Abstract
We develop a numerical method for solving three-dimensional problems of fluid filtration and absorption in a piecewise homogeneous medium by means of boundary integral equations. This method is applied to a simulation of the lymph flow in a lymph node. The lymph node is considered as a piecewise homogeneous domain containing porous media. The lymph flow is described by Darcy’s law. Taking into account the lymph absorption, we propose an integral representation for the velocity and pressure fields, where the lymph absorption imitates the lymph outflow from a lymph node through a system of capillaries. The original problem is reduced to a system of boundary integral equations, and a numerical algorithm for solving this system is provided. We simulate the lymph velocity and pressure as well as the total lymph flux. The method is verified by comparison with experimental data.
1. Introduction
In this paper, we develop a mathematical model of the filtration flow of a liquid in a piecewise homogeneous porous medium and apply it to the simulation of lymph flow in a lymph node.
The classical model of filtration flow relies on Darcy’s law, which is valid in a linear porous medium. The numerical methods for solving problems of natural convection in porous media go back to the work of Chan et al. [1], where the finite difference method (FDM) was applied. Hickox and Gartling [2,3] used the finite element method (FEM), and Prasad and Kulacki [4] applied the finite volume method (FVM). The finite element and finite volume methods have been widely developed for solving various problems, including those in complex inhomogeneous regions (see, for example, [5,6,7,8]).
The traditional application of the filtration flows models is to the problem of fluid flow in different soils. The typical examples are the water intake problem and the oil flow to oil wells. On the other hand, modeling the fluid convection in living organisms and, in particular, the modeling of the lymph flow has its own specifics. There are just a few works that have considered the lymph flow in the lymph node by means of direct numerical simulations. The fluid flow description by Darcy’s law in inhomogeneous regions (hydraulic conductivity is not constant) is due to Rose et al. [9]. In Moore et al. [10], a filtration domain was piecewise homogeneous, and the filtration flow was viscous, described by the Darcy–Brinkman law. The absorption of lymph into the blood in both papers was characterized by Starling’s law, which relates the divergence of the velocity field to the fluid pressure. In all these models, the FEM approach was used.
If the domain consists of several homogeneous subdomains, then the boundary integral equations method is preferable, and the main goal of this work is to apply this method to the lymph filtration problem. In fact, this method is effective if an integral representation for a solution over domain and subdomains boundaries exists. If the latter is true, then the approach has a number of advantages over the finite difference and finite element methods applied directly in the spatial domain. First, the dimension of the problem being solved is actually reduced. Two-dimensional integral equations (two-dimensional grids) are used instead of three-dimensional grids. Moreover, the differential equations outside the boundary surfaces and conservation laws for the numerical solutions are fulfilled. Finally, the integral representations of unknown functions allow us to calculate derivatives and various functionals without a significant loss of accuracy compared to the accuracy of numerical approximation of the functions themselves.
We can single out a number of papers in which the boundary element method was applied to the modeling of flows in porous media governed by Darcy’s law or the Darcy–Brinkman law [11]. The solution of a viscous fluid filtration problem governed by the Darcy–Brinkman law using the boundary elements method (BEM) was constructed in [12,13]. However, these papers only considered two-dimensional problems in a homogeneous domain with the boundary condition set only on the outer boundary. The method of fundamental solutions (MFS) was presented in [14,15,16] for two-dimensional problems. This method was similar to the boundary element method, except that the solution was a superposition of partial solutions, the functions of source points located outside the flow area. That allowed avoiding singularity in the boundary condition. In this case, the method of boundary integral equations was applied to flows in one homogeneous region.
The application of the boundary integral equations method to the filtration problems in piecewise homogeneous domains was developed in the works of Piven et al., where two-dimensional flows were studied. A systematic presentation of these results can be found in [17]. A three-dimensional model of the fluid filtration was developed by Lifanov et al. [18]. The authors considered the filtration flows in a piecewise homogeneous domain with different types of external and internal boundaries and with the conjugation conditions at the interfaces between media with different properties. However, in all these works the flows with viscosity and absorption were not considered. The latter is important for the correct modeling of lymph flows in a lymph node.
