Evaluation of the Antimicrobial Activity of Different Antibiotics Enhanced with Silver-Doped Hydroxyapatite Thin Films
Abstract
:1. Introduction
2. Materials and Methods
2.1. Tetracycline or Ciprofloxacin Embedded in Silver-Doped Hydroxyapatite Thin Films
2.2. Characterization Methods
2.3. Preliminary Observations of Antimicrobial Properties of the HAp, T-HAp, C-HAp, Ag:HAp, T-Ag:HAp and C-Ag:HAp Tetracycline (T) and Ciprofloxacin (C)
2.4. Antimicrobial Activity of the HAp, T-HAp, C-HAp, Ag:HAp, T-Ag:HAp and C-Ag:HAp, Tetracycline (T) and Ciprofloxacin (C)
2.5. Statistical Analysis
3. Results and Discussion
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Popa, C.L.; Prodan, A.M.; Ciobanu, C.S.; Predoi, D. The tolerability of dextran-coated iron oxide nanoparticles during in vivo observation of the rats. Gen. Physiol. Biophys. 2016, 35, 299–310. [Google Scholar] [CrossRef] [PubMed]
- Rajendran, A.; Barik, R.C.; Natarajan, D.; Kiran, M.S.; Pattanayak, D.K. Synthesis, phase stability of hydroxyapatite–silver composite with antimicrobial activity and cytocompatability. Ceram. Internat. 2014, 40, 10831–10838. [Google Scholar] [CrossRef]
- Jarcho, M. Calcium phosphate ceramics as hard tissue prosthetics. Clin. Orthop. Rel. Res. 1981, 157, 259–278. [Google Scholar] [CrossRef]
- Supova, M.; Suchy, T. Bio-nanoceramics and Bio-nanocomposites. In Handbook of Nanoceramic and Nanocomposite Coatings and Materials; Elsevier: Amsterdam, The Netherlands, 2015. [Google Scholar]
- Akhavan, A.; Sheikh, N.; Khoylou, F.; Naimian, F.; Ataeivarjovi, E. Synthesis of antimicrobial silver/hydroxyapatite nanocomposite by gamma irradiation. Radiat. Phys. Chem. 2014, 98, 46–50. [Google Scholar] [CrossRef]
- Hench, L.L. Bioceramics. J. Am. Ceram. Soc. 1998, 81, 1705–1728. [Google Scholar] [CrossRef]
- Woodward, J.R.; Hilldore, A.J.; Lan, S.K.; Park, C.J.; Morgan, A.W.; Eurell, J.A.C.; Clark, S.G.; Wheeler, M.B.; Jamison, R.D.; Johnson, A.J.W. The mechanical properties and osteoconductivity of hydroxyapatite bone scaffolds with multi-scale porosity. Biomaterials 2007, 28, 45–54. [Google Scholar] [CrossRef] [PubMed]
