The Influence of Ti and Al on the Evolution of Microstructure and Mechanical Properties in Medium-Entropy and High-Entropy Alloys Based on AlxTixCrFe2Ni2
Abstract
1. Introduction
2. Materials and Methods
3. Results
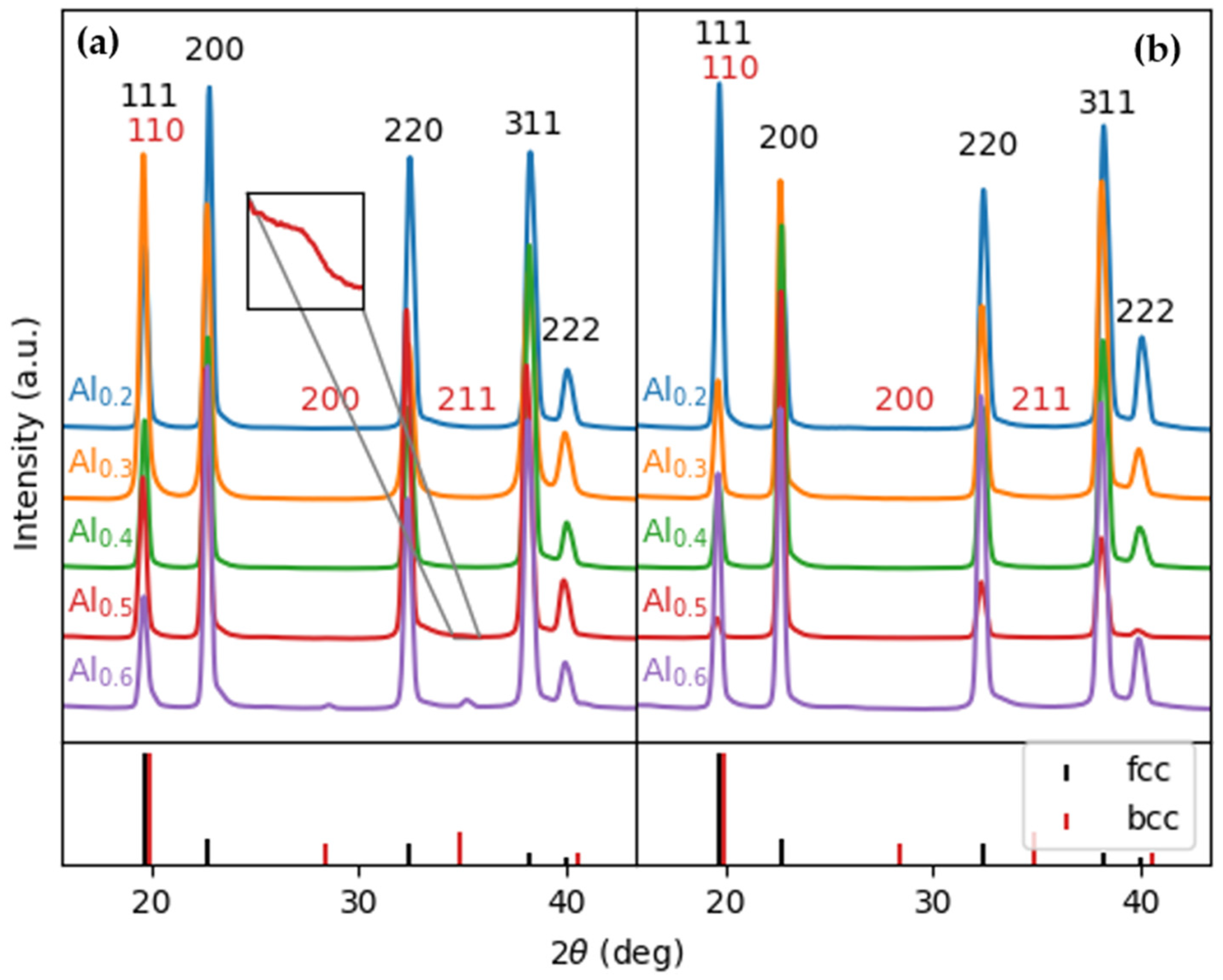
3.1. Characterization of Structural and Mechanical Properties of AlxCrFe2Ni2 Alloys
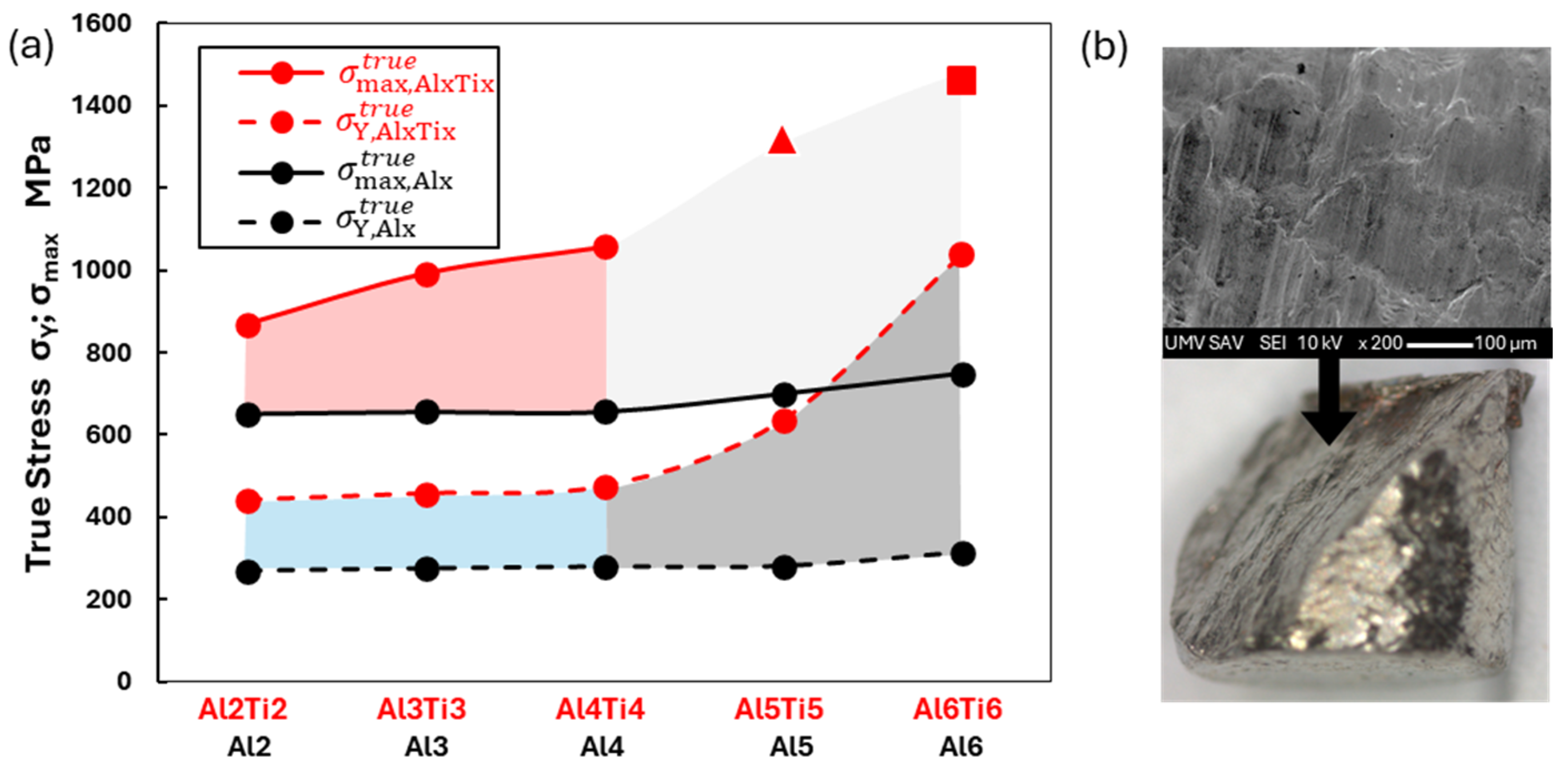
3.2. Characterization of Structural and Mechanical Properties of AlxTixCrFe2Ni2 Alloys
4. Discussion
4.1. Effect of Al and Ti Content on Microstructural Evolution
4.2. The Relationship Between Structure and Deformation Behavior
5. Conclusions
- Phase prediction based on thermodynamic calculations such as VEC, δ, ΔHmix, and others were in good agreement with the obtained structures for all studied alloys in the as-cast state.