In this paper, we use a simplified lymph node model consisting of two homogeneous domains: the outer one is the subcapsular sinus, the inner one is the T-cell and medullary zones. The inner region is characterized by a significantly higher hydraulic resistance and the presence of blood vessels absorbing lymph [19,20,21,22]. In the lymph nodes, the lymphatic and circulatory systems are conjugated. The overall fluid balance in the lymph node is determined by the pressure field, permeability, and location of blood vessels, in particular, high endothelial venules [20,22]. The structure of the lymph node is shown schematically in Figure 1.
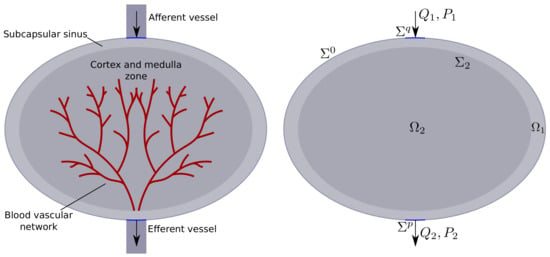
Figure 1.
Simplified schematic representation of the lymph nodes: an external subdomain with low hydraulic resistance and an internal subdomain where lymph is absorbed into the blood vessels.
In the previous works of the authors [23,24], a solution to the problem of fluid filtration in a piecewise homogeneous porous medium governed by the Darcy–Brinkman law was developed. In [23], we implemented the reduction in the boundary value problem in partial derivatives to a system of boundary integral equations and also proved the equivalence of the boundary value problem and a system of boundary integral equations. In [24], we constructed and verified a numerical scheme for solving a system of boundary integral equations with piecewise constant approximations and collocations.
In this paper, we consider the problem of a stationary three-dimensional filtration flow with absorption in a piecewise homogeneous medium. The filtration is described by Darcy’s law, and the absorption is described by Starling’s law. A characteristic feature of such a flow is a nonzero divergence. We propose an integral representation for the velocity and pressure fields of the filtration flow. We also develop numerical schemes and verify the numerical results by comparison with experimental data of the filtration in the lymph node.
2. Mathematical Model
We denote the filtration domain, bounded on the outside by a closed smooth surface . –boundary between the more permeable external subdomain and the less permeable internal subdomain . An example of the computational domain is shown in Figure 1 (on the right).
Fluid filtration in both subdomains is described by Darcy’s law for unknown fields of velocity and pressure p. In the external subdomain, the velocity satisfies the continuity equation. In the internal subdomain, Starling’s law describes the absorption of fluid in capillaries. This model is similar to one given in paper [9]. Thus, the filtration–absorption problem can be written as a system of partial differential equations (1).
The model parameters are —the coefficients of hydraulic conductivity in subdomains , , —the lymph dynamic viscosity, —the blood vessels’ absorption coefficient, A—the surface area of the blood vessels, —the blood pressure, —the mean difference in the oncotic pressure of blood and lymph, and —the oncotic reflectance.
The outer boundary is divided into three parts: the openings of the afferent (inlet) vessels with the given lymph inflow (), the openings of the efferent (output) vessels with the given pressure (), and the impenetrable outer border with zero lymph flow (). . The inner boundary is homogeneous. The pressure and the normal component of the velocity vector must be continuous on this boundary.
We assume that each of the surfaces , is oriented so that the outer side is considered positive. Let , , , be the unit vector of the outer (positive) normal to the surface at the point x. The following boundary conditions are set on the boundaries of the domains:
Here, is the known lymph flux, . is the known pressure on . An additional variable is introduced into the system of boundary conditions: is the unknown lymph flux on the surface , .
For simplicity, we introduce the notation:
We exclude the variable from the system (1) by applying the operator to the first equation of system (1) and substituting the second and third equations.
The pressure in the external subdomain satisfies the Laplace equation.
In the internal subdomain , the pressure satisfies the Helmholtz equation:
If , then .
2.1. Simple and Double Layer Potentials
The particular solutions of the Laplace and Helmholtz equations in the domain are the potentials of a simple and double layer on the boundary of the domain. The simple layer potential for the Helmholtz equation placed on the surface is the function
with , and h is the density of a simple layer potential, which is a function given on the surface .
