Multichromic Polymers Containing Alternating Bi(3-Methoxythiophene) and Triphenylamine Based Units with Para-Protective Substituents
Abstract
:1. Introduction
2. Experimental
2.1. Reagents and Equipment
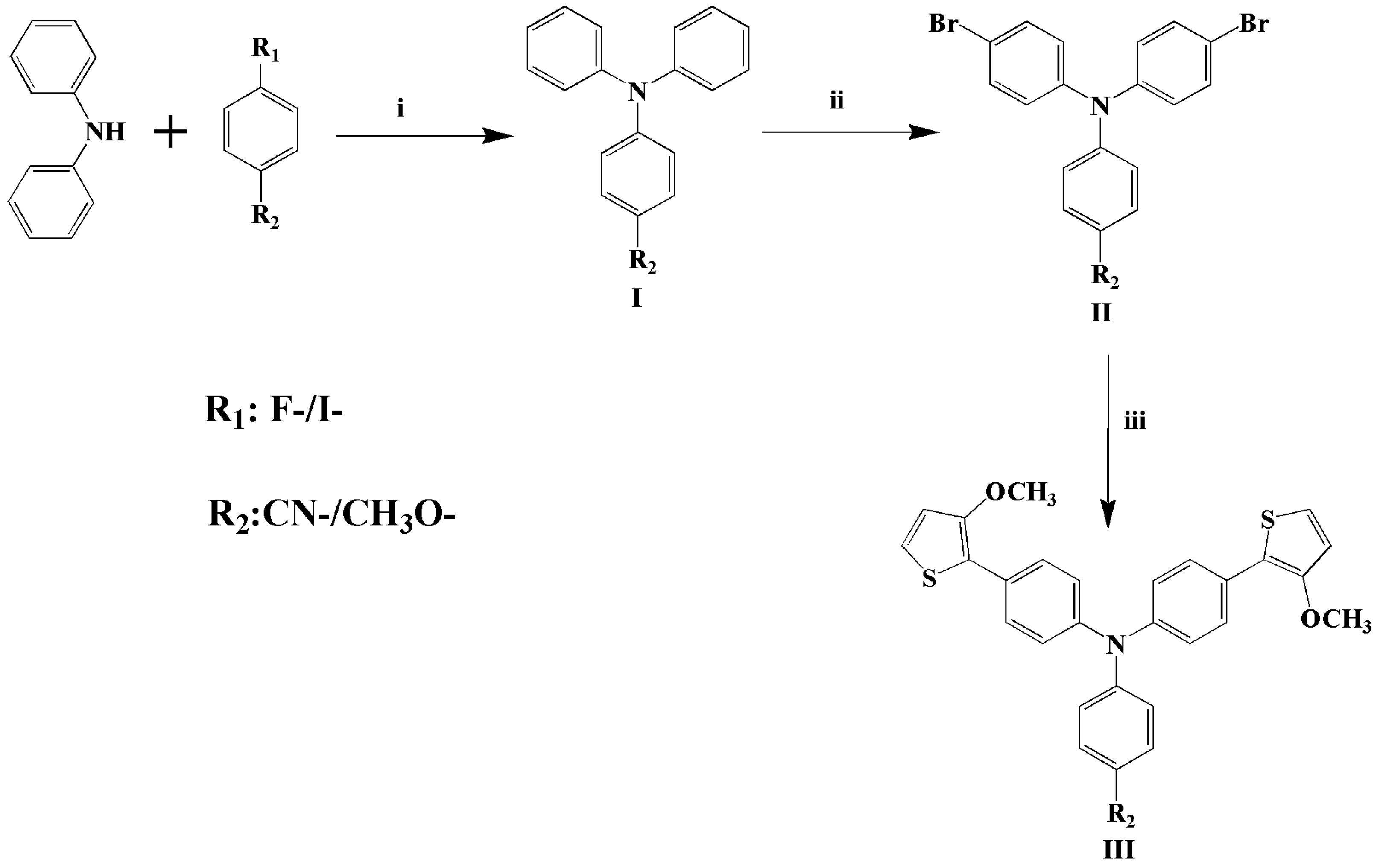
2.2. Synthesis of CMTPA and MMTPA
2.3. Analytical Test Method
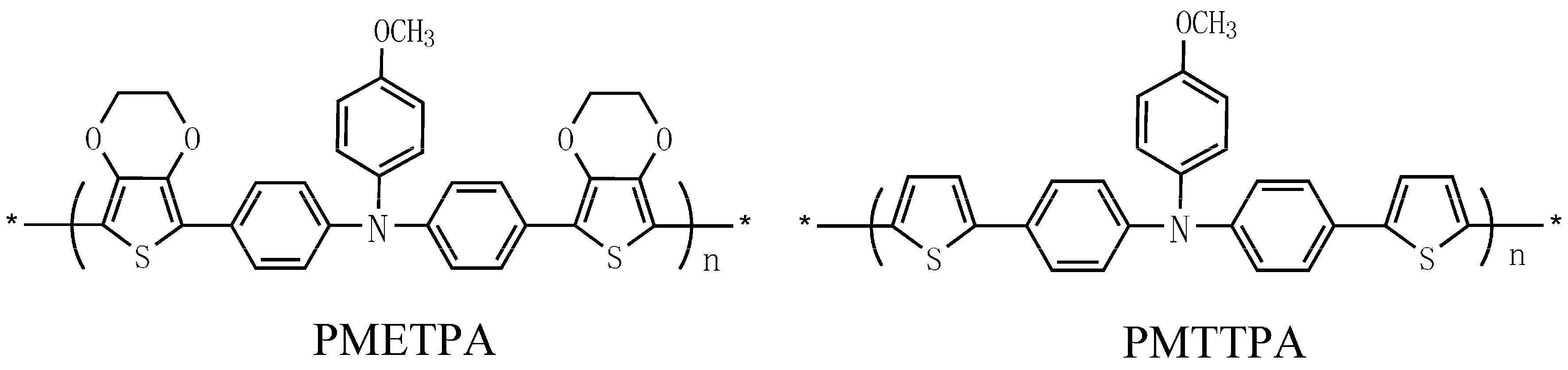
3. Results and Discussion
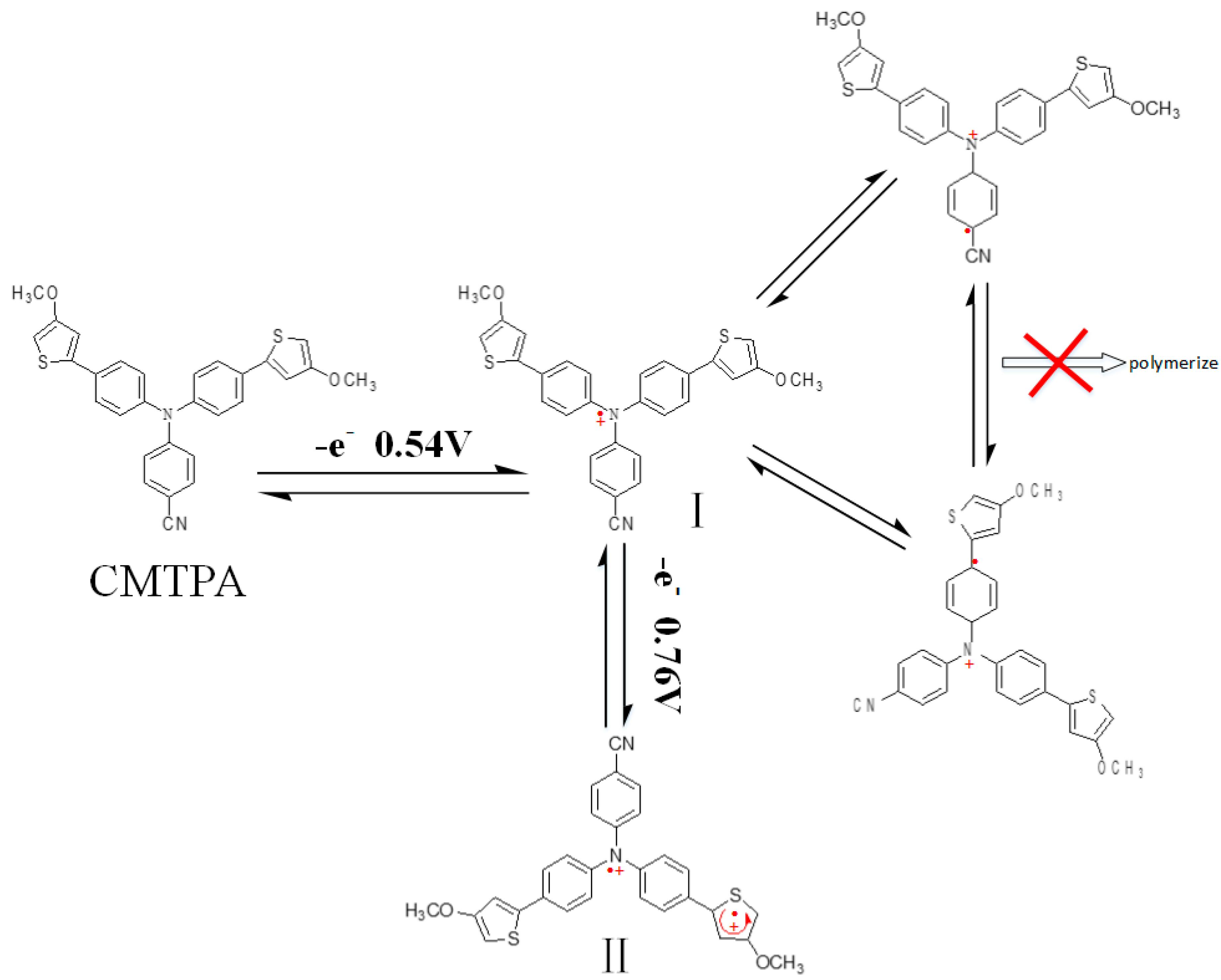
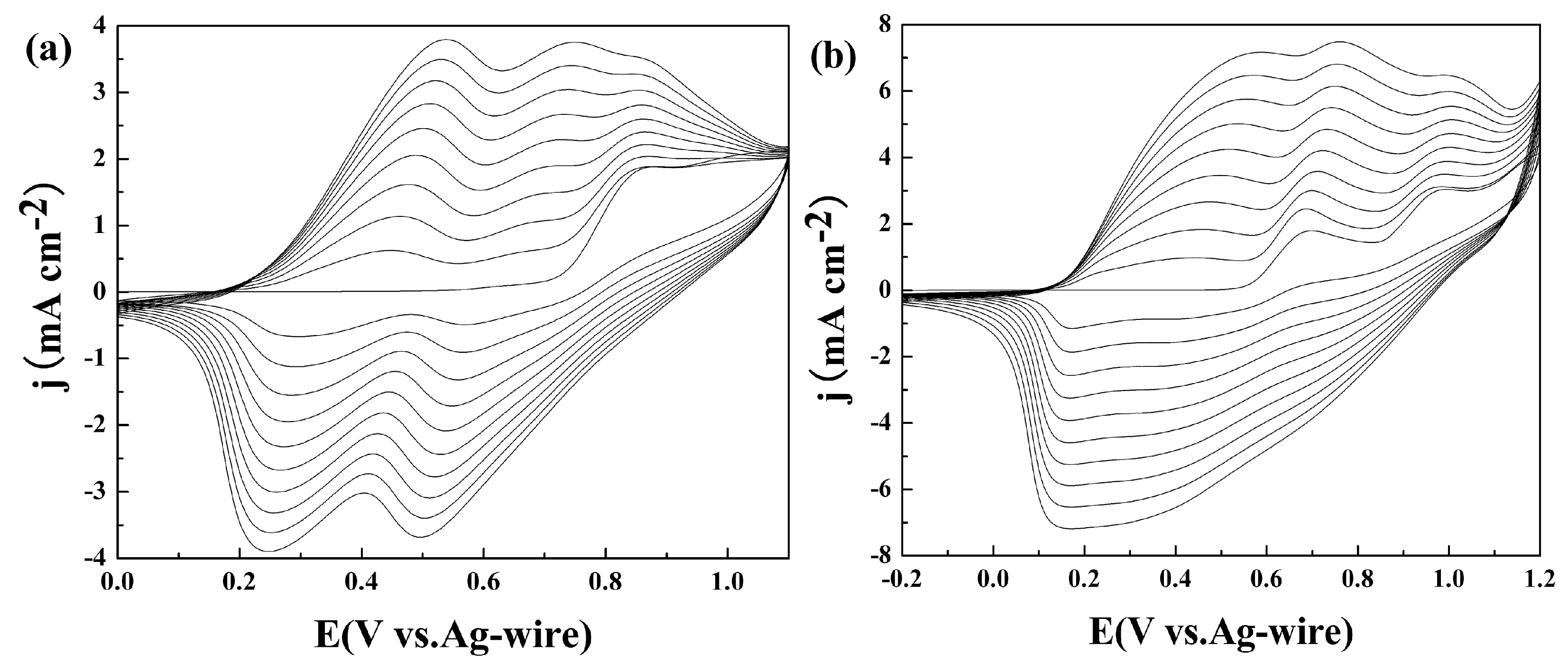
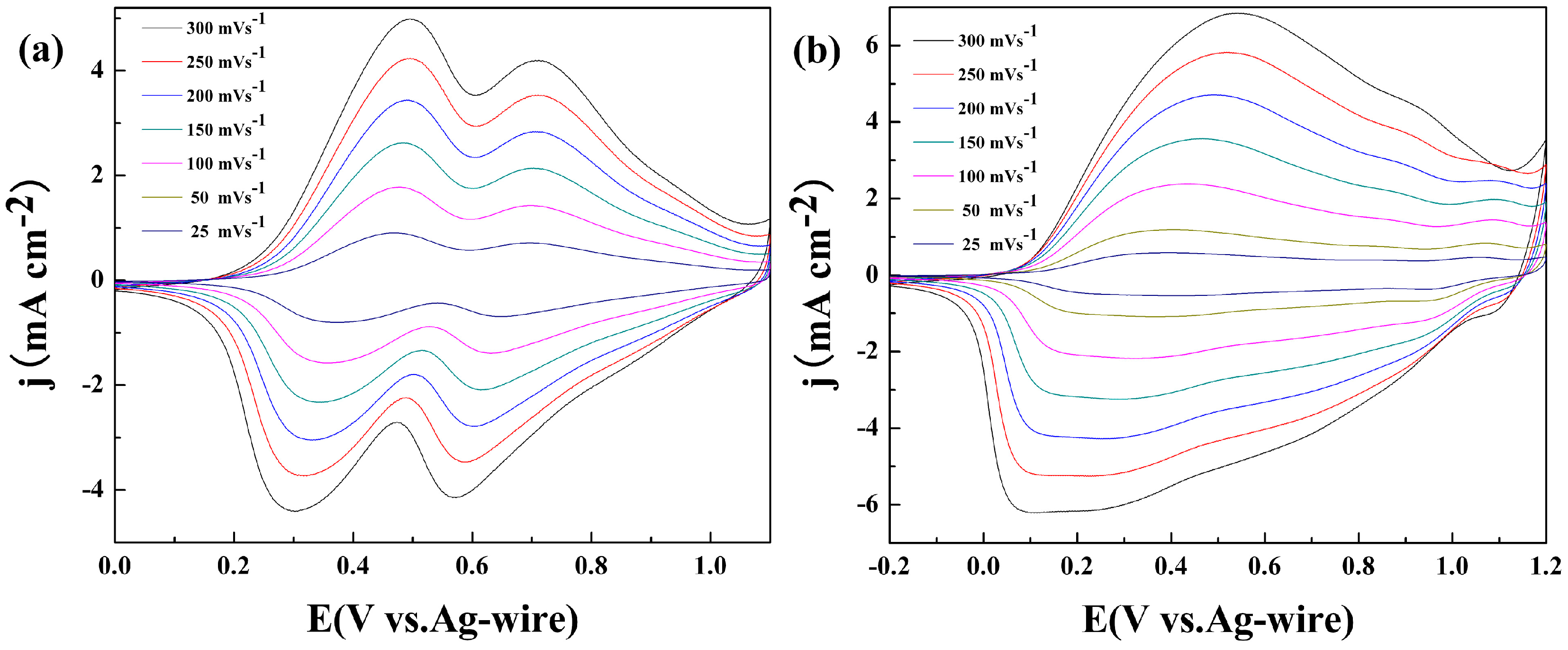
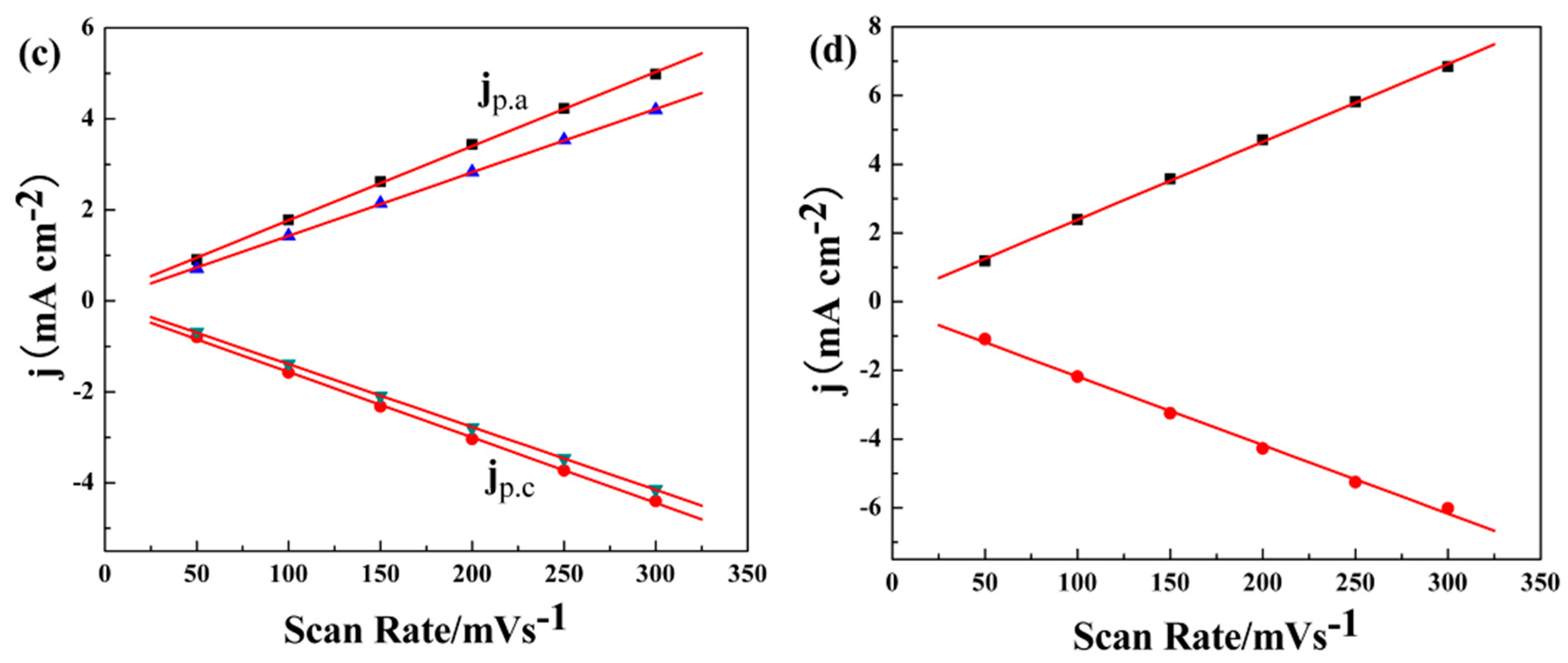
3.1. Electrochemical Polymerization
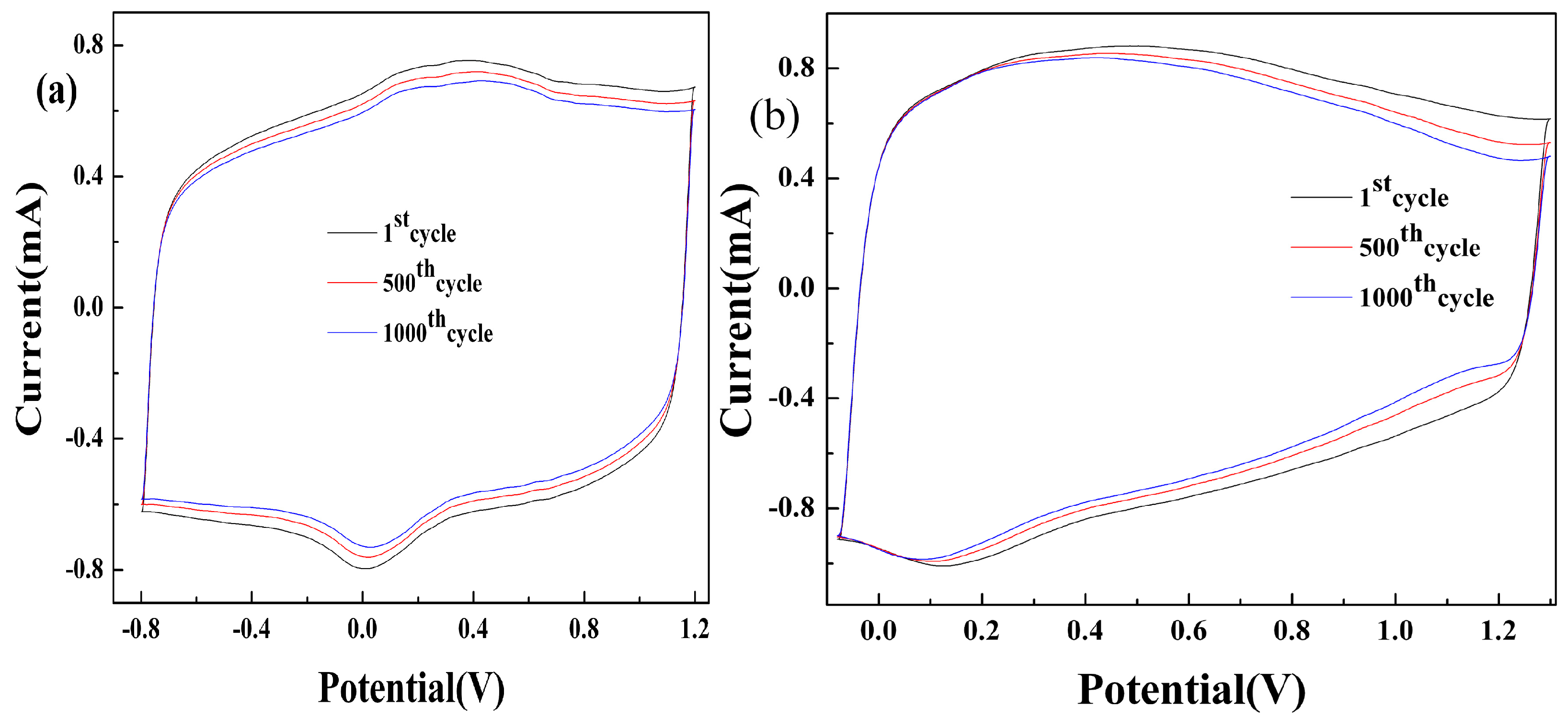
3.2. Redox Characterization of the Polymer
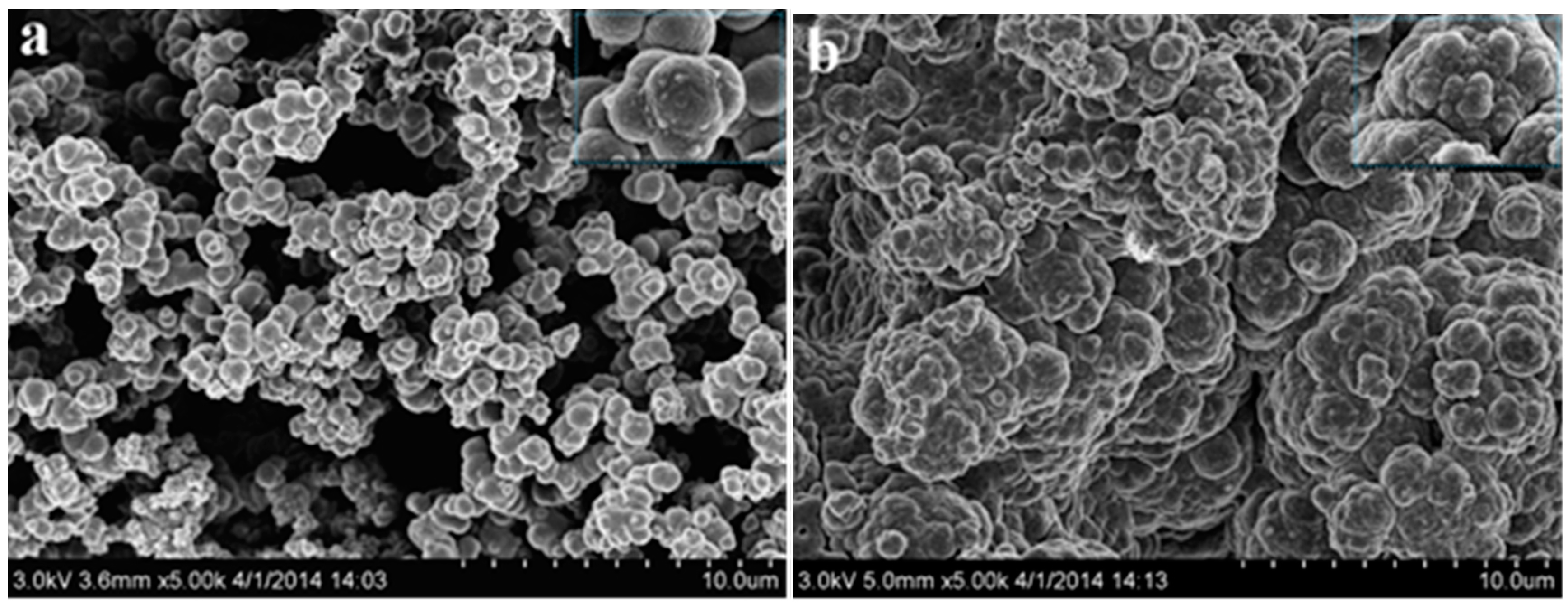
3.3. Morphology
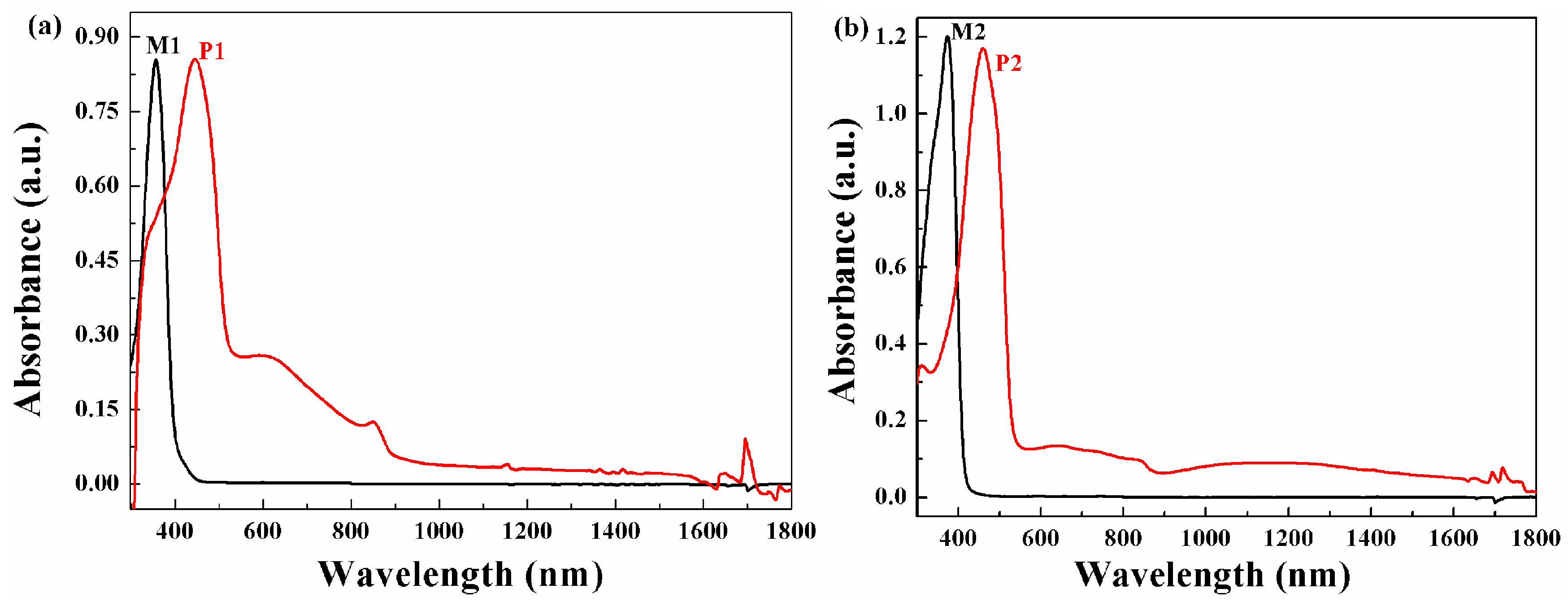
3.4. Optical Properties of the Monomers and Films
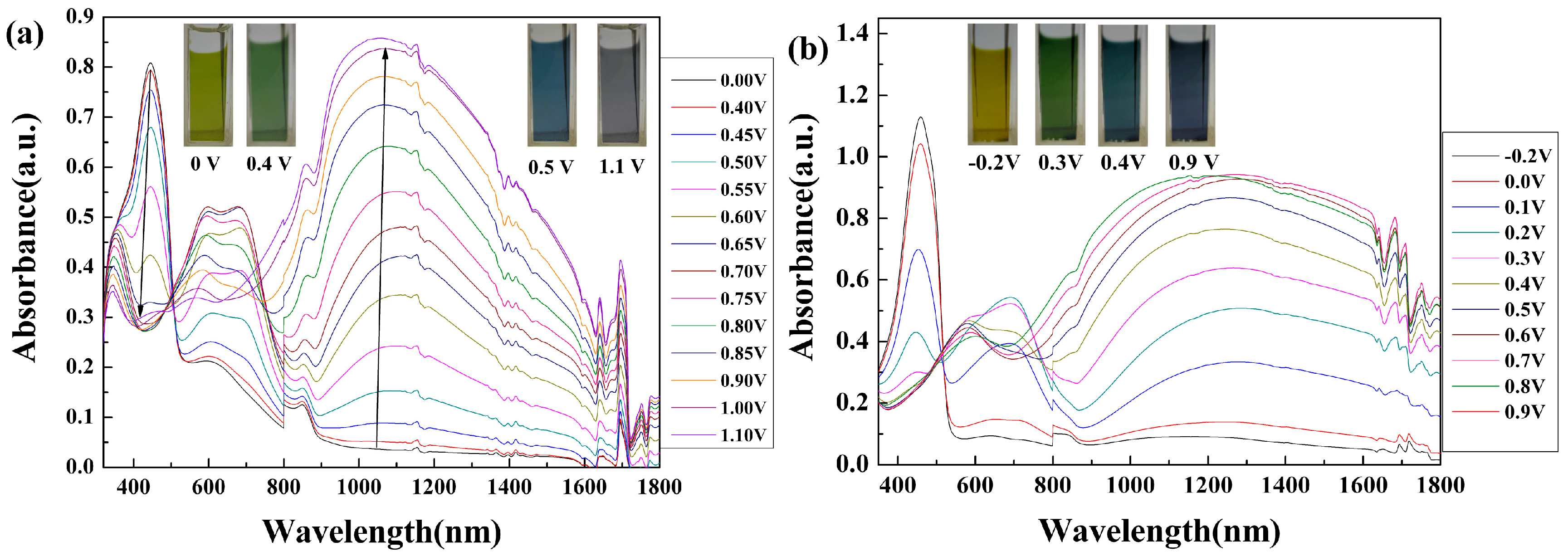
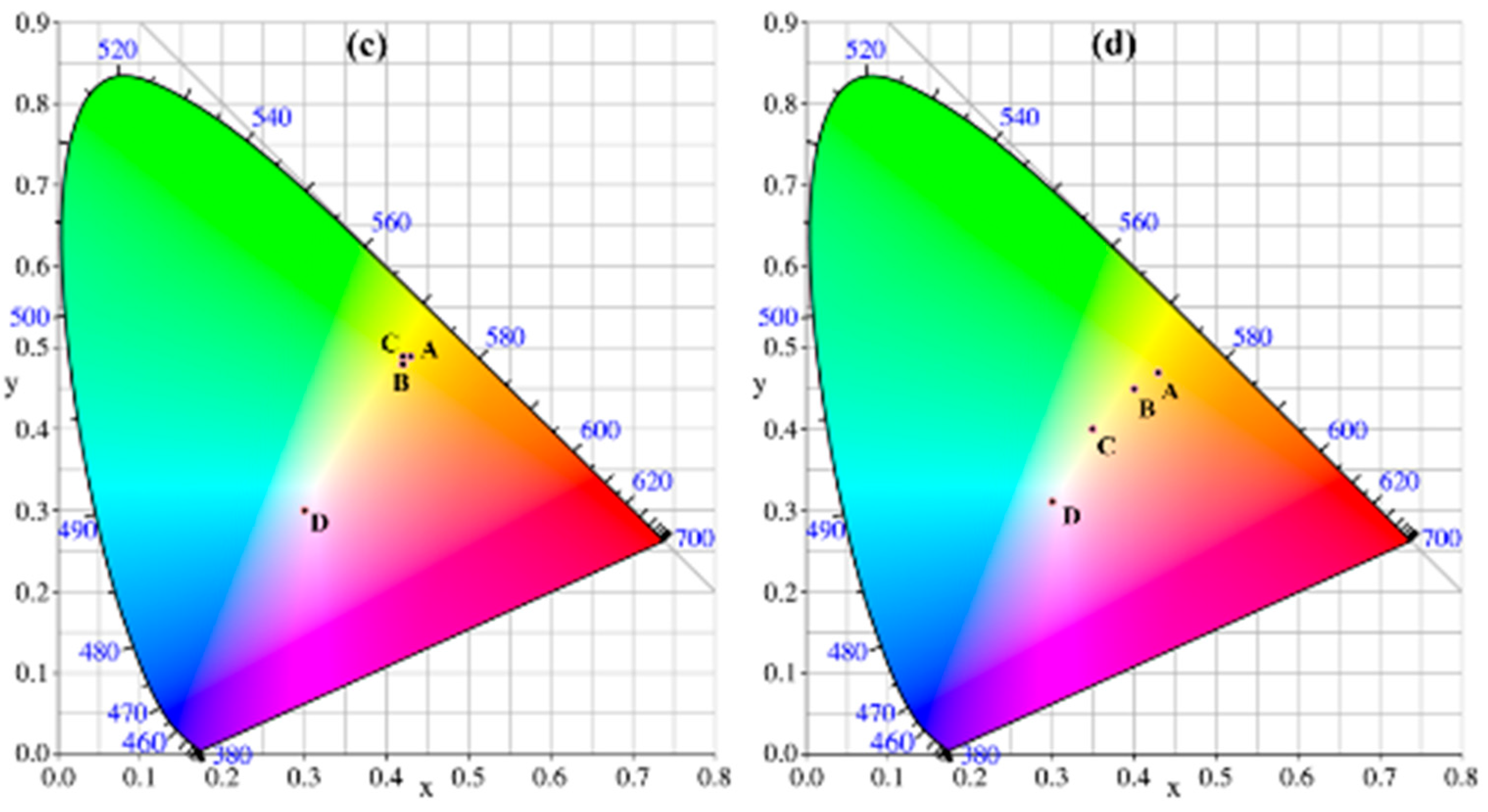
3.5. Spectroelectrochemistry
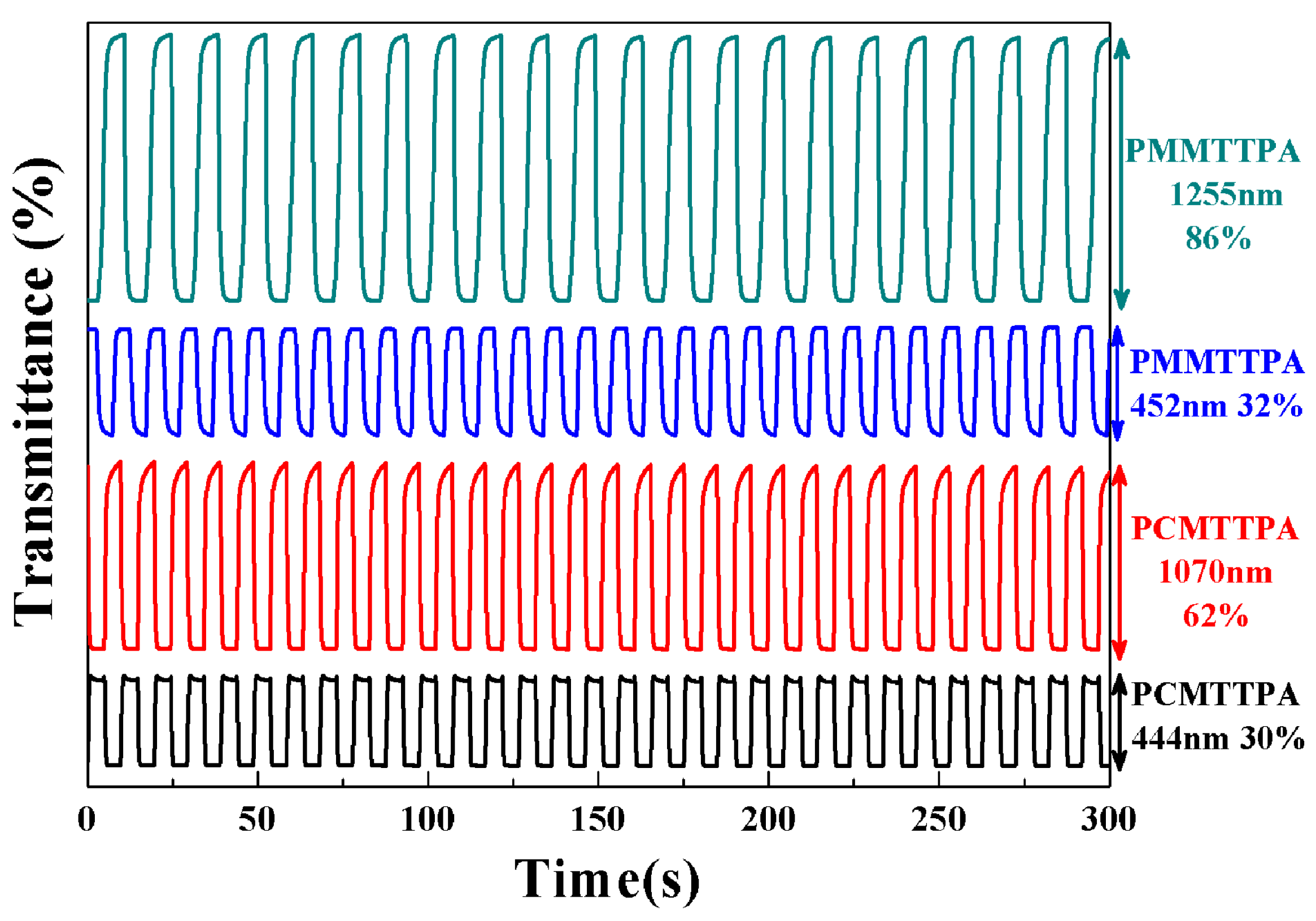
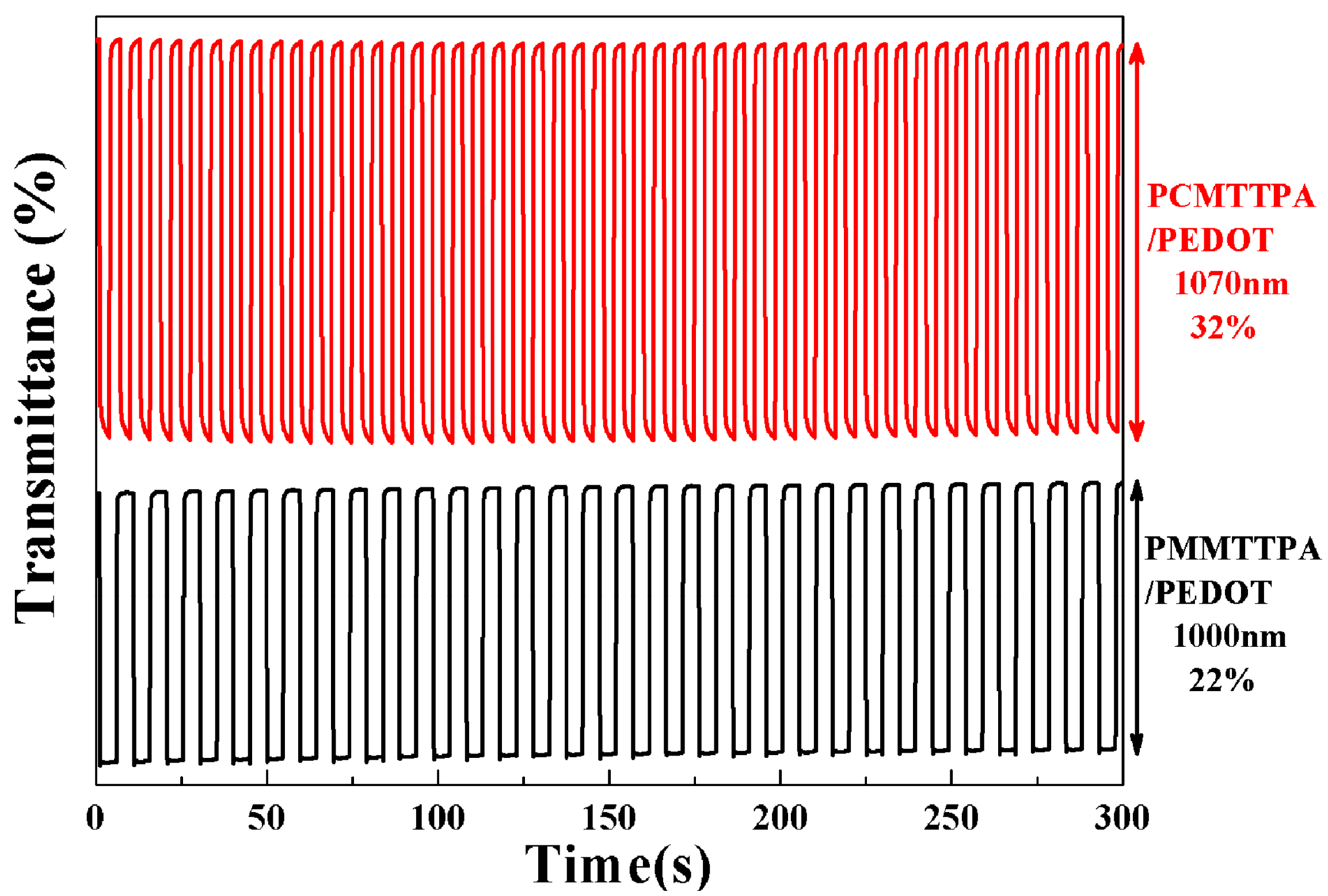
3.6. Electrochromic Switching of the Polymer Films in Solution
3.7. Spectroelectrochemical Properties of ECDs
3.8. Open Circuit Memory of ECDs
4. Conclusions
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Öktem, G.; Balan, A.; Baran, D.; Toppare, L. Donor-acceptor type random copolymers for full visible light absorption. Chem. Commun. 2011, 47, 3933–3935. [Google Scholar] [CrossRef] [PubMed]
- Yang, F.; Li, C.; Zhang, J.Q.; Feng, G.T.; Wei, Z.X.; Li, W.W. Methylated conjugated polymers based on diketopyrrolopyrrole and dithienothiophene for high performance field-effect transistors. Org. Electron. 2016, 37, 366–370. [Google Scholar] [CrossRef]