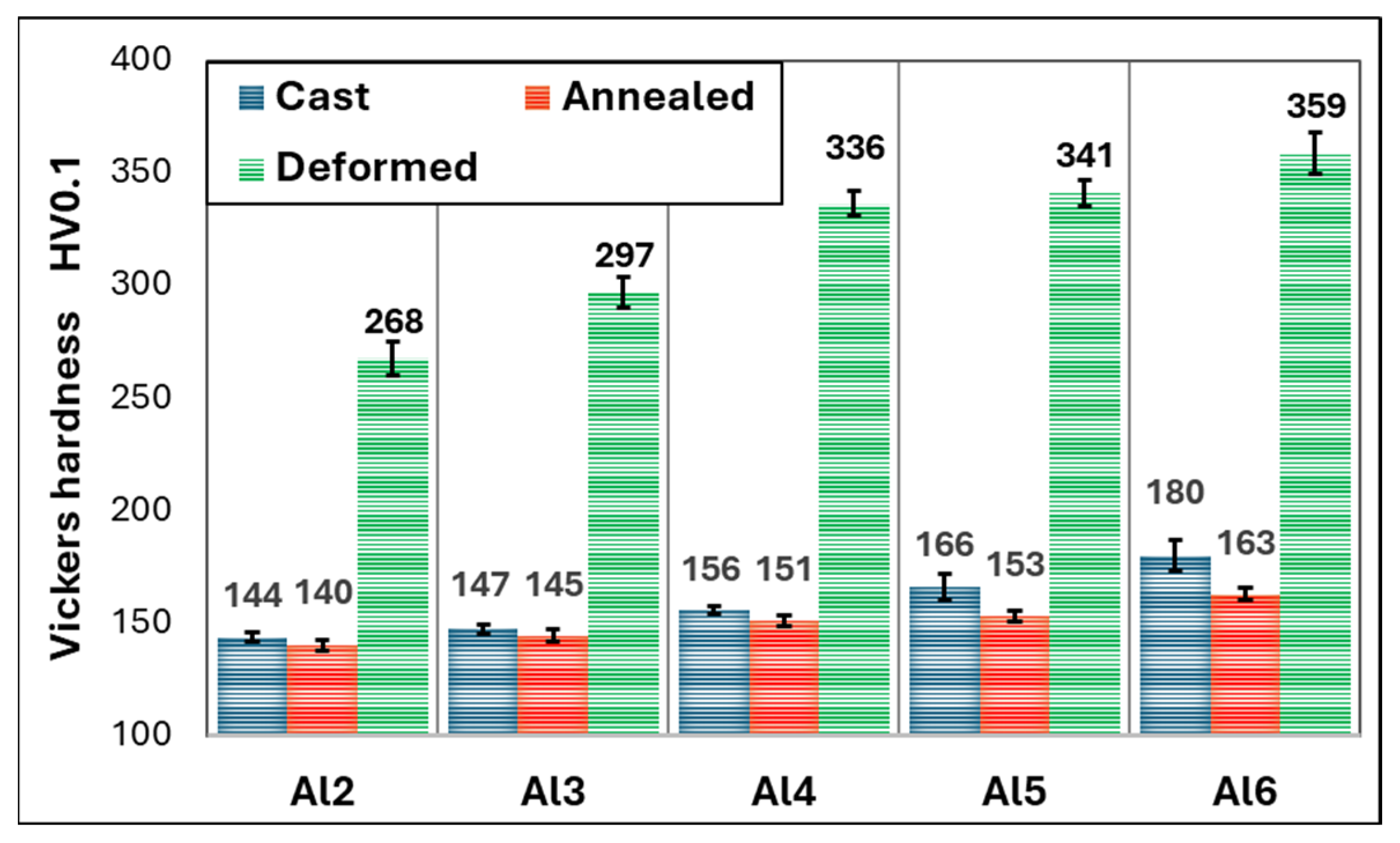
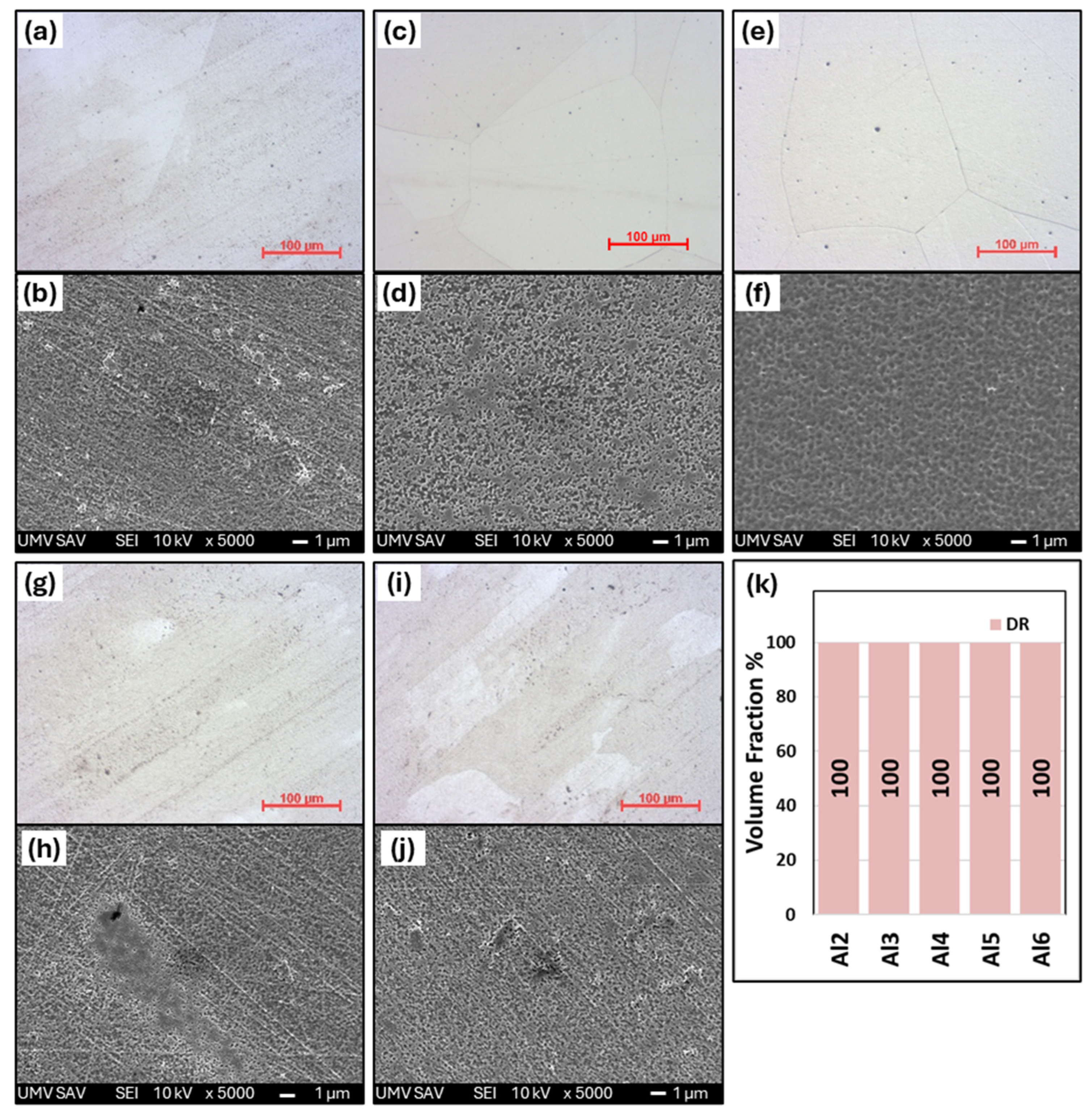
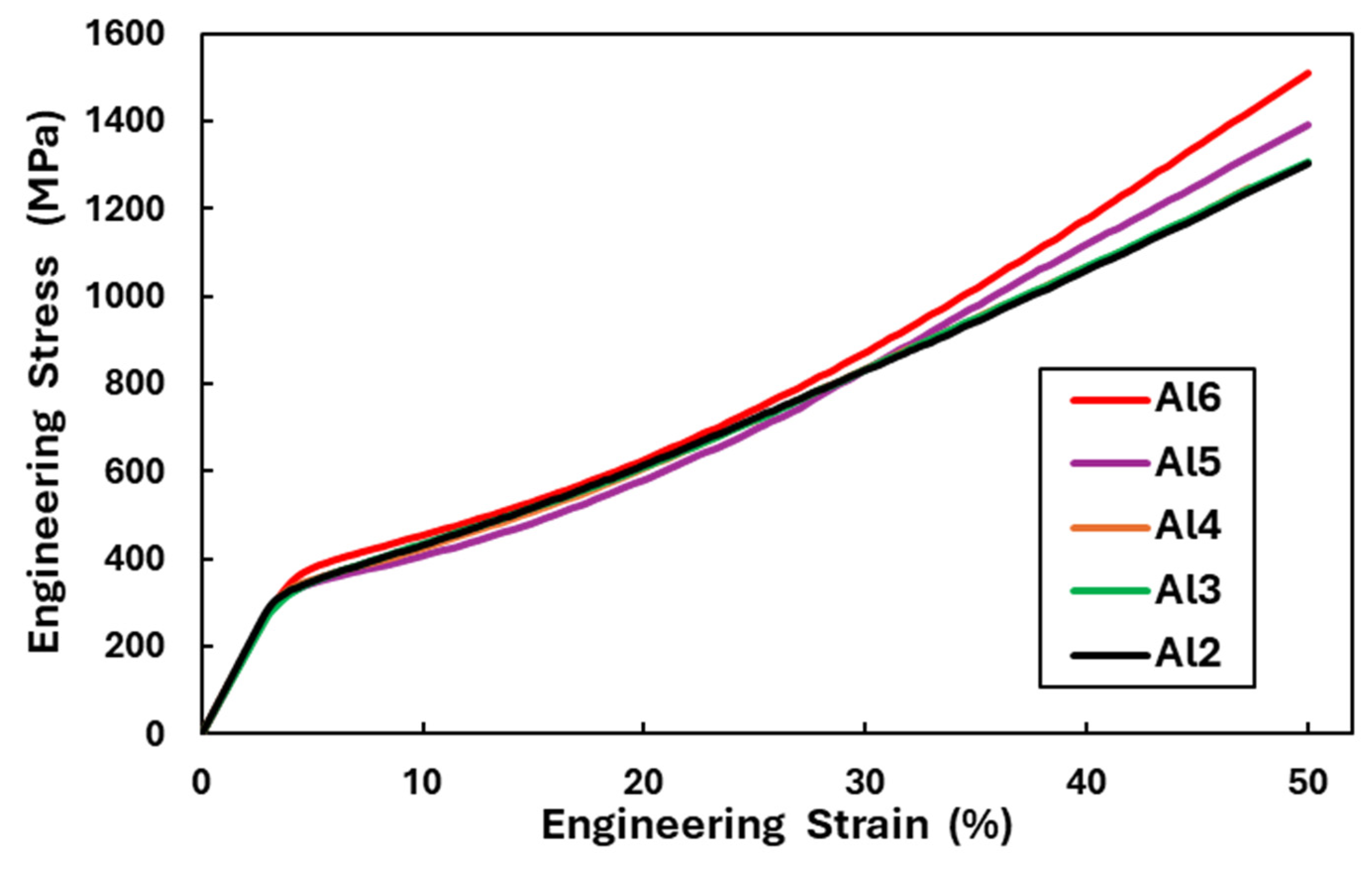
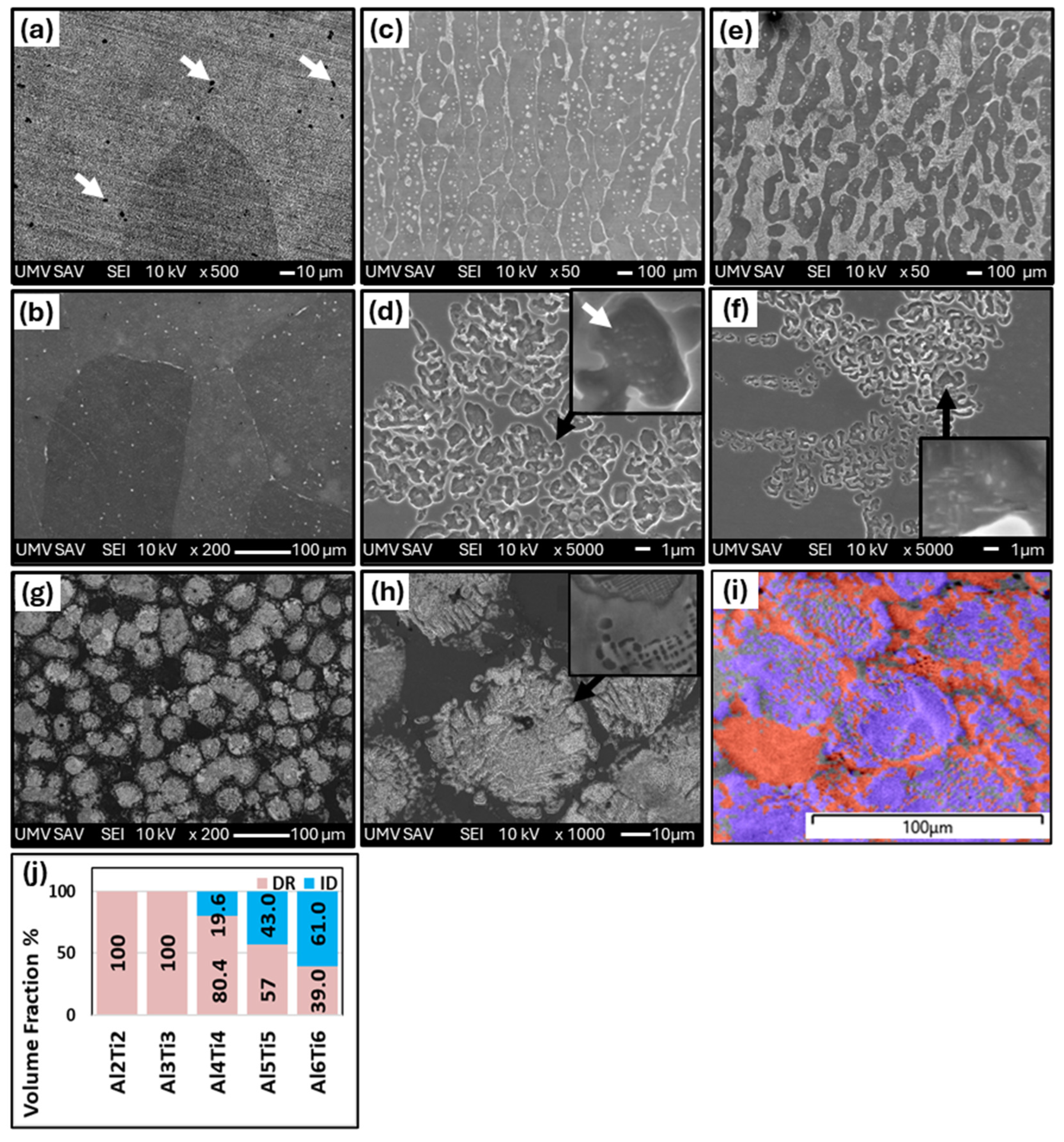
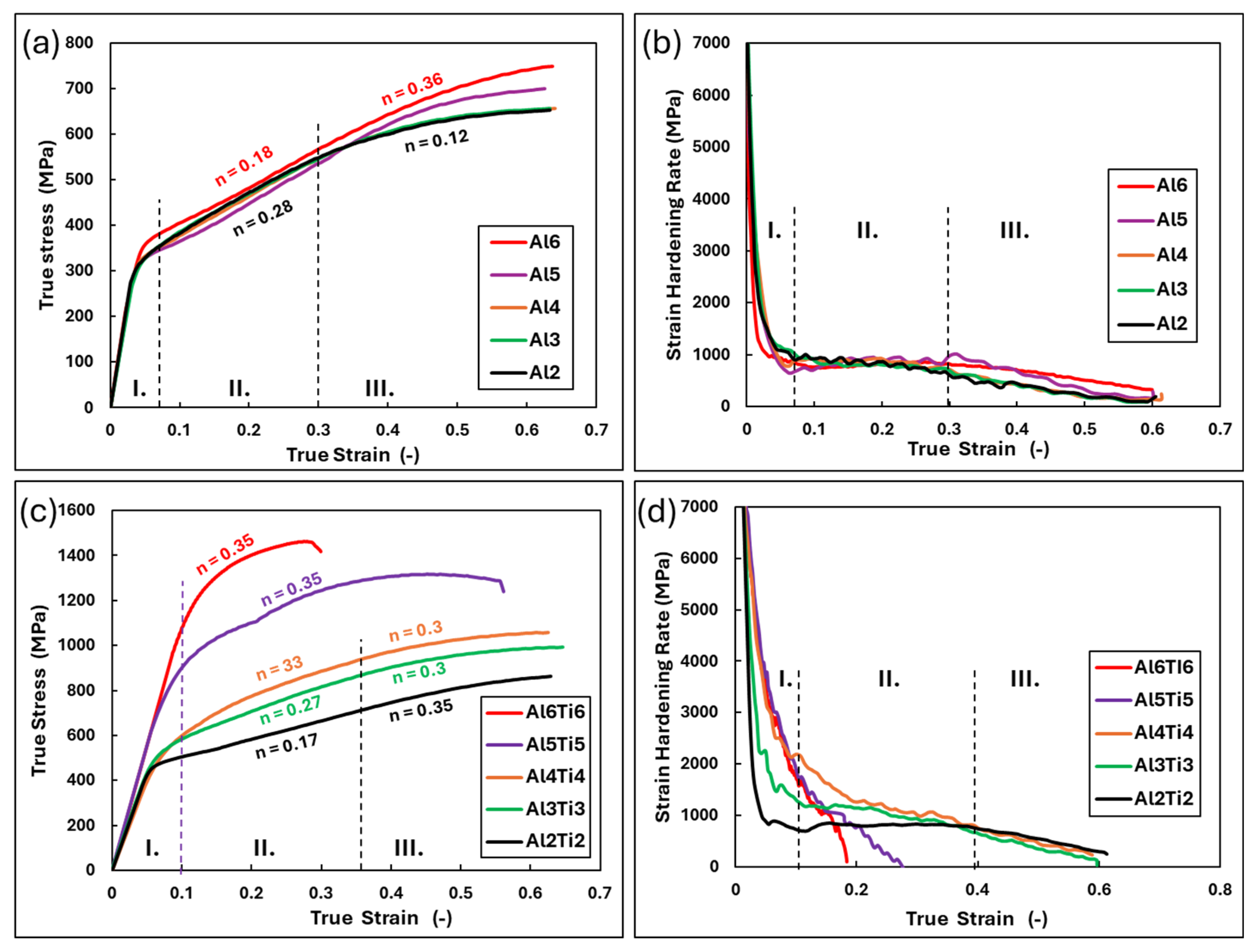
- The medium-entropy alloys AlxCrFe2Ni2 have a single-phase FCC structure in the as-cast state only up to an Al content of ≥3.85 at. %. As the Al content increases to 10.7 at. %, the structure transitions to a dual-phase FCC + BCC, with the volume fraction of BCC increasing to 8.8%. After homogenization annealing, all alloys reached a single-phase FCC structure, which is characterized by good ductility and a moderate-to-high strain-hardening exponent (n < 0.12; 0.36>). The Al0.6CrFe2Ni2 alloy, after 50% deformation, exhibited the highest compressive strength, , and a hardness of 359 HV0.1.
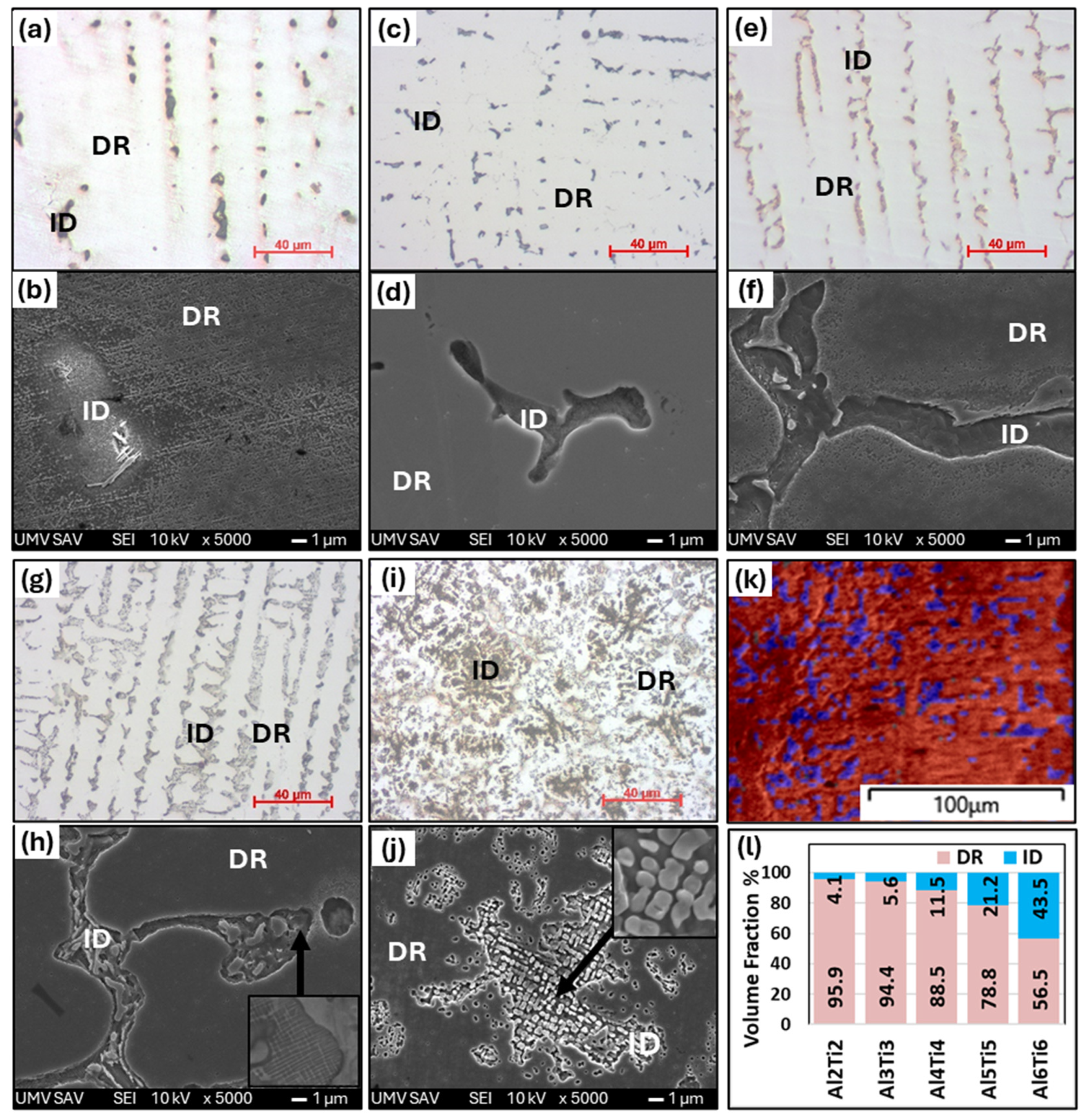
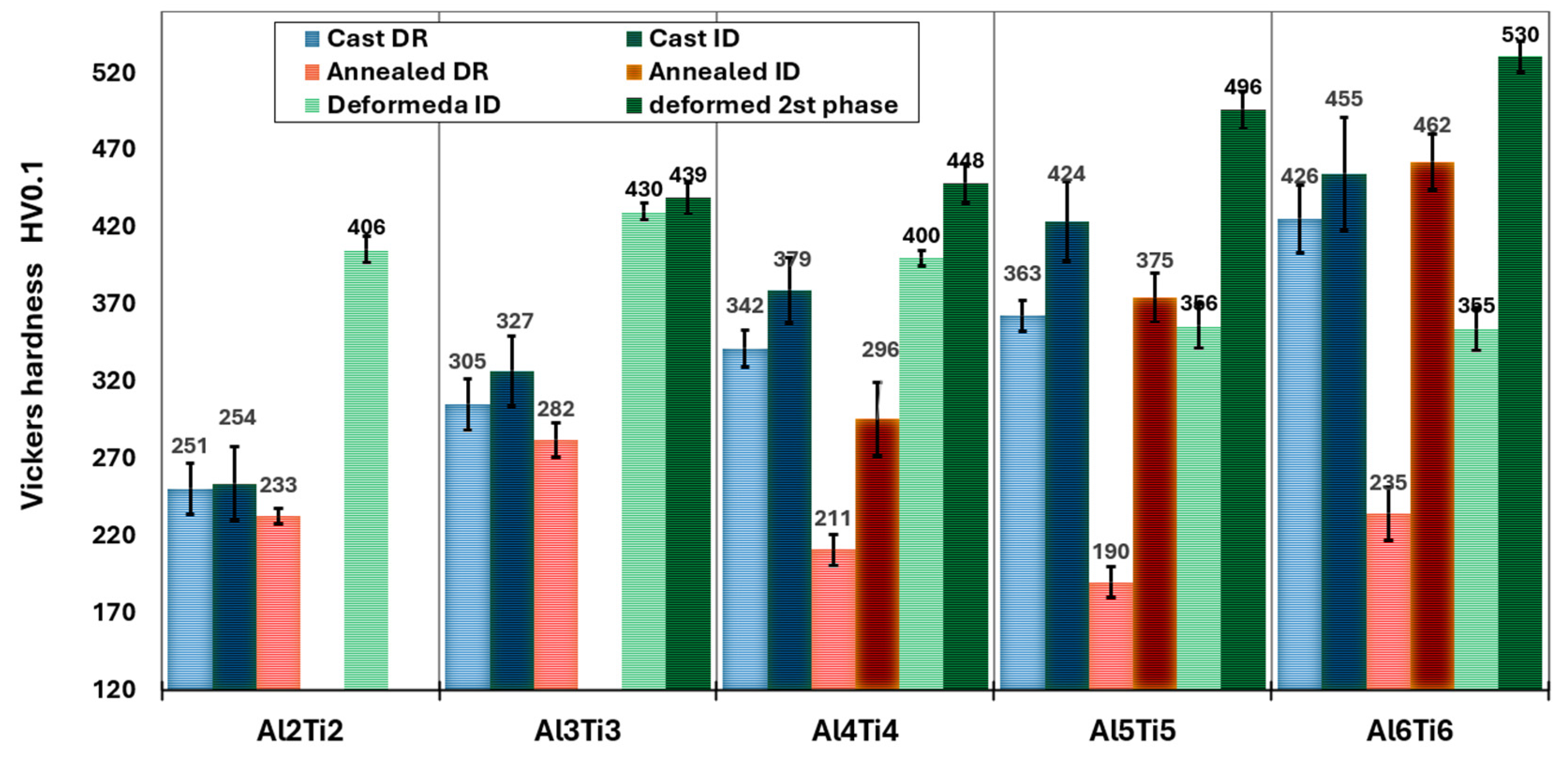
- Alloys of AlxTixCrFe2Ni2, alloyed with equal ratios of Al and Ti, exhibited a dendritic structure composed of FCC and BCC phases in the as-cast state across all concentrations. After homogenization annealing, only the Al2Ti2CrFe2Ni2 alloy exhibited a single-phase FCC structure. For the other alloys, as the Al and Ti content increased, the volume fraction of the BCC phase in the structure increased up to 61% for the Al0.6Ti0.6CrFe2Ni2 alloy. The highest compressive strength, , was achieved by the Al0.5Ti0.5CrFe2Ni2 alloy at 43% deformation. Premature failure of the alloy with the highest Al and Ti content (Al0.6Ti0.6CrFe2Ni2) was caused by a significant decrease in the strain hardening rate, which occurred at 26% deformation and .
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Liu, S.; Gao, M.C.; Liaw, P.K.; Zhang, Y. Microstructures and Mechanical Properties of AlxCrFeNiTi0.25 Alloys. J. Alloys Compd. 2015, 619, 610–615. [Google Scholar] [CrossRef]
- Bała, P.; Górecki, K.; Dziurka, R.; Kozieł, T. The Effect of Al and Ti Additions on Solid Solution Strengthening and Precipitation Hardening in CoNiFe Medium-Entropy Alloys. Materials 2023, 16, 6297. [Google Scholar] [CrossRef] [PubMed]
- Lee, W.; Oh, Y.; Jo, M.-G.; Han, H.N.; Kim, Y. Microstructures and Mechanical Properties of CrFeNi2Nbx Eutectic Multicomponent Alloys. J. Alloys Compd. 2021, 860, 158502. [Google Scholar] [CrossRef]
- Fu, W.; Zheng, W.; Huang, Y.; Guo, F.; Jiang, S.; Xue, P.; Ren, Y.; Fan, H.; Ning, Z.; Sun, J. Cryogenic Mechanical Behaviors of CrMnFeCoNi High-Entropy Alloy. Mater. Sci. Eng. A 2020, 789, 139579. [Google Scholar] [CrossRef]
- Tsai, M.-H.; Wang, C.-W.; Tsai, C.-W.; Shen, W.-J.; Yeh, J.-W.; Gan, J.-Y.; Wu, W.-W. Thermal Stability and Performance of NbSiTaTiZr High-Entropy Alloy Barrier for Copper Metallization. J. Electrochem. Soc. 2011, 158, H1161. [Google Scholar] [CrossRef]
