Self-Assembly of Lamellar/Micellar Block Copolymers Induced Through Their Rich Exposure to Various Solvent Vapors: An AFM Study
Abstract
1. Introduction
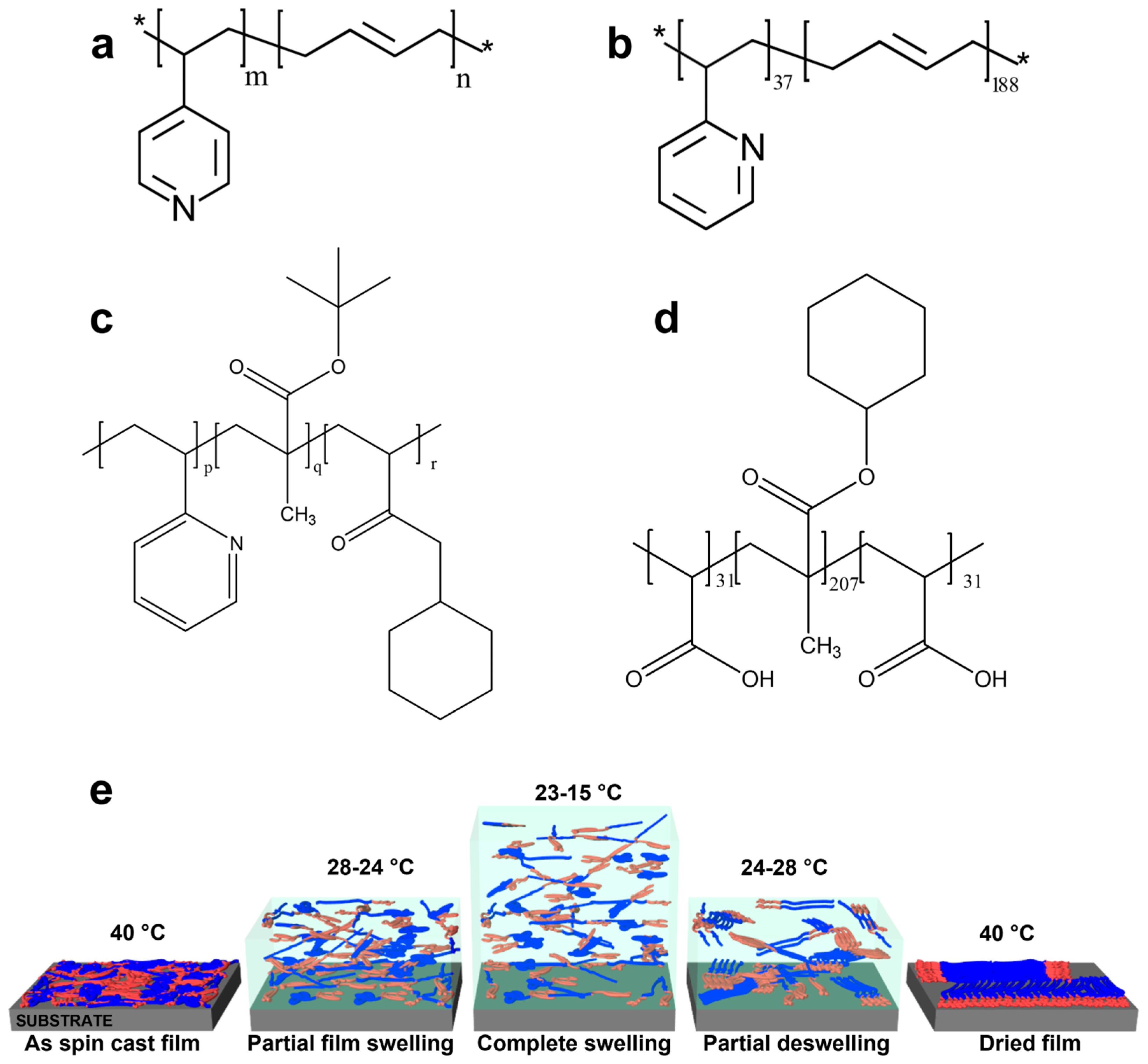
2. Materials and Methods
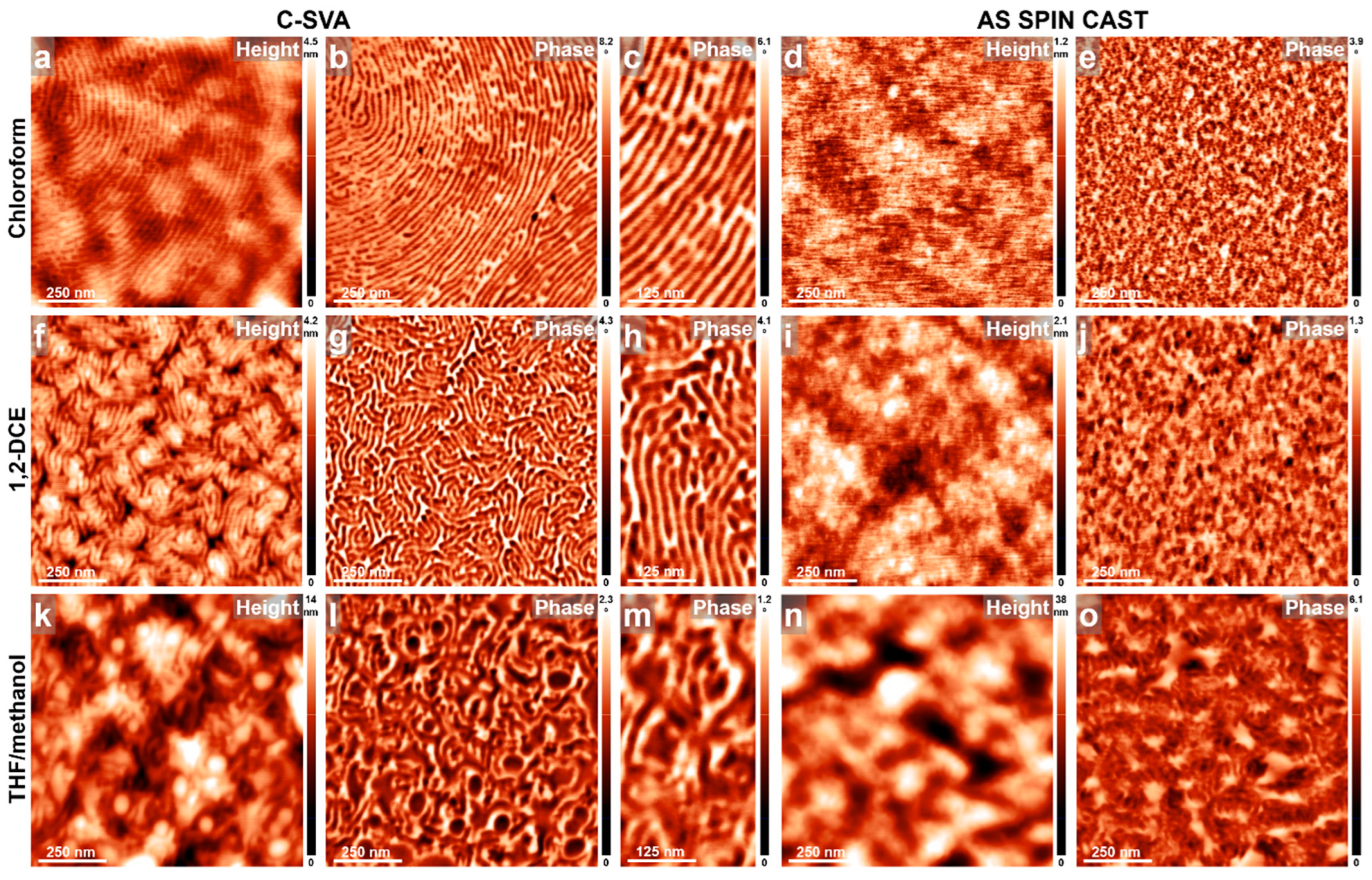
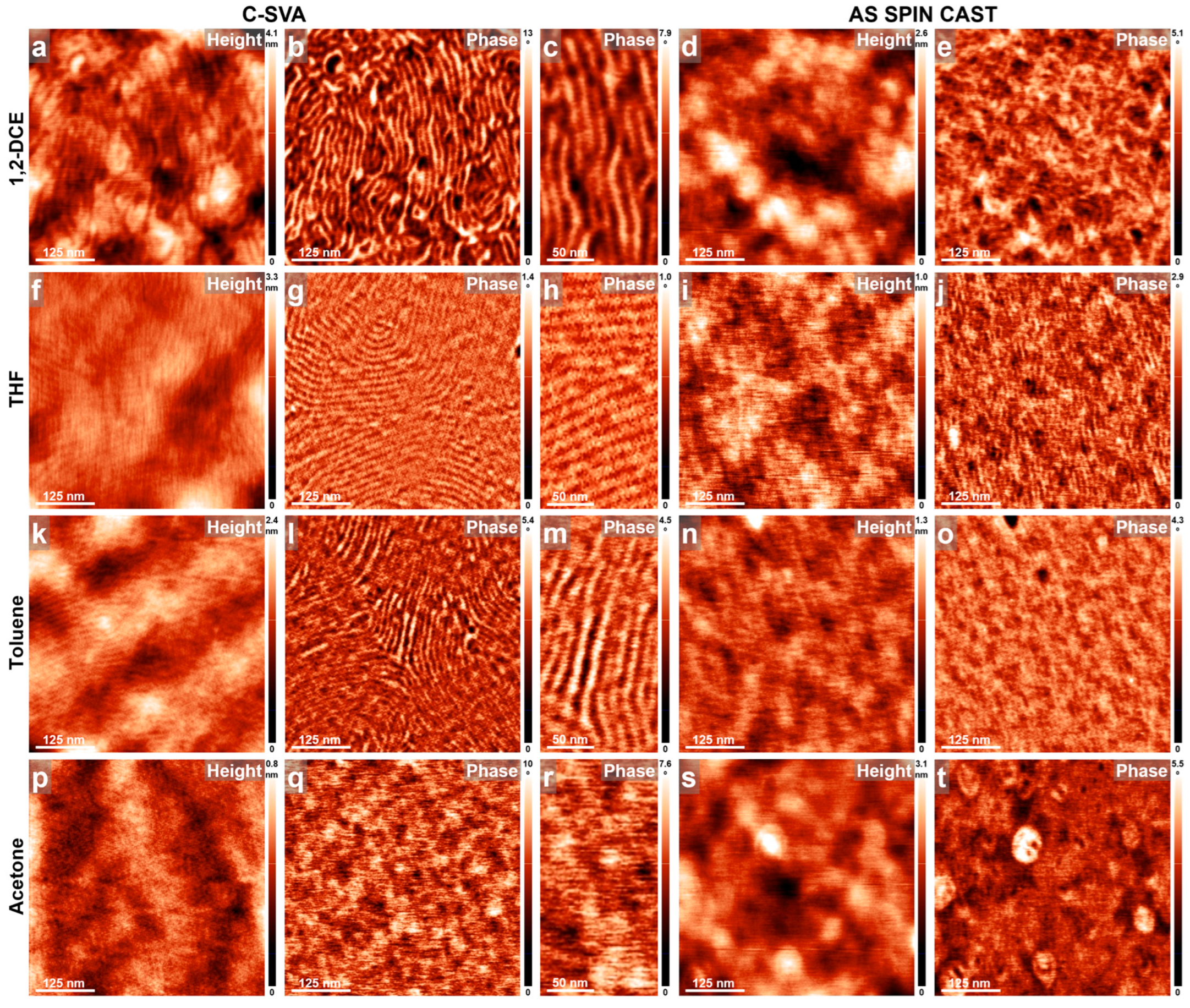
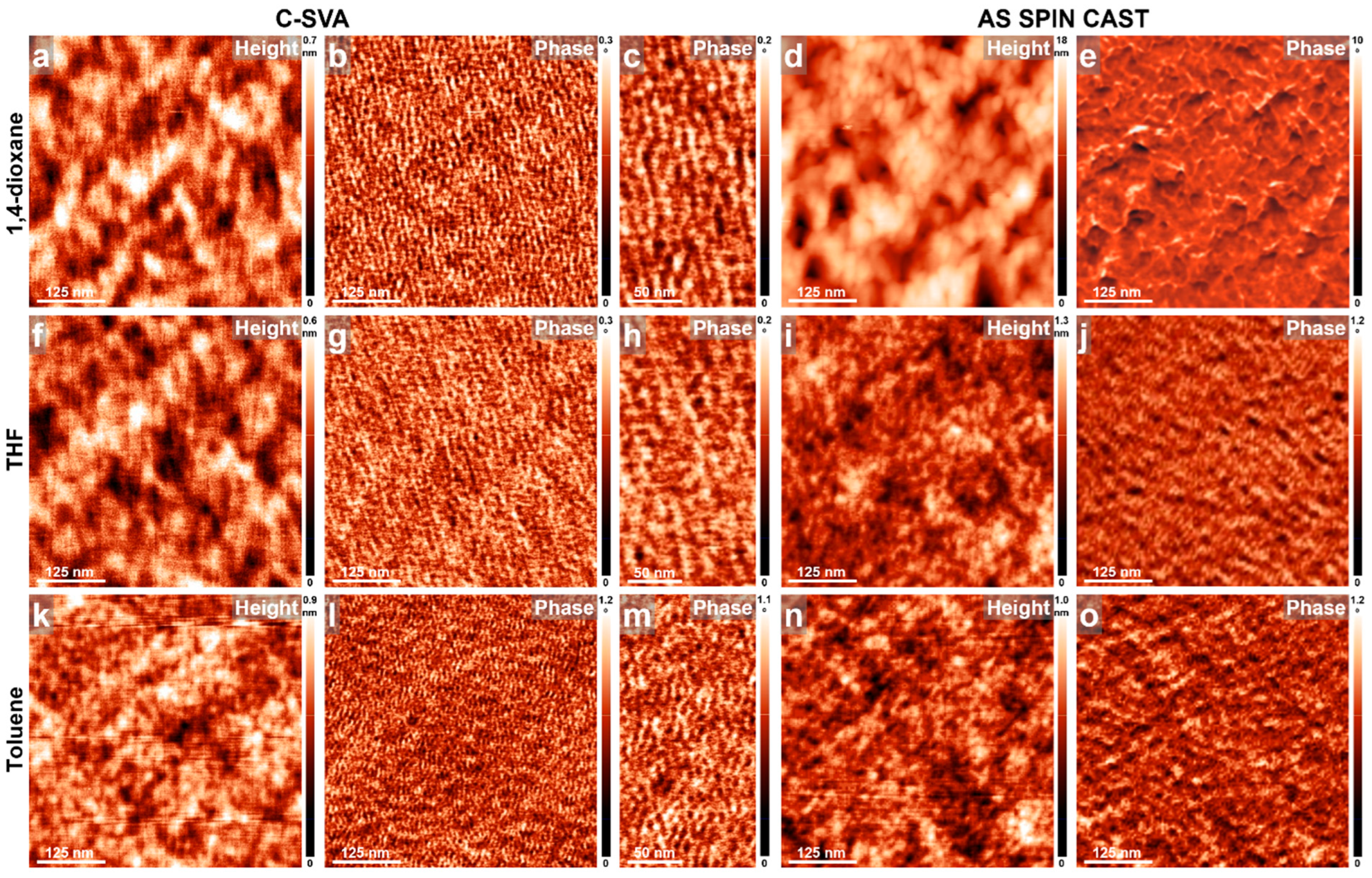
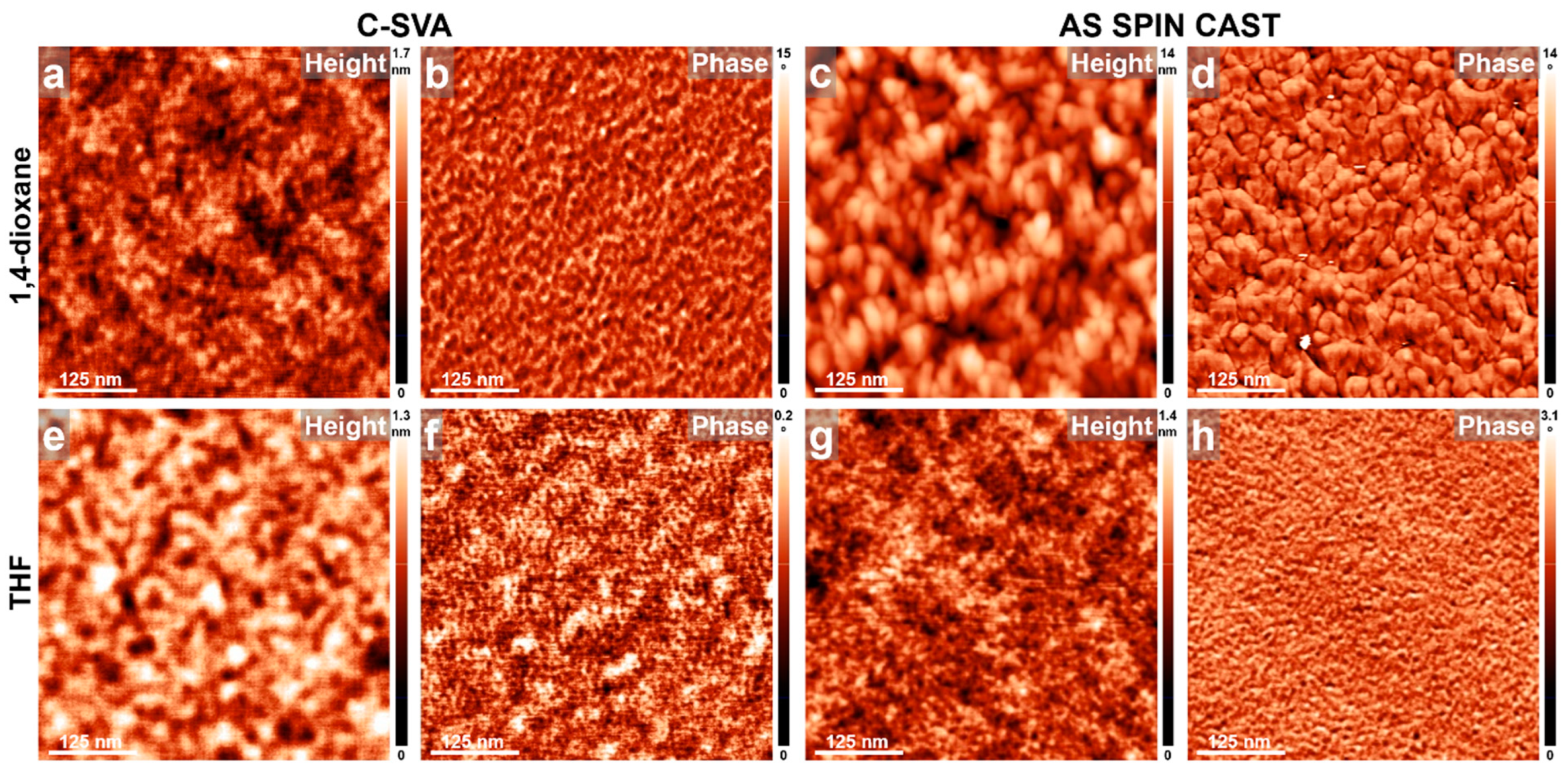
3. Results and Discussions
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Bates, F.S.; Fredrickson, G.H. Block Copolymers—Designer Soft Materials. Phys. Today 1999, 52, 32–38. [Google Scholar] [CrossRef]
- Bates, F.S.; Hillmyer, M.A.; Lodge, T.P.; Bates, C.M.; Delaney, K.T.; Fredrickson, G.H. Multiblock Polymers: Panacea or Pandora’s Box? Science 2012, 336, 434–440. [Google Scholar] [CrossRef] [PubMed]
- Polymeropoulos, G.; Zapsas, G.; Ntetsikas, K.; Bilalis, P.; Gnanou, Y.; Hadjichristidis, N. 50th Anniversary Perspective: Polymers with Complex Architectures. Macromolecules 2017, 50, 1253–1290. [Google Scholar] [CrossRef]
- Feng, H.; Lu, X.; Wang, W.; Kang, N.-G.; Mays, J.W. Block Copolymers: Synthesis, Self-Assembly, and Applications. Polymers 2017, 9, 494. [Google Scholar] [CrossRef] [PubMed]
- Morkved, T.L.; Wiltzius, P.; Jaeger, H.M.; Grier, D.G.; Witten, T.A. Mesoscopic Self-Assembly of Gold Islands on Deblock-Copolymer Films. Appl. Phys. Lett. 1994, 64, 422. [Google Scholar] [CrossRef]
- Spatz, J.P.; Roescher, A.; Sheiko, S.; Krausch, G.; Moller, M. Noble Metal Loaded Block Lonomers: Micelle Organization, Adsorption of Free Chains and Formation of Thin Films. Adv. Mater. 1995, 7, 731. [Google Scholar] [CrossRef]
- Park, M.; Harrison, C.; Chaikin, P.M.; Register, R.A.; Adamson, D.H. Block Copolymer Lithography: Periodic Arrays of ~1011 Holes in 1 Square Centimeter. Science 1997, 276, 1401–1404. [Google Scholar] [CrossRef]
- Thurn-Albrecht, T.; Schotter, J.; Kästle, G.A.; Emley, N.; Shibauchi, T.; Krusin-Elbaum, L.; Guarini, K.; Black, C.T.; Tuominen, M.T.; Russell, T.P. Ultrahigh-Density Nanowire Arrays Grown in Self-Assembled Diblock Copolymer Templates. Science 2000, 290, 2126–2129. [Google Scholar] [CrossRef]
- Lee, J.I.; Cho, S.H.; Park, S.-M.; Kim, J.K.; Kim, J.K.; Yu, J.-W.; Kim, Y.C.; Russell, T.P. Highly Aligned Ultrahigh Density Arrays of Conducting Polymer Nanorods Using Block Copolymer Templates. Nano Lett. 2008, 8, 2315–2320. [Google Scholar] [CrossRef]
- Kamcev, J.; Germack, D.S.; Nykypanchuk, D.; Grubbs, R.B.; Nam, C.-Y.; Black, C.T. Chemically Enhancing Block Copolymers for Block-Selective Synthesis of Self-Assembled Metal Oxide Nanostructures. ACS Nano 2013, 7, 339–346. [Google Scholar] [CrossRef]
- Yu, H.; Iyoda, T.; Ikeda, T. Photoinduced Alignment of Nanocylinders by Supramolecular Cooperative Motions. J. Am. Chem. Soc. 2006, 128, 11010–11011. [Google Scholar] [CrossRef]
