Experimental Study of the Discharging Process of Sorption Heat Storage Units Filled with 13X Zeolite
Abstract
1. Introduction
2. Materials and Methods
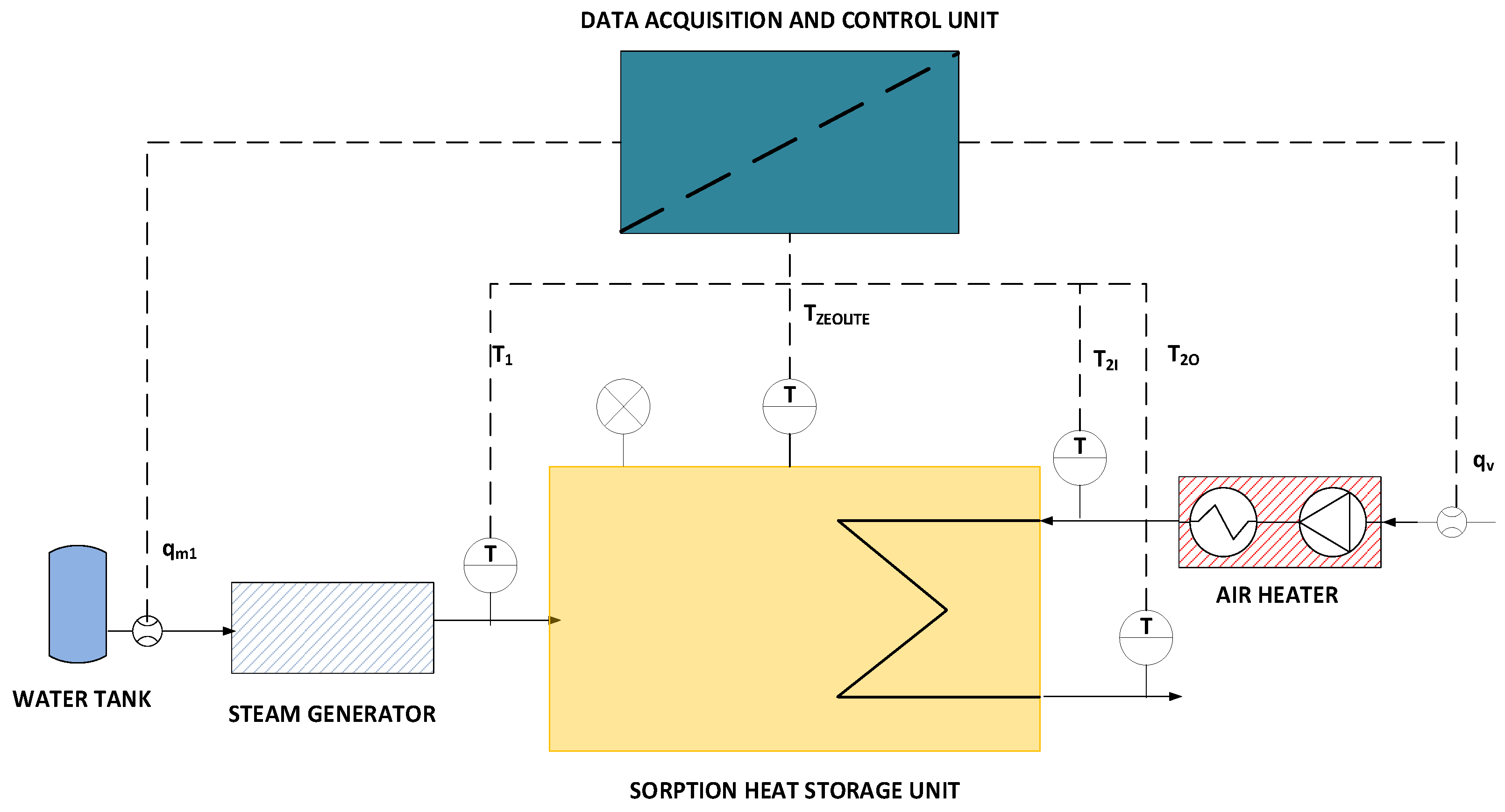
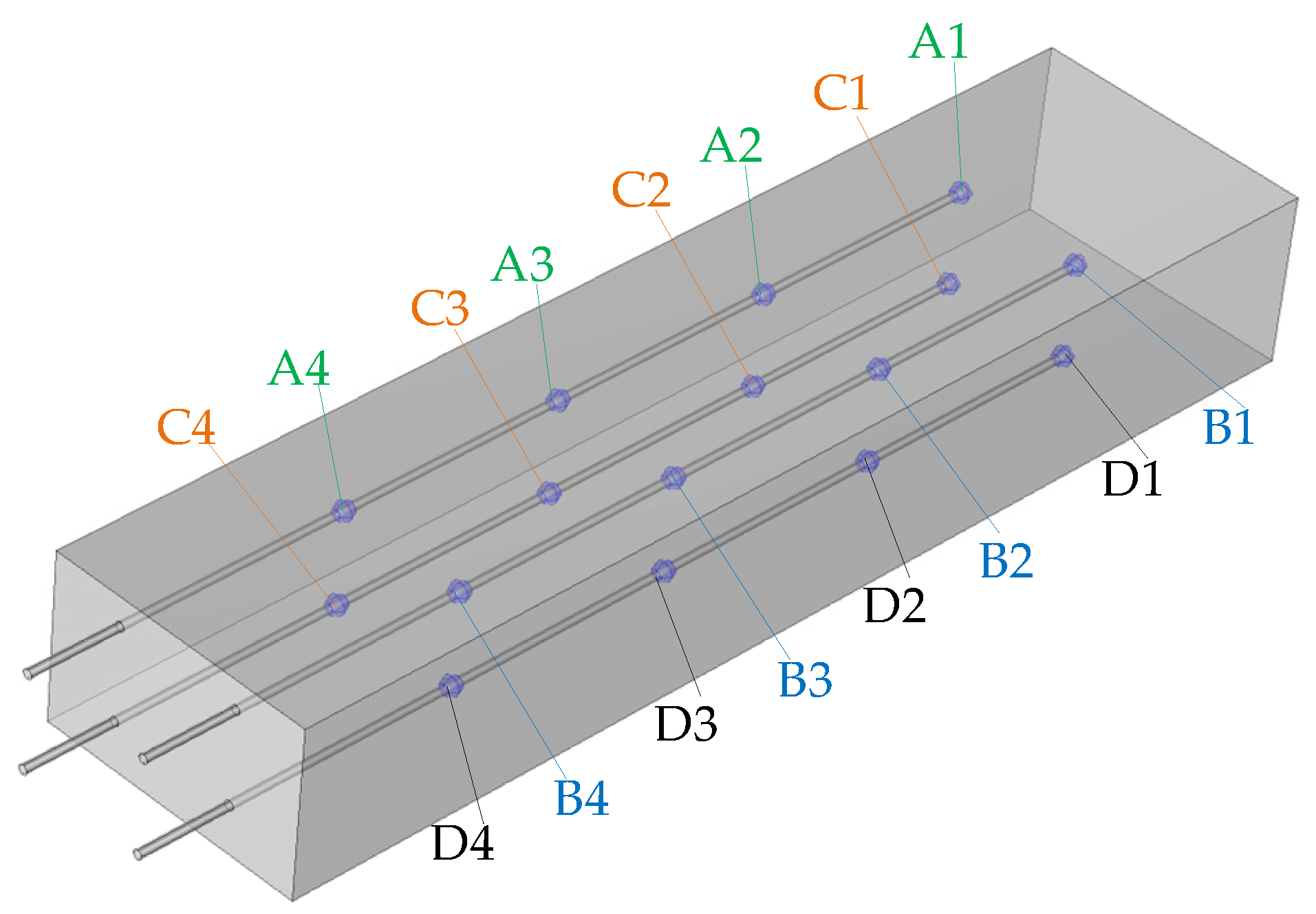