- Lin, L.; Chow, K.L.; Leng, Y. Study of hydroxyapatite osteoinductivity with an osteogenic differentiation of mesenchymal stem cells. J. Biomed. Mater. Res. Part A 2009, 89, 326–335. [Google Scholar] [CrossRef] [PubMed]
- Dorozhki, S.V. Calcium orthophosphates in dentistry. J. Mater. Sci. Mater. Med. 2013, 24, 1335–1363. [Google Scholar] [CrossRef] [PubMed]
- Zakharov, N.A.; Polunina, I.A.; Polunin, K.E.; Rakitina, N.M.; Kochetkova, E.I.; Sokolova, N.P.; Kalinnikov, V.T. Calcium hydroxyapatite for medical applications. Inorg. Mater. 2004, 40, 641–648. [Google Scholar] [CrossRef]
- Hara, T.; Hayashi, K.; Nakashima, Y.; Kanemaru, T.; Iwamoto, Y. The effect of hydroxyapatite coating on the bonding of bone to titanium implants in the femora of ovariectomisedrats. J. Bone Jt. Surg. 1999, 81, 705–709. [Google Scholar] [CrossRef]
- Dorozhkin, S.V. Calcium orthophosphate deposits: Preparation, properties and biomedical applications. Mater. Sci. Eng. C 2015, 55, 272–326. [Google Scholar] [CrossRef] [PubMed]
- Tredwin, C.J.; Georgiou, G.; Kim, H.W.; Knowles, J.C. Hydroxyapatite, fluor-hydroxyapatite and fluorapatite produced via the sol-gel method: Bonding to titanium and scanning electron microscopy. Dent. Mater. 2013, 29, 521–529. [Google Scholar] [CrossRef] [PubMed]
- Oron, A.; Agar, G.; Oron, U.; Stein, A. Enhancement of bony in-growth to metal implants by combining controlled hydroxyapatite coating and heat treatment. J. Biomed. Mater. Res. 2012, 100, 1668–1672. [Google Scholar] [CrossRef] [PubMed]
- Ripamonti, U.; Roden, L.C.; Renton, L.F. Osteoinductive hydroxyapatite-coated titanium implants. Biomaterials 2012, 33, 3813–3823. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Eliaz, N.; Xiang, Z.; Hsu, H.P.; Spector, M.; Hobbs, L.W. Early bone apposition in vivo on plasma-sprayed and electrochemically deposited hydroxyapatite coatings on titanium alloy. Biomaterials 2006, 27, 4192–4203. [Google Scholar] [CrossRef] [PubMed]
