Synthetic Biomaterials for Alveolar Bone Regeneration: A Systematic Review of Clinical Evidence
Abstract
1. Introduction
- 1.
- Do synthetic biomaterials achieve bone regeneration outcomes comparable to autografts, allografts, and xenografts?
- 2.
- How do clinical results vary according to the type of synthetic biomaterial used?
- 3.
- What complications, limitations, or methodological biases are reported in the current literature?
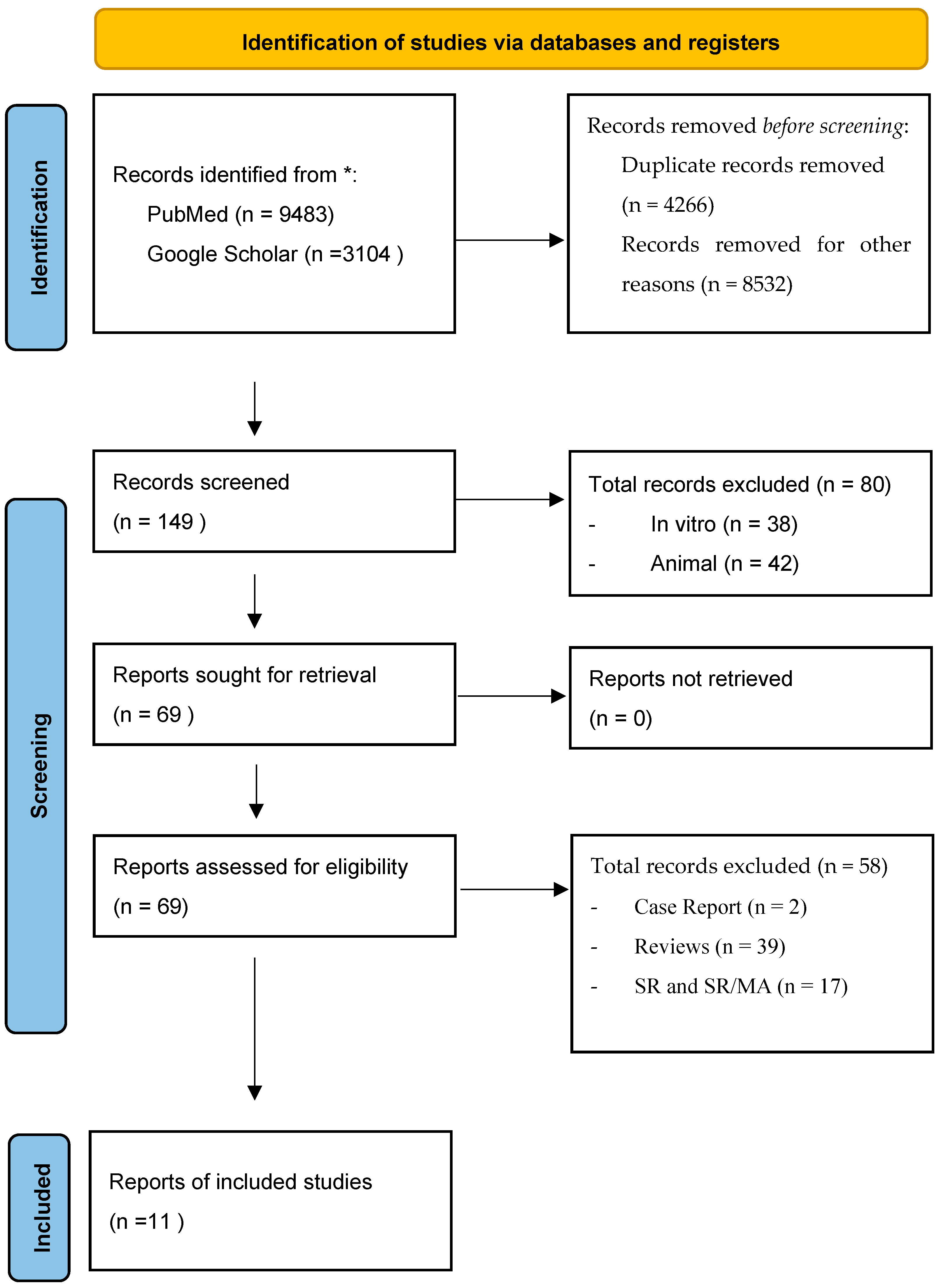
2. Materials and Methods
- (i).
- Randomized controlled trials (RCTs) or non-randomized comparative studies with a clearly defined control group;
- (ii).
- Standardized interventions involving synthetic biomaterials for alveolar bone volume regeneration;
- (iii).
- Comparable baseline characteristics among participants;
- (iv).
- Consistent and well-defined outcome measures (e.g., new bone formation, graft resorption, implant survival);
- (v).
- Adequate reporting of quantitative data (means, standard deviations, or confidence intervals) enabling statistical synthesis.
- Population: Patients with alveolar bone defects requiring regeneration, including those undergoing implant placement or other oral rehabilitations.
- Intervention: Use of synthetic biomaterials (e.g., hydroxyapatite, β-tricalcium phosphate, bioglass) for guided bone regeneration or ridge augmentation.
- Comparison: Autologous, allogenic, or xenogenic grafts, other regenerative techniques, or no treatment.
- Outcomes: Primary outcomes included bone volume gain, residual biomaterial, implant stability, infection rate, and complications. Secondary outcomes included histological integration and implant success.
(“bone regeneration” OR “guided bone regeneration” OR “dental bone graft”) AND (“synthetic biomaterials” OR “hydroxyapatite” OR “beta-tricalcium phosphate” OR “bioceramics” OR “bioactive glass”) AND (“dental implants” OR “oral surgery”).