- Wu, J.-M.; Lin, S.-J.; Yeh, J.-W.; Chen, S.-K.; Huang, Y.-S.; Chen, H.-C. Adhesive Wear Behavior of AlxCoCrCuFeNi High-Entropy Alloys as a Function of Aluminum Content. Wear 2006, 261, 513–519. [Google Scholar] [CrossRef]
- Chou, Y.L.; Wang, Y.C.; Yeh, J.W.; Shih, H.C. Pitting Corrosion of the High-Entropy Alloy Co1.5CrFeNi1.5Ti0.5Mo0.1 in Chloride-Containing Sulphate Solutions. Corros. Sci. 2010, 52, 3481–3491. [Google Scholar] [CrossRef]
- Lee, C.P.; Chang, C.C.; Chen, Y.Y.; Yeh, J.W.; Shih, H.C. Effect of the Aluminium Content of AlxCrFe1.5MnNi0.5 High-Entropy Alloys on the Corrosion Behaviour in Aqueous Environments. Corros. Sci. 2008, 50, 2053–2060. [Google Scholar] [CrossRef]
- Zhao, Y.L.; Meng, F.L.; Yang, T.; Luan, J.H.; Liu, S.F.; Yeli, G.M.; Lin, W.T.; Liu, W.H.; Liu, X.J.; Liu, C.T.; et al. Enhanced Helium Ion Irradiation Tolerance in a Fe-Co-Ni-Cr-Al-Ti High-Entropy Alloy with L12 Nanoparticles. J. Mater. Sci. Technol. 2023, 143, 169–177. [Google Scholar] [CrossRef]
- Csik, D.; Baranova, G.; Szaraz, D.; Dzunda, R.; Medvecky, L.; Saksl, K. Dual-Phase High Entropy Oxide Based on AlFeCoNiCu as an Advanced Anode Material for Lithium-Ion Batteries with Self-Healing Properties. In Proceedings of the 2023 13th European Space Power Conference, ESPC, Alicante, Spain, 2–6 October 2023; IEEE: New York, NY, USA, 2023. [Google Scholar]
- Csík, D.; Baranová, G.; Džunda, R.; Zalka, D.; Breitung, B.; Hagarová, M.; Saksl, K. High-Entropy Composite Coating Based on AlCrFeCoNi as an Anode Material for Li-Ion Batteries. Coatings 2023, 13, 1219. [Google Scholar] [CrossRef]
- Oroszová, L.; Csík, D.; Baranová, G.; Bortel, G.; Džunda, R.; Temleitner, L.; Hagarová, M.; Breitung, B.; Saksl, K. Utilizing High-Capacity Spinel-Structured High-Entropy Oxide (CrMnFeCoCu)3O4 as a Graphite Alternative in Lithium-Ion Batteries. Crystals 2024, 14, 218. [Google Scholar] [CrossRef]
- Nigutová, K.; Oroszová, L.; Molčanová, Z.; Csík, D.; Gáborová, K.; Möllmer, J.; Lange, M.; Saksl, K. Experimental Validation of Hydrogen Affinity as a Design Criterion for Alloys. Materials 2024, 17, 6106. [Google Scholar] [CrossRef] [PubMed]
- Mihaliková, M.; Zgodavová, K.; Bober, P.; Sütőová, A. Prediction of Bake Hardening Behavior of Selected Advanced High Strength Automotive Steels and Hailstone Failure Discussion. Metals 2019, 9, 1016. [Google Scholar] [CrossRef]
- Fedoriková, A.; Petroušek, P.; Kvačkaj, T.; Kočiško, R.; Zemko, M. Development of Mechanical Properties of Stainless Steel 316LN-IG after Cryo-Plastic Deformation. Materials 2023, 16, 6473. [Google Scholar] [CrossRef]
- Sas, J.; Kvačkaj, T.; Milkovič, O.; Zemko, M. Influence of Hot Plastic Deformation in γ and (γ + α) Area on the Structure and Mechanical Properties of High-Strength Low-Alloy (HSLA) Steel. Materials 2016, 9, 971. [Google Scholar] [CrossRef]
