Synthesis, Characterization, and Investigation of the Properties of a New Promising Poly(Azomethine) Organic Semiconductor Material
Abstract
1. Introduction
2. Experimental Section
2.1. Materials
2.2. Synthesis of Polymers P1 and P2
2.3. Instruments
3. Results and Discussion
3.1. Synthesis and Characterization
3.1.1. Synthesis
3.1.2. 1H NMR Analysis
3.1.3. GPC Analysis
3.1.4. Solubility Behavior
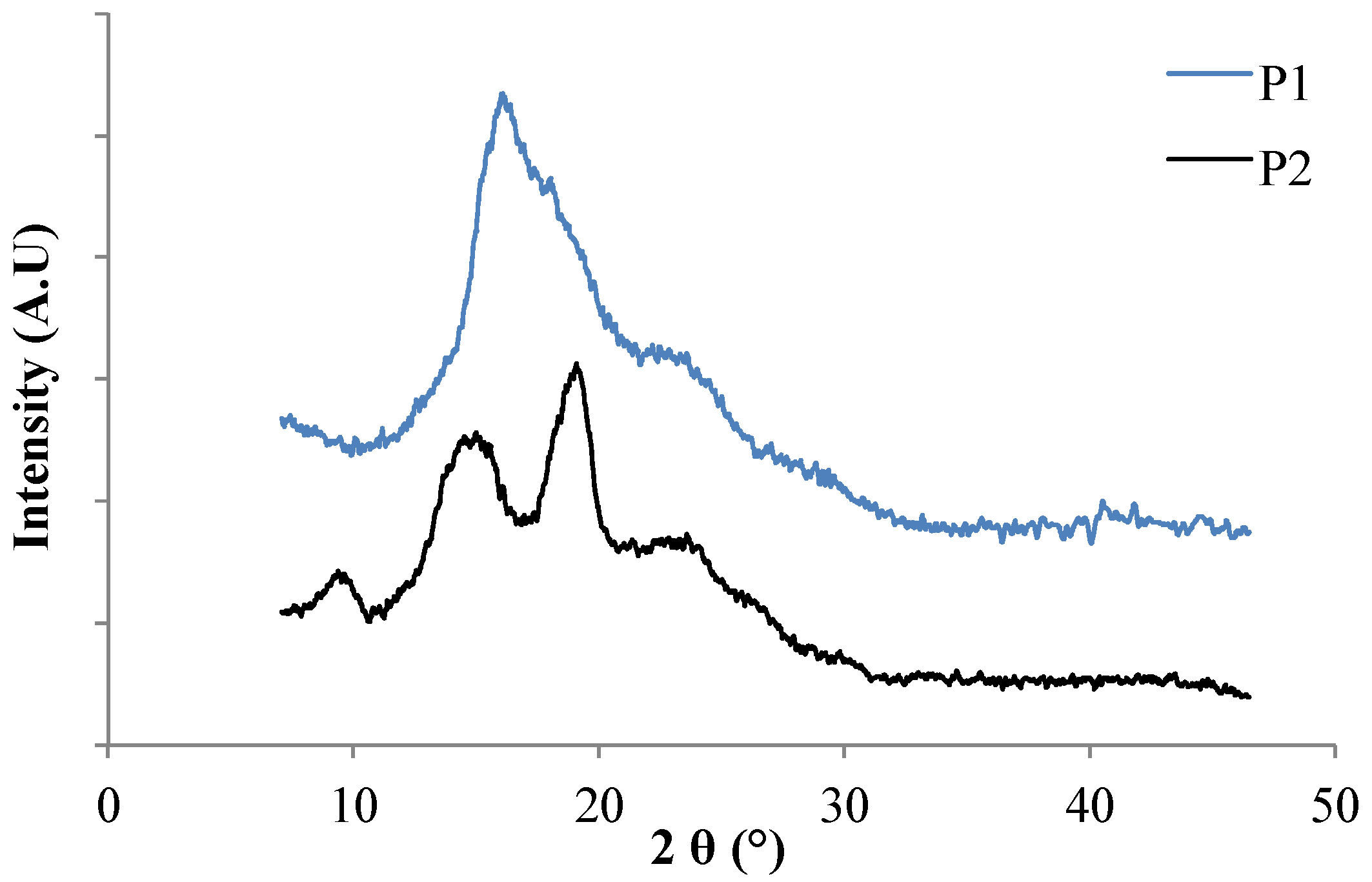
3.1.5. XRD Analysis
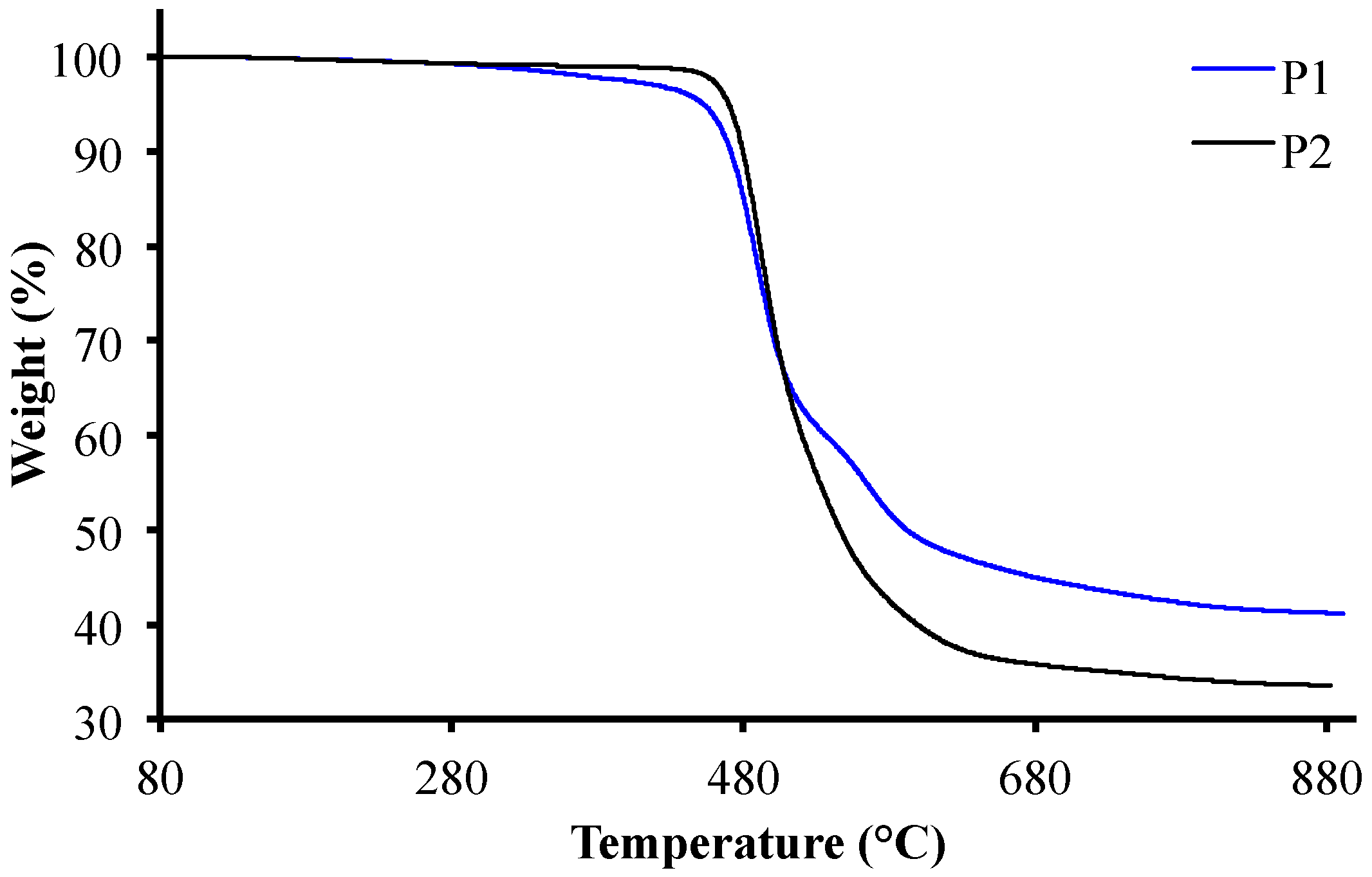
3.2. Thermal Properties
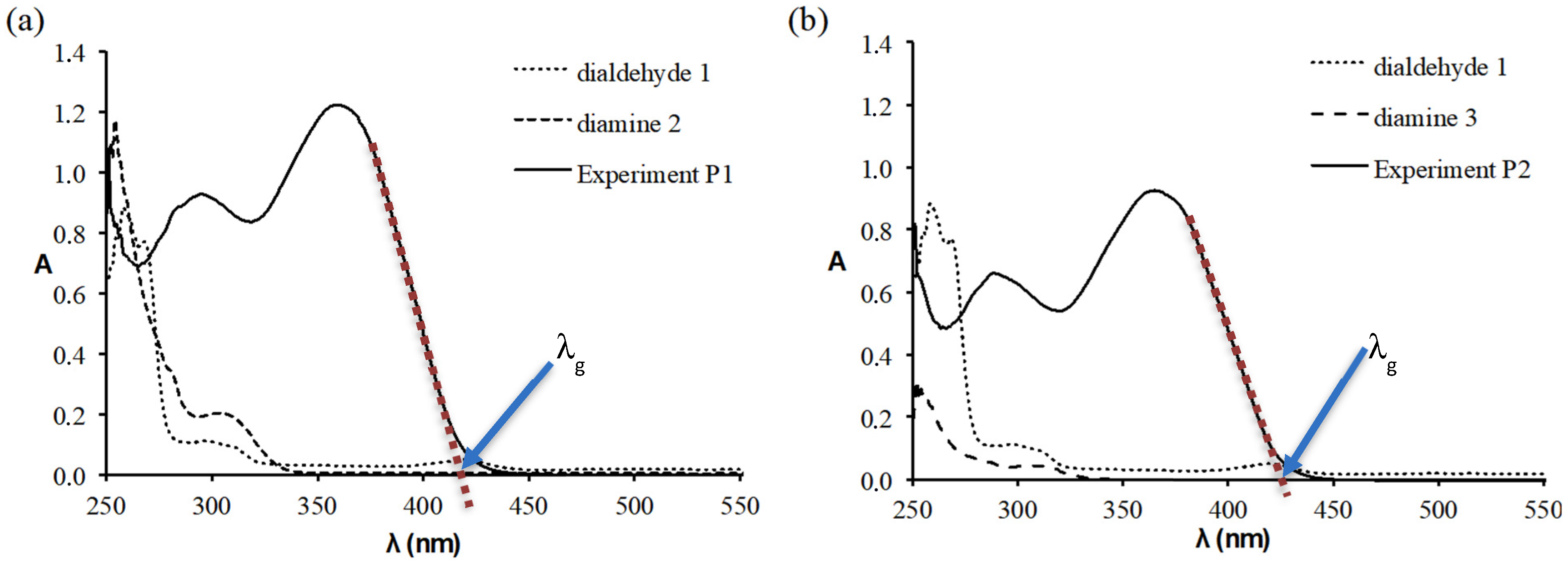
3.3. Optical Properties
3.4. Conductivity
4. Towards Semiconducting Organic Materials
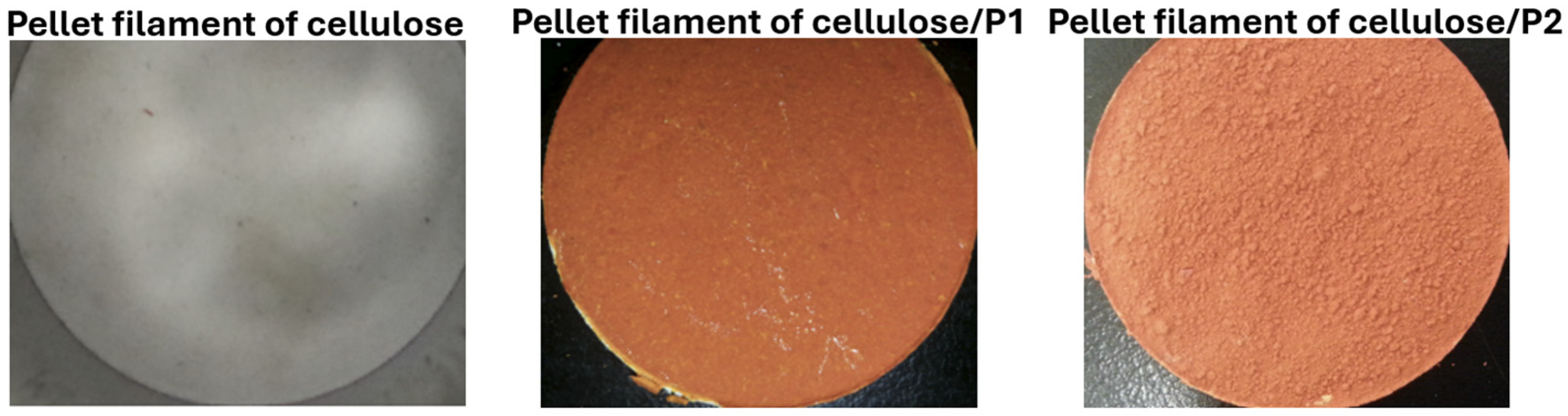
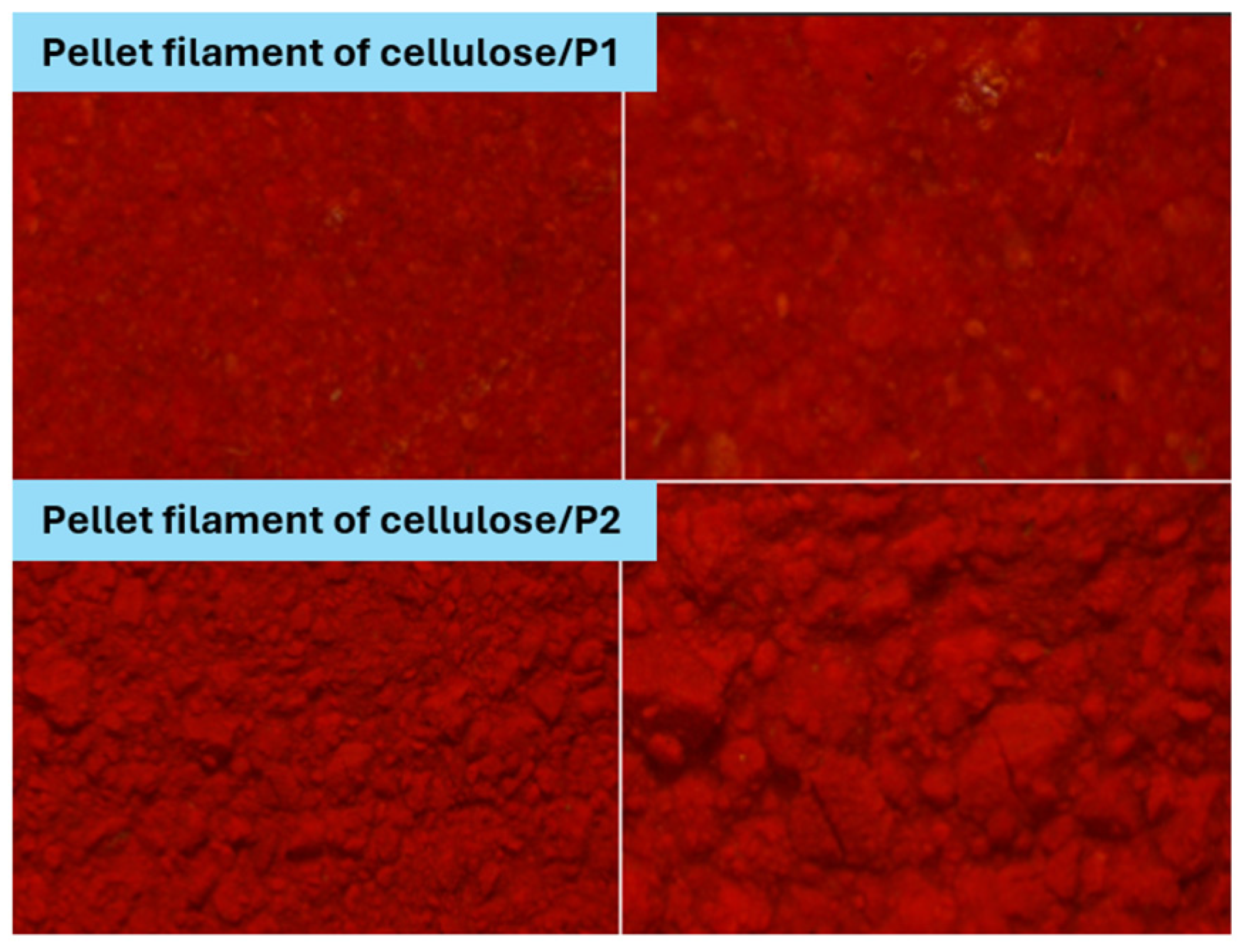
4.1. Cellulose Filaments as a Support
4.2. Determination of Conductivity
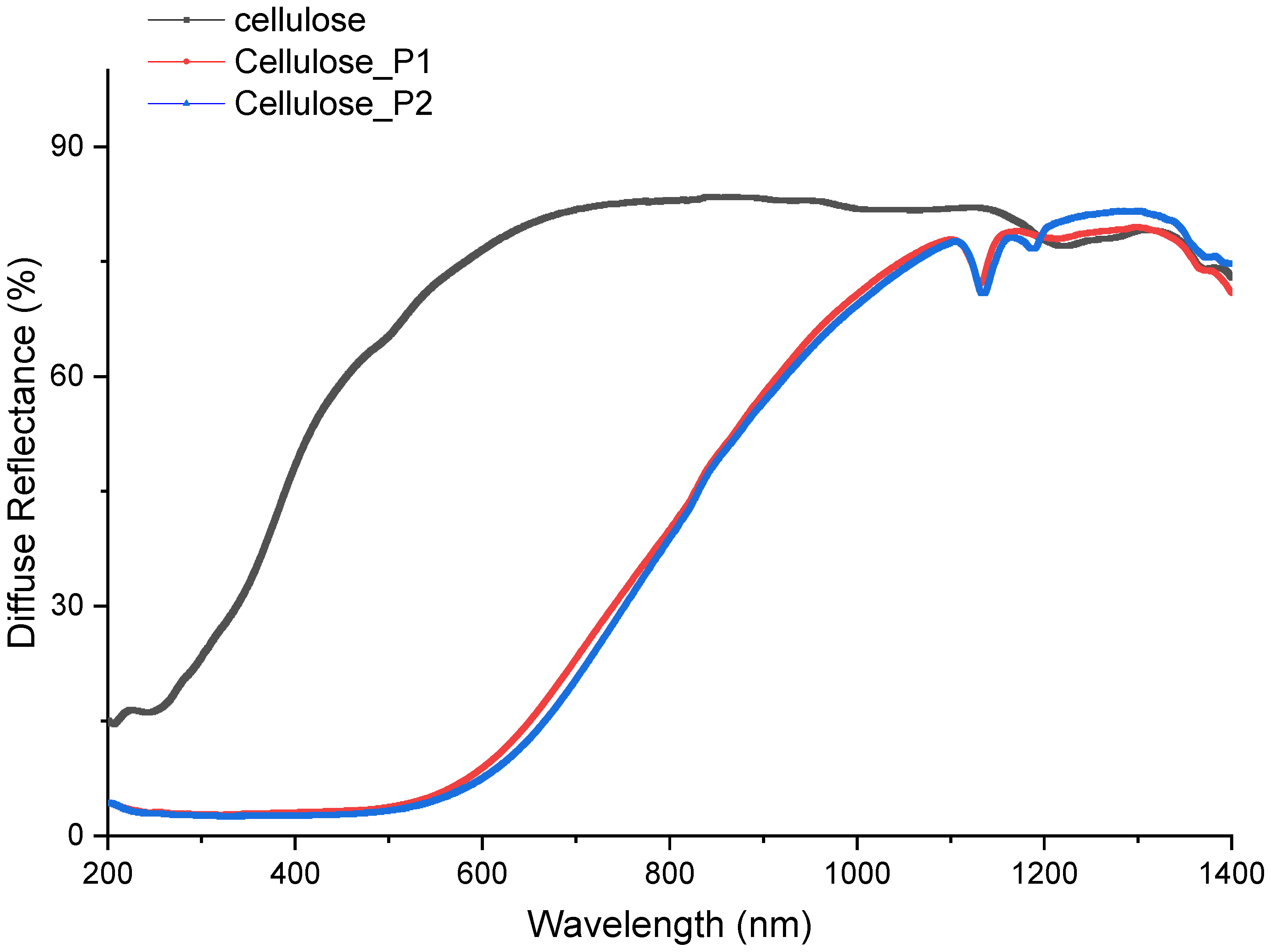
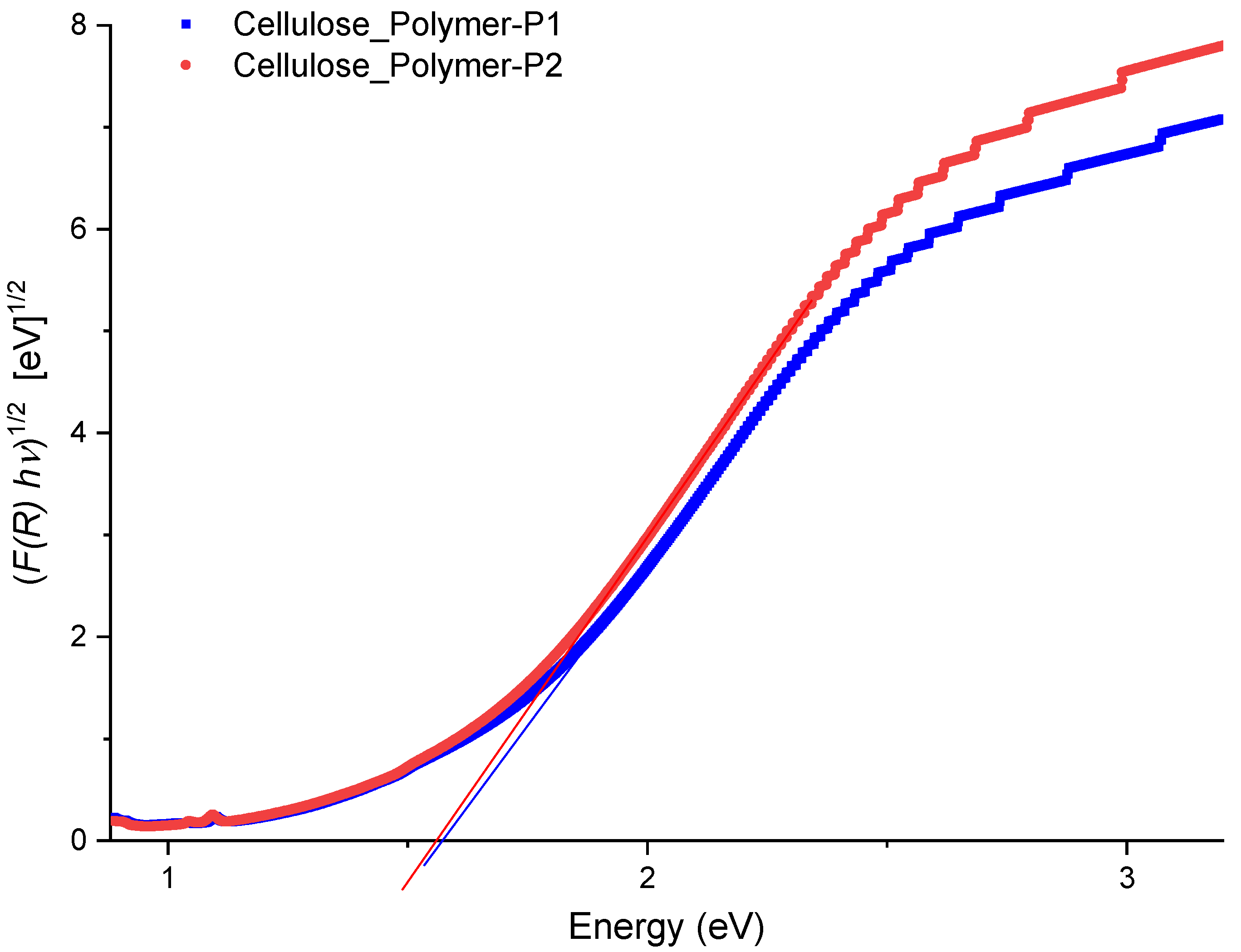
4.3. Diffuse Reflectance Spectroscopy
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Liu, C.; Shao, L.; Chen, S.; Hu, Z.; Cai, H.; Huang, F. Recent Progress in π-Conjugated Polymers for Organic Photovoltaics: Solar Cells and Photodetector. Prog. Polym. Sci. 2023, 143, 101711. [Google Scholar] [CrossRef]
- Pankow, R.M.; Thompson, B.C. The development of conjugated polymers as the cornerstone of organic electronics. Polymer 2020, 207, 122874. [Google Scholar] [CrossRef]
- Mdluli, S.B.; Ramoroka, M.E.; Yussuf, S.T.; Molibane, K.D.; John-Denk, V.S.; Iwuoha, E.I. π-Conjugated Polymers and Their Application in Organic and Hybrid Organic-Silicon Solar Cells. Polymers 2022, 14, 716. [Google Scholar] [CrossRef]
- Grigoras, M.; Catanescu, C.O. Imine Oligomers and Polymers. J. Macromol. Sci. Part C Polym. Rev. 2004, 44, 131–173. [Google Scholar]
