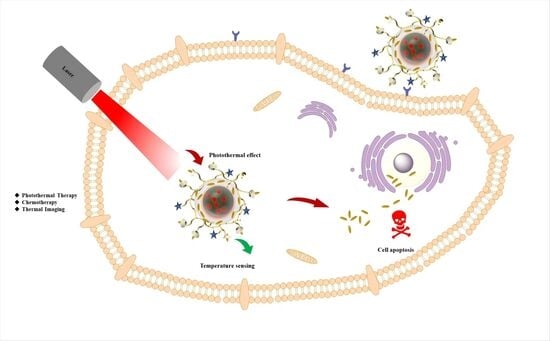
β-Cyclodextrin-Grafted Polypyrrole–Rhodamine B Nanoplatforms for Drug Delivery and Image-Guided Photothermal Therapy In Vitro
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Characterization
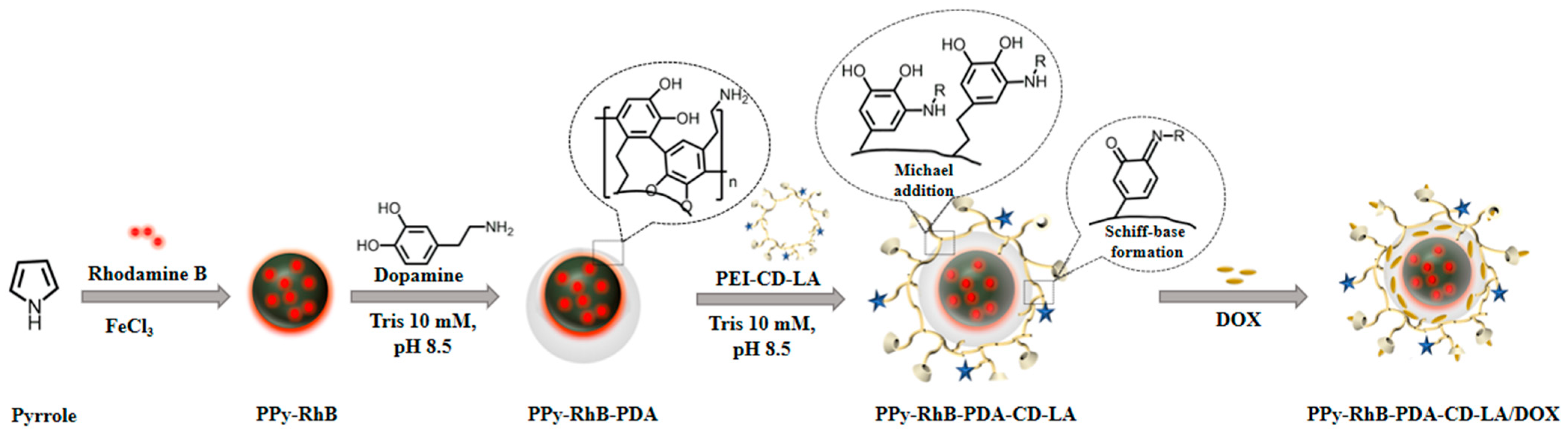
2.3. Preparation of PPy-RhB-PDA-CD-LA
2.4. In Vitro Photothermal Efficiency and Temperature Sensing
2.5. Drug Loading and Release
2.6. In Vitro Cytotoxicity Assay
2.7. Cellular Imaging Analysis
3. Results and Discussion
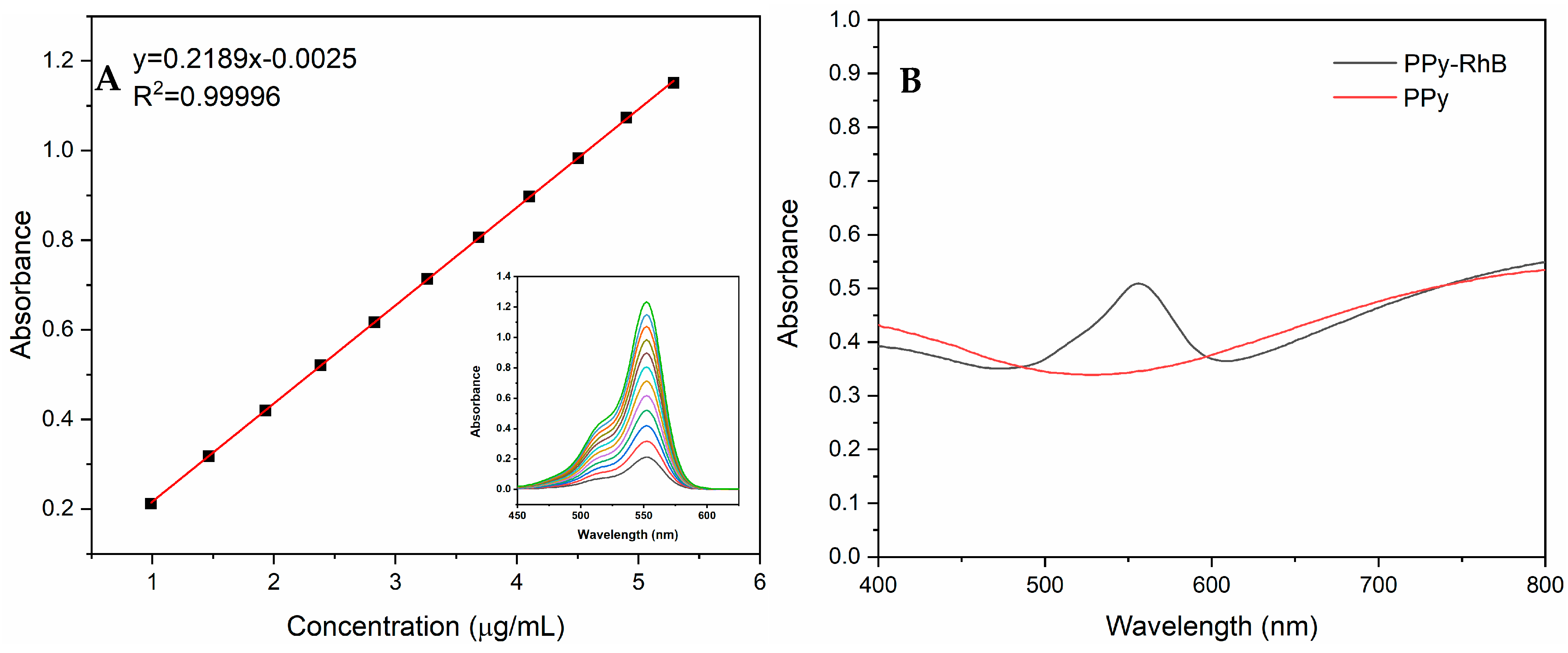
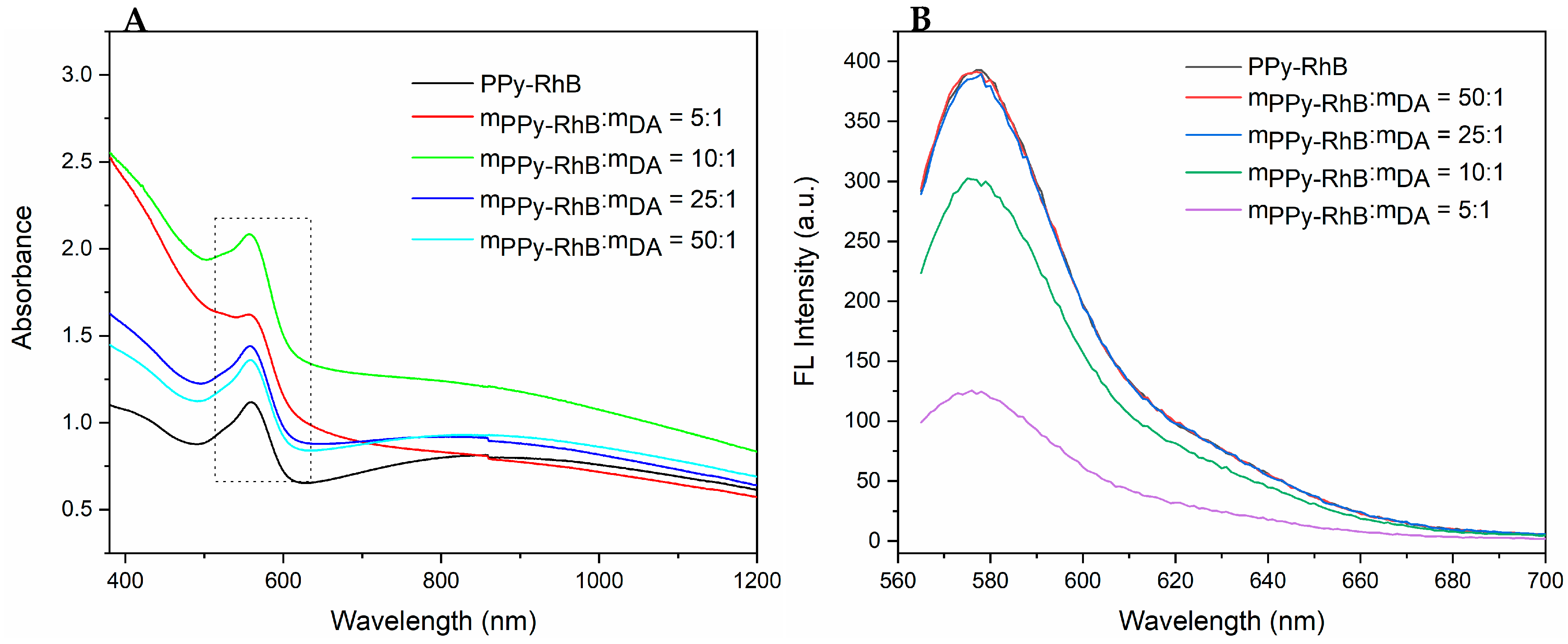
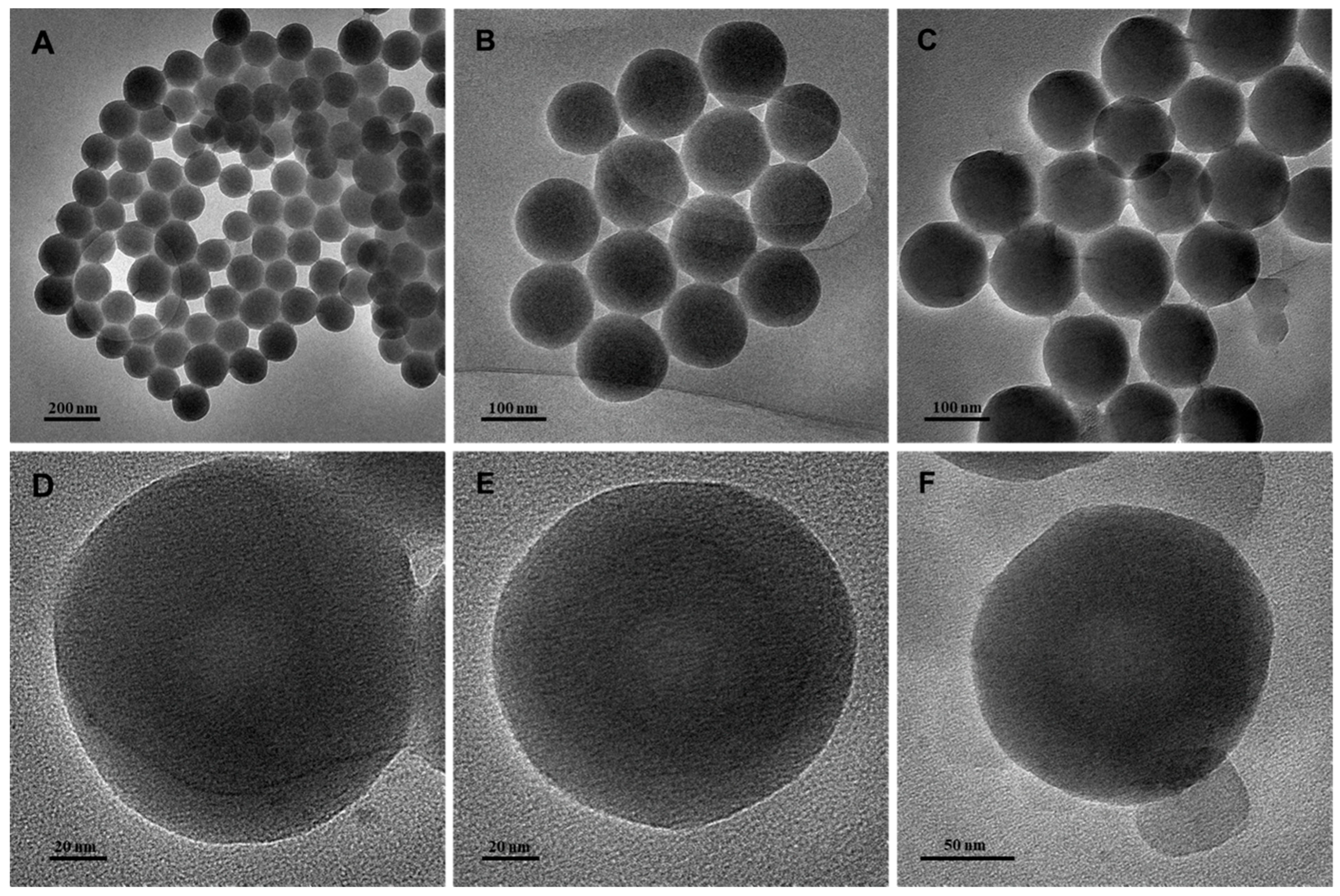
3.1. Characterization of PPy-RhB-PDA-CD-LA
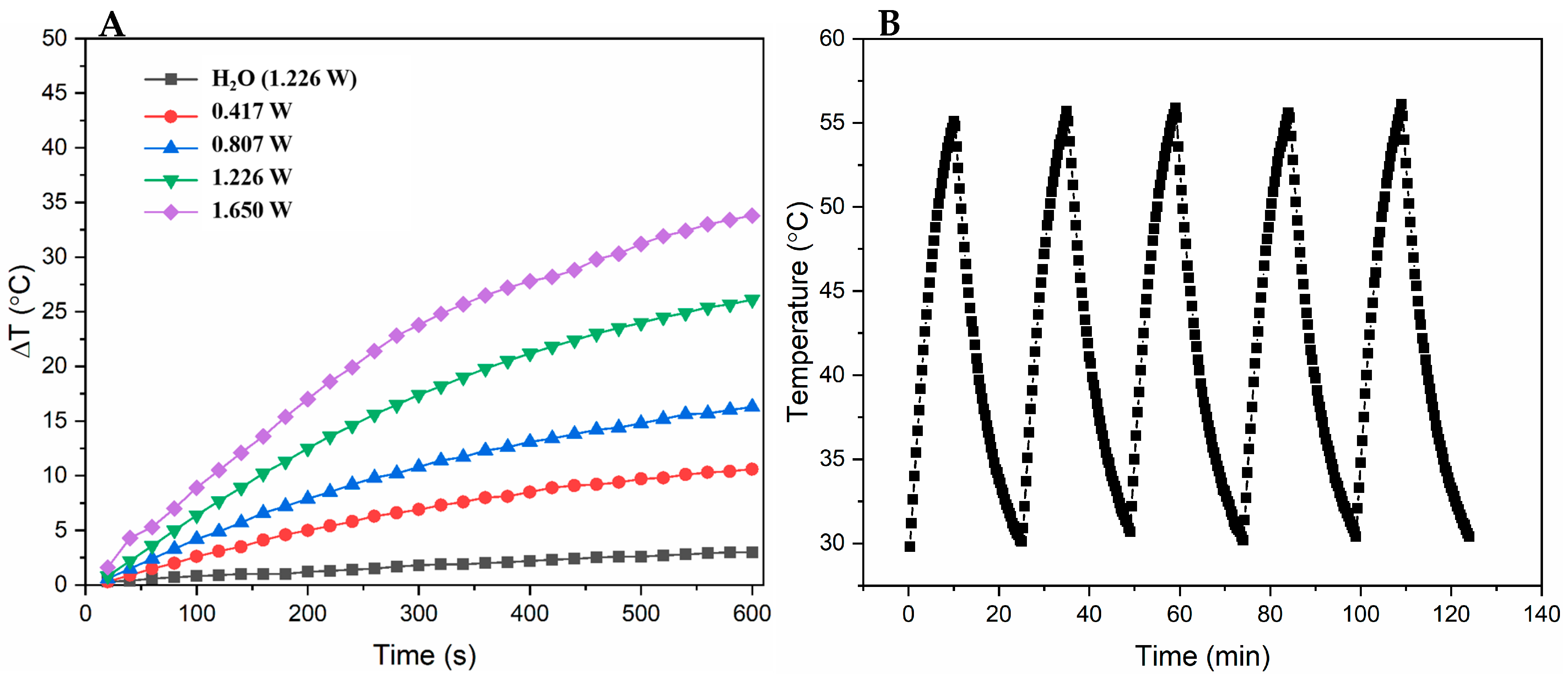
3.2. Photothermal Effects of PPy-RhB-PDA-CD-LA
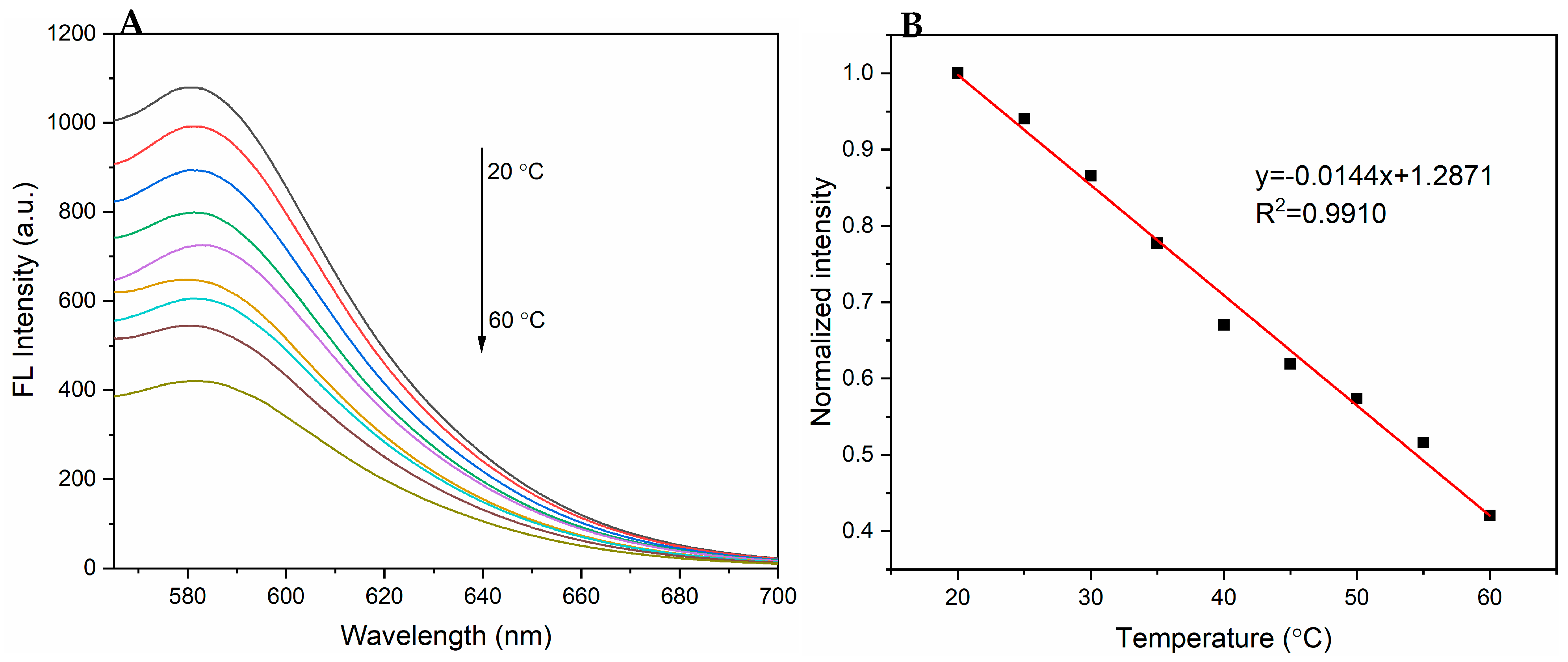
3.3. Temperature-Dependent Fluorescence of PPy-RhB-PDA-CD-LA
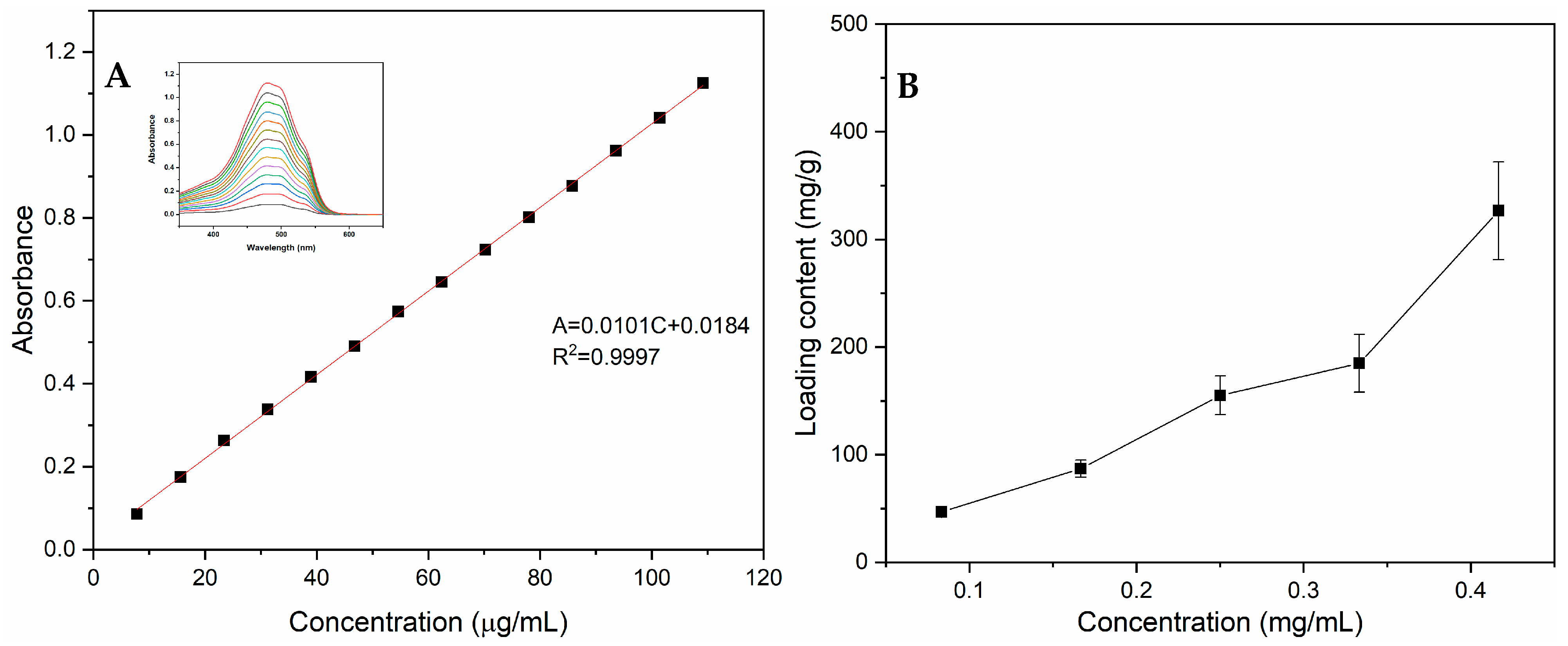
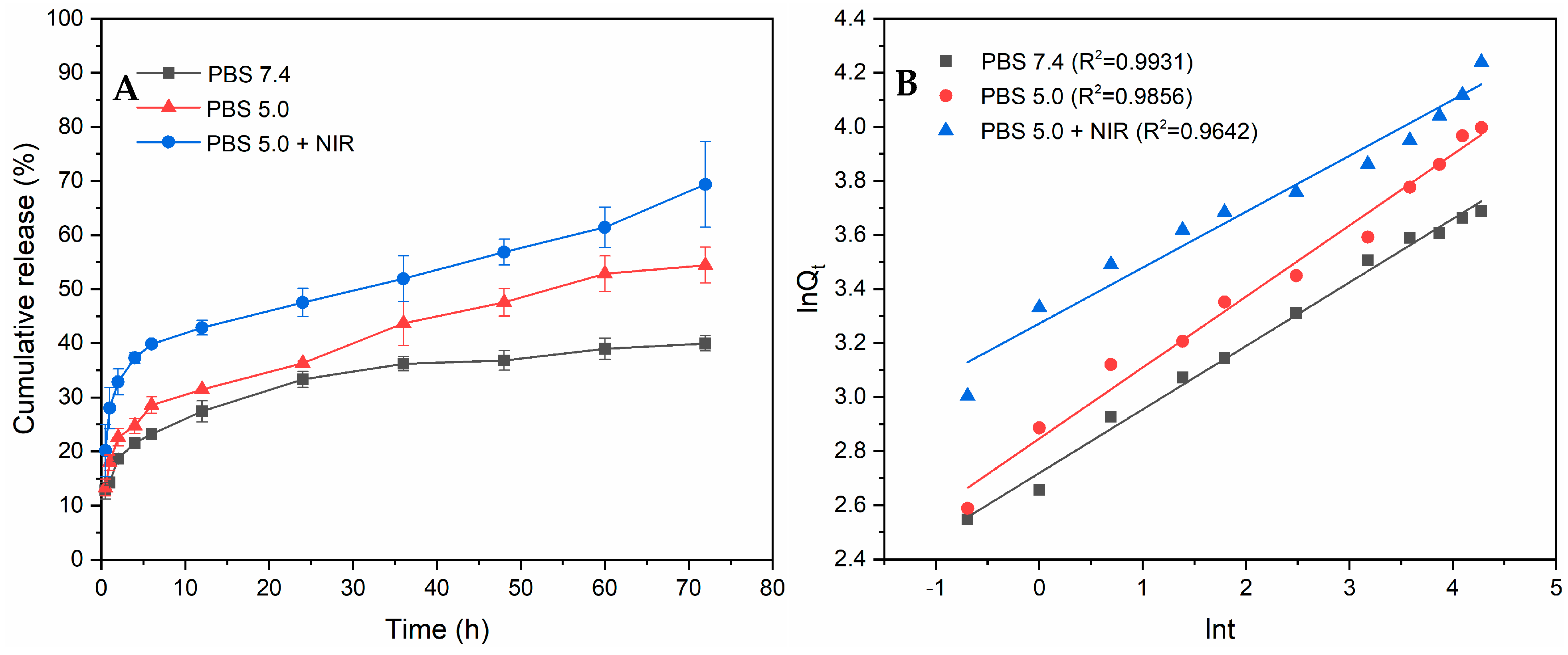
3.4. Drug Loading and Releasing Properties of PPy-RhB-PDA-CD-LA
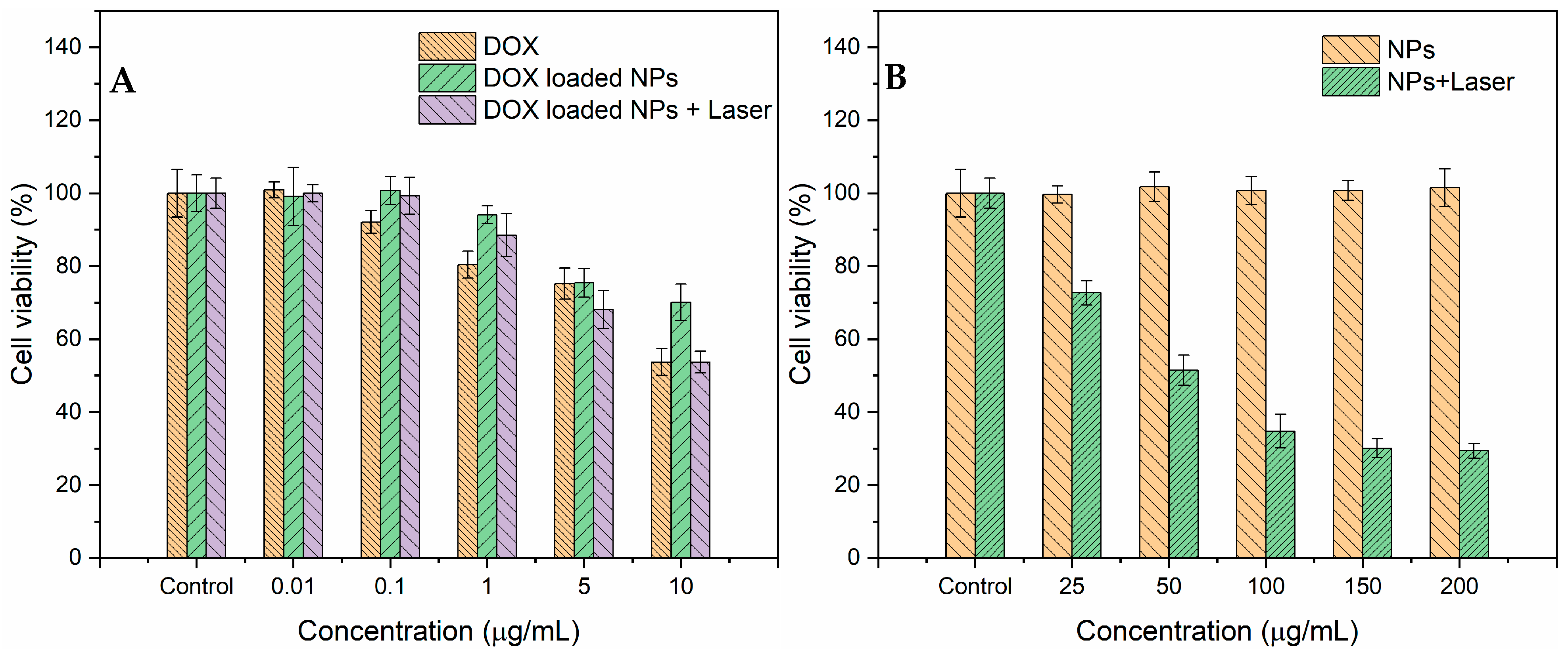
3.5. Cytotoxicity and Cell Photothermal Effect
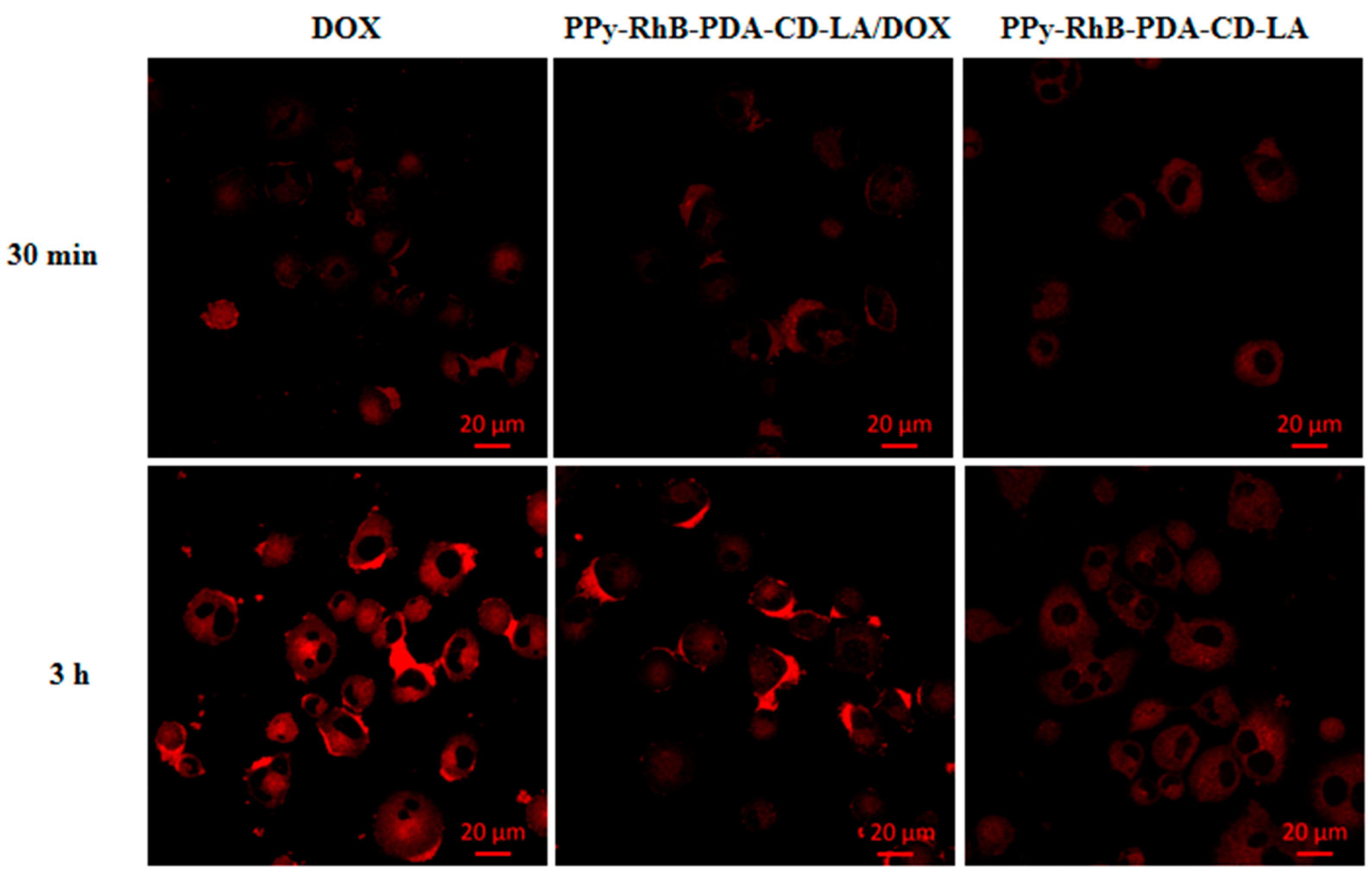
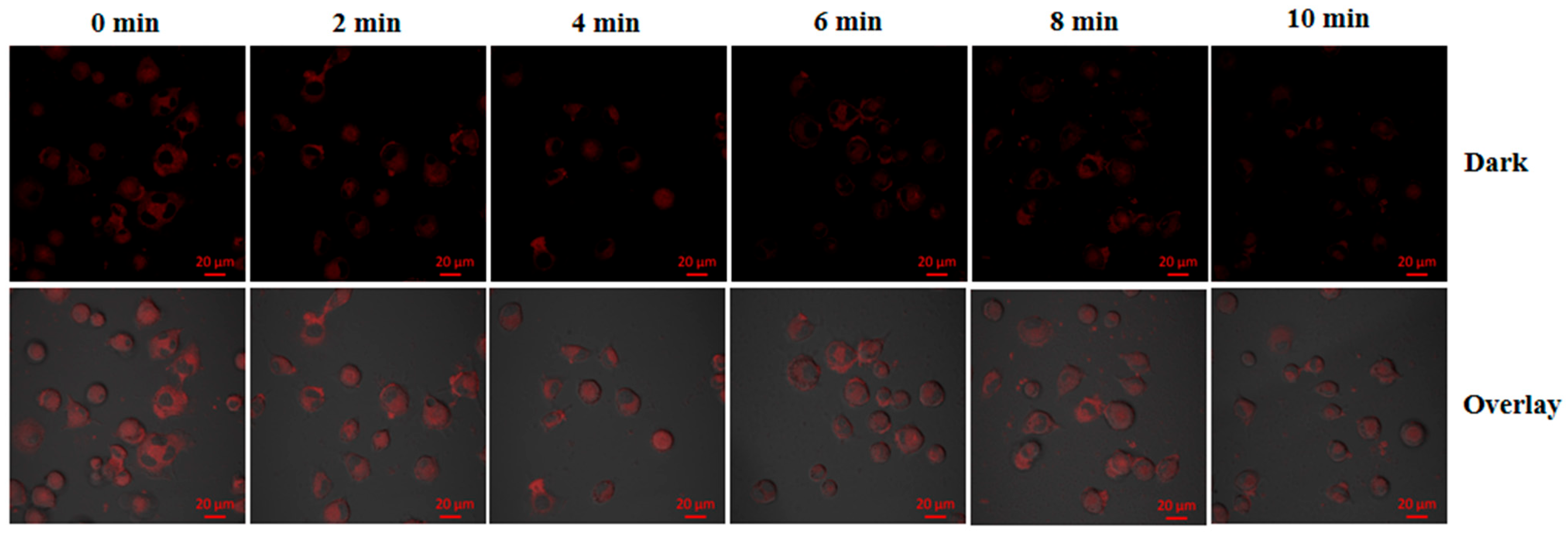
3.6. Cell Uptake and Intracellular Temperature Sensing
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Liu, B.; Zhou, H.; Tan, L.; Siu, K.T.H.; Guan, X.-Y. Exploring treatment options in cancer: Tumor treatment strategies. Signal Transduct. Target. Ther. 2024, 9, 175. [Google Scholar] [CrossRef]
- Anand, U.; Dey, A.; Chandel, A.K.S.; Sanyal, R.; Mishra, A.; Pandey, D.K.; De Falco, V.; Upadhyay, A.; Kandimalla, R.; Chaudhary, A.; et al. Cancer chemotherapy and beyond: Current status, drug candidates, associated risks and progress in targeted therapeutics. Genes Dis. 2023, 10, 1367–1401. [Google Scholar] [CrossRef]
- Yan, M.; Wu, S.; Wang, Y.; Liang, M.; Wang, M.; Hu, W.; Yu, G.; Mao, Z.; Huang, F.; Zhou, J. Recent Progress of Supramolecular Chemotherapy Based on Host–Guest Interactions. Adv. Mater. 2024, 36, 2304249. [Google Scholar] [CrossRef]
- Lee, H.; Kim, K.S.; Zare, I.; Bang, S.; Kang, H.S.; Moon, C.H.; Gwon, J.Y.; Seo, J.H.; Joo, H.; Cho, Y.; et al. Smart nanomaterials for multimodal theranostics and tissue regeneration. Coord. Chem. Rev. 2025, 541, 216801. [Google Scholar] [CrossRef]
