Size-Dependent Patterns of Seed Rain in Gaps in Temperate Secondary Forests, Northeast China
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Site
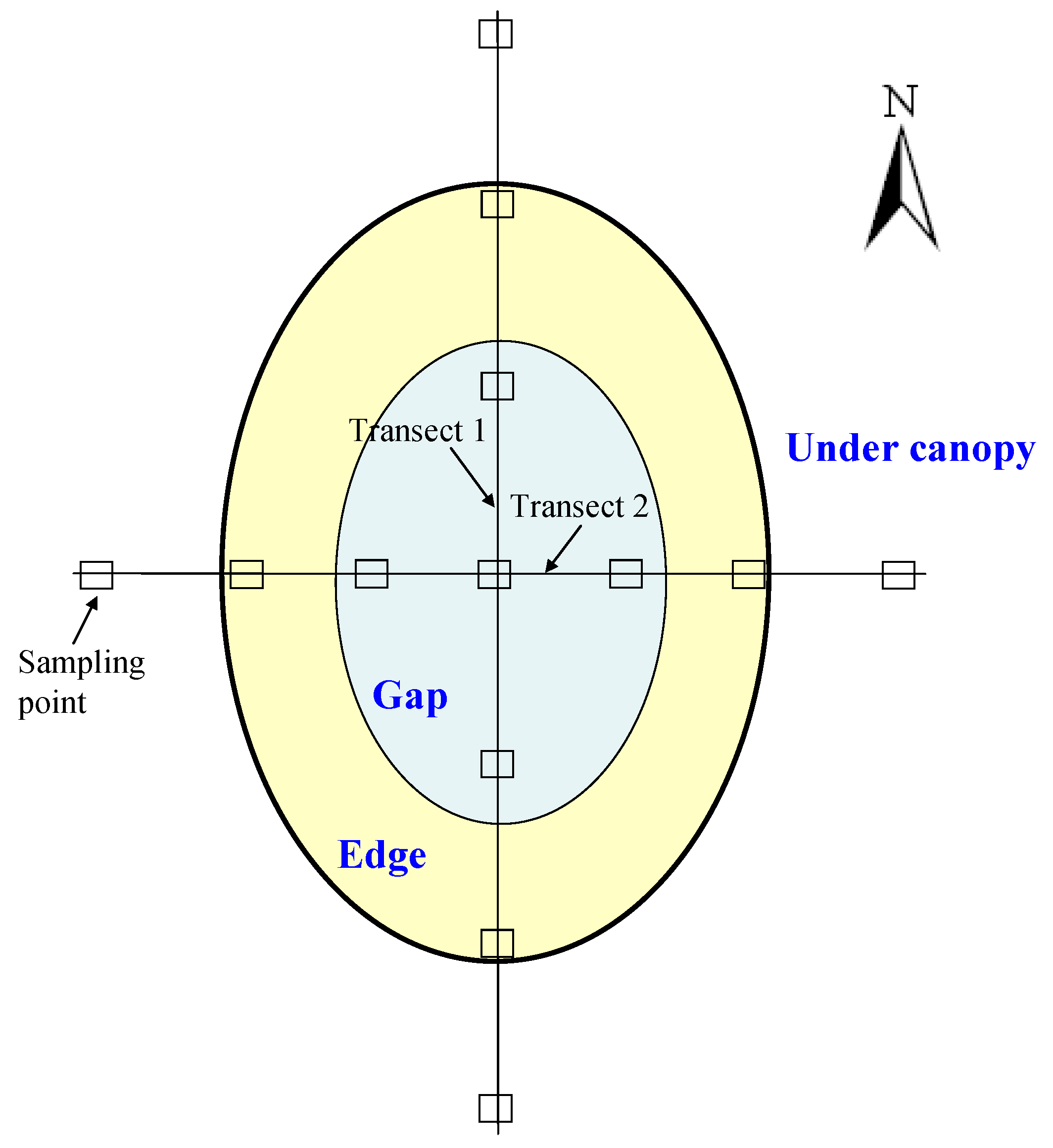
2.2. Sampling Points Setting
2.3. Seed Rain Investigation
2.4. Soil Seed Bank Sampling
2.5. Data Analysis
3. Results
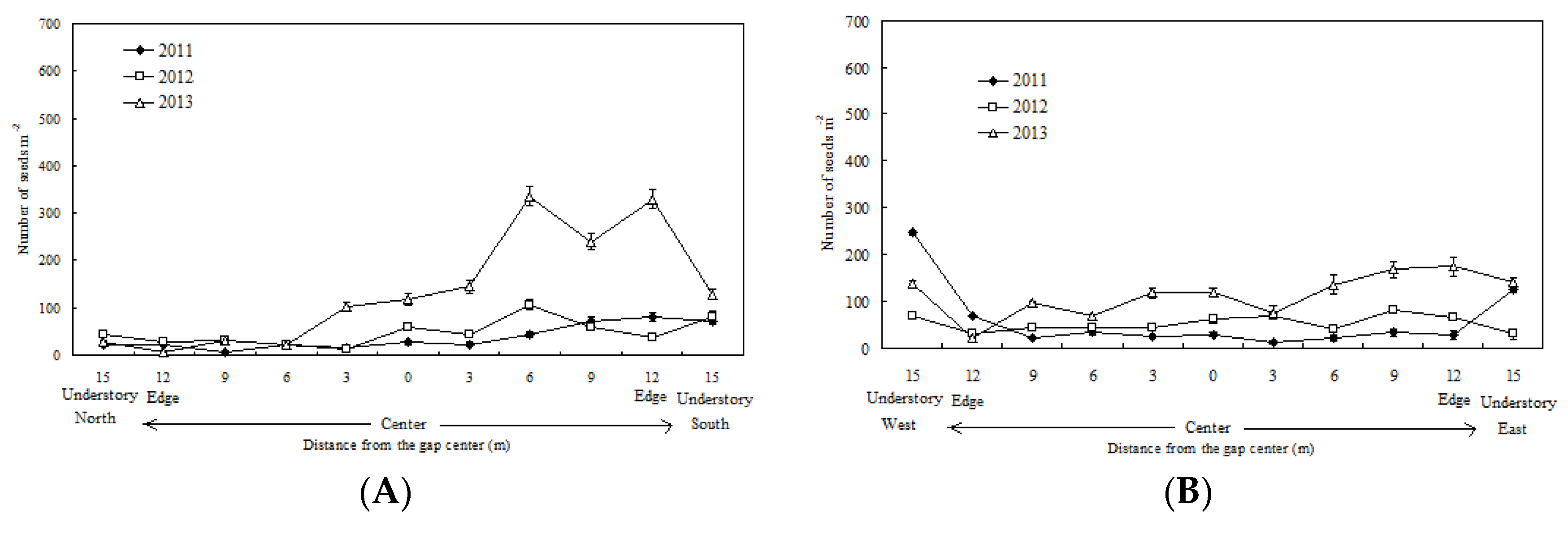
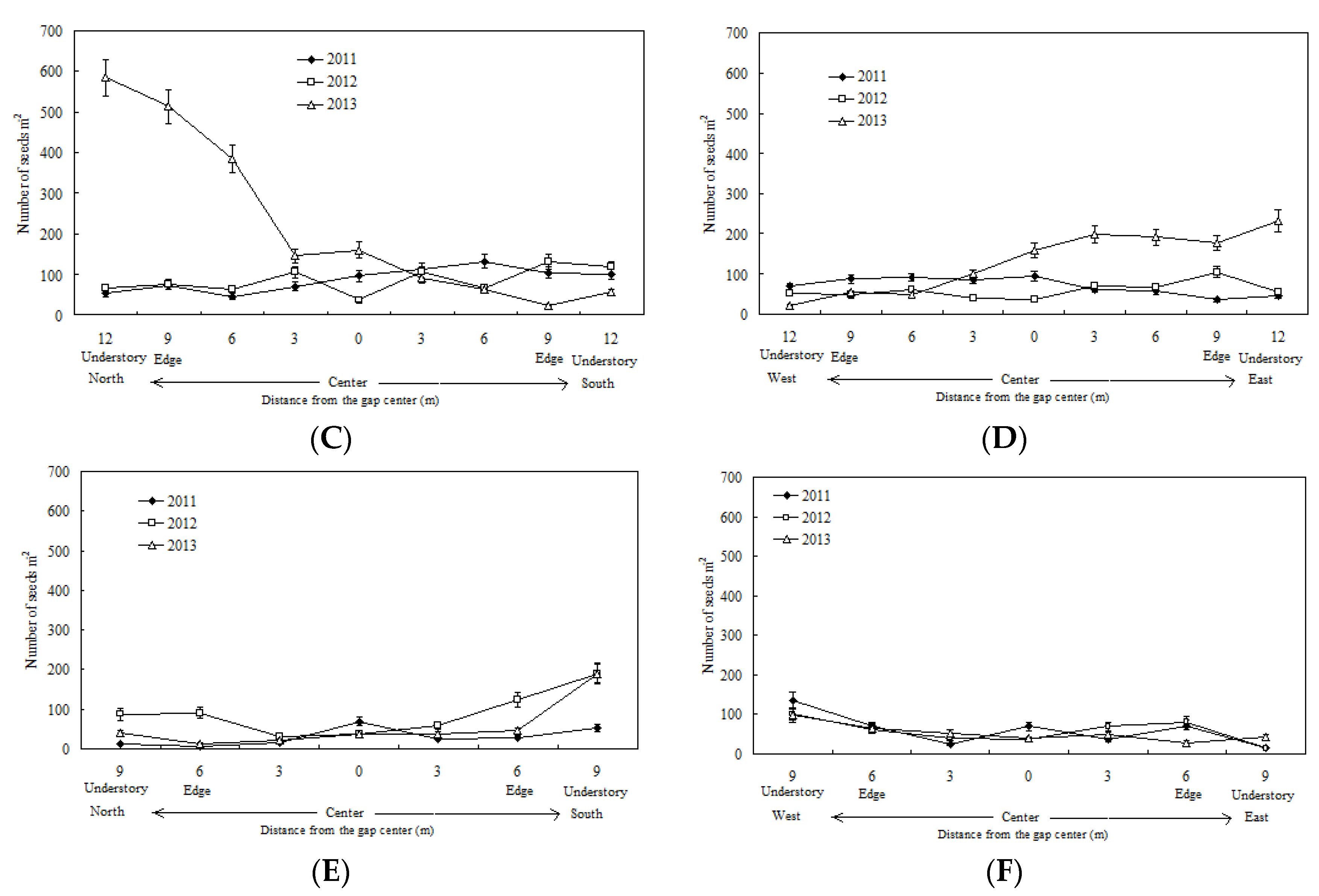
3.1. Spatiotemporal Variation in the Seed Rain and Its Relationship with Gap Size
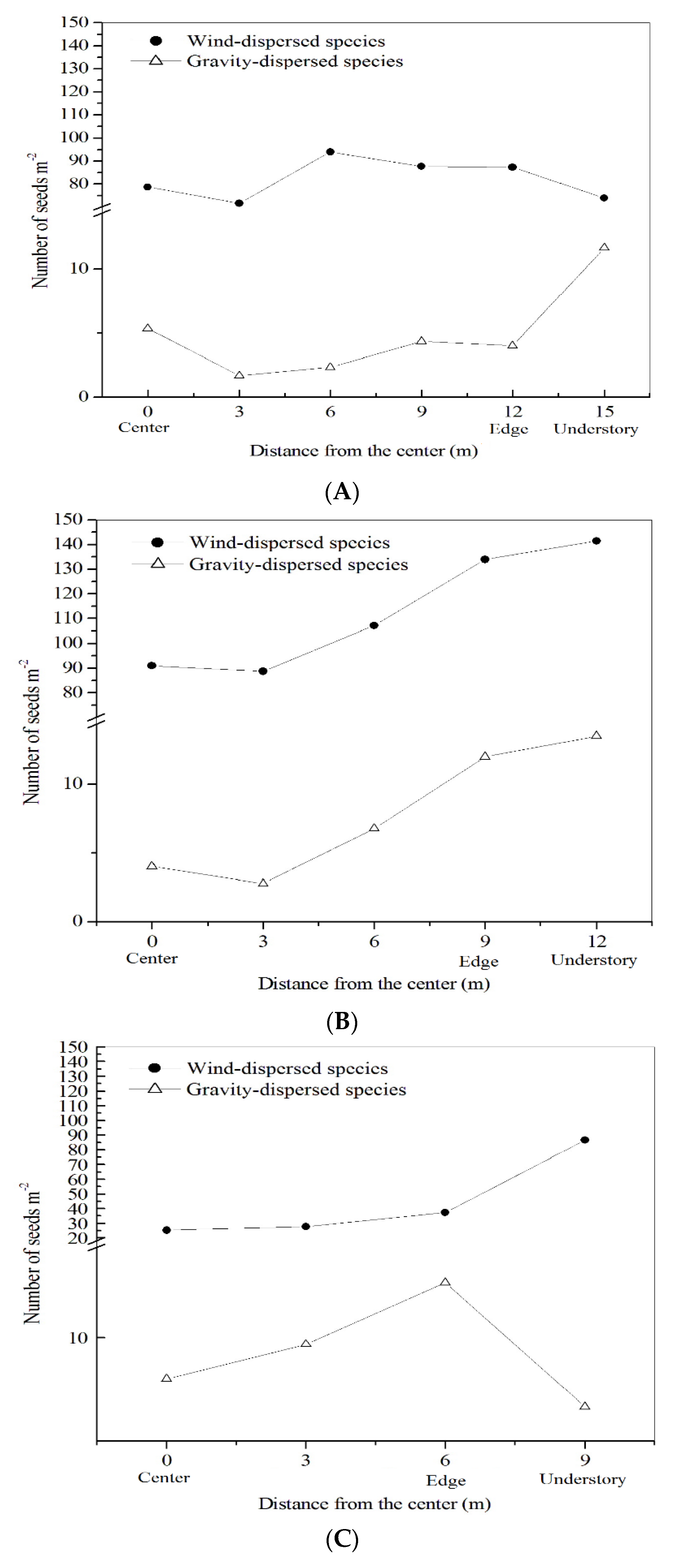
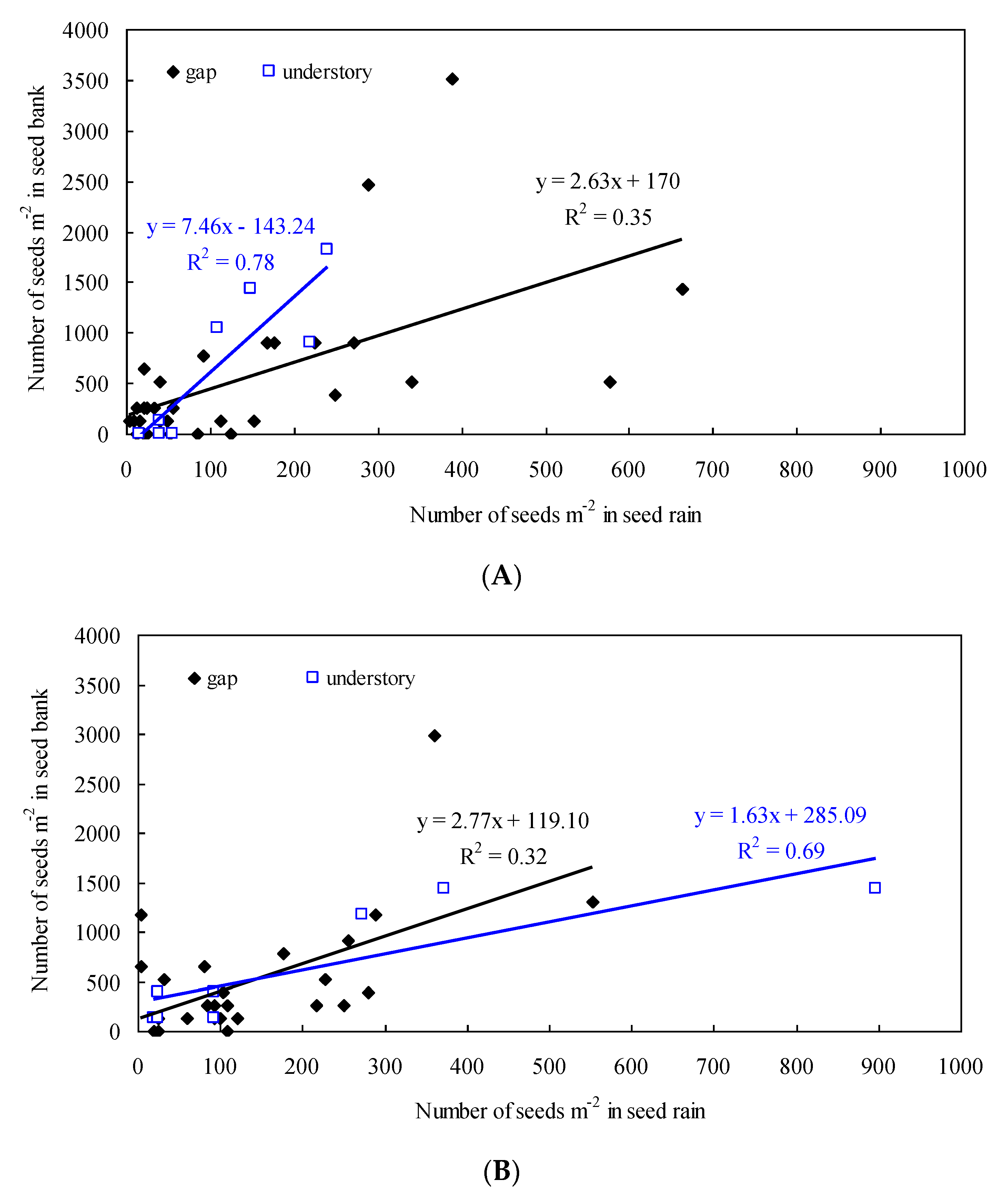
3.2. Relationship Between Seed Rain and Soil Seed Bank
4. Discussion
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Zhu, J.J.; Liu, S.R. Conception of secondary forest and its relation to ecological disturbance degree. Chin. J. Appl. Ecol. 2007, 267, 1085–1093, (In Chinese with English Abstract). [Google Scholar]
- Chen, X.W.; Li, B.L.; Lin, Z.S. The acceleration of succession for the restoration of the mixed-broadleaved Korean pine forests in Northeast China. For. Ecol. Manag. 2003, 117, 503–514. [Google Scholar] [CrossRef]
- Zhu, J.J.; Liu, Z.G. A review on disturbance ecology of forest. Chin. J. Appl. Ecol. 2004, 15, 1703–1710, (In Chinese with English Abstract). [Google Scholar]
- Chazdon, R.L. Beyond deforestation: Restoring forests and ecosystem services on degraded lands. Science 2008, 320, 1458–1460. [Google Scholar] [CrossRef] [PubMed]
- Yan, Q.L.; Zhu, J.J.; Zhang, J.P.; Yu, L.Z.; Hu, Z.B. Spatial distribution pattern of soil seed bank in canopy gaps of various sizes in temperate secondary forests, Northeast China. Plant Soil 2010, 329, 469–480. [Google Scholar] [CrossRef]