- Dianatdar, A.; Bose, R.K. Oxidative chemical vapor deposition for synthesis and processing of conjugated polymers: A critical review. J. Mater. Chem. C 2023, 11, 11776. [Google Scholar] [CrossRef]
- Gharahcheshmeh, M.H.; Chowdhury, K. Fabrication methods, pseudocapacitance characteristics, and integration of conjugated conducting polymers in electrochemical energy storage devices. Energy Adv. 2024, 3, 2668–2703. [Google Scholar] [CrossRef]
- Burroughes, J.H.; Bradley, D.D.C.; Brown, A.R.; Marks, R.N.; Mackay, K.; Friend, R.H.; Burns, P.L.; Holmes, A.B. Light-Emitting Diodes Based on Conjugated Polymers. Nature 1990, 347, 539–541. [Google Scholar]
- Sicard, L.; Navarathne, D.; Skalski, T.; Skene, W.G. On-Substrate Preparation of an Electroactive Conjugated Polyazomethine from Solution-Processable Monomers and Its Application in Electrochromic Devices. Adv. Funct. Mater. 2013, 23, 3549–3559. [Google Scholar] [CrossRef]
- Grucela-Zajac, M.; Bijak, K.; Kula, S.; Filapek, M.; Wiacek, M.; Janeczek, H.; Skorka, L.; Gasiorowski, J.; Hingerl, K.; Sariciftci, N.S.; et al. (Photo)Physical Properties of New Molecular Glasses End-Capped with Thiophene Rings Composed of Diimide and Imine Units. J. Phys. Chem. C Nanomater. Interfaces 2014, 118, 13070–13086. [Google Scholar]
- Sek, D.; Iwan, A.; Jarzabek, B.; Kaczmarczyk, B.; Kasperczyk, J.; Janeczek, H.; Mazurak, Z. Characterization and Optical Properties of Oligoazomethines with Triphenylamine Moieties Exhibiting Blue, Blue-Green and Green Light. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2009, 72, 1–10. [Google Scholar]
- Jung, S.-H.; Lee, T.-W.; Kim, Y.C.; Suh, D.H.; Cho, H.N. Synthesis and Characterization of Fluorene-Based Poly(Azomethines). Opt. Mater. 2002, 21, 169–173. [Google Scholar]