- Baþtan, F.E.; Özbek, Y.Y. Producing antibacterial silver-doped Hydroxyapatite powders with chemical precipitation and reshaping in a spray dryer. Mater. Technol. 2013, 47, 431–434. [Google Scholar]
- Ciobanu, C.S.; Popa, C.L.; Predoi, D. Sm:HAp Nanopowders Present Antibacterial Activity against Enterococcus faecalis. J. Nanomater. 2014, 2014. [Google Scholar] [CrossRef]
- Ciobanu, C.S.; Popa, C.L.; Predoi, D. Cerium doped hydroxyapatite nanoparticles synthesized by coprecipitation method. J. Serb. Chem. Soc. 2016, 81, 1–14. [Google Scholar] [CrossRef]
- Ciobanu, C.S.; Iconaru, S.L.; Le Coustumer, P.; Predoi, D. Vibrational investigations of silver-doped hydroxyapatite with antibacterial properties. J. Spectrosc. 2013, 2013. [Google Scholar] [CrossRef]
- Supova, M. Substituted hydroxyapatites for biomedical applications: A review. Ceram. Int. 2015, 41, 9203–9231. [Google Scholar] [CrossRef]
- Singh, M.; Singh, S.; Prasad, S.; Gambhir, I.S. Nanotechnology in medicine and antibacterial effect of silver nanoparticles. Dig. J. Nanomater. Biostruct. 2008, 3, 115–122. [Google Scholar]
- Gross, K. Hydroxyapatite–Synthesis of hydroxyapatite powders. J. Biomed. Mater. Res. 2002, 62, 228–236. [Google Scholar]
- Stanic, V.; Janackovic, D.; Dimitrijevic, S.; Tanaskovic, S.B.; Mitric, M.; Pavlovic, M.S.; Krstic, A.; Jovanovic, D.; Raičević, S. Synthesis of antimicrobial monophase silver-doped hydroxyapatite nanopowders for bone tissue engineering. Appl. Surf. Sci. 2011, 257, 4510–4518. [Google Scholar] [CrossRef]
- Harris, L.; Richards, R. Staphylococci and implant surfaces: A review. Injury 2006, 37, S3–S14. [Google Scholar] [CrossRef] [PubMed]
- Ciobanu, C.S.; Iconaru, S.L.; Popa, C.L.; Motelica-Heino, M.; Predoi, D. Evaluation of samarium doped hydroxyapatite, ceramics for medical application: Antimicrobial activity. J. Nanomater. 2015, 2015. [Google Scholar] [CrossRef]