- Xing, Y.; Li, Q.; Xue, B.; Mu, H.; Yang, Y.; Chen, L.; Guo, R.; Yu, S. Synthesis of magnetic carbon dot drug complex for targeting to nucleus in liver cancer therapy and fluorescence/magnetic resonance dual-modal imaging. Microchem. J. 2025, 213, 113719. [Google Scholar] [CrossRef]
- Liu, P.; Gan, N.; Li, Q.; Zeng, Z.; Zhong, J.; Wang, X.; Sun, Y.; Wu, D. Investigating the differences in β-Cyclodextrin derivatives/Hyperoside inclusion complexes: Dissolution properties, thermal stability, and antioxidant activity. Food Chem. 2025, 481, 144044. [Google Scholar] [CrossRef] [PubMed]
- Kali, G.; Haddadzadegan, S.; Bernkop-Schnürch, A. Cyclodextrins and derivatives in drug delivery: New developments, relevant clinical trials, and advanced products. Carbohydr. Polym. 2024, 324, 121500. [Google Scholar] [CrossRef]
- Devi, L.S.; Casadidio, C.; Gigliobianco, M.R.; Di Martino, P.; Censi, R. Multifunctionality of cyclodextrin-based polymeric nanoparticulate delivery systems for chemotherapeutics, combination therapy, and theranostics. Int. J. Pharm. 2024, 654, 123976. [Google Scholar] [CrossRef]
- Wen, Y.; Wang, J.; Zheng, W.; Zhu, J.; Song, X.; Chen, T.; Zhang, M.; Huang, Z.; Li, J. A supramolecular colloidal system based on folate-conjugated β-cyclodextrin polymer and indocyanine green for enhanced tumor-targeted cell imaging in 2D culture and 3D tumor spheroids. J. Colloid Interface Sci. 2024, 667, 259–268. [Google Scholar] [CrossRef]
- Man, S.; Liu, W.; Bi, J.; Bai, J.; Wu, Q.; Hu, B.; Hu, J.; Ma, L. Smart Mesoporous Silica Nanoparticles Loading Curcumin Inhibit Liver Cancer. J. Agric. Food Chem. 2024, 72, 25743–25754. [Google Scholar] [CrossRef]
- Hua, Z.; Zhang, X.; Xing, S.; Li, J.; Liang, D.; Chen, Y.; Abd El-Aty, A.M.; Zhu, B.-W.; Liu, D.; Tan, M. Design and preparation of multifunctional astaxanthin nanoparticles with good acid stability and hepatocyte-targeting ability for alcoholic liver injury alleviation. Mater. Today Nano 2024, 25, 100436. [Google Scholar] [CrossRef]