- Khuhawar, M.Y.; Mughal, M.A.; Channar, A.H. Synthesis and Characterization of Some New Schiff Base Polymers. Eur. Polym. J. 2004, 40, 805–809. [Google Scholar]
- Grigoras, M.; Catanescu, C.O.; Colotin, G. Poly(Schiff Base)S Containing 1,1′-Binaphthyl Moieties: Synthesis and Characterization. Macromol. Chem. Phys. 2001, 202, 2262–2266. [Google Scholar]
- Ghaemy, M.; Mighani, H.; Alizadeh, R. Synthesis and Characterization of Schiff-Base-Containing Polyamides. Chin. J. Polym. Sci. 2011, 29, 149–155. [Google Scholar]
- Ravikumar, L.; Pradeep, I.; Thangaiyan, T.; Mohan, R.; Balachandran, J. Synthesis, Characterization, and Conducting Properties of Poly(Thiourea Azomethines). Int. J. Polym. Mater. 2012, 61, 288–299. [Google Scholar]
- Bruma, M.; Fitch, J.W.; Cassidy, P.E. Hexafluoroisopropylidene-Containing Polymers for High-Performance Applications. J. Macromol. Sci. Part C Polym. Rev. 1996, 36, 119–159. [Google Scholar]
- Bruma, M.; Damaceanu, M.D.; Rusu, R.D. Study of Thin Films Made from Aromatic Polymers Containing Six-Member Imide Rings. High Perform. Polym. 2012, 24, 31–39. [Google Scholar]
- Giovanella, U.; Botta, C.; Galeotti, F.; Vercelli, B.; Battiato, S.; Pasini, M. Perfluorinated Polymer with Unexpectedly Efficient Deep Blue Electroluminescence for Full-Colour Oled Displays and Light Therapy Applications. J. Mater. Chem. C 2013, 1, 5322–5329. [Google Scholar]
- Olvera, L.I.; Guzmán-Gutiérrez, M.T.; Zolotukhin, M.G.; Fomine, S.; Cárdenas, J.; Ruiz-Trevino, F.A.; Villers, D.; Ezquerra, T.A.; Prokhorov, E. Novel High Molecular Weight Aromatic Fluorinated Polymers from One-Pot, Metal-Free Step Polymerizations. Macromolecules 2013, 46, 7245–7256. [Google Scholar]