- Leypold, C.F.; Reiher, M.; Brehm, G.; Schmitt, M.O.; Schneider, S.; Matousek, P.; Towrieb, M. Tetracycline and derivatives—Assignment of IR and Raman spectra via DFT calculations. Phys. Chem. Chem. Phys. 2003, 5, 1149–1157. [Google Scholar] [CrossRef]
- Bhongade, B.; Talath, S.; Dhaneshwar, S. A validated method for the quantitation of ciprofloxacin hydrochloride using diffuse reflectance infrared fourier transform spectroscopy. Int. J. Spectrosc. 2014, 2014. [Google Scholar] [CrossRef]
- Ciobanu, C.S.; Andronescu, E.; Predoi, D. BET and XRD studies on the hydroxyapatite and Europium doped hydroxyapatite. J. Optoelectron. Adv. Mater. 2011, 13, 821–824. [Google Scholar]
- Ciobanu, C.S.; Iconaru, S.L.; Pasuk, I.; Vasile, B.S.; Lupu, A.R.; Hermenean, A.; Dinischiotu, A.; Predoi, D. Structural properties of silver doped hydroxyapatite and their biocompatibility. Mater. Sci. Eng. C 2013, 33, 1395–1402. [Google Scholar] [CrossRef] [PubMed]
- Ciobanu, C.S.; Massuyeau, F.; Constantin, L.V.; Predoi, D. Structural and physical properties of antibacterial Ag-doped nano-hydroxyapatite synthesized at 100 °C. Nanoscale Res. Lett. 2011. [Google Scholar] [CrossRef] [PubMed]
- Iconaru, S.L.; Chapon, P.; Le Coustumer, P.; Predoi, D. Antimicrobial activity of thin solid films of silver doped hydroxyapatite prepared by sol-gel method. Sci. World J. 2014, 2014. [Google Scholar] [CrossRef] [PubMed]
- Wilkea, M.; Teichert, G.; Gemmab, R.; Pundt, A.; Kirchheim, R.; Romanus, H.; Schaa, P. Glow discharge optical emission spectroscopy for accurate and well resolved analysis of coatings and thin films. Thin Solid Films 2011, 520, 1660–1667. [Google Scholar] [CrossRef]
- Malherbe, J.; Martinez, H.; Fernández, B.; Pécheyran, C.; Donard, O.F.X. The effect of glow discharge sputtering on the analysis of metal oxide films. Spectrochim. Acta Part B Atomic Spectrosc. 2009, 64, 155–166. [Google Scholar] [CrossRef]
- Barinov, S.M.; Rau, J.V.; Cesaro, S.N.; Durisin, J.; Fadeeva, I.V.; Ferro, D.; Medvecky, L.; Trionfetti, G. Carbonate release from carbonated hydroxyapatite in the wide temperature rage. J. Mater. Sci. Mater. Med. 2006, 17, 597–604. [Google Scholar] [CrossRef] [PubMed]