2.1. Laboratory Setup
2.2. Operation and Control of the Laboratory Stand
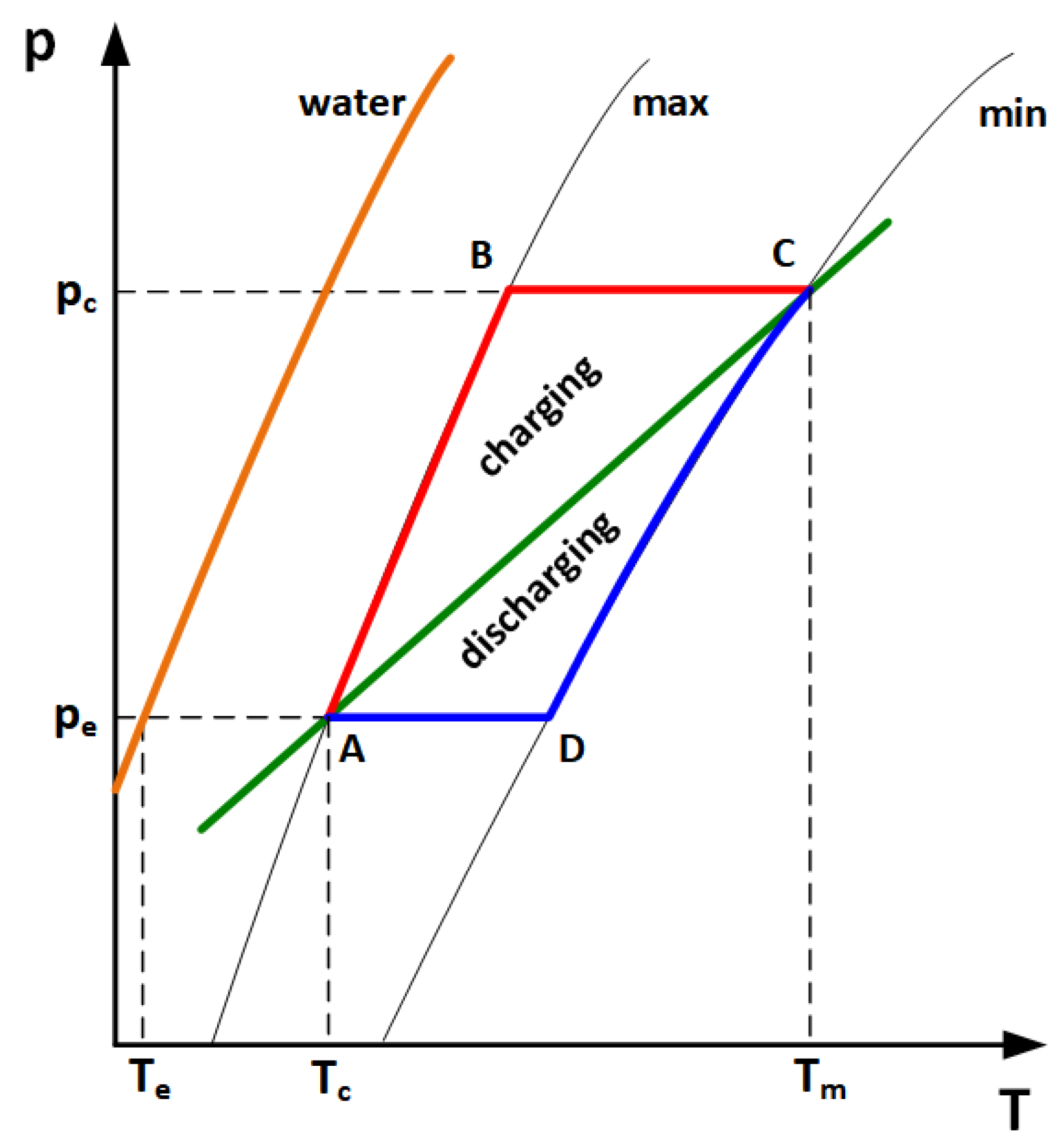
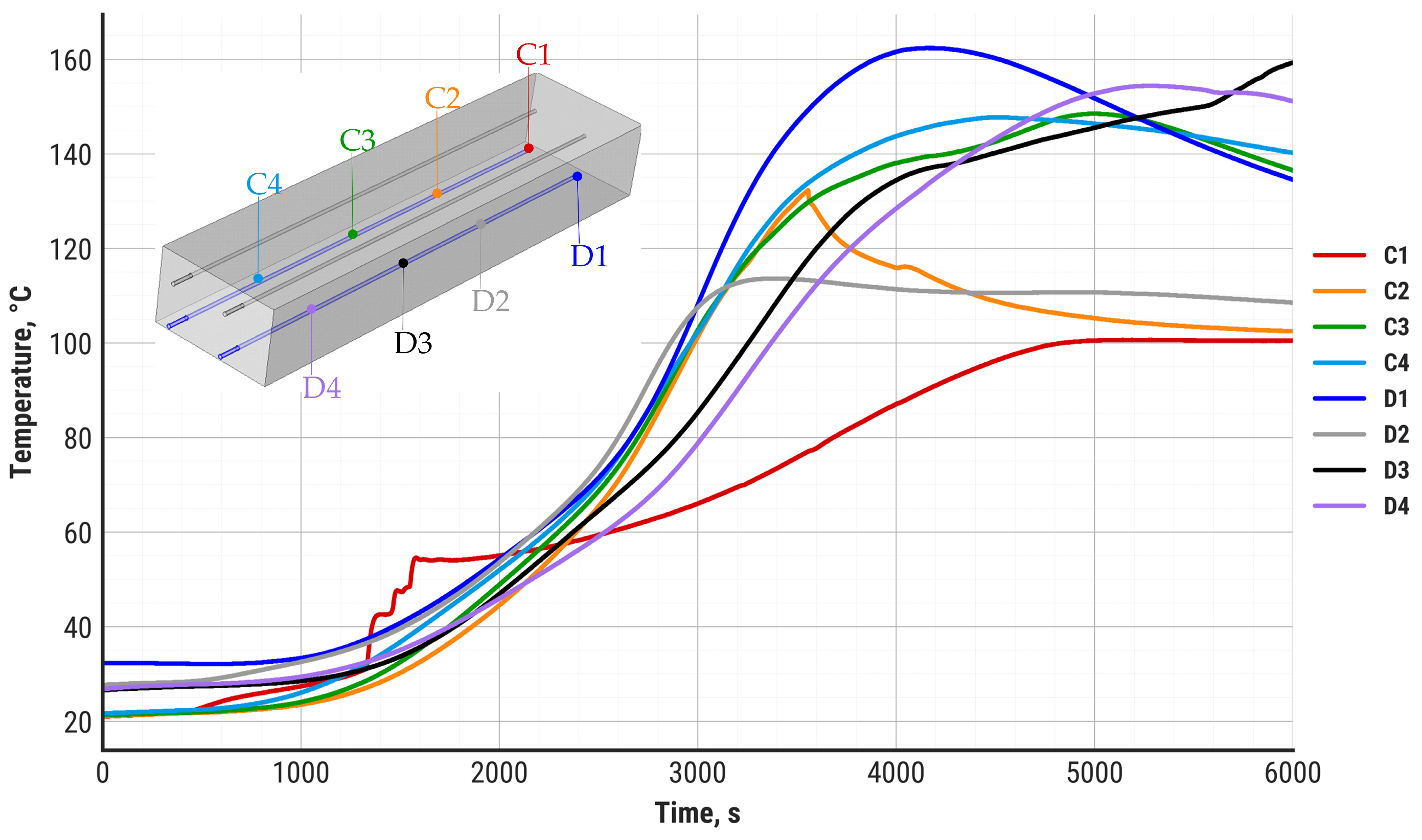
2.3. Experimental Methodology
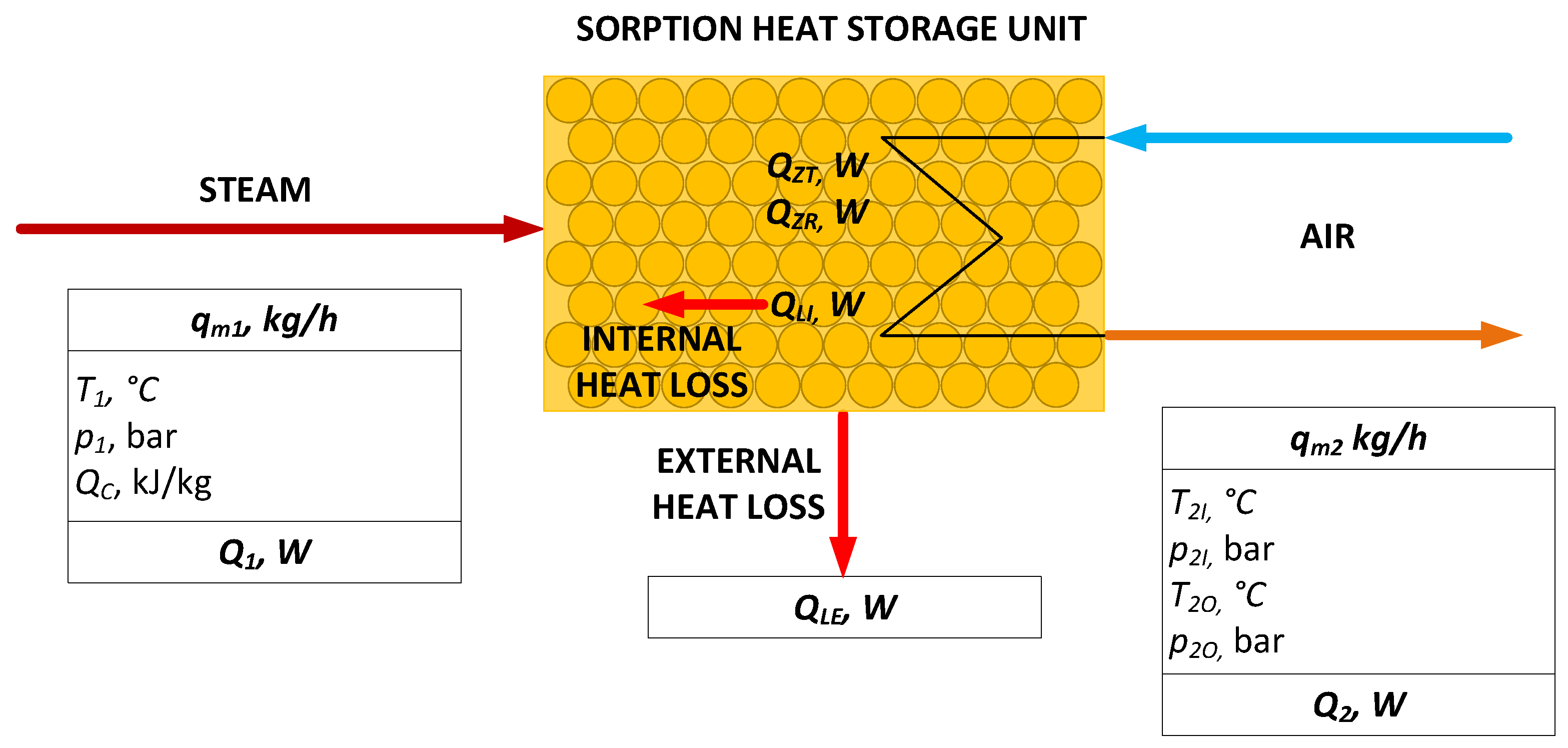
2.4. Heat Balance Model
3. Results and Discussion
4. Uncertainty Analysis
5. Conclusions
- The use of a control layer employing neural networks to optimize the steam flow rate improves the operational stability of the sorption thermal energy storage system.