- Calzolari, A.; Vercelli, B.; Ruini, A.; Virgili, T.; Pasini, M. Fluorine-Induced Enhancement of the Oxidation Stability and Deep-Blue Optical Activity in Conductive Polyfluorene Derivatives. J. Phys. Chem. C 2013, 117, 26760–26767. [Google Scholar]
- Puodziukynaite, E.; Burbulis, E.; Grazulevicius, J.V.; Jankauskas, V.; Undzenas, A.; Linonis, V. Aniline-Based Bis(Enamines) as New Amorphous Molecular Charge Transport Materials. Synth. Met. 2007, 157, 696–701. [Google Scholar] [CrossRef]
- Sosa-Gonzalez, W.E.; del Jesus, P.C.R.; de Jesus, A.V.M. Sulfonated Aromatic Copoly(Ether-Amide) Membranes: Preparation and Characterization for Possible Application in Polymer Electrolyte Membrane Fuel Cells. High Perform. Polym. 2014, 26, 997–1006. [Google Scholar] [CrossRef]
- Qian, G.; Benicewicz, B.C. Synthesis and Characterization of High Molecular Weight Hexafluoroisopropylidene-Containing Polybenzimidazole for High-Temperature Polymer Electrolyte Membrane Fuel Cells. J. Polym. Sci. Part A Polym. Chem. 2009, 47, 4064–4073. [Google Scholar]
- Medhioub, H.; Zerrouki, C.; Fourati, N.; Smaoui, H.; Guermazi, H.; Bonnet, J.J. Towards a Structural Characterization of an Epoxy Based Polymer Using Small-Angle X-Ray Scattering. J. Appl. Phys. 2007, 101, 043509. [Google Scholar] [CrossRef]
- Zerrouki, C.; Chassevent, M.; Fourati, N.; Tollens, E.; Bonnet, J.J. Réflexion Et Fluorescence X: Une Complémentarité Au Profit De La Caractérisation Des Surfaces. J. Phys. IV 2004, 118, 149–155. [Google Scholar] [CrossRef][Green Version]
- Ma, X.; Niu, H.; Wen, H.; Wang, S.; Lian, Y.; Jiang, X.; Wang, C.; Bai, X.; Wang, W. Synthesis, Electrochromic, Halochromic and Electro-Optical Properties of Polyazomethines with a Carbazole Core and Triarylamine Units Serving as Functional Groups. J. Mater. Chem. C 2015, 3, 3482–3493. [Google Scholar]