- Sano, M.; Nakamura, S.; Hara, M.; Nagano, S.; Shinohara, Y.; Amemiya, Y.; Seki, T. Pathways toward Photoinduced Alignment Switching in Liquid Crystalline Block Copolymer Films. Macromolecules 2014, 47, 7178–7186. [Google Scholar] [CrossRef]
- Komura, M.; Yoshitake, A.; Komiyama, H.; Iyoda, T. Control of Air-Interface-Induced Perpendicular Nanocylinder Orientation in Liquid Crystal Block Copolymer Films by a Surface-Covering Method. Macromolecules 2015, 48, 672–678. [Google Scholar] [CrossRef]
- Xia, S.; Song, L.; Chen, W.; Körstgens, V.; Opel, M.; Schwartzkopf, M.; Roth, S.V.; Müller-Buschbaum, P. Printed Thin Diblock Copolymer Films with Dense Magnetic Nanostructure. ACS Appl. Mater. Interfaces 2019, 11, 21935–21945. [Google Scholar] [CrossRef]
- Song, D.-P.; Jacucci, G.; Dundar, F.; Naik, A.; Fei, H.-F.; Vignolini, S.; Watkins, J.J. Photonic Resins: Designing Optical Appearance via Block Copolymer Self-Assembly. Macromolecules 2018, 51, 2395–2400. [Google Scholar] [CrossRef]
- Giraud, E.C.; Mokarian-Tabari, P.; Toolan, D.T.W.; Arnold, T.; Smith, A.J.; Howse, J.R.; Topham, P.D.; Morris, M.A. Highly Ordered Titanium Dioxide Nanostructures via a Simple One-Step Vapor-Inclusion Method in Block Copolymer Films. ACS Appl. Nano Mater. 2018, 1, 3426–3434. [Google Scholar] [CrossRef]
- Xiao, W.; Legros, P.; Chevallier, P.; Lagueux, J.; Oh, J.K.; Fortin, M.-A. Superparamagnetic Iron Oxide Nanoparticles Stabilized with Multidentate Block Copolymers for Optimal Vascular Contrast in T1-Weighted Magnetic Resonance Imaging. ACS Appl. Nano Mater. 2018, 1, 894–907. [Google Scholar] [CrossRef]
- Lee, S.; Lee, Y.; Kim, E.-M.; Nam, K.W.; Choi, I. Aqueous-Phase Synthesis of Hyaluronic Acid-Based Hydrogel Nanoparticles for Molecular Storage and Enzymatic Release. ACS Appl. Polym. Mater. 2020, 2, 342–350. [Google Scholar] [CrossRef]
- Phan, H.; Cavanagh, R.; Destouches, D.; Vacherot, F.; Brissault, B.; Taresco, V.; Penelle, J.; Couturaud, B. H2O2-Responsive Nanocarriers Prepared by RAFT-Mediated Polymerization-Induced Self-Assembly of N-(2-(Methylthio)Ethyl)Acrylamide for Biomedical Applications. ACS Appl. Polym. Mater. 2022, 4, 7778–7789. [Google Scholar] [CrossRef]
- Saha, B.; Bhattacharyya, S.; Mete, S.; Mukherjee, A.; De, P. Redox-Driven Disassembly of Polymer–Chlorambucil Polyprodrug: Delivery of Anticancer Nitrogen Mustard and DNA Alkylation. ACS Appl. Polym. Mater. 2019, 1, 2503–2515. [Google Scholar] [CrossRef]
- Arias-Zapata, J.; Garnier, J.D.; Mehedi, H.; Legrain, A.; Salem, B.; Cunge, G.; Zelsmann, M. Engineering Self-Assembly of a High-χ Block Copolymer for Large-Area Fabrication of Transistors Based on Functional Graphene Nanoribbon Arrays. Chem. Mater. 2019, 31, 3154–3162. [Google Scholar] [CrossRef]