- Sun, S.; Zhang, L.; Fei, S.; Tan, M. Enhanced anti-aging effect of nicotinamide mononucleotide encapsulated with reactive oxygen species responsive nanoparticles with liver and mitochondrial targeting abilities. Chem. Eng. J. 2024, 495, 153372. [Google Scholar] [CrossRef]
- Sathuvan, M.; Min, S.; Narayanan, K.; Gaur, A.; Hong, H.; Vivek, R.; Ganapathy, A.; Cheong, K.-L.; Kang, H.; Thangam, R. β-Cyclodextrin-based materials for 3D printing, cancer therapy, tissue engineering, and wound healing. Chem. Eng. J. 2024, 500, 157272. [Google Scholar] [CrossRef]
- Hong, S.; Li, Z.; Li, C.; Dong, C.; Shuang, S. β-Cyclodextrin grafted polypyrrole magnetic nanocomposites toward the targeted delivery and controlled release of doxorubicin. Appl. Surf. Sci. 2018, 427, 1189–1198. [Google Scholar] [CrossRef]
- Celi, N.; Cai, J.; Sun, H.; Feng, L.; Zhang, D.; Gong, D. Biohybrid Flexible Sperm-like Microrobot for Targeted Chemo-Photothermal Therapy. ACS Appl. Mater. Interfaces 2024, 16, 24341–24350. [Google Scholar] [CrossRef]
- Gianfaldoni, S.; Tchernev, G.; Wollina, U.; Fioranelli, M.; Roccia, M.G.; Gianfaldoni, R.; Lotti, T. An Overview of Laser in Dermatology: The Past, the Present and … the Future (?). Open Access Maced. J. Med. Sci. 2017, 5, 526–530. [Google Scholar] [CrossRef] [PubMed]
- Shen, S.; Qiu, J.; Huo, D.; Xia, Y. Nanomaterial-Enabled Photothermal Heating and Its Use for Cancer Therapy via Localized Hyperthermia. Small 2024, 20, 2305426. [Google Scholar] [CrossRef]
- Ain, N.U.; Rehman, Z.; Ibrahim, T.K.; Kebaili, I.; Boukhris, I.; Al-Buriahi, M.S. Organic dyes-based photothermal ablation of cancer cells: A review. Dye. Pigment. 2025, 237, 112687. [Google Scholar] [CrossRef]
- Zhang, M.; Xu, Z.; Chen, J.; Ju, Z.; Ma, Y.; Niu, Z.; Xu, Z.; Zhang, T.; Shi, F. Recent advances on nanomaterials-based photothermal sensing systems. TrAC Trends Anal. Chem. 2024, 177, 117801. [Google Scholar] [CrossRef]
- Liu, G.; Wang, Z.; Sun, W.; Lin, X.; Wang, R.; Li, C.; Zong, L.; Fu, Z.; Liu, H.; Xu, S. Robust emission in near-infrared II of lanthanide nanoprobes conjugated with Au (LNPs-Au) for temperature sensing and controlled photothermal therapy. Chem. Eng. J. 2023, 452, 139504. [Google Scholar] [CrossRef]
