. [Google Scholar]
- Lin, H.; He, Z.; Hao, J.; Tian, K.; Jia, X.; Kong, X.; Akbar, S.; Bei, Z.; Tian, X. Effect of N addition on home-field advantage of litter decomposition in subtropical forests. For. Ecol. Manag. 2017, 398, 216–225. [Google Scholar] [CrossRef]
- Raub, F.; Scheuermann, L.; Hoefer, H.; Brandl, R. No bottom-up effects of food addition on predators in a tropical forest. Basic Appl. Ecol. 2014, 15, 59–65. [Google Scholar] [CrossRef]
- Gan, H.; Zak, D.R.; Hunter, M.D. Chronic nitrogen deposition alters the structure and function of detrital food webs in a northern hardwood ecosystem. Ecol. Appl. 2013, 23, 1311–1321. [Google Scholar] [CrossRef] [PubMed]
- Eisenhauer, N.; Cesarz, S.; Koller, R.; Worm, K.; Reich, P.B. Global change belowground: Impacts of elevated CO2, nitrogen, and summer drought on soil food webs and biodiversity. Glob. Chang. Biol. 2012, 18, 435–447. [Google Scholar] [CrossRef]
- Preece, C.; Penuelas, J. Rhizodeposition under drought and consequences for soil communities and ecosystem resilience. Plant Soil 2016, 409, 1–17. [Google Scholar] [CrossRef] [Green Version]
- Cole, L.; Buckland, S.M.; Bardgett, R.D. Influence of disturbance and nitrogen addition on plant and soil animal diversity in grassland. Soil Biol. Biochem. 2008, 40, 505–514. [Google Scholar] [CrossRef]
- Jiang, M.; Wang, X.; Liusui, Y.; Sun, X.; Zhao, C.; Liu, H. Diversity and Abundance of Soil Animals as Influenced by Long-Term Fertilization in Grey Desert Soil, China. Sustainability 2015, 7, 10837–10853. [Google Scholar] [CrossRef] [Green Version]
- Zhu, X.; Zhu, B. Diversity and abundance of soil fauna as influenced by long-term fertilization in cropland of purple soil, China. Soil. Tillage Res. 2015, 146, 39–46. [Google Scholar] [CrossRef]
- Liu, X.; Duan, L.; Mo, J.; Du, E.; Shen, J.; Lu, X.; Zhang, Y.; Zhou, X.; He, C.; Zhang, F. Nitrogen deposition and its ecological impact in China: An overview. Environ. Pollut. 2011, 159, 2251–2264. [Google Scholar] [CrossRef]
- Ochoa-Hueso, R.; Rocha, I.; Stevens, C.J.; Manrique, E.; Luciañez, M.J. Simulated nitrogen deposition affects soil fauna from a semiarid Mediterranean ecosystem in central Spain. Biol. Fertil. Soils 2014, 50, 191–196. [Google Scholar] [CrossRef]
- Nkem, J.N.; Bruyn, L.A.L.D.; Hulugalle, N.R.; Grant, C.D. Changes in invertebrate populations over the growing cycle of an N-fertilised and unfertilised wheat crop in rotation with cotton in a grey Vertosol. Appl. Soil Ecol. 2002, 20, 69–74. [Google Scholar] [CrossRef]
- Xu, G.L.; Schleppi, P.; Li, M.H.; Fu, S.L. Negative responses of Collembola in a forest soil (Alptal, Switzerland) under experimentally increased N deposition. Environ. Pollut. 2009, 157, 2030–2036. [Google Scholar] [CrossRef] [PubMed]
- Yin, X.; Song, B.; Dong, W.; Xin, W.; Wang, Y. A review on the eco-geography of soil fauna in China. J. Geogr. Sci. 2010, 20, 333–346. [Google Scholar] [CrossRef]
- Potapov, A.M.; Goncharov, A.A.; Semenina, E.E.; Korotkevich, A.Y.; Tsurikov, S.M.; Rozanova, O.L.; Anichkin, A.E.; Zuev, A.G.; Samoylova, E.S.; Semenyuk, I.I. Arthropods in the subsoil: Abundance and vertical distribution as related to soil organic matter, microbial biomass and plant roots. Eur. J. Soil Biol. 2017, 82, 88–97. [Google Scholar] [CrossRef]
- Xu, G.L.; Mo, J.M.; Fu, S.L.; Gundersen, P.; Zhou, G.Y.; Xue, J.H. Response of soil fauna to simulated nitrogen deposition: A nursery experiment in subtropical China. Acta Sci. Circumstantiae 2007, 19, 603–609. [Google Scholar] [CrossRef] [Green Version]
- Morrien, E. Understanding soil food web dynamics, how close do we get? Soil Biol. Biochem. 2016, 102, 10–13. [Google Scholar] [CrossRef]