- Yan, Q.L.; Zhu, J.J.; Yu, L.Z. Seed regeneration potential of canopy gaps at early formation stage in temperate secondary forests, Northeast China. PLoS ONE 2012, 7, e39502. [Google Scholar] [CrossRef] [PubMed]
- Nathan, R.; Muller-Landau, H.C. Spatial patterns of seed dispersal, their determinants and consequences for recruitment. Trends Ecol. Evol. 2000, 15, 278–285. [Google Scholar] [CrossRef]
- Gagnon, J.L.; Jokela, E.J.; Moser, W.K.; Huber, D.A. Characteristics of gaps and natural regeneration in mature longleaf pine flatwoods ecosystems. For. Ecol. Manag. 2004, 187, 373–380. [Google Scholar] [CrossRef]
- Košulič, O.; Michalko, R.; Hula, V. Impact of canopy openness on spider communities: Implications for conservation management of formerly coppiced Oak forests. PLoS ONE 2016, 11, e0148585. [Google Scholar] [CrossRef]
- Greenberg, C.H. Response of reptile and amphibian communities to canopy gaps created by wind disturbance in the southern Appalachians. For. Ecol. Manag. 2001, 148, 135–144. [Google Scholar] [CrossRef]
- van Kuijk, M.; Anten, N.P.R.; Oomen, R.J.; van Bentum, D.W.; Werger, M.J.A. The limited importance of size-asymmetric light competition and growth of pioneer species in early secondary forest succession in Vietnam. Oecologia 2008, 157, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Bond, W.J.; Midgley, J.J. Ecology of sprouting in woody plants: The persistence niche. Trends Ecol. Evol. 2001, 16, 45–51. [Google Scholar] [CrossRef]
- Zhu, J.J.; Lu, D.L.; Zhang, W.D. Effects of gaps on regeneration of woody plants: A meta-analysis. J. For. Res. 2014, 25, 501–510. [Google Scholar] [CrossRef]
- Kern, C.C.; Burton, J.I.; Raymond, P.; D’Amato, A.W.; Keeton, W.S.; Royo, A.A.; Walters, M.B.; Webster, C.R.; Willis, J.L. Challenges facing gap-based silviculture and possible solutions for mesic northern forests in North America. Forestry 2017, 90, 4–17. [Google Scholar] [CrossRef]