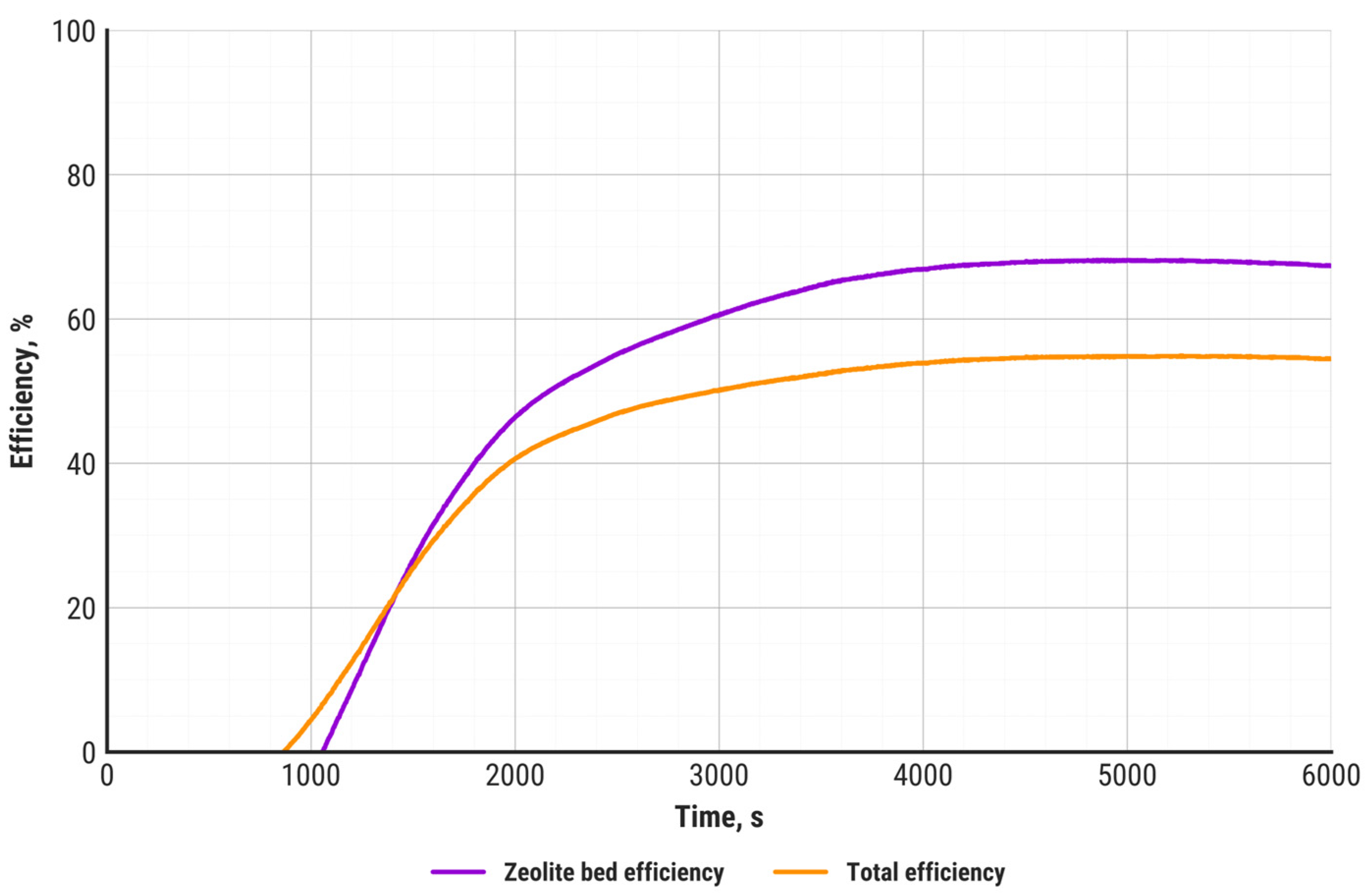
- The obtained total efficiency of the heat storage system and the zeolite bed efficiency are comparable to those reported by other researchers. The total storage efficiency was approximately 55%, while the zeolite bed efficiency was around 70%.