- Stel, B.; Gunkel, I.; Gu, X.; Russell, T.P.; De Yoreo, J.J.; Lingenfelder, M. Contrasting Chemistry of Block Copolymer Films Controls the Dynamics of Protein Self-Assembly at the Nanoscale. ACS Nano 2019, 13, 4018–4027. [Google Scholar] [CrossRef]
- Barreda, L.; Shen, Z.; Chen, Q.P.; Lodge, T.P.; Siepmann, J.I.; Hillmyer, M.A. Synthesis, Simulation, and Self-Assembly of a Model Amphiphile To Push the Limits of Block Polymer Nanopatterning. Nano Lett. 2019, 19, 4458–4462. [Google Scholar] [CrossRef] [PubMed]
- Chen, Q.P.; Barreda, L.; Oquendo, L.E.; Hillmyer, M.A.; Lodge, T.P.; Siepmann, J.I. Computational Design of High-χ Block Oligomers for Accessing 1 Nm Domains. ACS Nano 2018, 12, 4351–4361. [Google Scholar] [CrossRef] [PubMed]
- Jin, C.; Olsen, B.C.; Luber, E.J.; Buriak, J.M. Nanopatterning via Solvent Vapor Annealing of Block Copolymer Thin Films. Chem. Mater. 2017, 29, 176–188. [Google Scholar] [CrossRef]
- Li, W.; Müller, M. Defects in the Self-Assembly of Block Copolymers and Their Relevance for Directed Self-Assembly. Annu. Rev. Chem. Biomol. Eng. 2015, 6, 187–216. [Google Scholar] [CrossRef]
- Knoll, A.; Magerle, R.; Krausch, G. Phase Behavior in Thin Films of Cylinder-Forming ABA Block Copolymers: Experiments. J. Chem. Phys. 2004, 120, 1105–1116. [Google Scholar] [CrossRef]
- Harrison, C.; Angelescu, D.; Trawick, M.; Cheng, Z.; Huse, D.; Chaikin, P.; Vega, D.A.; Sebastian, J.; Register, R.; Adamson, D. Pattern Coarsening in a 2D Hexagonal System. Europhys. Lett. 2004, 67, 800. [Google Scholar] [CrossRef]
- Vega, D.A.; Harrison, C.K.; Angelescu, D.E.; Trawick, M.L.; Huse, D.A.; Chaikin, P.M.; Register, R.A. Ordering Mechanisms in Two-Dimensional Sphere-Forming Block Copolymers. Phys. Rev. E 2005, 71, 061803. [Google Scholar] [CrossRef]
- Handrea-Dragan, M.; Botiz, I. Multifunctional Structured Platforms: From Patterning of Polymer-Based Films to Their Subsequent Filling with Various Nanomaterials. Polymers 2021, 13, 445. [Google Scholar] [CrossRef]
- Anastasiadis, S.H.; Russell, T.P.; Satija, S.K.; Majkrzak, C.F. Neutron Reflectivity Studies of the Surface-Induced Ordering of Deblock Copolymer Films. Phys. Rev. Lett. 1989, 62, 1852. [Google Scholar] [CrossRef]
- Tuzar, Z.; Kratochvíl, P. Block and Graft Copolymer Micelles in Solution. Adv. Colloid Interface Sci. 1976, 6, 201–232. [Google Scholar] [CrossRef]
- Riess, G. Micellization of Block Copolymers. Prog. Polym. Sci. 2003, 28, 1107–1170. [Google Scholar] [CrossRef]
- Gohy, J.F. Block Copolymer Micelles. Adv. Polym. Sci. 2005, 190, 65–136. [Google Scholar]
- Atanase, L.I.; Riess, G. Micellization of Poly(2-Vinylpyrridine)-b-Poly(Cyclohexyl Methacrylate) (P2VP-b-PCHMA) Block Copolymers and Their Interpolymer Complex Formation in Non-Aqueous Medium. J. Colloid Interface Sci. 2019, 549, 171–178. [Google Scholar] [CrossRef] [PubMed]