- Zieger, S.L.J. Trophic Structure of Soil Animal Food Webs of Deciduous Forests as Analyzed by Stable Isotope Labeling. Ph.D. Thesis, Universität Göttingen, Göttingen, Germany, 2016. [Google Scholar]
- Leppälammi-Kujansuu, J.; Ostonen, I.; Strömgren, M.; Nilsson, L.O.; Kleja, D.B.; Sah, S.P.; Helmisaari, H.-S. Effects of long-term temperature and nutrient manipulation on Norway spruce fine roots and mycelia production. Plant Soil 2013, 366, 287–303. [Google Scholar] [CrossRef]
- Li, W.; Jin, C.; Guan, D.; Wang, Q.; Wang, A.; Yuan, F.; Wu, J. The effects of simulated nitrogen deposition on plant root traits: A meta-analysis. Soil Biol. Biochem. 2015, 82, 112–118. [Google Scholar] [CrossRef]
- Tsunoda, T.; Dam, N.M.V. Root chemical traits and their roles in belowground biotic interactions. Pedobiologia 2017, 65, 58–67. [Google Scholar] [CrossRef] [Green Version]
- Eissfeller, V.; Beyer, F.; Valtanen, K.; Hertel, D.; Maraun, M.; Polle, A.; Scheu, S. Incorporation of plant carbon and microbial nitrogen into the rhizosphere food web of beech and ash. Soil Biol. Biochem. 2013, 62, 76–81. [Google Scholar] [CrossRef]
- Sauvadet, M.; Chauvat, M.; Fanin, N.; Coulibaly, S.; Bertrand, I. Comparing the effects of litter quantity and quality on soil biota structure and functioning: Application to a cultivated soil in Northern France. Appl. Soil Ecol. 2016, 107, 261–271. [Google Scholar] [CrossRef]
- Sayer, E.J.; Sutcliffe, L.M.E.; Ross, R.I.C.; Tanner, E.V.J. Arthropod Abundance and Diversity in a Lowland Tropical Forest Floor in Panama: The Role of Habitat Space vs. Nutrient Concentrations. Biotropica 2010, 42, 194–200. [Google Scholar] [CrossRef]
- Ilieva-Makulec, K.; Olejniczak, I.; Szanser, M. Response of soil micro- and mesofauna to diversity and quality of plant litter. Eur. J. Soil Biol. 2006, 42, S244–S249. [Google Scholar] [CrossRef]
- Gilbert, K.J.; Fahey, T.J.; Maerz, J.C.; Sherman, R.E.; Bohlen, P.; Dombroskie, J.J.; Groffman, P.M.; Yavitt, J.B. Exploring carbon flow through the root channel in a temperate forest soil food web. Soil Biol. Biochem. 2014, 76, 45–52. [Google Scholar] [CrossRef]
- Melaniem, P.; Reinhard, L.; Stefan, S.; Mark, M. Compartmentalization of the soil animal food web as indicated by dual analysis of stable isotope ratios (15N/14N and 13C/12C). Soil Biol. Biochem. 2009, 41, 1221–1226. [Google Scholar]
- Wang, S.; Tan, Y.; Fan, H.; Ruan, H.; Zheng, A. Responses of soil microarthropods to inorganic and organic fertilizers in a poplar plantation in a coastal area of eastern China. Appl. Soil Ecol. 2015, 89, 69–75. [Google Scholar] [CrossRef]
- Liang, W.J.; Lou, Y.L.; Qi, L.; Shuang, Z.; Zhang, X.K.; Wang, J.K.; Coleman, D.; Fu, S.L.; Zou, X.M. Nematode faunal response to long-term application of nitrogen fertilizer and organic manure in Northeast China. Soil Biol. Biochem. 2009, 41, 883–890. [Google Scholar] [CrossRef]
- Zhao, J.; Wang, F.; Li, J.; Zou, B.; Wang, X.; Li, Z.; Fu, S. Effects of experimental nitrogen and/or phosphorus additions on soil nematode communities in a secondary tropical forest. Soil Biol. Biochem. 2014, 75, 1–10. [Google Scholar] [CrossRef]
- Boxman, A.W.; Blanck, K.; Brandrud, T.E.; Emmett, B.A.; Gundersen, P.; Hogervorst, R.F.; Kjonaas, O.J.; Persson, H.; Timmermann, V. Vegetation and soil biota response to experimentally-changed nitrogen inputs in coniferous forest ecosystems of the NITREX project. For. Ecol. Manag. 1998, 101, 65–79. [Google Scholar] [CrossRef]
- WIKIPEDIA. Köppen Climate Classification. 1884. Available online: https://en.wikipedia.org/wiki/Köppen_climate_classification (accessed on 3 January 2019).