3. Results
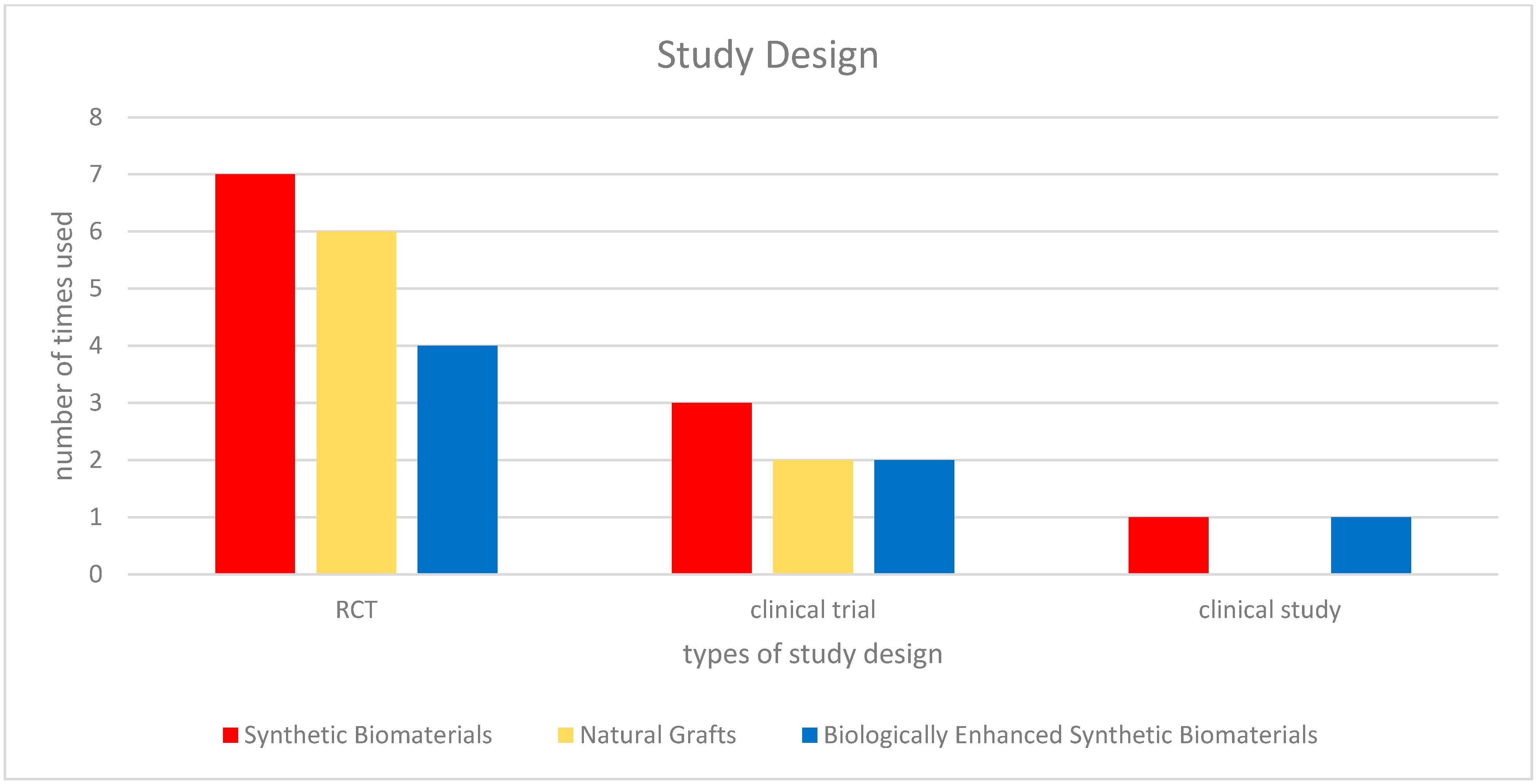
3.1. Bone Regeneration with Synthetic Biomaterials
3.2. Comparative Efficacy of Natural and Synthetic Grafts
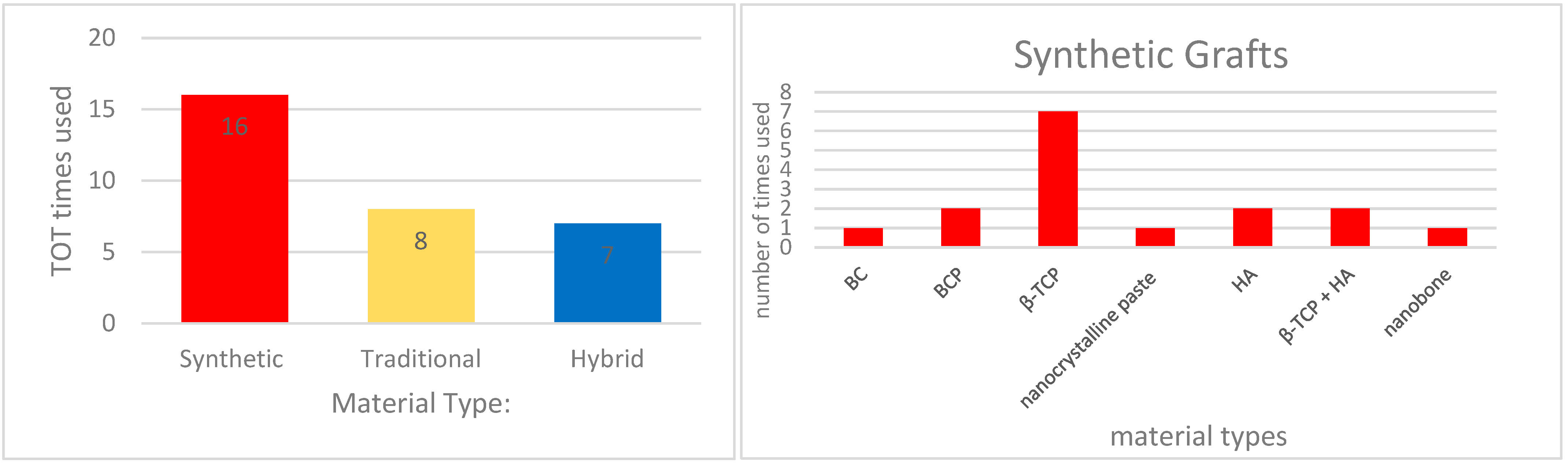
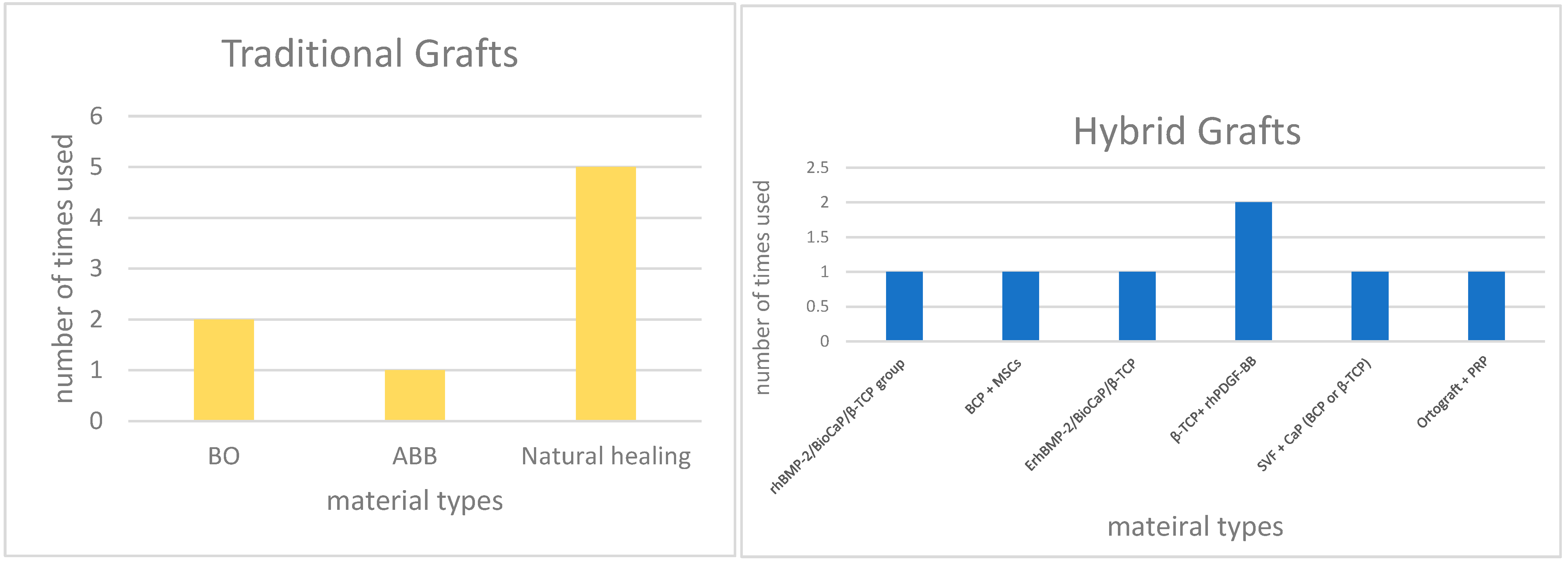
3.3. Variability Among Synthetic Biomaterials
3.4. Biologically Enhanced Synthetic Biomaterials
3.5. Limitations and Gaps
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Nkenke, E.; Neukam, F.W. Autogenous bone harvesting and grafting in advanced jaw resorption: Morbidity, resorption and implant survival. Eur. J. Oral Implantol. 2014, 7 (Suppl. 2), S203–S2017. [Google Scholar] [PubMed]
- Hämmerle, C.H.; Jung, R.E. Bone augmentation by means of barrier membranes. Periodontol 2000 2003, 33, 36–53. [Google Scholar] [CrossRef] [PubMed]
- Ferraz, M.P. Bone Grafts in Dental Medicine: An Overview of Autografts, Allografts and Synthetic Materials. Materials 2023, 16, 4117. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Baldi, D.; Pesce, P.; Musante, B.; Pera, F.; Fulcheri, E.; Romano, F.; Menini, M. Radiological and Histomorphometric Outcomes of Homologous Bone Graft in Postextractive Implant Sites: A 6-Year Retrospective Analysis. Implant. Dent. 2019, 28, 472–477. [Google Scholar] [CrossRef] [PubMed]
- Menini, M.; Canullo, L.; Iacono, R.; Triestino, A.; Caponio, V.C.A.; Savadori, P.; Pesce, P.; Pedetta, A.; Guerra, F. Effect of Different Graft Material Consistencies in the Treatment of Minimal Bone Dehiscence: A Retrospective Pilot Study. Dent. J. 2024, 12, 198. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Bose, S.; Roy, M.; Bandyopadhyay, A. Recent advances in bone tissue engineering scaffolds. Trends Biotechnol. 2012, 30, 546–554. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- De Angelis, N.; Pesce, P.; Poedjiastoeti, W.; Suwandi, T.; Tjandrawinata, R.; Bagnasco, F.; Menini, M. 3D-Printable Biopolymers for Socket Preservation Technique: Soft Tissues Response: A Pilot Randomised Clinical Study. Dent. J. 2024, 12, 321. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Erjavec, A.K.; Črešnar, K.P.; Švab, I.; Vuherer, T.; Žigon, M.; Brunčko, M. Determination of Shear Bond Strength between PEEK Composites and Veneering Composites for the Production of Dental Restorations. Materials 2023, 16, 3286. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Jung, J.M.; Kim, G.N.; Koh, Y.H.; Kim, H.E. Manufacturing and Characterization of Dental Crowns Made of 5-Mol% Yttria Stabilized Zirconia by Digital Light Processing. Materials 2023, 16, 1447. [Google Scholar] [CrossRef]
- Lakhkar, N.J.; Park, J.H.; Mordan, N.J.; Salih, V.; Wall, I.B.; Kim, H.W.; King, S.P.; Hanna, J.V.; Martin, R.A.; Addison, O.; et al. Titanium phosphate glass microspheres for bone tissue engineering. Acta Biomater. 2012, 8, 4181–4190. [Google Scholar] [CrossRef] [PubMed]