- Botiz, I. Prominent Processing Techniques to Manipulate Semiconducting Polymer Microstructures. J. Mater. Chem. C 2023, 11, 364–405. [Google Scholar] [CrossRef]
- Harant, A.W.; Bowman, C.N. Solvent Vapor Annealed Block Copolymer Films on Organosilane Self-Assembled Monolayers. J. Vac. Sci. Technol. B Microelectron. Nanometer Struct. Process. Meas. Phenom. 2005, 23, 1615–1621. [Google Scholar] [CrossRef]
- Xuan, Y.; Peng, J.; Cui, L.; Wang, H.; Li, B.; Han, Y. Morphology Development of Ultrathin Symmetric Diblock Copolymer Film via Solvent Vapor Treatment. Macromolecules 2004, 37, 7301–7307. [Google Scholar] [CrossRef]
- Kim, S.; Briber, R.M.; Karim, A.; Jones, R.L.; Kim, H.-C. Directed Self-Assembly of Thin Block Copolymer Films under Controlled Atmosphere. MRS Proc. 2006, 961, 1703. [Google Scholar] [CrossRef]
- Luo, M.; Epps, T.H.I. Directed Block Copolymer Thin Film Self-Assembly: Emerging Trends in Nanopattern Fabrication. Macromolecules 2013, 46, 7567–7579. [Google Scholar] [CrossRef]
- Sinturel, C.; Vayer, M.; Morris, M.; Hillmyer, M.A. Solvent Vapor Annealing of Block Polymer Thin Films. Macromolecules 2013, 46, 5399–5415. [Google Scholar] [CrossRef]
- Cavicchi, K.A.; Russell, T.P. Solvent Annealed Thin Films of Asymmetric Polyisoprene−Polylactide Diblock Copolymers. Macromolecules 2007, 40, 1181–1186. [Google Scholar] [CrossRef]
- Albert, J.N.L.; Bogart, T.D.; Lewis, R.L.; Beers, K.L.; Fasolka, M.J.; Hutchison, J.B.; Vogt, B.D.; Epps, T.H.I. Gradient Solvent Vapor Annealing of Block Copolymer Thin Films Using a Microfluidic Mixing Device. Nano Lett. 2011, 11, 1351–1357. [Google Scholar] [CrossRef]
- Jung, Y.S.; Ross, C.A. Solvent-Vapor-Induced Tunability of Self-Assembled Block Copolymer Patterns. Adv. Mater. 2009, 21, 2540–2545. [Google Scholar] [CrossRef]
- Jung, Y.S.; Ross, C.A. Orientation-Controlled Self-Assembled Nanolithography Using a Polystyrene−Polydimethylsiloxane Block Copolymer. Nano Lett. 2007, 7, 2046–2050. [Google Scholar] [CrossRef]
- Lodge, T.P.; Dalvi, M.C. Mechanisms of Chain Diffusion in Lamellar Block Copolymers. Phys. Rev. Lett. 1995, 75, 657–660. [Google Scholar] [CrossRef]
- Bates, F.S.; Fredrickson, G.H. Block Copolymer Thermodynamics: Theory and Experiment. Annu. Rev. Phys. Chem. 1990, 41, 525–557. [Google Scholar] [CrossRef]
- Leibler, L. Theory of Microphase Separation in Block Copolymers. Macromolecules 1980, 13, 1602–1617. [Google Scholar] [CrossRef]
- Knoll, A.; Horvat, A.; Lyakhova, K.S.; Krausch, G.; Sevink, G.J.A.; Zvelindovsky, A.V.; Magerle, R. Phase Behavior in Thin Films of Cylinder-Forming Block Copolymers. Phys. Rev. Lett. 2002, 89, 035501. [Google Scholar] [CrossRef]