- Kim, J.; Park, S.Y.; Han, G.; Chae, S.; Song, S.Y.; Shim, J.Y.; Bae, E.; Kim, N.; Kim, H.J.; Kim, J.Y.; et al. Conjugated polymers containing 6-(2-thienyl-)-4H-thieno[3,2-b]indole (TTI) and isoindigo for organic photovoltaics. Polymer 2016, 95, 36–44. [Google Scholar] [CrossRef]
- Kim, D.-M.; Cho, J.; Cho, C.-H.; Kim, K.B.; Kim, M.-Y.; Shim, Y.-B. Disposable all-solid-state pH and glucose sensors based on conductive polymer covered hierarchical AuZn Oxide. Biosens. Bioelectron. 2016, 79, 165–172. [Google Scholar] [CrossRef] [PubMed]
- Lv, X.J.; Yan, S.M.; Dai, Y.Y.; Ouyang, M.; Yang, Y.; Yu, P.F.; Zhang, C. Ion diffusion and electrochromic performance of poly(4,4′,4″-tris[4-(2-bithienyl)phenyl])amine) based on ion liquid as electrolyte. Electrochim. Acta 2015, 186, 85–94. [Google Scholar] [CrossRef]
- Christinelli, W.A.; Trench, A.B.; Pereira, E.C. Electrochromic properties of poly(o-methoxyaniline)-poly(3-thiophene acetic acid) layer by layer films. Sol. Energy Mater. Sol. C 2016, 157, 703–708. [Google Scholar] [CrossRef]
- Ikeda, T.; Higuchi, M. Electrochromic properties of polythiophene polyrotaxane film. Langmuir 2011, 27, 4184–4189. [Google Scholar] [CrossRef] [PubMed]
- Sapstead, R.M.; Corden, N.; Hillman, A.R. Latent fingerprint enhancement via conducting electrochromic copolymer films of pyrrole and 3,4-ethylenedioxythiophene on stainless steel. Electrochim. Acta 2015, 162, 119–128. [Google Scholar] [CrossRef]
- Carbas, B.B.; Önal, A. New fluorene-xanthene-based hybrid electrochromic and fluorescent polymers via donor-acceptor approach. Electrochim. Acta 2012, 66, 38–44. [Google Scholar] [CrossRef]
