Influence of Corrosion-Inhibiting Monolayers on the Bond Strength and Durability of Reinforced Concrete Structures Under Service Conditions
Abstract
1. Introduction
2. Materials and Methods
2.1. Inhibitory Monolayers
- Natural binding. Prior to treatment, the surface of the rebars underwent a chemical pickling process by immersion in a 1 M phosphoric acid solution for 5 min. Subsequently, the rebars were rinsed with distilled water to ensure the complete removal of any residual pickling solution. Once prepared, the rebars were immersed in a solution of the active compound for 10 min. This method does not involve any additional intervention that could alter the natural chemical adhesion process, which is driven by the attraction between opposite charges, thereby facilitating the formation of chemical bonds between the inhibitory compound and the metal surface.
- Electrolysis. Following the chemical pickling process and subsequent rinsing with distilled water, the rebars were immersed in the inhibitory solution. A constant current density of 0.5 ± 0.1 mA/cm2 was applied for 120 min using a regulated power supply unit (FAC-363B, Promax, Barcelona, Spain). Throughout the electrolysis process, the solution was maintained under constant agitation with a magnetic stirrer to ensure uniform monolayer formation. Additionally, a multimeter connected in series was incorporated to monitor and adjust the applied potential, ensuring current stability throughout the process.
2.2. Reinforced Concrete Specimens
2.3. Methods
2.3.1. Scanning Electron Microscopy Tests (SEM) and Energy Dispersive X-Ray Spectroscopy (EDX)
2.3.2. Bond Strength Test
2.3.3. Corrosion Test
3. Results and Discussion
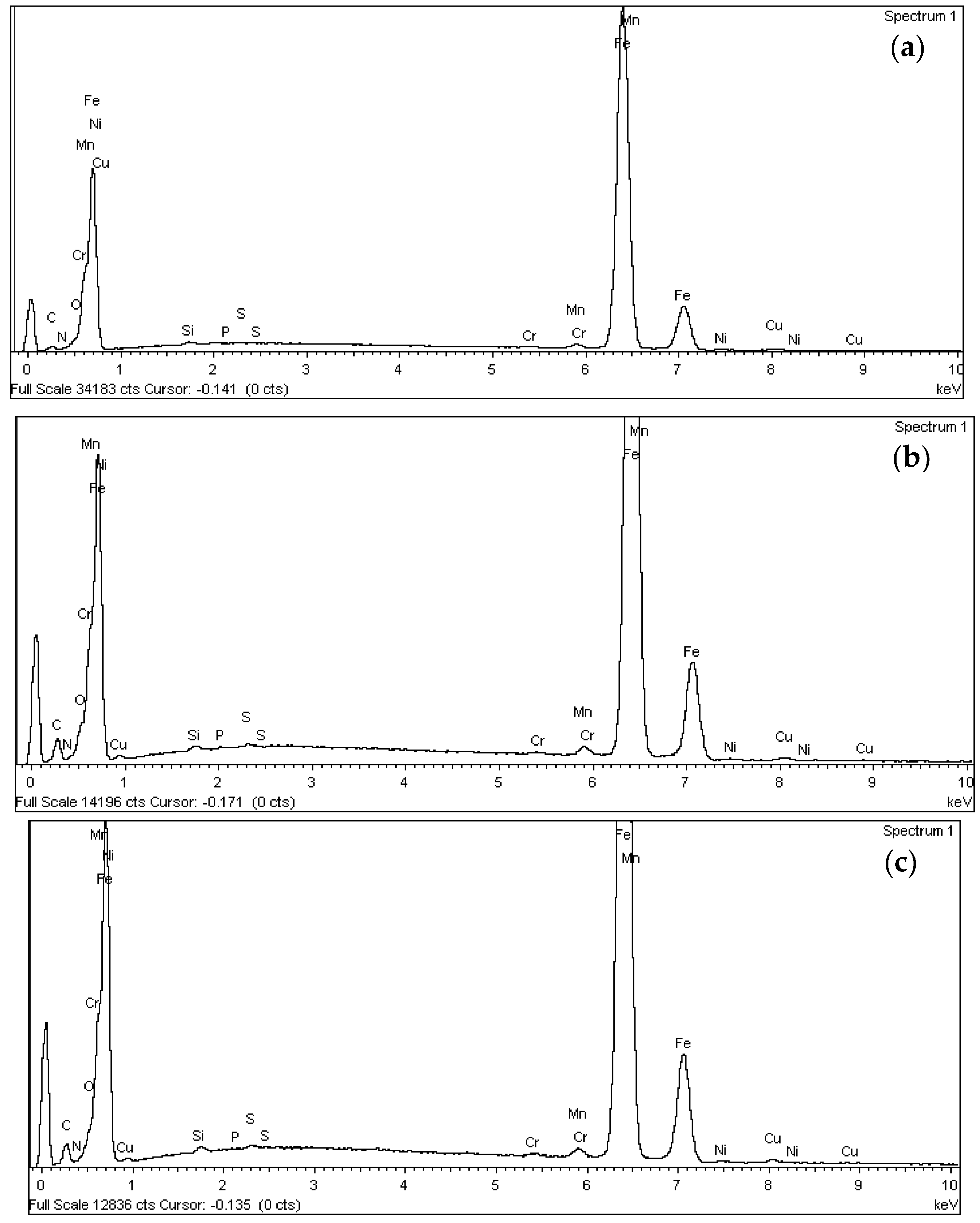
3.1. Scanning Electron Microscopy (SEM) and Energy Dispersive X-ray Spectroscopy (EDX)
- The iron (Fe) percentage decreases from 93.87% in non-treated rebar to an average of 80% after the application of the treatment. This result is particularly significant as it suggests a reduction in the exposed steel surface following treatment. Additionally, the values obtained for both techniques are very similar, indicating a comparable distribution of the inhibitory compound on the steel surface.