- Gotrik, K.W.; Hannon, A.F.; Son, J.G.; Keller, B.; Alexander-Katz, A.; Ross, C.A. Morphology Control in Block Copolymer Films Using Mixed Solvent Vapors. ACS Nano 2012, 6, 8052–8059. [Google Scholar] [CrossRef]
- Botiz, I.; Grozev, N.; Schlaad, H.; Reiter, G. The Influence of Protic Non-Solvents Present in the Environment on Structure Formation of Poly(γ-Benzyl-L-Glutamate in Organic Solvents. Soft Matter 2008, 4, 993–1002. [Google Scholar] [CrossRef] [PubMed]
- Babutan, I.; Todor-Boer, O.; Atanase, L.I.; Vulpoi, A.; Simon, S.; Botiz, I. Self-Assembly of Block Copolymers on Surfaces Exposed to Space-Confined Solvent Vapor Annealing. Polymer 2023, 273, 125881. [Google Scholar] [CrossRef]
- Babutan, I.; Todor-Boer, O.; Atanase, L.I.; Vulpoi, A.; Botiz, I. Crystallization of Poly(Ethylene Oxide)-Based Triblock Copolymers in Films Swollen-Rich in Solvent Vapors. Coatings 2023, 13, 918. [Google Scholar] [CrossRef]
- Babutan, I.; Todor-Boer, O.; Atanase, L.I.; Vulpoi, A.; Botiz, I. Self-Assembly of Block Copolymers in Thin Films Swollen-Rich in Solvent Vapors. Polymers 2023, 15, 1900. [Google Scholar] [CrossRef] [PubMed]
- Nakashima, K.; Bahadur, P. Aggregation of Water-Soluble Block Copolymers in Aqueous Solutions: Recent Trends. Adv. Colloid Interface Sci. 2006, 123–126, 75–96. [Google Scholar] [CrossRef]
- Cho, H.K.; Cheong, I.W.; Lee, J.M.; Kim, J.H. Polymeric Nanoparticles, Micelles and Polymersomes from Amphiphilic Block Copolymer. Korean J. Chem. Eng. 2000, 27, 731–740. [Google Scholar] [CrossRef]
- Kulthe, S.S.; Choudhari, Y.M.; Inamdar, N.N.; Mourya, V. Polymeric Micelles: Authoritative Aspects for Drug Delivery. Des. Monomers Polym. 2012, 15, 465–521. [Google Scholar] [CrossRef]
- Xu, W.; Ling, P.; Zhang, T. Polymeric Micelles, a Promising Drug Delivery System to Enhance Bioavailability of Poorly Water-Soluble Drugs. J. Drug Deliv. 2013, 340315, 1–15. [Google Scholar] [CrossRef]
- Ahmad, Z.; Shah, A.; Siddiq, M.; Kraatz, H.B. Polymeric Micelles as Drug Delivery Vehicles. RSC Adv. 2014, 4, 17028–17038. [Google Scholar] [CrossRef]
- Walther, A.; Goldmann, A.S.; Yelamanchili, R.S.; Drechsler, M.; Schmalz, H.; Eisenberg, A.; Müller, A.H.E. Multiple Morphologies, Phase Transitions, and Cross-Linking of Crew-Cut Aggregates of Polybutadiene-Block-Poly(2-Vinylpyridine) Diblock Copolymers. Macromolecules 2008, 41, 3254–3260. [Google Scholar] [CrossRef]
- Gu, X.; Gunkel, I.; Russell, T.P. Pattern Transfer Using Block Copolymers. Phil. Trans. R. Soc. A 2013, 371, 20120306. [Google Scholar] [CrossRef] [PubMed]
- Arges, C.G.; Kambe, Y.; Dolejsi, M.; Wu, G.-P.; Segal-Pertz, T.; Ren, J.; Cao, C.; Craig, G.S.W.; Nealey, P.F. Interconnected Ionic Domains Enhance Conductivity in Microphase Separated Block Copolymer Electrolytes. J. Mater. Chem. A 2017, 5, 5619–5629. [Google Scholar] [CrossRef]