- Iwan, A.; Boharewicz, B.; Tazbir, I.; Filapek, M. Enhanced Power Conversion Efficiency in Bulk Heterojunction Solar Cell Based on New Polyazomethine with Vinylene Moieties and [6,6]-Phenyl C61 Butyric Acid Methyl Ester by Adding 10-Camphorsulfonic Acid. Electrochim. Acta 2015, 159, 81–92. [Google Scholar]
- Birajdar, A.S.; Pawar, S.G.; Ghanwat, A.A.; Ubale, V.P. High-performance supercapacitive polyazomethines: Room temperature synthesis and their characterizations. J. Mol. Struct. 2024, 1299, 137173. [Google Scholar] [CrossRef]
- Ovdenko, V.; Vyshnevsky, D.; Davidenko, N.; Gryshchuk, L.; Pavlov, V. Synthesis, characterization, spectral properties and evaluation of the photophysical behavior of novel Congo Red based polymers. Opt. Mater. 2023, 135, 113268. [Google Scholar]
- Nilay Tezel, R.; Kaya, I. Synthesis and characterization of fluorescent and thermally stable poly(azomethine-ether)s: Optical and morphological properties. Polymer 2025, 323, 128169. [Google Scholar] [CrossRef]
- Marin, L.; Bejan, A.; Ailincai, D.; Belei, D. Poly(azomethine-phenothiazine)s with efficient emission in solid state. Eur. Polym. J. 2017, 95, 127–137. [Google Scholar]
- Capello, C.; Fischer, U.; Hungerbühler, K. What Is a Green Solvent? A Comprehensive Framework for the Environmental Assessment of Solvents. Green Chem. 2007, 9, 927–934. [Google Scholar]
- Hakami, A.A.; Alorfi, H.S.; Farghaly, T.A.; Hussein, M.A. Synthesis of new polyazomethine-based pyrazole moieties as promising biologically active macromolecules. Polym. Plast. Technol. Mater. 2024, 63, 2385–2396. [Google Scholar]
- Hakami, A.A.; AL-Sodies, S.; Alorfi, H.S.; Alnafisah, A.S.; Hussein, M.A. Novel branched Heteroaromatic Pyrazole-based Polyazomethines as anticancer agents. Results Chem. 2025, 14, 102129. [Google Scholar]