Moreover, under certain smoothness conditions of the density , the following formula is valid for the boundary values of the gradient of the simple layer potential [25]:
where is the so-called direct value of the gradient of a simple layer potential, determined by the formula
where the integral is considered in the sense of a principal value.
The double layer potential for the Helmholtz equation with density g set on the surface is the following integral operator:
The double layer potential with density , defined by Expression (11) at the points of the space , is a harmonic function on this set. The double layer potential can be extended on the surface from the external and internal domains and (see [25]). The boundary values of the function on the surface are given by the expression:
with being the direct value of the double layer potential obtained directly from Expression (11) at point .
If the double layer potential density satisfies a certain smoothness condition, then the vector field can be continued onto the surface from each of the domains and . For the boundary values of the gradient of the function , the formula is valid [25]:
The direct value of the double layer potential’s gradient at the boundary of a closed surface is the following expression:
The simple or double layer potential for the Laplace equation is a particular case of the Helmholtz potential with .
2.2. Boundary Integral Equations
Following the reasoning given in [23], we put a simple layer potential on the outer surface and a simple layer and double layer potentials on the inner boundary . Thus, the field of velocities and pressures in the regions , are sought in the following form:
Here, in , in , and is the domain indicator function for . The advantage of this representation is that the velocity and pressures are expressed by the same operators in different domains.
The velocity and pressure in the integral representations (15) and (16) satisfy system (1). The integral representations (15) and (16) are substituted into the boundary conditions (2)–(5). We construct a system of integral equations for the unknown densities of the simple and double layer potentials h, g in which each equation corresponds to one of the boundary conditions (2)–(5).
3. Numerical Method
We use the method of piecewise constant approximations and collocations for the numerical solution of the system of integral equations. This method has a low order of accuracy, but it also has such advantages as versatility, simplicity of implementation, and reliability. This is why the method is convenient for conducting computational experiments to test new mathematical models.
The surfaces , , are approximated by a system of triangular and rectangular cells with all the cells’ vertices lying on the surface being approximated.
Each cell has its local basis consisting of a normal vector and two tangential vectors , . We also set a collocation point on each cell , . The example of surface approximation is given in papers [24,26].
We approximate unknown functions by piecewise constant functions set on the approximate surfaces , . We set
for function h, and for function . These qualities are satisfied at the internal points of the cells. The is the cell edge.
3.1. Approximation of the Integral Operators
Let S be one of the surfaces , , and is its approximation. We approximate the direct values of the integral operators , , in collocation points , , and the following formulas:
Here, , and m is the number of subdomains where the direct value of the potentials are calculated. Note that if , the integrals are improper absolutely convergent, and , are singular considered as the principal value.
The direct values of the double layer potential gradient in collocation points are approximated the following way:
To calulate the integral , we separate the singularity and integrate it analytically. We decompose the function into
with . So, the integral is decomposed the following way:
To caluclate , we use the Biot–Savart law [27,28], and integral is improper convergent.
The integrals in expressions , , , and are calculated approximately by the rectangular method. Such a scheme for improper and singular integrals was described in the paper [24].
3.2. Approximation of the Boundary Equations
To calculate the approximate solution, we use the collocation method. Equations (17)–(20) must be fulfilled at collocation points , .
After solving the system of linear Equations (22)–(25), we calculate the velocity and pressure fields in point , by the approximations of Formulas (15) and (16):
with . The integrals in the expressions , , , and are proper in the inner points of the domains , . These integrals are also calculated by the rectangle method.
4. Results
The papers [29,30,31] contained the measurements of the lymph flow, pressure, and protein concentration in the popliteal lymph nodes of dogs. To verify the model, we used the experimental data given in these papers. We constructed a geometrical model approximating the lymph node and set the boundary conditions according to the experimental data. Then, we solved the parametrical optimization problem to fit the experiment.