- Park, S.; Kim, B.; Cirpan, A.; Russell, T.P. Preparation of Metallic Line Patterns from Functional Block Copolymers. Small 2009, 5, 1343–1348. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.; Kwak, J.; Choi, C.; Han, S.H.; Kim, J.K. Phase Behavior of Poly(2-Vinylpyridine)-Block-Poly(4-Vinylpyridine) Copolymers Containing Gold Nanoparticles. Macromolecules 2017, 50, 9373–9379. [Google Scholar] [CrossRef]
- Rahman, M.S.; Samal, S.; Lee, J.-S. Quantitative in Situ Coupling of Living Diblock Copolymers for the Preparation of Amphiphilic Coil−Rod−Coil Triblock Copolymer Poly(2-Vinylpyridine)-b-Poly(n-Hexyl Isocyanate)-b-Poly(2-Vinylpyridine). Macromolecules 2007, 40, 9279–9283. [Google Scholar] [CrossRef]
- Li, Z.; Zhao, W.; Liu, Y.; Rafailovich, M.H.; Sokolov, J.; Khougaz, K.; Eisenberg, A.; Lennox, R.B.; Krausch, G. Self-Ordering of Diblock Copolymers from Solution. J. Am. Chem. Soc. 1996, 118, 10892–10893. [Google Scholar] [CrossRef]
- Soum, A.; Fontanille, M.; Sigwalt, P. Anionic Polymerization of 2-Vinylpyridine Initiated by Symmetrical Organomagnesium Compounds in Tetrahydrofuran. J. Polym. Sci. Polym. Chem. Ed. 1977, 15, 659–673. [Google Scholar] [CrossRef]
- Atanase, L.I.; Riess, G. Stabilization of Non-Aqueous Emulsions by Poly(2-Vinylpyridine)-b-Poly(Butadiene) Block Copolymers. Colloids Surf. A Physicochem. Eng. Asp. 2014, 458, 19–24. [Google Scholar] [CrossRef]
- Jahanshahi, K.; Botiz, I.; Reiter, R.; Scherer, H.; Reiter, G. Reversible Nucleation, Growth, and Dissolution of Poly(γ-Benzyl l-Glutamate) Hexagonal Columnar Liquid Crystals by Addition and Removal of a Nonsolvent. Cryst. Growth Des. 2013, 13, 4490–4494. [Google Scholar] [CrossRef]
- Jahanshahi, K.; Botiz, I.; Reiter, R.; Thomann, R.; Heck, B.; Shokri, R.; Stille, W.; Reiter, G. Crystallization of Poly(γ-Benzyl L-Glutamate) in Thin Film Solutions: Structure and Pattern Formation. Macromolecules 2013, 46, 1470–1476. [Google Scholar] [CrossRef]
- Băbuțan, M.; Botiz, I. Morphological Characteristics of Biopolymer Thin Films Swollen-Rich in Solvent Vapors. Biomimetics 2024, 9, 396. [Google Scholar] [CrossRef] [PubMed]
- Cotoarbă, Y.; Todor-Boer, O.; Botiz, I. Crystals of Nonfullerene Acceptors Generated by Rich Exposure to Solvent Vapors. Cryst. Growth Des. 2025. [Google Scholar] [CrossRef]
- Todor-Boer, O.; Farcău, C.; Botiz, I. Large Enhancement of Photoluminescence Obtained in Thin Polyfluorene Films of Optimized Microstructure. Polymers 2024, 16, 2278. [Google Scholar] [CrossRef] [PubMed]
- Flory, P.J. Statistical Thermodynamics of Polymer Solutions. In Principles of Polymer Chemistry; Cornell University Press: Ithaca, NY, USA, 1953; pp. 495–540. [Google Scholar]
- Tseng, H.; Wong, P.; Lloyd, D.R.; Barlow, J.W. Thermodynamic Interaction in Polybutadiene/Solute Systems by Inverse Gas Chromatography. Polym. Eng. Sci. 1987, 27, 1141–1147. [Google Scholar] [CrossRef]