- Yao, Z.-F.; Li, Q.-Y.; Wu, H.-T.; Ding., Y.-F.; Wang., Z.-Y.; Lu., Y.; Wang., J.-Y.; Pei., J. Building crystal structures of conjugated polymers through X-ray diffraction and molecular modeling. SmartMat 2021, 2, 378–387. [Google Scholar] [CrossRef]
- Niu, H.; Huang, Y.; Bai, X.; Li, X.; Zhang, G. Study on Crystallization, Thermal Stability and Hole Transport Properties of Conjugated Polyazomethine Materials Containing 4,4′-Bisamine-Triphenylamine. Mater. Chem. Phys. 2004, 86, 33–37. [Google Scholar] [CrossRef]
- Damaceanu, M.-D.; Rusu, R.-D.; Nicolescu, A.; Bruma, M.; Rusanov, A.L. Organosoluble Asymmetric Aromatic Polyamides Bearing Pendent Phenoxy Groups. Polym. Int. 2011, 60, 1248–1258. [Google Scholar]
- Sonker, E.; Tiwari, R.; Kumar, K.; Krishnamoorthi, S. Electrical properties of new polyazomethines. SN Appl. Sci. 2020, 2, 1123. [Google Scholar] [CrossRef]
- Hafeez, A.; Akhtera, Z.; Gallagher, J.F.; Siddiqui, H.M. Liquid phase synthesis of aromatic poly(azomethine)s, their physicochemical properties, and measurement of ex situ electrical conductivity of pelletized powdered samples. Des. Monomers Polym. 2017, 20, 74–88. [Google Scholar] [CrossRef]
- El-Shekeil, A.G.; Al-Yusufy, F.A.; Saknidy, S. DC Conductivity of some Polyazomethines. Polym. Int. 1997, 42, 39–44. [Google Scholar]
- Li, X.; Li, C.; Li, S. Synthesis, characterization and electrical properties of soluble conjugated poly-Schiff bases. Synth. Met. 1993, 60, 285–288. [Google Scholar] [CrossRef]
- Gharahcheshmeh, M.H.; Robinson, M.T.; Gleason, E.F.; Gleason, K.K. Optimizing the Optoelectronic Properties of Face-On Oriented Poly(3,4-Ethylenedioxythiophene) via Water-Assisted Oxidative Chemical Vapor Deposition. Adv. Funct. Mater. 2021, 31, 2008712. [Google Scholar] [CrossRef]