- Zhou, P.; Wan, Z.Q.; Liu, Y.N.; Jia, C.Y.; Weng, X.L.; Xie, J.L.; Deng, L.J. Synthesis and electrochromic properties of a novel conducting polymer film based on dithiafulvenyl-triphenylamine-di(N-carbazole). Electrochem. Acta 2016, 190, 1015–1024. [Google Scholar] [CrossRef]
- Dyer, A.L.; Craig, M.R.; Babiarz, J.E.; Kiyak, K.; Reynolds, J.R. Orange and red to transmissive electrochromic polymers based on electron-rich dioxythiophenes. Macromolecules 2010, 43, 4460–4467. [Google Scholar] [CrossRef]
- Mi, S.; Wu, J.C.; Liu, J.; Xu, Z.P.; Wu, X.M.; Luo, G.; Zheng, J.M.; Xu, C.Y. AIEE-Active and electrochromic bifunctional polymer and a device composed thereof synchronously achieve electrochemical fluorescence switching and electrochromic switching. ACS Appl. Mater. Interfaces 2015, 7, 27511–27517. [Google Scholar] [CrossRef] [PubMed]
- Tremblay, M.H.; Skalski, T.; Gautier, Y.; Pianezzola, G.; Skene, W.G. Investigation of triphenylamine-thiophene-azomethine derivatives: Toward understanding their electrochromic behavior. J. Phys. Chem. C 2016, 120, 9081–9087. [Google Scholar] [CrossRef]
- Data, P.; Motyka, R.; Lapkowski, M.; Suwinski, J. Spectroelectrochemical analysis of charge carriers as a way of improving poly(p-phenylene)-based electrochromic windows. J. Phys. Chem. C 2015, 119, 20188–20200. [Google Scholar] [CrossRef]
- Amb, C.M.; Dyer, A.L.; Reynolds, J.R. Navigating the color palette of solution-processable electrochromic polymers. Chem. Mater. 2011, 23, 397–415. [Google Scholar] [CrossRef]