- Grime, J.P. Plant Strategies, Vegetation Processes, and Ecosystem Properties, 2nd ed.; John Wiley & Sons Ltd.: Chichester, UK, 2001. [Google Scholar]
- Wang, B.C.; Smith, T.B. Closing the seed dispersal loop. Trends Ecol. Evol. 2002, 17, 379–385. [Google Scholar] [CrossRef]
- Martini, A.M.Z.; dos Santos, F.A.M. Effects of distinct types of disturbance on seed rain in the Atlantic forest of NE Brazil. Plant Ecol. 2007, 190, 81–95. [Google Scholar] [CrossRef]
- de Andrés, E.G.; Camarero, J.J.; Martínez, I.; Coll, L. Uncoupled spatiotemporal patterns of seed dispersal and regeneration in Pyrenean silver fir populations. For. Ecol. Manag. 2014, 319, 18–28. [Google Scholar] [CrossRef]
- Evans, K.L.; Gaston, K.J. Can the evolutionary-rates hypothesis explain species-energy relationships? Funct. Ecol. 2005, 19, 899–915. [Google Scholar] [CrossRef]
- Busing, R.T.; White, P.S. Species diversity and small-scale disturbance in an old-growth temperate forest: A consideration of gap partitioning concepts. Oikos 1997, 78, 562–568. [Google Scholar] [CrossRef]
- Forrester, J.A.; Leopold, D.J. Extant and potential vegetation of an old-growth maritime Ilex opaca forest. Plant Ecol. 2006, 183, 349–359. [Google Scholar] [CrossRef]
- Bohrer, G.; Katul, G.G.; Nathan, R.; Walko, R.L.; Avissar, R. Effects of canopy heterogeneity, seed abscission and inertia on wind-driven dispersal kernels of tree seeds. J. Ecol. 2008, 96, 569–580. [Google Scholar] [CrossRef]
- Loiselle, B.A.; Ribbens, E.; Vargas, O. Spatial and temporal variation of seed rain in a tropical lowland wet forest. Biotropica 1996, 28, 82–95. [Google Scholar] [CrossRef]