- Wang, W.-R.; Wang, W.-L.; Wang, S.-C.; Tsai, Y.-C.; Lai, C.-H.; Yeh, J.-W. Effects of Al Addition on the Microstructure and Mechanical Property of AlxCoCrFeNi High-Entropy Alloys. Intermetallics 2012, 26, 44–51. [Google Scholar] [CrossRef]
- Zhou, Y.J.; Zhang, Y.; Wang, Y.L.; Chen, G.L. Solid Solution Alloys of AlCoCrFeNiTix with Excellent Room-Temperature Mechanical Properties. Appl. Phys. Lett. 2007, 90, 181904. [Google Scholar] [CrossRef]
- Ma, S.G.; Jiao, Z.M.; Qiao, J.W.; Yang, H.J.; Zhang, Y.; Wang, Z.H. Strain Rate Effects on the Dynamic Mechanical Properties of the AlCrCuFeNi2 High-Entropy Alloy. Mater. Sci. Eng. A 2016, 649, 35–38. [Google Scholar] [CrossRef]
- Wang, X.; Liu, J.; Zhang, Y.; Zhang, A.; Hou, H.; Miao, Z.; Hongliang, D. Microstructure and Mechanical Property of Novel Nanoparticles Strengthened AlCrCuFeNi Dual-Phase High Entropy Alloy. Mater. Today Commun. 2022, 32, 104155. [Google Scholar] [CrossRef]
- Wei, R.; Zhang, K.; Chen, L.; Han, Z.; Wang, T.; Chen, C.; Jiang, J.; Hu, T.; Li, F. Novel Co-Free High Performance TRIP and TWIP Medium-Entropy Alloys at Cryogenic Temperatures. J. Mater. Sci. Technol. 2020, 57, 153–158. [Google Scholar] [CrossRef]
- Guo, S.; Ng, C.; Liu, C.T. Anomalous Solidification Microstructures in Co-Free AlxCrCuFeNi2 High-Entropy Alloys. J. Alloys Compd. 2013, 557, 77–81. [Google Scholar] [CrossRef]
- Borkar, T.; Gwalani, B.; Choudhuri, D.; Mikler, C.V.; Yannetta, C.J.; Chen, X.; Ramanujan, R.V.; Styles, M.J.; Gibson, M.A.; Banerjee, R. A Combinatorial Assessment of AlxCrCuFeNi2 (0 < x < 1.5) Complex Concentrated Alloys: Microstructure, Microhardness, and Magnetic Properties. Acta Mater. 2016, 116, 63–76. [Google Scholar] [CrossRef]
- Liu, X.X.; Ma, S.G.; Song, W.D.; Zhao, D.; Wang, Z.H. Microstructure Evolution and Mechanical Response of Co-Free Ni2CrFeAl0.3Tix High-Entropy Alloys. J. Alloys Compd. 2023, 931, 167523. [Google Scholar] [CrossRef]
- Wei, C.; Li, L.; Lu, Y.; Du, X.; Wang, T. Evolution of Microstructure and Mechanical Properties of As-Cast AlxCrFe2Ni2 High-Entropy Alloys with Al Content. Met. Mater. Trans. A 2021, 52, 1850–1860. [Google Scholar] [CrossRef]
- Luo, S.; Wang, Z.; Nagaumi, H.; Wu, Z. Tailoring the Microstructure and Mechanical Property of Additively Manufactured AlCrCuFeNi3.0 High-Entropy Alloy through Heat Treatment. Mater. Sci. Eng. A 2022, 844, 143192. [Google Scholar] [CrossRef]
- Ren, B.; Zhao, R.-F.; Liu, Z.-X.; Guan, S.-K.; Zhang, H.-S. Microstructure and Properties of Al0.3CrFe1.5MnNi0.5Tix and Al0.3CrFe1.5MnNi0.5Six High-Entropy Alloys. Rare Met. 2014, 33, 149–154. [Google Scholar] [CrossRef]