- The oxygen (O) content on the surface increases from 0.81% to values above 5%. This increase is attributed to the inhibitory compound, which introduces oxygen through the carboxyl group. This behaviour confirms the presence of the compound on the metal surface, demonstrating its effective adsorption.
- The presence of carbon (C) on the surface increases from 3.22% to values close to 12%, representing, along with iron (Fe), the most significant variation observed. This increase can be attributed to 4-aminobenzoic acid, which consists of an aromatic ring primarily composed of carbon and hydrogen, characteristic elements of organic compounds. This variation further reinforces the evidence of the compound’s presence on the metal surface.
- Regarding nitrogen (N), an element contributed by the amino group of the inhibitory compound, its percentage increases from 0.01% to an average value of 0.80%. This increase provides further evidence of the presence and distribution of the compound on the steel surface.
- No significant differences were observed in the content of the remaining analysed components.
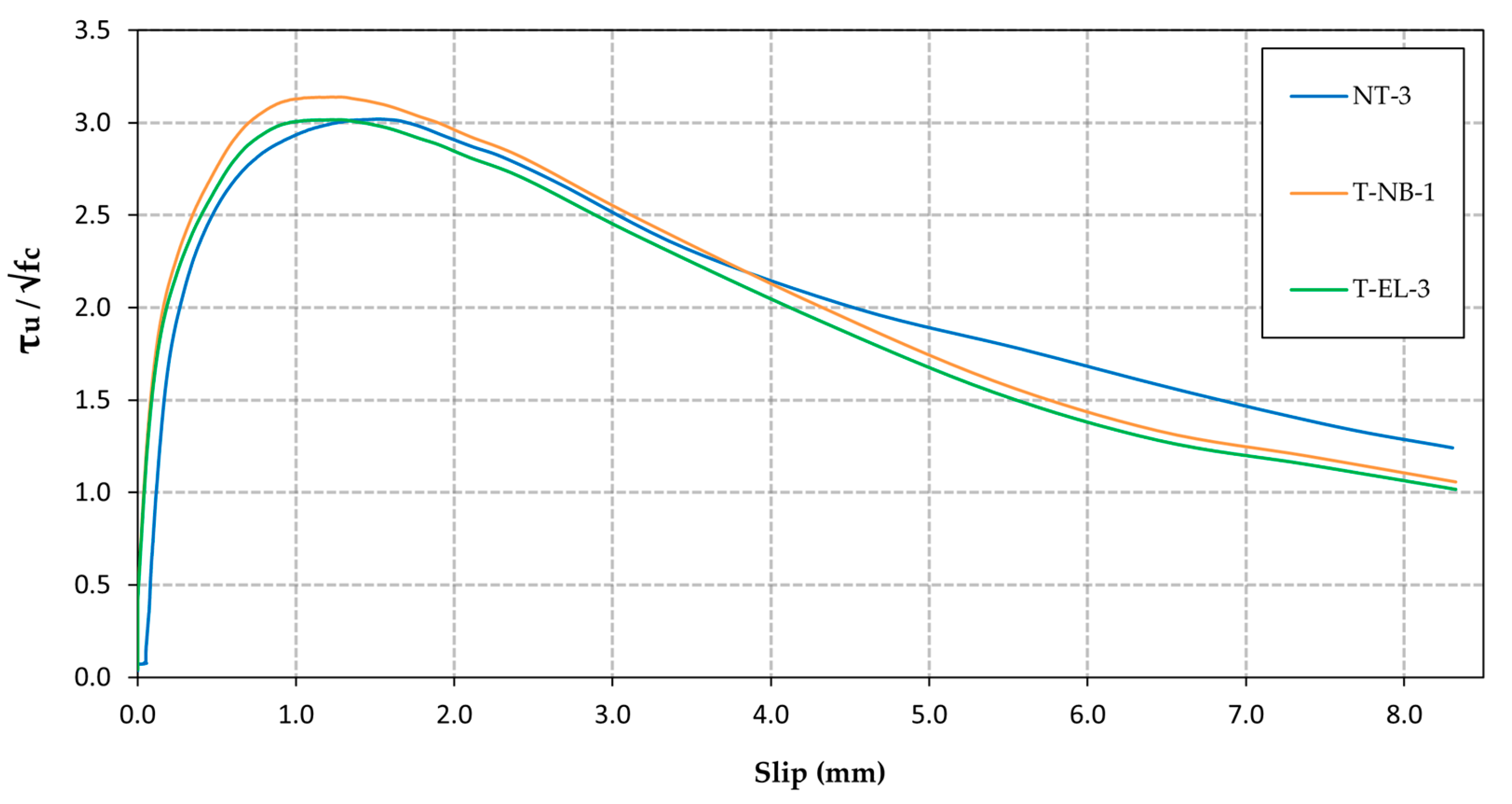
3.2. Bond Strength
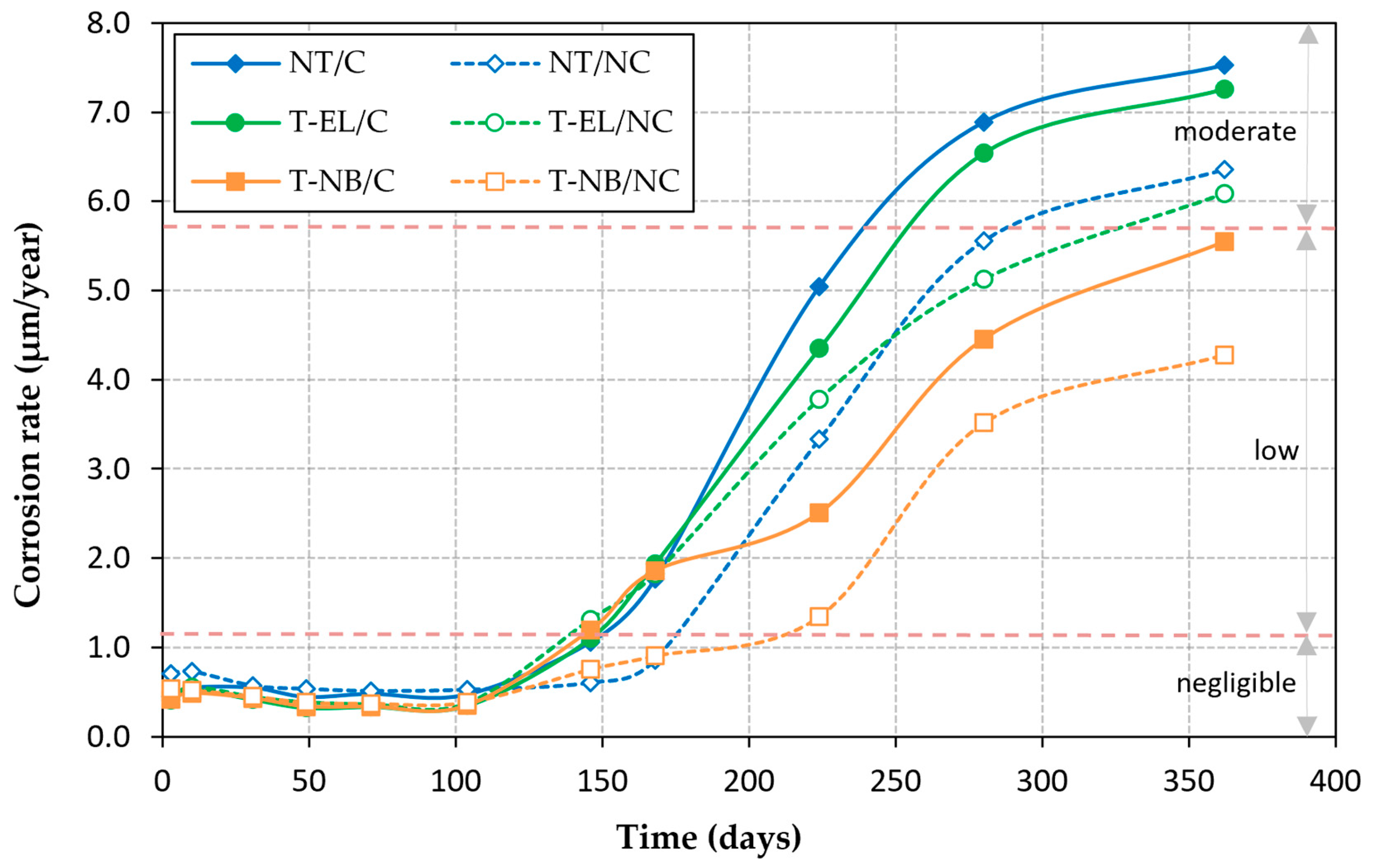
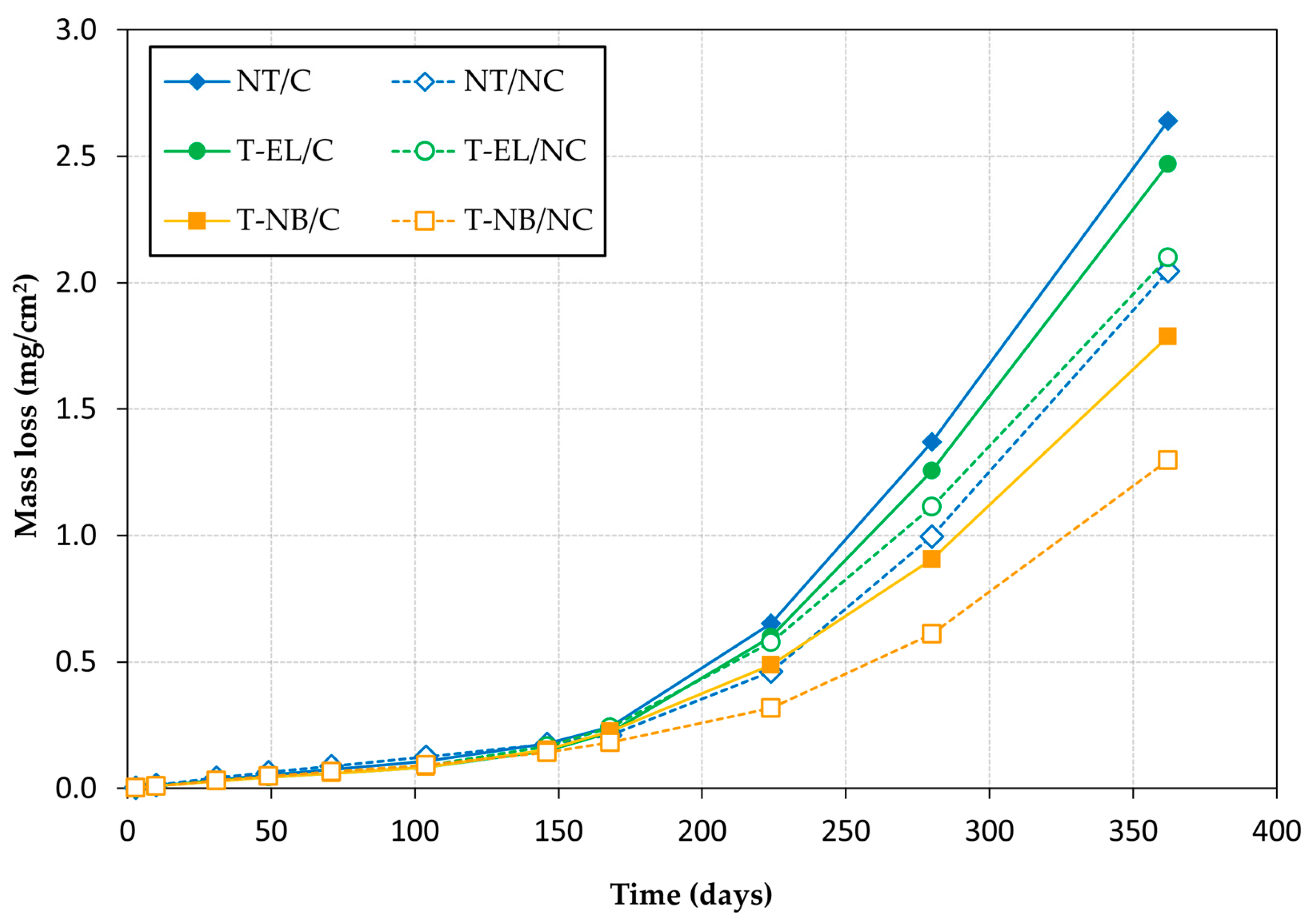
3.3. Corrosion Rate
4. Conclusions
- The Scanning Electron Microscopy (SEM) tests show that the exposed steel surface decreases after the application of the inhibitor monolayer. The distribution of this layer on the steel surface is similar for both techniques analysed in the study: natural binding and electrolysis.
- The application of the inhibitor monolayer by natural binding or electrolysis does not affect the bond strength between reinforcement and concrete, exhibiting behaviour similar to that of the untreated rebars.
- The application of load–unload cycles adversely affects the passive layer that protects the reinforcement from corrosion, accelerating corrosion kinetics. In specimens submerged in seawater for one year, the steel mass loss in rebars that had previously been subjected to cyclic loading was higher than in those that had not: 18% in NT rebars, 29% in T-NB rebars, and 19% in T-EL rebars.
- The rebars treated with the inhibitor exhibited less corrosion than the untreated rebars. This reduction in corrosion is particularly significant in the rebars treated with the natural fixation method of the inhibitor monolayer, where, after one year of testing, the steel section loss was 32% lower than in the untreated rebars for the loaded specimens and 37% lower for the unloaded specimens.