- Mangano, F.G.; Iezzi, G.; Shibli, J.A.; Pires, J.T.; Luongo, G.; Piattelli, A.; Mangano, C. Early bone formation around immediately loaded implants with nanostructured calcium-incorporated and machined surface: A randomized, controlled histologic and histomorphometric study in the human posterior maxilla. Clin. Oral Investig. 2017, 21, 2603–2611, Erratum in Clin. Oral Investig. 2021, 25, 6473. [Google Scholar] [CrossRef] [PubMed]
- Pesce, P.; Canullo, L.; Testori, T.; Mastroianni, A.; Fabbro, M.D.; Menini, M. The clinical effect of bone perforations in periodontal regeneration and alveolar socket preservation: A systematic review with meta-analysis. Clin. Oral Investig. 2025, 29, 64. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- De Angelis, N.; Kassim, Z.H.; Mohd Yusof, E.; Yumang, C.; Menini, M. Bone Augmentation Techniques with Customized Titanium Meshes: A Systematic Review of Randomized Clinical Trials. Open Dent. J. 2023, 17, 1–9. [Google Scholar] [CrossRef]
- Klijn, R.J.; Meijer, G.J.; Bronkhorst, E.M.; Jansen, J.A. A meta-analysis of histomorphometric results and graft healing time of various biomaterials compared to autologous bone used as sinus floor augmentation material in humans. Tissue Eng. Part. B Rev. 2010, 16, 493–507. [Google Scholar] [CrossRef] [PubMed]
- Miron, R.J.; Zhang, Y.F. Osteoinduction: A review of old concepts with new standards. J. Dent. Res. 2012, 91, 736–744. [Google Scholar] [CrossRef] [PubMed]
- Sterne, J.A.C.; Savović, J.; Page, M.J.; Elbers, R.G.; Blencowe, N.S.; Boutron, I.; Cates, C.J.; Cheng, H.Y.; Corbett, M.S.; Eldridge, S.M.; et al. RoB 2: A revised tool for assessing risk of bias in randomised trials. BMJ 2019, 366, l4898. [Google Scholar] [CrossRef] [PubMed]
- Tomas, M.; Karl, M.; Čandrlić, M.; Matijević, M.; Juzbašić, M.; Peloza, O.C.; Radetić, A.T.J.; Kuiš, D.; Vidaković, B.; Ivanišević, Z.; et al. A Histologic, Histomorphometric, and Immunohistochemical Evaluation of Anorganic Bovine Bone and Injectable Biphasic Calcium Phosphate in Humans: A Randomized Clinical Trial. Int. J. Mol. Sci. 2023, 24, 5539. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Page, M.J.; McKenzie, J.E.; Bossuyt, P.M.; Boutron, I.; Hoffmann, T.C.; Mulrow, C.D.; Shamseer, L.; Tetzlaff, J.M.; Akl, E.A.; Brennan, S.E.; et al. The PRISMA 2020 statement: An updated guideline for reporting systematic reviews. BMJ 2021, 372, n71. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Wang, Y.; Fu, G.; Zhang, J.; Xu, Y.; Shen, M.; Yi, Z.; Lan, J.; Li, Q.; Zhao, Y.; Wu, R.; et al. Bioceramics for Guided Bone Regeneration: A Multicenter Randomized Controlled Trial. Clin. Implant. Dent. Relat. Res. 2025, 27, e13437. [Google Scholar] [CrossRef] [PubMed]
- Prins, H.J.; Schulten, E.A.; Ten Bruggenkate, C.M.; Klein-Nulend, J.; Helder, M.N. Bone Regeneration Using the Freshly Isolated Autologous Stromal Vascular Fraction of Adipose Tissue in Combination With Calcium Phosphate Ceramics. Stem Cells Transl. Med. 2016, 5, 1362–1374. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Gjerde, C.; Mustafa, K.; Hellem, S.; Rojewski, M.; Gjengedal, H.; Yassin, M.A.; Feng, X.; Skaale, S.; Berge, T.; Rosen, A.; et al. Cell therapy induced regeneration of severely atrophied mandibular bone in a clinical trial. Stem Cell Res. Ther. 2018, 9, 213. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Sarment, D.P.; Cooke, J.W.; Miller, S.E.; Jin, Q.; McGuire, M.K.; Kao, R.T.; McClain, P.K.; McAllister, B.S.; Lynch, S.E.; Giannobile, W.V. Effect of rhPDGF-BB on bone turnover during periodontal repair. J. Clin. Periodontol. 2006, 33, 135–140. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Nagaveni, N.B.; Praveen, R.B.; Umashankar, K.V.; Pranav, B.; Sreedevi, R.; Radhika, N.B. Efficacy of platelet-rich-plasma (PRP) in bone regeneration after cyst enucleation in pediatric patients—A clinical study. J. Clin. Pediatr. Dent. 2010, 35, 81–87. [Google Scholar] [CrossRef] [PubMed]
- Gupta, A.K.; Arora, K.S.; Aggarwal, P.; Kaur, K.; Mohapatra, S.; Pareek, S. Evaluation of biphasic hydroxapatite and β-tricalcium phosphate as a bone graft material in the treatment of periodontal vertical bony defects—A clinical and digital radiological measurement study. Indian J. Dent. Res. 2022, 33, 152–157. [Google Scholar] [CrossRef] [PubMed]
- Canuto, R.A.; Pol, R.; Martinasso, G.; Muzio, G.; Gallesio, G.; Mozzati, M. Hydroxyapatite paste Ostim, without elevation of full-thickness flaps, improves alveolar healing stimulating BMP- and VEGF-mediated signal pathways: An experimental study in humans. Clin. Oral Implant. Res. 2013, 24 (Suppl. A100), 42–48. [Google Scholar] [CrossRef] [PubMed]
- Troedhan, A.; Schlichting, I.; Kurrek, A.; Wainwright, M. Primary implant stability in augmented sinuslift-sites after completed bone regeneration: A randomized controlled clinical study comparing four subantrally inserted biomaterials. Sci. Rep. 2014, 4, 5877. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Sun, Y.; Xu, C.; Wang, M.; Wei, L.; Pieterse, H.; Wu, Y.; Liu, Y. Radiographic and histological evaluation of bone formation induced by rhBMP-2-incorporated biomimetic calcium phosphate material in clinical alveolar sockets preservation. Int. J. Implant. Dent. 2023, 9, 37. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Wei, L.; Sun, Y.; Yu, D.; Pieterse, H.; Wismeijer, D.; Liu, Y.; Wu, Y. The Clinical Efficacy and Safety of ErhBMP-2/BioCaP/β-TCP as a Novel Bone Substitute Using the Tooth-Extraction-Socket-Healing Model: A Proof-of-Concept Randomized Controlled Trial. J. Clin. Periodontol. 2025, 52, 299–309. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Alkaabi, S.A.; Alsabri, G.A.; NatsirKalla, D.S.; Alavi, S.A.; Mueller, W.E.G.; Forouzanfar, T.; Helder, M.N. A Systematic Review on Regenerative Alveolar Graft Materials in Clinical Trials: Risk of Bias and Meta-Analysis. J. Plast. Reconstr. Aesthetic Surg. 2022, 75, 356–365. [Google Scholar] [CrossRef]
- Inchingolo, A.M.; Patano, A.; Di Pede, C.; Inchingolo, A.D.; Palmieri, G.; de Ruvo, E.; Campanelli, M.; Buongiorno, S.; Carpentiere, V.; Piras, F.; et al. Autologous Tooth Graft: Innovative Biomaterial for Bone Regeneration. Tooth Transformer® and the Role of Microbiota in Regenerative Dentistry. A Systematic Review. J. Funct. Biomater. 2023, 14, 132. [Google Scholar] [CrossRef]