- Yuan, Y.; Zhang, G.-Q.; Lu, F.; Tong, Q.-X.; Yang, Q.-D.; Mo, H.-W.; Ng, T.-W.; Lo, M.-F.; Guo, Z.-Q.; Wu, C.; et al. A versatile triphenylamine/fluoranthene-based derivative as a nondoped green-emitting, hole-transporting interlayer for electroluminescent devices. Chem. Asian J. 2013, 8, 1253–1258. [Google Scholar] [CrossRef] [PubMed]
- Ye, J.; Chen, Z.; Wang, K.; An, F.-F.; Yuan, Y.; Chen, W.-C.; Yang, Q.D.; Zhang, X.H.; Lee, C.-S. A bipolar transporter as an efficient green fluorescent emitter and host for red phosphors in multi- and single-layer organic light-emitting diodes. Chem. Eur. J. 2014, 20, 13762–13769. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.Q.; Liang, Y.; Zhu, J.Y.; Bai, X.D.; Jiang, X.K.; Zhang, Q. High coloration efficiency and fast switching speed of poly(amic acid-imide)s containing triphenylamine in acidic electrolyte. RSC Adv. 2015, 5, 11071–11076. [Google Scholar] [CrossRef]
- Wang, H.-M.; Hsiao, S.-H. Multicolor electrocromic poly(amide-imide)s with N,N-diphenyl-N′N′-di-4-tert-butylphenyl-1,4-phenylenediamine moieties. Polym. Chem. 2010, 1, 1013–1023. [Google Scholar] [CrossRef]
- Petr, A.; Kvarnström, C.; Dunsch, L.; Ivaska, A. Electrochemical synthesis of electroactive polytriphenylamine. Synth. Met. 2000, 108, 245–247. [Google Scholar] [CrossRef]
- Lin, H.-Y.; Liu, G.-S. Poly(triphenylamine)s derived from oxidative coupling reaction: Substituent effects on the polymerization, electrochemical, and electro-optical properties. J. Polym. Sci. Part A Polym. Chem. 2009, 47, 285–294. [Google Scholar] [CrossRef]
- Chang, H.-W.; Lin, K.-H.; Chueh, C.-C.; Liou, G.-S.; Chen, W.-C. New P-type of poly(4-methoxy-triphenylamine)s derived by coupling reactions: Synthesis, electrochromic behaviors, and hole mobility. J. Polym. Sci. Part A Polym. Chem. 2009, 47, 4037–4050. [Google Scholar] [CrossRef]