- Jones, F.A.; Chen, J.; Weng, G.J.; Hubbell, S.P. A genetic evaluation of seed dispersal in the Neotropical tree Jacaranda copaia (Bignoniaceae). Am. Nat. 2005, 166, 543–555. [Google Scholar] [CrossRef] [PubMed]
- Denslow, J.S.; Diaz, A.E.G. Seed rain to tree-fall gaps in a Neotropical rainforest. Can. J. For. Res. 1990, 20, 642–648. [Google Scholar] [CrossRef]
- Du, Y.J.; Mi, X.C.; Ma, K.P. Comparison of seed rain and seed limitation between community understory and gaps in a subtropical evergreen forest. Acta Oecol. 2012, 44, 11–19. [Google Scholar] [CrossRef]
- Denslow, J.S. Gap partitioning among tropical rainforest trees. Biotropica 1980, 12, 47–55. [Google Scholar] [CrossRef]
- Gray, A.N.; Spies, T.A. Gap size, within-gap position and canopy structure effects on conifer seedling establishment. J. Ecol. 1996, 84, 635–645. [Google Scholar] [CrossRef]
- Schnitzer, S.A.; Mascaro, J.; Carson, W.P. Treefall gaps and the maintenance of plant species diversity in tropical forests. In Tropical Forest Community Ecology; Carson, W.P., Schnitzer, S.A., Eds.; Blackwell Publishing Ltd.: Oxford, UK, 2008; pp. 196–209. [Google Scholar]
- Zang, R.G.; Zhang, W.Y.; Ding, Y. Seed dynamics in relation to gaps in a tropical montane rainforest of Hainan Island, South China: (I) seed rain. J. Integr. Plant Biol. 2007, 49, 1565–1572. [Google Scholar] [CrossRef]
- Liu, G.X.; Mao, P.S.; Wang, Y.W.; Han, J.G. Effects of adult neighbour and gap size on seedling emergence and early growth of Bromus inermis Leyss. Ecol. Res. 2008, 23, 197–205. [Google Scholar] [CrossRef]
- Li, B.H.; Hao, Z.Q.; Bin, Y.; Zhang, J.; Wang, M. Seed rain dynamics reveals strong dispersal limitation, different reproductive strategies and responses to climate in a temperate forest in northeast China. J. Veg. Sci. 2012, 23, 271–279. [Google Scholar] [CrossRef]