- Peng, M.; Kaczmarek, A.M.; Van Hecke, K. Ratiometric Thermometers Based on Rhodamine B and Fluorescein Dye-Incorporated (Nano) Cyclodextrin Metal–Organic Frameworks. ACS Appl. Mater. Interfaces 2022, 14, 14367–14379. [Google Scholar] [CrossRef]
- Li, M.; Xuan, Y.; Zhang, W.; Zhang, S.; An, J. Polydopamine-containing nano-systems for cancer multi-mode diagnoses and therapies: A review. Int. J. Biol. Macromol. 2023, 247, 125826. [Google Scholar] [CrossRef]
- Wang, X.-H.; Chen, X.-Q.; Peng, H.-S.; Wei, X.-F.; Wang, X.-J.; Cheng, K.; Liu, Y.-A.; Yang, W. Facile synthesis of polypyrrole–rhodamine B nanoparticles for self-monitored photothermal therapy of cancer cells. J. Mater. Chem. B 2020, 8, 1033–1039. [Google Scholar] [CrossRef]
- Huang, N.; Zhang, S.; Yang, L.; Liu, M.; Li, H.; Zhang, Y.; Yao, S. Multifunctional Electrochemical Platforms Based on the Michael Addition/Schiff Base Reaction of Polydopamine Modified Reduced Graphene Oxide: Construction and Application. ACS Appl. Mater. Interfaces 2015, 7, 17935–17946. [Google Scholar] [CrossRef]
- Huang, L.; Wang, Y.; Ling, X.; Chaurasiya, B.; Yang, C.; Du, Y.; Tu, J.; Xiong, Y.; Sun, C. Efficient delivery of paclitaxel into ASGPR over-expressed cancer cells using reversibly stabilized multifunctional pullulan nanoparticles. Carbohydr. Polym. 2017, 159, 178–187. [Google Scholar] [CrossRef] [PubMed]
- Wei, Q.; Zhang, F.; Li, J.; Li, B.; Zhao, C. Oxidant-induced dopamine polymerization for multifunctional coatings. Polym. Chem. 2010, 1, 1430–1433. [Google Scholar] [CrossRef]
- Cho, S.; Kim, S.-H. Hydroxide ion-mediated synthesis of monodisperse dopamine–melanin nanospheres. J. Colloid Interface Sci. 2015, 458, 87–93. [Google Scholar] [CrossRef] [PubMed]
- Wu, M.; Wang, T.; Müller, L.; Müller, F.A. Adjustable synthesis of polydopamine nanospheres and their nucleation and growth. Colloids Surf. A Physicochem. Eng. Asp. 2020, 603, 125196. [Google Scholar] [CrossRef]
- Ball, V.; Frari, D.D.; Toniazzo, V.; Ruch, D. Kinetics of polydopamine film deposition as a function of pH and dopamine concentration: Insights in the polydopamine deposition mechanism. J. Colloid Interface Sci. 2012, 386, 366–372. [Google Scholar] [CrossRef] [PubMed]