- Li, Y.-Q.; Fang, R.-C.; Zheng, A.-M.; Chu, Y.-Y.; Tao, X.; Xu, H.-H.; Ding, S.-J.; Shen, Y.-Z. Nonvolatile memory devices based on polyimides bearing noncoplanar twisted biphenyl units containing carbazole and triphenylamine side-chain groups. J. Mater. Chem. 2011, 21, 15643–15654. [Google Scholar] [CrossRef]
- Ma, X.-C.; Niu, H.J.; Wen, H.-L.; Wang, S.H.; Lian, Y.-F.; Jiang, X.-K.; Wang, C.; Bai, X.-D.; Wang, W. Synthesis, electrochromic, halochromic and electro-optical properties of polyazomethines with a carbazole core and triarylamine units serving as functional groups. J. Mater. Chem. C 2015, 3, 3482–3493. [Google Scholar] [CrossRef]
- Lin, K.-W.; Ming, S.L.; Zhen, S.J.; Zhao, Y.; Lu, B.-Y.; Xu, J.-K. Molecular design of DBT/DBF hybrid thiophenes π-conjugated systems and comparative study of their electropolymerization and optoelectroic properties: From comonomers to electrochromic polymers. Polym. Chem. 2015, 6, 4575–4587. [Google Scholar] [CrossRef]
- Lu, B.Y.; Zhen, S.J.; Ming, S.L.; Xu, J.-K.; Zhao, G.-Q. Effect of electrolytes on the electropolymerization and optoelectronic properties of poly(methylselenophene). RSC Adv. 2015, 5, 70649–70660. [Google Scholar] [CrossRef]
- Tao, Y.J.; Zhang, K.; Zhang, Z.Y.; Cheng, H.F.; Jiao, C.L.; Zhao, Y.L.; Xu, W.F. Enhanced electrochromic properties of donor-acceptor polymers via TiO2 composite. Polymer 2016, 91, 98–105. [Google Scholar] [CrossRef]
- Sai, M.; Wu, J.C.; Liu, J.; Zheng, J.M.; Xu, C.Y. Donor-π-bridge-acceptor fluorescent polymers based on thiophene and triphenylamine derivatives as solution processable electrochromic materials. Org. Electron. 2015, 23, 116–123. [Google Scholar]