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Roger-Lund, S.; Darkwa, J.; Worall, M.; Calautit, J.; Boukhanouf, R. A Review of Thermochemical Energy Storage Systems for District Heating in the UK. Energies 2024, 17, 3389. [Google Scholar] [CrossRef]
- Barrasso, M.; Langella, G.; Amoresano, A.; Iodice, P. Latest Advances in Thermal Energy Storage for Solar Plants. Processes 2023, 11, 1832. [Google Scholar] [CrossRef]
- Zeng, Y.; Zhou, T.; Wang, T.; Zhang, M.; Zhang, S.; Yang, H. Long-Duration Energy Storage: A Critical Enabler for Renewable Integration and Decarbonization. Energies 2025, 18, 466. [Google Scholar] [CrossRef]
- Babaei, M.; Nick, H.M. Performance of low-enthalpy geothermal systems: Interplay of spatially correlated heterogeneity and well-doublet spacings. Appl. Energy 2019, 253, 113569. [Google Scholar] [CrossRef]
- Sitka, A.; Szulc, P.; Smykowski, D.; Tietze, T.; Anwajler, B.; Pytlik, B.; Jodkowski, W.; Redzicki, R. The Impact of Binary Salt Blends’ Composition on Their Thermophysical Properties for Innovative Heat Storage Materials. J. Manuf. Mater. Process. 2024, 8, 208. [Google Scholar] [CrossRef]
- Pfleger, N.; Bauer, T.; Martin, C.; Eck, M.; Wörner, A. Thermal energy storage—Overview and specific insight into nitrate salts for sensible and latent heat storage. Beilstein J. Nanotechnol. 2015, 6, 1487–1497. [Google Scholar] [CrossRef] [PubMed]
- Vasta, S.; Brancato, V.; La Rosa, D.; Palomba, V.; Restuccia, G.; Sapienza, A.; Frazzica, A. Adsorption heat storage: State-of-the-art and future perspectives. Nanomaterials 2018, 8, 522. [Google Scholar] [CrossRef]
- Mahon, H.; O’Connor, D.; Friedrich, D.; Hughes, B. A review of thermal energy storage technologies for seasonal loops. Energy 2022, 239, 122207. [Google Scholar] [CrossRef]
- Rolka, P.; Nowakowska, H.; Lackowski, M. Analysis of the Properties and Thermal Behavior of Low-Temperature Phase Change Materials (PCMs) That Can Be Applied in Heating and Ventilation Systems. Materials 2024, 17, 5573. [Google Scholar] [CrossRef]
- Liu, L.; Hammami, N.; Bigot, D.; Malet-Damour, B.; Habas, J.P. Multi-Scale Study of a Phase Change Material on a Tropical Island for Evaluating Its Impact on Human Comfort in the Building Sector. Materials 2024, 17, 3241. [Google Scholar] [CrossRef]
- Wang, T.; Jin, Y.; Alam, F. Study on Phase Change Materials’ Heat Transfer Characteristics of Medium Temperature Solar Energy Collection System. Materials 2024, 17, 5159. [Google Scholar] [CrossRef]
- Marcos, J.D.; Golpour, I.; Barbero, R.; Rovira, A. Decarbonizing European Industry: A Novel Technology to Heat Supply Using Waste and Renewable Energy. Appl. Sci. 2024, 14, 8994. [Google Scholar] [CrossRef]
- Mellouli, S.; Alqahtani, T.; Askri, F.; Algarni, S.; Alshammari, B.M.; Kolsi, L. New hybrid thermal energy storage unit using dual hydrides with cascaded PCMs: Application to CSP plants. Case Stud. Therm. Eng. 2024, 60, 104628. [Google Scholar] [CrossRef]
- Aneke, M.; Wang, M. Energy storage technologies and real life applications—A state of the art review. Appl. Energy 2016, 179, 350–377. [Google Scholar] [CrossRef]
- Aydin, D.; Casey, S.P.; Riffat, S. The latest advancements on thermochemical heat storage systems. Renew. Sustain. Energy Rev. 2015, 41, 356–367. [Google Scholar] [CrossRef]
- Zhang, Y.; Wang, R.; Li, T.; Zhao, Y. Thermochemical characterizations of novel vermiculite-LiCl composite sorbents for low-temperature heat storage. Energies 2016, 9, 854. [Google Scholar] [CrossRef]
- Farulla, G.A.; Cellura, M.; Guarino, F.; Ferraro, M. A review of thermochemical energy storage systems for power grid support. Appl. Sci. 2020, 10, 3142. [Google Scholar] [CrossRef]
- Zhang, Y.; Wang, R. Sorption thermal energy storage: Concept, process, applications and perspectives. Energy Storage Mater. 2020, 27, 352–369. [Google Scholar] [CrossRef]
- Hayatina, I.; Auckaili, A.; Farid, M. Review on the Life Cycle Assessment of Thermal Energy Storage Used in Building Applications. Energies 2023, 16, 1170. [Google Scholar] [CrossRef]
- Ametta, M.; Maggio, G.; Vasta, S. Thermodynamic Evaluation of the Potential of a Sorption Storage System for Renewables and Waste Heat Integration. Appl. Sci. 2025, 15, 1951. [Google Scholar] [CrossRef]