- Wang, Z.; Baker, I.; Guo, W.; Poplawsky, J.D. The Effect of Carbon on the Microstructures, Mechanical Properties, and Deformation Mechanisms of Thermo-Mechanically Treated Fe40.4Ni11.3Mn34.8Al7.5Cr6 High Entropy Alloys. Acta Mater. 2017, 126, 346–360. [Google Scholar] [CrossRef]
- Lu, W.; Luo, X.; Yang, Y.; Le, W.; Huang, B.; Li, P. Co-Free Non-Equilibrium Medium-Entropy Alloy with Outstanding Tensile Properties. J. Alloys Compd. 2020, 833, 155074. [Google Scholar] [CrossRef]
- He, J.Y.; Liu, W.H.; Wang, H.; Wu, Y.; Liu, X.J.; Nieh, T.G.; Lu, Z.P. Effects of Al Addition on Structural Evolution and Tensile Properties of the FeCoNiCrMn High-Entropy Alloy System. Acta Mater. 2014, 62, 105–113. [Google Scholar] [CrossRef]
- Kao, Y.-F.; Chen, T.-J.; Chen, S.-K.; Yeh, J.-W. Microstructure and Mechanical Property of As-Cast, -Homogenized, and -Deformed AlxCoCrFeNi (0 ≤ x ≤ 2) High-Entropy Alloys. J. Alloys Compd. 2009, 488, 57–64. [Google Scholar] [CrossRef]
- Górecki, K.; Bała, P.; Bednarczyk, W.; Kawałko, J. Cryogenic Behaviour of the Al5Ti5Co35Ni35Fe20 Multi-Principal Component Alloy. Mater. Sci. Eng. A 2019, 745, 346–352. [Google Scholar] [CrossRef]
- Górecki, K.; Bala, P.; Cios, G.; Koziel, T.; Stępień, M.; Wieczerzak, K. The Influence of Cooling Rate During Crystallization on the Effective Partitioning Coefficient in High-Entropy Alloys from Al-Ti-Co-Ni-Fe System. Met. Mater. Trans. A 2016, 47, 3257–3262. [Google Scholar] [CrossRef]
- Zhang, M.; Ma, Y.; Dong, W.; Liu, X.; Lu, Y.; Zhang, Y.; Li, R.; Wang, Y.; Yu, P.; Gao, Y.; et al. Phase Evolution, Microstructure, and Mechanical Behaviors of the CrFeNiAlxTiy Medium-Entropy Alloys. Mater. Sci. Eng. A 2020, 771, 138566. [Google Scholar] [CrossRef]
- Fan, J.; Fu, L.; Sun, Y.; Xu, F.; Ding, Y.; Wen, M.; Shan, A. Unveiling the Precipitation Behavior and Mechanical Properties of Co-Free Ni47-xFe30Cr12Mn8AlxTi3 High-Entropy Alloys. J. Mater. Sci. Technol. 2022, 118, 25–34. [Google Scholar] [CrossRef]
- Chen, M.-R.; Lin, S.-J.; Yeh, J.-W.; Chen, S.-K.; Huang, Y.-S.; Tu, C.-P. Microstructure and Properties of Al0.5CoCrCuFeNiTix (X = 0–2.0) High-Entropy Alloys. Mater. Trans. 2006, 47, 1395–1401. [Google Scholar] [CrossRef]
- Wang, M.; Wen, Z.; Liang, L.; Chen, W.; Mo, H.; Wei, G.; Zhao, Y. Excellent Combination of Compressive Strength and Strain of AlCrFeNi MPEAs via Adding Ti and V. J. Alloys Compd. 2023, 947, 169560. [Google Scholar] [CrossRef]
- Lu, W.; Yan, K.; Luo, X.; Wang, Y.; Hou, L.; Li, P.; Huang, B.; Yang, Y. Superb Strength and Ductility Balance of a Co-Free Medium-Entropy Alloy with Dual Heterogeneous Structures. J. Mater. Sci. Technol. 2022, 98, 197–204. [Google Scholar] [CrossRef]