- Motta, C.; Cavagnetto, D.; Amoroso, F.; Baldi, I.; Mussano, F. Bioactive Glass for Periodontal Regeneration: A Systematic Review. BMC Oral Health 2023, 23, 264. [Google Scholar] [CrossRef]
- Shamsoddin, E.; Houshmand, B.; Golabgiran, M. Biomaterial Selection for Bone Augmentation in Implant Dentistry: A Systematic Review. J. Adv. Pharm. Technol. Res. 2019, 10, 46. [Google Scholar] [CrossRef]
- Papageorgiou, S.N.; Papageorgiou, P.N.; Deschner, J.; Götz, W. Comparative Effectiveness of Natural and Synthetic Bone Grafts in Oral and Maxillofacial Surgery Prior to Insertion of Dental Implants: Systematic Review and Network Meta-Analysis of Parallel and Cluster Randomized Controlled Trials. J. Dent. 2016, 48, 1–8. [Google Scholar] [CrossRef]
- Jasser, R.A.L.; AlSubaie, A.; AlShehri, F. Effectiveness of Beta-Tricalcium Phosphate in Comparison with Other Materials in Treating Periodontal Infra-Bony Defects around Natural Teeth: A Systematic Review and Meta-Analysis. BMC Oral Health 2021, 21, 219. [Google Scholar] [CrossRef]
- Tavelli, L.; Chen, C.; Barootchi, S.; Kim, D.M. Efficacy of Biologics for the Treatment of Periodontal Infrabony Defects: An American Academy of Periodontology Best Evidence Systematic Review and Network Meta-analysis. J. Periodontol. 2022, 93, 1803–1826. [Google Scholar] [CrossRef]
- Castillo-Paz, A.M.; Correa-Piña, B.A.; Martinez-Hernandez, H.D.; Gomez-Vazquez, O.M.; Cañon-Davila, D.F.; Zubieta-Otero, L.F.; Londoño-Restrepo, S.M.; Perez-Torrero, E.; Rodriguez-Garcia, M.E. Influence of the Changes in the Bone Mineral Density on the Guided Bone Regeneration Using Bioinspired Grafts: A Systematic Review and Meta-Analysis. Biomed. Mater. Devices 2023, 1, 162–178. [Google Scholar] [CrossRef]
- Atieh, M.A.; Alsabeeha, N.H.; Payne, A.G.; Ali, S.; Faggion, C.M.J.; Esposito, M. Interventions for Replacing Missing Teeth: Alveolar Ridge Preservation Techniques for Dental Implant Site Development. Cochrane Database Syst. Rev. 2021, 2021. [Google Scholar] [CrossRef]
- AlKudmani, H.; ALJasser, R.; Andreana, S. Is Bone Graft or Guided Bone Regeneration Needed When Placing Immediate Dental Implants? A Systematic Review. Implant. Dent. 2017, 26, 936–944. [Google Scholar] [CrossRef]
- Čandrlić, M.; Tomas, M.; Matijević, M.; Kačarević, Ž.P.; Bićanić, M.; Udiljak, Ž.; Butorac Prpić, I.; Miškulin, I.; Čandrlić, S.; Včev, A. Regeneration of Buccal Wall Defects after Tooth Extraction with Biphasic Calcium Phosphate in Injectable Form vs. Bovine Xenograft: A Randomized Controlled Clinical Trial. Dent. J. 2023, 11, 223. [Google Scholar] [CrossRef] [PubMed]
- Troeltzsch, M.; Troeltzsch, M.; Kauffmann, P.; Gruber, R.; Brockmeyer, P.; Moser, N.; Rau, A.; Schliephake, H. Clinical efficacy of grafting materials in alveolar ridge augmentation: A systematic review. J. Craniomaxillofac. Surg. 2016, 44, 1618–1629. [Google Scholar] [CrossRef] [PubMed]
- Esposito, M.; Grusovin, M.G.; Coulthard, P.; Worthington, H.V. The efficacy of various bone augmentation procedures for dental implants: A Cochrane systematic review of randomized controlled clinical trials. Int. J. Oral Maxillofac. Implants 2006, 21, 696–710. [Google Scholar] [PubMed]