- Cabeza, L.F.; Solé, A.; Barreneche, C. Review on sorption materials and technologies for heat pumps and thermal energy storage. Renew. Energy 2017, 110, 3–39. [Google Scholar] [CrossRef]
- Zbair, M.; Bennici, S. Survey summary on salts hydrates and composites used in thermochemical sorption heat storage: A review. Energies 2021, 14, 3105. [Google Scholar] [CrossRef]
- Jose, A.; Mathew, T.; Fernández-Navas, N.; Querebillo, C.J. Porous Inorganic Nanomaterials: Their Evolution towards Hierarchical Porous Nanostructures. Micro 2024, 4, 229–280. [Google Scholar] [CrossRef]
- de Gennaro, B.; Cappi, A.; de Gennaro, M.; Bianco, N.; Langella, A.; Cappelletti, P.; Marocco, A.; Aprea, P.; Pansini, M. Use of Zeolites in the Capture and Storage of Thermal Energy by Water Desorption—Adsorption Cycles. Materials 2022, 15, 5574. [Google Scholar] [CrossRef]
- Saadat, F.; Hashmi, A.R.; Zheng, X.; Pan, Q.; Wang, B.; Gan, Z. Progress in zeolite–water adsorption technologies for energy-efficient utilization. Energy 2024, 308, 133001. [Google Scholar] [CrossRef]
- de Antonellis, S.; Colombo, L.P.M.; Castellazzi, P.; Rossetti, A.; Marocco, L. System integration analysis of a zeolite 13x thermal energy storage. Energy Built Environ. 2024, 5, 568–579. [Google Scholar] [CrossRef]
- Feng, C.; E, J.; Han, W.; Deng, Y.; Zhang, B.; Zhao, X.; Han, D. Key technology and application analysis of zeolite adsorption for energy storage and heat-mass transfer process: A review. Renew. Sustain. Energy Rev. 2021, 144, 110954. [Google Scholar] [CrossRef]
- Gaeini, M.; Zondag, H.A.; Rindt, C.C.M. Effect of kinetics on the thermal performance of a sorption heat storage reactor. Appl. Therm. Eng. 2016, 102, 520–531. [Google Scholar] [CrossRef]
- Mette, B.; Kerskes, H.; Drück, H.; Müller-Steinhagen, H. Experimental and numerical investigations on the water vapor adsorption isotherms and kinetics of binderless zeolite 13X. Int. J. Heat Mass Transf. 2014, 71, 555–561. [Google Scholar] [CrossRef]
- Tatsidjodoung, P.; Le Pierrès, N.; Heintz, J.; Lagre, D.; Luo, L.; Durier, F. Experimental and numerical investigations of a zeolite 13X/water reactor for solar heat storage in buildings. Energy Convers. Manag. 2016, 108, 488–500. [Google Scholar] [CrossRef]
- Hu, P.; Wang, S.; Wang, J.; Jiang, S.; Sun, Y.; Ma, Z. Scale-up of open zeolite bed reactors for sorption energy storage: Theory and experiment. Energy Build. 2022, 264, 112077. [Google Scholar] [CrossRef]
- Kang, Z.; Wang, C.; Tan, R.; Liu, S.; Wang, F. Numerical study of an energy storage unit based on zeolite-water adsorption for mobilized thermal energy storage. J. Energy Storage 2024, 98, 113092. [Google Scholar] [CrossRef]
- Chen, D.; Chen, X.; Ma, Z.; Wang, Y.; Roskilly, A.P.; Zhou, J. Experimental Study of LiCl/LiBr-Zeolite Composite Adsorbent for Thermochemical Heat Storage. Buildings 2022, 12, 2001. [Google Scholar] [CrossRef]
- Yu, M.; Liu, W.; Lin, Y.; Gao, N.; Zhang, X.; Jiang, L. Experimental Study on Heat Release Performance for Sorption Thermal Battery Based on Wave Analysis Method. Sustainability 2024, 16, 6654. [Google Scholar] [CrossRef]
- Abohamzeh, E.; Frey, G. Numerical Investigation of the Adsorption Process of Zeolite/Water in a Thermochemical Reactor for Seasonal Heat Storage. Energies 2022, 15, 5944. [Google Scholar] [CrossRef]
- Yue, X.; Xu, Y.; Zhou, X.; Xu, D.; Chen, H. Study on the Performance of a Solar Heating System with Seasonal and Cascade Thermal-Energy Storage. Energies 2022, 15, 7733. [Google Scholar] [CrossRef]
- Hironaka, S.; Ooga, S.; Hanaki, M.; Wijayanta, A.T.; Fukai, J. High potency of application on an open direct-contact thermal storage using humid air. Int. J. Refrig. 2024, 168, 18–28. [Google Scholar] [CrossRef]
- de Antonellis, S.; Marocco, L.D.; Tomaino, G.; Romano, F.; Calabrese, L.; Freni, A. Experimental analysis of a sorption thermal energy storage for air heating and dehumidification in electric vehicles. Next Energy 2024, 5, 100170. [Google Scholar] [CrossRef]
- Narwal, K.; Kempers, R.; O’Brien, P.G. Enhanced energy storage density in thermal energy storage systems simultaneously heated with solar radiation and industrial waste heat. Results Eng. 2025, 25, 103792. [Google Scholar] [CrossRef]