- Yeh, J.-W. Overview of High-Entropy Alloys. In High-Entropy Alloys: Fundamentals and Applications; Gao, M.C., Yeh, J.-W., Liaw, P.K., Zhang, Y., Eds.; Springer International Publishing: Cham, Switzerland, 2016; pp. 1–19. ISBN 978-3-319-27013-5. [Google Scholar]
- Guo, S.; Liu, C.T. Phase Stability in High Entropy Alloys: Formation of Solid-Solution Phase or Amorphous Phase. Prog. Nat. Sci. Mater. Int. 2011, 21, 433–446. [Google Scholar] [CrossRef]
- Guo, S.; Ng, C.; Lu, J.; Liu, C.T. Effect of Valence Electron Concentration on Stability of Fcc or Bcc Phase in High Entropy Alloys. J. Appl. Phys. 2011, 109, 103505. [Google Scholar] [CrossRef]
- Guo, S. Phase Selection Rules for Cast High Entropy Alloys: An Overview. Mater. Sci. Technol. 2015, 31, 1223–1230. [Google Scholar] [CrossRef]
- Yang, X.; Zhang, Y. Prediction of High-Entropy Stabilized Solid-Solution in Multi-Component Alloys. Mater. Chem. Phys. 2012, 132, 233–238. [Google Scholar] [CrossRef]
- Czarkowski, P.; Krawczynska, A.T.; Slesinski, R.; Brynk, T.; Budniak, J.; Lewandowska, M.; Kurzydlowski, K.J. Low Temperature Mechanical Properties of 316L Type Stainless Steel after Hydrostatic Extrusion. Fusion Eng. Des. 2011, 86, 2517–2521. [Google Scholar] [CrossRef]
- Chen, Y.; Deng, H.W.; Xie, Z.M.; Wang, M.M.; Yang, J.F.; Zhang, T.; Xiong, Y.; Liu, R.; Wang, X.P.; Fang, Q.F.; et al. Tailoring Microstructures and Tensile Properties of a Precipitation-Strengthened (FeCoNi)94Ti6 Medium-Entropy Alloy. J. Alloys Compd. 2020, 828, 154457. [Google Scholar] [CrossRef]
- Lu, W.; Luo, X.; Yang, Y.; Yan, K.; Huang, B.; Li, P. Nano-Precipitates Strengthened Non-Equiatomic Medium-Entropy Alloy with Outstanding Tensile Properties. Mater. Sci. Eng. A 2020, 780, 139218. [Google Scholar] [CrossRef]
- Dong, Y.; Gao, X.; Lu, Y.; Wang, T.; Li, T. A Multi-Component AlCrFe2Ni2 Alloy with Excellent Mechanical Properties. Mater. Lett. 2016, 169, 62–64. [Google Scholar] [CrossRef]
- Pavlina, E.J.; Van Tyne, C.J. Correlation of Yield Strength and Tensile Strength with Hardness for Steels. J. Mater. Eng. Perform. 2008, 17, 888–893. [Google Scholar] [CrossRef]




| Microstructure | Lattice Constant (Å) | |||||
|---|---|---|---|---|---|---|
| Samples | Al2 | Al3 | Al4 | Al5 | Al6 | |
| as-cast | FCC | 3.592 | 3.603 | 3.593 | 3.607 | 3.599 |
| BCC | - | - | - | 3.333 | 2.877 | |
| as-annealed | FCC | 3.588 | 3.600 | 3.593 | 3.602 | 3.600 |
| Alloys | Region | Chemical Composition (at. %) | |||
|---|---|---|---|---|---|
| Al | Cr | Fe | Ni | ||
| Al0.2CrFe2Ni2 | Nominal | 3.85 | 19.23 | 38.46 | 38.46 |
| Actual | 3.52 | 18.39 | 38.53 | 39.57 | |
| Al0.3CrFe2Ni2 | Nominal | 5.66 | 18.87 | 37.74 | 37.74 |
| Actual | 5.00 | 19.09 | 39.12 | 36.79 | |
| Dendrite | 4.60 | 18.23 | 39.64 | 37.52 | |
| Inter-dendrite | 18.18 | 5.57 | 12.17 | 45.25 | |