- Chan, H.L.; Lin, G.H.; Fu, J.H.; Wang, H.L. Alterations in bone quality after socket preservation with grafting materials: A systematic review. Int. J. Oral Maxillofac. Implants. 2013, 28, 710–720. [Google Scholar] [CrossRef] [PubMed]
- Lin, Y.; He, J.-f.; Zhang, X.; Wang, H.-m. Intraoperative Factors Associated with Free Flap Failure in the Head and Neck Region: A Four-Year Retrospective Study of 216 Patients and Review of the Literature. Int. J. Oral Maxillofac. Surg. 2019, 48, 447–451. [Google Scholar] [CrossRef] [PubMed]
- Zhou, J.; Zhang, Z.; Joseph, J.; Zhang, X.; Ferdows, B.E.; Patel, D.N.; Chen, W.; Banfi, G.; Molinaro, R.; Cosco, D.; et al. Biomaterials and nanomedicine for bone regeneration: Progress and future prospects. Exploration 2021, 1, 20210011. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Wu, H.; Wei, X.; Liu, Y.; Dong, H.; Tang, Z.; Wang, N.; Bao, S.; Wu, Z.; Shi, L.; Zheng, X.; et al. Dynamic degradation patterns of porous polycaprolactone/β-tricalcium phosphate composites orchestrate macrophage responses and immunoregulatory bone regeneration. Bioact. Mater. 2022, 21, 595–611. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
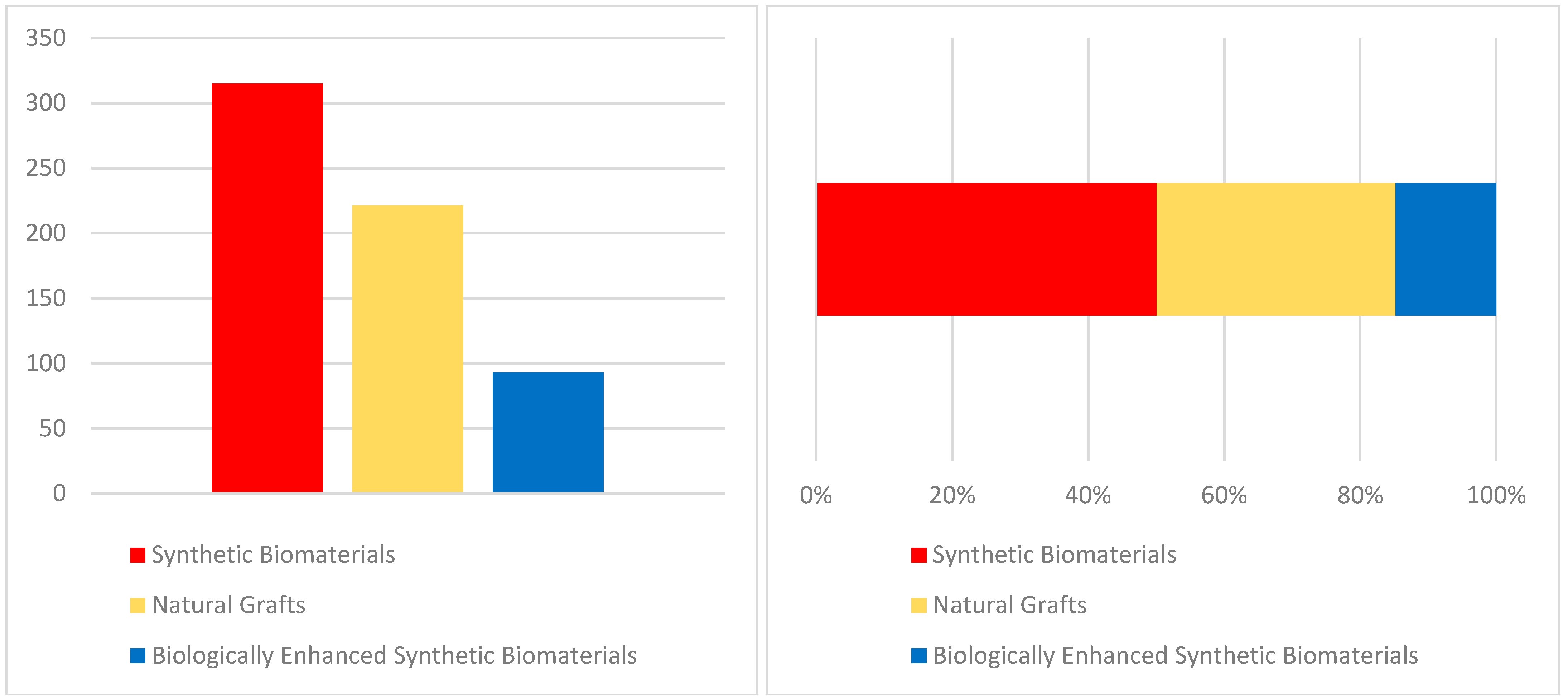
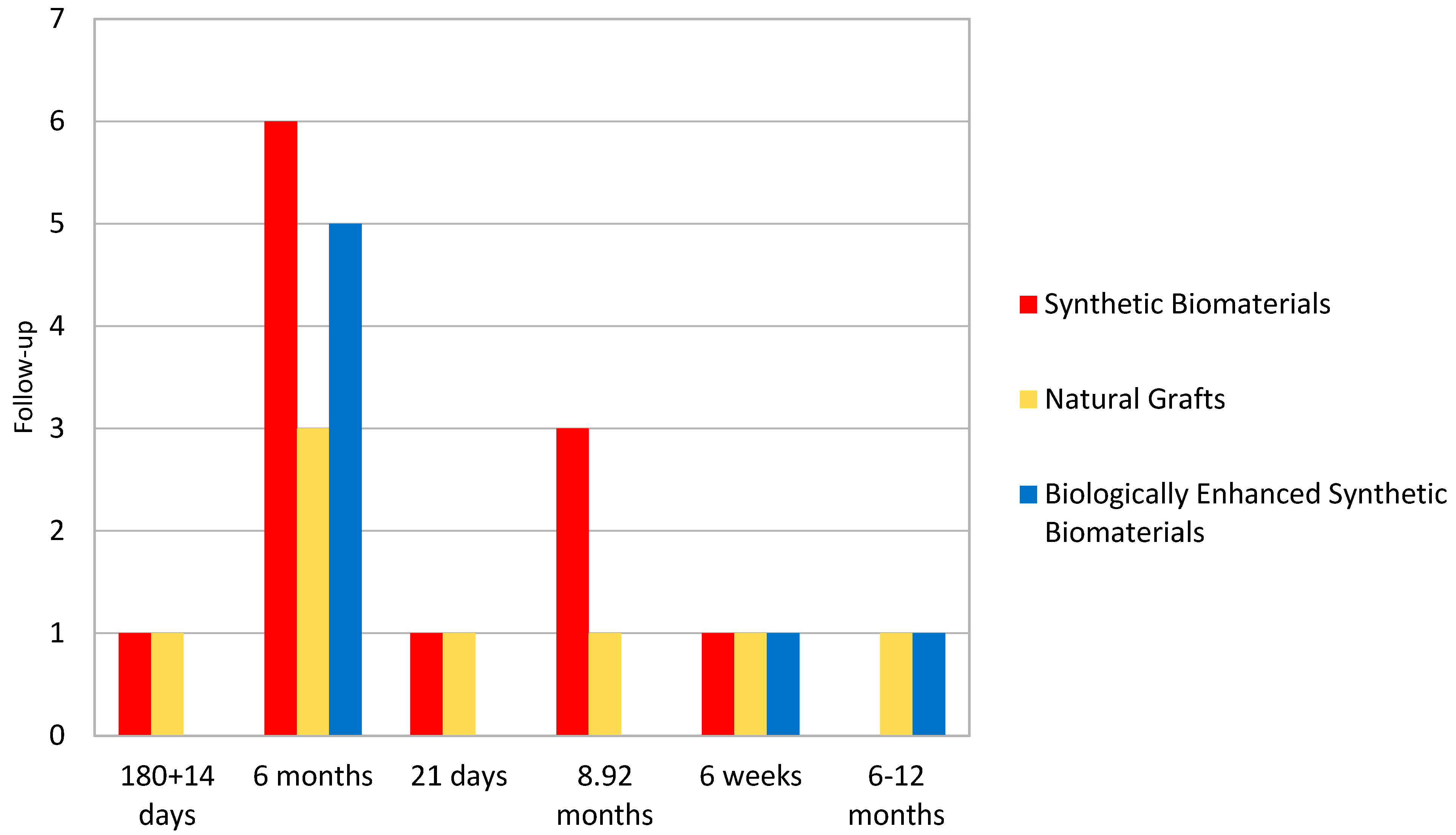
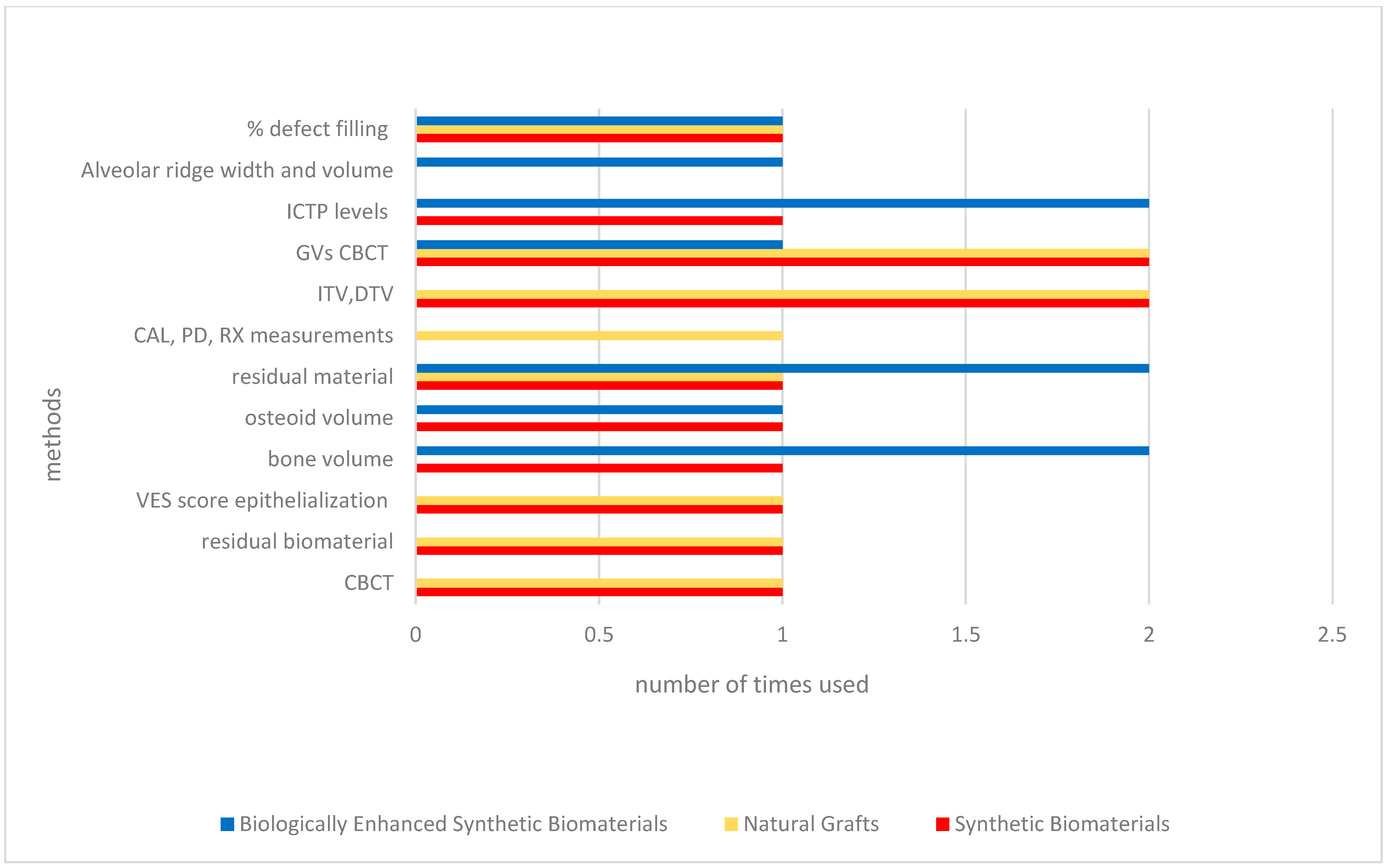
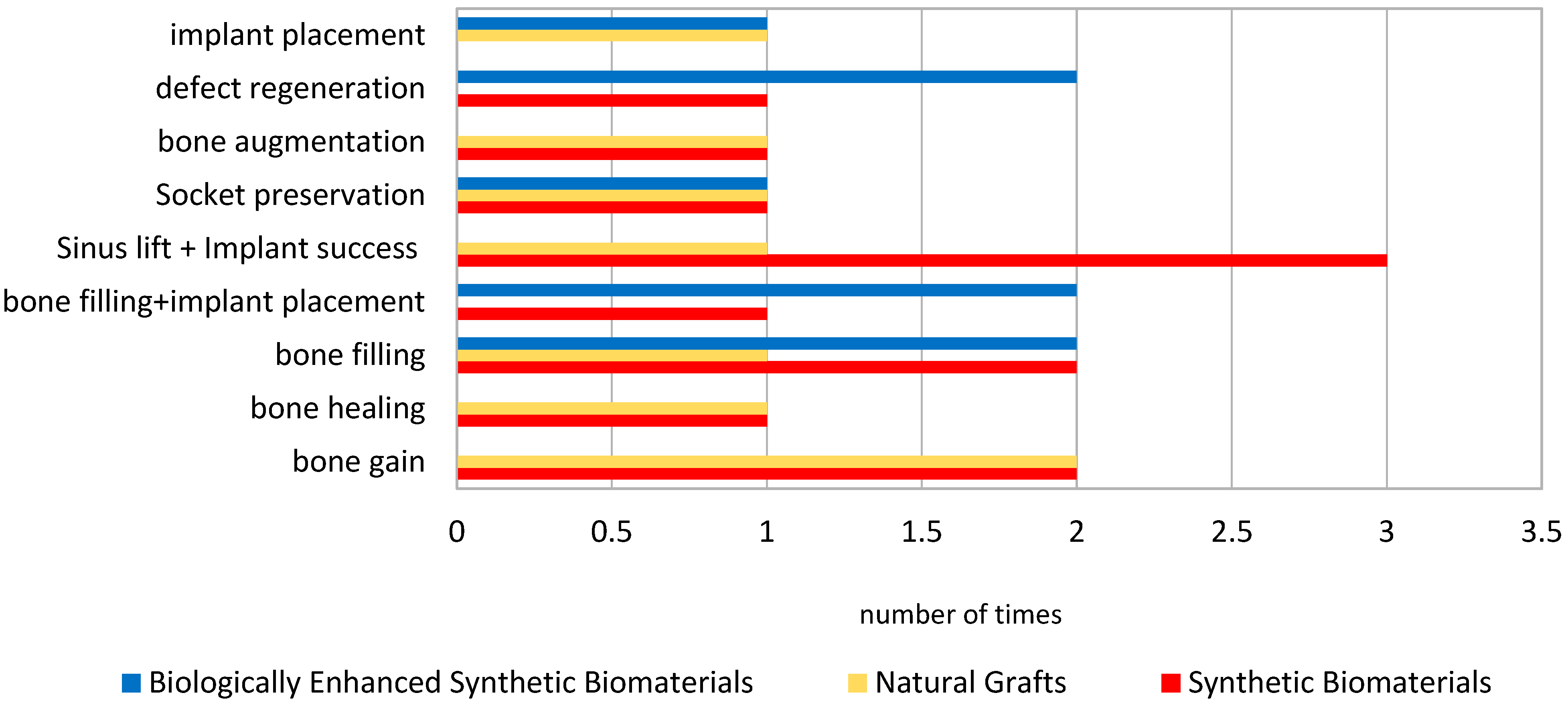
| Author | Type of Intervention | Comparison/Control | Outcomes Measured | Conclusions |
|---|---|---|---|---|
| Tomas MK et al. [17] | Effectiveness comparison among different materials | ABB (Bio-oss®) and Injectable BCP (maxresorb® inject—16.5% biphasic granules and 83.5% nano-HA gel) | Differences between newly formed bone, residual biomaterial and soft tissue | No significant difference found between the groups |
| Wang YF et al. [19] | GBR during implant placement | Novel bioceramic (BC) versus a control xenograft (BO) | CBCT images at T1 and T2 measuring horizontal buccal bone thicknesss and width change Soft tissue gealing status | Novel BC material is safe and effective, efficacy comparable to xenograft bone |
| Prins HjS et al. [20] | 6 bilaterally treated 4 unilateral | β-TCP and BCP ceramics combined with SVF versus β-TCP and BCP without SVF | Micro-CT and quantitative histomorphometric evaluations on both unilateral and bilaterally treated patients | In study biopsies (with SVF) bone and osteoid % were higher Pared analysis on bilaterally treated patients revealed higher bone and osteoid % when treated adding SVF |
| Gjerde CM et al. [21] | Bone regeneration using MSCs | BCP granules with MSCs versus BCP alone | Average bone healing, increased bone width and volume were statistically relevant | MSCs induce significant formation of new bone |
| Sarment DP et al. [22] | Periodontal regenerative surgery | rhPDGF (+0.3 mg/mL) with β-TCP, rhPDGF (+1.0 mg/mL) with β-TCP, versus β-TCP alone | Evaluate release of the ICTP into periodontal wound fluid | rhPDGF-BB has a direct effect on ICTP released from the wound |