- Rossi, S.; Morin, H.; Laprise, D.; Gionest, F. Testing masting mechanisms of boreal forest species at different stand densities. Oikos 2012, 121, 665–674. [Google Scholar] [CrossRef]
- Yang, K.; Zhu, J.J.; Yan, Q.L.; Zhang, J.X. Soil enzyme activities as potential indicators of soluble organic nitrogen pools in forest ecosystems of Northeast China. Ann. For. Sci. 2012, 69, 795–803. [Google Scholar] [CrossRef]
- Chen, D.K.; Zhou, X.F.; Zhu, N. Natural Secondary Forest-Structure, Function, Dynamics and Management; Northeast Forestry University Press: Harbin, China, 1994. (In Chinese) [Google Scholar]
- Hu, L.L.; Mao, Z.H.; Zhu, J.J.; Liu, Z.G.; Chen, G.H. Classification and ordination of secondary forests in montane zone of eastern Liaoning Province. Acta Ecol. Sin. 2005, 25, 2848–2854, (In Chinese with English Abstract). [Google Scholar]
- Zhu, J.J.; Zhang, G.Q.; Wang, G.G.; Yan, Q.L.; Lu, D.L.; Li, X.F.; Zheng, X. On the size of forest gaps: Can their lower and upper limits be objectively defined? Agric. For. Meteorol. 2015, 213, 64–76. [Google Scholar] [CrossRef]
- Hu, L.L.; Zhu, J.J. Determination of the tridimensional shape of canopy gaps using two hemispherical photographs. Agric. For. Meteorol. 2009, 149, 862–872. [Google Scholar] [CrossRef]
- Thompson, K.; Band, S.R.; Hodgson, J.G. Seed size and shape predict persistence in soil. Funct. Ecol. 1993, 7, 236–241. [Google Scholar] [CrossRef]
- Gang, Q.; Yan, Q.L.; Zhu, J.J. Effects of thinning on early seed regeneration of two broadleaved tree species in larch plantations: Implication for converting pure larch plantations into larch-broadleaved mixed forests. Forestry 2015, 88, 573–585. [Google Scholar] [CrossRef]