- Arichi, S.; Matsuura, H.; Tanimoto, Y.; Murata, H. Studies of Poly-2-Vinylpyridine. II. Solubilities in Various Solvents. Bull. Chem. Soc. Jpn. 1966, 39, 434–439. [Google Scholar] [CrossRef]
- Ren, Z.B.; Liu, J.; Chen, Y.P.; Chen, M.; Qian, D.J. Facile Fabrication of Porous Pure and Ag Nanoparticle-Doped Poly(4-Vinylpyridine) Films at the Liquid–Liquid Interfaces. Chin. Chem. Lett. 2011, 22, 867–870. [Google Scholar] [CrossRef]
- Raczkowska, J.; Stetsyshyn, Y.; Awsiuk, K.; Zemła, J.; Kostruba, A.; Harhay, K.; Marzec, M.; Bernasik, A.; Lishchynskyi, O.; Ohar, H.; et al. Temperature-Responsive Properties of Poly(4-Vinylpyridine) Coatings: Influence of Temperature on the Wettability, Morphology, and Protein Adsorption. RSC Adv. 2016, 6, 87469–87477. [Google Scholar] [CrossRef]
- Chung, T.-M.; Wang, H.-F.; Lin, T.; Chiang, Y.-W.; Chen, Y.-C.; Ko, B.-T.; Ho, R.-M. Helical Phase Driven by Solvent Evaporation in Self-Assembly of Poly(4-Vinylpyridine)-Block-Poly(l-Lactide) Chiral Block Copolymers. Macromolecules 2012, 45, 9727–9733. [Google Scholar] [CrossRef]
- Li, H.-J.; Tsiang, R.C.-C. Preparation and Characterization of a Linear Poly(4-Vinyl Pyridine)-b-Polybutadiene-b-Poly(4-Vinylpyridine) Using a t-Butyllithium/m-Diisopropenylbenzene Diadduct as a Dicarbanion Initiator. Polymer 2000, 41, 5601–5610. [Google Scholar] [CrossRef]
- Burkert, S.; Bittrich, E.; Kuntzsch, M.; Müller, M.; Eichhorn, K.-J.; Bellmann, C.; Uhlmann, P.; Stamm, M. Protein Resistance of PNIPAAm Brushes: Application to Switchable Protein Adsorption. Langmuir 2010, 26, 1786–1795. [Google Scholar] [CrossRef]
- Ansarifar, M.A.; Luckham, P.F. Measurement of the Interaction Force Profiles between Block Copolymers of Poly(2-Vinylpyridine)/Poly(t-Butylstyrene) in a Good Solvent. Polymer 1988, 29, 329–335. [Google Scholar] [CrossRef]
- Chuang, V.P.; Ross, C.A.; Gwyther, J.; Manners, I. Self-Assembled Nanoscale Ring Arrays from a Polystyrene-b-Polyferrocenylsilane-b-Poly(2-Vinylpyridine)Triblock Terpolymer Thin Film. Adv. Mater. 2009, 21, 3789–3793. [Google Scholar] [CrossRef]
- Elbs, H.; Krausch, G. Ellipsometric Determination of Flory-Huggins Interaction Parameters in Solution. Polymer 2004, 45, 7935–7942. [Google Scholar] [CrossRef]
- Oss-Ronen, L.; Schmidt, J.; Abetz, V.; Radulescu, A.; Cohen, Y.; Talmon, Y. Characterization of Block Copolymer Self-Assembly: From Solution to Nanoporous Membranes. Macromolecules 2012, 45, 9631–9642. [Google Scholar] [CrossRef]
- Larison, T.; Stefik, M. Persistent Micelle Corona Chemistry Enables Constant Micelle Core Size with Independent Control of Functionality and Polyelectrolyte Response. Langmuir 2021, 37, 9817–9825. [Google Scholar] [CrossRef] [PubMed]