- Yang, X.J.; Wang, M.; Zhao, J.S.; Cui, C.S.; Wang, S.H.; Liu, J.F. Multichromic polymers containing alternating bithiophenes derivatives and 4-cyanotriphenylamine unit and their application for electrochromic devices. J. Electroanal. Chem. 2014, 715, 1–10. [Google Scholar] [CrossRef]
- Xu, C.X.; Zhao, J.S.; Cui, C.S.; Wang, M.; Kong, Y.; Zhang, X.X. Triphenylamine-based multielectrochromic material and its neutral green electrochromic devices. J. Electroanal. Chem. 2012, 682, 29–36. [Google Scholar] [CrossRef]
- Zhao, J.S.; Yang, X.J.; Wang, S.H.; Yu, J.S. Two new near-infrared switchable electrochromic bithiophenes derivatives based on 4-methoxytriphenylamine unit and their application for electrochromic devices. ESC J. Solid State Sci. Technol. 2014, 3, R121–R130. [Google Scholar] [CrossRef]
- Xu, Z.; Wang, M.; Zhao, J.S.; Cui, C.S.; Fan, W.Y.; Liu, J.F. Donor-acceptor type neutral green polymers containing 2,3-di(5-methylfuran-2-yl) quinoxaline acceptor and different thiophene donors. Electrochem. Acta 2014, 125, 241–249. [Google Scholar] [CrossRef]
- Zhao, H.; Tang, D.D.; Zhao, J.S.; Wang, M.; Dou, J.M. Two novel ambipolar donor-acceptor type electrochromic polymers with the realization of RGB (red-green-blue) display in one polymer. RSC Adv. 2014, 4, 61537–61547. [Google Scholar] [CrossRef]
- Akpmar, H.Z.; Udum, Y.A.; Toppare, L. Multichromic and soluble conjugated polymers containing thiazolothiazole unit for electrochromic applications. Eur. Polym. J. 2015, 63, 255–261. [Google Scholar] [CrossRef]