- Simpson, R.L.; Leck, M.A.; Parker, V.T. Seed banks: General concepts and methodological issues. In Ecology of Soil Seed Bank; Leck, M.A., Parker, V.T., Simpson, R.L., Eds.; Academic Press: San Diego, CA, USA, 1989; pp. 3–8. [Google Scholar]
- Wu, Z.Y.; Raven, P.H.; Hong, D.Y. Flora of China; Science Press: Beijing, China; Missouri Botanical Garden Press: St. Louis, MO, USA, 2013; pp. 11–52. [Google Scholar]
- Romermann, C.; Tackenberg, O.; Jackel, A.K.; Poschlod, P. Eutrophication and fragmentation are related to species’ rate of decline but not to species rarity: Results from a functional approach. Biodivers. Conserv. 2008, 17, 591–604. [Google Scholar] [CrossRef]
- Sokal, R.R.; Sneath, P.H.A. Principles of Numerical Taxonomy; W.H. Freeman and Company: San Francisco, CA, USA, 1963. [Google Scholar]
- Gurevitch, J.; Chester, S.T. Analysis of repeated measures experiments. Ecology 1986, 67, 251–255. [Google Scholar] [CrossRef]
- Kern, C.C.; Reich, P.B.; Montgomery, R.A.; Strong, T.F. Do deer and shrubs override canopy gap size effects on growth and survival of yellow birch, northern red oak, eastern white pine, and eastern hemlock seedlings? For. Ecol. Manag. 2012, 267, 134–143. [Google Scholar] [CrossRef]
- Puerta-Piñero, C.; Muller-Landau, H.C.; Calderón, O.; Wright, S.J. Seed arrival in tropical forest tree fall gaps. Ecology 2013, 94, 1552–1562. [Google Scholar] [CrossRef]
| Gap Size Category | Forest Gap | Area (m2) | Altitude (m) | Slope (°) | Height of Gap Border Trees (m) | DBH of Gap Border Trees (cm) | Vegetation Composition of Gap Border Trees (%) | Quadrats Along the South-North Direction of Gap | Quadrats Along the East-West Direction of Gap | Canopy Openness (%) | |