| Nagaveni NBP et al. [23] | Bone regeneration after cystectomy | adding PRP to a bone graft in the versus without PRP | After 6 months, defect bone fills show 94% filling in test group and 47% in the control group | PRP application improves bone height regeneration |
| Gupta AKA et al. [24] | Periodontal regenerative surgery | β-TCP versus open flap debridement only | Probing pocket depth, Clinical attachment level, Radiological measurements | β-TCP reduces probing depth almost by 75% and gain clinical attachment |
| Canuto RAP et al. [25] | Bone regeneration for post-extractive socket treatment using the graft materials without elevation of full-thickness flaps | Ostim®-filled socket vs. without Ostim® | Clinical control (VAS score) and biological parameters (synthesis of pro-osteogenic factors, as BMP-4, BMP-7, alkaline phosphatase, osteocalcin) | Ostim® increases the production of positive molecules for socket healing but causes greater pain at day 1 |
| Troedhan AS et al. [26] | Bone regeneration and implant insertion | Bio-Oss®, Nano-Bone, easy-graft CLASSIC, easy-graft CRYSTAL in alternative to natural subantral bone | Drill Torque Value (DTV) and Implant-insertion Torque Value (ITV) | Statistical supremacy of easy-graft CRYSTAL |
| Sun Y et al. [27] | Socket preservation and bone formation | rhBMP-2/BioCaP/beta-TCP, beta-TCP, natural healing | Radiographic evaluation (change in GVs in CBCT scans) and Histomorphologic (% of new bone, residual material areas, unmineralized tissue area) | hBMP-2/BioCaP/beta-TCP is a promising substitute |
| Wei LS et al. [28] | Tooth-extraction-healing model before implant placement surgery | ErhBMP-2/BioCaP/beta-TCP, beta-TCP, natural healing | % of new bone, residual material areas, unmineralized tissue area | ErhBMP-2/BioCaP/β-TCP is a promising substitute |
| Risk of Bias Arising from the Randomization Process | Effect of Assignment to Intervention | Risk of Bias Due to Missing Outcome Data | Risk of Bias in Measurement of the Outcome | Risk of Bias in Selection of the Reported Result | Overall Risk-of-Bias Judgment | |
|---|---|---|---|---|---|---|
| Tomas MK et al. (2023) [17] | Some Concerns | |||||
| Wang YF et al. (2025) [19] | Low Risk | |||||
| Prins HJS et al. (2016) [20] | High Risk of bias | |||||
| Gjerde CM et al. (2018) [21] | Some Concerns | |||||
| Sarment DP et al. (2006) [22] | Some Concerns | |||||
| Nagaveni NBP et al. (2010) [23] | Some Concerns | |||||
| Gupta AKA et al. (2022) [24] | Some Concerns | |||||
| Canuto RAP et al. (2013) [25] | Some Concerns | |||||
| Troedhan AS et al. (2014) [26] | Low Risk of bias | |||||
| Sun Y et al. (2023) [27] | Some Concerns | |||||
| Wei LS et al. (2025) [28] | Low Risk of bias |
 green = low risk of bias;
green = low risk of bias;  yellow = some concerns;
yellow = some concerns;  red = high risk of bias. A study was categorized as high-risk or having some concerns if it met these criteria in at least one domain.