- Muñoz-Bonilla, A.; Fernández-García, M.; Cerrada, M.L.; Mantovani, G.; Haddleton, D.M. Aggregation and Solubilization of Organic Solvents and Petrol/Gasoline in Water Mediated by Block Copolymers. Eur. Polym. J. 2007, 43, 4583–4592. [Google Scholar] [CrossRef]
- Muñoz-Bonilla, A.; Haddleton, D.M.; Cerrada, M.L.; Fernández-García, M. Synthesis of Poly(Di[Methylamine]Ethyl Methacrylate)-b-Poly(Cyclohexyl Methacrylate)-b-Poly(Di[Methylamine]Ethyl Methacrylate) Amphiphilic Triblock Copolymers by ATRP: Condensed-Phase and Solution Properties. J. Polym. Sci. Part A Polym. Chem. 2008, 46, 85–92. [Google Scholar] [CrossRef]
- Han, T.; Cao, W.; Xu, Z.; Adibnia, V.; Olgiati, M.; Valtiner, M.; Ma, L.; Zhang, C.; Ma, M.; Luo, J.; et al. Hydration Layer Structure Modulates Superlubrication by Trivalent La3+ Electrolytes. Sci. Adv. 2023, 9, eadf3902. [Google Scholar] [CrossRef]
- Yu, J.; Jackson, N.E.; Xu, X.; Morgenstern, Y.; Kaufman, Y.; Ruths, M.; de Pablo, J.J.; Tirrell, M. Multivalent Counterions Diminish the Lubricity of Polyelectrolyte Brushes. Science 2018, 360, 1434–1438. [Google Scholar] [CrossRef]
- Wan, X.; Zhao, Y.; Li, Z.; Li, L. Emerging Polymeric Electrospun Fibers: From Structural Diversity to Application in Flexible Bioelectronics and Tissue Engineering. Exploration 2022, 2, 20210029. [Google Scholar] [CrossRef]
- Sun, Z.; Ou, Q.; Dong, C.; Zhou, J.; Hu, H.; Li, C.; Huang, Z. Conducting Polymer Hydrogels Based on Supramolecular Strategies for Wearable Sensors. Exploration 2024, 4, 20220167. [Google Scholar] [CrossRef] [PubMed]
- Han, T.; Zhang, S.; Zhang, C. Unlocking the Secrets behind Liquid Superlubricity: A State-of-the-Art Review on Phenomena and Mechanisms. Friction 2022, 10, 1137–1165. [Google Scholar] [CrossRef]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Babutan, I.; Atanase, L.I.; Botiz, I. Self-Assembly of Lamellar/Micellar Block Copolymers Induced Through Their Rich Exposure to Various Solvent Vapors: An AFM Study. Materials 2025, 18, 1759. https://doi.org/10.3390/ma18081759
Babutan I, Atanase LI, Botiz I. Self-Assembly of Lamellar/Micellar Block Copolymers Induced Through Their Rich Exposure to Various Solvent Vapors: An AFM Study. Materials. 2025; 18(8):1759. https://doi.org/10.3390/ma18081759
Chicago/Turabian StyleBabutan, Iulia, Leonard Ionut Atanase, and Ioan Botiz. 2025. "Self-Assembly of Lamellar/Micellar Block Copolymers Induced Through Their Rich Exposure to Various Solvent Vapors: An AFM Study" Materials 18, no. 8: 1759. https://doi.org/10.3390/ma18081759
APA StyleBabutan, I., Atanase, L. I., & Botiz, I. (2025). Self-Assembly of Lamellar/Micellar Block Copolymers Induced Through Their Rich Exposure to Various Solvent Vapors: An AFM Study. Materials, 18(8), 1759. https://doi.org/10.3390/ma18081759