- Tlili, A.; Attia, G.; Khaoulani, S.; Zerrouki, C.; Yaakoubi, N.; Othmane, A.; Fourati, N. Rethinking the use of redox probes for the detection of electroactive proteins with electrochemical sensors modified with molecularly imprinted polymers. Biosens. Bioelectron. 2025, 271, 117105. [Google Scholar] [CrossRef] [PubMed]
- Kubelka, P. New contributions to the optics of intensely light-scattering materials, Part I. J. Opt. Soc. Am. 1948, 38, 448–457. [Google Scholar] [CrossRef]
- Tauc, J. Amorphous and Liquid Semiconductors; Springer Science & Business Media: Berlin/Heidelberg, Germany, 2012. [Google Scholar]
- Khlifi, N.; Zerrouki, C.; Fourati, N.; Guermazi, H.; Guermazi, S. Investigation of structural and optical properties of TM-doped CuO NPs: Correlation with their photocatalytic efficiency in sunlight-induced pollutant degradation. Measurement 2024, 237, 115209. [Google Scholar] [CrossRef]


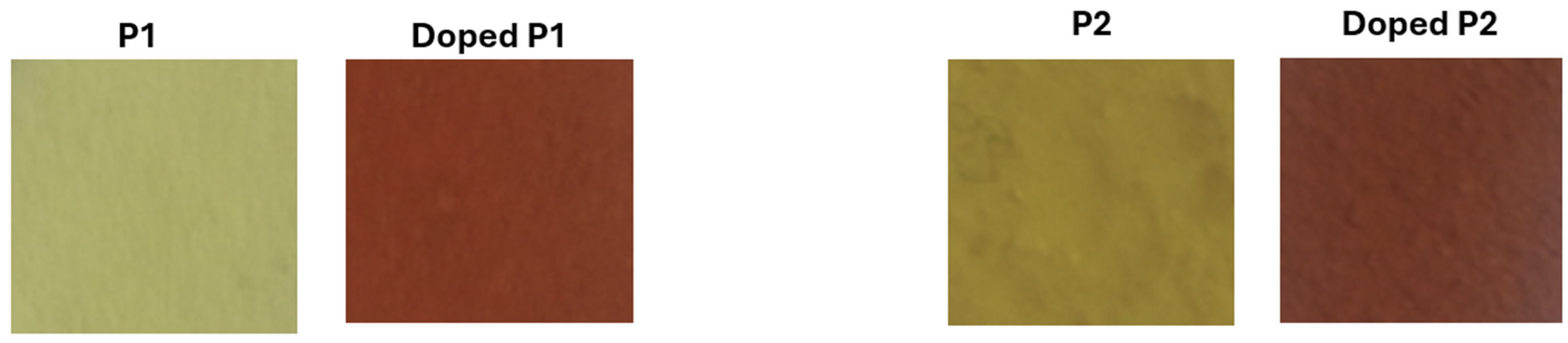
| Polymer | Mw a (g/mol) | Mn b (g/mol) | Mw/Mn (PDI) c | DP d |
|---|---|---|---|---|
| P1 | 4903 | 3184 | 1.54 | 8 |
| P2 | 2324 | 1979 | 1.17 | 5 |
| Polymer | CHCl3 | THF | DMF | DMAc | NMP |
|---|---|---|---|---|---|
| P1 | ++ | ++ | ++ | ++ | ++ |
| P2 | ++ | ++ | -- | ++ | ++ |
| UV/Vis Extracted Parameters | |||
|---|---|---|---|
| λabs-max (nm) | λg (nm) | Eg (eV) | |
| P1 | 360 | 420 | 2.95 |
| P2 | 367 | 425 | 2.92 |
| Conductivity | (S/cm) | |
|---|---|---|
| Doping Agent | P1 | P2 |
| Without doping | 1 × 10−8 | 3 × 10−9 |
| HCl | 2.6 × 10−3 | 1.2 × 10−3 |
| p-TsOH | 3.2 × 10−5 | - |
| ACS | 2 × 10−8 | 4 × 10−9 |
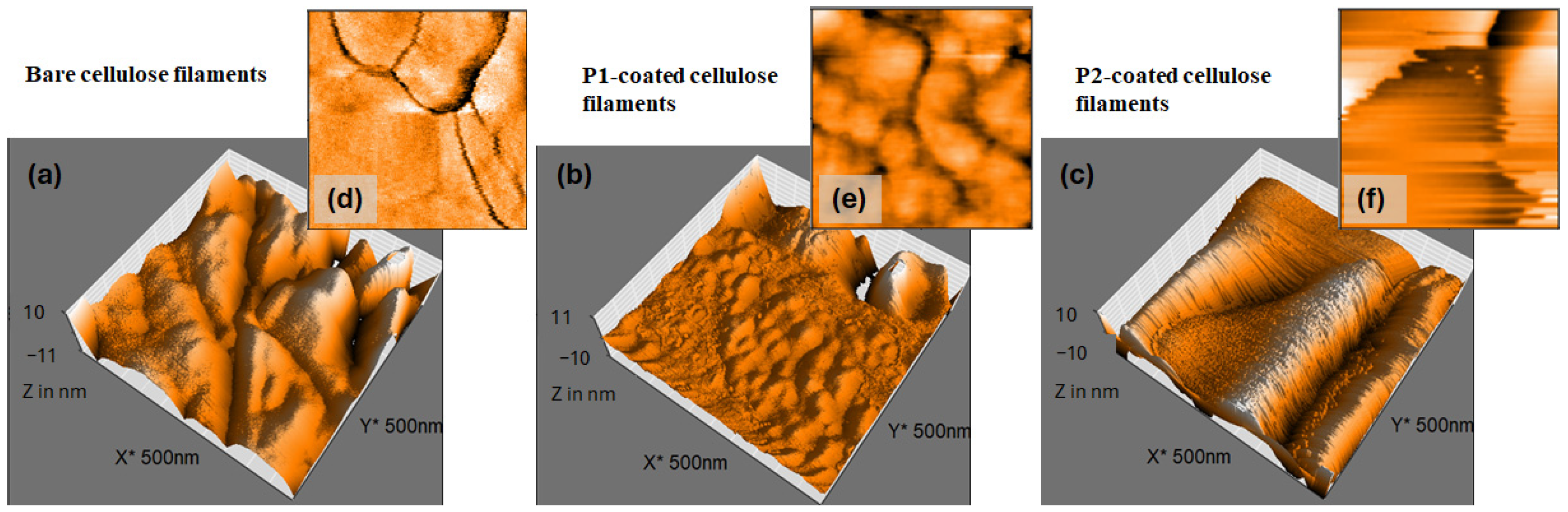
| Roughness Parameters | |||
|---|---|---|---|