- Hussain, M.A.; Shah, A.; Jantan, I.; Tahir, M.N.; Shah, M.R.; Ahmed, R.; Bukhari, S.N.A. One pot light assisted green synthesis, storage and antimicrobial activity of dextran stabilized silver nanoparticles. J. Nanobiotechnol. 2014, 12, 1–6. [Google Scholar] [CrossRef] [PubMed]
- Yuan, Q.; Qin, C.; Wu, J.; Xu, A.; Zhang, Z.; Liao, J.; Lin, S.; Ren, X.; Zhang, P. Synthesis and characterization of Cerium-doped hydroxyapatite/polylactic acid composite coatings on metal substrates. Mater. Chem. Phys. 2016, 182, 365–371. [Google Scholar] [CrossRef]
- Koutsopoulos, S. Synthesis and characterization of hydroxyapatite crystals: A review study on the analytical methods. J. Biomed. Mater. Res. 2002, 62, 600–612. [Google Scholar] [CrossRef] [PubMed]
- Fowler, B.O. Infrared studies of apatites. I. Vibrational assignments for calcium, strontium, and barium hydroxyapatites utilizing isotopic substitution. Inorg. Chem. 1974, 13, 194–207. [Google Scholar] [CrossRef]
- Klee, W.E.; Engel, G. Infrared spectra of the phosphate ions in various apatites. J. Inorg. Nucl. Chem. 1970, 32, 1837–1843. [Google Scholar] [CrossRef]
- Joris, S.J.; Amberg, C.H. Nature of deficiency in nonstoichiometric hydroxyapatites. II. Spectroscopic studies of calcium and strontium hydroxyapatites. J. Phys. Chem. 1971, 75, 3172–3178. [Google Scholar] [CrossRef]
- Baddiel, C.B.; Berry, E.E. Spectra-structure correlations in hydroxyapatite and fluorapatite. Spectrochim. Acta A 1966, 22, 1407–1416. [Google Scholar] [CrossRef]
- Berzina-Cimdina, L.; Borodajenko, N. Research of calcium phosphates using fourier transform infrared spectroscopy. In Infrared Spectroscopy-Materials Science, Engineering and Technology; Theophile, T., Ed.; InTech: Ocala, FL, USA, 2012. [Google Scholar]
- Gunasekaran, S.; Varadhan, S.R.; Karunanidhi, N. Qualitative analysis on the infrared bands of tetracycline and ampicillin. Proc. Indian Natl. Sci. Acad. Part A Phys. Sci. 1996, 62, 309–316. [Google Scholar]