| Al0.4CrFe2Ni2 | Nominal | 7.41 | 18.52 | 37.04 | 37.04 |
| Actual | 7.54 | 17.72 | 36.33 | 38.42 | |
| Dendrite | 5.75 | 17.04 | 38.72 | 38.50 | |
| Inter-dendrite | 18.12 | 10.10 | 26.88 | 44.90 | |
| Al0.5CrFe2Ni2 | Nominal | 9.09 | 18.18 | 36.36 | 36.36 |
| Actual | 8.29 | 17.77 | 36.63 | 37.31 | |
| Dendrite | 7.76 | 18.10 | 36.54 | 37.60 | |
| Inter-dendrite | 17.21 | 8.51 | 22.17 | 52.11 | |
| Al0.6CrFe2Ni2 | Nominal | 10.71 | 17.86 | 35.71 | 35.71 |
| Actual | 10.13 | 17.80 | 35.93 | 36.14 | |
| Dendrite | 8.79 | 17.33 | 38.03 | 35.86 | |
| Inter-dendrite | 24.18 | 11.34 | 17.34 | 47.14 | |
| Alloys | Region | Chemical Composition (at. %) | |||
|---|---|---|---|---|---|
| Al | Cr | Fe | Ni | ||
| Al0.2CrFe2Ni2 | Actual | 3.41 | 18.56 | 37.77 | 40.26 |
| Al0.3CrFe2Ni2 | Actual | 4.86 | 18.98 | 36.78 | 39.38 |
| Al0.4CrFe2Ni2 | Actual | 6.94 | 17.77 | 36.36 | 38.94 |
| Al0.5CrFe2Ni2 | Actual | 8.00 | 18.52 | 35.09 | 38.39 |
| Al0.6CrFe2Ni2 | Actual | 10.00 | 17.56 | 35.51 | 36.93 |
| Al2 | Al3 | Al4 | Al5 | Al6 | |
|---|---|---|---|---|---|
| 270 | 286 | 295 | 303 | 327 | |
| 1302 | 1309 | 1316 | 1390 | 1511 |
| Microstructure | Lattice Constant (Å) | |||||
|---|---|---|---|---|---|---|
| Samples | Al2 | Al3 | Al4 | Al5 | Al6 | |
| as-cast | FCC | 3.602 | 3.611 | 3.611 | 3.602 | 3.617 |
| BCC 1 | - | 3.385 | 2.932 | 2.923 | 2.887 | |
| BCC 2 | - | - | - | - | 3.011 | |
| as-annealed | FCC | 3.607 | 3.620 | 3.617 | 3.628 | 3.612 |
| BCC 1 | - | - | 2.927 | 2.935 | 2.867 | |
| BCC 2 | - | - | - | - | 2.943 | |
| Region | Al | Ti | Cr | Fe | Ni | |
|---|---|---|---|---|---|---|
| Al0.2Ti0.2CrFe2Ni2 | Nominal | 3.70 | 3.70 | 18.52 | 37.04 | 37.04 |
| Actual | 3.58 | 3.63 | 17.90 | 35.15 | 39.74 | |
| Dendrite | 3.15 | 1.93 | 17.64 | 37.17 | 40.10 | |
| Inter-dendrite | 2.63 | 13.86 | 8.94 | 17.97 | 56.59 | |
| Al0.3Ti0.3CrFe2Ni2 | Nominal | 5.36 | 5.36 | 17.86 | 35.71 | 35.71 |
| Actual | 5.41 | 5.56 | 17.76 | 36.05 | 35.22 | |
| Dendrite | 5.87 | 5.08 | 18.49 | 34.76 | 35.81 | |
| Inter-dendrite | 18.18 | 18.83 | 5.57 | 12.17 | 45.25 | |
| Al0.4Ti0.4CrFe2Ni2 | Nominal | 6.90 | 6.90 | 17.24 | 34.48 | 34.48 |
| Actual | 6.92 | 7.04 | 17.66 | 34.53 | 33.85 | |
| Dendrite | 6.75 | 6.76 | 18.07 | 34.77 | 33.66 | |
| Inter-dendrite | 18.92 | 18.13 | 5.04 | 13.25 | 44.65 | |
| Al0.5Ti0.5CrFe2Ni2 | Nominal | 8.33 | 8.33 | 16.67 | 33.33 | 33.33 |
| Actual | 8.28 | 8.89 | 16.53 | 32.66 | 33.63 | |
| Dendrite | 4.80 | 8.44 | 18.85 | 35.91 | 32.00 | |
| Inter-dendrite | 19.23 | 19.79 | 4.04 | 12.70 | 44.23 | |
| Al0.6Ti0.6CrFe2Ni2 | Nominal | 9.68 | 9.68 | 16.13 | 32.26 | 32.26 |
| Actual | 10.03 | 9.72 | 16.14 | 29.62 | 34.51 | |
| Dendrite | 5.44 | 5.69 | 17.73 | 37.54 | 33.59 | |
| Inter-dendrite | 17.62 | 13.95 | 6.12 | 12.80 | 49.50 |