- Johannes, K.; Kuznik, F.; Hubert, J.L.; Durier, F.; Obrecht, C. Design and characterisation of a high powered energy dense zeolite thermal energy storage system for buildings. Appl. Energy 2015, 159, 80–86. [Google Scholar] [CrossRef]
- Kuznik, F.; Gondre, D.; Johannes, K.; Obrecht, C.; David, D. Numerical modelling and investigations on a full-scale zeolite 13X open heat storage for buildings. Renew. Energy 2019, 132, 761–772. [Google Scholar] [CrossRef]
- Gao, S.; Wang, S.; Hu, P.; Wang, J.; Sun, Y.; Ma, Z. Performance of sorption thermal energy storage in zeolite bed reactors: Analytical solution and experiment. J. Energy Storage 2023, 64, 107154. [Google Scholar] [CrossRef]
- Gao, S.; Wang, S.; Sun, Y.; Wang, J.; Hu, P.; Shang, J.; Ma, Z.; Liang, Y. Effect of charging operating conditions on open zeolite/water vapor sorption thermal energy storage system. Renew. Energy 2023, 215, 119033. [Google Scholar] [CrossRef]
- Kim, H.; Hong, S.K.; Kang, J.G.; Moon, S.W.; Kim Gho Yoon, S.; Wei Djae Moon, H.; Kim, B.S. Exploration adsorption characteristics of zeolite 13X depending on humidity and flow rate in sorption thermal energy storage applications. Int. J. Heat Mass Transf. 2024, 221, 125049. [Google Scholar] [CrossRef]
- Zettl, B.; Englmair, G.; Steinmaurer, G. Development of a revolving drum reactor for open-sorption heat storage processes. Appl. Therm. Eng. 2014, 70, 42–49. [Google Scholar] [CrossRef]
- Finck, C.; Henquet, E.; van Soest, C.; Oversloot, H.; de Jong, A.J.; Cuypers, R.; van T’Spijker, H. Experimental results of a 3 kWh thermochemical heat storage module for space heating application. Energy Procedia 2014, 48, 320–326. [Google Scholar] [CrossRef]
- van Alebeek, R.; Scapino, L.; Beving, M.A.J.M.; Gaeini, M.; Rindt, C.C.M.; Zondag, H.A. Investigation of a household-scale open sorption energy storage system based on the zeolite 13X/water reacting pair. Appl. Therm. Eng. 2018, 139, 325–333. [Google Scholar] [CrossRef]
- Gaeini, M.; Javed, M.R.; Ouwerkerk, H.; Zondag, H.A.; Rindt, C.C.M. Realization of a 4kW thermochemical segmented reactor in household scale for seasonal heat storage. Energy Procedia 2017, 135, 105–114. [Google Scholar] [CrossRef]
- Di Palo, M.; Sabatelli, V.; Buzzi, F.; Gabbrielli, R. Experimental and numerical assessment of a novel all-in-one adsorption thermal storage with zeolite for thermal solar applications. Appl. Sci. 2020, 10, 8517. [Google Scholar] [CrossRef]
- Schreiber, H.; Lanzerath, F.; Reinert, C.; Grüntgens, C.; Bardow, A. Heat lost or stored: Experimental analysis of adsorption thermal energy storage. Appl. Therm. Eng. 2016, 106, 981–991. [Google Scholar] [CrossRef]
- Abedin, A.H.; Rosen, M.A. A Critical Review of Thermochemical Energy Storage Systems. Open Renew. Energy J. 2011, 4, 42–46. [Google Scholar] [CrossRef]
- Scapino, L.; Zondag, H.A.; Van Bael, J.; Diriken, J.; Rindt, C.C.M. Sorption heat storage for long-term low-temperature applications: A review on the advancements at material and prototype scale. Appl. Energy 2017, 190, 920–948. [Google Scholar] [CrossRef]
- Wu, H.; Salles, F.; Zajac, J. A critical review of solid materials for low-temperature thermochemical storage of solar energy based on solid-vapour adsorption in view of space heating uses. Molecules 2019, 24, 945. [Google Scholar] [CrossRef]
- Mikhaeil, M.; Gaderer, M.; Dawoud, B. Experimental Investigation of the Adsorption and Desorption Kinetics on an Open-Structured Asymmetric Plate Heat Exchanger; Matching Between Small-Scale and Full-Scale Results. Front. Energy Res. 2022, 10, 818486. [Google Scholar] [CrossRef]
- Banaei, A.; Zanj, A. A review on the challenges of using zeolite 13x as heat storage systems for the residential sector. Energies 2021, 14, 8062. [Google Scholar] [CrossRef]
- Tietze, T.; Szulc, P.; Smykowski, D.; Sitka, A.; Redzicki, R. Application of phase change material and artificial neural networks for smoothing of heat flux fluctuations. Energies 2021, 14, 3531. [Google Scholar] [CrossRef]
- Gabbrielli, R.; Buzzi, F.P.; Di Paco, F.; Di Palo, M.; Arcieri, G.; Sabatelli, V. Thermochemical energy storage with zeolite 13X: Results from a full-scale solar heat application. Appl. Therm. Eng. 2025, 279, 127608. [Google Scholar] [CrossRef]