- Wang, S.; Chen, H.Y.; Tan, Y.; Fan, H.; Ruan, H. Fertilizer regime impacts on abundance and diversity of soil fauna across a poplar plantation chronosequence in coastal Eastern China. Sci. Rep. 2016, 6, 20816. [Google Scholar] [CrossRef] [Green Version]
- Zhu, J.; Wang, Q.; He, N.; Smith, M.D.; Elser, J.J.; Du, J.; Yuan, G.; Yu, G.; Yu, Q. Imbalanced atmospheric nitrogen and phosphorus depositions in China: Implications for nutrient limitation. J. Geophys. Res. Biogeosci. 2016, 121, 1605–1616. [Google Scholar] [CrossRef] [Green Version]
- Ramnarine, R.; Voroney, R.P.; WagnerRiddle, C.; Dunfield, K.E. Carbonate removal by acid fumigation for measuring the δ13C of soil organic carbon. Can. J. Soil Sci. 2011, 91, 247–250. [Google Scholar] [CrossRef]
- Wotherspoon, A.; Voroney, R.P.; Thevathasan, N.V.; Gordon, A.M. Comparison of Three Methods for Measurement of Soil Organic Carbon. Commun. Soil Sci. Plant Anal. 2015, 46, 362–374. [Google Scholar] [CrossRef]
- Xu, X.; Niu, S.; Sherry, R.A.; Zhou, X.; Zhou, J.; Luo, Y. Interannual variability in responses of belowground net primary productivity (NPP) and NPP partitioning to long-term warming and clipping in a tallgrass prairie. Glob. Chang. Biol. 2012, 18, 1648–1656. [Google Scholar] [CrossRef]
- Derner, J.D.; Briske, D.D. Does a tradeoff exist between morphological and physiological root plasticity? A comparison of grass growth forms. Acta Oecol. 1999, 20, 519–526. [Google Scholar] [CrossRef]
- Gao, Y.Z.; Giese, M.; Lin, S.; Sattelmacher, B.; Zhao, Y.; Brueck, H. Belowground net primary productivity and biomass allocation of a grassland in Inner Mongolia is affected by grazing intensity. Plant Soil 2008, 307, 41–50. [Google Scholar] [CrossRef]
- Yang, B.; Zhang, W.; Xu, H.; Wang, S.; Xu, X.; Fan, H.; Chen, H.Y.H.; Ruan, H. Effects of soil fauna on leaf litter decomposition under different land uses in eastern coast of China. J. For. Res. 2018, 29, 973–982. [Google Scholar] [CrossRef]
- Wang, G.B.; Deng, F.F.; Xu, W.H.; Chen, H.Y.H.; Ruan, H.H. Poplar plantations in coastal China: Towards the identification of the best rotation age for optimal soil carbon sequestration. Soil Use Manag. 2016, 32, 303–310. [Google Scholar] [CrossRef]
- Wallwork, J.A. The Distribution and Diversity of Soil Fauna. Q. Rev. Biol. 1977, 52, 319. [Google Scholar]
- Wu, H.; Lu, M.; Lu, X.; Guan, Q.; He, X. Interactions between earthworms and mesofauna has no significant effect on emissions of CO2 and N2O from soil. Soil Biol. Biochem. 2015, 88, 294–297. [Google Scholar] [CrossRef]
- Bokhorst, S.; Huiskes, A.; Convey, P.; Pmvan, B.; Aerts, R. Climate change effects on soil arthropod communities from the Falkland Islands and the Maritime Antarctic. Soil Biol. Biochem. 2008, 40, 1547–1556. [Google Scholar] [CrossRef]