- Socrates, G. Infrared Characteristic Group Frequencies–Tables and Charts, 2nd ed.; John Wiley & Sons: Chichester, UK, 1994; p. 366. [Google Scholar]
- Sahoo, S.; Chakraborti, C.K.; Behera, P.K. Spectroscopic investigations of a ciprofloxacin/hpmc mucoadhesive suspension. Int. J. Appl. Pharm. 2012, 4, 1–8. [Google Scholar]
- Trigoulet, N.; Hashimoto, T.; Molchan, I.S.; Skeldon, P.; Thompson, G.E.; Tempez, A.; Chapon, P. Surface topography effects on glow discharge depth profiling analysis. Surf. Interface Anal. 2010, 42, 328–333. [Google Scholar] [CrossRef]
- Groza, A.; Surmeian, A.; Diplasu, C.; Luculescu, C.; Chapon, P.; Tempez, A.; Ganciu, M. Physico-chemical processes occurring during polymerization of liquid polydimethylsiloxane films on metal substrates under atmospheric pressure air corona discharges. Surf. Coat. Technol. 2012, 212, 145–151. [Google Scholar] [CrossRef]
- Nath, S.; Kalmodia, S.; Basu, B. Densification, phase stability and in vitro biocompatibility property of hydroxyapatite-10 wt % silver composites. J. Mater. Sci. Mater. Med. 2010, 21, 1273–1287. [Google Scholar] [CrossRef] [PubMed]
- Kim, T.N.; Feng, Q.L.; Kim, J.O.; Wu, J.; Wang, H.; Chen, G.C.; Cui, F.Z. Antimicrobial effects of metal ions (Ag+, Cu2+, Zn2+) in hydroxyapatite. J. Mater. Sci. Mater. Med. 1998, 9, 129–134. [Google Scholar] [CrossRef] [PubMed]
- Zeng, Y.X.; Pei, X.B.; Yang, S.Y.; Qin, H.; Cai, H.; Hu, S.; Sui, L.; Wan, Q.; Wang, J. Graphene oxide/hydroxyapatite composite coatings fabricated by electrochemical deposition. Surf. Coat. Technol. 2016, 286, 72–79. [Google Scholar] [CrossRef]
- Karami, N.; Nowrouzian, F.; Adlerberth, I.; Wold, A.E. Tetracycline resistance in escherichia coli and persistence in the infantile colonic microbiota. Antimicrob. Agents Chemother. 2006, 50, 156–161. [Google Scholar] [CrossRef] [PubMed]
- Draenert, K.; Wiese, F.G.; Garde, U.; Draenert, Y.; Helber, U.; Boerner, M. Synthetische knochenersatzwerkstoffe auf HA-und TCP-Basis. Trauma Berufskrankh. 2001, 3, 293–300. [Google Scholar] [CrossRef]