The fluid exchange between the lymph node and the circulatory system is described by Starling’s law (1), the third equation. The value of parameter was set at 0.88, according to [10]. The experimental data presented in [31] contained the blood pressure and the protein concentration in the blood and the lymph . The oncotic pressure was calculated by the formula , with kg/mol as the molar mass of proteins, as the universal gas constant, and K as the absolute temperature. Given these data, we calculated .
The experiment was carried out on the lymph node with one inlet opening with a fixed flow and one outlet opening with fixed pressure (see Figure 1 on the right). The external subdomain was ellipsoid with radii mm, and mm. The internal subdomain was ellipsoid with radii mm, and mm. The afferent and efferent lymphatic vessels were considered to be cylindrical with the radius mm and the centers and located on the upper and bottom poles of , respectively.
The surfaces and were approximated as follows:
We approximated the velocity functions and on the parts of the boundary and , so that the flow through the openings had a parabolic profile. The pressure on the outlet was assumed to be constant.
The function on part of surface was approximated in a similar way.
The known experiment did not contain the complete description of all the parameters that arise in the mathematical formulation of the problem, since it is extremely difficult to calculate such measurements. Therefore we used the following approach: part of the experimental data were used as the initial parameters of model, and the other part of the data were used for validation of the model. We denoted the model parameters . We approximated the boundary integral Equations (17)–(20) by a numerical scheme (22)–(25) and solved the corresponding linear system with parameters and boundary conditions (28) with the values and taken from paper [31] (Table 1).

Table 1.
Parameters providing optimal approximation to the experimental data.
To compare the mathematical model prediction with the experimental data values of the outlet flow and inlet pressure , we calculated the pressure on and the flow on using the following formulas:
The article [31] provided data on several animals (greyhound dogs) with a number of experimental values for each animal. Assuming that different animals corresponded to different values of the parameters, we calculated the optimal values of the parameters that minimized the functional for all experiments for each animal.
Here, and were the i-th experiment data values, and and were calculated by the numerical model for the i-th experiment boundary condition values. To solve the optimization problem, we used the Nelder–Mead method.
The values of the functional as well as the optimal values of the parameters are given in Table 1. In the experiment, the value of varied, while had a small variation, and was constant. So, the comparison of the simulation results with the experimental data can be represented as the dependence of and on (see Figure 2 and Figure 3).

Figure 2.
Pressure values at the inlet opening depending on pressure at the outlet opening. IE—integral equation model, Exp—experimental data.

Figure 3.
Flow values at the outlet opening depending on pressure at the outlet opening. IE—integral equation model, Exp—experimental data.
The value of the deviation of the results from the experiment demonstrates that the model accurately approximated the data for four animals.
5. Discussion
In this paper, we proposed a model of the fluid filtration flow in a piecewise homogeneous domain containing porous medium and applied it to the simulation of the lymph flow in the lymph node. We also assumed that the fluid was absorbed in the inner subdomain. A mixed boundary condition was set on the outer boundary of the domain (the pressure was set on the outlet opening, the flow was set on the inlet opening, and the impermeability condition was set on the remaining part of the boundary). At the interface between the media, the conjugation conditions were set (the continuity of the pressure and the normal component of the fluid velocity). An integral representation of the velocity and pressure fields in terms of the potentials of a simple layer and double layer at the domain boundary was proposed. The boundary value problem was reduced to a system of boundary integral equations for unknown densities of simple layer and double layer potentials.
A numerical solution scheme based on the method of piecewise constant approximations and collocations was constructed for the system of integral equations. The solution of the approximated system of boundary integral equations allowed calculation of the velocity and pressure fields in any inner point of the domain.
The model predictions were also compared with the existing experimental data on lymph filtration in the lymph node. An agreement between the values of the total flow characteristics obtained by numerical simulation and the experimental data was reached. The analysis of the obtained test results, in particular, showed the applicability of the developed numerical model for the simulation of the considered class of filtration flows.
The results of this work were used for the development of an artificial neural network model describing the lymph node drainage function, which was described in [32].