- Danaei, M.; Dehghankhold, M.; Ataei, S.; Hasanzadeh Davarani, F.; Javanmard, R.; Dokhani, A.; Khorasani, S.; Mozafari, M.R. Impact of Particle Size and Polydispersity Index on the Clinical Applications of Lipidic Nanocarrier Systems. Pharmaceutics 2018, 10, 57. [Google Scholar] [CrossRef]
- Zha, Z.; Yue, X.; Ren, Q.; Dai, Z. Uniform polypyrrole nanoparticles with high photothermal conversion efficiency for photothermal ablation of cancer cells. Adv. Mater. (Deerfield Beach Fla.) 2013, 25, 777–782. [Google Scholar] [CrossRef]
- Wang, W.; Wang, J.; Yan, Y. Novel polyamide nanofiltration membrane PEI-(β-CD@g-C3N5)/TMC based on microfiltration substrate for efficient separation of lithium and magnesium. Desalination 2024, 592, 118136. [Google Scholar] [CrossRef]
- Prakash, A.; Yadav, S.; Saxena, P.S.; Srivastava, A. Development of folate-conjugated polypyrrole nanoparticles incorporated with nitrogen-doped carbon quantum dots for targeted bioimaging and photothermal therapy. Talanta 2024, 278, 126528. [Google Scholar] [CrossRef]
- Zhang, H.; Wu, Y.; Gan, Z.; Yang, Y.; Liu, Y.; Tang, P.; Wu, D. Accurate intracellular and in vivo temperature sensing based on CuInS2/ZnS QD micelles. J. Mater. Chem. B 2019, 7, 2835–2844. [Google Scholar] [CrossRef] [PubMed]
- Lin, Q.; Zhou, P.; Zeng, X.; Lin, M.; Gao, M.; Zhao, C.; Lin, C.; Lin, T.; Wu, X. Modulations of high-quality white emission and fluorescence temperature sensing in La/Dy/Eu tri-doped KNN transparent-ferroelectric ceramics. Ceram. Int. 2024, 51, 16719–16726. [Google Scholar] [CrossRef]
- Xie, N.; Huang, J.; Yang, X.; He, X.; Liu, J.; Huang, J.; Fang, H.; Wang, K. Scallop-Inspired DNA Nanomachine: A Ratiometric Nanothermometer for Intracellular Temperature Sensing. Anal. Chem. 2017, 89, 12115–12122. [Google Scholar] [CrossRef]
- Xu, Y.; Yang, Y.; Lin, S.; Xiao, L. Red-Emitting Carbon Nanodot-Based Wide-Range Responsive Nanothermometer for Intracellular Temperature Sensing. Anal. Chem. 2020, 92, 15632–15638. [Google Scholar] [CrossRef]