- Mocanu, A.; Furtos, G.; Rapuntean, S.; Horovitz, O.; Flore, C.; Garbo, C.; Danisteanua, A.; Rapunteanc, G.; Prejmerean, C.; Tomoaia-Cotisel, M. Synthesis, characterization and antimicrobial effects of composites based on multisubstituted hydroxyapatite and silver nanoparticles. Appl. Surf. Sci. 2014, 298, 225–235. [Google Scholar] [CrossRef]
- Katsikogianni, M.; Missirilis, Y.F. Concise review of mechanisms of bacterial adhesion to biomaterials and of techniques used in estimating bacteria–material interactions. Eur. Cells Mater. 2004, 8, 37–57. [Google Scholar]
- Popa, C.L.; Deniaud, A.; Michaud-Soret, I.; Guégan, R.; Motelica-Heino, M.; Predoi, D. Structural and biological assessment of zinc doped hydroxyapatite nanoparticles. J. Nanomater. 2016, 2016. [Google Scholar] [CrossRef]
- Kotoka, R.; Yamoah, N.K.; Mensah-Darkwa, K.; Moses, T.; Kumar, D. Electrochemical corrosion behavior of silver doped tricalcium phosphate coatings on magnesium for biomedical application. Surf. Coat. Technol. 2016, 292, 99–109. [Google Scholar] [CrossRef]
- Kim, J.S.; Kuk, E.; Yu, K.N.; Kim, J.H.; Park, S.J.; Lee, H.J.; Kim, S.H.; Park, Y.K.; Park, Y.H.; Hwang, C.Y.; et al. Antimicrobial effects of silver nanoparticles. Nanomed. Nanotechnol. Biol. Med. 2007, 3, 95–101. [Google Scholar] [CrossRef] [PubMed]
- Chaki, T.K.; Wang, P.E. Densification and strengthening of silver-reinforced hydroxyapatite-matrix composite prepared by sintering. J. Mater. Sci. Mater. Med. 1994, 5, 533–542. [Google Scholar] [CrossRef]
- Asmus, S.M.F.; Sakakuoorand, S.; Pezzotti, G. Hydroxyapatite toughened by silver inclusions. J. Comput. Mater. 2003, 37, 2117–2129. [Google Scholar] [CrossRef]
- Feng, Q.L.; Kim, T.N.; Wu, J.; Park, E.S.; Kim, J.O.; Lim, D.Y.; Cui, F.Z. Antibacterial effects of Ag-HAp thin films on alumina substrates. Thin Solid Films 1998, 335, 214–219. [Google Scholar] [CrossRef]
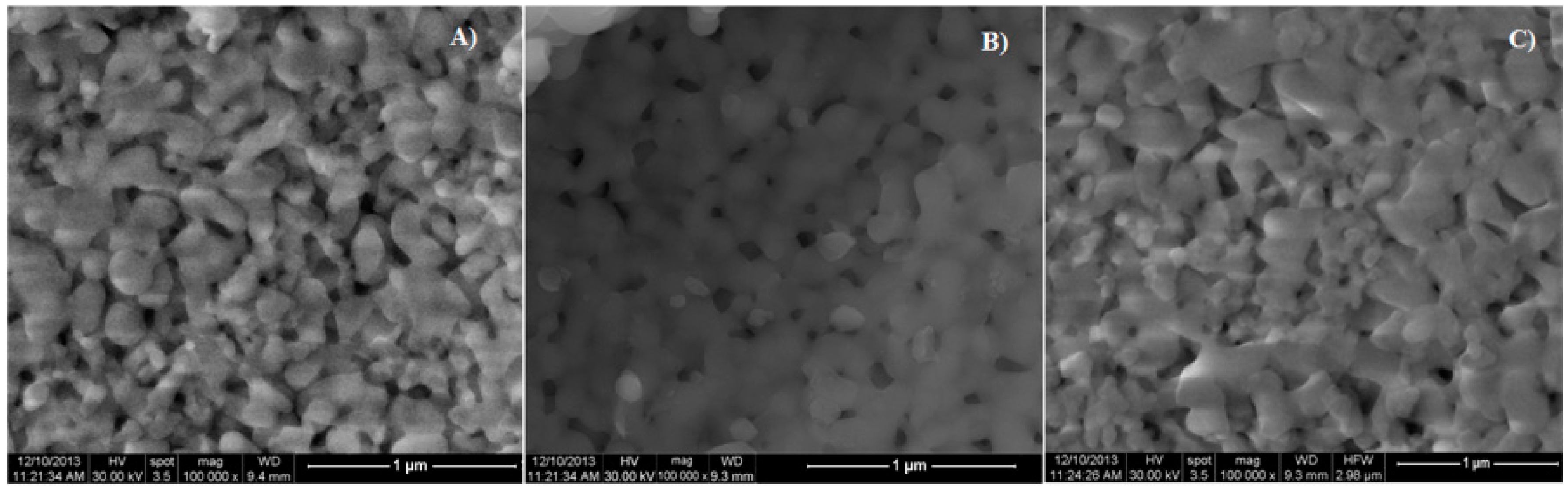
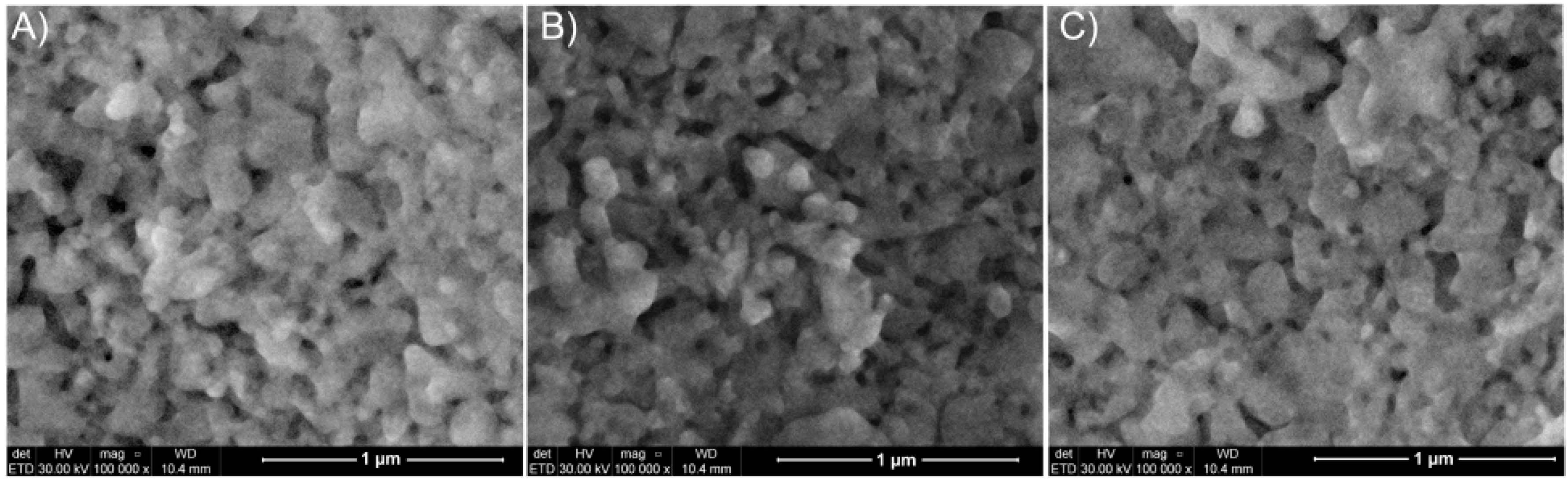