- The inhibitor monolayer provides less corrosion protection when the treatment is applied by electrolysis than when it is applied by natural binding. However, since in each case the concrete strength of the test specimens was not the same, it is not possible to attribute this poorer performance solely to the treatment application technique, and further research is needed to determine the real impact of the application method.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Yuvaraj, S.; Nirmalkumar, K.; Rajesh Kumar, V.; Gayathri, R.; Mukilan, K.; Shubikksha, S. Influence of corrosion inhibitors in reinforced concrete—A state of art of review. Mater. Today Proc. 2022, 68, 2406–2412. [Google Scholar]
- Geng, Z.; Shi, J. Passivation behavior of steel in novel low-carbon binders with belitic calcium sulfoaluminate cement. Cem. Concr. Compos. 2024, 152, 105686. [Google Scholar]
- Wang, D.; Yue, Y.; Qian, J. Effect of carbonation on the corrosion behavior of steel rebar embedded in magnesium phosphate cement. Compos. Part B Eng. 2024, 268, 111088. [Google Scholar]
- Lliso-Ferrando, J.R.; Gasch, I.; Martínez-Ibernón, A.; Valcuende Payá, M.O. Effect of macrocell currents on rebar corrosion in reinforced concrete structures exposed to a marine environment. Ocean Eng. 2022, 257, 1–12. [Google Scholar]
- Lliso-Ferrando, J.R.; Soto Camino, J.; Gasch, I.; Valcuende Payá, M.O. Significance of macrocell currents in reinforced concrete columns partially immersed in seawater. Constr. Build. Mater. 2023, 389, 131739. [Google Scholar]
- Lliso-Ferrando, J.R.; Gasch, I.; Martínez-Ibernón, A.; Valcuende Payá, M.O. Macrocell Corrosion Currents in Simulated Con-crete Pore Solution and Reinforced Concrete. Int. J. Concr. Struct. Mater. 2023, 17, 15. [Google Scholar] [CrossRef]
- Echeverría Boan, M.; Rodríguez, A.; Abreu, C.M.; Echeverría, C.A. Unraveling the impact of chloride and sulfate ions collection on atmospheric corrosion of steel. Corrosion 2013, 69, 1217–1224. [Google Scholar]
- Parangusan, H.; Bhadra, J.; Al-Thani, N. A review of passivity breakdown on metal surfaces: Influence of chloride- and sul-fide-ion concentrations, temperature, and pH. Emerg. Mater. 2021, 4, 1187–1203. [Google Scholar]
- Calavera, J.; Delibes, A.; Izquierdo, J.M.; González, G. Influencia de la Oxidación y de las Manchas de Mortero Sobre la Adherencia de Armaduras de Hormigón; Intemac: Madrid, Spain, 1979. [Google Scholar]
- Ndukwe, A.I. Green inhibitors for corrosion of metals in acidic media: A review. Acad. J. Manuf. Eng. 2022, 20, 36–50. [Google Scholar]
- El Ibrahimi, B.; Jmiai, A.; Bazzi, L.; El Issami, S. Amino acids and their derivatives as corrosion inhibitors for metals and alloys. Arab. J. Chem. 2020, 13, 740–771. [Google Scholar]
- Zhao, R.; Xu, W.; Yu, Q.; Niu, L. Synergistic effect of SAMs of S-containing amino acids and surfactant on corrosion inhibition of 316L stainless steel in 0.5 M NaCl solution. J. Mol. Liq. 2020, 318, 114322. [Google Scholar]
- Sadeghi, N.; Sharma, A. Pull-out test for studying bond strength in corrosion affected reinforced concrete structures—A review. Otto-Graf-J. 2019, 18, 259–272. [Google Scholar]
- Tondolo, F. Bond behaviour with reinforcement corrosion. Constr. Build. Mater. 2015, 93, 926–932. [Google Scholar]
- Rilem. Technical Recommendations for the Testing and Use of Construction Materials; CRC Press: London, UK, 2018. [Google Scholar]
- Adasooriya, N.D.; Samarakoon, S.; Gudmestad, O.T. Corrosion propagation phase and bond strength degradation of rein-forced concrete structures: State of the art. Int. J. Comput. Methods Exp. Meas. 2018, 6, 499–514. [Google Scholar]