red = high risk of bias. A study was categorized as high-risk or having some concerns if it met these criteria in at least one domain.| Author | Subjects | Measurements | Results | |
|---|---|---|---|---|
| Section 3.1 | Wang YF et al. [19] | novel bioceramic (BC), control xenograft (BO) for GBR | horizontal bone thickness (HT) at T1 (post-operative) and T2 (180 ± 14 days postoperatively) | HT at T1 BC: 6.053 ± 1.262 mm BO: at 5.907 ± 1.269 mm. HT at T2 BC: 5.775 ± 1.345 mm BO: 5.273 ± 1.285 mm |
| Tomas MK et al. [17] | ABB (Bio-oss®), Injectable BCP (maxresorb® inject) | % of residual biomaterial | BCP: 28.61 ± 11.38% ABB: 31.72 ± 15.52% | |
| Canuto RAP et al. [25] | Ostim pastes | VAS score Pro-osteogenic factors augmentation | VES: 5 at day 2 Increase VEGF, IL-10, IL-1β expression | |
| Gupta AKA et al. [24] | biphasic hydroxyapatite BHA and β-tricalcium phosphate, OFD | probing depth clinical attachment % bone filling in RX | probing depth reduction BHA—β-TCP: 44.70% OFD: 31.37% clinical attachment gain BHA—β-TCP: 41.07% OFD: 29.87% % bone filling BHA—β-TCP: 42.57% OFD: 20.04% |
| Author | Subjects | Measurements | Results | |
|---|---|---|---|---|
| Section 3.2 | Wang YF et al. [19] | novel bioceramic (BC), control xenograft (BO) for GBR | buccal bone thickness T1 (post-operative) and T2 (180 ± 14 days postoperatively) % post-operative heling status at T1 (14 days postoperative) and T2 (30 days postoperative) | buccal bone thickness at T1 BO: 3.633 ± 1.105 mm BC: 3.421 ± 1.227 mm buccal bone thickness at T2 BO: 2.042 ± 1.097 mm BC: 2.476 ± 1.141 mm No inflammation at T1 BO: 90.79% BC: 96.05% No inflammation at T2 BO: 98.68% BC: 97.37% |
| Tomas MK et al. [17] | ABB (Bio-oss®), Injectable BCP (maxresorb® inject) | % newly formed bone (NB) % soft tissue (ST) | new bone ABB: 41.73 ± 13.99% ABB: 39.91 ± 8.40% soft tissue ABB: 26.54 ± 7.25% BCP: 31.49 ± 11.09%) | |
| Troedhan AS et al. [26] | Bio-Oss®, natural bone (NB) | DTV and ITV (Ncm) | DTV mean value BO: 12.7 Ncm NB: 10,2 Ncm ITV mean value BO: 26.2 Ncm NB: 22.2 Ncm | |
| Gjerde CM et al. [21] | BCP/MSCs | width and volume of alveolar ridge | average bone width increase 4.05 mm average bone volume increase 887.23 ± 365.01 mm3 |
| Author | Subjects | Measurements | Results | |
|---|---|---|---|---|
| Section 3.3 | Troedhan AS et al. [26] | easy-graft CRYSTAL, easy-graft CLASSIC, NanoBone, Bio-Oss, natural healing (NB) | DTV and ITV (Ncm) chemical composition and behavior | DTV mean value NB: 10.2 Ncm Bio-Oss: 12.7 Ncm NanoBone: 17.5 Ncm easy-graft CLASSIC: 20.3 Ncm Easy-graft CRYSTAL: 23.8 Ncm ITV mean value NB: 22.2 Ncm Bio-Oss: 26.2 Ncm NanoBone: 33.3 Ncm easy-graft CLASSIC: 45.9 Ncm easy-graft CRYSTAL: 56.6 Ncm chemical composition Bio-Oss: anorganic bovine bone easy-graft CRYSTAL: 40% beta-tricalcium phosphate (β-TCP) and 60% hydroxyapatite (HA), coated by a 10-micrometer layer of polylactic-co-glycolic acid (PLGA). easy-graft CLASSIC: pure β-TCP particles. NanoBone: Nanocrystalline HA embedded in a SiO2 matrix |
| Author | Subjects | Measurements | Results | |
|---|---|---|---|---|
| Section 3.4 | Sun Y et al. [27] | rhBMP-2/BioCaP/β-TCP, β-TCP, natural healing | % newly formed bone (NB) % residual material (RM) % unmineralized tissue area (UT) | NB rhBMP-2/BioCaP/β-TCP: 21.18% ± 7.62% β-TCP: 13.44% ± 6.03% natural healing: 9.49% ± 0.08% RM rhBMP-2/BioCaP/β-TCP: 10.04% ± 4.57% β-TCP: 20.60% ± 9.54% UT rhBMP-2/BioCaP/β-TCP: 68.78% ± 7.67% β-TCP: 65.96% ± 12.64% natural healing: 90.38% ± 7.5% |
| WEI LS et al. [28] | ErBMP-2/BioCaP/β-TCP, β-TCP, natural healing | % newly formed bone (NB) % residual material (RM) % unmineralized tissue area (UT) | NB ErhBMP-2/BioCaP/β-TCP: 7.72% ± 6.01% β-TCP: 2.96% ± 2.23% natural healing: 8.37% ± 6.31% RM ErhBMP-2/BioCaP/β-TCP: 10.90% ± 4.04% β-TCP: 15.73% ± 4.52% UT ErhBMP-2/BioCaP/β-TCP: 81.38% ± 4.81% β-TCP: 81.32% ± 4.70% natural healing: 91.63% ± 6.31% | |
| Sarment DP et al. [22] | β-TCP, β-TCP + rhPDGF-BB 0.3 mg/mL β-TCP + rhPDGF-BB 1.0 mg/ml | release of ICTP (pg/site) | ICTP levels (overall AUC values) rhPDGF-BB 0.3 mg/mL: 641.7 ± 164.1 pg/10 s rhPDGF-BB 1.0 mg/mL: 672.8 ± 183.0 pg/10 s β-TCP: 437.5 ± 287.1 | |
| Prins HjS et al. [20] | BCP, β-TCP, BCP/SVF β-TCP/SVF | mm vertical bone augmentation % bone volume % graft volume % bone volume proportion | bone augmentation β-TCP: 10.261.5 mm β-TCP/SVF: 9.9 ± 1.3 mm BCP: 12.4 ± 1.6 mm BCP/SVF: 12.1 ± 1.6 mm bone volume SVF sides: 19.5% ± 3.8% control sides: 13.7% ± 4.4% graft volume SVF sides: 10.5% ± 3.6% control sides: 14.0% ± 3.6% bone volume proportion SVF sides: 15.2% ± 4.7% control sides: 13.3% ± 3.0% | |
| Nagaveni NBP et al. [23] | Ortograft/PRP, Ortograft | % filling defect mm reduction defect | Ortograft/PRP: 9.5 ± 1.0 mm to 1.3 ± 2.1 mm (94% defect filled) Ortograft: reached 47% defect fill only at the 6th month |
| Author | Subjects | Measurements | Results | |
|---|---|---|---|---|
| Section 3.5 | Troedhan AS et al. [26] | DTV and ITV | Ncm | DTV: insufficient average differences (2–5 Ncm) between materials and bone ITV: highly significant differences 4 Ncm difference between native bone and Bio-Oss 34 Ncm in easy-graft CRYSTAL-augmented sites DTV and ITV specific to the Q2-implant |
| Gjerde CM et al. [21] | critical number of cells or a specific cell-to-biomaterial ratio (BCP) seems to be a key factor influencing bone formation | |||
| Prins HjS et al. [20] | minor discrepancies between the histomorphometric results and micro-CT findings regarding bone and graft volumes | |||
| Sun Y et al. [27] | CBCT data may not be sufficient for a comprehensive analysis (revealed significant differences in grayscale value) | |||
| Tomas MK et al. [17] | newly formed bone, residual biomaterial and soft tissue show p = 0.629, p = 0.485, p = 0.113, respectively, = p-value should be p > 0.05 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bozza, B.; Pesce, P.; Baldi, D.; Bagnasco, F.; Migliorati, M.; De Angelis, N. Synthetic Biomaterials for Alveolar Bone Regeneration: A Systematic Review of Clinical Evidence. Materials 2025, 18, 5328. https://doi.org/10.3390/ma18235328
Bozza B, Pesce P, Baldi D, Bagnasco F, Migliorati M, De Angelis N. Synthetic Biomaterials for Alveolar Bone Regeneration: A Systematic Review of Clinical Evidence. Materials. 2025; 18(23):5328. https://doi.org/10.3390/ma18235328
Chicago/Turabian StyleBozza, Beatrice, Paolo Pesce, Domenico Baldi, Francesco Bagnasco, Marco Migliorati, and Nicola De Angelis. 2025. "Synthetic Biomaterials for Alveolar Bone Regeneration: A Systematic Review of Clinical Evidence" Materials 18, no. 23: 5328. https://doi.org/10.3390/ma18235328
APA StyleBozza, B., Pesce, P., Baldi, D., Bagnasco, F., Migliorati, M., & De Angelis, N. (2025). Synthetic Biomaterials for Alveolar Bone Regeneration: A Systematic Review of Clinical Evidence. Materials, 18(23), 5328. https://doi.org/10.3390/ma18235328