| Arithmetic Average Sa (nm) | Root Mean Square Sq (nm) | Peak-to-Valley Height Sz (nm) | |
| Cell filaments | (6.1 ± 0.2) | (8.0 ± 0.3) | (86.1 ± 1.3) (nm) |
| P1-coated cell filaments | (2.6 ± 0.2) | (4.6 ± 0.3) | (44.6 ± 1.1) (nm) |
| P2-coated cell filaments | (2.8 ± 0.2) | (4.2 ± 0.2) | (1.01 ± 0.09) (µm) |
| Conductivity | (S/cm) | |
|---|---|---|
| Polymer | Doped Polymer | Polymer-Coated Cellulose Filaments |
| P1 | 2.6 × 10−3 | 2.4 × 10−3 |
| P2 | 1.2 × 10−3 | 1.3 × 10−3 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ismaili, J.; Zerrouki, C.; Fourati, N.; Leroy-Lhez, S.; Montplaisir, D.; Villandier, N.; Zerrouki, R. Synthesis, Characterization, and Investigation of the Properties of a New Promising Poly(Azomethine) Organic Semiconductor Material. Materials 2025, 18, 1658. https://doi.org/10.3390/ma18071658
Ismaili J, Zerrouki C, Fourati N, Leroy-Lhez S, Montplaisir D, Villandier N, Zerrouki R. Synthesis, Characterization, and Investigation of the Properties of a New Promising Poly(Azomethine) Organic Semiconductor Material. Materials. 2025; 18(7):1658. https://doi.org/10.3390/ma18071658
Chicago/Turabian StyleIsmaili, Jihane, Chouki Zerrouki, Najla Fourati, Stephanie Leroy-Lhez, Daniel Montplaisir, Nicolas Villandier, and Rachida Zerrouki. 2025. "Synthesis, Characterization, and Investigation of the Properties of a New Promising Poly(Azomethine) Organic Semiconductor Material" Materials 18, no. 7: 1658. https://doi.org/10.3390/ma18071658
APA StyleIsmaili, J., Zerrouki, C., Fourati, N., Leroy-Lhez, S., Montplaisir, D., Villandier, N., & Zerrouki, R. (2025). Synthesis, Characterization, and Investigation of the Properties of a New Promising Poly(Azomethine) Organic Semiconductor Material. Materials, 18(7), 1658. https://doi.org/10.3390/ma18071658