|---|---|---|---|---|---|---|---|---|---|---|---|
| In Gap | Under Canopy | ||||||||||
| Large gaps | G1 | 346.5 | 756 | 16 | 16.33 ± 0.65 | 14.36 ± 1.16 | Fraxinus rhynchophylla (43), Acer mono (20), Quercus mongolica (18) | 11 | 10 | 24 | 17 |
| G2 | 323.3 | 760 | 15 | 17.58 ± 0.45 | 16.17 ± 1.98 | Acer mono (37), Fraxinus rhynchophylla (20), Quercus mongolica (18) | 11 | 10 | 22 | 16 | |
| G3 | 286.2 | 743 | 15 | 16.35 ± 1.21 | 18.13 ± 1.32 | Acer mono (35), Quercus mongolica (25), Juglans mandshurica Maxim. (15) | 11 | 10 | 21 | 18 | |
| Medium gaps | G4 | 196.9 | 752 | 17 | 16.47 ± 1.22 | 13.24 ± 1.75 | Quercus mongolica (56), Fraxinus rhynchophylla (21), Acer mono (15) | 9 | 8 | 19 | 15 |
| G5 | 176.8 | 741 | 17 | 17.55 ± 1.07 | 12.54 ± 1.71 | Acer mono (34), Fraxinus rhynchophylla (30), Quercus mongolica (16) | 9 | 8 | 19 | 16 | |
| G6 | 162.3 | 758 | 18 | 16.75 ± 0.63 | 11.77 ± 1.09 | Fraxinus rhynchophylla (31), Acer mono (36), Juglans mandshurica (15) | 9 | 8 | 17 | 13 | |
| G7 | 150.6 | 753 | 19 | 17.01 ± 1.34 | 12.34 ± 2.65 | Acer mono (44), Fraxinus rhynchophylla (21), Tilia tuan Szyszyl. (15) | 9 | 8 | 16 | 13 | |
| Small gaps | G8 | 121.8 | 748 | 16 | 15.73 ± 0.98 | 12.13 ± 1.78 | Fraxinus rhynchophylla (45), Acer mono (24), Juglans mandshurica (15) | 7 | 6 | 15 | 12 |
| G9 | 98.7 | 755 | 16 | 16.15 ± 1.16 | 10.62 ± 1.39 | Acer mono (47), Fraxinus rhynchophylla (25), Ulmus laciniata (Trautv.) Mayr (15) | 7 | 6 | 14 | 11 | |
| G10 | 51.5 | 743 | 18 | 15.58 ± 0.71 | 10.32 ± 1.77 | Quercus mongolica (36), Acer mono (26), Fraxinus rhynchophylla (15) | 7 | 6 | 13 | 11 | |