- Bell, I.H.; Wronski, J.; Quoilin, S.; Lemort, V. Pure and pseudo-pure fluid thermophysical property evaluation and the open-source thermophysical property library coolprop. Ind. Eng. Chem. Res. 2014, 53, 2498–2508. [Google Scholar] [CrossRef]
- Churchill, S.W.; Chu, H.H.S. Correlating equations for laminar and turbulent free convection from a vertical plate. Int. J. Heat Mass Transf. 1975, 18, 1323–1329. [Google Scholar] [CrossRef]





| Authors | Storage Shape | Storage Dimensions | Zeolite Amount | Adsorbate Temperature During Delivery | Maximum Temperature at the Outlet of the Working Medium | Heat Exchange System |
|---|---|---|---|---|---|---|
| [37] | column | H: 350 mm D: 30 mm | H: 200 mm D: 30 mm | - | - | No |
| [38] | box | H: 126 mm L: 268 mm W: 221 mm | 35 × 268 × 221 mm | 21 °C | 37 °C | No |
| [39] | box | b.d. | 90 mm × 90 mm × 50 mm | - | - | No |
| [40,41] | cylinders | 4.16 × 2.2 × 1.6 m | 80 kg | 20 | 57 | No |
| [31,42] | column | H: 100 mm D: 44 mm | H: 100 mm D: 38 mm | 22 | 53 | No |
| [31,43] | column | H: 168 mm D: 86 mm | H: 120 mm D: 76 mm | 18 | 37 | No |
| [44] | column | H: 1673 mm D:159.3 mm | H: 300 mm D:159.3 mm | - | - | No |
| [45] | cylinder | H: 300 mm D: 700 mm | 45.15 kg | 25 | 60 | No |
| [46] | box | 1000 mm × 300 mm × 33 mm | 41 kg | 20 | 54 | No |
| [47,48] | box | 500 mm × 459 mm × 500 mm | 62.5 dm3 | 13 | 35 | No |
| [49] | box | - | 200 dm3 | 17 | 36 | Yes |
| [50] | column | - | 10 kg | 40 | 70 | Yes |
| Component | Main Parameters |
|---|---|
| Air heater: Leister Mistral 6 (Leister, Kaegiswil, Switzerland) | 6–21 m3/h (20 °C), 40–500 °C |
| Steam generator: Batistella Barbara 31 (Batistella, Rossano, Italy) | 1450 W, 2.8 bar |
| Air flowmeter: MV 106 (Mass Flow Online, Bronkhorst, The Netherlands) | Max 500 L/min, accuracy: ±(1% RD + 0.5% FS) |
| Thermocouples type J (Termoaparatura Wrocław, Wrocław, Poland) | −40–750 °C, 55 µV/°C. |
| Parameter | Value |
|---|---|
| Air mass flow rate, kg/h | 14.4 |
| Inlet air temperature, °C | 40 |
| Zeolite bed mass, g | 2270 |
| Average steam flow rate, kg/h | 1.04 |
| Parameter | Value |
|---|---|
| Air mass flow rate, kg/h | 14.4 |
| Inlet air temperature, °C | 40 |
| Zeolite bed mass, g | 2270 |
| Average steam flow rate, kg/h | 1.04 |
| Zeolite thickness, mm | 100 |
| Casing (steel) thickness, mm | 5 |
| Insulation thickness, m | 50 |
| Characteristic dimension of casing, mm | 300 |
| Zeolite thermal conductivity, W/(m∙K) | 0.07 |
| Insulation thermal conductivity, W/(m∙K) | 0.07 |
| Parameter | Value | |
|---|---|---|
| Steam | qm1, kg/h | 1.12 |
| T1, °C | 130 | |
| p1, bar | 2.8 | |
| Q1, W | 21.6 | |
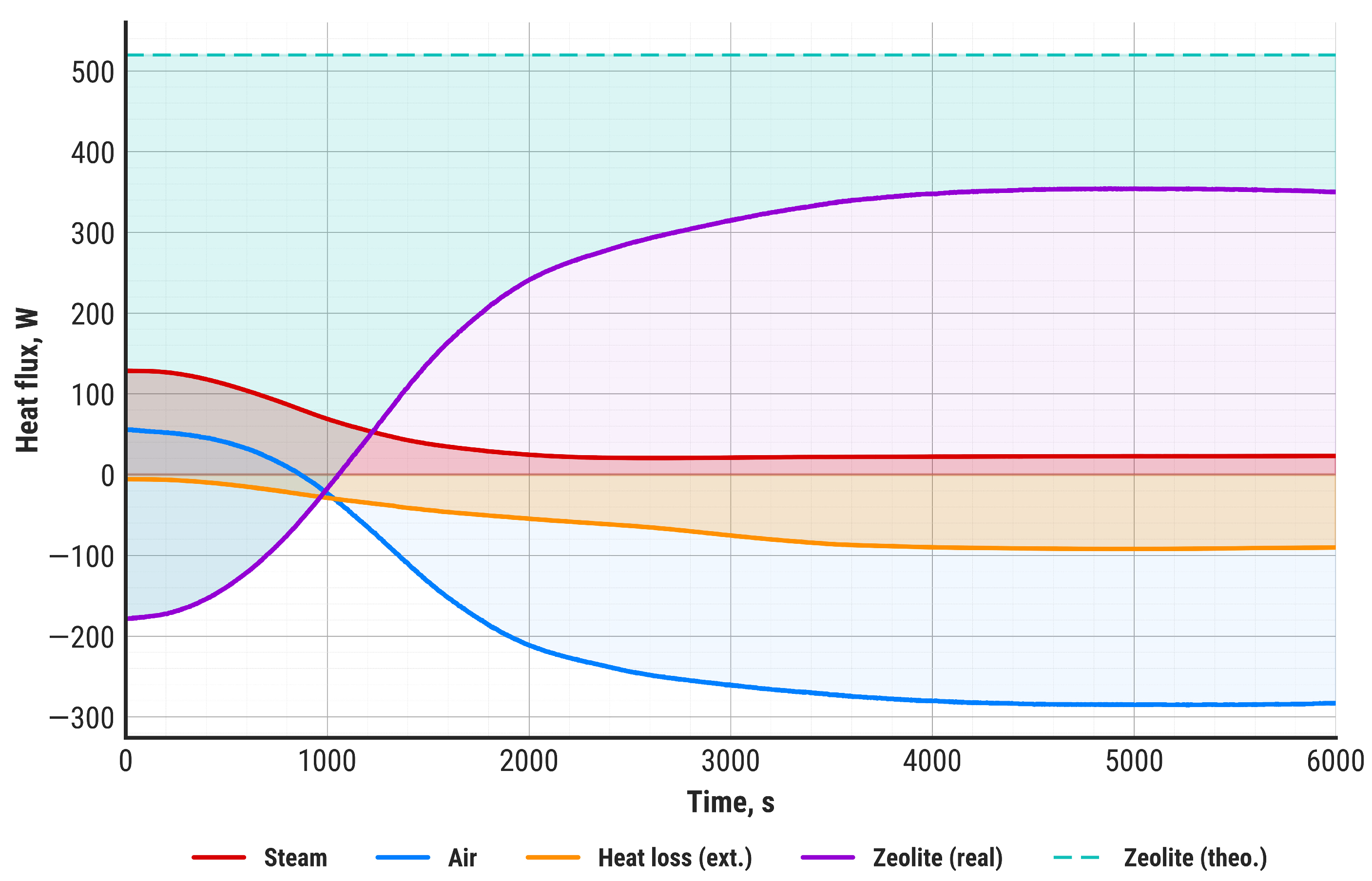
| TES | QZT, W | 565.1 |
| QZR, W | 399.6 | |
| QLI, W | 165.5 | |
| QLE, W | 91.9 | |
| Air | qm2, kg/h | 14.4 |
| T2I, °C | 40 | |
| T2O, °C | 2 | |
| Q2, W | 307.7 | |
| ηT, % | 54.4 | |
| ηZ, % | 70.1 |
| Parameter | Value |
|---|---|
| Viscosity, heat capacity, thermal conductivity, thermal expansion coefficient | ±1% |
| Mass flow rate | ±1% |
| Temperature | ±2 °C |
| Dimensions | ±1 mm |
| Zeolite bed efficiency | ±3.98% |
| Total discharge efficiency | ±1.00% |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pytlik, B.; Smykowski, D.; Szulc, P.; Tietze, T.; Anwajler, B.; Chorążyczewski, A. Experimental Study of the Discharging Process of Sorption Heat Storage Units Filled with 13X Zeolite. Materials 2025, 18, 5327. https://doi.org/10.3390/ma18235327
Pytlik B, Smykowski D, Szulc P, Tietze T, Anwajler B, Chorążyczewski A. Experimental Study of the Discharging Process of Sorption Heat Storage Units Filled with 13X Zeolite. Materials. 2025; 18(23):5327. https://doi.org/10.3390/ma18235327
Chicago/Turabian StylePytlik, Beata, Daniel Smykowski, Piotr Szulc, Tomasz Tietze, Beata Anwajler, and Artur Chorążyczewski. 2025. "Experimental Study of the Discharging Process of Sorption Heat Storage Units Filled with 13X Zeolite" Materials 18, no. 23: 5327. https://doi.org/10.3390/ma18235327
APA StylePytlik, B., Smykowski, D., Szulc, P., Tietze, T., Anwajler, B., & Chorążyczewski, A. (2025). Experimental Study of the Discharging Process of Sorption Heat Storage Units Filled with 13X Zeolite. Materials, 18(23), 5327. https://doi.org/10.3390/ma18235327