Author Contributions
Conceptualization, A.S. and R.T.; methodology, A.S.; software, R.T.; validation, R.T.; investigation, R.T.; writing—original draft preparation, R.T.; writing—review and editing, A.S.; visualization, R.T.; supervision, A.S. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the Moscow Center for Fundamental and Applied Mathematics at INM RAS (agreement with the Ministry of Education and Science of the Russian Federation No. 075-15-2019-1624) and partially funded by the Russian Science Foundation (grant number 18-11-00171).
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Data is contained within the article.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Chan, B.; Ivey, C.; Barry, J. Natural convection in enclosed porous media with rectangular boundaries. Heat Transf. 1970, 92, 21–27. [Google Scholar] [CrossRef]
- Hickox, C.; Gartling, D. A numerical study of natural convection in a horizontal porous layer subjected to an end-to-end temperature difference. Heat Transf. 1981, 103, 797–802. [Google Scholar] [CrossRef]
- Gartling, D.K.; Hickox, C.E. MARIAH: A Finite-Element Computer Program for Incompressible Porous Flow Problems. Theoretical Background; NASA STI/Recon Technical Report N; NASA: Washington, DC, USA, 1982; Volume 83, p. 22562. [Google Scholar]
- Prasad, V.; Kulacki, F. Convective heat transfer in a rectangular porous cavity—effect of aspect ratio on flow structure and heat transfer. Heat Transf. 1984, 106, 158–165. [Google Scholar] [CrossRef]
- Chen, Z.; Huan, G.; Ma, Y. Computational Methods for Multiphase Flows in Porous Media; SIAM: Philadelphia, PA, USA, 2006. [Google Scholar]
- Lacroix, S.; Vassilevski, Y.; Wheeler, J.; Wheeler, M. Iterative solution methods for modeling multiphase flow in porous media fully implicitly. SIAM J. Sci. Comput. 2003, 25, 905–926. [Google Scholar] [CrossRef]
- Nikitin, K.; Novikov, K.; Vassilevski, Y. Nonlinear finite volume method with discrete maximum principle for the two-phase flow model. Lobachevskii J. Math. 2016, 37, 570–581. [Google Scholar] [CrossRef]
- Terekhov, K.M.; Vassilevski, Y.V. Finite volume method for coupled subsurface flow problems, I: Darcy problem. J. Comput. Phys. 2019, 395, 298–306. [Google Scholar] [CrossRef]
- Cooper, L.J.; Heppell, J.P.; Clough, G.F.; Ganapathisubramani, B.; Roose, T. An image-based model of fluid flow through lymph nodes. Bull. Math. Biol. 2016, 78, 52–71. [Google Scholar] [CrossRef] [PubMed]
- Jafarnejad, M.; Woodruff, M.C.; Zawieja, D.C.; Carroll, M.C.; Moore, J., Jr. Modeling lymph flow and fluid exchange with blood vessels in lymph nodes. Lymphat. Res. Biol. 2015, 13, 234–247. [Google Scholar] [CrossRef] [PubMed]
- Brinkman, H.C. A calculation of the viscous force exerted by a flowing fluid on a dense swarm of particles. Flow Turbul. Combust. 1949, 1, 27–34. [Google Scholar] [CrossRef]
- Mardanov, R.; Zaripov, S.; Sharafutdinov, V.; Dunnett, S.J. A Stokes–Brinkman model of the fluid flow in a periodic cell with a porous body using the boundary element method. Eng. Anal. Bound. Elem. 2018, 88, 54–63. [Google Scholar] [CrossRef]
- Nishad, C.S.; Chandra, A.; Karmakar, T.; Sekhar, G.R. A non-primitive boundary element technique for modeling flow through non-deformable porous medium using Brinkman equation. Meccanica 2018, 53, 2333–2352. [Google Scholar] [CrossRef]
- Karageorghis, A.; Lesnic, D.; Marin, L. The method of fundamental solutions for Brinkman flows. Part I. Exterior domains. J. Eng. Math. 2021, 126, 19. [Google Scholar] [CrossRef]