- Li, Y.; Chen, Y.; Xu, C.; Xu, H.; Zou, X.; Chen, H.Y.H.; Ruan, H. The abundance and community structure of soil arthropods in reclaimed coastal saline soil of managed poplar plantations. Geoderma 2018, 327, 130–137. [Google Scholar] [CrossRef]
- Yin, W. Subtropical Soil Animals in China (in Chinese); Science Press: Beijing, China, 2000. [Google Scholar]
- Yin, W. Chinese subtropical soil animals (in Chinese); Science Press: Beijing, China, 1992. [Google Scholar]
- Whittaker, R.H. Evolution and Measurement of Species Diversity. Taxon 1972, 21, 213–251. [Google Scholar] [CrossRef]
- Sjursen, H.; Michelsen, A.; Jonasson, S. Effects of long-term soil warming and fertilisation on microarthropod abundances in three sub-arctic ecosystems. Appl. Soil Ecol. 2005, 30, 148–161. [Google Scholar] [CrossRef]
- Van, d.W.A.; Rhem, G.; Korevaar, H.; Schouten, A.J.; Gajmjagers, O.A.; Rutgers, M.; Mulder, C. Dissimilar response of plant and soil biota communities to long-term nutrient addition in grasslands. Biol. Fertil. Soils 2009, 45, 663–667. [Google Scholar]
- Cusack, D.F.; Firestone, M.K. Changes in microbial community characteristics and soil organic matter with nitrogen additions in two tropical forests. Ecology 2011, 92, 621–632. [Google Scholar] [CrossRef] [PubMed]
- Wall, D.H.; Bardgett, R.D.; Kelly, E. Biodiversity in the dark. Nat. Geosci. 2010, 3, 297–298. [Google Scholar] [CrossRef]
- Kudrin, A.A.; Tsurikov, S.M.; Tiunov, A.V. Trophic position of microbivorous and predatory soil nematodes in a boreal forest as indicated by stable isotope analysis. Soil Biol. Biochem. 2015, 86, 193–200. [Google Scholar] [CrossRef]
- Li, J.; Wen, Y.C.; Li, X.H.; Li, Y.T.; Yang, X.D.; Lin, Z.; Song, Z.Z.; Cooper, J.M.; Zhao, B.Q. Soil labile organic carbon fractions and soil organic carbon stocks as affected by long-term organic and mineral fertilization regimes in the North China Plain. Soil Tillage Res. 2018, 175, 281–290. [Google Scholar] [CrossRef]
- Fenn, M.E.; Baron, J.S.; Allen, E.B.; Rueth, H.M.; Nydick, K.R.; Geiser, L.; Bowman, W.D.; Sickman, J.O.; Meixner, T.; Johnson, D.W. Ecological Effects of Nitrogen Deposition in the Western United States. Bioscience 2003, 53, 404–420. [Google Scholar] [CrossRef]
- Fu, G.; Shen, Z.X. Response of alpine soils to nitrogen addition on the Tibetan Plateau: A meta-analysis. Appl. Soil Ecol. 2017, 114, 99–104. [Google Scholar] [CrossRef]
- Lu, X.; Mo, J.; Gilliam, F.S.; Zhou, G.; Fang, Y. Effects of experimental nitrogen additions on plant diversity in an old-growth tropical forest. Glob. Chang. Biol. 2010, 16, 2688–2700. [Google Scholar] [CrossRef] [Green Version]
- Xu, C.; Geng, Q.; Guo, L.; Li, Q.; Cheng, X.; Luo, Y.; Ruan, H.; Xu, X. Variation in Soil Carbon Content in Response to Long-Term Nitrogen Addition in Poplar Plantations (Populus Deltoids) on the East Coast of China. in preparation.