- Nie, G.-M.; Yang, H.-J.; Chen, J.; Bai, Z.M. A novel high-quality electrochromic material from 3,4-ethylenedioxythiophene bis-substituted fluorene. Org. Electron. 2012, 13, 2167–2176. [Google Scholar] [CrossRef]
- Ponnapati, R.; Felipe, M.-J.; Muthalagu, V.; Puno, K.; Wolff, B.; Advincula, R. Conjugated polymer network films of poly(p-phenylene vinylene) with hole-transporting carbazole pendants: Dual photoluminescence and electrochromic behavior. ACS. Appl. Mater. Interfaces 2014, 4, 1211–1218. [Google Scholar] [CrossRef] [PubMed]


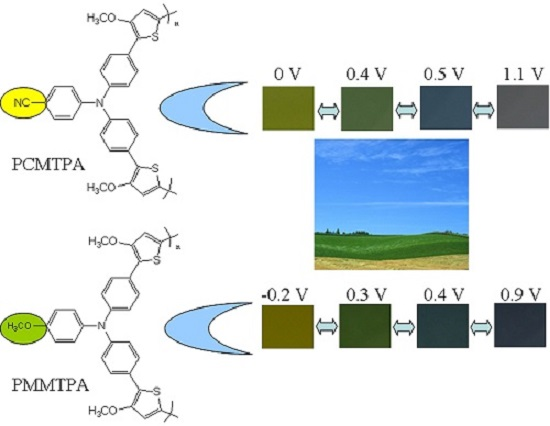
| Compounds | Eonset, vs. (Ag-wire) (V) | λmax (nm)/λonset (nm) | Eg a (eV) | HOMO b (eV) | LUMO c (eV) |
|---|---|---|---|---|---|
| CMTPA | 0.58 | 357/445 | 3.12 | −5.01 | −1.89 |
| MMTPA | 0.45 | 373/463 | 3.01 | −4.88 | −1.87 |
| METPA d | 0.46 | 371/418 | 2.96 | −4.86 | −1.90 |
| MTTPA d | 0.49 | 363/415 | 2.98 | −4.89 | −1.91 |
| PCMTPA | 0.16 | 445/607 | 2.39 | −4.59 | −2.2 |
| PMMTPA | 0.09 | 463 | 2.35 | −4.52 | −2.17 |
| PMETPA d | 0.11 | 454/533 | 2.32 | −4.51 | −2.19 |
| PMTTPA d | 0.18 | 435/522 | 2.37 | −4.58 | −2.21 |
| Polymers | E, vs. (Ag wire) (V) | L | a | b | Colors |
|---|---|---|---|---|---|
| PCMTPA | 0 | 61.08 | −9.50 | 56.56 | yellow |
| 0.4 | 63.01 | −8.60 | 62.15 | yellowish green | |
| 0.5 | 61.94 | −11.31 | 57.42 | steel blue | |
| 1.1 | 53.76 | 4.98 | −7.10 | gray | |
| PMMTPA | −0.2 | 82.58 | −4.07 | 70.32 | bright yellow |
| 0.3 | 78.71 | −7.69 | 54.84 | dark yellowish green | |
| 0.4 | 72.69 | −12.22 | 25.59 | dark blue green color | |
| 0.9 | 69.25 | 1.36 | −6.67 | dark blue |
| Compounds | Wavelength (nm) | ∆T% | Response Time (t, s) | CE (cm2·C−1) |