- Nayak, J.; Sood, A.; Kulharia, M.; Sahoo, S.K.; Kumar, R. Structural Distortion of β-Cyclodextrin Plays a Key Role in the pH-dependent Host–Guest Chemistry with Doxorubicin, Evident by the Electrochemical and Molecular Dynamics Approach. J. Chem. Inf. Model. 2023, 63, 2975–2982. [Google Scholar] [CrossRef]
- Wang, Y.; Wu, Y.; Li, K.; Shen, S.; Liu, Z.; Wu, D. Ultralong Circulating Lollipop-Like Nanoparticles Assembled with Gossypol, Doxorubicin, and Polydopamine via π–π Stacking for Synergistic Tumor Therapy. Adv. Funct. Mater. 2019, 29, 1805582. [Google Scholar] [CrossRef]
- Lv, L.; Cheng, H.; Wang, Z.; Miao, Z.; Zhang, F.; Chen, J.; Wang, G.; Tao, L.; Zhou, J.; Zhang, H.; et al. “Carrier–drug” layer-by-layer hybrid assembly of biocompatible polydopamine nanoparticles to amplify photo-chemotherapy. Nanoscale 2022, 14, 13740–13754. [Google Scholar] [CrossRef]
- Abdollahi, E.; Haddadi-Asl, V.; Ahmadi, H.; Shirjandi, M.; Khanipour, F. Investigation of Release Kinetics of DOX from Polydopamine Nanocapsules Prepared by Hard Template Method. Macromol. Mater. Eng. 2025, 310, 2400261. [Google Scholar] [CrossRef]
- Keawchaoon, L.; Yoksan, R. Preparation, characterization and in vitro release study of carvacrol-loaded chitosan nanoparticles. Colloids Surf. B Biointerfaces 2011, 84, 163–171. [Google Scholar] [CrossRef]
- Lakshani, N.; Wijerathne, H.S.; Sandaruwan, C.; Kottegoda, N.; Karunarathne, V. Release Kinetic Models and Release Mechanisms of Controlled-Release and Slow-Release Fertilizers. ACS Agric. Sci. Technol. 2023, 3, 939–956. [Google Scholar] [CrossRef]
- Li, J.; Li, X.; Liu, P. Doxorubicin-doxorubicin conjugate prodrug as drug self-delivery system for intracellular pH-triggered slow release. Colloids Surf. B Biointerfaces 2020, 185, 110608. [Google Scholar] [CrossRef]
- Liu, M.; Sun, Y.; Teh, D.B.L.; Zhang, Y.; Cao, D.; Mei, Q. Nanothermometry for cellular temperature monitoring and disease diagnostics. Interdiscip. Med. 2024, 2, e20230059. [Google Scholar] [CrossRef]
- Pun, S.H.; Bellocq, N.C.; Liu, A.; Jensen, G.; Machemer, T.; Quijano, E.; Schluep, T.; Wen, S.; Engler, H.; Heidel, J.; et al. Cyclodextrin-modified polyethylenimine polymers for gene delivery. Bioconjugate Chem. 2004, 15, 831–840. [Google Scholar] [CrossRef]