- Leiderman, K.; Olson, S.D. Swimming in a two-dimensional Brinkman fluid: Computational modeling and regularized solutions. Phys. Fluids 2016, 28, 021902. [Google Scholar] [CrossRef]
- Martins, N.F.; Rebelo, M. Meshfree methods for nonhomogeneous Brinkman flows. Comput. Math. Appl. 2014, 68, 872–886. [Google Scholar] [CrossRef]
- Piven, V. Mathematical Models of Fluid Filtration; Orel State University: Oryol, Russia, 2015. (In Russian) [Google Scholar]
- Lifanov, I.; Piven, V.; Stavtsev, S. Mathematical modelling of the three-dimensional boundary value problem of the discharge of the well system in a homogeneous layer. Russ. J. Numer. Anal. Math. Model. 2002, 17, 99–112. [Google Scholar] [CrossRef]
- Kumar, V.; Scandella, E.; Danuser, R.; Onder, L.; Nitschké, M.; Fukui, Y.; Halin, C.; Ludewig, B.; Stein, J.V. Global lymphoid tissue remodeling during a viral infection is orchestrated by a B cell–lymphotoxin-dependent pathway. Blood 2010, 115, 4725–4733. [Google Scholar] [CrossRef] [PubMed]
- Kelch, I.D.; Bogle, G.; Sands, G.B.; Phillips, A.R.; LeGrice, I.J.; Dunbar, P.R. Organ-wide 3D-imaging and topological analysis of the continuous microvascular network in a murine lymph node. Sci. Rep. 2015, 5, 16534. [Google Scholar] [CrossRef] [PubMed]
- Harisinghani, M.G. Atlas of Lymph Node Anatomy; Springer Science & Business Media: Berlin/Heidelberg, Germany, 2012. [Google Scholar]
- Wiig, H.; Swartz, M.A. Interstitial fluid and lymph formation and transport: Physiological regulation and roles in inflammation and cancer. Physiol. Rev. 2012, 92, 1005–1060. [Google Scholar] [CrossRef] [PubMed]
- Setukha, A.; Tretyakova, R.; Bocharov, G. Methods of potential theory in a filtration problem for a viscous fluid. Differ. Equat. 2019, 55, 1182–1197. [Google Scholar] [CrossRef]
- Setukha, A.; Tretyakova, R. Numerical Solution of a Stationary Filtration Problem of Viscous Fluid in a Piecewise Homogeneous Porous Medium by Applying the Boundary Integral Equation Method. Comput. Math. Math. Phys. 2020, 60, 2076–2093. [Google Scholar] [CrossRef]
- Colton, D.; Kress, R. Integral Equation Methods in Scattering Theory; Wiley: New York, NY, USA, 1983. [Google Scholar]
- Tretiakova, R. Filtration of Viscous Fluid in Homogeneous Domain with Mixed Boundary Condition. Lobachevskii J. Math. 2021, 42, 1465–1474. [Google Scholar] [CrossRef]
- Lifanov, I.K. Singular Integral Equations and Discrete Vortices; VSP: Utrecht, The Netherlands, 1996. [Google Scholar]
- Katz, J.; Plotkin, A. Low-Speed Aerodynamics; Cambridge University Press: Cambridge, MA, USA, 2001; Volume 13. [Google Scholar]
- Adair, T.; Moffatt, D.; Paulsen, A.; Guyton, A. Quantitation of changes in lymph protein concentration during lymph node transit. Am. J. Physiol. Heart Circ. Physiol. 1982, 243, H351–H359. [Google Scholar] [CrossRef] [PubMed]
- Adair, T.; Guyton, A. Modification of lymph by lymph nodes. II. Effect of increased lymph node venous blood pressure. Am. J. Physiol. Heart Circ. Physiol. 1983, 245, H616–H622. [Google Scholar] [CrossRef]
- Adair, T.; Guyton, A. Modification of lymph by lymph nodes. III. Effect of increased lymph hydrostatic pressure. Am. J. Physiol. Heart Circ. Physiol. 1985, 249, H777–H782. [Google Scholar] [CrossRef] [PubMed]
- Tretiakova, R.; Setukha, A.; Savinkov, R.; Grebennikov, D.; Bocharov, G. Mathematical Modeling of Lymph Node Drainage Function by Neural Network. Mathematics 2021, 9, 3093. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).