|---|---|---|---|---|
| PCMTPA | 444 | 30 | 0.8 | 112 |
| 1070 | 62 | 4.5 | 124 | |
| PMMTPA | 452 | 32 | 1.1 | 145 |
| 1255 | 86 | 3.6 | 164 | |
| PMTTPA a | 430 | 28.7 | 0.9 | 177 |
| 1280 | 55.5 | 0.4 | 305 | |
| PMETPA a | 454 | 43.7 | 1.3 | 230 |
| 1200 | 77.6 | 1.47 | 201 |
| Compounds | Optical Contrast (ΔT%) | Response Time (s) | Color | Coloration Efficiency (cm2·C−1) |
|---|---|---|---|---|
| PCMTPA/PEDOT | 32 (1070 nm) | 0.6 (1070 nm) | yellow/blue | 301 (1070 nm) |
| PMMTPA/PEDOT | 22 (440 nm) | 1.80 (440 nm) | green/blue | 235 (440 nm) |
| PMETPA/PEDOT a | 29.8 (640 nm) | 0.40 (640 nm) | yellowish green/blue | 270 (640 nm) |
| PMTTPA/PEDOT a | 30.3 (600 nm) | 1.80 (600 nm) | yellow/stone-blue | 517 (600 nm) |
© 2016 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC-BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hou, Y.; Kong, L.; Ju, X.; Liu, X.; Zhao, J.; Niu, Q. Multichromic Polymers Containing Alternating Bi(3-Methoxythiophene) and Triphenylamine Based Units with Para-Protective Substituents. Materials 2016, 9, 779. https://doi.org/10.3390/ma9090779
Hou Y, Kong L, Ju X, Liu X, Zhao J, Niu Q. Multichromic Polymers Containing Alternating Bi(3-Methoxythiophene) and Triphenylamine Based Units with Para-Protective Substituents. Materials. 2016; 9(9):779. https://doi.org/10.3390/ma9090779
Chicago/Turabian StyleHou, Yingfei, Lingqian Kong, Xiuping Ju, Xiaoli Liu, Jinsheng Zhao, and Qingshan Niu. 2016. "Multichromic Polymers Containing Alternating Bi(3-Methoxythiophene) and Triphenylamine Based Units with Para-Protective Substituents" Materials 9, no. 9: 779. https://doi.org/10.3390/ma9090779