- Zhang, J.; Sun, H.; Ma, P.X. Host-guest interaction mediated polymeric assemblies: Multifunctional nanoparticles for drug and gene delivery. ACS Nano 2010, 4, 1049–1059. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Liu, G.; Liu, W.; Wang, Y.; Xu, M.; Wang, B. Turn-on fluorometric β-carotene assay based on competitive host-guest interaction between rhodamine 6G and β-carotene with a graphene oxide functionalized with a β-cyclodextrin-modified polyethyleneimine. Microchim. Acta 2016, 183, 1161–1168. [Google Scholar] [CrossRef]
- Hua, Z.; Zhang, X.; Chen, Y.; Liu, R.; Li, Y.; Li, J.; Liu, D.; Tan, M. A bifunctional hepatocyte-mitochondrion targeting nanosystem for effective astaxanthin delivery to the liver. Food Chem. 2023, 424, 136439. [Google Scholar] [CrossRef] [PubMed]
- Ping, Y.; Liu, C.; Zhang, Z.; Liu, K.L.; Chen, J.; Li, J. Chitosan-graft-(PEI-β-cyclodextrin) copolymers and their supramolecular PEGylation for DNA and siRNA delivery. Biomaterials 2011, 32, 8328–8341. [Google Scholar] [CrossRef]
- Liang, H.; Yang, K.; Yang, Y.; Hong, Z.; Li, S.; Chen, Q.; Li, J.; Song, X.; Yang, H. A Lanthanide Upconversion Nanothermometer for Precise Temperature Mapping on Immune Cell Membrane. Nano Lett. 2022, 22, 9045–9053. [Google Scholar] [CrossRef]
- Meng, L.; Jiang, S.; Song, M.; Yan, F.; Zhang, W.; Xu, B.; Tian, W. TICT-Based Near-Infrared Ratiometric Organic Fluorescent Thermometer for Intracellular Temperature Sensing. ACS Appl. Mater. Interfaces 2020, 12, 26842–26851. [Google Scholar] [CrossRef] [PubMed]
- Silva, S.F.V.; Figueiredo, G.; Pereira, R.F.P.; de Zea Bermudez, V.; Fu, L.; André, P.S.; Carneiro Neto, A.N.; Ferreira, R.A.S. Time-gated multi-dimensional luminescence thermometry via carbon dots for precise temperature mobile sensing. Nanoscale 2024, 16, 20532–20541. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Xiao, X.; Wei, Z.; Chen, Y. A Ratio Fluorescence Thermometer Based on Carbon Dots & Lanthanide Functionalized Metal-Organic Frameworks. Z. Anorg. Allg. Chem. 2022, 648, e202100323. [Google Scholar]