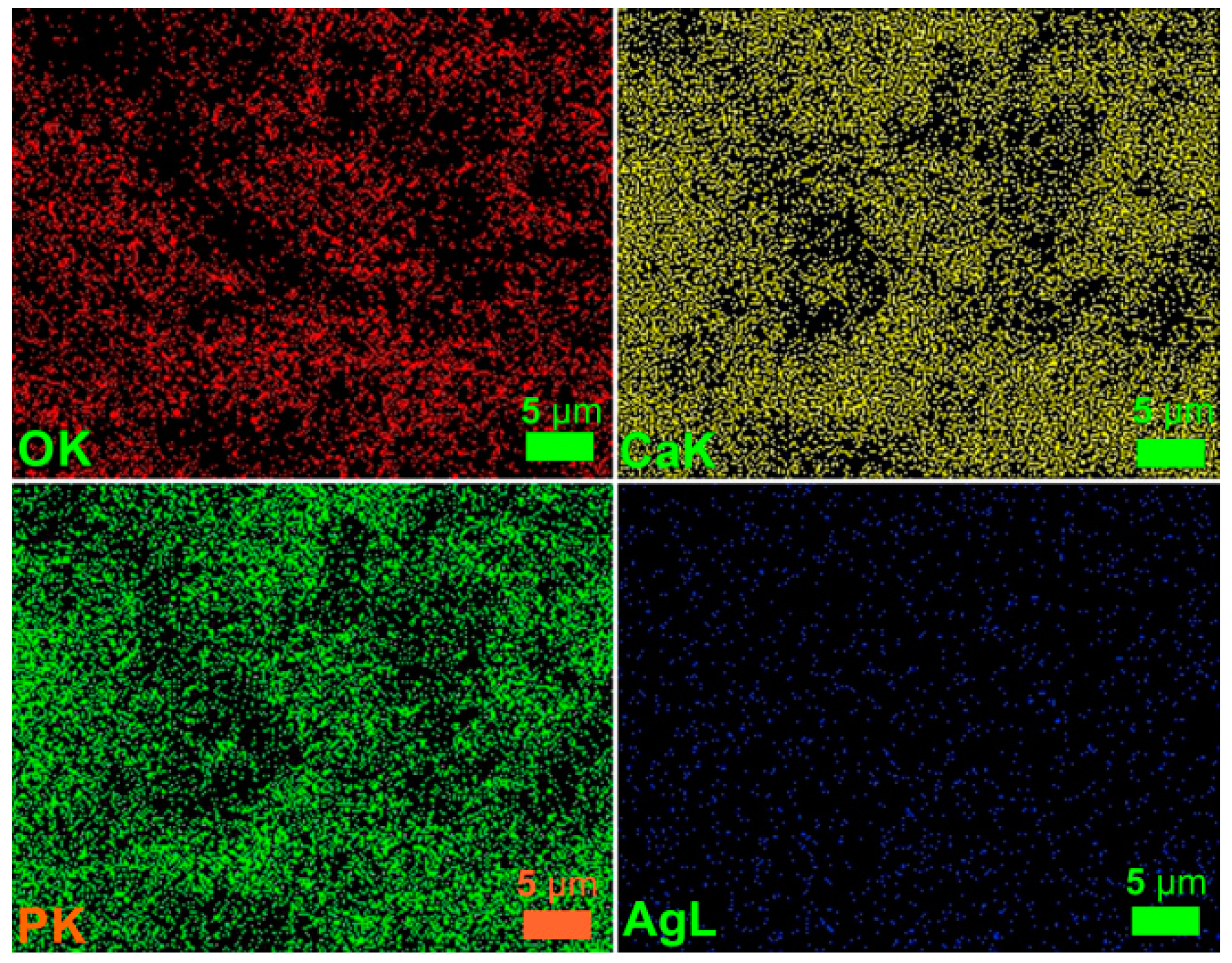
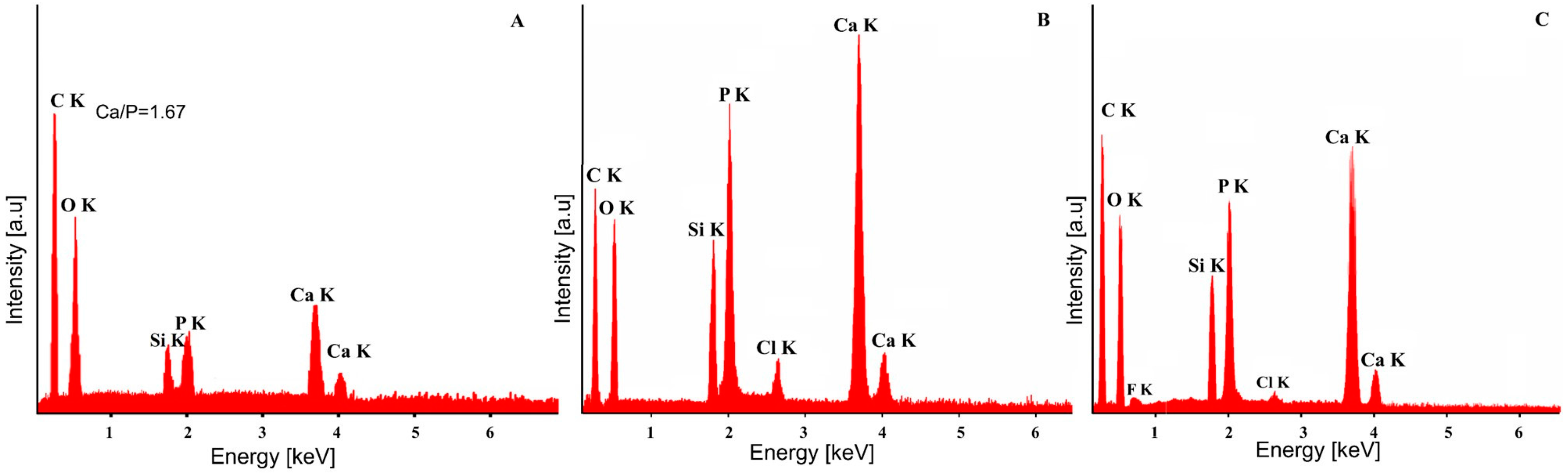
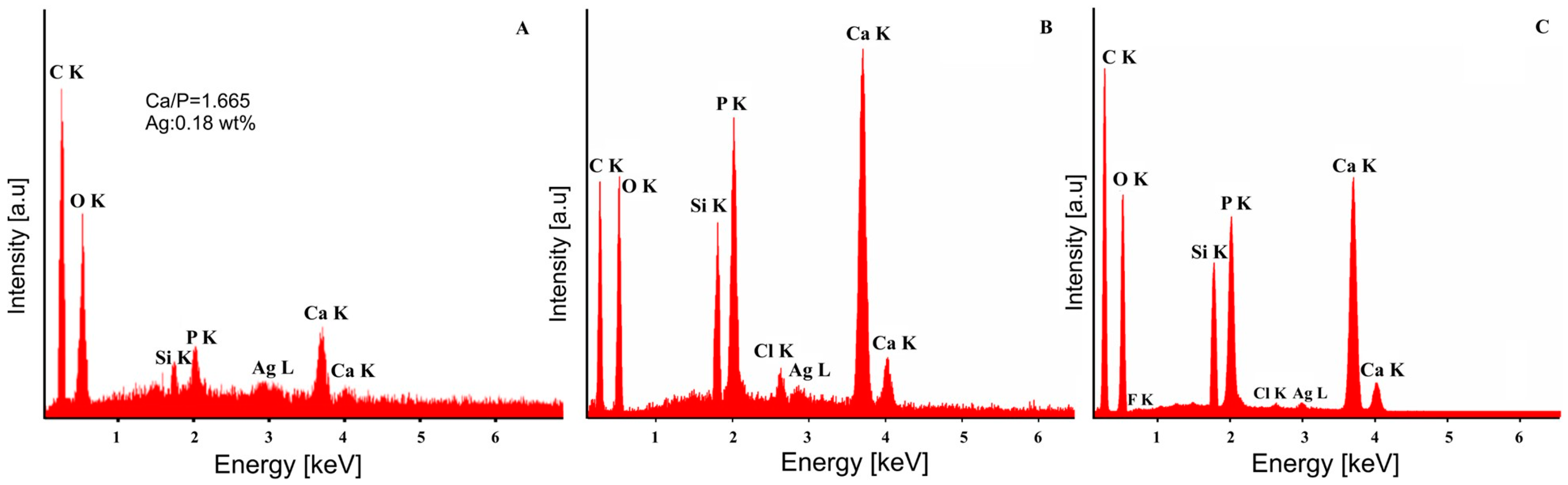
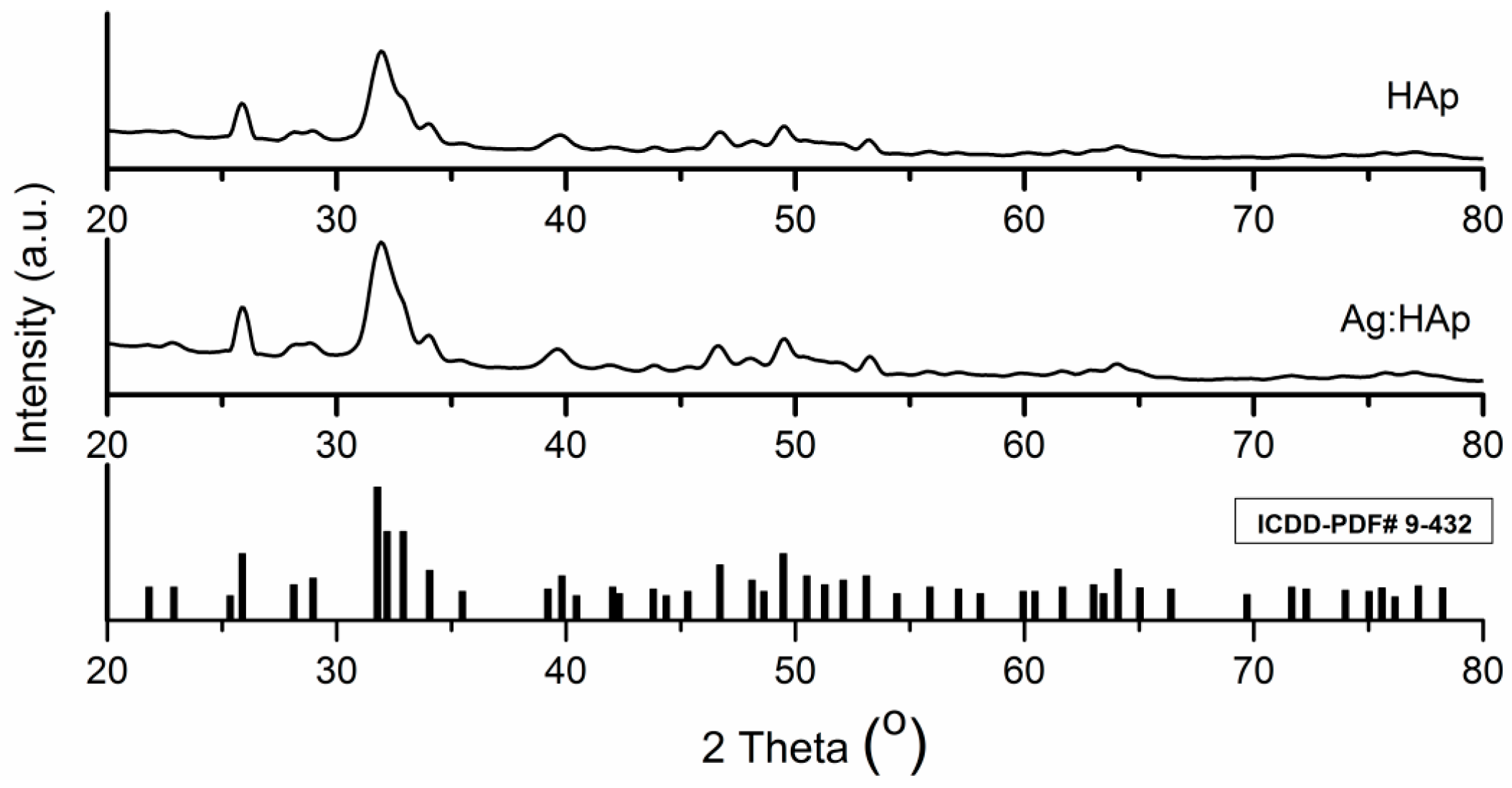
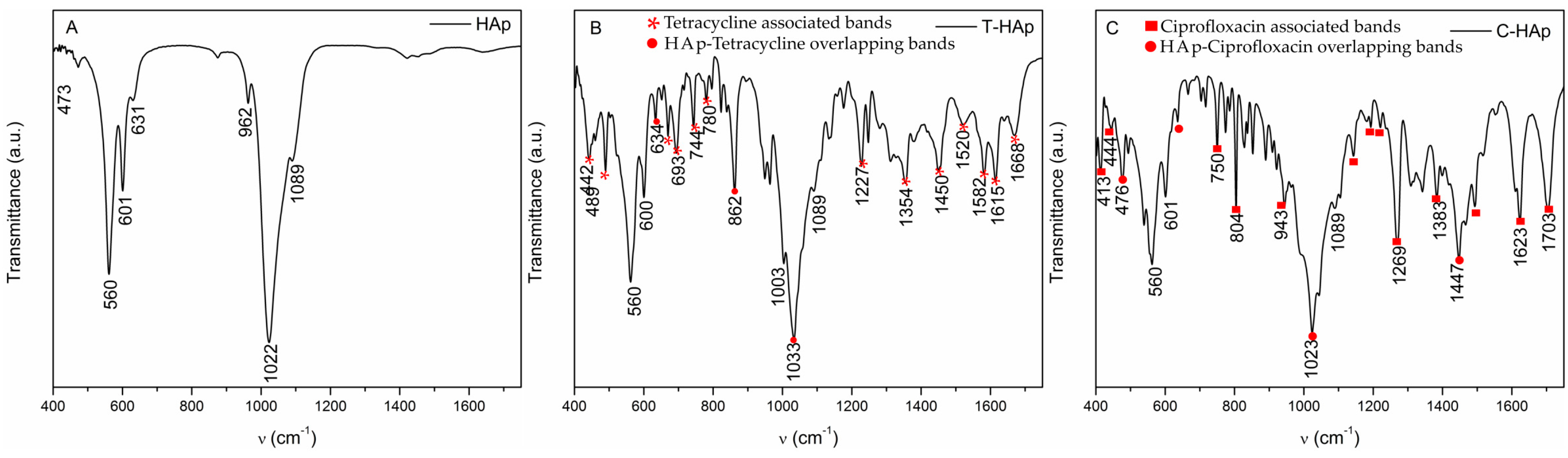
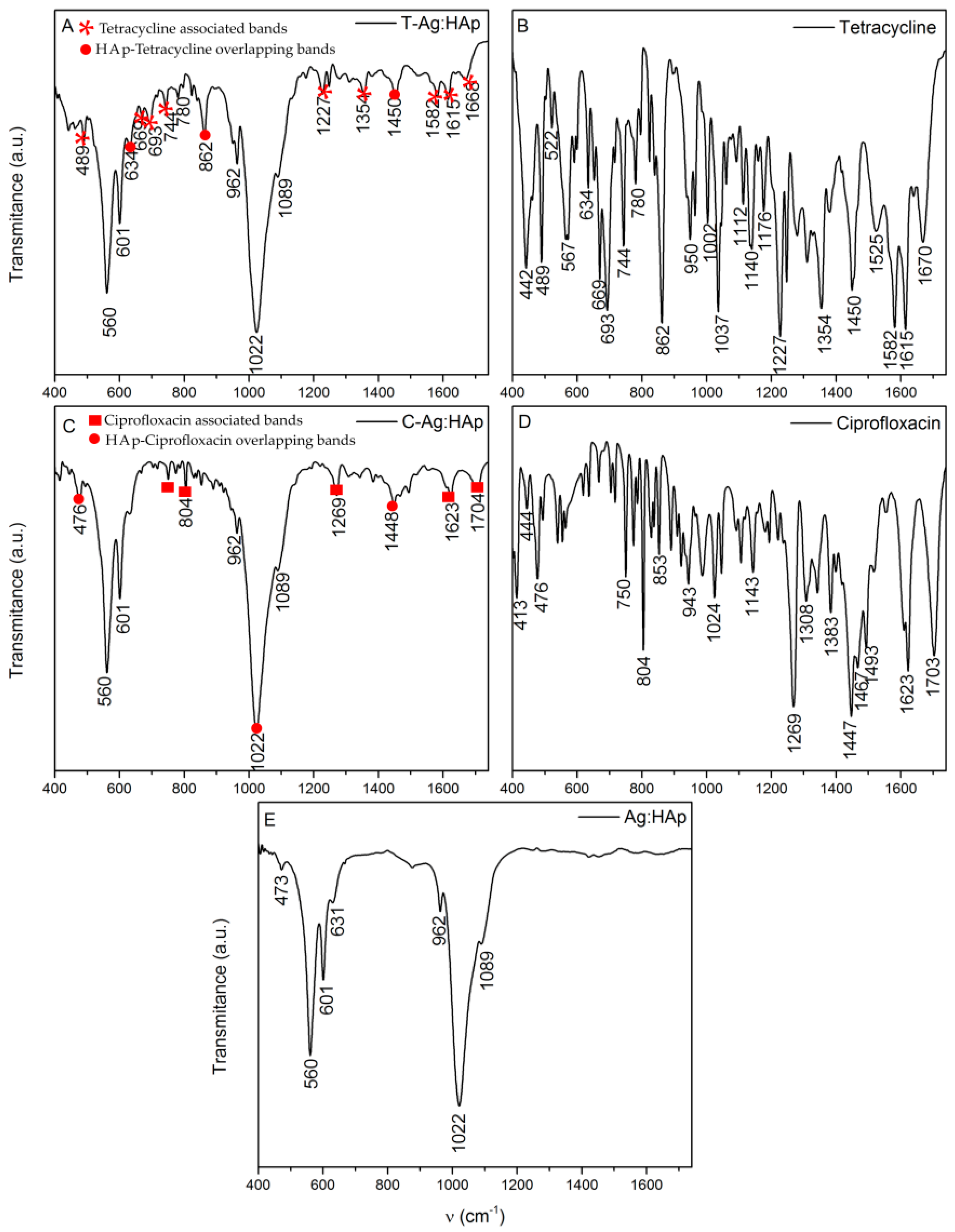
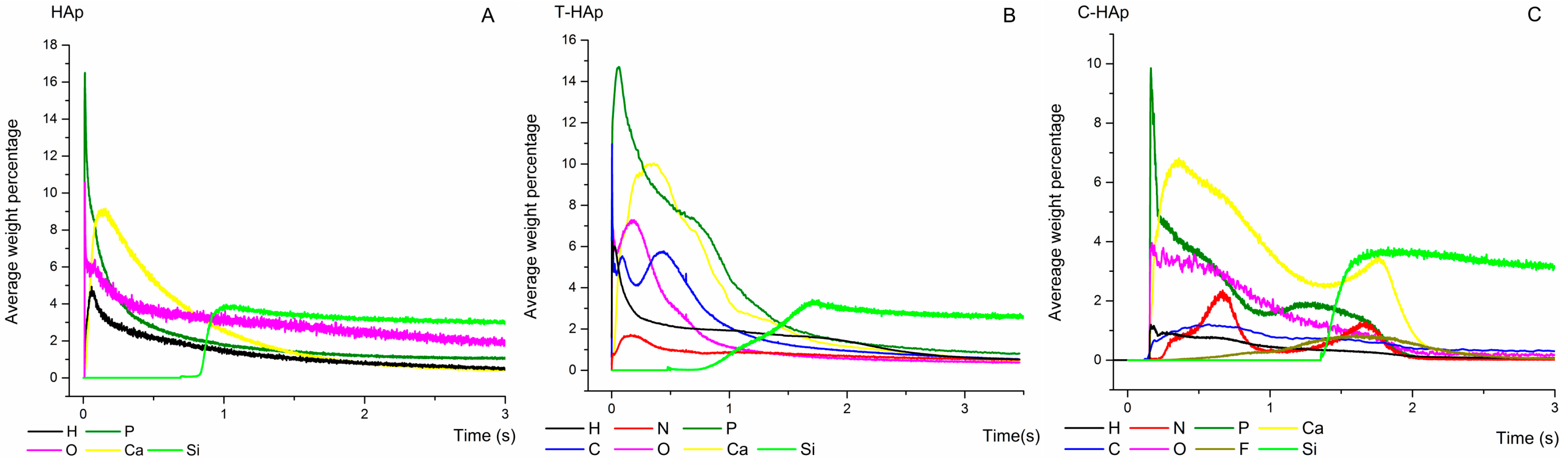
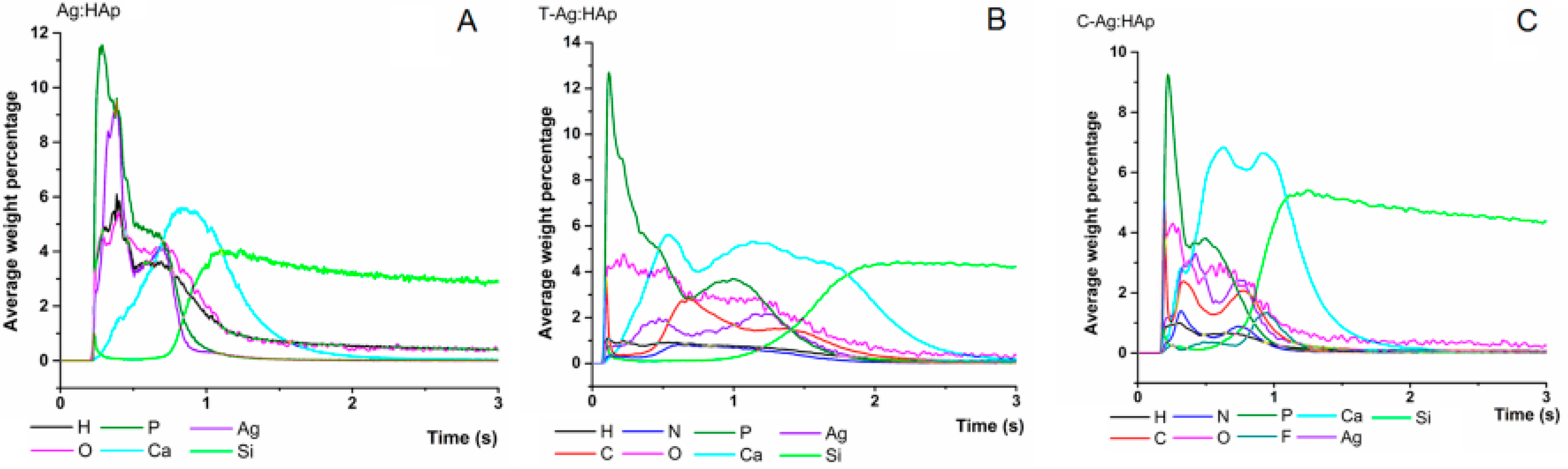

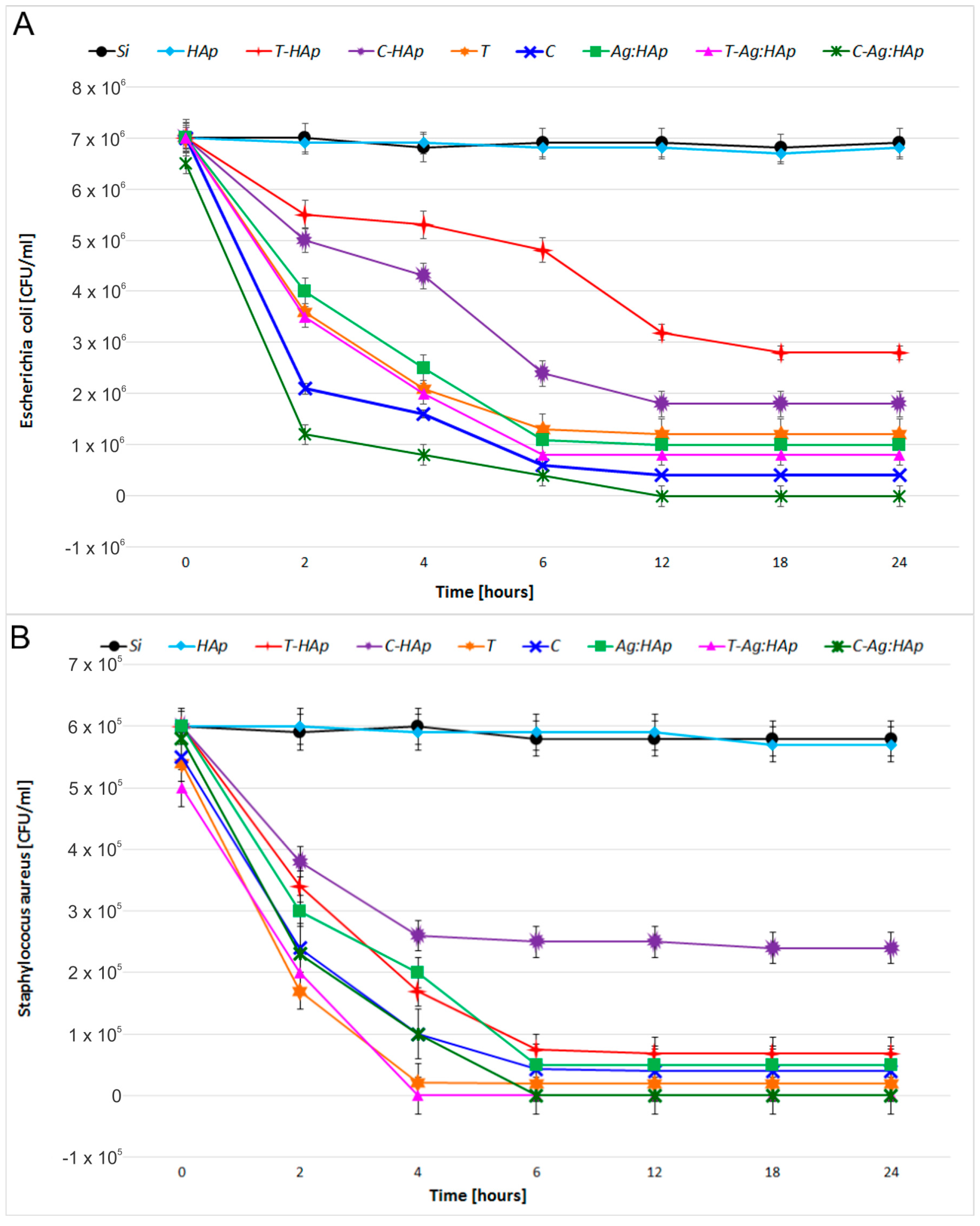
| Bacterial Strains | Thin Films Samples | Mean Zone of Inhibition (mm) |
|---|---|---|
| E. coli ATCC 25922 | Si (control) | 0 |
| HAp | 0 | |
| T-HAp | 17 ± 0.3 | |
| C-HAp | 19 ± 0.5 | |
| Ag:HAp | 23 ± 0.6 | |
| T-Ag:HAp | 25 ± 0.7 | |
| C-Ag:HAp | 28 ± 0.2 | |
| T | 20 ± 0.4 | |
| C | 26 ± 0.3 | |
| S. aureus 0364 | Si (control) | 0 |
| HAp | 0 | |
| T-HAp | 26 ± 0.5 | |
| C-HAp | 24 ± 0.5 | |
| Ag:HAp | 30 ± 0.6 | |
| T-Ag:HAp | 40 ± 0.7 | |
| C-Ag:HAp | 35 ± 0.4 | |
| T | 33 ± 0.5 | |
| C | 31 ± 0.7 |
© 2016 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC-BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Predoi, D.; Popa, C.L.; Chapon, P.; Groza, A.; Iconaru, S.L. Evaluation of the Antimicrobial Activity of Different Antibiotics Enhanced with Silver-Doped Hydroxyapatite Thin Films. Materials 2016, 9, 778. https://doi.org/10.3390/ma9090778
Predoi D, Popa CL, Chapon P, Groza A, Iconaru SL. Evaluation of the Antimicrobial Activity of Different Antibiotics Enhanced with Silver-Doped Hydroxyapatite Thin Films. Materials. 2016; 9(9):778. https://doi.org/10.3390/ma9090778
Chicago/Turabian StylePredoi, Daniela, Cristina Liana Popa, Patrick Chapon, Andreea Groza, and Simona Liliana Iconaru. 2016. "Evaluation of the Antimicrobial Activity of Different Antibiotics Enhanced with Silver-Doped Hydroxyapatite Thin Films" Materials 9, no. 9: 778. https://doi.org/10.3390/ma9090778
APA StylePredoi, D., Popa, C. L., Chapon, P., Groza, A., & Iconaru, S. L. (2016). Evaluation of the Antimicrobial Activity of Different Antibiotics Enhanced with Silver-Doped Hydroxyapatite Thin Films. Materials, 9(9), 778. https://doi.org/10.3390/ma9090778