- Wei, C.; Zheng, H.; Li, Q.; Lü, X.; Yu, Q.; Zhang, H.; Chen, Q.; He, N.; Kardol, P.; Liang, W. Nitrogen Addition Regulates Soil Nematode Community Composition through Ammonium Suppression. PLoS ONE 2012, 7, e43384. [Google Scholar] [CrossRef] [PubMed]
- Guo, J.H.; Liu, X.J.; Zhang, Y.; Shen, J.L.; Han, W.X.; Zhang, W.F.; Christie, P.; Goulding, K.W.T.; Vitousek, P.M.; Zhang, F.S. Significant Acidification in Major Chinese Croplands. Science 2010, 327, 1008–1010. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Collange, B.; Navarrete, M.; Peyre, G.; Mateille, T.; Tchamitchian, M. Root-knot nematode (Meloidogyne) management in vegetable crop production: The challenge of an agronomic system analysis. Crop Prot. 2011, 30, 1251–1262. [Google Scholar] [CrossRef] [Green Version]
- Pinho, P.; Theobald, M.R.; Dias, T.; Tang, Y.S.; Cruz, C.; Martinsloução, M.A.; Máguas, C.; Sutton, M.; Branquinho, C. Critical loads of nitrogen deposition and critical levels of atmospheric ammonia for semi-natural Mediterranean evergreen woodlands. Biogeosciences 2012, 9, 1205–1215. [Google Scholar] [CrossRef] [Green Version]
- Yang, J.-Y.; Fan, J. Review of study on mineralization, saturation and cycle of Nitrogen in forest ecosystems. J. For. Res. 2003, 14, 239–243. [Google Scholar]
- Van Diepen, L.T.A.; Lilleskov, E.A.; Pregitzer, K.S.; Miller, R.M. Simulated Nitrogen Deposition Causes a Decline of Intra- and Extraradical Abundance of Arbuscular Mycorrhizal Fungi and Changes in Microbial Community Structure in Northern Hardwood Forests. Ecosystems 2010, 13, 683–695. [Google Scholar] [CrossRef]
- Högberg, M.N.; Briones, M.J.I.; Keel, S.G.; Metcalfe, D.B.; Campbell, C.; Midwood, A.J.; Thornton, B.; Hurry, V.; Linder, S.; Näsholm, T. Quantification of effects of season and nitrogen supply on tree below-ground carbon transfer to ectomycorrhizal fungi and other soil organisms in a boreal pine forest. New Phytol. 2010, 187, 485–493. [Google Scholar] [CrossRef]
- Doblasmiranda, E.; Sánchezpiñero, F.; Gonzálezmegías, A. Vertical distribution of soil macrofauna in an arid ecosystem: Are litter and belowground compartmentalized habitats? Pedobiologia 2009, 52, 361–373. [Google Scholar] [CrossRef]
- Wei, H.; Liu, W.; Zhang, J.; Qin, Z. Effects of simulated acid rain on soil fauna community composition and their ecological niches. Environ. Pollut. 2016, 220, 460–468. [Google Scholar] [CrossRef]
- Rusek, J.; Marshall, V.G. Impacts of Airborne Pollutants on Soil Fauna. Annu. Rev. Ecol. Syst. 2000, 31, 395–423. [Google Scholar] [CrossRef]
- Wang, S.; Ruan, H. Effects of soil mesofauna and microclimate on nitrogen dynamics in leaf litter decomposition along an elevation gradient. Afr. J. Biotechnol. 2011, 10, 6732–6742. [Google Scholar]
- Li, Q.; Bai, H.; Liang, W.; Xia, J.; Wan, S.; Wh, V.D.P. Nitrogen addition and warming independently influence the belowground micro-food web in a temperate steppe. PLoS ONE 2013, 8, e60441. [Google Scholar] [CrossRef] [PubMed]
- Hishi, T.; Fujimaki, R.; McGonigle, T.P.; Takeda, H. Relationships among fine roots, fungal hyphae and soil microarthropods among different soil microhabitats in a temperate coniferous forest of Chamaecyparis obtusa. Eur. J. Soil Biol. 2008, 44, 473–477. [Google Scholar] [CrossRef]
- Bradford, M.A. Re-visioning soil food webs. Soil Biol. Biochem. 2016, 102, 1–3. [Google Scholar] [CrossRef]