| Region | Al | Ti | Cr | Fe | Ni | |
|---|---|---|---|---|---|---|
| Al0.2Ti0.2CrFe2Ni2 | Matrix | 3.58 | 3.63 | 17.90 | 35.15 | 39.74 |
| Black particle | 9.37 | 87.71 | 1.23 | 1.69 | - | |
| Al0.3Ti0.3CrFe2Ni2 | Matrix | 4.89 | 5.18 | 17.66 | 36.16 | 36.10 |
| Al0.4Ti0.4CrFe2Ni2 | Matrix | 6.02 | 4.25 | 18.59 | 36.80 | 34.35 |
| Phase | 8.11 | 13.92 | 12.59 | 24.65 | 40.75 | |
| Al0.5Ti0.5CrFe2Ni2 | Matrix | 6.49 | 4.19 | 18.27 | 37.40 | 33.67 |
| Phase | 5.51 | 19.67 | 9.74 | 19.98 | 44.80 | |
| Al0.6Ti0.6CrFe2Ni2 | Matrix | 6.40 | 4.78 | 17.74 | 38.05 | 33.03 |
| Phase | 15.36 | 9.93 | 9.32 | 21.95 | 43.44 |
| Al2Ti2 | Al3Ti3 | Al4Ti4 | Al5Ti5 | Al6Ti6 | |
|---|---|---|---|---|---|
| 447 | 475 | 488 | 669 | 1190 | |
| 1760 | 2010 | 2129 | 2239 | 1942 |
| Alloys | δ % | ΔHmix, kJ mol−1 | ΔSmix, J mol−1 K−1 | Ω, - | VEC - | Tm °C |
|---|---|---|---|---|---|---|
| Al2 | 2.77 | −5.62 | 1.16 R | 3.10 | 8.25 | 1592 |
| Al3 | 3.27 | −6.28 | 1.20 R | 2.86 | 8.10 | 1586 |
| Al4 | 3.94 | −7.57 | 1.24 R | 2.40 | 8.04 | 1554 |
| Al5 | 4.11 | −7.86 | 1.25 R | 2.33 | 7.98 | 1548 |
| Al6 | 4.48 | −8.64 | 1.27 R | 2.14 | 7.86 | 1533 |
| Al2Ti2 | 4.18 | −8.65 | 1.28 R | 2.23 | 8.11 | 1593 |
| Al3Ti3 | 5.02 | −10.68 | 1.36 R | 1.90 | 7.86 | 1583 |
| Al4Ti4 | 5.54 | −12.38 | 1.41 R | 1.69 | 7.70 | 1572 |
| Al5Ti5 | 6.00 | −14.30 | 1.45 R | 1.50 | 7.57 | 1555 |
| Al6Ti6 | 6.28 | −15.83 | 1.48 R | 1.36 | 7.48 | 1538 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kočiško, R.; Petroušek, P.; Saksl, K.; Petryshynets, I.; Milkovič, O.; Csík, D. The Influence of Ti and Al on the Evolution of Microstructure and Mechanical Properties in Medium-Entropy and High-Entropy Alloys Based on AlxTixCrFe2Ni2. Materials 2025, 18, 1382. https://doi.org/10.3390/ma18061382
Kočiško R, Petroušek P, Saksl K, Petryshynets I, Milkovič O, Csík D. The Influence of Ti and Al on the Evolution of Microstructure and Mechanical Properties in Medium-Entropy and High-Entropy Alloys Based on AlxTixCrFe2Ni2. Materials. 2025; 18(6):1382. https://doi.org/10.3390/ma18061382
Chicago/Turabian StyleKočiško, Róbert, Patrik Petroušek, Karel Saksl, Ivan Petryshynets, Ondrej Milkovič, and Dávid Csík. 2025. "The Influence of Ti and Al on the Evolution of Microstructure and Mechanical Properties in Medium-Entropy and High-Entropy Alloys Based on AlxTixCrFe2Ni2" Materials 18, no. 6: 1382. https://doi.org/10.3390/ma18061382
APA StyleKočiško, R., Petroušek, P., Saksl, K., Petryshynets, I., Milkovič, O., & Csík, D. (2025). The Influence of Ti and Al on the Evolution of Microstructure and Mechanical Properties in Medium-Entropy and High-Entropy Alloys Based on AlxTixCrFe2Ni2. Materials, 18(6), 1382. https://doi.org/10.3390/ma18061382