- Bošnjak, J.; Sharma, A.; Öttl, C. Modified beam-end test setup to study the bond behavior of reinforcement in concrete after fire. Mater. Struct. 2018, 51, 1–10. [Google Scholar]
- Fazayel, A.S.; Khorasani, M.; Sarabi, A.A. The effect of functionalized polycarboxylate structures as corrosion inhibitors in a simulated concrete pore solution. Appl. Surf. Sci. 2018, 441, 895–913. [Google Scholar]
- Yoo, S.H.; Kim, Y.W.; Chung, K.; Kim, N.K.; Kim, J.S. Corrosion Inhibition Properties of Triazine Derivatives Containing Carboxylic Acid and Amine Groups in 1.0 M HCl Solution. Ind. Eng. Chem. Res. 2013, 52, 10880–10889. [Google Scholar] [CrossRef]
- Okey, N.C.; Obasi, N.L.; Ejikeme, P.M.; Ndinteh, D.T.; Romasami, P.; Sherif, E.M.; Akpan, E.D.; Ebenso, E.E. Evaluation of some amino benzoic acid and 4-aminoantipyrine derived Schiff bases as corrosion inhibitors for steel in acidic medium: Systhesis, experimental and computational studies. J. Mol. Liq. 2020, 315, 113773. [Google Scholar]
- Imjjad, A.; Abbiche, K.; Mellaoui, M.D.; Jmiai, A.; El Baraka, N.; Taleb, A.A.; Bazzi, I.; El Issami, S.; Hilali, M.; Said, R.B.; et al. Corrosion inhibition of mild steel by aminobenzoic acid isomers in hydrochloric acid solution: Efficiency and adsorption mechanisms. Appl. Surf. Sci. 2022, 576, 151780. [Google Scholar]
- Bazzi, A.; Abbiche, K.; Izzaouihda, S.; Acharjee, N.; Hanane, Z.; Marakchi, K.; Komiha, N.; El Issami, S.; Bazzi, L.; Hilali, M. Inhibition efficiency and adsorption mechanism of 4-aminobenzoic acid for copper corrosion in nitric acid medium: A combined experimental and theoretical investigation. Struct. Chem. 2021, 32, 2183–2198. [Google Scholar]
- UNE-EN 12390-3:2003; Testing Hardened Concrete—Part 3: Compressive Strength of Test Specimens. AENOR: Madrid, Spain, 2003.
- EN 10080:2006; Steel for the Reinforcement of Concrete–Weldable Reinforcing Steel–General. European Committee for Standardization (CEN): Brussels, Belgium, 2006.
- Zhang, W.; Liu, Z.; Liu, Q.; Zhang, W. Investigation of bond behavior between waterborne epoxy-coated steel reinforcing bars (WECR) and concrete. J. Build. Eng. 2023, 76, 107328. [Google Scholar] [CrossRef]
- Lindorf, A.; Lemnitzer, L.; Curbach, M. Experimental investigations on bond behaviour of reinforced concrete under transverse tensión and repeated loading. Eng. Struct. 2009, 31, 1469–1476. [Google Scholar]
- Katz, A. Bond to concrete of FRP rebars after cyclic loading. J. Compos. Constr. 2000, 4, 137–144. [Google Scholar]
- Macioski, G.; Souza, D.J.; Capraro Brandao, A.P.; de Medeiros, M.H.F. Análisis de la corrosión de barras de acero en función de la variación del pH del medio. Alconpat 2016, 6, 223–234. [Google Scholar] [CrossRef][Green Version]
- ACI 318-19; Building Code Requirements for Structural Concrete and Commentary. American Concrete Institute (ACI): Farmington Hills, MI, USA, 2019.
- Fédération Internationale du Béton (fib). Bulletin No. 1: Bond of Reinforcement in Concrete; FIB: Lausanne, Switzerland, 2000. [Google Scholar]
- UNE 112072:2011; Corrosión de Armaduras en Estructuras de Hormigón. Medida de la Velocidad de Corrosión de Armaduras en Probetas y Estructuras de Hormigón Armado. Método de la Resistencia de Polarización. UNE: Madrid, Spain, 2011.
- ASTM STP 1065; Corrosion Rates of Steel in Concrete. Berke, N.S., Chaker, V., Whiting, D.A., Eds.; American Society for Testing and Materials: Philadelphia, PA, USA, 1990.