- Pollierer, M.M.; Langel, R.; Körner, C.; Maraun, M.; Scheu, S. The underestimated importance of belowground carbon input for forest soil animal food webs. Ecol. Lett. 2007, 10, 729–736. [Google Scholar] [CrossRef] [PubMed]
- Mcnaughton, S.J.; Oesterheld, M.; Frank, D.A.; Williams, K.J. Ecosystem-level patterns of primary productivity and herbivory in terrestrial habitats. Nature 1989, 341, 142–144. [Google Scholar] [CrossRef]
- Du, E.; de Vries, W. Nitrogen-induced new net primary production and carbon sequestration in global forests. Environ. Pollut. 2018, 242, 1476–1487. [Google Scholar] [CrossRef]
| Variables | N Addition (N) | Time (T) | N × T | |||
|---|---|---|---|---|---|---|
| df | F, p | df | F, p | df | F, p | |
| Litter mass | 4 | 0.41 | 1 | 311.77 *** | 4 | 0.58 |
| Soil temperature | 4 | 0.49 | 15 | 1582.24 *** | 60 | 0.29 |
| Soil moisture | 4 | 0.67 | 15 | 233.25 *** | 60 | 0.38 |
| Variables | N Addition (N) | |
|---|---|---|
| df | F, p | |
| Litter C:N ratio | 4 | 1.68 |
| TN | 4 | 0.96 |
| SOC | 4 | 1.67 |
| Variables | N Addition (N) | Soil Depth (depth) | N × Depth | ||||
|---|---|---|---|---|---|---|---|
| df | F, p | df | F, p | df | F, p | ||
| Total soil fauna | Dtotal | 4 | 8.11 ** | 2 | 308.52 *** | 8 | 2.77 * |
| Gtotal | 4 | 1.91 | 2 | 10.63 * | 8 | 2.19 | |
| Htotal | 4 | 1.33 | 2 | 6.35 | 8 | 1.78 | |
| Phytophagous soil fauna | Dp | 4 | 5.91 * | 2 | 118.38 *** | 8 | 1.75 |
| Gp | 4 | 1.74 | 2 | 8.13 * | 8 | 2.79* | |
| Hp | 4 | 2.28 | 2 | 4.94 | 8 | 2.81 | |
| Fine root biomass | Live root biomass | 4 | 1.84 | 2 | 13.42 *** | 8 | 0.72 |
| Dead root biomass | 4 | 2.54 | 2 | 8.9 ** | 8 | 1.39 | |
| Total root biomass | 4 | 1.64 | 2 | 17.32 *** | 8 | 0.88 | |
| Dependent | Model | Variables | Regression | r2 and p |
|---|---|---|---|---|
| Dp | A-1 | Total root | Dp = 2.79 + 0.72 total root | 0.48 *** |
| A-2 | Total root, dead root | Dp = 2.95 + 0.63 total root+0.11 dead root | 0.53 *** | |
| Dtotal | B-1 | Total root | Dtotal = 3.03 + 0.67 total root | 0.42 *** |
| B-2 | Total root, dead root | Dtotal = 3.20 + 0.58 total root+0.12 dead root | 0.49 *** |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bian, H.; Geng, Q.; Xiao, H.; Shen, C.; Li, Q.; Cheng, X.; Luo, Y.; Ruan, H.; Xu, X. Fine Root Biomass Mediates Soil Fauna Community in Response to Nitrogen Addition in Poplar Plantations (Populus deltoids) on the East Coast of China. Forests 2019, 10, 122. https://doi.org/10.3390/f10020122
Bian H, Geng Q, Xiao H, Shen C, Li Q, Cheng X, Luo Y, Ruan H, Xu X. Fine Root Biomass Mediates Soil Fauna Community in Response to Nitrogen Addition in Poplar Plantations (Populus deltoids) on the East Coast of China. Forests. 2019; 10(2):122. https://doi.org/10.3390/f10020122
Chicago/Turabian StyleBian, Haixue, Qinghong Geng, Hanran Xiao, Caiqin Shen, Qian Li, Xiaoli Cheng, Yiqi Luo, Honghua Ruan, and Xia Xu. 2019. "Fine Root Biomass Mediates Soil Fauna Community in Response to Nitrogen Addition in Poplar Plantations (Populus deltoids) on the East Coast of China" Forests 10, no. 2: 122. https://doi.org/10.3390/f10020122
APA StyleBian, H., Geng, Q., Xiao, H., Shen, C., Li, Q., Cheng, X., Luo, Y., Ruan, H., & Xu, X. (2019). Fine Root Biomass Mediates Soil Fauna Community in Response to Nitrogen Addition in Poplar Plantations (Populus deltoids) on the East Coast of China. Forests, 10(2), 122. https://doi.org/10.3390/f10020122