- Wang, Y.; Liang, S.; Mei, M.; Zhao, Q.; She, G.; Shi, W.; Mu, L. Sensitive and Stable Thermometer Based on the Long Fluorescence Lifetime of Au Nanoclusters for Mitochondria. Anal. Chem. 2021, 93, 15072–15079. [Google Scholar] [CrossRef]
- Sun, M.; Li, P.; Wang, M.; Liang, Y.; Yang, X.; Pang, S. Carbonized polymer dots-silver nanoclusters nanocomposite with dual-emission for property ratiometric fluorescence and visual detection of temperature. Diam. Relat. Mater. 2024, 141, 110559. [Google Scholar] [CrossRef]
- Savchuk, O.A.; Silvestre, O.F.; Adão, R.M.R.; Nieder, J.B. GFP fluorescence peak fraction analysis based nanothermometer for the assessment of exothermal mitochondria activity in live cells. Sci. Rep. 2019, 9, 7535. [Google Scholar] [CrossRef]
- Liu, Y.; Ai, K.; Liu, J.; Deng, M.; He, Y.; Lu, L. Dopamine-Melanin Colloidal Nanospheres: An Efficient Near-Infrared Photothermal Therapeutic Agent for In Vivo Cancer Therapy. Adv. Mater. 2013, 25, 1353–1359. [Google Scholar] [CrossRef]


| mPPy-RhB:mDA | 1:0 | 50:1 | 25:1 | 10:1 | 5:1 |
| Size (nm) | 129.6 | 133.2 | 131.7 | 133.4 | 131.9 |
| PDI | 0.096 | 0.109 | 0.101 | 0.123 | 0.087 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hong, S.; Jiao, Y.; Li, R.; Lei, P.; Dong, C.; Guo, S.; Shuang, S. β-Cyclodextrin-Grafted Polypyrrole–Rhodamine B Nanoplatforms for Drug Delivery and Image-Guided Photothermal Therapy In Vitro. Materials 2025, 18, 5313. https://doi.org/10.3390/ma18235313
Hong S, Jiao Y, Li R, Lei P, Dong C, Guo S, Shuang S. β-Cyclodextrin-Grafted Polypyrrole–Rhodamine B Nanoplatforms for Drug Delivery and Image-Guided Photothermal Therapy In Vitro. Materials. 2025; 18(23):5313. https://doi.org/10.3390/ma18235313
Chicago/Turabian StyleHong, Shasha, Yuan Jiao, Ruyu Li, Peng Lei, Chuan Dong, Shang Guo, and Shaomin Shuang. 2025. "β-Cyclodextrin-Grafted Polypyrrole–Rhodamine B Nanoplatforms for Drug Delivery and Image-Guided Photothermal Therapy In Vitro" Materials 18, no. 23: 5313. https://doi.org/10.3390/ma18235313
APA StyleHong, S., Jiao, Y., Li, R., Lei, P., Dong, C., Guo, S., & Shuang, S. (2025). β-Cyclodextrin-Grafted Polypyrrole–Rhodamine B Nanoplatforms for Drug Delivery and Image-Guided Photothermal Therapy In Vitro. Materials, 18(23), 5313. https://doi.org/10.3390/ma18235313