- RILEM Technical Committee TC-154. Electrochemical Techniques for Measuring Metallic Corrosion; RILEM Publications: Paris, France, 2003. [Google Scholar]

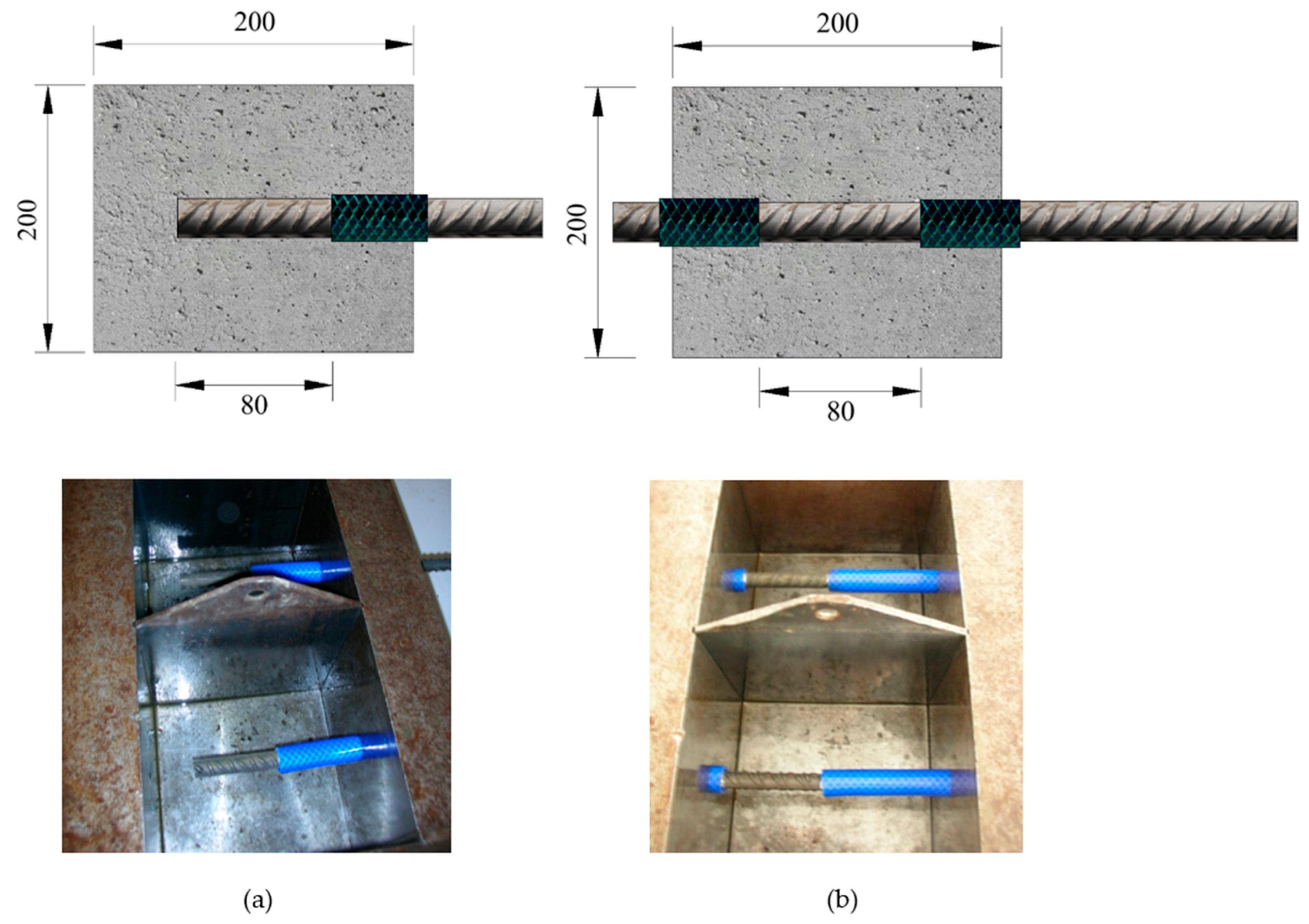
| Cement (kg/m3) | Water (kg/m3) | w/c | Admixture (kg/m3) | Sand (kg/m3) | Gravel (kg/m3) |
|---|---|---|---|---|---|
| 340.0 | 187.0 | 0.55 | 2.7 | 1,108.3 | 738.8 |
| Batch | Specimen 1 | Specimen 2 | Mean |
|---|---|---|---|
| NT | 51.51 | 52.70 | 52.11 |
| T-NB | 48.09 | 49.99 | 49.04 |
| T-EL | 43.71 | 46.79 | 45.25 |
| No. Batches | Test | |||||
|---|---|---|---|---|---|---|
| Bond (Pull-out) | Corrosion Rate | Compressive Strength | ||||
| Specimen | No. Specimens | Specimen | No. Specimens | Specimen | No. Specimens | |
| 3 (1 per treatment) | Cubic (Figure 2a) (200 mm) | 24 (8 per batch) | Cubic (Figure 2b) (200 mm) | 24 (8 per batch) | Cylindrical (∅150 mm) | 6 (2 per batch) |
| Element | No Treatment | Natural Binding | Electrolysis | |||
|---|---|---|---|---|---|---|
| Weight (%) | Mole Fraction | Weight (%) | Mole Fraction | Weight (%) | Mole Fraction | |
| Fe | 93.87 | 0.8223 | 80.08 | 0.5055 | 79.91 | 0.5005 |
| C | 3.22 | 0.1311 | 11.53 | 0.3384 | 11.93 | 0.3475 |
| Mn | 0.86 | 0.0076 | 0.93 | 0.0060 | 0.84 | 0.0053 |
| O | 0.81 | 0.0248 | 5.52 | 0.1216 | 5.33 | 0.1165 |
| Cu | 0.46 | 0.0036 | 0.46 | 0.0026 | 0.47 | 0.0026 |
| Si | 0.31 | 0.0053 | 0.28 | 0.0035 | 0.27 | 0.0033 |
| Ni | 0.19 | 0.0016 | 0.11 | 0.0007 | 0.15 | 0.0009 |
| Cr | 0.14 | 0.0013 | 0.16 | 0.0011 | 0.11 | 0.0007 |
| P | 0.10 | 0.0016 | 0.13 | 0.0015 | 0.12 | 0.0013 |
| S | 0.04 | 0.0006 | 0.06 | 0.0015 | 0.04 | 0.0004 |
| N | 0.01 | 0.0002 | 0.74 | 0.0186 | 0.84 | 0.0210 |
| Total | 100 | 1 | 100 | 1 | 100 | 1 |
| Rebar Treatment | Batch | Specimen | Maximum Load (kN) | τu (MPa) | fc (MPa) | Mode of Failure | |
|---|---|---|---|---|---|---|---|
| No treatment | NT | NT-1 | 84.12 | 20.92 | 2.90 | Pull-out | |
| NT | NT-2 | 90.64 | 22.54 | 3.12 | Pull-out | ||
| NT | NT-3 | 87.80 | 21.83 | 3.02 | Pull-out | ||
| NT | NT-4 | 88.33 | 21.97 | 52.11 | 3.04 | Pull-out | |
| NT | NT-5 | 84.33 | 20.97 | 2.91 | Pull-out | ||
| NT | NT-6 | 79.68 | 19.81 | 2.74 | Pull-out | ||
| NT | NT-7 | 88.43 | 21.99 | 3.05 | Pull-out | ||