| Species | Family | Life Form | Propagule Type | Appendages | Abundance Class | Common Dispersal Type for the Primary Dispersal | Viability Rate of Seeds after 1-year Burial |
|---|---|---|---|---|---|---|---|
| Quercus mongolica | Fagaceae | Tree | Nut | None | Rare | Gravity | - |
| Bothrocaryum controversum (Hemsl.) Pojark. | Cornaceae | Tree | Drupe | None | Rare | Gravity | - |
| Fraxinus rhynchophylla | Oleaceae | Tree | Samara | Wing | Rare | Wind | - |
| Fraxinus mandschurica Rupr. | Oleaceae | Tree | Samara | Wing | Common | Wind | 10% [40] |
| Juglans mandshurica | Juglandaceae | Tree | Nut | None | Common | Gravity | 82% [40] |
| Acer mono | Aceraceae | Tree | Samara | Wing | Common | Wind | - |
| Acer pseudosieboldianum (Pax) Kom. | Aceraceae | Tree | Samara | Wing | Rare | Wind | - |
| Phellodendron amurense Rupr. | Rutaceae | Tree | Seed | None | Rare | Gravity | - |
| Betula costata Trautv. | Betulaceae | Tree | Nutlet | Wing | Common | Wind | - |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yan, Q.; Gang, Q.; Zhu, J. Size-Dependent Patterns of Seed Rain in Gaps in Temperate Secondary Forests, Northeast China. Forests 2019, 10, 123. https://doi.org/10.3390/f10020123
Yan Q, Gang Q, Zhu J. Size-Dependent Patterns of Seed Rain in Gaps in Temperate Secondary Forests, Northeast China. Forests. 2019; 10(2):123. https://doi.org/10.3390/f10020123
Chicago/Turabian StyleYan, Qiaoling, Qun Gang, and Jiaojun Zhu. 2019. "Size-Dependent Patterns of Seed Rain in Gaps in Temperate Secondary Forests, Northeast China" Forests 10, no. 2: 123. https://doi.org/10.3390/f10020123
APA StyleYan, Q., Gang, Q., & Zhu, J. (2019). Size-Dependent Patterns of Seed Rain in Gaps in Temperate Secondary Forests, Northeast China. Forests, 10(2), 123. https://doi.org/10.3390/f10020123