| NT | NT-8 | 102.15 | 25.40 | 3.52 | Pull-out | ||
| Natural binding | T-NB | T-NB-1 | 87.45 | 21.75 | 3.11 | Pull-out | |
| T-NB | T-NB-2 | 87.42 | 21.74 | 3.10 | Pull-out | ||
| T-NB | T-NB-3 | 82.89 | 20.61 | 2.94 | Pull-out | ||
| T-NB | T-NB-4 | 89.25 | 22.19 | 49.04 | 3.17 | Pull-out | |
| T-NB | T-NB-5 | 81.33 | 20.23 | 2.89 | Pull-out | ||
| T-NB | T-NB-6 | 78.57 | 19.54 | 2.79 | Pull-out | ||
| T-NB | T-NB-7 | 83.63 | 20.80 | 2.97 | Pull-out | ||
| T-NB | T-NB-8 | 73.81 | 18.36 | 2.62 | Pull-out | ||
| Electrolysis | T-EL | T-EL-1 | 84.77 | 21.08 | 3.13 | Pull-out | |
| T-EL | T-EL-2 | 75.15 | 18.69 | 2.78 | Pull-out | ||
| T-EL | T-EL-3 | 80.54 | 20.03 | 2.98 | Pull-out | ||
| T-EL | T-EL-4 | 83.49 | 20.76 | 45.25 | 3.09 | Pull-out | |
| T-EL | T-EL-5 | 83.75 | 20.83 | 3.10 | Pull-out | ||
| T-EL | T-EL-6 | 71.34 | 17.74 | 2.64 | Pull-out | ||
| T-EL | T-EL-7 | 82.70 | 20.57 | 3.06 | Pull-out | ||
| T-EL | T-EL-8 | 72.91 | 18.13 | 2.70 | Pull-out |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Monzón-Bello, P.; Vengut-Tro, R.; Soto-Camino, J.; Valcuende-Payá, M.O. Influence of Corrosion-Inhibiting Monolayers on the Bond Strength and Durability of Reinforced Concrete Structures Under Service Conditions. Materials 2025, 18, 1656. https://doi.org/10.3390/ma18071656
Monzón-Bello P, Vengut-Tro R, Soto-Camino J, Valcuende-Payá MO. Influence of Corrosion-Inhibiting Monolayers on the Bond Strength and Durability of Reinforced Concrete Structures Under Service Conditions. Materials. 2025; 18(7):1656. https://doi.org/10.3390/ma18071656
Chicago/Turabian StyleMonzón-Bello, Pablo, Roberto Vengut-Tro, Juan Soto-Camino, and Manuel Octavio Valcuende-Payá. 2025. "Influence of Corrosion-Inhibiting Monolayers on the Bond Strength and Durability of Reinforced Concrete Structures Under Service Conditions" Materials 18, no. 7: 1656. https://doi.org/10.3390/ma18071656
APA StyleMonzón-Bello, P., Vengut-Tro, R., Soto-Camino, J., & Valcuende-Payá, M. O. (2025). Influence of Corrosion-Inhibiting Monolayers on the Bond Strength and Durability of Reinforced Concrete Structures Under Service Conditions. Materials, 18(7), 1656. https://doi.org/10.3390/ma18071656






